Abstract
Tianeptine (7-[([3-chloro-6,11]-dihydro-6-methyldibenzo[c,f][1,2]thiazepin-11-yl) amino] heptanoic acid S, S dioxide) is a tricyclic compound prescribed as an antidepressant in European countries, but is not currently approved for use in the United States. There are few published case reports of tianeptine intoxication. Presented is the first case of acute toxicity associated with the intravenous use of tianeptine. A 36-year-old male intentionally injected tianeptine powder intravenously to “help him see into the future”. He became unresponsive and a bystander called emergency medical services. Upon arrival to the Emergency Department, excessive constriction of the pupils, sedation, and a respiratory rate of 6 respirations per minute (rpm) were noted. Blood and urine were collected ~2 h post admission. The patient's serum ethanol concentration was 133 mg/dL. His toxicity was successfully reversed with two doses of naloxone 0.4 mg IV. He was started on a naloxone infusion at 0.2 mg/h and discharged 13 h after admittance awake, alert and oriented. The patient's urine sample screened negative for common drugs of abuse and total tricyclic antidepressants. A high performance liquid chromatography tandem mass spectrometry method was developed and validated to quantify tianeptine in urine. The calibration range was 1–100 ng/mL with linear regression correlation (r2) of 0.9996 or greater. The limit of quantitation was administratively set at 1 ng/mL. The bias of the assay was determined to be within ±20% of the target value for each quality control specimen. The intra-day and inter-day precision did not exceed 15% coefficient of variation for each quality control specimen. Matrix effects, absolute recovery, carryover and specificity were also evaluated. The patient's tianeptine urine concentration was determined to be 2 ng/mL.
Introduction
Tianeptine (7-[([3-chloro-6,11]-dihydro-6-methyldibenzo[c,f][1,2]thiazepin-11-yl) amino] heptanoic acid S, S dioxide) (Figure 1) is a tricyclic compound prescribed as an antidepressant in European countries, but is not currently approved for use in the United States (1). It is currently being sold on certain websites in the United States as a nootropic or smart drug/cognitive enhancer. Tianeptine has a similar anxiolytic efficacy profile to other tricyclic antidepressants (TCAs), but it has differing mechanisms of action. Instead of blocking the reuptake of serotonin or norepinephrine and thereby enhancing synaptic concentrations of these neurotransmitters, tianeptine's mechanism of action remains elusive (2). Originally thought to be a serotonin reuptake enhancer, newer studies suggest that tianeptine's possible dual activation of the mu and delta opioid receptors is the initial molecular event responsible for tianeptine's modulation of the glutamatergic system (2, 3). The activation of these receptors are also thought to be responsible for causing many of the known acute and chronic effects of this compound, including its antidepressant/anxiety actions (2, 3). Tianeptine also differs from most TCAs in that it is not primarily metabolized by the hepatic cytochrome p450 system, indicating less likelihood of drug–drug interactions (4). The major metabolic pathway is β-oxidation and the principal metabolites are propanoic acid sidechain (MC3, inactive metabolite) and pentanoic acid sidechain (MC5, active metabolite). Less than 3% of the dose is excreted unchanged in urine and MC5 half-life is 7.2 h. (4–6). Previously published methods for the detection of tianeptine use high performance liquid chromatography with UV or fluorescence detection, and one case used liquid chromatography with photodiode array mass spectrometry detection (1, 7, 8). There are limited published case reports of tianeptine intoxication. A case study is presented of an acute toxicity associated with the intravenous use and the identification and quantitation of tianeptine in urine using a high performance liquid chromatography tandem mass spectrometry (HPLC–MS-MS) method.
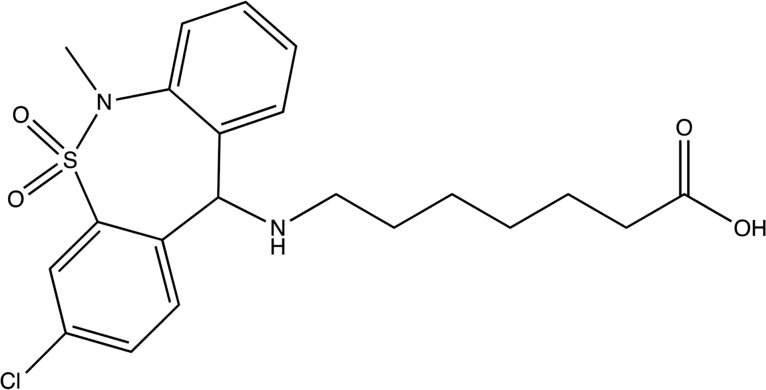
Figure 1.
Structure of tianeptine.
Case History
A 36-year-old male intentionally injected tianeptine powder dissolved in water intravenously to “help him see into the future”. He became unresponsive and a bystander called emergency medical services. He was administered naloxone 1 mg IV due to suspected heroin use by medical personal and was transported to the Emergency Department (ED) around midnight. Upon arrival to the ED, excessive constriction of the pupils, sedation and a respiratory rate of 6 respirations per minute (rpm) were noted. His toxicity was successfully reversed with two doses of naloxone 0.4 mg IV and he was placed on a naloxone infusion at 0.2 mg/h. Blood and urine were collected ~2 h post admission.
Initial vital signs included an electrocardiogram that gave a QRS 104 ms with QT of 461 ms. Initial serum laboratory results were sodium 140 mEq/L, potassium 3.4 mEq/L, bicarbonate 23 mg/dL, chloride 101 mEq/L, glucose 190 mg/dL, anion gap 16, AST 51 IU/L, ALT 69 IU/L. The patient's serum ethanol concentration was also determined to be 133 mg/dL. The patient remained stable on the naloxone infusion, and was titrated off and discontinued 9 h after his arrival. He was observed for another 4 h, and at the time of discharge, he was alert, awake and oriented. Vital signs were blood pressure 94/43 mm Hg, heart rate 72 bpm, respiratory rate 12 rpm, pulse oximetry 98% on room air and temperature 36.5°C. A urine specimen was sent to the Virginia Commonwealth University Health System FIRM laboratory for analysis of drugs of abuse and tianeptine. Serum was not forwarded to the FIRM laboratory for additional testing. The patient's urine sample screened negative for common drugs of abuse and total TCAs. The urine creatinine was determined to be 0.11 mg/dL.
Experimental
Reagents
The primary reference materials for tianeptine and its internal standard (ISTD) protriptyline were purchased from Cayman Chemical Company (Ann Arbor, MI) and Cerilliant Corporation (Round Rock, TX), respectively. Protriptyline was chosen as the ISTD as it is used in the FIRM laboratory's TCA assay and in a published method for the detection of tianeptine in post-mortem samples (1). Ammonium formate, acetonitrile, methanol and water were purchased from Fisher Scientific (Hanover Park, IL). Clean Screen FAStTM solid phase extraction (SPE) columns were purchased from United Chemical Technologies (Bristol, PA). In-house ultrapure air was supplied to the UCT Positive Pressure Manifold. In-house certified drug-free urine provided the matrix for all prepared calibrators, and quality control (QC) specimens.
Sample preparation
Appropriate volumes of the tianeptine working solution prepared in methanol were added to urine to obtain a seven point calibration curve with a range of 1–100 ng/mL. The following QC urine specimens were prepared and analyzed with the test specimen: limit of quantification quality control (LOQ), target concentration of 1 ng/mL; low control (LQC), target concentration of 3 ng/mL; medium control (MQC), target concentration of 30 ng/mL; and a high control (HQC), target concentration of 75 ng/mL. A drug-free control (negative control) that did not contain tianeptine but did contain the ISTD, protriptyline, and a double negative control that did not contain tianeptine or ISTD were also run with each sample batch. All QC samples were stored at −20°C until analysis.
Specimen extraction
Tianeptine was isolated from urine by use of Clean Screen FAStTM SPE columns. Briefly, to 200 μL aliquots of calibrators, QC specimens or patient samples, 20 μL of 100 ng/mL working solution in methanol of the ISTD for a total of 10 ng was added followed by the addition of 200 μL of 50:50 acetonitrile:distilled water. Samples were mixed using a vortex mixer. The samples were transferred to Clean Screen FAStTM SPE columns and rapidly aspirated into auto-sampler vials with air in an UCT Positive Pressure Manifold. Vials were then capped and placed on the HPLC–MS-MS for analysis.
Instrumental analysis
The HPLC–MS-MS analysis of tianeptine was performed on a Waters Xevo TQD LC–MS-MS attached to an Acquity HPLC® System controlled by MassLynx software (Milford, MA). Chromatographic separation (Figure 2) was performed on an Allure Biphenyl 5 μm 100 × 2.5 mm2 column (Restek, Bellefonte, PA). The column temperature was 40°C and 10 µL was the sample injection volume. The mobile phase consisted of A: water and 20 mM ammonium formate and B: methanol and 20 mM ammonium formate. The following gradient was used: 0.00–1.5 min at 95% A and 5% B, 1.5–3 min at 60% A and 40% B, 3–3.5 min at 100% B and then return to 95% A and 5% B at 3.6 min. The flow rate was 0.6 mL/min. The source temperature was set at 150°C with a capillary voltage of 3.00 kV. The desolvation temperature was set at 600°C with a gas flow rate of 650 L/h. The cone flow rate was set at 100 L/h. The acquisition mode used was multiple reaction monitoring. The retention times (min), cone voltage (V), transition ions (m/z) and corresponding collection energies (eV) for the compounds can be found in Table I. The total run time for the analytical method was 4.0 min.
Figure 2.
Chromatographic separation of the MQC (30 ng/mL tianeptine and 10 ng/mL protriptyline, the ISTD).
Table I.
HPLC–MS-MS acquisition parameters
| Analyte | RT (min) | Cone (V) | Transition ions (m/z) | CE (eV) |
|---|---|---|---|---|
| Tianetpine | 2.95 | 40 | 437 > 292 | 15 |
| 437 > 228 | 39 | |||
| Protriptyline | 2.86 | 28 | 264 > 191 | 43 |
| 264 > 154 | 19 |
The analytical method was evaluated over five analytical runs on 5 separate days. For each day the linear regression correlation coefficients (r2) for the tianeptine calibration curve were 0.9996 or better. The LOQ and the lower limit of detection (LOD) were administratively set at a concentration of 1 ng/mL. The LOD had a response at least ten times the signal to noise ratio of the response to drug-free pooled urine. QC specimens were extracted and analyzed in triplicate for each analytical run and were used to determine the bias and precision. The bias of the assay for the LOQ (1 ng/mL) ranged from 4 to 13%, at the LQC (3 ng/mL) from −15 to 9%, at the MQC (30 ng/mL) from −15 to 10%, and at the HQC (75 ng/mL) from 2 to −15%. The intra-day precision for the four QC specimens ranged between −2 and 9% with a coefficient of variation of <9%. Sample carryover was evaluated using two different procedures. For the carryover study, the high calibrator containing 100 ng/mL was followed by a drug-free urine injection. Tianeptine was not detected in the injected drug-free urine sample. An additional procedure to evaluate possible carryover during analysis was performed by injecting an extracted HQC (75 ng/mL) immediately followed by injecting an extracted LQC (3 ng/mL). Lack of carryover was confirmed as the LQC did not demonstrate a significant quantified bias.
Results and Discussion
To the authors’ knowledge, this is the first reported case of intravenous use of tianeptine by intentional injection. There are several previously reported cases (1, 8–15) that present the oral abuse of tianeptine. The patient's tianeptine urine concentration was determined to be 2 ng/mL. Urine concentrations of ~2,000 and 3,200 ng/mL have been reported in fatal tianeptine intoxications (1, 12). Both cases involved the oral ingestion of multiple tablets of tianeptine. The authors are unaware of reported normal therapeutic range either for steady state or a single therapeutic dose in blood or in urine. The patient's ECG was normal, but complications can occur in TCA intoxications with normal ECG readings (9). Initial serum laboratory results were within normal ranges with the exception of an elevated ALT concentration of 69 IU/L, with the normal range being 7–55 IU/L. The initial suspected overdose was attributed to heroin due to the presence of intravenous paraphernalia, and the excessive constriction of the pupils, the sedated state of the patient, and his low respiratory rate. It has been suggested that tianeptine may affect the opioid receptors and produce symptoms similar to an opioid overdose. Naloxone and other opioid antagonists have been suggested as treatment for tianeptine poisoning (9). The relatively low tianeptine urine concentration may be due to the extensive metabolism of tianeptine, <3% of the dose is excreted unchanged in urine (4–6), the acute exposure ~2 h prior to collections as well as the low urine creatinine concentration of 0.11 mg/dL (normal range: 20–400 mg/dL).
Conclusion
Tianeptine is currently being marketed as a cognitive enhancer in the United States, and there are limited published case reports of tianeptine intoxication. The presented case is the first known case of intravenous use of tianeptine. The administration of naloxone appears to have been effective in blocking the toxic effects of tianeptine. The developed HPLC–MS-MS method was robust and reliable for the detection and quantification of tianeptine in the patient's urine sample. The low tianeptine urine concentration (2 ng/mL) in the patient's sample is most likely due to the extensive metabolism of tianeptine.
Funding
This project was supported in part by the National Institute on Health (NIH) Center for Drug Abuse grant P30DA033934.
References
- 1. Proenca P., Teixiera H., Pinheiro J., Monsanto P., Vieira D. (2007) Fatal Intoxication with tianeptine (Stablon). Forensic Science International, 170, 200–203. [DOI] [PubMed] [Google Scholar]
- 2. Gassaway M.M., Rives M.L., Kruegel A.C., Javitch J.A., Sames D. (2014) The atypical antidepressant and neurorestorative agent tianeptine is a u-opioid receptor agonist. Translational Psychiatry, 4, e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McEwen B.S., Chattarji S., Diamond D.M., Jay T.M., Reagan L.P., Svenningsson P., et al. (2010) The neurobiological properties of Tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Molecular Psychiatry, 15, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grislain L., Gele P., Bertrand M., Luijten W., Bromet N., Salvadori C., et al. (1990) The metabolic pathways of tianpetine, a new antidepressant, in healthy volunteers. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 18, 804–808. [PubMed] [Google Scholar]
- 5. Wagstaff A.J., Ormrod D., Spencer C.M. (2001) Tianeptine: a review of its use in depressive disorders. CNS Drugs, 15, 231–259. [DOI] [PubMed] [Google Scholar]
- 6. Wilde M.I., Benfield P. (1995) Tianeptine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depression and coexisting anxiety and depression. Drugs, 49, 411–439. [DOI] [PubMed] [Google Scholar]
- 7. Gaulier J.M., Marquet E., Lacassie R., Desroches G., Lachatre G. (2000) High-performance liquid chromatographic determination of tianeptine in plasma to pharmacokinetic studies. Journal of Chromatography B, Biomedical Sciences and Applications, 748, 407–414. [DOI] [PubMed] [Google Scholar]
- 8. Ulu S.T. (2006) Determination of tianeptine in human plasma using high-performance liquid chromatography with fluorescence detection. Journal of Chromatography, 834, 62–67. [DOI] [PubMed] [Google Scholar]
- 9. Ari M., Oktar S., Duru M. (2010) Amitriptyline and tianeptine poisoning treated by naloxone. Human and Experimental Toxicology, 29, 793–795. [DOI] [PubMed] [Google Scholar]
- 10. Guillem E., Lepine J.P. (2003) Does addiction to antidepressants exist? About a case of one addiction to tianeptine. L'Encephale, 29, 456–459. [PubMed] [Google Scholar]
- 11. Kisa C., Bulbul D., Aydemir C., Goka E. (2007) Is it possible to be dependent to Tianeptine, an antidepressant? A case report. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 31, 776–778. [DOI] [PubMed] [Google Scholar]
- 12. Lechowicz W. (2000) Complex intoxication by tramadol and tianeptine: a case report. Z Zagadnieñ Nauk Sdowych, 44, 130–139. [Google Scholar]
- 13. Leterme L., Singlan Y., Auclair V., Le boisselier R., Firma V. (2003) Misuse of tianeptine: five cases of abuse. Annals of Internal Medicine, 154, 58–63. [PubMed] [Google Scholar]
- 14. Saatcioglu O., Erim R., Cakmak D. (2006) A case of tianeptine abuse. Turkish Journal of Psychiatry, 17, 72–75. [PubMed] [Google Scholar]
- 15. Vandel P., Regina W., Bonin B., Sechter D., Bizourad P. (1999) Abuse of tianeptine. A case report. L'Encephale, 25, 672–673. [PubMed] [Google Scholar]