Abstract
In this analysis, we used social network and spatial data to examine associations between people's drug injection status and their social and/or spatial proximity to others who injected drugs. We recruited 503 rural Kentucky residents who used drugs to participate in the Social Networks among Appalachian People (SNAP) Study (2008–2010). Interviewer-administered surveys collected information on recent (past 6 months) sex, drug-use, and social-support network members (n = 897 ties). Using network simulations, we determined a threshold for the association between social proximity to others who injected drugs and recent injection status (“socially proximal” was defined by a shortest path ≤2). We defined “geographically proximal” as the median road-network distance between pairs of individuals who both injected drugs (≤7 miles (≤11.2 km)). Logistic regression was used to determine the independent and joint associations between the number of socially and/or geographically proximal injecting peers and a person's injection status. After adjustment, the odds of recent injection increased by 0.4% for each injecting peer who was geographically proximal but not socially proximal, 12% for each geographically and socially proximal injecting peer, and 22% for each injecting peer who was socially proximal but not geographically proximal. When implementing network-based interventions which promote cessation of injection drug use, investigators should consider collecting sociometric network data to examine whether the intervention diffuses through the network and whether there are additive or threshold effects.
Keywords: homophily; injection drug use; shortest path; social network analysis; social networks; spatial analysis
The human immunodeficiency virus (HIV)/hepatitis C virus (HCV) “risk environment” has been characterized as a dynamic interplay between social network factors and spatial/structural factors (1–4). However, most research on HIV/HCV risk behaviors among people who use drugs has examined the risk environment from only 1 perspective. From a network perspective, people are more likely to inject drugs if they 1) are introduced to injection by a friend, sex partner, or relative (5–8); 2) are exposed to other people who inject (i.e., drug partners, sex partners, friends, or family members) (6, 9–14); 3) live in social environments where drug use and injection are normative (6, 11, 14) or there is increased communication about injection drug use (14); or 4) perceive a shorter social distance between themselves and others who inject (14). From a spatial perspective, drug availability, price, and purity vary by location and can influence a person's likelihood of injecting and/or frequency of injection (15–18). Among people who inject drugs, injection risk practices are shaped by aspects of both the built environment (i.e., location of/distance to prevention services/materials, availability of/access to transportation) and the social environment (i.e., neighborhood disadvantage, unemployment rates, poverty) (19–21).
Few studies have assessed the combined effect of social network and spatial/structural factors on injection status; however, there is a rationale for doing so. Because people who are socially connected may also be in close geographic proximity to one another (22), risk behaviors may cluster among individuals within networks because of their shared physical and/or social environment, network norms/relationships, or both. Similarly, spatial clustering of risk behaviors may be partially attributed to network relationships and social norms. Most research on the “risk environment” has been conducted in urban settings, and it is unknown whether findings can be generalized to rural settings, where geographic mobility, population density, and network structure may differ. The current analysis addresses these gaps. We used a combined approach to understand the relative influence of network and spatial components of the risk environment in a rural setting. We examined 1) whether a person's injection status was associated with social and/or geographic proximity to others who did and did not inject drugs, 2) the social distance at which peer injection status remained associated with a person's injection status, and 3) whether a person's odds of injecting drugs increased with each additional socially and/or geographically proximal injecting peer.
METHODS
Sample
The data for this analysis were collected through baseline assessments (2008–2010) in the Social Networks among Appalachian People (SNAP) Study, an ongoing longitudinal study which examines risk factors for HIV, HCV, and sexually transmitted infections in a cohort of 503 persons who use drugs in rural eastern Kentucky. The methods of the SNAP Study have been described in detail elsewhere (23). Briefly, participants were recruited using respondent-driven sampling (24); interviewer-administered surveys collected information on demographic characteristics and risk behaviors; and a social network inventory collected information about recent (past 6 months) drug-use, sex, and social-support network members. The protocol was approved by the Institutional Review Board at the University of Kentucky, and a Certificate of Confidentiality was obtained from the National Institutes of Health. Participants were compensated $50.
Network data
Participants provided the first name and last initial of each person with whom they had had sex, with whom they had used drugs, or on whom they had counted for emotional, financial, material, or informational support in the last 6 months. Participants could list up to 8 names for each name-generating question, for a maximum of 24 network members. For each name listed, data on demographic characteristics were also collected. Network member names and demographic characteristics were cross-referenced with those of other study participants and network members named by other study participants. If at least 1 individual indicated a relationship with another participant, a tie was considered to be present (i.e., undirected ties). If tie confirmation was questionable, community-based interviewers were queried for their knowledge of existing network linkages. This resulted in 897 ties, 34% of which were recruitment ties and 22% of which were ties to peers people reported injecting drugs with.
Analytical approach
Network analysis
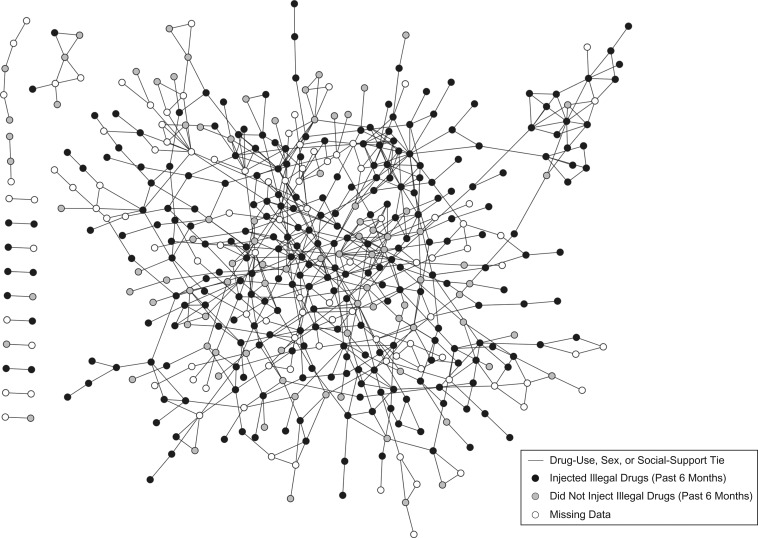
Figure 1 displays all confirmed ties (i.e., drug-use, sex, and social-support ties) by injection status (past 6 months), mapped using the open-source software Cytoscape (25). We calculated the geodesic distance, or the shortest path between each pair of individuals in the data set, using the R (R Foundation for Statistical Computing, Vienna, Austria) statistical system (http://cran.r-project.org/). Because isolates have infinite geodesic distances, isolates were excluded from this analysis. Using network simulations, we 1) examined whether peer injection status was associated with an individual's injection status, beyond what could be explained by chance, and 2) identified a threshold for the association between peer injection status and recent injection status. To do so, we compared the observed network with a null distribution (1,000 randomly generated networks with the same network topology (i.e., arrangement of nodes) and injection prevalence, but with injection status distributed randomly) using R (26). We calculated the risk that an individual injected drugs given that a network member injected for pairs of individuals separated by 1–6 degrees in the observed and simulated networks. The risk ratios calculated in each of the 1,000 simulated networks were ranked, and the 25th and 976th observations were taken to represent the 95% confidence limits for the null distribution. If the observed risk ratio was not included within these bounds, it was considered significantly different from what could be expected by chance (P < 0.05) (Table 1). Based on these network simulations, which suggested that a person's injection status was associated with the injection status of others who were either directly or indirectly connected to him/her via 1 intermediary individual (P < 0.05), we created a binary variable to represent “socially proximal” peers (i.e., those separated by ≤2 degrees).
Figure 1.
The SNAP Study network, comprised of individuals with at least 1 drug-use, sex, or social-support tie to other SNAP participants (n = 464), 2008–2010. SNAP, Social Networks among Appalachian People.
Table 1.
Risk of Injection Drug Use Given That a Network Member Injected Drugs for Pairs of Individuals Separated by 1–6 Degrees in Observed (SNAP Study, 2008–2010) and Simulated Networks
| Shortest Path (Geodesic Distance)a | Observed RR for Injection (Past 6 Months) | 95% CI for Expected RR Based on the Null Distribution |
|---|---|---|
| 1 | 1.24 | 0.87, 1.13 |
| 2 | 1.10 | 0.93, 1.08 |
| 3 | 1.00 | 0.95, 1.05 |
| 4 | 0.97 | 0.96, 1.03 |
| 5 | 0.99 | 0.97, 1.02 |
| 6 | 1.00 | 0.98, 1.02 |
Abbreviations: CI, confidence interval; RR, risk ratio; SNAP, Social Networks among Appalachian People.
a Number of persons in the shortest path of persons between any 2 individuals.
Spatial analysis
Participants’ residential addresses were geocoded using ArcGIS 10.1 (ESRI, Redlands, California) and ESRI's North American Street Map data (27). Participants who did not provide complete addresses or whose addresses could not be geocoded were not analyzed (final n = 491). Participants whose addresses could not be geocoded were not significantly different from those whose addresses were geocoded in terms of age, race, sex, or education. Using the network analyst extension in ArcGIS 10.1 and the North America Detailed Streets layer package (27), we computed the shortest road-network distance between each pair of participants using their residential addresses. Based on the median distance between pairs of individuals who both injected drugs, we used 7 miles (11.2 km) as a cutpoint to distinguish between geographically proximal and distant pairs. Notably, all participants included in the network analysis described above, with the exception of those who could not be geocoded, were included in the spatial analysis. To test the sensitivity of our findings to the criteria used to define “geographically proximal,” we repeated our analysis using a threshold informed by the average road-network distance between recruiter-recruit pairs (≤4 miles (≤6.4 km)) (24). Although effect sizes varied slightly, the significance and implications of the study findings did not differ when the cutpoint used to define “geographically proximal” was altered.
Combined analysis
Using the cutpoints defined above for “socially proximal” (shortest path ≤2 degrees) and “geographically proximal” (≤7 miles (≤11.2 km)), we created variables to represent the number of peers who did and who did not inject drugs who were 1) socially proximal, 2) geographically proximal, 3) socially but not geographically proximal, 4) geographically but not socially proximal, and 5) both socially and geographically proximal. This resulted in 10 different summary measures representing counts of network members: 5 for peers who injected drugs and 5 for peers who did not inject drugs. Measures were descriptively compared with χ2 statistics and t tests (for categorical and continuous variables, respectively), and multivariable logistic regression was performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina) (28) to determine the independent and joint associations between socially and geographically proximal injecting peers and injection status.
RESULTS
Baseline characteristics have been previously described (23, 24, 29, 30). Specific to this sample, the median age was 31 years (interquartile range, 26–38), 57% were male, 74% reported injecting illegal drugs in the past 6 months, and 43% were anti-HCV-positive based on a third-generation enzyme immunoassay using dried blood-spot specimens. Among those who reported injecting illegal drugs in the past 6 months, all reported using opiates, 93% reported injecting opiates of some kind, and 92% reported injecting prescription pain relievers. Other drugs that participants reported injecting included cocaine (41%), heroin (11%), cocaine and prescription opiates together (8%), prescription stimulants (5%), prescription benzodiazepines (4%), methamphetamine (2%), heroin and cocaine together (1%), and “some other opiate” (4%).
Spatial analysis
As Table 2 shows, the mean geographic distance between SNAP participants was 9.96 miles (15.9 km), and the mean distance between pairs of SNAP participants who injected drugs (8.73 miles (14.0 km)) was significantly less than the mean distance between pairs of SNAP participants who did not inject drugs (9.82 miles (15.7 km)) (P < 0.0001).
Table 2.
Geographic and Social Distances Between Pairs of Participants in the SNAP Study, Overall and Among Pairs in Which Both Participants Injected Drugs, Pairs in Which Neither Injected Drugs, and Pairs in Which One Person Injected But the Other Did Not, 2008–2010
| Distance | No. of Persons | Mean | Median (IQRa) |
|---|---|---|---|
| Geographic distance, milesb | |||
| Overall | 234,530 | 9.96 | 7.27 (3.56–12.69) |
| Among pairs in which: | |||
| Both participants injected | 16,180 | 8.73 | 7.04 (2.83–12.59) |
| Both participants did not inject | 20,789 | 9.82 | 6.98 (3.44–12.31) |
| One participant injected and the other did not | 206,561 | 10.07 | 7.32 (3.64–12.73) |
| Among pairs not socially connected (i.e., social distance = infinity) | 68,322 | 12.69 | 9.54 (5.06–18.21) |
| Among pairs who were socially connected | 175,208 | 8.89 | 6.59 (3.12–11.35) |
| Pairs who were directly connected (1 degree of separation) | 1,380 | 6.19 | 2.97 (0.27–8.25) |
| Pairs who were indirectly connected (2 degrees of separation) | 3,332 | 7.24 | 4.59 (1.36–9.31) |
| Pairs separated by the maximum social distance (16 degrees of separation) | 2 | 13.67 | 13.67 (13.67–13.67) |
| Social proximity (shortest path between participantsc), degrees of separation | |||
| Overall | 175,208 | 6.59 | 7 (5–8) |
| Among pairs in which: | |||
| Both participants injected | 15,059 | 6.44 | 6 (5–8) |
| Both participants did not inject | 13,303 | 6.69 | 7 (5–8) |
| One participant injected and the other did not | 146,846 | 6.60 | 7 (5–8) |
Abbreviations: IQR, interquartile range; SNAP, Social Networks Among Appalachian People.
a 25th–75th percentiles.
b 1 mile = 1.6 km.
c Number of persons in the shortest path of persons between any 2 individuals.
Network analysis
The mean shortest path between participants was 6.59 degrees (Table 2). Figure 1 displays all SNAP participants with at least 1 drug-use, sex, or social-support tie to another SNAP participant. As Table 1 shows, the observed risk of injection was 1.24 times higher for study participants who were directly connected to another participant who reported injecting drugs (i.e., 1 degree of separation) and 1.10 times higher for those connected by 2 degrees to another such participant (P < 0.05). Notably, 76.9% and 79.0% of individuals were connected to 1 or more people who injected drugs by 1 degree and 2 degrees, respectively.
Combined analysis
As can be seen in Table 2, the geographic distance between SNAP participants increased as the social distance between pairs increased. For example, the mean distance between SNAP participants who were not socially connected was significantly greater than that for those who were socially connected (12.69 miles (20.3 km) vs. 8.89 miles (14.2 km); P < 0.0001), and it increased with social distance (P < 0.0001 for linear trend); people who were directly connected were a mean of 6.19 miles (9.9 km) apart, as opposed to 13.67 miles (21.9 km) apart for those separated by the maximum shortest path. Further, for each unit increase in shortest path length, the geographic distance between peers increased by an average of 0.52 miles (0.8 km) (P < 0.0001; data not shown in tables). Web Figure 1 (available at https://academic.oup.com/aje) displays the intersection between social and geographic distances for 1) all pairs, 2) pairs in which both persons injected, 3) pairs in which both persons did not inject, and 4) pairs in which one person injected and the other did not.
As Table 3 shows, the odds of injection increased with each additional injecting peer; however, the odds of injection did not decrease with each additional noninjecting peer. Having socially proximal injecting peers was more strongly associated with injection drug use than having geographically proximal injecting peers (i.e., 19% increased odds of injection for each additional peer vs. 0.2% increased odds). Further, when injecting peers were categorized on the basis of their social and geographic proximity, having peers who injected drugs who were socially but not geographically proximal was associated with the greatest per-unit increase in the odds of injection (odds ratio = 1.21, 95% confidence interval: 1.12, 1.31). The odds of injection increased by 17% (odds ratio = 1.17, 95% confidence interval: 1.12, 1.23) for each additional geographically and socially proximal injecting peer.
Table 3.
Odds of Injection Drug Use Among Participants Based on Their Geographic and Social Proximity to Other Participants Who Did and Did Not Inject Drugs (n = 491), SNAP Study, 2008–2010
| Injection of Drugs in Past 6 Months | Odds of Injecting Drugs in Past 6 Months vs. Not Injecting in Past 6 Months | |||||
|---|---|---|---|---|---|---|
| Did Not Inject (n = 229) | Injected (n = 262) | |||||
| Mean | 95% CIa | Mean | 95% CIa | Odds Ratio | 95% CIb | |
| No. of noninjecting peers who were: | ||||||
| Socially proximal but not geographically proximal | 1.25 | 1.02, 1.48 | 1.16 | 1.00, 1.32 | 0.97 | 0.86, 1.08 |
| Geographically proximal but not socially proximal | 58.33 | 51.56, 65.11 | 81.90 | 76.43, 87.38 | 1.01 | 1.006, 1.013 |
| Both socially proximal and geographically proximal | 2.08 | 1.75, 2.41 | 2.24 | 1.94, 2.55 | 1.03 | 0.96, 1.10 |
| No. of injecting peers who were: | ||||||
| Socially proximal but not geographically proximal | 1.31 | 0.98, 1.65 | 2.82 | 2.38, 3.26 | 1.21 | 1.12, 1.31 |
| Geographically proximal but not socially proximal | 92.66 | 81.77, 103.60 | 126.10 | 117.50, 134.80 | 1.005 | 1.003, 1.008 |
| Both geographically proximal and socially proximal | 2.53 | 2.08, 2.98 | 5.40 | 4.76, 6.05 | 1.17 | 1.12, 1.23 |
| Geographically proximal | 125.00 | 114.60, 135.4 | 139.00 | 130.10, 147.90 | 1.002 | 1.000, 1.005 |
| Socially proximal | 3.84 | 3.28, 4.41 | 8.23 | 7.46, 9.00 | 1.19 | 1.14, 1.24 |
Abbreviations: CI, confidence interval; SNAP, Social Networks among Appalachian People.
a 95% CIs were calculated using the TTEST procedure in SAS 9.4 (28). The CI for the mean specifies a range of values within which the mean lies, and it is given by , where s is the sample deviation of the observations and N is the number of valid observations. The t value in the formula is computed based on the number of degrees of freedom being N − 1 and the P value being 1 − α/2, where α is the confidence level, 0.95.
b 95% CIs were calculated using the LOGISTIC procedure in SAS 9.4, and each is the Wald CI of an individual odds ratio. The Wald CI for βj is given by , where zp is the 100th percentile of the standard normal distribution. is the maximum likelihood estimate of βj, and is the standard error estimate of .
Based on our assessment of potential confounders (Table 4), we adjusted for anti-HCV status in the final multivariable model (Table 5, model 3). After adjustment, the odds of injection increased by 0.4% for each additional geographically but not socially proximal injecting peer, 12% for each additional geographically and socially proximal injecting peer, and 22% for each socially but not geographically proximal injecting peer. Although each additional injecting peer was associated with a corresponding increase in the odds of injection, socially proximal peers had greater weight, and those who were socially but not geographically proximal had the greatest weight. Further, we observed an interaction between the number of injecting peers who were geographically but not socially proximal and the number who were socially but not geographically proximal (Table 5, model 2).
Table 4.
Demographic and Behavioral Correlates of Illegal Drug Injection in the Past 6 Months (n = 491), SNAP Study, 2008–2010
| Correlate | Injection of Drugs in Past 6 Months | 2-Sided P Valuea | |||||
|---|---|---|---|---|---|---|---|
| Did Not Inject (n = 229) | Injected (n = 262) | ||||||
| Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | ||
| Age, years | 35.5 (9.2) | 31.7 (8.0) | 0.02 | ||||
| Monthly income from employment, dollars | 369.8 (900.0) | 344.1 (755.7) | 0.73 | ||||
| Duration of education, completed months | 132.8 (24.1) | 134.7 (24.1) | 0.39 | ||||
| Male sex | 124 | 54.2 | 157 | 59.9 | 0.20 | ||
| Use of opiates in past 6 months | 228 | 99.6 | 262 | 100 | 0.28 | ||
| History of a drug-related overdose | 68 | 29.7 | 74 | 28.2 | 0.72 | ||
| Ever having witnessed an overdose | 126 | 55.0 | 160 | 61.1 | 0.18 | ||
| Anti-HCV-positive | 56 | 24.5 | 154 | 58.8 | <0.01 | ||
Abbreviations: HCV, hepatitis C virus; SD, standard deviation; SNAP, Social Networks among Appalachian People.
a 2-sided P values were calculated using the t test (for continuous variables) and the χ2 test statistic (for categorical and binary variables).
Table 5.
Adjusted Odds of Having Injected Illegal Drugs in the Past 6 Months (Final Multivariable Models; n = 491), SNAP Study, 2008–2010a,b
| Model 1c | Model 2d | Model 3e | ||||
|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | Adjusted OR | 95% CI | Adjusted OR | 95% CI | |
| Anti-HCV-positive | 3.82 | 2.52, 5.80 | ||||
| No. of peers who inject drugs | ||||||
| Socially proximal but not geographically proximal | 1.29 | 1.18, 1.41 | 1.19 | 1.08, 1.32 | 1.22 | 1.12, 1.34 |
| Both socially and geographically proximal | 1.12 | 1.05, 1.19 | ||||
| Geographically proximal but not socially proximal | 1.007 | 1.005, 1.010 | 1.006 | 1.003, 1.009 | 1.004 | 1.001, 1.007 |
| Interactionf | 1.002 | 1.0002, 1.003 | ||||
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; OR, odds ratio; SNAP, Social Networks among Appalachian People.
a The adjusted ORs were calculated using the LOGISTIC procedure in SAS 9.4 (28) and represent the OR for each model parameter, given the other parameters included in the model.
b The 95% CIs were calculated using the LOGISTIC procedure in SAS 9.4 (28) and represent the Wald CI for an individual OR, given the other parameters included in the model.
c Model 1 accounted for only 2 parameters: 1) the number of injecting peers who were socially but not geographically proximal and 2) the number of injecting peers who were geographically but not socially proximal.
d Model 2 accounted for 3 parameters: 1) the number of injecting peers who were socially but not geographically proximal, 2) the number of injecting peers who were geographically but not socially proximal, and 3) the interaction between the two. The P value for the interaction was 0.025. Because of this interaction, as the number of injecting peers who were geographically but not socially proximal increased, the additional odds of injection associated with each additional socially proximal but not geographically proximal injecting peer were reduced only slightly.
e Model 3 accounted for 1) the number of injecting peers who were socially but not geographically proximal, 2) the number of injecting peers who were geographically but not socially proximal, 3) the number of injecting peers who were both socially and geographically proximal, and 4) anti-HCV status, the only individual-level parameter which retained statistical significance after adjustment for the number of socially and/or geographically proximal injecting peers.
f Interaction between the number of injecting peers who were socially but not geographically proximal and the number of injecting peers who were geographically but not socially proximal.
DISCUSSION
HCV is prevalent in this cohort (31), and there has been a recent increase in the number of acute HCV infections reported in the Appalachian states (32). Since many of these infections are attributed to injection drug use among people living in nonurban settings (32), it is important to understand the social context of injection drug use so that efforts can be made to reduce future HCV transmission and other adverse health outcomes associated with injection drug use. In this sample, an individual's injection status was positively and statistically significantly associated with having more socially proximal peers who injected drugs, and the association extended beyond direct ties to persons who were separated by ≤2 degrees. This is consistent with other studies in which researchers reported 1) a threshold of 3 degrees of influence for smoking, obesity, happiness, and loneliness (33–36) and 2) an increased prevalence of syphilis among persons directly or indirectly connected via peer-recruitment linkages to other study participants with syphilis (37).
Our findings have public health relevance, as interventions that change one person's injection behaviors could have cascading effects on the behavior of others who are socially connected to that person by up to 2 degrees. Because an individual's injection status is associated with the number of peers who inject drugs within 2 degrees of separation, it is possible that cessation of injection may similarly be associated with social norms among people who are socially proximal. For example, a person's likelihood of cessation may be additive (i.e., may increase with each additional peer who stops injecting) or may be dependent on a threshold of influence (i.e., may occur only when a certain number or proportion of network members quit). As with smoking cessation (33), a person's decision to stop injecting drugs may reflect the decisions of others who are directly and indirectly connected to him/her.
Although network-based interventions can be more effective than individual interventions in promoting behavior changes (38, 39), most current network-based interventions for reducing drug use and sexual risk behaviors are not designed to evaluate either of these processes. In most cases, intervention studies enroll a group of index participants who are randomized to either the intervention group or the control group. Index participants are asked to refer risk partners, who complete a survey but are not exposed directly to the intervention; their only exposure to the intervention is through the index participant who recruited them. As a result, researchers who implement most peer-driven interventions are not able to examine the cascading impact of an intervention beyond direct ties to include persons connected by ≥2 degrees of separation. In the absence of more detailed network data, this study design can only capture diffusion of the intervention from index participants to the network members they recruit. Further, the absence of more detailed information on the relationships between participants precludes the ability to test for additive effects (i.e., where one's likelihood of behavior change increases as more network members change their behaviors or are exposed to the intervention) or threshold effects (i.e., where individual behavior change occurs only when the behaviors of a set number or proportion of social network members also change or when a set number or proportion of network members are exposed to the intervention). Future network-based interventions designed to reduce injection risk behaviors or promote injection cessation might benefit from the collection of sociometric network data so that both of these aspects can be examined to more accurately assess or measure how behavior change diffuses through a network following the intervention. Network interventions in other settings have also shown that diffusion of an intervention may vary based on who receives the intervention (i.e., nominated peers vs. randomly selected individuals vs. highly connected individuals) (40).
Because the ties in our sample were undirected, we were unable to make any causal inferences; however, the association between a person's injection status and the number of injecting peers was much stronger for peers who were socially proximal than for those who were geographically proximal. This suggests that in this sample, a person's injection status was more strongly associated with the behaviors of those with whom he/she interacted (directly or indirectly) than with the prevalence of injection drug use among peers in close geographic proximity who he/she may have been less likely to interact with. Further, the statistical interaction we observed in model 2 of Table 5 suggests that in contexts where injection drug use is more prevalent (i.e., more injecting peers who are geographically proximal but with whom the person does not interact socially), the added effect of each additional socially proximal injecting peer is slightly smaller than it is in contexts where there are fewer people in close geographic proximity who inject.
Our findings also have implications for evaluating the adoption of safer syringe practices following recent legal changes in Kentucky. In response to the recent HIV outbreak in neighboring rural Indiana among persons injecting prescription opioids (41) and the high HCV incidence in Kentucky (42), Senate Bill 192 was passed into law in Kentucky on March 25, 2015. This bill permits the provision of sterile syringes via local health departments (43). Although Kentucky law still prohibits the sale or distribution of drug paraphernalia (i.e., syringes) (44, 45), under this bill, syringes obtained through syringe exchange programs (SEPs) are exempt from this classification. Among persons enrolled in our cohort (prior to the bill's passage), 30% of those who reported injecting illicit drugs in the past 6 months reported having done so with a dirty needle (i.e., one that someone else had used first and that had not been sterilized or cleaned with bleach before use), and over half (54%) of those who reported using a dirty needle reported doing so because there was only 1 needle available. Even though pharmacists are permitted to sell syringes, they are legally required to collect the following information for each syringe sale: 1) purchaser's name and address, 2) quantity of syringes/needles purchased, 3) purchase date, and 4) planned use of the syringes/needles (45). Further, these records are required to be maintained for at least 2 years and are available for inspection by law enforcement officers. As a result of these legal restrictions and other barriers (i.e., judgmental attitudes among some staff in pharmacies, needing to lie about being diabetic, inconvenient hours (46)), fewer than 10% of SNAP participants who reported injection drug use (past 6 months) reported obtaining syringes from pharmacies. In the context of recent legal changes and the launch of SEPs in Kentucky and neighboring West Virginia, it will be important to examine how geographic and social proximity to other persons who use SEPs (i.e., access to syringes and harm reduction materials/services) are associated with behavior changes among those who do not use SEPs (i.e., through diffusion of behavioral norms and/or secondary exchange) and similarly whether SEP use is influenced by geographic and/or social proximity to others who use SEPs.
A few limitations should be acknowledged. In order to examine whether the association between peer injection status and one's own injection status differed by geographic and social proximity to each peer and whether geographic and social proximity to peers acted independently or interacted, we created binary variables to categorize peers on the basis of their geographic proximity (≤7 miles (≤11.2 km) vs. >7 miles (>11.2 km)), social proximity (≤2 degrees of separation vs. >2), and injection status in the past 6 months (yes vs. no). Notably, the resulting variables used in our analyses were count variables, which represented the total number of peers based on their geographic proximity, social proximity, and injection status. One advantage of categorizing peers using thresholds for the proximity measures is that it simplifies the interpretation of study findings and can inform the development of practical intervention strategies for altering injection practices. However, in simplifying our analytical approach, it is also possible that some nuances were masked.
The generalizability of our findings is also limited. First, the geographic landscape and the primary mode of transportation in the study area are 2 key variables which are context-dependent. This study was conducted in a mountainous region of eastern Kentucky, where public transportation is unavailable and the primary mode of transportation is driving. In this study sample, 37% of participants had independent access to transportation (i.e., had a valid driver's license and owned a car). Based on data from the US Census, the average commuting time to work was 21 minutes for persons living in the area. In this study's setting, 7 miles or 11.2 km (the median distance between pairs of individuals who injected drugs) corresponds to an approximately 11- to 19-minute drive, depending on whether main roads or local roads are used (47). Notably, our analytical approach was strengthened by the fact that we used road network distances rather than Euclidean distances. This is an important strength, given that the mountainous landscape of this region can result in dramatically different findings when Euclidian and road-network distance measures are used. Because roads typically wind around mountains rather than cut through them, road-network distances tend to be longer than Euclidean distances in this context.
Finally, because this network represented only ties among SNAP participants, it is possible that some individuals may have been connected to others who injected drugs but their social connection was not captured in our analysis because these individuals were not part of the SNAP sample. It is also possible that persons who were omitted from the network analysis due to their not being SNAP participants may have been bridges between other SNAP participants, thereby reducing the social distance between some pairs of SNAP participants. Despite these limitations, the networks in this study represented real-life linkages (some of which were peer-recruitment linkages), similar to those that would be generated with a network-driven intervention used in the field. Consequently, our findings demonstrate the feasibility of reaching persons who inject illegal drugs to target them with interventions that promote injection cessation. In this setting, where the network association with injection status was greater than the spatial/geographic association, network-based interventions which target drug injectors who are more centrally located in the network to promote injection cessation could result in larger reductions in the prevalence of injection in the network overall, via diffusion of the intervention message and changes in injection norms.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, Boston University, Boston, Massachusetts (Abby E. Rudolph); Department of Epidemiology, College of Public Health, University of Kentucky, Lexington, Kentucky (April M. Young); and Center on Drug and Alcohol Research, Department of Behavioral Science, College of Medicine, University of Kentucky, Lexington, Kentucky (April M. Young, Jennifer R. Havens).
Funding for this study was provided by the National Institute of Drug Abuse (grants R01 DA024598 and R01 DA033862 (Principal Investigator: J.R.H.) and grant K01 DA033879 (Principal Investigator: A.E.R.)).
Conflict of interest: none declared.
REFERENCES
- 1. Rhodes T, Singer M, Bourgois P, et al. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61(5):1026–1044. [DOI] [PubMed] [Google Scholar]
- 2. Brouwer KC, Rusch ML, Weeks JR, et al. Spatial epidemiology of HIV among injection drug users in Tijuana, Mexico. Ann Assoc Am Geogr. 2012;102(5):1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De P, Singh AE, Wong T, et al. Sexual network analysis of a gonorrhoea outbreak. Sex Transm Infect. 2004;80(4):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez AN, Lorvick J, Kral AH. Activity spaces among injection drug users in San Francisco. Int J Drug Policy. 2014;25(3):516–524. [DOI] [PubMed] [Google Scholar]
- 5. Stenbacka M. Initiation into intravenous drug abuse. Acta Psychiatr Scand. 1990;81(5):459–462. [DOI] [PubMed] [Google Scholar]
- 6. Sherman SG, Smith L, Laney G, et al. Social influences on the transition to injection drug use among young heroin sniffers: a qualitative analysis. Int J Drug Policy. 2002;13(2):113–120. [Google Scholar]
- 7. Eaves CS. Heroin use among female adolescents: the role of partner influence in path of initiation and route of administration. Am J Drug Alcohol Abuse. 2004;30(1):21–38. [DOI] [PubMed] [Google Scholar]
- 8. Laurie ML, Green KL. Health risks and opportunities for harm reduction among injection-drug-using clients of Saskatoon's needle exchange program. Can J Public Health. 2000;91(5):350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abelson J, Treloar C, Crawford J, et al. Some characteristics of early‐onset injection drug users prior to and at the time of their first injection. Addiction. 2006;101(4):548–555. [DOI] [PubMed] [Google Scholar]
- 10. Bravo MJ, Barrio G, de La Fuente L, et al. Reasons for selecting an initial route of heroin administration and for subsequent transitions during a severe HIV epidemic. Addiction. 2003;98(6):749–760. [DOI] [PubMed] [Google Scholar]
- 11. Neaigus A, Miller M, Friedman SR, et al. Potential risk factors for the transition to injecting among non‐injecting heroin users: a comparison of former injectors and never injectors. Addiction. 2001;96(6):847–860. [DOI] [PubMed] [Google Scholar]
- 12. van Ameijden EJ, Van Den Hoek JA, Hartgers C, et al. Risk factors for the transition from noninjection to injection drug use and accompanying AIDS risk behavior in a cohort of drug users. Am J Epidemiol. 1994;139(12):1153–1163. [DOI] [PubMed] [Google Scholar]
- 13. Sherman SG, Fuller CM, Shah N, et al. Correlates of initiation of injection drug use among young drug users in Baltimore, Maryland: the need for early intervention. J Psychoactive Drugs. 2005;37(4):437–443. [DOI] [PubMed] [Google Scholar]
- 14. Neaigus A, Gyarmathy VA, Miller M, et al. Transitions to injecting drug use among noninjecting heroin users: social network influence and individual susceptibility. J Acquir Immune Defic Syndr. 2006;41(4):493–503. [DOI] [PubMed] [Google Scholar]
- 15. Firestone M, Fischer B. A qualitative exploration of prescription opioid injection among street-based drug users in Toronto: behaviours, preferences and drug availability. Harm Reduct J. 2008;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhodes T, Ball A, Stimson GV, et al. HIV infection associated with drug injecting in the newly independent states, eastern Europe: the social and economic context of epidemics. Addiction. 1999;94(9):1323–1336. [DOI] [PubMed] [Google Scholar]
- 17. Latkin C, Glass GE, Duncan T. Using geographic information systems to assess spatial patterns of drug use, selection bias and attrition among a sample of injection drug users. Drug Alcohol Depend. 1998;50(2):167–175. [DOI] [PubMed] [Google Scholar]
- 18. Kruse GR, Barbour R, Heimer R, et al. Drug choice, spatial distribution, HIV risk, and HIV prevalence among injection drug users in St. Petersburg, Russia. Harm Reduct J. 2009;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhodes T, Simic M. Transition and the HIV risk environment. BMJ. 2005;331(7510):220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galea S, Ahern J, Vlahov D. Contextual determinants of drug use risk behavior: a theoretic framework. J Urban Health. 2003;80(suppl 3):iii50–iii58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bluthenthal RN, Do DP, Finch B, et al. Community characteristics associated with HIV risk among injection drug users in the San Francisco Bay Area: a multilevel analysis. J Urban Health. 2007;84(5):653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rothenberg R, Muth SQ, Malone S, et al. Social and geographic distance in HIV risk. Sex Transm Dis. 2005;32(8):506. [DOI] [PubMed] [Google Scholar]
- 23. Young AM, Jonas AB, Mullins UL, et al. Network structure and the risk for HIV transmission among rural drug users. AIDS Behav. 2012;17(7):2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Young AM, Rudolph AE, Quillen D, et al. Spatial, temporal and relational patterns in respondent-driven sampling: evidence from a social network study of rural drug users. J Epidemiol Community Health. 2014;68(8):792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smoot ME, Ono K, Ruscheinski J, et al. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szabo G, Barabasi AL Network effects in service usage. 2007. http://lanl.arxiv.org/abs/physics/0611177. Submitted November 18, 2006. Accessed February 29, 2012.
- 27. ESRI North America detailed streets. 2012. http://www.arcgis.com/home/item.html?id=f38b87cc295541fb88513d1ed7cec9fd. Updated September 25, 2014. Accessed September 1, 2013.
- 28. SAS Institute Inc Base SAS® 9.4 Procedures Guide: Statistical Procedures. 2nd ed Cary, NC: SAS Institute Inc.; 2013. [Google Scholar]
- 29. Stephens DB, Havens JR. Predictors of alcohol use among rural drug users after disclosure of hepatitis C virus status. J Stud Alcohol Drugs. 2013;74(3):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jonas AB, Young AM, Oser CB, et al. OxyContin® as currency: OxyContin® use and increased social capital among rural Appalachian drug users. Soc Sci Med. 2012;74(10):1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Havens JR, Lofwall MR, Frost SD, et al. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. Am J Public Health. 2013;103(1):e44–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 33. Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358(21):2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–379. [DOI] [PubMed] [Google Scholar]
- 35. Fowler JH, Christakis NA. Dynamic spread of happiness in a large social network: longitudinal analysis over 20 years in the Framingham Heart Study. BMJ. 2008;337:a2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cacioppo JT, Fowler JH, Christakis NA. Alone in the crowd: the structure and spread of loneliness in a large social network. J Pers Soc Psychol. 2009;97(6):977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rudolph AE, Gaines TL, Lozada R, et al. Evaluating outcome-correlated recruitment and geographic recruitment bias in a respondent-driven sample of people who inject drugs in Tijuana, Mexico. AIDS Behav. 2014;18(12):2325–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Latkin CA. Outreach in natural settings: the use of peer leaders for HIV prevention among injecting drug users’ networks. Public Health Rep. 1998;113(suppl 1):151–159. [PMC free article] [PubMed] [Google Scholar]
- 39. Valente TW. Network interventions. Science. 2012;337(6090):49–53. [DOI] [PubMed] [Google Scholar]
- 40. Kim DA, Hwong AR, Stafford D, et al. Social network targeting to maximise population behaviour change: a cluster randomised controlled trial. Lancet. 2015;386(9989):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weinbaum C, Lyerla R, Margolis HS. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR Recomm Rep. 2003;52(RR-1):1–36. [PubMed] [Google Scholar]
- 42. Division of Viral Hepatitis, Centers for Disease Control and Prevention Viral Hepatitis Surveillance—United States, 2013 Atlanta, GA: Centers for Disease Control and Prevention; 2013. https://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf. Accessed December 2, 2016. [Google Scholar]
- 43. Kentucky Senate Senate Bill 192. Passed March 25, 2015. www.lrc.ky.gov/record/15rs/SB192/bill.doc. Accessed July 1, 2016.
- 44. Kentucky Legislative Research Commission 218A.500. Definitions for KRS 218A.500 and 218A.510—Unlawful Practices—Substance Abuse Treatment Outreach Program—Informing Peace Officer About Presence of Needles or Other Sharp Objects Before Search—Penalties Frankfort, KY: Legislative Research Commission; 2010. http://www.lrc.ky.gov/krs/218a00/500.pdf. Updated April 26, 2010. Accessed July 1, 2016. [Google Scholar]
- 45. Kentucky Legislative Research Commission 217.177. Sale and Disposal of Hypodermic Syringes or Needles Frankfort, KY: Legislative Research Commission; 2005. http://www.lrc.ky.gov/krs/217-00/177.pdf. Updated June 20, 2005. Accessed July 1, 2016. [Google Scholar]
- 46. Reich W, Compton WM, Horton JC, et al. Injection drug users report good access to pharmacy sale of syringes. J Am Pharm Assoc (Wash). 2002;42(6 suppl 2):S68–S72. [DOI] [PubMed] [Google Scholar]
- 47. Google Maps Kentucky [street map]. 2016. https://www.google.com/maps. Accessed July 1, 2016.