Abstract
Rett syndrome (RTT) is an X-linked neurodevelopmental disorder caused by mutations in the gene encoding methyl CpG binding protein 2 (MeCP2) that occur sporadically in 1:10,000 female births. RTT is characterized by a period of largely normal development followed by regression in language and motor skills at 6–18 months of age. Mecp2 mutant mice recapitulate many of the clinical features of RTT, but the majority of behavioral assessments have been conducted in male Mecp2 hemizygous null mice as offspring of heterozygous dams. Given that RTT patients are predominantly female, we conducted a systematic analysis of developmental milestones, sensory abilities, and motor deficits, following the longitudinal decline of function from early postnatal to adult ages in female Mecp2 heterozygotes of the conventional Bird line (Mecp2tm1.1bird-/+), as compared to their female wildtype littermate controls. Further, we assessed the impact of postnatal maternal environment on developmental milestones and behavioral phenotypes. Cross-fostering to CD1 dams accelerated several developmental milestones independent of genotype, and induced earlier onset of weight gain in adult female Mecp2tm1.1bird-/+ mice. Cross-fostering improved the sensitivity of a number of motor behaviors that resulted in observable deficits in Mecp2tm1.1bird-/+ mice at much earlier (6–7 weeks) ages than were previously reported (6–9 months). Our findings indicate that female Mecp2tm1.1bird-/+ mice recapitulate many of the motor aspects of RTT syndrome earlier than previously appreciated. In addition, rearing conditions may impact the phenotypic severity and improve the ability to detect genotype differences in female Mecp2 mutant mice.
Introduction
Mutations in MECP2 have been associated with several neurodevelopmental syndromes including classic and variant forms of Rett syndrome (RTT) in females and severe neonatal encephalopathy and intellectual disability in males (1). Cases of classical RTT are characterized by a period of relatively normal early postnatal development for the first 6 to 18 months of life. This is followed by the progressive loss of purposeful hand use and speech along with the development of stereotypical hand movements and gait abnormalities. Additional abnormalities that are observed in the majority of cases include seizures, intellectual disability, gastrointestinal disturbances, low bone density, and decreased muscle tone (1–3).
MeCP2-related syndromes are influenced by the type and location of mutation in MECP2, X chromosome inactivation in females, and additional genetic modifying factors (4–6). Sporadic mutations in MECP2 account for up to 95% of classic Rett syndrome cases (4,7,8). However, even within classic RTT, a range of MECP2 mutations have been associated with varying clinical symptoms and degree of severity (4–6). MECP2 is located on the X chromosome and consequently is subject to X chromosome inactivation in females. X chromosome inactivation occurs on a cell-to-cell basis and while random, can show degrees of skewing that alter the ratio of maternal to paternal expression. Most cases of the classic RTT syndrome show random X inactivation patterns, but cases of skewing that impact phenotype severity have also been documented (5,6,9). However, the pattern of inactivation in peripheral lymphocytes does not always explain the clinical discordance between identical mutations nor correlate with disease severity (9), indicating a role for additional genetic and/or environmental modifiers.
A variety of mouse models have been utilized to examine the role of MeCP2 in brain development and phenotypes relevant to RTT (10,11,12). Genetic manipulations in mice include whole exon deletions (13,14), specific DNA binding domain deletions (15), and a variety of knock-in point and truncation mutations that model human mutations (16–21). RTT mouse models share phenotypic development of abnormal breathing, gait abnormalities, tremors, EEG abnormalities, and in some cases cognitive deficits, social abnormalities, and changes in anxiety behaviors (10). Until recently, the majority of behavioral characterization in RTT mouse models was conducted in males as they show an early-onset phenotype that recapitulates many aspects of RTT. However, the rapid, early progression of symptoms and early death (between 5 and 12 weeks of age) (13,14) fails to capture the delayed onset of symptoms and the mosaic nature of MECP2 expression that occurs in RTT females. Early work suggested that symptoms were delayed until four to six months of age in heterozygous females on a standard C57BL/6J background (13,14). More recent work in female heterozygous mouse models has revealed mixed findings on the earliest age of phenotypes, depending on backgrounds strain and mutation type (11,15,22–24). Comprehensive assessment of female RTT mouse models is critical for translation of findings to RTT in human females. Consequently, we evaluated a wide range of behavioral measures across development to phenotype Mecp2tm1.1bird-/+ female mice on a standard C57BL/6J background.
As Mecp2tm1.1bird-/y males die before reaching sexual maturity, heterozygous females are mated to wildtype males to maintain a colony and obtain RTT model mice. This breeding scheme results in mutant female dams that produce smaller litters and often neglect and cannibalize their offspring (24,25). To counteract these problems, researchers have used a variety of different genetic backgrounds including CD1, 129/C57BL6, F1 hybrids of FVB/Nx129S6/SvEv and C57BL/6x129S6/SvEv (11,14,24). While these approaches may improve litter size and genetic robustness, the potential confounding effects of maternal care derived from mutant females and the variations in background strain could impact a number of downstream behavioral and molecular assays ascribed to Mecp2 genotype. Particularly, the early postnatal environment can dramatically shape a broad range of behavioral traits in the offspring including those related to anxiety, maternal care, social interactions and cognition (26–32).
Cross-fostering has previously been utilized to both examine and minimize the impact of maternal environment on offspring phenotype (33–37). The strain-dependent variation in maternal behavior can alter neuroendocrine (38,39) and anxiety-related behaviors (39,40) in wildtype offspring, or modulate the phenotype of serotonin receptor mutant animals (40,41). Maternal phenotypes contribute a combination of environmental factors relevant from in utero to weaning that can influence phenotypes of offspring into adulthood. For example, maternal genotype can alter both the prenatal (shown by embryo transfer) and postnatal environment, leading to behavioral alterations in both the infant and adult (5-HT1A or 1B) receptor knockout mice (42,43). Oxytocin null male mice show increased aggression behaviors, a phenotype that was more severe for offspring from oxytocin null homozygous than heterozygous dams (44), suggesting a compounding effect of offspring genotype and maternal environment. Cross-fostering can potentially mitigate a variety of postnatal maternal environmental factors that may be deficient in Mecp2tm1.1bird-/+ dams including lactation, milk quality, pup retrieval, nursing behavior, or microbiota. However, altering postnatal maternal environment by cross-fostering may produce significant alterations in the behavioral and physiological phenotype of the offspring (45).
In order to rigorously define the phenotypes of Mecp2tm1.1bird-/+ female mice (Mecp2-/+) independent of maternal environmental effects, we designed this study to compare Mecp2 mutant mice and their wildtype littermates who were reared by their own Mecp2 mutant mothers, versus Mecp2 mutant mice and their wildtype littermates who were cross-fostered and reared by CD1 foster mothers, on a battery of developmental milestones and behavioral assays. CD1 dams were chosen for their high quality of maternal care (35), and previous work showing inter and intra strain cross fostering did not alter strain specific differences in maternal care (33,35). A limited behavioral examination was conducted on the male offspring for comparison. The two rearing conditions, i.e. cross-fostered versus biological dam, allows for the separate examination of the impact of maternal care, genotype and any genotype by early life environment interactions. Our findings indicate that female Mecp2-/+ mice develop impairments in motor function earlier than previously demonstrated. Some of these impairments were more significant in cross-fostered animals, due to enhanced performance in wildtype littermates. Fostering did not impact male Mecp2-/y survival but did slightly delay the onset of hind limb clasping behavior in Mecp2 mutants of both sexes. Overall, we propose that cross-fostering with non-mutant dams such as CD1 may be an advantageous approach for separating environmental impacts of maternal care from true Mecp2 genotype effects in multiple RTT mouse models.
Results
Cross-fostering does not alter mutant pup survival
To directly investigate the impact of maternal genotype on RTT mouse model phenotypes, we compared Mecp2tm1.1bird-/+ offspring on a C57BL/6J background that were allowed to remain with their mutant birth mother to offspring that were born to Mecp2 dams but cross-fostered to CD1 dams within 48 h of birth. CD1 mice were chosen as foster mothers based on their increased maternal care and decreased frequency of cannibalization (24). A total of 113 pups were born from 14 Mecp2tm1.1bird-/+ dams. Within the first 24 h after birth, while all pups were still with their birth mother, 19 pups were cannibalized including one entire litter. A total of 53 pups from eight litters were successfully cross-fostered to CD1 dams and no additional pups were lost after fostering. For the biological dam raised cohort, a total of 41 pups from five litters were used and three additional pups were cannibalized by PND 6, for a final count of 39 biological dam raised pups for developmental milestone analysis. Litter size ranged from 3 to 12 pups (M = 6.6 pups per litter) for all fostered animals and from 7 to 10 pups (M = 7.8 pups per litter) for biological dam raised litters (t(10) = -0.990, P = 0.350). For fostered litters there was the expected ratio of genotypes (X2(3) = 3.679, P = 0.298) for a total of 15 Mecp2+/+, 11 Mecp2-/+, 9 Mecp2+/y, and 18 Mecp2-/y pups. Similarly, for biological dam raised litters there were the expected genotype ratios (X2(3) = 2.744, P = 0.433) for a total of 13 Mecp2+/+, 9 Mecp2-/+, 11 Mecp2+/y, and 6 Mecp2-/y pups. When considering both fostered and biological dam reared litters, there was no difference in the representation of genotypes between the two experimental conditions (X2(3) = 4.517, P = 0.211). Together, these results demonstrate that fostering did not specifically alter the distribution of sex or genotype.
Developmental milestones are accelerated by fostering independent of genotype
To test the hypothesis that early developmental milestones may be affected by both genotype and fostering, all pups were assessed for developmental milestones on postnatal days (PND) 6, 8, 10, 12, 14, 16, 18 and 20. All statistical analyses included tests for rearing condition, genotype, developmental day, and litter size effects (Fig. 1). Measures of physical development were significantly accelerated by cross-fostering, including body weight and body length. The time to first pinnae detachment, eye opening, and fur development also occurred earlier in most of the fostered compared to biological dam raised conditions (Fig. 1). Several measures of sensory development and motor reflex (cliff avoidance, auditory startle, grasping, bar hold, horizontal screen score) also differed between fostered compared to biological dam raised mice within individual genotypes across time points. Together, these results suggest a general acceleration in development in pups reared by CD1 mothers as compared to pups reared by their own Mecp2 dams, independent of genotype.
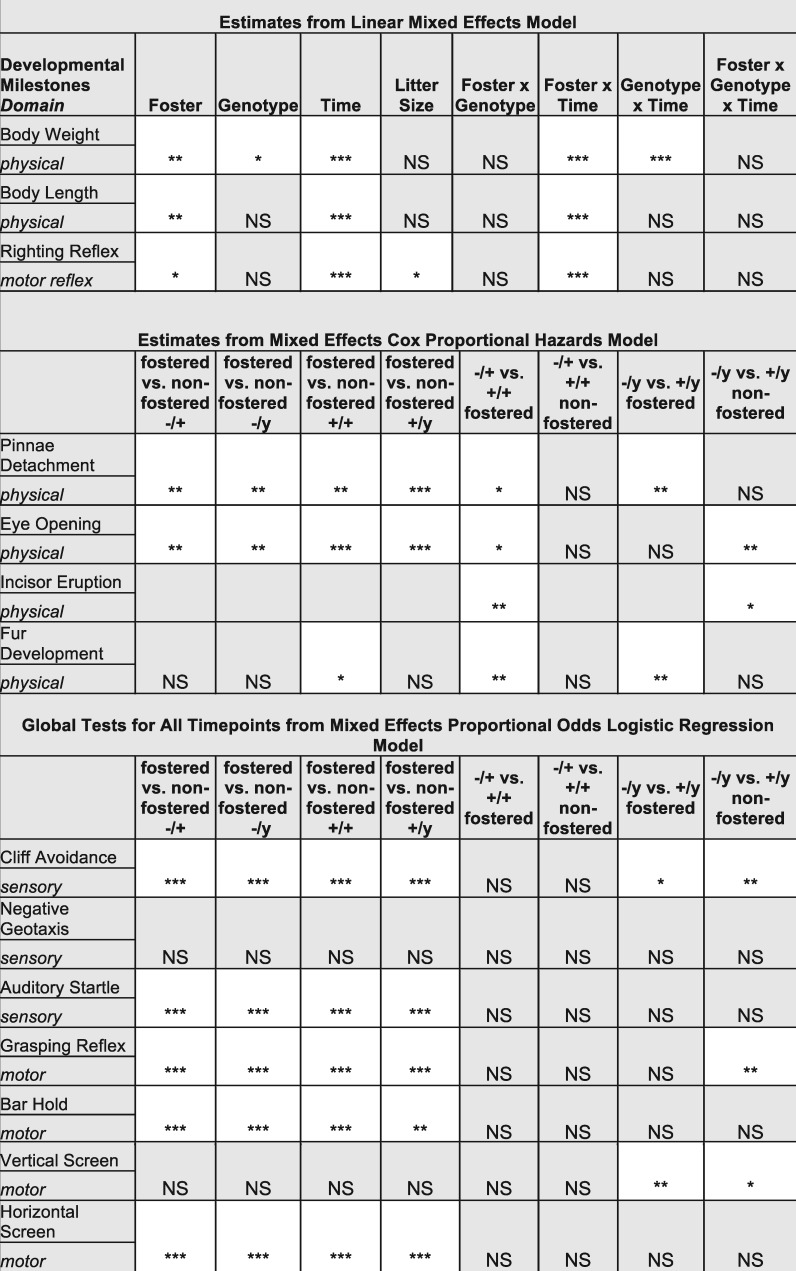
Figure 1.
Summary of significant results for developmental milestone tests, performed from postnatal days (PND) 6–20. Developmental milestones are shown by domain (physical, motor, sensory, in italics). Statistically significant comparisons are highlighted in white, with *P < 0.05, **P < 0.01, and ***P < 0.001 using the statistical models described for each subsection. NS, not significant. Fostered animals: 15 Mecp2+/+, 11 Mecp2-/+, 9 Mecp2+/y, and 18 Mecp2-/y; Non-fostered animals: 13 Mecp2+/+, 9 Mecp2-/+, 11 Mecp2+/y, and 6 Mecp2-/y.
Fostering also altered the impact of genotype for both males and females on several measures, one of the most pronounced being body weight (Fig. 2A and B). By PND 20 in females biological dam raised Mecp2-/+ mice were significantly smaller than biological dam raised Mecp2+/+ mice (P = 0.015). At this time point, there was not a significant difference in body weight between fostered Mecp2-/+ and Mecp2+/+ mice (P = 0.091), indicating that the female body weight phenotype may be more sensitive to early life maternal care differences. By PND20 Mecp2-/y weighed less than Mecp2+/y mice for both fostered (P < 0.001) and biological dam raised (P < 0.001) conditions. This indicates fostering was not able to correct the weight loss observed in male mutants. Fostering increased body length, but did not interact with genotype (Supplementary Material, Fig. S1A and B).
Figure 2.
Cross-fostering accelerated developmental milestones in both genotypes. (A). Body weight in grams across postnatal days (PND) 6 to 20 for fostered and biological dam female wildtype and mutant mice. Overall body weight differences were observed across timepoints (grey *P < 0.05 Benjamini-Hochberg corrected t-tests foster vs biological dam). Body weights differed between biological dam Mecp2-/+ and Mecp2+/+ mice on PND 20 (black *P < 0.05 estimates from linear mixed effects model). (B) Body weight in grams across PDN 6 to 20 for fostered and biological dam male wildtype and mutant mice. Overall body weight differences were observed across timepoints (grey *P < 0.05 Benjamini-Hochberg corrected t-tests foster vs biological dam). Body weights differed between biological dam Mecp2-/y and Mecp2+/y mice on PND 20 (black *P < 0.05 estimates from linear mixed effects model). (C) Rating score from 0 (none) to 3 (fully present) for fostered and biological dam female wildtype and mutant mice from PND 6–20. Only fostered mutants showed a significant delay in pinnae detachment (*P < 0.05 Cox Proportional Hazard Model, time to first development). (D) Rating score for fostered and biological dam male wildtype and mutant mice from PND 6–20. Only fostered mutants showed a significant delay in pinnae detachment (black *P < 0.05 Cox Proportional Hazard Model, time to first development). Fostered animals: 15 Mecp2+/+, 11 Mecp2-/+, 9 Mecp2+/y, and 18 Mecp2-/y; Non-fostered animals: 13 Mecp2+/+, 9 Mecp2-/+, 11 Mecp2+/y, and 6 Mecp2-/y.
Importantly, fostering resulted in a slight delay in the mutants or an acceleration of the wildtypes for the time to first occurrence of a number of physical milestones including the time to first pinnae detachment (Fig. 2C and D), eye opening, and incisor eruption (Fig. 1, Supplementary Material, Fig. S1C and D). Specifically, fostered Mecp2-/y mice showed a delay in time to pinnae detachment (Figs 1 and 2D) and fur development compared to fostered Mecp2+/y mice (Fig. 1). For sensory and motor developmental milestones there were no significant genotype effects for either fostering condition in females (Fig. 1). In males, both fostered and biological dam raised Mecp2-/y mice differed significantly from Mecp2+/y mice on cliff avoidance and vertical screen tests (Supplementary Material, Fig. S1E and F) consistent with previous observations of early developmental abnormalities in male RTT mouse models (22) (Fig. 1). Together, these results suggest that fostering accelerated physical milestone development without globally altering sensory or motor development. In several physical measures better maternal care and nutrition may allow for more normal development in wildtype animals, thereby increasing sensitivity of the tests to detect developmental delays in mutant animals.
Fostering does not alter the Mecp2 mutant male death phenotype
Mecp2 tm1.1bird-/y males have been reported to die between 5 and 10 weeks of age (11). To determine the potential impact of fostering on survival, a Cox proportional hazards model was used to compare the hazard of death by genotype and fostering status. As expected, Mecp2-/y males showed a significantly higher death rate than Mecp2+/y males, both for cross-fostered (Hazard Ratio = 42.7, P = 0.013) and biological dam raised (Hazard Ratio = 48.3, P = 0.013) litters. No significant difference in death was seen by fostering status in Mecp2-/y males (Hazard Ratio = 0.35, P = 0.222) or Mecp2+/y males (Hazard Ratio = 0.39, P = 0.682) indicating that fostering failed to significantly alter the death rates in the male mutant mice (Supplementary Material, Fig. S2).
Body weight and hind limb clasping are significantly impacted by fostering
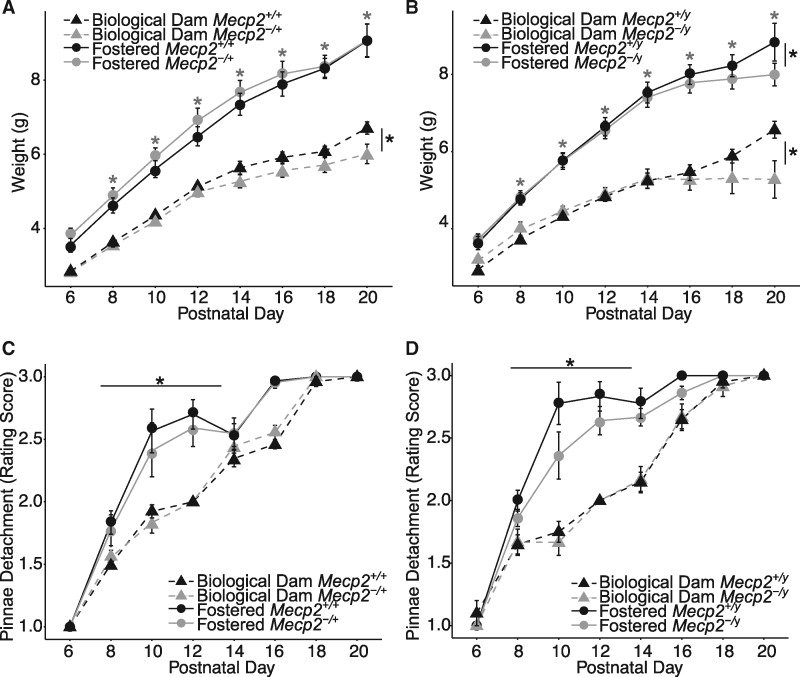
Previous work in multiple RTT mouse models has demonstrated differences in body weight and the occurrence of hind limb clasping behaviors in both males (13,24,46–48) and females (12,15,18,22–24,49–51). To longitudinally test the impact of fostering and genotype on body weight and hind limb clasping phenotypes, weekly assessments were performed on both male and female offspring. Fostered female weights were significantly higher in Mecp2-/+ than Mecp2+/+ mice at all-time points after PND 49 (Supplementary Material, Table S1 and Fig. 3A). Likewise, body weights were significantly higher in fostered Mecp2-/+ mice than in biological dam raised Mecp2-/+ mice at PND 49–98, indicating that fostering accelerates the progressive weight gain observed in the female mutant mice. For instance, in biological dam raised mice, significantly elevated body weights in Mecp2-/+ compared to Mecp2+/+ mice do not occur until after PND 84.
Figure 3.
Cross-fostering modifies body weight and hind limb clasping phenotypes. (A) Body weight assessed weekly for fostered and biological dam raised Mecp2-/+ and wildtype females. (B) Body weight assessed weekly for fostered and biological dam raised Mecp2-/y and wildtype males. Both fostered and biological dam males show lower body weight than their respective wildtype littermates. (C) Biological dam Mecp2-/+ females show earlier onset of hind limb clasping than fostered Mecp2-/+ females. (D) Biological dam Mecp2-/y males show earlier onset of hind limb clasping than fostered Mecp2-/y males. Grey * denote significant differences between fostered mutants and wildtypes. Black * denote significant differences between biological dam mutants and wildtypes. Grey + denote significant differences between wildtype fostered and biological dam conditions. Black + denote significant differences between mutant fostered and biological dam conditions. Fostered animals: 15 Mecp2+/+, 11 Mecp2-/+, 9 Mecp2+/y, and 18 Mecp2-/y; Non-fostered animals: 13 Mecp2+/+, 9 Mecp2-/+, 11 Mecp2+/y, and 6 Mecp2-/y. Body weight was assessed using linear mixed effects model. Clasping score was analyzed with mixed effects proportional odds logistic regression model and time to first clasping onset was assessed using a mixed effects Cox proportional hazards model.
Interestingly, fostering in early life also significantly impacted body weight of wildtype females, specifically at the later time points. In contrast, male body weights were significantly lower in Mecp2-/y mice than in Mecp2+/y mice with the same fostering status at all-time points between PND 28 and 49. At PND 49, weights were significantly higher in fostered MeCP2-/y mice than in biological dam raised Mecp2-/y mice (P = 0.011). These findings indicate that fostering has minimal impact overall on the reduced body weights of Mecp2-/y mice except near the end of life (Supplementary Material, Table S1 and Fig. 3B).
For the development of hind limb clasping behavior, both sexes showed a delay in clasping onset with fostering. Biological dam raised Mecp2-/+ females developed significant hind limb clasping significantly earlier than fostered Mecp2-/+ females (PND 56 vs 63) (Fig. 2C) (mixed effects Cox proportional hazards model, Hazard Ratio = 0.14, P = 0.021). Similarly, clasping was significantly higher in Mecp2-/y compared to Mecp2+/y biological dam raised males by PND 28 but not significantly different in fostered males until PND 42 (Fig. 3D). When directly compared, fostered Mecp2-/y mice had a significantly longer time to clasping onset than biological dam raised Mecp2-/y mice (Hazard Ratio = 0.07, P = 0.003). Together, these findings indicate that fostering impacts both the long-term weight gain trajectories of female Mecp2 mutant mice, and moderately delays the onset of at least one neurologic symptom (hind limb clasping) in both sexes.
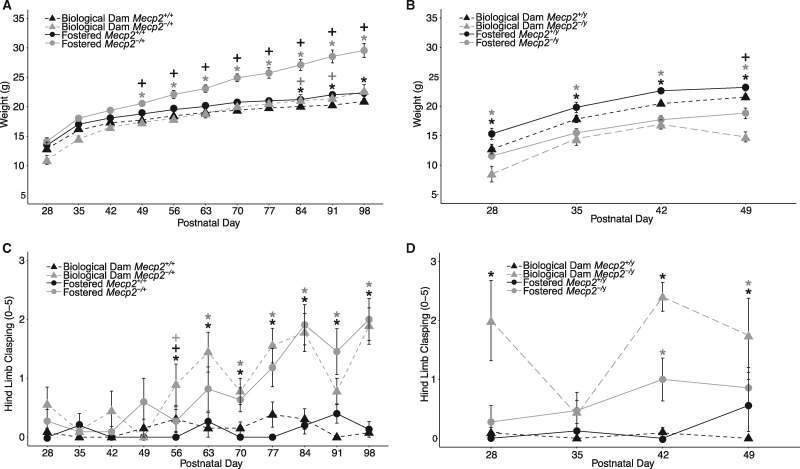
Fostering improves sensitivity to detect female deficits associated with Mecp2 mutation in the open field exploration test
To test how fostering and genotype interact in an exploratory motor task, we evaluated measures of horizontal, vertical, and total activity performance of female mice in a novel open field (Fig. 4A–D, Supplementary Material, Table S1). For both horizontal activity and center time, fostering moderated the genotype effect such that only fostered Mecp2+/+ and Mecp2-/+ mice significantly differed. These effects were largely independent of any impact of fostering on anxiety-like behavior. There was a significant difference between genotypes on time spent in the open arm and open arm entries of the elevated plus-maze but not in light ↔ dark exploration time in dark chamber (Supplementary Materials, Fig. S3, Table S1), but none of these measures was impacted by fostering. Together, these findings suggest that fostering amplifies the motor and exploration differences between wildtype and mutant female mice independent of anxiety-like behavior, improving the sensitivity to detect genotype differences.
Figure 4.
Cross-fostering improves sensitivity to detect deficits in open field exploration test. (A) Fostered Mecp2-/+ show lower horizontal activity during open field exploration compared to fostered wildtype littermates. Open field was conducted from PND 49–63. (B) Fostered Mecp2-/+ showed lower vertical activity compared to wild-type littermates. Fostered and biological dam wildtypes also showed differences. (C) No differences were observed for total activity. (D) Fostered Mecp2-/+ spent less time in the center of the open field compared to fostered wildtype littermates. *P < 0.05 Tukey’s post hoc test. Fostered animals: 15 Mecp2+/+ and 11 Mecp2-/+; Non-fostered animals: 13 Mecp2+/+ and 9 Mecp2-/+.
Fostering alters sensitivity to detect motor impairments in female Mecp2 mutants
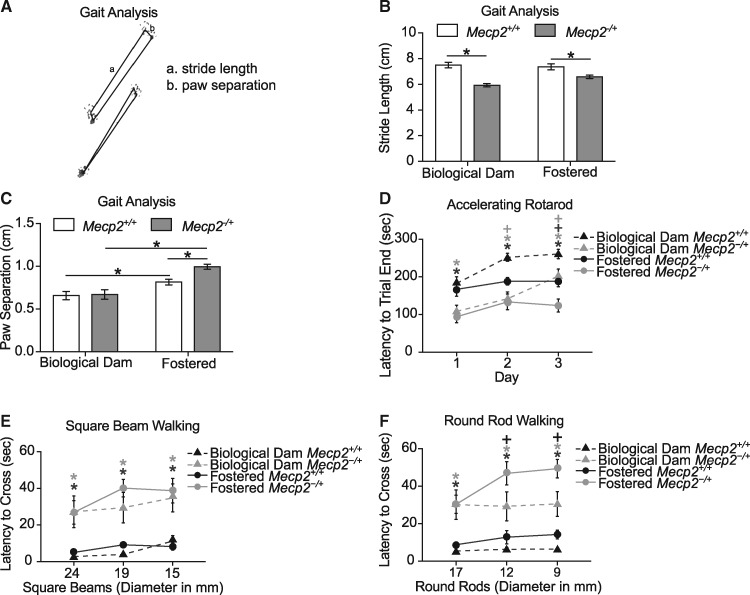
Previous work in various lines of Mecp2 mutant mice have identified abnormalities in motor function reminiscent of motor impairments observed in girls with Rett Syndrome (12,22,49,50,52–55). To test the hypothesis that fostering may also affect the sensitivity to detect motor deficits in female mutants, we performed four different motor tasks: gait analysis, accelerating rotarod latency to trial end, and balance beam crossing of square beams and round rods.
Gait was analyzed using a standard footprint analysis method (56), and measures of stride length, paw separation, front base and hind base were assessed and compared between conditions (Fig. 5A). Genotype altered stride length, paw separation, and front base measures (Fig. 5B and C, Supplementary Materials, Fig. S4, Table S1). Fostering showed a moderate impact on paw separation, a measure of motor coordination. Fostering also increased paw separation in both wildtype and mutant animals relative to biological dam raised animals and increased the differences between Mecp2+/+ and Mecp2-/+ mice such that genotype effects were only observed in fostered animals (Fig. 5C). At the time of gait analysis the fostered mutant mice were already significantly heavier than fostered wildtypes and biological dam raised mutants (Fig. 3A). Consequently, to evaluate the impact of increased body weight on measures of gait analysis bodyweight was added in as a covariate to the analysis (Supplementary Material, Table S1). Body weight as a covariate was significant only for the gait analysis measure of paw separation. Further examination did not reveal any direct correlation between an individual animal’s body weight and any measure of gait for any genotype or fostering condition (Supplementary Material, Table S2). These results suggest that increased body weight may influence but does not fully account for the improved sensitivity for detecting differences in paw separation in fostered Mecp2-/+ mice.
Figure 5.
Mecp2 mutant females showed motor impairments on multiple measures. (A) Gait analysis conducted by footprint test (PND 52-66). (B) Mecp2-/+ mice showed a shorter stride length when compared to wildtype littermates, in both rearing conditions. (C) Fostered Mecp2-/+ mice show greater paw separation than wildtype littermates than biological dam Mecp2-/+ mice. Fostered wildtypes also showed greater separation compared to biological dam wildtypes. * denote significant Tukey’s corrected posthoc differences between groups. (D) Both fostered and biological dam Mecp2-/+ mice show impaired performance on accelerating rotarod (PND 63–71). Three trials a day were averaged over three days and latency to fall was examined using a mixed model repeated measures ANOVA with day as the repeated measure. (E) Both fostered and biological dam Mecp2-/+ mice show longer latency to cross square beams of decreasing width (PND 80–94). (F) Both fostered and biological dam Mecp2-/+ mice show longer latency to cross round rods of decreasing diameter. Balance beam walking, on a succession square beams of decreasing width (E), followed by a succession of round rods of decreasing diameter (F), were assessed using a mixed model repeated measures ANOVA with beam as the repeated measure and genotype and fostering as factors. Results using body weight as a covariate are shown in Supplementary Material, Table S1 and correlations with body weight are in Supplementary Material, Table S2. All posthoc tests were Benjamini-Hochberg corrected comparisons: Grey * denote significant differences between fostered mutants and wildtypes, Black * denote significant differences between biological dam mutants and wildtypes, Grey + denote significant differences between wildtype fostered and biological dam conditions, Black + denote significant differences between mutant fostered and biological dam conditions. Fostered animals: 15 Mecp2+/+ and 11 Mecp2-/+; Non-fostered animals: 13 Mecp2+/+ and 9 Mecp2-/+.
Motor coordination and learning were further assessed using the accelerating rotarod (three trials per day, averaged, for three consecutive days). There was a main effect of fostering and an interaction between fostering and day of testing but no interaction with genotype (Supplementary Material, Table S1), indicating that fostering did impact motor behavior in both genotypes. Overall, Mecp2-/+ mice showed impairments across time relative to wildtype littermates (Fig. 5D) as expected based on previous studies (22,49,50,52–55). Fostered compared to biological dam raised mice also differed on several of the testing days indicating that fostering can shift motor performance, and Mecp2+/+ biological dam raised mice actually outperformed fostered wildtype mice on day two and three (Benjamini-Hochberg corrected posthocs P = 0.006 and 0.0001, respectively). Body weight (collected the same week as testing) as a covariate was not significant, but did alter the statistical effect of fostering such that the main effect was no longer significant but the interaction between fostering and day reach significance (Supplementary Material, Table S1). There was no correlation between body weight for any condition and individual animal performance on any day of testing (Supplementary Material, Table S2).
As a comparison test of motor coordination we evaluated the time required to transverse a series of square beams and round rods. All animals were tested on a series of three square beams of decreasing diameter (increasing difficulty) and then the next day tested on three rods of decreasing diameter (increasing difficulty). Both fostered and biological dam raised MeCP2-/+ mice showed longer latencies to cross compared to MeCP2+/+ mice for all three beams (all P values < 0.0001) (Fig. 5E). Body weight was a significant covariate for this analysis and significantly impacted the main effect of genotype (Supplementary Material, Table S1), but was not correlated for any specific condition with latency to cross any of the beams (Supplementary Material, Table S2). Together, this indicates body weight in both fostering conditions may contribute on top of motor impairments to the longer latencies in the mutant animals. Significant impairments were also observed in fostered and biological dam raised MeCP2-/+ mice for all three rod diameters (all ps <0.05) (Fig. 5F). Fostered MeCP2-/+ mice were slower to cross on rods with diameters of 12 and 9 mm than biological dam raised MeCP2-/+ mice, but there were no differences between fostered and biological dam raised wildtype mice on any of the rods. To examine the hypothesis that the larger impairment in fostered MeCP2-/+ mice could be due to their increased body weight, we added body weight (collected on the week of testing) as a covariate to the analysis. Body weight was not a significant covariate and did not impact the three way significant interaction between fostering, genotype and rod (Supplementary Material, Table S2). In addition, on an individual animal level, body weight and rod walking latency for each rod did not correlate for any condition (Supplementary Material, Table S2). Overall, these findings demonstrate general Mecp2-/+ female motor impairments, including abnormalities in gait and motor coordination, which are largely independent from body weight changes. In addition, fostering enhanced the sensitivity of at least some of these measures to detect genotype differences.
Social approach is intact in Mecp2-/+ mice and not altered by fostering
Only one previous study examined sociability in Mecp2tm1.1bird-/+ females, identifying moderate but significant impairments in social behavior that depended on background strain (12). In order to assess sociability in female Mecp2-/+ mice on a standard C57BL6/J background, we employed our three-chambered social approach task (57). During the test phase of the task the subject mouse is given a choice between a novel object and a novel, sex-matched 129S1/SvImJ stranger mouse. Both chamber time (time spent in the chamber containing the novel mouse versus time spent in the chamber containing the novel object) and sniff time (time spent sniffing the novel object versus time sniffing the novel mouse) were examined as measures of sociability (57). This is an all-or-none, yes-or-no assay which determines whether significant sociability is detectable within a genotype or treatment group (57). Both fostered and biological dam raised Mecp2+/+ and Mecp2-/+ mice displayed significant sociability, spending more time in the chamber with the novel mouse and more time sniffing the novel mouse, as compared to the novel object (Supplementary Material, Fig. S5A and B). To assess any potential motor impairment, during both the habituation and testing phase we examined total entries and distance travelled (Supplementary Material, Fig. S5). During the habituation and test sessions, Mecp2-/+ mice displayed fewer total entries and less distance traveled than Mecp2+/+ littermate controls, for both rearing conditions (Supplementary Materials, Fig. S5, Table S1). Overall the motor impairments observed in this task appear not to impair sociability during the test phase, indicating that Mecp2-/+ mice show intact sociability despite the onset of motor impairments.
Short-term memory is intact in Mecp2-/+ mice and not altered by fostering
Previous examinations of spatial memory in Mecp2 mutant females have reported mixed findings depending on mutation type and background strain (12,24,49,52,55,58). The majority of this work used the Morris water maze and fear conditioning cognitive assays, which are sensitive to motor impairments, as they require either strenuous motor activity (swimming) or use outcome measures potentially confounded by lack of movement (freezing). Consequently, we sought to examine cognitive function using novel object recognition and novel object location memory tasks that are non-stressful and require only minimal exploratory movement (59). Novel object recognition and object location memory engage different brain regions and are thought to tap into distinct memory processes such that deficits in one task can be observed independent of the other (60–62). Consequently, we tested short-term memory for both novel objects and spatial location using the two tasks sequentially in the same cohort of animals as previously described (60). Deficits have previously been found in a variation of the novel object recognition paradigm in Mecp2tm1.1Jae/+ at older ages (PND336–420) (52) and in young adult (PND34–36) Mecp2R168X-/+ (49).
Novel object recognition memory testing consisted of a 10 min training session followed 60 min later by a 5-min test session in which one of the training objects was replaced by a novel object (Supplementary Material, Fig. S6A). During habituation to the empty arena there were differences between fostered Mecp2+/+ and Mecp2-/+ mice at days 1 and 3. By the end of habituation day 4, all groups showed equivalent movement (Supplementary Materials, Fig. S7B, Table S1). There were no differences between conditions for the training phase. During the test phase, mutant mice generally explored less than wildtypes, however no individual posthoc comparisons were significant, indicating that total exploration differences had a minimal impact on test preference (Supplementary Material, Fig. S6C and D). When comparing the exploration of the familiar versus novel objects for each condition (Supplementary Material, Fig. S6E, there were significant preferences for the novel object for fostered Mecp2-/+ (P = 0.010) and biological dam raised Mecp2-/+ (P = 0.015) and marginally significant preferences for fostered Mecp2+/+ (P = 0.083) and biological dam raised Mecp2+/+ (P = 0.055) mice. When adjusting for the total exploration differences with a discrimination index, there were no differences between groups (Supplementary Material, Fig. S6F).
Object location recognition memory consisted of a 10 min training session followed 60 min later by a 5 min test session in which one of the training objects was moved to a novel location (Supplementary Material, Fig. S7A). There were no differences in habituation or total exploration during training or testing (Supplementary Material, Fig. S8B–D). However, during testing, there was a significant preference for the moved object in the cross-fostered Mecp2-/+ (P = 0.001), cross-fostered Mecp2+/+ (P = 0.001), biological dam raised Mecp2+/+ (P = 0.012), and a trend for a preference in biological dam raised Mecp2-/+ (P = 0.079) (Supplementary Material, Fig. S7F). When examining the discrimination index, all groups showed significant and equivalent preferences for the moved object (Supplementary Material, Fig. S7F). Together, these findings indicate normal short-term memory in Mecp2-/+ females, independent of rearing condition, indicating that the basic cognitive function is intact at this age.
Discussion
Mecp2 mutant mouse lines are the current leading preclinical model for RTT syndrome. Maintaining a colony of Mecp2 mutant mice requires using heterozygous mutant dams because the male mutants are sterile (13). Previous reports have indicated poor overall maternal care (24,25) in Mecp2 mutant females, and even in wildtype mice, reduced expression of MeCP2 in the hypothalamus is correlated with lower levels of maternal behavior (63). Furthermore, Mecp2tm1.1bird-/+ females show dramatic failures in learned pup retrieval with higher errors and longer retrieval latencies than their wildtype counterparts (64). In this study, although we did not directly measure maternal behaviors, we sought to directly compare phenotypes of offspring reared by their biological Mecp2 mutant dams versus offspring cross-fostered to CD1 females, a strain with high reproductive success. This approach permitted us to examine the impact of maternal environment on offspring, the direct effects of Mecp2 genotype isolated from postnatal maternal environmental factors, and any gene by environment interaction between the two. Overall, our findings indicate that cross-fostering can significantly alter the time course of the acquisition of some developmental milestones and a subset of adult phenotypes, predominantly body weight, activity, and motor behaviors. We also demonstrate that cross-fostering improves the sensitivity to detect differences in several behavioral tasks between female Mecp2 mutants and their littermate controls.
The maternal postnatal environment is multifaceted and includes potential differences in maternal behaviors, lactation, microbiota, or milk quality, all of which may have distinct impacts on pup development. Strain dependent differences have been observed in nursing type, licking behavior, nest building, pup retrieval and pup contact (36,65). Milk composition can also subtlety vary in amino acid composition between different mouse strains (66,67) and influence offspring growth trajectories (68). During lactation at least some hormones and growth factors present in breast milk can be absorbed by nursing offspring and transferred into the pup’s circulation in biological active forms (69), potentially altering pup development. Cross-fostering can also shift the offspring’s microbiota towards that of the foster mother compared to the birth mother, an effect that can last into adulthood for the offspring (34). A limitation of our study is that it was only designed to examine the effects of cross-fostering on Mecp2 mutant mice, not to directly determine which of the multi-faceted components of maternal care were important. Another limitation of our study is that no direct comparison of CD1 and C57BL6/J dams have been made for these distinct maternal factors. Previous work using Mecp2tm1.1bird-/+ mice on a CD1 background strain found a 30% reduction in cannibalism compared to the standard C57BL6/J background, supporting the robust maternal care of the CD1 strain (24).
Cross-fostering to dams of different mouse strains has previously been shown to alter developmental milestone progression, with the dams that show higher levels of maternal care producing offspring with accelerated development (70). This is consistent with our results showing that cross-fostering accelerated some physical developmental milestones in both male and female offspring. Milestones that were reached earlier in the cross-fostered offspring included body weight, body length, pinnae detachment, eye opening, incisor eruption, and fur development. Milestones that were unaltered by rearing conditions included negative geotaxis and vertical screen score. Several studies have reported enhanced maternal care early in life can alter brain development and behavior into adulthood (26–32,39,71). Innate variability of maternal behaviors in rats can alter the stress response system in the offspring for the lifetime of the animal (26,71,72). Cross-fostering at birth can reverse both behavioral (71) and DNA methylation changes (26) related to poor maternal care. In multiple tests in our study, including open field, paw separation in footprint gait analysis, and round rod walking, cross-fostering improved or altered behavior to an extent that allowed differences between genotypes to be more easily detectable. Together, our findings indicate that rearing by Mecp2-/+ mutant dams may negatively impact wildtype littermates of Mecp2 mutant mice, making true genotype-dependent phenotypic differences more difficult to assess. This is consistent with findings in other neurodevelopmental models in which rearing conditions by mutant dams can negatively impact phenotypic outcomes in the offspring (73). Consequently, future pre-clinical work with Rett syndrome models could benefit from the cross-fostering of Mecp2 offspring.
One of the most consistent findings in heterozygous female Mecp2 mutant mice of varying genetic mutations is development of impaired motor function. Impairments have been reported across several different training protocols and background strains, multiple groups in Mecp2 mutant females for Mecp2tm1.1bird-/+, Mecp2tm1.1Jae/+, Mecp2R168X-/+ mice on accelerating rotarod (22,49,50,52–55), although most testing occurs at ages beyond 6 months. Previous work has also demonstrated impairments in grid walking and open field locomotion in Mecp2tm1.1bird-/+ females on F1 hybrid backgrounds (12). In this study, both fostered and biological dam raised Mecp2-/+ mice showed motor impairments at ages younger than typically examined, and several of these tasks show larger genotype differences in the fostered animals. Fostering appears to accentuate differences between genotypes in the case of paw separation, but not for other gait measures and may stem at least in part from differences in body weight. Both fostered and biological dam raised Mecp2-/+ mice showed impairments on accelerating rotarod performance, but on the final day of testing this effect was more actually more pronounced for biological dam raised Mecp2-/+ mice. In contrast, for the rod walking task, fostered Mecp2-/+ mice showed a larger impairment relative to biological dam raised Mecp2-/+ mice for the two smaller diameter rods (12 and 9 mm). Overall, fostering appears to accentuate the impairments in Mecp2-/+ mice on some of the motor tasks often by improving performance of the wildtype littermates, allowing detection of genotype effects earlier than previously reported.
Anxiety testing in MeCP2 deficient mouse models has largely indicated decreases in anxiety-like behaviors relative to wildtype performance. Similar to our findings, previous work with female Mecp2tm1.1bird-/+ and Mecp2tm1.1Jae/+ mice of different ages and background strains have shown increased time in the open arm of the elevated plus-maze (12,55). Measures of light ↔ dark box exploration at PND42 in Mecp2tm1.1bird-/+ on two different hybrid backgrounds also reported increased time in the light compartment (12). We failed to find differences in the light ↔ dark box exploration in our experiments, indicating this task may be either less sensitive to alterations in anxiety or measure different aspects of anxiety behavior than elevated plus-maze. Differences in open field behavior, a measure of motor function, exploration, and anxiety-like behaviors were found only in fostered Mecp2tm1.1bird-/+ mice and were partially due to changes in wildtype performance. Deficits detected in the present study using the Mecp2tm1.1bird-/+ mice are generally consistent with previous findings of a general hypoactivity across MeCP2 mutant models, but occurred at earlier ages in the fostered Mecp2tm1.1bird-/+ mice than previously reported (12,22,53,54,58).
One of the most striking effects of fostering on phenotype occurred for body weight. Changes in body weight in different lines of Mecp2 mutant females depend on both mutation type, background strain and additional factors with heterozygous females reported to show both decreases (22,49), increases (12,16), and normal body weight across development and into adulthood (12,15,23,24,50). Mecp2tm1.1bird-/y males also show either obesity on a B6129S6F1 background (46–48) or weight loss on a C57BL/6 (11) or CD1(23) background. In our study, biological dam raised Mecp2-/+ and Mecp2+/+ mice showed genotype differences in body weight differences at PND 20 prior to weaning. In contrast, the fostered Mecp2-/+ and Mecp2+/+ mice did not show genotype differences in body weight at this early developmental time point, suggesting the female heterozygous genotype may be more sensitive to the early maternal environment. The differential impact of fostering on the female mutants continued into adulthood with fostered Mecp2-/+ mice showing an increased weight gain across life. Increased body weight in the fostered Mecp2-/+ occurred earlier than previously observed weight increases in Mecp2-/+ on a CD1 background (24) (PND 49 vs PND 119), suggesting a long-term interaction between the C57BL/6 strain background and maternal environment. Environmental reprograming of the stress response has been linked to early life changes in gene expression and DNA methylation in the hypothalamus (26,74) and expression of MeCP2 in hypothalamic nuclei appears to regulate body weight and metabolism. Mecp2tm1.1bird-/y males on a C57BL/6:129/SvJ background become overweight (46–48) with increased amounts of inguinal white adipose tissue, increased circulating leptin levels, but normal food intake (48). Treatment of Mecp2tm1.1bird-/y males with a single dose of leptin failed to show the reduction in food intake observed in wildtypes and failed to activate Pomc expression in the hypothalamus. Interestingly, these mice also showed an increase in leptin receptor expression and increased activation of the downstream signaling molecules in the leptin cascade, indicating a failure in the final activation steps in regulating Pomc gene expression (48). Deletion of Mecp2 specifically in POMC neurons in the hypothalamus of male mice resulted in increased body weight, higher fat composition, higher food intake, and lower utilization of fat as an energy source. These animals also showed increased DNA methylation over the Pomc promoter and decreased Pomc expression in hypothalamus at four months of age (75). Similarly, male mice with deletion of Mecp2 in Sim1-expressing hypothalamic neurons were obese and showed alterations in aggression, social behaviors and feeding. These animals also showed normal basal metabolic rates and activity levels but increased food consumption and by 42 weeks of age, increased leptin levels and increased Crh and Bdnf expression (76). While beyond the scope of the current study, our data suggest that fostering may alter hypothalamic function in Mecp2 mutant females by regulating epigenetic mechanisms downstream of leptin signaling.
Sociability has not been extensively examined in Mecp2 mutant female mouse models. We found that sociability appears to be intact and not influenced by fostering status in the Mecp2-/+ mice at the age tested. Previous work with male Mecp2 mice had observed both impairments and enhancements in social behavior, but many of these findings could have been confounded by severe motor impairments (77–79). Overall the reduce exploratory activity observed during both phases of this task do not appear to impair social preference during the test phase, indicating intact sociability despite the onset of motor impairments in the mutant females. Previous work with Mecp2tm1.1bird-/+ mice on both FVB/Nx129S6/SvEv and C57BL/6x129S6/SvEv F1 hybrid backgrounds showed reduced sociability scores at two timepoints in the three chamber social approach test. However, the Mecp2 mutant females on both backgrounds did show significant sociability, as defined in this all-or-none assay as more investigation of the novel mouse than of the novel object, at 12 weeks of age. At the older age (22 weeks), mutants on the FVB background showed a preference while the B6 background did not (12). Fostering had little impact on sociability in our experiments, consistent with previous observations in the BTBR inbred strain of mice (80). In comparison to our data, these findings indicate that background strain but not early maternal experience, may be a particularly important factor when evaluating social deficits in Mecp2 mutant mouse models.
Cognitive function has been assessed using a number of different learning and memory tasks in several female RTT mouse models. We found that both short-term novel object recognition and object location memory were intact in both fostered and biological dam raised Mecp2-/+ mice at the age tested. Slight differences in total exploration time and distance traveled during habitation reflect the development of motor impairments, but did not impair the discrimination index on either task. Variations of the novel object recognition paradigm have also been examined in Mecp2tm1.1Jae/+ and Mecp2R168X-/+ mice. In PND336–420 Mecp2tm1.1Jae/+ females deficits were found in novel object recognition 24 h after two 5 min training sessions (separated by 24 h). These deficits were rescued by reintroduction of Mecp2 using a viral vector, indicating that cognitive impairments in this case were reversible (52). Similarly, young adult (PND34–36) Mecp2R168X-/+ mice show intact short-term (60 min) but impaired long-term (24 h) novel object recognition memory (49). Future work with Mecp2-/+ mice to test long term (24 h) memory for both novel object recognition and object location memory could further examine cognitive abilities.
In our study the majority of Mecp2-/+ mice under both rearing conditions display significant body weight and motor symptom development by PND 63, indicating that they can successfully be utilized as a preclinical model for RTT syndrome. Cognitive and social behaviors were unaltered by fostering or genotype in the present studies, indicating that these tasks may not serve as robust preclinical endpoints at these younger ages (PND 62–85). For the purposes of therapeutic development, focus may best remain on phenotypic symptoms (such as hind limb clasping and body weight changes) and motor impairments. Similar body weight and hind limb clasping phenotypes develop in Mecp2-/+ mice on a CD1 background although at later ages than observed here (24), suggesting that early maternal environment and background strain are both important for future pre-clinical studies. While maintaining Mecp2 mutant mice on a CD1 background has an advantage of not requiring the additional manipulations for cross fostering, behavioral phenotyping is much less common in this strain. Use of the standard C57BL/6J background has several advantages for both behavioral and mechanistic studies including ease of ordering breeding pairs (directly available from Jackson Laboratories), extensively characterized genomic information, and ability to cross to a number of other different mutant lines of animals available on the same background. Fostering Mecp2-/+ mice increased the differences between Mecp2-/+ and wildtype controls in a number of tasks (open field activity, paw separation, round rod walking), making identification of deficits easier at a younger age of testing. Since one of the largest impacts of fostering was on the development of excessive weight gain, future work could investigate how early life environment alters metabolism, feeding behavior, and hypothalamic function, all areas implicated in Rett syndrome.
Materials and Methods
Cross fostering (PND 0–2)
Mecp2 heterozygous females (Mecp2-/+, B6.129P2(C)-Mecp2tm1.1Bird/J stock number 003890) were maintained on a pure C57BL/6J background by breeding heterozygous females to wildtype C57BL/6J males (Jax strain 000664). For cross-fostering experiments, Mecp2-/+ females were paired with wildtype C57BL/6J males for 2 weeks. CD1 male and female mice, also maintained on a CD1 background, were also paired over the same time period so that litters would coincide. All males were then removed from the mating cages. For fostered pups, within the first 48 h of birth the entire litter from Mecp2-/+dams were removed from their birth mother and placed with a CD1 dam that had given birth to a litter within the previous 24–48 h. All but two the CD1 offspring were euthanized upon cross fostering and all pups remained with the CD1 dam until weaning at postnatal day (PND) 21. For litters raised by their biological dams, all pups remained with the Mecp2-/+dam until weaning at PND 21. Mice were maintained in a conventional temperature controlled vivarium, with ad libitum access to food and water, with the lights on from 7 AM to 7 PM. Behavioral testing was conducted in adjacent testing rooms during the light phase of the circadian cycle. All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Developmental milestones (PND 6–20)
Developmental milestones (81–83) were measured on PND 6, 8, 10, 12, 14, 16, 18, and 20. All measures were conducted by an experimenter blind to genotype. Body weight and body length (nose to anus) were measured using a scale (grams) and ruler (cm). Pinnae detachment, ear detachment, eye opening, incisor eruption, and fur development were each rated on a scale of 0 (not present) to 3 (fully present). Cliff avoidance was tested by placing each pup near the edge of a clipboard, gently nudging it towards the edge, and scoring avoidance on a rating scale from 0 (no edge avoidance) to 3 (complete turning and backing away from the edge). Righting reflex was tested by placing each pup on its back, releasing it, and measuring the time for it to fully flip over onto four paws for two trials on each developmental day. Failures to flip over in the righting test were recorded as a maximum score of 30 s. Grasping reflex was tested by brushing the forepaws with a cotton tipped applicator and rating the grasping reflex from 0 (none) to 3 (strong). Auditory startle was tested using a rating scale from 0 (no response) to 3 (large flinch and turn) in response to a finger snap near the pup’s head. Bar holding was tested by placing each pup’s front paws on a cotton tipped applicator stick and gently lifting up. Scoring consisted of a rating scale from 0 (immediate fall) to 3 (stay on and climb up). Level screen reflex was tested by placing each pup on a screen, gently pulling the tail, and rating the level of resistance (0 = none to 3= strong). Vertical screen reflex was tested by placing each pup on a screen at a 90° angle and rating the pup’s ability to remain on the screen from 0 (immediate fall) to 3 (hold on and climb to top). Negative geotaxis was tested by placing each pup, facing downwards, on a screen angled at 45° from parallel, and scored on a scale from 0 (no turning) to 3 (complete turning and climbing to the top of the screen).
Adult behavioral battery
Behaviors were assessed in young adult mice from PND 45 to 94. Mice had free access to food and water and lights were maintained on a 12:12-h light/dark cycle, with all behavioral testing performed during the light portion of the cycle.
Body weight and clasping score (weekly PND 45–94)
Weekly body weights and hind limb clasping were assessed beginning from PND 28. Hind limb clasping was rated on a scale from 0 (no clasping behavior) to 5 (both hind limbs tucked in and abdomen contracted) (21).
Elevated plus-maze (PND 45–59)
Each mouse was individually placed in the center area of a black Plexiglas automated elevated plus-maze (Med-Associates, St. Albans City, VT), under 300 lux illumination for a 5-min test session (82,84,85). The entries per arm, total entries, and the time spent in each arm were recorded and compared between conditions (83).
Light ↔ dark exploration (PND 47–61)
The light ↔ dark exploration task was performed as previously described (82). Briefly, the automated photocell-equipped apparatus was made of two Plexiglas compartments separated by a partition with a small opening. One compartment was transparent and illuminated by an overhead light (400 lux). The other compartment was made of black Plexiglas and closed on top. Each mouse was placed into the center of the light compartment and allowed to freely explore for 10 min. The number of transitions between light and dark sides and time spent in each compartment were recorded and compared between conditions.
Open field exploration (PND 49–63)
Exploratory behavior was assessed in a novel open field as previously described (82). Each mouse was placed in an automated VersaMax Animal Activity Monitoring System (AccuScan Instruments, Columbus, OH) and allowed to freely explore for 30 min. The number of horizontal and vertical beam breaks was used as a measure of horizontal and vertical activity respectively. Total distance traveled was used as a measure of total activity. Time spent in the center of the chamber compared to along the edges was recorded and compared between conditions.
Gait analysis (PND 52–66)
On the day prior to analysis all animals were habituated to restraint and paint application. On test day each animal was restrained, and the front feet were painted with blue while the back feet were painted with red paint (Sargent Art 22-3350 Washable Paint). The mouse was then allowed to walk down a straight alleyway lined with drawing paper (Strathmore). Colored footprints were analyzed for stride length (distance between successive forelimb and successive hind limb prints), hind-base (distance between the right and left hind prints), front-base (distance between right and left front prints), and paw separation (distance between the forepaw and hind paw placement) (56). Two to three footprints were analyzed per animal.
Accelerating rotarod (PND 63–71)
Animals were given three trials per day with 1 h inter-trial intervals, repeated over three consecutive days (82,83). Each trial consisted of placing the animal on the rotarod apparatus (Ugo Basile mouse accelerating rotarod) and starting the rotation at 4 revolutions per minute (rpm). After the mouse was successfully walking, the rotarod was accelerated from 4 to 40 rpm over 5 min (82). The latency to fall from the rotating beam to the flange on the floor of the apparatus was recorded for each animal. The trial was terminated in cases where the mouse clutched the beam without walking, for five consecutive turns, or a maximum of 300 s had elapsed (82,83).
Beam and rod walking (PND 80–94)
A beam walking motor task was conducted as previously described (56). 59 cm long square dowels were suspended 68 cm above a cushioned landing pad. A goal box at the end of the beam consisted of a 12cm diameter cylinder to provide motivation to cross the beam. Each mouse was placed at the end of the beam and the time to cross to the goal box on the other end was measured. On the day prior to testing all animals were given four practice trials on the largest diameter square beam in order to become accustom to the procedure (56). On the test day each animal was sequentially tested on three square beams (24, 19 and 15 mm) and three round rods (17, 12 and 9 mm). Testing sequence was based on presentations of decreasing diameter to present increasing levels of difficulty. Each mouse was given two trials on each beam, separated by approximately 30 min. The time to transverse the beam was recorded and averaged across the two trials for each beam. A maximum time of 60 s was assigned to individuals that failed to cross the beam in that duration. In the small number of cases where mice fell from the beam, a score of 60 s was assigned.
Social approach (PND 67–81)
Sociability was assessed using the three chambered social approach task as previously described (83,57,86). The testing apparatus consisted of three connected chambers made of white matte Plexiglas, separated by two sliding doors. Prior to testing, the subject mouse was placed in the empty center chamber with the doors closed for 5 min. Doors were then removed to allow free access to all three empty chambers for 10 min. Distance traveled and the number of entries into the two side chambers were automatically scored using EthoVison XT tracking software (Noldus Information Technology Inc. Leesburg, VA). The mouse was then confined to the center chamber and a novel female 129Sv/ImJ mouse was placed in an inverted wire pencil cup in one chamber. An empty inverted wire pencil cup was simultaneously placed in the other chamber, to serve as the novel object. The partition doors were opened and the subject mouse was given free access to all three chambers for 10 min. Time spent in the chamber containing the novel mouse versus time spent in the chamber containing the novel object was automatically recorded by the EthoVision tracking system. Time spent sniffing the novel mouse and time spent sniffing the novel object were similarly automatically collected. The number of entries into each chamber, the total distance traveled were simultaneously scored using EithoVision XT (83,57,87). The sensitivity of this yes-or-no binary assay, originally developed by the Crawley team, is sufficient to determine sociability, defined as more time with the novel mouse than with the novel object within genotype or treatment group. This test is not sufficiently sensitive to compare the time spent with the novel mouse across genotypes or treatments. Ratio and index measures are not appropriate for this assay, which usually is directly influenced by exploratory activity levels that introduce artifacts in interpreting social scores.
Short-term memory novel object recognition and object location recognition (PND 62–76 and 70–85 respectively)
Novel object recognition testing was performed as described previously (59,60). Briefly, all animals were handled for 2 min per day by the experimenter for three days. All animals were then habituated to the empty training arena for 10 min per day for four days. For the training session each animal was placed into the arena with two identical objects for 10 min. A 5 min testing session occurred 60 min after training. For Novel Object Recognition testing, one of the training objects was replaced with a novel object. For Object Location Recognition testing, one of the training objects was placed in a novel location. Time spent exploring each object was scored by an experimenter blind to all conditions. A discrimination index was calculated as follows: (time exploring the novel object – time exploring familiar object)/total object exploration time *100. Animals exploring less than 3 s total for both objects during training or testing were excluded from further analysis (88).
Statistical Analyses
For assessment of the developmental milestone data, continuous outcomes were analyzed using linear mixed effects models, including fixed effects for genotype, fostering, time, all two- and three- way interactions among these factors, litter size, and random intercepts for mouse and litter. Pinna detachment, eye opening, incisor eruption, and fur development were analyzed using mixed effects Cox proportional hazards models of the time to full development; these models included fixed effects for genotype, fostering, the genotype-fostering interaction, and litter size and a random slope for litter. Incisor development was only analyzed in fostered mice, as no biological dam raised mouse of any genotype developed full incisors by 20 days. Other ordered categorical outcomes were analyzed using mixed effects proportional odds logistic regression models, including fixed effects for genotype, fostering, time, all two-way interactions among these factors, litter size, and random intercepts for mouse and litter. For level screen scores, only days 8 through 14 were analyzed, as there was insufficient variability in scores at other time points for modeling.
Survival of cross-fostered and biological dam raised Mecp2+/y and Mecp2-/y males was estimated for each genotype-fostering combination using the Kaplan-Meier method. Differences in the hazard of death between different genotypes and fostering conditions were modeled with a Cox proportional hazards model, with parameters estimated via Firth’s penalized maximum likelihood (89), due to the absence of any deaths in the Mecp2+/y group. The model included the effects for genotype, fostering status, the interaction between genotype and fostering status, and a gamma frailty parameter for litter. The analysis was conducted using R, version 3.3.1 (90), with Firth penalized maximum likelihood estimation using the R package coxphf, version 1.11 (91).
Post-weaning weight was analyzed using a linear mixed effects model, including fixed effects for genotype, fostering, time, all two- and three- way interactions among these factors, litter size, and random intercepts for mouse and litter. Clasping score was analyzed using a mixed effects proportional odds logistic regression model, including fixed effects for genotype, fostering, time, all two-way interactions among these factors, litter size, and random intercepts for mouse and litter. Time to first observation of clasping behavior in mutant mice was modeled using a mixed effects Cox proportional hazards models of the time to full development; this model included fixed effects for fostering and litter size and a random intercept for litter.
All other analyses included parametric independent, two-tailed t-tests or ANOVA models with either Tukey’s or Benjamini-Hochberg corrected multiple posthoc tests. Repeated measure tasks were accessed using a mixed model repeated measures ANOVA with a repeated measures and genotype and fostering as factors. Additional comparisons between motor performance and body weight were performed by adding body weight (collected from the week of task performance) as a covariate to the statistical model. Pearson’s correlations between body weight and task performance for each condition (foster x genotype) were also examined using Benjamini-Hochberg corrected P-values for multiple comparisons. All analyses were conducted using R, version 3.3.1 (90) or prism version 7. Linear mixed effects modeling was conducted using the R package lme4, version 1.1-12 (92), mixed effects Cox proportional hazards modeling was conducted using the R package coxme, version 2.2-5 (93), and mixed effects proportional odds logistic regression modeling was conducted using the R packages ordinal, version 2015.6-28 (94).
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
AVC, JML and JNC designed experiments and wrote the manuscript. AVC and MP conducted behavioral experiments. BDJ conducted statistical analyzes and wrote the manuscript. AVC, AC, AN, and DHY assisted with behavioral scoring and data analysis.
AVC, JML, JNC, BDJ were supported by the National Institutes of Health [T32MH073124-06 to AVC, 3RO1NS081913-11S1 to JML, 5R01NS081913-14 to JML, U54HD079125 to JNC and BDJ, UL1TR000002 to BDJ, 1R01NS085709 to JNC]; and JML by the International Rett Syndrome Foundation. We would like to thank Tatiana Kazdoba and Prescott Leach for their helpful discussion and insights on the project.
Conflict of Interest statement. None declared.
Funding
National Institutes of Health [T32MH073124-06, 3RO1NS081913-11S1, 5R01NS081913-14, U54HD079125, UL1TR000002, 1R01NS085709], International Rett Syndrome Foundation.
References
- 1. Erlandson A., Hagberg B. (2005) MECP2 abnormality phenotypes: clinicopathologic area with broad variability. J. Child Neurol., 20, 727–732. [DOI] [PubMed] [Google Scholar]
- 2. Hagberg B., Witt-Engerström I. (1986) Rett syndrome: a suggested staging system for describing impairment profile with increasing age towards adolescence. Am. J. Med. Genet. Suppl., 1, 47–59. [DOI] [PubMed] [Google Scholar]
- 3. Hagberg B. (2005) Rett syndrome: long-term clinical follow-up experiences over four decades. J. Child Neurol., 20, 722–727. [DOI] [PubMed] [Google Scholar]
- 4. Neul J.L., Fang P., Barrish J., Lane J., Caeg E.B., Smith E.O., Zoghbi H., Percy A., Glaze D.G. (2008) Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology, 70, 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffbuhr K.C., Moses L.M., Jerdonek M.A., Naidu S., Hoffman E.P. (2002) Associations between MeCP2 mutations, X-chromosome inactivation, and phenotype. Ment. Retard. Dev. Disabil. Res. Rev., 8, 99–105. [DOI] [PubMed] [Google Scholar]
- 6. Hoffbuhr K., Devaney J.M., LaFleur B., Sirianni N., Scacheri C., Giron J., Schuette J., Innis J., Marino M., Philippart M., et al. (2001) MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology, 56, 1486–1495. [DOI] [PubMed] [Google Scholar]
- 7. Trappe R., Laccone F., Cobilanschi J., Meins M., Huppke P., Hanefeld F., Engel W. (2001) MECP2 mutations in sporadic cases of Rett syndrome are almost exclusively of paternal origin. Am. J. Hum. Genet., 68, 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bienvenu T., Carrié A., de Roux N., Vinet M.C., Jonveaux P., Couvert P., Villard L., Arzimanoglou A., Beldjord C., Fontes M., et al. (2000) MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum. Mol. Genet., 9, 1377–1384. [DOI] [PubMed] [Google Scholar]
- 9. Bao X., Jiang S., Song F., Pan H., Li M., Wu X.-R. (2008) X chromosome inactivation in Rett Syndrome and its correlations with MECP2 mutations and phenotype. J. Child Neurol., 23, 22–25. [DOI] [PubMed] [Google Scholar]
- 10. Katz D.M., Berger-Sweeney J.E., Eubanks J.H., Justice M.J., Neul J.L., Pozzo-Miller L., Blue M.E., Christian D., Crawley J.N., Giustetto M., et al. (2012) Preclinical research in Rett syndrome: setting the foundation for translational success. Dis. Model. Mech., 5, 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samaco R.C., McGraw C.M., Ward C.S., Sun Y., Neul J.L., Zoghbi H.Y. (2013) Female Mecp2(+/-) mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum. Mol. Genet.., 22, 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patrizi A., Picard N., Simon A.J., Gunner G., Centofante E., Andrews N.A., Fagiolini M. (2016) Chronic administration of the N-methyl-D-aspartate receptor antagonist ketamine improves Rett syndrome phenotype. Biol. Psychiat., 79, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. (2001) A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet., 27, 322–326. [DOI] [PubMed] [Google Scholar]
- 14. Chen R.Z., Akbarian S., Tudor M., Jaenisch R. (2001) Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet., 27, 327–331. [DOI] [PubMed] [Google Scholar]
- 15. Pelka G.J., Watson C.M., Radziewic T., Hayward M., Lahooti H., Christodoulou J., Tam P.P.L. (2006) Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain, 129, 887–898. [DOI] [PubMed] [Google Scholar]
- 16. Shahbazian M., Young J., Yuva-Paylor L., Spencer C., Antalffy B., Noebels J., Armstrong D., Paylor R., Zoghbi H. (2002) Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron, 35, 243–254. [DOI] [PubMed] [Google Scholar]
- 17. Brown K., Selfridge J., Lagger S., Connelly J., De Sousa D., Kerr A., Webb S., Guy J., Merusi C., Koerner M.V., et al. (2016) The molecular basis of variable phenotypic severity among common missense mutations causing Rett syndrome. Hum. Mol. Genet., 25, 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goffin D., Allen M., Zhang L., Amorim M., Wang I.-T.J., Reyes A.-R.S., Mercado-Berton A., Ong C., Cohen S., Hu L., et al. (2012) Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nat. Neurosci., 15, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brendel C., Belakhov V., Werner H., Wegener E., Gärtner J., Nudelman I., Baasov T., Huppke P. (2011) Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. J. Mol. Med. (Berl), 89, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jentarra G.M., Olfers S.L., Rice S.G., Srivastava N., Homanics G.E., Blue M., Naidu S., Narayanan V. (2010) Abnormalities of cell packing density and dendritic complexity in the MeCP2 A140V mouse model of Rett syndrome/X-linked mental retardation. BMC Neurosci., 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yasui D.H., Gonzales M.L., Aflatooni J.O., Crary F.K., Hu D.J., Gavino B.J., Golub M.S., Vincent J.B., Schanen N.C., Olson C.O., et al. (2014) Mice with an isoform-ablating Mecp2exon 1 mutation recapitulate the neurologic deficits of Rett syndrome. Hum. Mol. Genet., 23, 2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santos M., Silva-Fernandes A., Oliveira P., Sousa N., Maciel P. (2007) Evidence for abnormal early development in a mouse model of Rett syndrome. Genes, Brain Behav., 6, 277–286. [DOI] [PubMed] [Google Scholar]
- 23. Picker J.D., Yang R., Ricceri L., Berger-Sweeney J. (2006) An altered neonatal behavioral phenotype in Mecp2 mutant mice. Neuroreport, 17, 541–544. [DOI] [PubMed] [Google Scholar]
- 24. Cobolli Gigli C., Scaramuzza L., Gandaglia A., Bellini E., Gabaglio M., Parolaro D., Kilstrup-Nielsen C., Landsberger N., Bedogni F. (2016) MeCP2 Related Studies Benefit from the Use of CD1 as Genetic Background. PLoS One, 11, e0153473.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jugloff D.G.M., Logan R., Eubanks J.H. (2006) Breeding and maintenance of an Mecp2-deficient mouse model of Rett syndrome. J. Neurosci. Methods, 154, 89–95. [DOI] [PubMed] [Google Scholar]
- 26. Weaver I.C.G., Cervoni N., Champagne F.A., Alessio A.C.D., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. (2004) Epigenetic programming by maternal behavior. 7, 847–854. [DOI] [PubMed] [Google Scholar]
- 27. Singh-Taylor A., Korosi A., Molet J., Gunn B.G., Baram T.Z. (2015) Synaptic rewiring of stress-sensitive neurons by early-life experience: A mechanism for resilience?. Neurobiol. Stress, 1, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fenoglio K. a., Brunson K.L., Baram T.Z. (2006) Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Front. Neuroendocrinol., 27, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L.Y., Babayan A.H., Andres A.L., Gall C.M., Lynch G., Baram T.Z., Chen Y., Krama E.A. (2012) Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol. Psychiatry, 18, 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meaney M.J., Aitken D.H., Bhatnagar S., Sapolsky R.M. (1991) Postnatal handling attenuates certain neuroendocrine, anatomical, and cognitive dysfunctions associated with aging in female rats. Neurobiol. Aging, 12, 31–38. [DOI] [PubMed] [Google Scholar]
- 31. Meaney M.J., Aitken D.H., Bodnoff S.R., Iny L.J., Sapolsky R.M. (1985) The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry, 9, 731–734. [DOI] [PubMed] [Google Scholar]
- 32. Anacker C., Donnell K.J.O., Meaney M.J. (2014) Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin. Neurosci., 16, 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bartolomucci A., Gioiosa L., Chirieleison A., Ceresini G., Parmigiani S., Palanza P. (2004) Cross fostering in mice: behavioral and physiological carry-over effects in adulthood. Genes. Brain. Behav., 3, 115–122. [DOI] [PubMed] [Google Scholar]
- 34. Daft J.G., Ptacek T., Kumar R., Morrow C., Lorenz R.G. (2015) Cross-fostering immediately after birth induces a permanent microbiota shift that is shaped by the nursing mother. Microbiome, 3, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Veen R., Abrous D.N., Ronald de Kloet E., Piazza P.V., Koehl M. (2008) Impact of intra- and interstrain cross-fostering on mouse maternal care. Genes, Brain Behav., 7, 184–192. [DOI] [PubMed] [Google Scholar]
- 36. Shoji H., Kato K. (2006) Maternal behavior of primiparous females in inbred strains of mice: A detailed descriptive analysis. Physiol. Behav., 89, 320–328. [DOI] [PubMed] [Google Scholar]
- 37. Shoji H., Kato K. (2009) Maternal care affects the development of maternal behavior in inbred mice. Dev. Psychobiol., 51, 345–357. [DOI] [PubMed] [Google Scholar]
- 38. Prakash P., Merali Z., Kolajova M., Tannenbaum B.M., Anisman H. (2006) Maternal factors and monoamine changes in stress-resilient and susceptible mice: Cross-fostering effects. Brain Res., 1111, 122–133. [DOI] [PubMed] [Google Scholar]
- 39. Priebe K., Romeo R.D., Francis D.D., Sisti H.M., Mueller A., McEwen B.S., Brake W.G. (2005) Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: a cross-fostering study. Dev. Psychobiol., 47, 398–407. [DOI] [PubMed] [Google Scholar]
- 40. Carola V., Frazzetto G., Gross C. (2006) Identifying interactions between genes and early environment in the mouse. Genes, Brain Behav., 5, 189–199. [DOI] [PubMed] [Google Scholar]
- 41. Carola V., Frazzetto G., Pascucci T., Audero E., Puglisi-Allegra S., Cabib S., Lesch K.P., Gross C. (2008) Identifying Molecular Substrates in a Mouse Model of the Serotonin Transporter x Environment Risk Factor for Anxiety and Depression. Biol. Psychiatry, 63, 840–846. [DOI] [PubMed] [Google Scholar]
- 42. Weller A., Leguisamo A.C., Towns L., Ramboz S., Bagiella E., Hofer M., Hen R., Brunner D. (2003) Maternal effects in infant and adult phenotypes of 5HT1A and 5HT1B receptor knockout mice. Dev. Psychobiol., 42, 194–205. [DOI] [PubMed] [Google Scholar]
- 43. Gleason G., Liu B., Bruening S., Zupan B., Auerbach A., Mark W., Oh J.-E., Gal-Toth J., Lee F., Toth M. (2010) The serotonin1A receptor gene as a genetic and prenatal maternal environmental factor in anxiety. Proc. Natl. Acad. Sci., 107, 7592–7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winslow J.T., Hearn E.F., Ferguson J., Young L.J., Matzuk M.M., Insel T.R. (2000) Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm. Behav., 37, 145–155. [DOI] [PubMed] [Google Scholar]
- 45. Gleason G., Zupan B., Toth M. (2011) Maternal genetic mutations as gestational and early life influences in producing psychiatric disease-like phenotypes in mice. Front. Psychiatry, 2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pitcher M.R., Ward C.S., Arvide E.M., Chapleau C.A., Pozzo-Miller L., Hoeflich A., Sivaramakrishnan M., Saenger S., Metzger F., Neul J.L. (2013) Insulinotropic treatments exacerbate metabolic syndrome in mice lacking MeCP2 function. Hum. Mol. Genet., 22, 2626–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ward C.S., Arvide E.M., Huang T.-W., Yoo J., Noebels J.L., Neul J.L. (2011) MeCP2 is critical within HoxB1-derived tissues of mice for normal lifespan. J. Neurosci., 31, 10359–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Torres-Andrade R., Moldenhauer R., Gutierrez-Bertín N., Soto-Covasich J., Mancilla-Medina C., Ehrenfeld C., Kerr B. (2014) The increase in body weight induced by lack of methyl CpG binding protein-2 is associated with altered leptin signalling in the hypothalamus. Exp. Physiol., 99, 1229–1240. [DOI] [PubMed] [Google Scholar]
- 49. Schaevitz L.R., Gómez N.B., Zhen D.P., Berger-Sweeney J.E. (2013) MeCP2 R168X male and female mutant mice exhibit Rett-like behavioral deficits. Genes. Brain. Behav., 12, 732–740. [DOI] [PubMed] [Google Scholar]
- 50. Nag N., Berger-Sweeney J.E. (2007) Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiol. Dis., 26, 473–480. [DOI] [PubMed] [Google Scholar]
- 51. Sun Z., Evans J., Bhagwate A., Middha S., Bockol M., Yan H., Kocher J.-P. (2014) CAP-miRSeq: a comprehensive analysis pipeline for microRNA sequencing data. BMC Genomics, 15, 423.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garg S.K., Lioy D.T., Cheval H., McGann J.C., Bissonnette J.M., Murtha M.J., Foust K.D., Kaspar B.K., Bird A., Mandel G. (2013) Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. J. Neurosci., 33, 13612–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jugloff D.G.M., Vandamme K., Logan R., Visanji N.P., Brotchie J.M., Eubanks J.H. (2008) Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Hum. Mol. Genet., 17, 1386–1396. [DOI] [PubMed] [Google Scholar]
- 54. Kondo M., Gray L.J., Pelka G.J., Christodoulou J., Tam P.P.L., Hannan A.J. (2008) Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome–Mecp2 gene dosage effects and BDNF expression. Eur. J. Neurosci., 27, 3342–3350. [DOI] [PubMed] [Google Scholar]
- 55. Stearns N.A., Schaevitz L.R., Bowling H., Nag N., Berger U.V., Berger-Sweeney J. (2007) Behavioral and anatomical abnormalities in Mecp2 mutant mice: A model for Rett syndrome. Neuroscience, 146, 907–921. [DOI] [PubMed] [Google Scholar]
- 56. Carter R.J., Morton J., Dunnett S.B. (2001) Motor coordination and balance in rodents. Curr. Protoc. Neurosci., Chapter 8, Unit 8.12–Uni1.14. [DOI] [PubMed] [Google Scholar]
- 57. Yang M., Silverman J.L., Crawley J.N. (2011) Automated three-chambered social approach task for mice. Curr Protoc Neurosci., Chapter 8, Unit 8 26, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lonetti G., Angelucci A., Morando L., Boggio E.M., Giustetto M., Pizzorusso T. (2010) Early Environmental Enrichment Moderates the Behavioral and Synaptic Phenotype of MeCP2 Null Mice. Biol. Psychiatry, 67, 657–665. [DOI] [PubMed] [Google Scholar]
- 59. Vogel-Ciernia A., Wood M.A. (2014) Examining object location and object recognition memory in mice. Curr. Protoc. Neurosci., 2014, 8.31.1–8.31.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vogel-Ciernia A., Matheos D.P., Barrett R.M., Kramár E. a., Azzawi S., Chen Y., Magnan C.N., Zeller M., Sylvain A., Haettig J., et al. (2013) The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat. Neurosci., 16, 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haettig J., Stefanko D.P., Multani M.L., Figueroa D.X., McQuown S.C., Wood M.A., Mcquown C. (2011) HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn. Mem., 18, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stefanko D., Barrett R., Ly A., Reolon G., Wood M. (2009) Modulation of long-term memory for object recognition via HDAC inhibition. Proc. Natl. Acad. Sci., 106, 9447–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mashoodh R., Franks B., Curley J.P., Champagne F.A. (2012) Paternal social enrichment effects on maternal behavior and offspring growth. Proc. Natl. Acad. Sci. U. S. A, 109, 17232–17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Krishnan K., Lau B.Y.B., Ewall G., Huang Z.J., Shea S.D., Amir R.E., Veyver I.B., Van den, Zoghbi H.Y., Chahrour M., Zoghbi H.Y., et al. (2017) MECP2 regulates cortical plasticity underlying a learned behaviour in adult female mice. Nat. Commun., 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Champagne F.A., Curley J.P., Keverne E.B., Bateson P.P.G. (2007) Natural variations in postpartum maternal care in inbred and outbred mice. Physiol. Behav., 91, 325–334. [DOI] [PubMed] [Google Scholar]
- 66. Meier H., Hoag W.G., McBurney J.J., Myers D.D. (1967) Chemical characterization of imbred strain mouse milk. II. Total fatty acids and fatty acid analyses. Proc. Soc. Exp. Biol. Med., 124, 633–636. [DOI] [PubMed] [Google Scholar]
- 67. Meier H., Hoag W.G., McBurney J.J. (1965) Chemical Characterization of Inbred-Strain Mouse Milk. I. Gross Composition and Amino Acid Analysis. J. Nutr., 85, 305–308. [DOI] [PubMed] [Google Scholar]
- 68. Nagasawa H., Naito T., Kataoka K. (1989) Relationship between Milk Composition and Pup’s Growth in Mice. Exp. Biol. Med., 191, 78–81. [DOI] [PubMed] [Google Scholar]
- 69. Koldovsky O. (1996) The Potential Physiological Significance of Milk-Borne Hormonally Active Substances for the Neonate. J. Mammary Gland Biol. Neoplasia, 1, 317–322. [DOI] [PubMed] [Google Scholar]
- 70. Carlier M., Roubertoux P., Cohen-Salmon C. (1983) Early development in mice: I. Genotype and post-natal maternal effects. Physiol. Behav., 30, 837–844. [DOI] [PubMed] [Google Scholar]
- 71. Francis D., Diorio J., Liu D., Meaney M.J. (1999) Nongenomic Transmission Across Generations of Maternal Behavior and Stress Responses in the Rat. Science, 286, 1155–1158. [DOI] [PubMed] [Google Scholar]
- 72. Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky P.M., Meaney M.J. (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277, 1659–1662. [DOI] [PubMed] [Google Scholar]
- 73. Grier M.D., Carson R.P., Lagrange A.H. (2015) Of mothers and myelin: Aberrant myelination phenotypes in mouse model of Angelman syndrome are dependent on maternal and dietary influences. Behav. Brain Res., 291, 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meaney M.J., Szyf M. (2005) Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci., 7, 103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang X., Lacza Z., Sun Y.E., Han W. (2014) Leptin resistance and obesity in mice with deletion of methyl-CpG-binding protein 2 (MeCP2) in hypothalamic pro-opiomelanocortin (POMC) neurons. Diabetologia, 57, 236–245. [DOI] [PubMed] [Google Scholar]
- 76. Fyffe S.L., Neul J.L., Samaco R.C., Chao H.-T., Ben-Shachar S., Moretti P., McGill B.E., Goulding E.H., Sullivan E., Tecott L.H., et al. (2008) Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron, 59, 947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pearson B.L., Defensor E.B., Pobbe R.L.H., Yamamoto L.H.L., Bolivar V.J., Blanchard D.C., Blanchard R.J. (2012) Mecp2 Truncation in Male Mice Promotes Affiliative Social Behavior. Behav. Genet., 42, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schaevitz L.R., Moriuchi J.M., Nag N., Mellot T.J., Berger-Sweeney J. (2010) Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiol. Behav., 100, 255–263. [DOI] [PubMed] [Google Scholar]
- 79. Moretti P., Bouwknecht J.A., Teague R., Paylor R., Zoghbi H.Y. (2005) Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet., 14, 205–220. [DOI] [PubMed] [Google Scholar]
- 80. Yang M., Zhodzishsky V., Crawley J.N. (2007) Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int. J. Dev. Neurosci., 25, 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fox W.M. (1965) Reflex-ontogeny and behavioural development of the mouse. Anim. Behav., 13, 234–241. [DOI] [PubMed] [Google Scholar]
- 82. Brielmaier J., Matteson P.G., Silverman J.L., Senerth J.M., Kelly S., Genestine M., Millonig J.H., DiCicco-Bloom E., Crawley J.N. (2012) Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One, 7, 40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang M., Bozdagi O., Scattoni M.L., Wohr M., Roullet F.I., Katz a. M., Abrams D.N., Kalikhman D., Simon H., Woldeyohannes L., et al. (2012) Reduced Excitatory Neurotransmission and Mild Autism-Relevant Phenotypes in Adolescent Shank3 Null Mutant Mice. J. Neurosci., 32, 6525–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang M., Lewis F.C., Sarvi M.S., Foley G.M., Crawley J.N. (2015) 16p11.2 Deletion mice display cognitive deficits in touchscreen learning and novelty recognition tasks. Learn. Mem., 22, 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kazdoba T.M., Hagerman R.J., Zolkowska D., Rogawski M.A., Crawley J.N. (2016) Evaluation of the neuroactive steroid ganaxolone on social and repetitive behaviors in the BTBR mouse model of autism. Psychopharmacology (Berl), 233, 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Silverman J.L., Pride M.C., Hayes J.E., Puhger K.R., Butler-Struben H.M., Baker S., Crawley J.N. (2015) GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology, 40, 2228–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nadler J.J., Moy S.S., Dold G. (2004) Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav., 5, 303–314. [DOI] [PubMed] [Google Scholar]
- 88. Vogel-Ciernia A., Wood M.A. (2013) Neuron-specific chromatin remodeling: A missing link in epigenetic mechanisms underlying synaptic plasticity, memory, and intellectual disability disorders. Neuropharmacology, 0, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Firth D. (1993) Bias reduction of maximum likelihood estimates. Biometrika, 80, 27–38. [Google Scholar]
- 90. R Development Core Team (2011) R: a language and environment for statistical computing.
- 91. Ploner M., Heinze G. (2015) coxphf: Cox regression with Firth’s penalized likelihood.
- 92. Bates D., Mächler M., Bolker B., Walker S. (2015) Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw., 67, 1–48. [Google Scholar]
- 93. Therneau T.M. (2015) Mixed Effects Cox Models.
- 94. Christensen R.H.B. (2015) Ordinal - Regression Models for Ordinal Data.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.