Abstract
Background: Circulating levels of fibroblast growth factor 23 (FGF23) increase progressively and correlate with systemic inflammation in chronic kidney disease (CKD). The aim of this study was to identify and characterize the causal relationship between FGF23 and inflammation in CKD.
Methods: Circulating FGF23 and inflammatory cytokines were correlated in healthy subjects and patients with varying levels of CKD. In addition, FGF23 expression in blood and solid organs was measured in normal mice that were exposed acutely (one time) or chronically (2-week) to low-dose lipopolysaccharide (LPS); chronic exposure being either sustained (subcutaneous pellets), intermittent (daily injections) or combined sustained plus acute (subcutaneous pellets plus acute injection on the day of sacrifice). Blood was analyzed for both terminal (cFGF23) and intact (iFGF23) FGF23 levels. Solid tissues were investigated with immunohistochemistry, enzyme-linked immunosorbent assay and reverse transcription polymerase chain reaction.
Results: FGF23 levels correlated significantly with neutrophil gelatinase–associated lipocalin (r = 0.72, P < 0.001), C-reactive protein (r = 0.38, P < 0.001), tumor necrosis factor-α (r = 0.32, P = 0.001) and interleukin-6 (r = 0.48, P < 0.001). Acute LPS administration increased tissue FGF23 mRNA and plasma levels of cFGF23 but not iFGF23. Neither chronic sustained nor chronic pulsatile LPS increased the tissue or circulating levels of FGF23. However, acute on chronic LPS raised tissue FGF23 mRNA and both circulating cFG23 and iFGF23. Interestingly, the spleen was the major source of FGF23.
Conclusion: Acute on chronic exposure to LPS stimulates FGF23 production in a normal mouse model of inflammation. We provide the first evidence that the spleen, under these conditions, contributes substantially to elevated circulating FGF23 levels.
Keywords: CKD spleen, FGF23, inflammation
INTRODUCTION
The circulating levels of the phosphaturic hormone fibroblast growth factor 23 (FGF23) are a strong predictor of cardiovascular disease (CVD) and mortality both in the general population [1] and in patients with chronic kidney disease (CKD) [2, 3], who, remarkably, can experience hormone levels as high as 100-fold greater than normal [4]. The mechanism(s) linking FGF23 with the adverse outcome, however, remains uncertain partially because of limited knowledge about regulation of this hormone. For example, while initial reports suggested that circulating phosphorus is the main stimulus for FGF23 production in CKD, this notion has been challenged by more recent studies showing that a low-phosphorus diet does not lower the FGF23 levels in mice with CKD [5] and that high phosphorus in the medium of bone cell cultures does not regulate FGF23 gene expression [6].
Recently, systemic inflammation has been proposed as a possible regulator of FGF23 in CKD based on the observation that hormone levels correlate significantly with those of the inflammatory markers C-reactive protein (CRP), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (P < 0.001 for each) in CKD patients and with IL-6 (P < 0.01), TNF receptor 1 (P = 0.05) and vascular cell adhesion molecule 1 (P = 0.04) in the general population in univariate and multivariable adjusted linear regression analysis [7, 8]. This possibility is particularly attractive since systemic inflammation is common in CKD [9] and, like FGF23, it is a nontraditional risk factor for CVD. Possible involvement of inflammation in FGF23 regulation also prompts the envisioning of pathogenic links among FGF23, inflammation and CVD, although the role of FGF23 in this paradigm remains unknown. In this study, we explored the relationship between inflammation and FGF23 using (a) cross-sectional analysis of individuals with diabetes and variable levels of diabetic nephropathy from the Family Investigation of Nephropathy of Diabetes (FIND) study and (b) investigation of mouse models of lipopolysaccharide (LPS)-induced acute and chronic inflammation. We hypothesize that LPS-induced inflammation increases systemic FGF23 levels.
MATERIALS AND METHODS
Human studies/clinical cross-sectional analysis
Study population
The study included 115 Mexican American subjects recruited from our institution who participated in the FIND study [10, 11] for whom baseline plasma samples were available in a local −80°C repository. FIND was a multicenter study with family-based linkage and a case–control design that aimed to identify genetic determinants of diabetic nephropathy in predefined ethnic groups. Available clinical information included the presence of type 2 diabetes mellitus (T2DM), severity of diabetic nephropathy based on the level of albuminuria from absent to being the cause of end-stage renal disease (ESRD), demographic characteristics, body mass index (BMI) and estimated glomerular filtration rate [eGFR by the Modification of Diet in Renal Disease (MDRD) equation]. The Office of the Institutional Review Board of the University of texas Health Sciences Center at San Antonio (UTHSCSA) and the Research & Development Office of the Audie L. Murphy VA Hospital authorized the study.
Data collection and measurements
Plasma FGF23 was measured by a human C-terminal-FGF23 (cFGF23) enzyme-linked immunosorbent assay (ELISA) kit (Immutopics, San Clemente, CA, USA) that detects both intact FGF23 (iFGF23) and its C-terminal fragments [12]. Neutrophil gelatinase–associated lipocalin (NGAL), TNF-α, IL-6 and CRP were analyzed because these inflammatory markers are most commonly elevated and associated with increased CVD and risk of death in CKD patients [13–15]. NGAL, TNF-α and IL-6 were measured using the Quantikine Immunoassays (R&D System, Minneapolis, MN; for TNF-α and IL-6, lower detection limits 0.19 and 0.11 pg/mL, intra-assay CV 3.1–8.7 and 5.5–9.8% and interassay CV 7.4–10.4 and 5.5–11.2%, respectively). Serum calcium, phosphorus, albumin and high-sensitivity CRP (lower detection limits < 0.07 mg/L, intra-assay CV 0.76–4.08% and interassay CV 1.6–4.9%) were measured in stored blood samples using standard methods by the centralized laboratory of the VA hospital.
Animals studies
Experimental design
The animal studies were reviewed and approved by the Institutional Animal Care, Use and Ethics Committee of UTHSCSA. Six-week-old male FVB/NJ mice were housed in an environmentally controlled animal care facility and maintained on 12 h:12 h light–dark cycles with free access to water and food pellets (18% protein, 6% fat, 0.95% calcium, 0.66% nonphytate phosphorus) under pathogen-free conditions.
Chronic inflammation is highly prevalent in CKD and one of the proposed possible mechanisms is translocation of bacterial endotoxins across the gut wall barrier [16]. To simulate this condition, bacterial LPS was administered systemically following four distinct protocols: (1) Acute inflammation: mice were injected intraperitoneally (i.p.) with a single dose (3.3 μg/kg body weight) of LPS (Escherichia coli serotype 0127:B8; Sigma, St. Louis, MO, USA), then anesthetized after 2, 3 and 5 h (n = 4/time point), exsanguinated by cardiac puncture and necropsied. This LPS dose is known to cause mild inflammation without producing septic acute kidney injury [17], which is a possible cause of increased FGF23. (2) Sustained chronic inflammation: anesthetized mice were implanted subcutaneously (s.q.) with time-release pellets (Innovative Research of America, Sarasota, FL, USA) containing LPS or placebo (n = 5/group). The LPS pellets constantly deliver 2 mg/kg/day LPS for 2 weeks and induce chronic inflammation with minimal animal stress and without development of LPS tolerance [18]. Mice also received daily vehicle injection and were sacrificed after 2 weeks. (3) Intermittent chronic inflammation: the sporadic inflammatory bursts that characterize chronic inflammation in CKD were mimicked with daily i.p. injections of 3.3 μg/kg body weight LPS for 2 weeks, in addition to s.q. vehicle pellets. Mice were sacrificed 5 h after the last i.p. injection. (4) Combined sustained plus intermittent chronic inflammation: mice were implanted s.q. with LPS pellets and administered daily vehicle injection. After 2 weeks they received a single i.p. injection of 3.3 μg/kg LPS, followed 5 h later by isoflurane anesthesia, exsanguination by cardiac puncture and necropsy for organ collection.
Histopathology
To assess the acute or chronic renal toxicity resulting from exposure to LPS, renal tissue was fixed in formalin, stained with hematoxylin and eosin and examined under ×10 and ×40 magnification. No tubular injury was detected in either group, including proximal tubule brush border abnormality, epithelial cell necrosis, tubular atrophy, interstitial infiltrate or fibrosis, while mild hypercellularity of unknown significance and origin was noted in ∼20% of glomeruli in the chronic sustained and acute on chronic inflammation groups.
Biochemical measurements
FGF23 levels were analyzed in plasma by both a murine iFGF23 ELISA kit that measures the intact active protein exclusively and a cFGF23 ELISA kit (Immutopics, Carlsbad, CA, USA), since these two assays provide different and complementary information. TNF-α and NGAL were measured using R & D Systems Quantikine ELISA kits.
Immunostaining of the bone
Since bone cells have classically been considered the source of circulating FGF23 [19], bones were fixed in 10% formalin, decalcified and embedded in paraffin. To detect FGF23 in osteocytes, 5-μm-thick deparaffinized sections were treated with peroxidase followed by protein block. After overnight incubation with rat anti-FGF23 antibody (1:400), a polymer detection system was used as we have previously described [20, 21].
Measurement of FGF23 protein in tissue lysate
Heart and spleen tissues were homogenized in 50 mM Tris, pH 7.4, 100 mM NaCl, 2 mM EDTA, 1% NP40, 10 mM NaO vanadate (Sigma Scientific) with the addition of protease inhibitors (Roche). Bone lysates were prepared as we have described previously [22]. Lysates were quantitated for FGF23 using mouse/rat FGF-23 (C-terminal) (Immutopics).
Quantitative real-time PCR
Total RNA was extracted from frozen spleen tissue using Trizol Reagent (Ambion, Life Technologies) according to the manufactures instructions. RNA was quantitated using a Nanodrop 1000 spectrophotometer (Thermo Scientific). cDNA synthesis was prepared using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems). Real-time PCR primers PPM03722F-200 (for FGF23) and PPM02945B-200 (for actin) (Qiagen).
Measurement of nuclear factor-κB (NF-κB) activity
The DNA-binding activity of NF-κB was assayed using a nonradioactive ELISA-based colorimetric assay kit (item no. 10011223; Cayman Chemical, Ann Arbor, MI, USA). The assay was done according to the manufacturer's protocol.
Statistical analysis
Continuous variables were described using mean ± standard deviation (SD) or median with interquartile range (IQR) as appropriate, while categorical variables were described with percentages. Pearson's correlations were used to assess linear associations among NGAL, FGF23, diabetes status, demographic profile, renal function, bone mineral metabolism and inflammation. Multiple regression models (standard method) were used to examine the strength of these associations after adjustment for known confounding factors, including age, sex, BMI, phosphorus, albumin, renal function and CRP. Analysis of variance (ANOVA) and Tukey's post hoc analysis were performed to analyze the response of FGF23 to LPS exposures. SPSS version 21 (IBM, Armonk, NY, USA) and Stata (StataCorp, College Station, TX, USA) were used for statistical analysis.
RESULTS
Human studies/cross-sectional analysis
Demographic and biochemical parameters
Table 1 shows the characteristics of the whole population and its subdivision into five groups based on status of diabetes mellitus and on severity of renal disease. Groups with more severe renal disease displayed progressively lower eGFR, BMI and serum albumin, but higher serum FGF-23 and phosphorus. Conversely, serum TNF-α, IL-6, CRP and NGAL had a biphasic pattern, with the lowest levels in the T2DM and microalbuminuria groups and the highest in the ESRD group. Sex distribution and serum calcium were not different among the groups.
Table 1.
Baseline characteristics of study participants by diabetes status and level of renal dysfunction
| Variables | Normal range | All (n = 115) | Healthy controls (n = 25) | T2DM (n = 30) | T2DM + micro- albuminuria (n = 9) | T2DM + macro-albuminuria (n = 23) | T2DM + ESRD (n = 28) | P-value |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age (years), mean ± SD | 57.6 ± 12.9 | 49 ± 16.1 | 60.5 ± 11.9 | 61.7 ± 12.8 | 60.2 ± 10.9 | 58.6 ± 9.5 | 0.005 | |
| Male (%) | 56 | 68 | 66 | 55 | 43 | 50 | 0.33 | |
| BMI (kg/m2), mean ± SD | 19–25 | 30.9 ± 6.6 | 32.3 ± 7.5 | 33.2 ± 7.3 | 32 ± 4.9 | 30.3 ± 5.4 | 27.1 ± 4.4 | 0.004 |
| Serum chemistry, mean ± SD | ||||||||
| GFR (mL/min) | <90 | 61.4 ± 42.6 | 94.3 ± 18.6 | 94.2 ± 25.7 | 75.1 ± 23.3 | 46.1 ± 23.6 | 5 ± 0 | <0.001 |
| Calcium (mg/dL) | 8.4–10.2 | 9.1 ± 0.6 | 8.9 ± 0.5 | 9.3 ± 0.3 | 9.3 ± 0.4 | 9.0 ± 0.3 | 8.9 ± 0.9 | 0.11 |
| Phosphorus (mg/dL) | 3–5.5 | 4.1 ± 1.3 | 3.7 ± 0.5 | 3.5 ± 0.5 | 3.5 ± 0.8 | 4.1 ± 1.3 | 5.3 ± 1.6 | <0.001 |
| Albumin (g/dL) | 3.3–4.0 | 3.74 ± 0.5 | 3.8 ± 0.4 | 3.9 ± 0.4 | 3.9 ± 0.3 | 3.5 ± 0.5 | 3.5 ± 0.6 | 0.007 |
| Inflammatory markers, median (IQR) | ||||||||
| CRP (mg/dL) | <0.3 | 0.38 (0.17–0.8) | 0.41 (0.17–0.79) | 0.29 (0.12–0.65) | 0.15 (0.1–0.4) | 0.35 (0.2–0.57) | 0.78 (0.23–1.32) | 0.001 |
| FGF23 (RU/mL) | ≤180 | 86.4 (32.9–482) | 32.9 (20.4–52.7) | 36.1 (21.6–89.3) | 69.8 (44.7–86.4) | 121.8 (54.5–243) | 2014.5 (867–4396) | <0.001 |
| TNF-α (pg/mL) | 4.14 (3.37–7.94) | 3.85 (3.0–4.85) | 3.24 (1.0–3.78) | 3.66 (3.47–4.5) | 4.33 (3.72–10.6) | 8.4 (5.12–12.9) | 0.01 | |
| IL-6 (pg/mL) | 2.04 (1.21–4.14) | 1.31 (0.82–2.54) | 1.65 (0.91–2.88) | 1.75 (1.39–2.19) | 1.6 (1.22–3.26) | 5.0 (2.63–6.99) | <0.001 | |
| NGAL (ng/mL) | 100–200 | 192 (110–419) | 150.9 (117–208) | 108.8 (76.1–212) | 114.6 (108–236) | 195.7 (159–291) | 1284.9 (829–1562) | <0.001 |
aDetermined by the MDRD equation.
P: significance level, comparison of group means with one-way ANOVA.
Correlation and multiple regression analysis
As shown in Table 2, plasma FGF23 had the strongest positive correlation with plasma NGAL followed by serum phosphorus, IL-6, CRP and TNF-α, while it correlated inversely with eGFR and albumin. Interestingly, in multiple regression (standard model), the association between FGF23 as a dependent variable and NGAL persisted (β = 0.27, P = 0.016) (Table 3) even after controlling for age, sex, BMI, eGFR, albuminuria, phosphorus, albumin, TNF-α, IL-6 and CRP, and it was even stronger (β = 0.78, P = 0.006) when the analysis was confined to the 28 subjects with ESRD.
Table 2.
Association of plasma NGAL and FGF23 concentrations with specific biochemical and inflammatory parameters
| Variables | GFRa | Calcium | Phosphorus | Albumin | log10CRP | log10FGF23 | log10TNF-α | log10IL-6 |
|---|---|---|---|---|---|---|---|---|
| Calcium | 0.12 | |||||||
| Phosphorus | −0.62* | 0.06 | ||||||
| Albumin | 0.26*** | 0.61* | −0.14 | |||||
| log10CRP | −0.22** | −0.17 | 0.15 | −0.32* | ||||
| log10FGF23 | −0.72* | −0.18 | 0.57* | −0.34* | 0.38* | |||
| log10TNF-α | −0.45* | −0.16 | 0.28*** | −0.16 | 0.06 | 0.32*** | ||
| log10IL-6 | −0.43* | 0.17 | 0.32*** | −0.3*** | 0.46* | 0.48* | 0.36* | |
| log10NGAL | −0.72* | −0.05 | 0.57* | −0.16 | 0.37* | 0.71* | 0.37* | 0.42* |
aDetermined by the MDRD equation.
Pearson's correlation coefficient: *P < 0.001, **P < 0.05, ***P < 0.01.
Table 3.
Multivariable linear regression analysis model with FGF23 as the dependent variable
| log10FGF23 | β coef. | SE | P-value | 95% CI |
|---|---|---|---|---|
| Age | −0.14 | 0.005 | 0.09 | −0.02–0.001 |
| Sex | 0.053 | 0.115 | 0.5 | −0.14–0.3 |
| eGFR | −0.427 | 0.002 | 0.001 | −0.013 to −0.003 |
| log10 BMI | 0.003 | 0.73 | 0.9 | −1.4–1.4 |
| Phosphorus | 0.14 | 0.06 | 0.13 | −0.03–0.2 |
| Albumin | −0.09 | 0.12 | 0.25 | −0.4–0.1 |
| log10CRP | 0.08 | 0.14 | 0.36 | −0.15–0.4 |
| log10IL-6 | 0.11 | 0.19 | 0.19 | −0.13–0.6 |
| log10TNFα | −0.04 | 0.15 | 0.6 | −0.37–0.21 |
| log10NGAL | 0.27 | 0.18 | 0.016 | 0.08–0.78 |
coef., coefficient; SE, standard error; CI, confidence interval.
Studies in mice
Effects of acute LPS treatment on FGF23 plasma levels
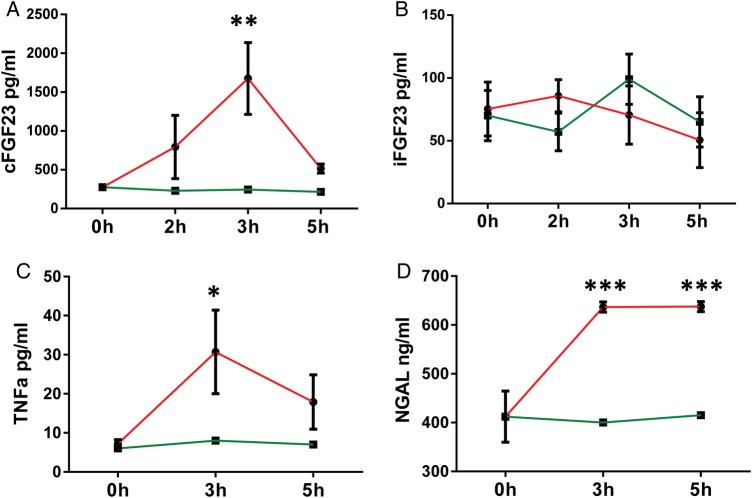
A single i.p. injection of LPS in mice showed a trend toward higher plasma cFGF23 after 2 h (LPS versus placebo: 793 ± 406 versus 230 ± 40 pg/mL; P = ns), significantly higher levels after 3 h (1676 ± 463 versus 245 ± 10 pg/mL; P = 0.003) and a decrease toward baseline after 5 h (515 ± 58 versus 215 ± 7 pg/mL; P = ns; Figure 1A), while it did not affect the plasma levels of iFGF23 (Figure 1B). Plasma TNF-α responded similarly, with an increase after 3 h (LPS versus placebo: 30.7 ± 10.7 versus 8 ± 1.5 pg/mL; P = 0.03) and a return toward baseline after 5 h (17.9 ± 6.9 versus 7 ± 0.6 pg/mL; P = ns; Figure 1C), while plasma NGAL responded in a more sustained manner by rising to 636 ± 10.49 versus 400 ± 15 ng/mL (P < 0.001) after 3 h and staying at 637.5 ± 10.4 versus 415 ± 10 ng/mL (P < 0.001) after 5 h (Figure 1D). Because the clinical cross-sectional analysis showed a strong independent association of FGF23 with NGAL (see above), we tested in mice if NGAL may be a direct stimulus for FGF23 production. Plasma cFGF23 levels, however, did not change 3 or 5 h after i.p. injection of 2, 10 or 25 μg NGAL (5 mice/dose/time point) (not shown).
FIGURE 1.
Effect of acute inflammation on FGF3 regulation: acute inflammation was induced by a single injection (i.p.) of 3.3 μg/kg LPS and mice were sacrificed at hour 3 and 5. Plasma levels of (A) C-terminal FGF23, (B) intact FGF23, (C) TNF-α and (D) NGAL. Data are presented as mean ± SD, n = 4 per group. * P < 0.05 versus control, ** P < 0.01 versus control on ANOVA. Green line = vehicle, red line = LPS.
Effects of sustained, intermittent and combined sustained plus intermittent chronic LPS treatment on FGF23 plasma levels
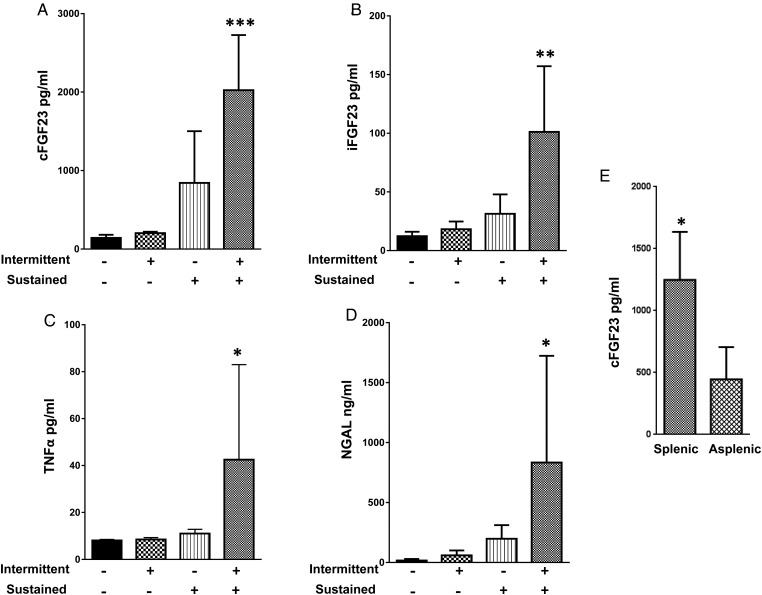
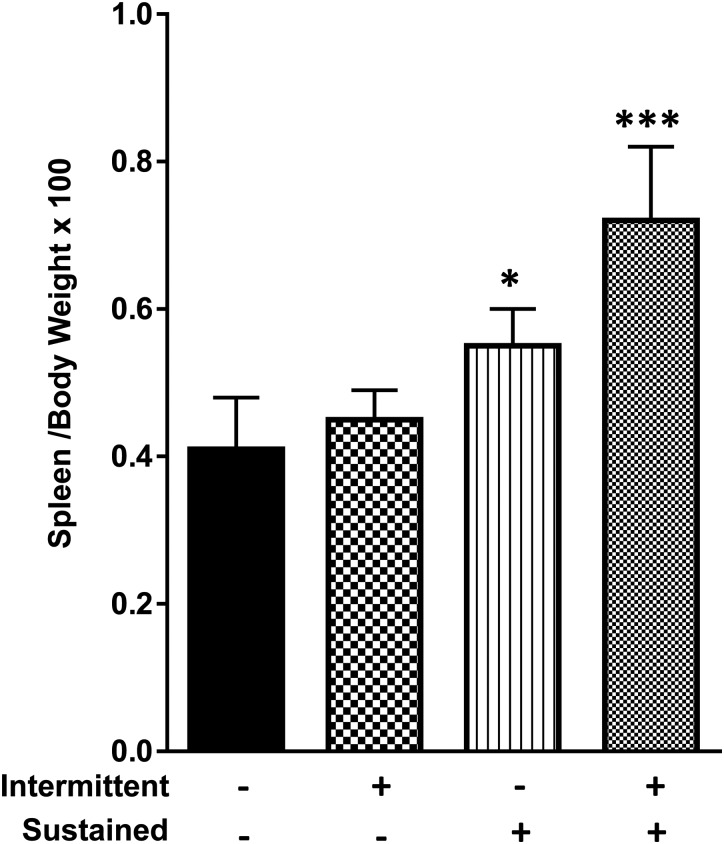
Plasma FGF23 was studied in two mouse models of chronic inflammation consisting of either sustained (s.q. pellets) or intermittent (daily i.p. injection) treatment with LPS or placebo for 2 weeks, and in one mouse model of acute on chronic inflammation (LPS s.q. pellets plus a single LPS i.p. injection on the day of sacrifice). The plasma levels of cFGF23, iFGF23, TNF-α and NGAL (Figure 2A–D) were unchanged in both the sustained and intermittent chronic LPS models, although the spleen weight was increased at necropsy in the sustained chronic LPS mice, suggesting the presence of inflammation (Figure 3A). Conversely, the combined sustained plus intermittent chronic LPS model displayed a large rise in the plasma levels of both cFGF23 (LPS versus placebo: 2024 ± 703 versus 142 ± 40 pg/mL; P < 0.001) and iFGF23 (101.2 ± 55.9 versus 12.3 ± 3.8 pg/mL; P = 0.001), as opposed to the exclusive increase in cFGF23 in the acute LPS treatment experiment. Also, TNF-α (42.4 ± 40.5 versus 8.12 ± 0.37; P = 0.05) and NGAL (834 ± 890 versus 16.4 ± 12.5 ng/mL; P = 0.03) were markedly higher in this model (Figure 2C and D).
FIGURE 2.
Effect of chronic intermittent, chronic sustained and chronic sustained plus intermittent LPS treatment on FGF23 expression. Chronic intermittent treatment consisted of a 3.3 μg/kg i.p. LPS injection daily for 2 weeks. Chronic sustained treatment consisted of subcutaneous sustained-release LPS pellets delivering 2 mg/kg/day for 2 weeks. Chronic sustained plus intermittent treatment consisted of sustained-release LPS pellets as above plus 3.3 μg/kg i.p. LPS on the day of sacrifice. All mice were sacrificed 5 h after the last injection. Plasma levels of (A) C-terminal FGF23, (B) intact FGF23, (C) TNF-α and (D) NGAL. Data are presented as mean ± SD, n = 5 per group. * P < 0.05 versus control, ** P < 0.01 versus control, *** P < 0.001 versus control on ANOVA. (E) Effect of chronic sustained plus intermittent LPS treatment on FGF23 expression in mice with and without spleen, n = 5 per group. * P = 0.02 versus asplenic mice.
FIGURE 3.
Spleen weight of animals controlled for body weight treated with chronic intermittent, chronic sustained and chronic sustained plus intermittent LPS treatment. Data are presented as mean ± SD, n = 5 per group. * = P < 0.05 versus control, *** P < 0.001 versus control.
Effects of LPS treatment on FGF23 expression in bone, heart and spleen tissues
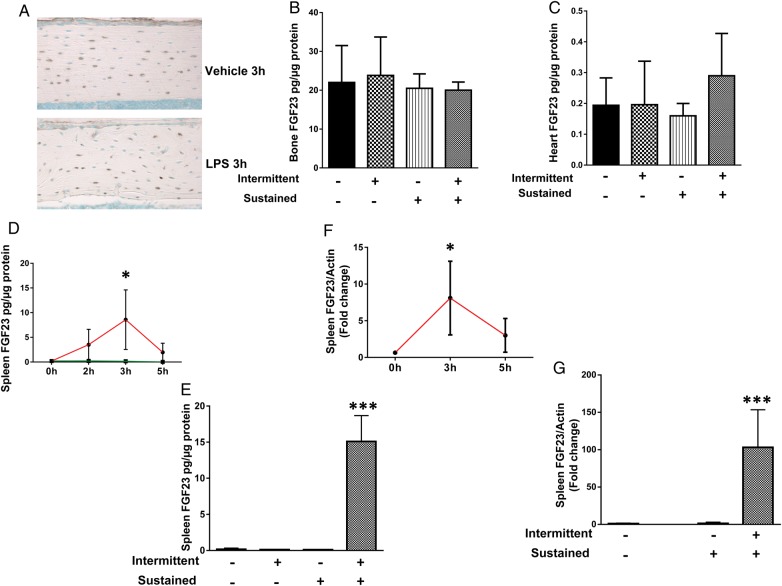
Bone and heart tissues were studied as possible sources of circulating FGF23 based on prior reports [19, 23], while the spleen was studied because of increased organ weight in the sustained chronic and combined sustained plus intermittent chronic LPS groups (Figure 3). Immunohistochemistry of cortical bone showed basal FGF23 expression in osteocytes (positive cells stain brown) of control mice but no significant increase in protein expression after acute LPS exposure (Figure 4A). Cortical bone lysates from control mice also showed basal FGF23, protein levels, and similar levels were observed in a sustained chronic, intermittent chronic and combined sustained plus intermittent chronic LPS groups (Figure 4B). The heart yielded similar findings with detectable levels of FGF23 but without differences among treatment groups (Figure 4C). In contrast, the concentration of FGF23 protein was high in spleen lysates from the acute LPS treatment experiment (3 h after acute injection) (Figure 4D) and from the acute on chronic group (Figure 4E). These observations were correlated with concomitant increases in FGF23 mRNA expression, indicating that increased transcription is responsible for the higher FGF23 protein concentration (Figure 4F and G). To confirm that the spleen provides a source of circulating FG23, we repeated the combined sustained and intermittent LPS treatment, the most intensive FGF23-inducing maneuver, in both mice with spleen or without spleen. The plasma cFGF23 levels were significantly lower in the asplenic mice compared with mice with spleen (Figure 2E), although these were somewhat higher than for control mice from the previous experiments, suggesting that spleen contributes significantly to the circulating FGF23 levels in response to LPS treatment.
FIGURE 4.
(A) Bone FGF23 protein expression on immunohistochemistry from acute experiment. (B) Bone lysate FGF23 protein expression by ELISA from chronic experiment. (C) Heart lysate FGF23 protein expression by ELISA from chronic experiment. (D) Spleen lysate FGF23 protein by ELISA in acute experiment. (E) spleen expression of FGF23 mRNA from acute experiment. (F) spleen lysate FGF23 protein by ELISA from chronic experiment. (G) spleen expression of FGF23 mRNA from chronic experiment. Data are presented as mean ± SD, n = 5 per group. * P < 0.05 versus control, *** P < 0.001 versus control in ANOVA.
Effects of LPS exposure on NF-κB activation
It is known that LPS acts through binding to the toll-like receptor 4 (TLR-4), followed by downstream activation of the transcription factor NF-κB [24]. Since recent evidence implicates NF-κB in the regulation of FGF23 synthesis [25], we proceeded to investigate the DNA-binding activity of the NF-κB p65 subunit in spleen nuclear extracts from the experimental groups described above. No increase in activity was observed in the acute inflammation group (Figure 5A). However, the acute on chronic LPS group exclusively displayed increased activity of NF-κB p65 in the spleen tissue (Figure 5B).
FIGURE 5.
DNA-binding activity of the NF-κb present in the nuclear extract of the spleen measured by colorimetric ELISA assay: (A) the acute experiment, (B) the chronic experiment. Data are presented as mean ± SD, n = 5 per group. ** P < 0.01 versus control, in ANOVA.
DISCUSSION
The present study confirms the existence of an association between the circulating levels of FGF23 and systemic inflammation in CKD patients. In addition, the study offers an original and plausible explanation for this association by providing the evidence that normal mice treated with combined sustained and intermittent chronic LPS treatment, a model of chronic inflammation in CKD, display high production rates and circulating levels of FGF23. Furthermore, our study demonstrates for the first time that the spleen contributes a significant portion of increased circulating FGF23 levels induced by LPS.
FGF23 has emerged as an important predictor of rapid progression of renal disease, CVD and all-cause mortality in CKD patients [2, 3, 26]. Uncertainties linger about the mechanisms implicated in the regulation of FGF23 levels in CKD patients. As already mentioned in the introduction, the role of phosphorus retention has been somewhat challenged by recent experimental and clinical studies [4]. In the last few years, a direct correlation has been noted between circulating FGF23 and various inflammatory markers, including CRP, TNF-α and IL-6, in patients with CKD [7, 8, 27, 28]. However, mechanistic studies addressing the association between inflammation and FGF23 are scarce [25, 29].
CKD coexists with a complex form of systemic inflammation, whereby sustained smoldering inflammation is intermittently aggravated by sporadic and variably intense inflammatory bursts [9]; some authors point to low-grade endotoxemia of intestinal origin as a possible cause [16]. These observations led us to explore the relationship between inflammation and FGF23 by treating normal mice either acutely or chronically with LPS with distinct protocols. We found that a single acute injection of LPS caused a transient increase in plasma cFGF23, but not in iFGF23 levels. Since cFGF23 assay detects both c-fragment and intact FGF23, increased cFGF23 levels suggest increased production of FGF23, also supported by the evidence of increased expression of FGF23 mRNA in the spleen tissue of these mice. Furthermore, no increase in iFGF23 levels indirectly implies simultaneous increased cleavage of FGF23 into the C and N fragments. These results are in agreement with a recently published study by David et al. [28], showing that induction of acute inflammation with a single injection of Brucella abortus or IL-1β results in increased cFGF23 but not iFGF23. The proposed mechanism of these findings was simultaneous increase in production and cleavage of FGF23 [29].
Interestingly, in chronic experiments, only the combined sustained plus intermittent LPS treatment yielded a significant increase in both cFGF23 and iFGF23 levels. This effect was not equivalent to that elicited by a single acute injection of LPS, since the combination of sustained plus intermittent LPS caused an increase in both cFGF23 and iFGF23 and, additionally, the FGF23 plasma levels were up for at last 5 h (last time tested) when compared with the transient increase at 3 h observed in the acute experiment. We do not believe that the increase in FGF23 following combined sustained and intermittent LPS is attributable to the increase in total LPS dose, because in an additional experiment sustained administration of an LPS dose that was twice as high (4 mg/kg/day) for 3 weeks did not increase the FGF23 levels (data not shown). Furthermore, absence of stimulation in the intermittent and sustained chronic groups is not explained by endotoxin tolerance [30] since our combined sustained and intermittent group had the opposite response, i.e. stimulation of FGF23 synthesis. Nonetheless, the combined sustained and intermittent LPS exposure scenario is the closest simulation of inflammation in CKD patients, i.e. smoldering inflammation intermittently aggravated by sporadic bursts. Our results are consistent with prior evidence of increased plasma cFGF23 and iFGF23 in mice receiving daily injection of IL-1β for 4 days [29]. These authors explained their observation as the result of chronic inflammation-induced saturation and/or a reduction in activity of the FGF23 cleavage enzymes.
The most notable observation of our study was that the spleen was the major source of FGF23 synthesis in both the acute and the combined sustained plus intermittent chronic LPS models. This was supported by reduced circulating FGF23 levels in asplenic mice compared with those with intact spleen. We treated the mice with relatively low-dose LPS to avoid confounding of results from concomitant renal injury [18]. Perhaps this dose and/or timing of LPS administration was not sufficient to elicit production of FGF23 in the bone. However, the possibility that the bone also contributed to circulating FGF23 cannot be totally excluded and, perhaps due to the low level of expression, FGF23 may not have been detected by immunostaining or ELISA. Our results are consistent with a recent study where acute treatment with high-dose LPS (50 μg/kg) increased the spleen expression of FGF23 mRNA [25]. The latter study, however, did not report splenic FGF23 protein expression or effects of chronic LPS administration. Under physiologic conditions, FGF23 is mainly expressed by bone osteocytes and osteoblasts, but much lower concentrations are also detected in other tissues, including heart, thymus, skeletal muscle, brain and liver [19]. Our observation, along with prior evidence that splenocytes express the FGF23 receptor [19], leads us to speculate on possible regulatory effects of FGF23 on either the native or acquired immune system. This possibility is further supported by evidence that normal thymus expresses FGF23 and that FGF23 and klotho double-knockout mice experience both thymus atrophy and a reduced number of splenocytes [31, 32]. Our study sheds some light in this area, but much more work remains to be done to clarify the significance of FGF23 in inflammation.
TLR and NF-κB activation are thought to mediate LPS-induced production of FGF23 [25]. Treatment of dendritic cells in culture with a TLR-2 agonist increased FGF23 expression, while the use of a NF-κB inhibitor blocked LPS-induced synthesis of FGF23 [25]. In the present study, mice treated with a combination of sustained plus intermittent chronic LPS showed increased expression of FGF23 mRNA and increased NF-κB activity in splenic nuclear extracts, as well as increased plasma levels of both cFGF23 and iFGF23. Conversely, mice that received a single acute LPS injection showed increased expression of FGF23 mRNA but no change of NF-κB in splenic nuclear extracts and an isolated increase of cFGF23 in plasma. Taken together, these observations suggest that activation of NF-κB reduces the activity of the FGF23 cleavage system in mice exposed to a combination of constant and intermittent chronic LPS.
Consistent with previous studies, we demonstrate the association of FG23 with inflammatory cytokines CRP, IL-6 and TNF-α in our clinical study. The novelty of this observation is the independent relationship of FGF23 with the acute phase reactant NGAL. Similar to FGF23, serum and urinary NGAL levels increase immediately in acute kidney injury [33] and gradually with the progression of CKD, and predict the progression of renal disease and mortality in this population [34–38]. These characteristics and our observation on circulating NGAL and FGF23 suggest that these two proteins may regulate each other, or that an undefined upstream effector(s) may regulate both. Since injection of normal mice with NGAL did not affect FGF23 levels, and LPS injection caused sustained elevation of NGAL at 3 and 5 h but only a transient increase in cFGF23 levels at 3 h, the latter is the most likely explanation for our clinical observation. NF-κB has been shown to induce NGAL [39] and FGF gene expression [25, 29], and since NF-κB activity [40, 41] has been shown to be upregulated in CKD in association with oxidative stress and inflammation, it may be the upstream signal regulating FGF23 and NGAL. Further studies are required to determine whether FGF23 and NGAL collectively play a pathological role in the development of CVD associated with CKD.
Our study has several limitations. We do not provide the specific mechanism responsible for synthesis of FGF23 by LPS administration. However, by the significant circumstantial evidence of upregulation of NF-κB activity, we make a strong case regarding its role in FGF23 synthesis by inflammation. In our clinic study, we only measured a limited set of markers of mineral metabolism, which precludes us from examining the full impact of this functional system on the relationship between NGAL and FGF23 levels. Second, the cross-sectional design of the study precludes inference about possible effects of the NGAL–FGF23 association on clinical outcome.
In conclusion, LPS-induced inflammation stimulates FGF23 synthesis, possibly by NF-κB activation. It remains to be explored if the same is the case for other modes of inflammation. Moreover, we demonstrate that spleen is the major source of the increase in circulating FGF23. These results suggest a potential new mechanism for the increase in FGF23 in CKD patients since CKD is a state of heightened inflammation. Further studies are required to exploit this observation by finding new ways to reduce FGF23 levels in CKD patients.
FUNDING
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR001120 and by NIH-NIDDK U24DK07616 (sub-award 25732-14) to S.B. and in part by NIH-NCCAM AT004490 to P.F. This study was also supported in part by the NIH/NIA grant 5 R01 AG045040 to S.L.W.
CONFLICT OF INTEREST STATEMENT
No financial conflict of interest exists.
REFERENCES
- 1. Mirza MA, Larsson A, Lind L et al. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 2009; 205: 385–390 [DOI] [PubMed] [Google Scholar]
- 2. Gutierrez OM, Mannstadt M, Isakova T et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gutierrez OM, Januzzi JL, Isakova T et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119: 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 2012; 82: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang S, Gillihan R, He N et al. Dietary phosphate restriction suppresses phosphaturia but does not prevent FGF23 elevation in a mouse model of chronic kidney disease. Kidney Int 2013; 84: 713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu S, Tang W, Zhou J et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 2006; 17: 1305–1315 [DOI] [PubMed] [Google Scholar]
- 7. Mendoza JM, Isakova T, Ricardo AC et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 2012; 7: 1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gutierrez OM, Wolf M, Taylor EN. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin J Am Soc Nephrol 2011; 6: 2871–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol 2001; 12: 1549–1557 [DOI] [PubMed] [Google Scholar]
- 10. Arar NH, Plaetke R, Arar MY et al. Incorporating the contextual assessment approach to regimens used in genetic family studies. Genet Med 2002; 4: 451–463 [DOI] [PubMed] [Google Scholar]
- 11. Knowler WC, Coresh J, Elston RC et al. The family investigation of nephropathy and diabetes (FIND): design and methods. J Diabetes Complic 2005; 19: 1–9 [DOI] [PubMed] [Google Scholar]
- 12. Jonsson KB, Zahradnik R, Larsson T et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 2003; 348: 1656–1663 [DOI] [PubMed] [Google Scholar]
- 13. Barreto DV, Barreto FC, Liabeuf S et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int 2010; 77: 550–556 [DOI] [PubMed] [Google Scholar]
- 14. Wanner C, Zimmermann J, Schwedler S et al. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl 2002; 80: 99–102 [DOI] [PubMed] [Google Scholar]
- 15. Stenvinkel P, Heimburger O, Paultre F et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 1999; 55: 1899–1911 [DOI] [PubMed] [Google Scholar]
- 16. Lau WL, Kalantar-Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron 2015; 130: 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhargava R, Altmann CJ, Andres-Hernando A et al. Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-alpha antibodies. PLoS One 2013; 8: e79037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith BJ, Lightfoot SA, Lerner MR et al. Induction of cardiovascular pathology in a novel model of low-grade chronic inflammation. Cardiovasc Pathol 2009; 18: 1–10 [DOI] [PubMed] [Google Scholar]
- 19. Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012; 92: 131–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ubaidus S, Li M, Sultana S et al. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc (Tokyo) 2009; 58: 381–392 [DOI] [PubMed] [Google Scholar]
- 21. Harris SE, MacDougall M, Horn D et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone 2012; 50: 42–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abboud SL, Ghosh-Choudhury N, Liu LC et al. Osteoblast-specific targeting of soluble colony-stimulating factor-1 increases cortical bone thickness in mice. J Bone Miner Res 2003; 18: 1386–1394 [DOI] [PubMed] [Google Scholar]
- 23. Yan L, Mathew L, Chellan B et al. S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner. Arterioscler Thromb Vasc Biol 2014; 34: 1399–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34: 637–650 [DOI] [PubMed] [Google Scholar]
- 25. Masuda Y, Ohta H, Morita Y et al. Expression of FGF23 in activated dendritic cells and macrophages in response to immunological stimuli in mice. Biol Pharm Bull 2015; 38: 687–693 [DOI] [PubMed] [Google Scholar]
- 26. Kendrick J, Cheung AK, Kaufman JS et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22: 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manghat P, Fraser WD, Wierzbicki AS et al. Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos Int 2010; 21: 1853–1861 [DOI] [PubMed] [Google Scholar]
- 28. Jean G, Terrat JC, Vanel T et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 2009; 24: 2792–2796 [DOI] [PubMed] [Google Scholar]
- 29. David V, Martin A, Isakova T et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 2016; 89: 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lopez-Collazo E, del Fresno C. Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit Care 2013; 17: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakatani T, Sarraj B, Ohnishi M et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (FGF23)-mediated regulation of systemic phosphate homeostasis. FASEB J 2009; 23: 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okada S, Yoshida T, Hong Z et al. Impairment of B lymphopoiesis in precocious aging (klotho) mice. Int Immunol 2000; 12: 861–871 [DOI] [PubMed] [Google Scholar]
- 33. Mori K, Lee HT, Rapoport D et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 2005; 115: 610–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malyszko J, Malyszko JS, Bachorzewska-Gajewska H et al. Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant Proc 2009; 41: 158–161 [DOI] [PubMed] [Google Scholar]
- 35. Bolignano D, Coppolino G, Campo S et al. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 2007; 27: 373–378 [DOI] [PubMed] [Google Scholar]
- 36. Ding H, He Y, Li K et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol 2007; 123: 227–234 [DOI] [PubMed] [Google Scholar]
- 37. Yang YH, He XJ, Chen SR et al. Changes of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: one year observational follow-up study. Endocrine 2009; 36: 45–51 [DOI] [PubMed] [Google Scholar]
- 38. Lindberg S, Jensen JS, Mogelvang R et al. Plasma neutrophil gelatinase-associated lipocalinin in the general population: association with inflammation and prognosis. Arterioscler Thromb Vasc Biol 2014; 34: 2135–2142 [DOI] [PubMed] [Google Scholar]
- 39. Volpe V, Raia Z, Sanguigno L et al. NGAL controls the metastatic potential of anaplastic thyroid carcinoma cells. J Clin Endocrinol Metab 2013; 98: 228–235 [DOI] [PubMed] [Google Scholar]
- 40. Pedruzzi LM, Cardozo LF, Daleprane JB et al. Systemic inflammation and oxidative stress in hemodialysis patients are associated with down-regulation of Nrf2. J Nephrol 2015; 28: 495–501 [DOI] [PubMed] [Google Scholar]
- 41. Hueso M, Torras J, Carrera M et al. Chronic kidney disease is associated with an increase of intimal dendritic cells in a comparative autopsy study. J Inflamm (Lond) 2015; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]