Abstract
Background:
Type 2 diabetes mellitus may alter the effect of physical activity on physical and cognitive function.
Methods:
The Lifestyle Interventions and Independence for Elders (LIFE) trial randomized controlled clinical trial of physical activity intervention (walking, resistance training, and flexibility exercises) enrolled adults aged 70–89 years who were sedentary and non-demented and who had functional limitations. Standardized measures of physical and cognitive function were collected an average of 2 years post-randomization. Differences between the intervention and control groups from 415 individuals with diabetes and 1,061 individuals without diabetes were contrasted with analyses of covariance.
Results:
At 24 months, assignment to the physical activity intervention resulted in 0.019 m/s relatively faster average 400-meter gait speeds (p = .007 overall) both for individuals with and without diabetes (intervention × diabetes interaction p = .99). No benefits were seen on scores from a physical performance battery. Performance on cognitive tests was better among participants assigned to the physical activity intervention compared with control only for those with diabetes, particularly for global cognitive function (p = .02) and delayed memory (p = .005), with mean [95% confidence intervals] for benefit from physical activity intervention of 0.114 [0.007,0.111] and 0.208 [0.030,0.387] standard deviations, respectively.
Conclusions:
Physical activity intervention improved the gait speed of older, sedentary individuals with and without diabetes. The cognitive function benefits occurred among participants with, but not without, diabetes. The mechanisms through which physical activity affects physical and cognitive function in older adults may differ for individuals by diabetes status.
Keywords: Behavioral intervention, Type 2 diabetes, Clinical trials
One-quarter of U.S. adults who are 65 years or older have Type 2 diabetes mellitus (1). This accelerates their development of many age-related conditions and geriatric syndromes (2–4). Physical inactivity is an important component of the geriatric syndrome in diabetes (5). Sedentary lifestyle in older adults is associated with poorer control of diabetes and increased risk for comorbidities related to diabetes and insulin resistance (3,6). While treatment guidelines generally recommend that sedentary individuals with diabetes increase their physical activity (7), these recommendations may be of increasing importance later in life.
Declines in mobility and cognitive function are two features of the geriatric syndrome that diabetes is known to accelerate. It reduces mobility by lowering muscle quality and increasing neuropathy and peripheral vascular disease (8). It accelerates cognitive decline by disrupting energy metabolism in the brain, restricting its blood flow, and increasing atrophy and cerebrovascular disease (9). Physical activity may be expected to protect mobility and cognitive function. However, there is no clear evidence that structured physical activity programs to increase physical function benefit physical and cognitive function in older sedentary individuals with diabetes. These individuals face additional disease-related barriers towards increasing their physical activity including increased rates of hospitalization, sarcopenia, frailty, and complications such as arthritis, impaired vision, heart disease, and neuropathy that may diminish the effectiveness of interventions (2,8).
We conducted an exploratory analysis of data from a randomized controlled clinical trial of a physical activity intervention that included large numbers of older individuals with and without diabetes and standardized assessments of physical and cognitive function. The Lifestyle Interventions and Independence for Elders (LIFE) trial found that physical activity intervention tailored to older individuals preserved mobility, but had no overall effects on cognitive function (10,11). We hypothesized that the intervention may have different effects on physical and cognitive function, depending on individuals’ diabetes status.
Methods
LIFE was an eight-center, single-blinded, randomized controlled trial of an intervention to increase physical activity versus a health education control condition (12,13, Supplementary Appendix). Participants were sedentary, aged 70–89 years, and could walk 400 meters in 15 minutes despite lower extremity functional limitations. LIFE was approved by all sites’ institutional review boards; informed consent was obtained from all participants.
Interventions
The physical activity intervention focused on walking, strength, flexibility, and balance training (10,12). Each week, participants were to attend two center-based visits and perform home-based activity 3–4 times, with goals of 30 minutes of walking at moderate intensity, 10 minutes of primarily lower extremity strength training with ankle weights, 10 minutes of balance training, and large muscle group flexibility exercises.
The health education group attended weekly workshops during the first 26 weeks of the intervention and monthly sessions thereafter (bi-monthly attendance was optional). Topics included travel safety, age appropriate preventive services, and nutrition, with 5–10 minutes of upper extremity stretching and flexibility exercises.
Measures
Diabetes was defined by self-report, current use of medications, or fasting glucose ≥126mg/dL at enrollment. The Short Physical Performance Battery (SPPB) consists of a 4-meter walk at usual pace, a timed repeated chair stand, and three increasingly more difficult standing balance tests (14). The total score ranges from 0 (worst) to 12 (best). Gait speed was calculated from a 400-meter walk test (10). For those who did not complete the walk, it was based on the portion of the walk that was completed.
A neuropsychological battery was administered by masked interviewers at baseline and 24 months (11). Three computer-based cognitive tests were administered at baseline and at either 18 or 30 months, depending on when participants were enrolled (11,15). The interviewer-administered tests included the Modified MiniMental State Exam (3MSE), a test of global cognitive function; the Wechsler Adult Intelligence Scale-III Digit Symbol Coding test (DSC), a test of psychomotor speed, attention, and working memory; and the Hopkins Verbal Learning Test-Revised (HVLT-D), a test of delayed recall. The computer tests added sensitivity for speed of processing and executive function: 1-back and 2-back tasks, the Eriksen Flanker task, and a task-switching paradigm. Higher scores reflect better performance on the 3MSE, DSC, HVLT-D, and n-back tests; lower scores reflect better performance on the Eriksen Flanker task and task-switching tests.
Self-reported demographic characteristics, medical and hospitalization history, body mass index, and medication use were collected at baseline. Physical activity (min/wk) was assessed with the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (16). Hospitalizations were based on self-report.
Statistical Analysis
Baseline risk factors and measures of physical and cognitive function were compared between intervention and diabetes groups using analyses of variance and logistic regression. A 1% winsorization was used to reduce the impact of extreme cognitive function scores: scores below the 1st percentile were replaced by the 1st percentile and scores above the 99th percentile were replaced by the 99th percentile. Individual scores were standardized by dividing their difference from the baseline cohort-wide mean by the standard deviation and ordered so that positive scores reflected better performance. A composite, which we loosely refer to as executive function, was formed by averaging standardized measures from the computer-administered battery (11). Analyses of covariance were used to assess mean follow-up physical and cognitive measures, with adjustment for baseline values and inclusion of age, education, sex, and race. Additional covariates were included in supporting analyses.
Results
Our analyses were based on the 1,476 (90.3%) of 1,635 participants who provided post-randomization physical and cognitive function data. Compared with those missing assessments, participants included in analyses had faster baseline mean 400-meter gait speeds (p < .001) and better performance on the 1-back cognitive function test (p = .03), but did not differ markedly (p > .05) for any other baseline factors (Table 1). They also did not differ on the prevalence of diabetes (p = .56) or intervention assignment (p = .38).
Table 1.
Risk Factors and Baseline Cognitive and Physical Function Scores for Sedentary Older Adults With and Without Diabetes by Intervention Assignment: N (percent) or mean (SD)
| Physical Activity | Health Education | Physical Activity vs Health Education | Diabetes vs No Diabetes | |||
|---|---|---|---|---|---|---|
| Diabetes, N = 199 | No Diabetes N = 536 | Diabetes, N = 216 | No Diabetes, N = 525 | p-Value | p-Value | |
| Covariates | ||||||
| Age, y | ||||||
| 70–79 | 134 (67.3) | 294 (54.8) | 143 (66.2) | 270 (51.4) | .32 | <.001 |
| 80–89 | 65 (32.7) | 242 (45.2) | 73 (33.8) | 255 (48.6) | ||
| Sex | ||||||
| Female | 126 (63.3) | 370 (69.0) | 134 (62.0) | 369 (70.3) | .87 | .01 |
| Male | 73 (36.7) | 166 (31.0) | 82 (38.0) | 156 (29.7) | ||
| Education | ||||||
| High school or less | 94 (47.2) | 183 (34.1) | 79 (36.6) | 183 (34.9) | .35 | .01 |
| Beyond high school | 105 (52.8) | 353 (65.9) | 137 (63.4) | 342 (65.1) | ||
| Race/Ethnicity | ||||||
| African American | 47 (23.6) | 105 (19.6) | 40 (18.5) | 74 (14.1) | ||
| Non-Hispanic white | 137 (68.8) | 405 (75.6) | 159 (73.6) | 422 (80.4) | .03 | .02 |
| Other | 15 (7.5) | 26 (4.8) | 17 (7.9) | 29 (5.5) | ||
| Body mass index, kg/m2 | 31.8 (0.4) | 29.5 (0.2) | 31.9 (0.4) | 29.5 (0.3) | .84 | <.001 |
| Prior cardiovascular disease | ||||||
| No | 123 (61.8) | 402 (75.0) | 142 (65.7) | 374 (71.2) | .45 | <.001 |
| Yes | 76 (36.2) | 134 (25.0) | 74 (34.3) | 151 (28.8) | ||
| Hypertension, Miss = 1 | ||||||
| No | 33 (16.6) | 130 (24.2) | 32 (14.9) | 130 (24.8) | .90 | <.001 |
| Yes | 166 (83.4) | 406 (75.8) | 183 (85.1) | 395 (75.2) | ||
| CHAMPS, min/wk | 77.7 (136.7) | 74.9 (122.5) | 77.6 (113.7) | 86.8 (138.8) | .21 | .68 |
| Apo-E4 gene, Miss = 151 | ||||||
| 0 alleles | 149 (82.3) | 360 (75.5) | 156 (78.8) | 353 (75.3) | .165 | .05 |
| 1 or 2 alleles | 32 (17.7) | 117 (24.5) | 42 (21.2) | 116 (24.7) | ||
| Physical Function | ||||||
| SPPB | 7.46 (1.56) | 7.38 (1.59) | 7.31 (1.62) | 7.13 (1.83) | .07 | .11 |
| 400-meter gait speed, m/s | 0.82 (0.17) | 0.83 (0.17) | 0.81 (0.17) | 0.83 (0.16) | .38 | .04 |
| Cognitive Function* | ||||||
| 3MSE | 91.5 (0.4) | 91.7 (0.2) | 91.4 (0.4) | 91.9 (0.2) | .73 | .30 |
| DSC | 45.1 (0.9) | 46.3 (0.6) | 45.7 (0.9) | 47.5 (0.6) | .13 | .05 |
| HVLT-D | 7.4 (0.2) | 7.9 (0.1) | 7.4 (0.2) | 7.8 (0.1) | .51 | .005 |
| n-back task, % correct | ||||||
| 1-back | 0.80 (0.01) | 0.82 (0.01) | 0.82 (0.01) | 0.82 (0.01) | .56 | .18 |
| 2-back | 0.51 (0.01) | 0.51 (0.01) | 0.50 (0.01) | 0.51 (0.01) | .76 | .80 |
| Task switching, ms | ||||||
| No switch | 1,466 (53) | 1,460 (34) | 1,419 (48) | 1,404 (33) | .18 | .83 |
| Switch | 2,408 (77) | 2,447 (49) | 2,304 (67) | 2,363 (49) | .11 | .42 |
| Eriksen Flanker, ms | ||||||
| Congruent | 662 (13) | 643 (8) | 681 (15) | 639 (8) | .11 | .005 |
| Incongruent | 732 (16) | 716 (10) | 766 (19) | 708 (10) | .71 | .006 |
Notes: 3MSE = Modified MiniMental State Exam; CHAMPS = Community Healthy Activities Model Program for Seniors; DSC = Digit Symbol Coding; HVLT-D = Hopkins Verbal Learning Test-Revised; SPPB = Short Physical Performance Battery.
*Higher scores reflect better performance for the SPPB (0 to 12), 3MSE (0 to 100), DSC (0 to 133), HVLT-D (0 to 12), n-back tests (0 to 100). Higher scores reflect worse performance for the task switching and Flanker tasks.
Compared with others in our analyses, participants with diabetes were younger and had less formal education. They included a higher prevalence of males, African Americans, cardiovascular disease, and hypertension (Table 1; all p < .05). They also had higher mean body mass indices and slightly lower mean 400-meter gait speeds. Their mean performances on the DSC, HVLT-D, and flanker tasks were worse. The balance between intervention groups afforded by randomization for the full LIFE cohort was maintained in the subset included in our analyses for all factors in Table 1 (all p > .05), except race/ethnicity: there were slightly more African Americans assigned to the physical activity compared with health education intervention (p = .03).
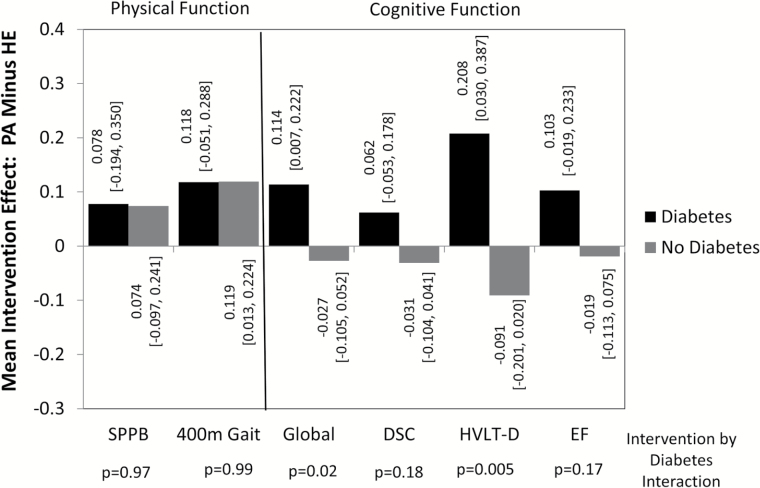
Table 2 and Figure 1 present our primary findings. At 24 months, with covariate adjustment for baseline scores, age, education, gender, and race/ethnicity, overall mean SPPB scores did not differ between intervention groups: the mean [95% confidence interval] difference (physical activity minus health education) was 0.12 [−0.11,0.34] SPPB units (p = .32). Overall differences in 400-meter gait speeds between intervention groups were significant (p = .007). Physical activity intervention participants with diabetes averaged 0.019 [−0.008,0.047] m/s faster gaits; those without diabetes averaged 0.019 [0.002,0.036] m/s faster gaits. Figure 1 presents results in standard deviation units to facilitate comparisons across measures: intervention effects were similar for participants with and without diabetes, with p-values for tests of interaction p = .97 (SPPB) and p = .99 (400-meter gait speed). Analyses based on 4-meter gait yielded results similar to those for 400-meter gait.
Table 2.
Physical and Standardized Cognitive Function Measures at 2 Years for Participants With and Without Diabetes: Mean (SE) With Adjustment for Age, Education, Gender, Race/Ethnicity, and Baseline Value
| Physical Activity | Health Education | PA vs HE | PA vs HE | |||
|---|---|---|---|---|---|---|
| Diabetes | No Diabetes | Diabetes | No Diabetes | Mean [95% CI] | p-Value | |
| SPPB | 7.84 (0.16) | 8.02 (0.10) | 7.72 (0.15) | 7.91 (0.10) | 0.12 [−0.11,0.34] | .32 |
| 400-meter gait, m/s | 0.788 (0.010) | 0.795 (0.006) | 0.769 (0.010) | 0.776 (0.006) | 0.019 [0.005,0.034] | .007 |
| 3MSE, SDs | 0.028 (0.047) | −0.086 (0.028) | −0.121 (0.045) | −0.059 (0.029) | 0.023 [−0.44,0.90] | .51 |
| DSC, SDs | 0.010 (0.043) | −0.010 (0.026) | −0.053 (0.041) | 0.022 (0.026) | −0.005 [−0.067,0.056] | .87 |
| HVLT-D, SDs | −0.018 (0.066) | −0.226 (0.040) | −0.227 (0.063) | −0.135 (0.040) | −0.007 [−0.102,0.087] | .87 |
| Executive function, SDs | 0.070 (0.054) | −0.037 (0.034) | 0.033 (0.053) | 0.018 (0.034) | 0.016 [−0.064,0.095] | .70 |
Notes: 3MSE = Modified MiniMental State Exam; CI = confidence interval; DSC = Digit Symbol Coding; HE = health education; HVLT-D = Hopkins Verbal Learning Test-Revised; PA = physical activity; SD = standard deviation; SE = standard error; SPPB = Short Physical Performance Battery.
Figure 1.
Mean [95% confidence interval] intervention effects (Physical Activity minus Health Education) in standard deviation units for physical function (Short Physical Performance Battery and 400-meter gait) and cognitive function (global composite, digit symbol coding, delayed memory, and executive function) scores with covariate adjustment for age, education, gender, race/ethnicity, and baseline scores.
There were no overall differences between intervention groups for any of the cognitive function measures (Table 2, all p > .50). However, as seen in Figure 1, for each cognitive function measure, the mean differences between physical activity and health education participants were positive for participants with diabetes and negative for those without diabetes, with tests for interactions reaching statistical significance for 3MSE (p = .02) and HVLT-D (p = .005). For these measures, 95% confidence intervals for the mean difference between physical activity and health education participants with diabetes excluded zero: for 3MSE the mean intervention effect was 0.114 [0.007,0.222] standard deviations and for HVLT-D it was 0.208 [0.030,0.387] standard deviations. The 95% confidence intervals for intervention effects included zero among participants with diabetes for the other two cognitive function tests, and for all four tests among those without diabetes. Additional covariate adjustment for all factors in Table 1 (ie, including body mass index, prior cardiovascular disease, hypertension, CHAMPS score, and Apo-E4 genotype) attenuated the interactions for the cognitive function tests slightly. For 3MSE, the interaction no longer reached statistical significance (p = .07). For HVLT-D, it remained highly significant (p = .007). This additional covariate adjustment had no material impact on interactions for other measures.
Differences between participants with and without diabetes in estimated 2-year mean intervention effects (Figure 1) ranged from about 0.1 to 0.3 standard deviations. When baseline cognitive functions were regressed against participants’ age, estimated slopes ranged from −0.025 SD/y for executive function to −0.043 SD/y for DSC test scores. Thus, the estimated intervention effects exceed the magnitude of cognitive decline associated with a difference of several years in age along these regression slopes.
We explored several potential correlates of intervention benefits (Supplementary Table). Attendance in physical activity and health education intervention sessions was similar for participants with and without diabetes (interaction p = .70). CHAMPS physical activity increased similarly at 12 and 24 months among physical activity intervention participants both with and without diabetes. Changes in mean body mass index and blood pressure over time were small in both intervention groups and did not vary by diabetes status. The hospitalization rate was similar between intervention groups for individuals with diabetes. It was slightly higher among physical activity compared with health education participants without diabetes, but not significantly (p = .15). The annual hospitalization rate was negatively associated with 3MSE and HVLT-D scores (both p < .001); including this as a covariate did not alter interactions between diabetes and intervention assignment (3MSE interaction p = .03; HVLT-D interaction p = .007). Adding interactions between intervention assignment and hospitalization rates also did not affect results. Insulin use among participants with diabetes was balanced between intervention groups.
Discussion
The LIFE physical activity intervention significantly increased physical activity and lowered the incidence of major mobility disability (10). During the prior LIFE pilot trial, the physical activity intervention also improved gait speeds and SPPB performance over 1 year (17), but resulted in no overall benefit for cognitive function (11). Our present analyses add the following. First, there was no difference in the effects of the physical activity intervention on 400-meter gait speeds for individuals with and without diabetes. Second, while there were no overall benefits of the intervention on cognitive function, among individuals with diabetes the physical activity intervention resulted in better global cognitive function and delayed memory. Finally, covariate adjustment for hypertension and history of cardiovascular disease did not affect results and we could not attribute differential benefits for individuals with diabetes to differences in markers of adherence (intervention session attendance, participation in physical activity), changes in body mass index, hospitalizations, or insulin use.
Physical Function
Diabetes and its primary risk factor obesity are both strongly associated with impaired mobility function and mobility disability (18–21). Many trials in persons with diabetes have assessed the effects of exercise training on metabolic control and disease risk factors, however we are aware of no reports that the response to exercise differs in persons on the basis of Type 2 diabetes status alone (22). We are also aware of no other study that evaluated the effect of exercise on the risk of mobility disability in older persons with Type 2 diabetes. A number of studies have examined the effect of exercise on changes in self-reported physical functioning in diabetes and have shown significant improvements associated with exercise (23,24). Most have been relatively short in duration and focused on middle-aged participants. The Action for Health in Diabetes (Look AHEAD) trial studied lifestyle intervention designed to reduce caloric intake and increase physical activity in persons with Type 2 diabetes, ages 45–76 years. Its intervention was associated with superior maintenance of self-reported physical function over 4 years (25). Mediation analysis showed that both exercise and weight loss contributed to the benefit. Gait speed was measured in a subset of participants at 8 and 9 years. The intervention benefited gait speeds in both the younger and older participants (25). In the LIFE-pilot study, obesity attenuated the benefits on gait speed and SPPB scores that the physical activity intervention provided (26). A community-based exercise intervention trial in overweight and obese older adults showed that the mobility benefits of exercise were not as well sustained unless they were also paired with weight loss (27). LIFE participants with Type 2 diabetes were heavier than nonparticipants, but had a similar experience with respect to mobility function to those without the disease. The LIFE primary results manuscript included an assessment of the comparability of intervention effects on its primary outcome, incidence of major mobility, across participants grouped by diabetes, insulin resistance, and normal, which were comparable (interaction p = .41) (10).
Cognitive Function
There is not consistent evidence that aerobic exercise benefits the cognitive function of older individuals who are not cognitively impaired (28). Evidence that physical activity interventions differentially benefit cognition among individuals with diabetes is limited and mixed. Baker, et al., reported the effects of a 6-month trial of aerobic exercise in 28 adults (ages 57–83 years) who were either newly diagnosed with diabetes (21%) or prediabetes (29). Compared to a stretching intervention, the physical activity intervention was associated with relative improvements in executive function, but not memory. The Look AHEAD trial found no cognitive benefit of 8–9 years of its lifestyle intervention in 978 adults (aged 45–76 years) with Type 2 diabetes (30). Cohort studies have reported weak associations between physical activity and better cognitive function among individuals with diabetes (31,32). While our findings that physical activity may benefit cognitive function in an older, inactive, and more physically vulnerable cohort with diabetes is most consistent with Baker, et al., we saw evidence of benefit for memory, and less evidence of benefit for executive function. Differences among the cohorts, timeframes, targeted physical activities, and cognitive measures among studies may account for the different findings.
The potential benefit of the LIFE physical activity intervention for cognitive function of individuals with diabetes contrasts with the lack of benefit for those without diabetes. The benefits LIFE has reported for mobility (10) and those we report for gait speed may be attributed to improvements in factors such as inflammation, neuropathy, and vascular function, pathways that cognitive and physical function share but which were not assessed in LIFE (33,34). It may be that cognitive deficits in individuals with diabetes are more closely associated with inflammation and vascular dysfunction than for those without diabetes, enhancing the potency of the LIFE intervention for people with diabetes. Many participants with diabetes had hypertension, heart disease, and obesity: it is possible that the benefits in cognitive function the physical activity intervention provided for these individuals reflected the contribution of a broader set of metabolic and vascular factors. It is possible that physical activity intervention benefited cognitive function by improving diabetes control (35) and that its benefits for physical function were through pathways (eg, increased strength) that did not influence cognitive function in individuals without diabetes. Another possible mechanism is through energy metabolism. Glucose is the primary source of energy for brain, however among individuals with diabetes for whom glucose-based metabolism is less reliable, the brain may adapt to have greater efficiency to draw on alternative energy sources (36,37). In older individuals who have less robust blood-brain barriers, it is possible that physical activity bouts may compete with the brain for glucose resources, leading to lower glucose levels in the brain that potentially counteract benefits from other pathways (eg, inflammation and vascular function) that improve physical function (38). If participants with diabetes had adapted to draw more efficiently on alternatives to glucose-based energy, perhaps they were less susceptible to this phenomenon and more able to accrue benefits from other pathways. This can account for the slightly (nonsignificantly) lower cognitive function seen in the physical activity intervention group.
Potential Mediators of Cognitive Benefits
We saw no evidence that the intervention differentially benefited individuals with diabetes due to increased adherence to the intervention, changes in weight and blood pressure, or differences in insulin use. Hospitalization rates were similar between intervention groups for participants with diabetes, but were slightly higher among physical activity intervention participants compared to health education participants among those without diabetes (39). While higher rates of hospitalization were associated with poorer levels of cognitive function during follow-up, the differential intervention effects we saw on cognition depending on diabetes status could not be attributed to differences in hospitalization rates.
Limitations
Our analyses were initiated to describe the experience of individuals with diabetes in the trial and were not a pre-specified comparison: this enhances the possibility of a chance finding. They were exploratory and require confirmation. As volunteers to a randomized clinical trial, LIFE participants may not be representative of general populations. While other trials of lifestyle interventions have reported beneficial effects on cognitive function within 2 years (40,41), this may be a relatively short span of time to produce cognitive benefits. The mean effect on gait speed that we report (0.019 m/s) is modest, however it falls within the range reported as minimally significant in the LIFE-P trial (0.018 to 0.027 m/s) (42). Diabetes is often underdiagnosed, however excluding participants (N = 38) identified only through testing did not alter findings.
Conclusions
Based on exploratory analysis from the LIFE trial, physical activity interventions may benefit both gait speed and cognitive function in older physically vulnerable and inactive individuals with diabetes, but only gait speed among those without diabetes.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
This work was supported by National Institutes of Health (UO1AG22376, U01AG022376-05A2S, P30AG028740, P30AG21332, 1P30AG031679, P30AG024827, P30AG021342, UL1RR025744, 1R24HD065688-01A1, K07AG3587) and partially supported by USDA (58-1950-7-707). Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the United States Department of Agriculture.
Supplementary Material
References
- 1. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi:10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 2. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: consensus report. Diabetes Care. 2012;35:2650–2664. doi:10.1111/jgs.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bianchi L, Zuliani G, Volpato S. Physical disability in the elderly with diabetes: epidemiology and mechanisms. Curr Diab Rep. 2013;13:824–830. doi:10.1007/s11892-013-0424-6 [DOI] [PubMed] [Google Scholar]
- 4. Araki A, Ito H. Diabetes mellitus and geriatric syndromes. Geriatr Gerontol Int. 2009;9:105–114. doi:10.1111/j.1447-0594.2008.00495.x [DOI] [PubMed] [Google Scholar]
- 5. Abdelhafiz AH, Sinclair AJ. Diabetes, nutrition, and exercise. Clin Geriatr Med. 2015;31:439–451. doi:10.1016/j.cger.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 6. Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985). 2011;111: 1497–1504. doi:10.1152/japplphysiol.00420.2011 [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes Care. 2015;38(Suppl):S20–30. doi:10.2337/dc15-S007 [DOI] [PubMed] [Google Scholar]
- 8. Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–1277. [DOI] [PubMed] [Google Scholar]
- 9. Biessels GJ, Deary IJ, Ryan CM. Cognition in diabetes: a lifespan perspective. Lancet Neurology. 2008;7:184–190. [DOI] [PubMed] [Google Scholar]
- 10. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults. JAMA. 2014;311:2387–2396. doi:10.1136/bmj.i245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sink K, Espeland MA, Castro C, et al. Effect of physical activity on cognitive outcomes in sedentary older adults. JAMA. 2015;314:781–790. doi:10.1001/jama.2015.9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fielding RA, Rejeski WJ, Blair SN, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Bio Sci Med Sci. 2011;66:1226–1237. doi:10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle Interventions and Independence for Elders study: recruitment and baseline characteristics. J Geront A Bio Sci Med Sci. 2013;68:1549–1558. doi:10.1093/gerona/glt064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi:10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espeland MA, Katula JA, Rushing J, et al. Performance of a computer-based assessment of cognitive function measures in two cohorts of seniors. Int J Geriatr Psychiatry. 2013;28:1239–1250. doi:10.1002/gps.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M465–M470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance. J Geront A Biol Sci Med Sci. 2006;61:1157–1165. [DOI] [PubMed] [Google Scholar]
- 18. Figaro MK, Kritchevsky SB, Resnick HE, et al. Diabetes, inflammation, and functional decline in older adults. Diabetes Care. 2006;29:2039–2045. doi:10.2337/dc06-0245 [DOI] [PubMed] [Google Scholar]
- 19. Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes. Diabetes Care. 2007;30:1507–1512. doi:10.2337/dc06-2537 [DOI] [PubMed] [Google Scholar]
- 20. Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11:568–579. doi:10.1111/j.1467-789X.2009.00703.x [DOI] [PubMed] [Google Scholar]
- 21. Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13:46–51. doi:10.1097/MCO.0b013e32833309cf [DOI] [PubMed] [Google Scholar]
- 22. Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord. 2013;14:77–86. doi:10.1007/s11154-012-9234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicolucci A, Balducci S, Cardelli P, et al. Improvement of quality of life with supervised exercise training in subjects with type 2 diabetes mellitus. Arch Intern Med. 2011;171:1951–1953. doi:10.1001/archinternmed.2011.561 [DOI] [PubMed] [Google Scholar]
- 24. Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. NEJM. 2012;366:1209–1217. doi:10.1056/NEJMoa1110294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Houston DK, Leng X, Bray GA, et al. Impact of a long-term intensive lifestyle intervention on physical function. Obesity. 2015;23:77–84. doi:10.1002/oby.20944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manini TM, Newman AB, Fielding R, et al. Effects of exercise on mobility in obese and nonobese older adults. Obesity. 2010;18: 1168–1175. doi:10.1038/oby.2009.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rejeski WJ, Brubaker PH, Goff DC, Jr, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171:880–886. doi:10.1001/archinternmed.2010.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognition in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015;4:CD005381. doi:10.1002/14651858.CD005381.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baker LD, Frank LL, Foster-Schubert K, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22:569–579. doi:10.3233/JAD-2010-100768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Espeland MA, Rapp SR, Bray GA, et al. Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci. 2014;69:1101–1108. doi:10.1093/gerona/glu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colberg SR, Somma CT, Sechrist SR. Physical activity participation may offset some of the negative impact of diabetes on cognitive function. J Am Med Dir Assoc. 2008;9:434–438. doi:10.1016/j.jamda.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 32. Devore EE, Kang JH, Okereke O, Grodstein F. Physical activity levels and cognitive function in women with type 2 diabetes. Am J Epidemiol. 2009;170:1040–1047. doi:10.1093/aje/kwp224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newman AB, Arnold AM, Naydeck BL, et al. Successful aging: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163: 2315–2322. doi:10.1001/archinte.163.19.2315 [DOI] [PubMed] [Google Scholar]
- 34. Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi:10.1016/j.arr.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balducci S, Zanuso S, Cardelli P, et al. Changes in physical fitness predict improvements in modifiable risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study. Diabetes Care. 2012;35:1347–1354. doi:10.2337/dc11-1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amaral AI. Effects of hypoglycaemia on neuronal metabolism in the adult brain: role of alternative substrates to glucose. J Inherit Metab Dis. 2013;36:621–634. doi:10.1007/s10545-012-9553-3 [DOI] [PubMed] [Google Scholar]
- 37. Sickmann HM, Waagepetersen HS. Effects of diabetes on brain metabolism–is brain glycogen a significant player? Metab Brain Dis. 2015;30: 335–343. doi:10.1007/s11011-014-9546-z [DOI] [PubMed] [Google Scholar]
- 38. De Feo P, Gallai V, Mazzotta G, et al. Modest decrements in plasma glucose concentration cause early impairment in cognitive function and later activation of glucose counterregulation in the absence of hypoglycemic symptoms in normal man. J Clin Invest. 1988;82:436–444. doi:10.1172/JCI113616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marsh AP, Applegate WB, Guralnik JM, et al. Hospitalization during a physical activity intervention in older adults at risk of mobility disability. J Am Geriatr Soc. 2016;5:933–43. doi:10.1111/jgs.14114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people. Lancet. 2015;385: 2255–2263. doi:10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 41. Iuliano E, di Cagno A, Aquino G, et al. Effects of different types of physical activity on cognitive functions and attention in older people. Experiment Gerontol. 2015;70:105–110. doi:10.1016/j.exger.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 42. Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.