Abstract
Background: Guideline-concordant local therapy options for early breast cancer include lumpectomy plus whole breast irradiation (Lump+WBI), lumpectomy plus brachytherapy, mastectomy alone, mastectomy plus reconstruction, and, in older women, lumpectomy alone. We performed a comparative examination of each treatment’s complications and cost to assess their relative values.
Methods: Using the MarketScan database of younger women with private insurance and the SEER-Medicare database of older women with public insurance, we identified 105 211 women with early breast cancer diagnosed between 2000 and 2011. We used diagnosis and procedural codes to identify treatment complications within 24 months of diagnosis and compared complications by treatment using two-sided logistic regression. Mean total and complication-related cost, relative to Lump+WBI, were calculated from a payer’s perspective and adjusted for differences in covariables using linear regression. All statistical tests were two-sided.
Results: Lump+WBI was the most commonly used treatment. Mastectomy plus reconstruction was associated with nearly twice the complication risk of Lump+WBI (Marketscan: 54.3% vs 29.6%, relative risk [RR] = 1.87, 95% confidence interval [CI] = 1.82 to 1.91, P < .001; SEER-Medicare: 66.1% vs 37.6%, RR = 1.75, 95% CI = 1.69 to 1.82, P < .001) and was also associated with higher adjusted total cost (Marketscan: $22 481 greater than Lump+WBI; SEER-Medicare: $1748 greater) and complication-related cost (Marketscan: $9017 greater; SEER-Medicare: $2092 greater). Brachytherapy had modestly higher total cost and complications than WBI. Lumpectomy alone entailed lower cost and complications in the SEER-Medicare cohort only.
Conclusions: Mastectomy plus reconstruction results in substantially higher complications and cost than other guideline-concordant treatment options for early breast cancer. These findings are relevant to patients evaluating their local therapy options and to value-based population health management.
Achieving value for patients, defined as the quality of outcomes per dollar spent, has captured the attention of health care policy makers as a way to improve care and reduce costs across whole populations (1,2). The case for value-based care is especially compelling in oncology, where both underuse of effective and inexpensive therapies and overuse of costly but equally effective or marginally better interventions are prevalent (1,3). Likewise, breast cancer is a cogent target within oncology as it now accounts for the highest number of new cancer cases in the United States and requires substantial societal resources for care of those diagnosed with the disease (4). Of the nearly 250 000 breast cancer patients diagnosed this year (4), over 60% will present with localized, early-stage disease (5) for which the National Comprehensive Cancer Network (NCCN) Guidelines identify several evidence-based local management options including mastectomy (Mast alone), mastectomy plus reconstruction (Mast+Recon), lumpectomy plus whole breast irradiation (Lump+WBI), and, in certain cases, lumpectomy plus brachytherapy (Lump+Brachy) or lumpectomy followed by endocrine therapy alone without radiation (Lump alone) (6).
Evidence suggests that real-world decision-making among these options is currently driven by patient and provider preferences, geography, and accessibility to certain treatments (7–10). Incorporating the risk of complications, cost, and considerations of value into the clinical decision may be a more rational approach to ensuring affordable, effective care for this broad and diverse patient population. Because the local treatment options above are associated with similar survival, their costs, complications, and quality of life implications underpin the value calculation (11,12). And yet, little research has sought to quantify and compare the expense and toxicities of these therapies in contemporary practice. To address this gap in knowledge, we compared the costs and complications associated with each local strategy using two complementary databases consisting of younger women with private insurance (MarketScan) and older women with public insurance (SEER-Medicare) diagnosed with breast cancer between 2000 and 2011.
Methods
Data Sources
The MarketScan Commercial Claims and Encounters database (Truven Health Analytics, Ann Arbor, MI) is a convenience sample of individual-level medical and drug insurance claims for patients under the age of 65 years. The claims are derived from 45 large employers and more than 100 health insurers that provide private insurance to employees, spouses, and dependents. Healthcare is provided under a variety of fee-for-service, capitated, and partially capitated health plans, including preferred provider organizations and health maintenance organizations. In 2002, the data set was expanded to include small- and medium-sized firms.
The Surveillance, Epidemiology, and End Results (SEER)–Medicare database captures clinical, pathological, and Medicare claims data for incident cancers diagnosed in Medicare beneficiaries who reside within one of 16 geographic areas accounting for 26% of the US population. The case ascertainment rate is approximately 98%, and 93% of patients in SEER are successfully linked to Medicare claims (13).
Study Subjects
A validated, claims-based algorithm was used to identify incident breast cancer cases in the MarketScan data from 2000 to 2011 (Supplementary Table 1, available online) (14). This algorithm has a published sensitivity of 82% to 87% and a specificity exceeding 99% for early-stage breast cancer (14,15). SEER data were used to identify incident breast cancer cases in the SEER-Medicare cohort during the same years. To enable determination of prediagnosis comorbidity and postdiagnosis treatments and outcomes, the cohorts were limited to patients with complete insurance coverage from 12 months prior through 24 months after diagnosis. In addition, as the intent was to assess the value of treatments appropriate for early breast cancer, we excluded patients treated with neoadjuvant chemotherapy or postmastectomy radiation, both of which are generally used for more advanced stage disease. Staging data are not available in MarketScan claims, so diagnosis codes were used to exclude patients with distant metastasis (Supplementary Table 2, available online). In contrast, staging data are present in the SEER-Medicare cohort and were used to limit this cohort to patients with early cancer, defined as stage T1-2 N0-1 M0 (16). The analytic cohorts were further limited to patients treated with either Lump+WBI, Lump+Brachy, Lump alone, Mast alone, and Mast+Recon. This approach yielded 44 344 patients from the MarketScan data (median age = 53 years) and 60 867 patients from the SEER-Medicare database (median age = 75 years) (Supplementary Table 1, available online).
Definitions of Study Variables
Breast surgery and radiation were classified using claims within one year of diagnosis as in prior studies (Supplementary Table 2, available online) (17). The most extensive breast surgery (lumpectomy vs mastectomy) within the first year after diagnosis was considered the definitive surgery. Oncologic breast surgeries in the second year of diagnosis were considered secondary surgeries not part of the initial treatment course. The Lump+WBI group was limited to those patients who received at least 15 unique external beam radiation treatments without concomitant brachytherapy. The Lump+Brachy group was limited to patients who received brachytherapy without concomitant external beam radiation. The Mast+Recon group included patients with mastectomy within one year of diagnosis and a code for breast reconstruction within two years of diagnosis (7).
Additional treatment variables derived from claims included receipt of chemotherapy, trastuzumab, axillary surgery, and/or contralateral mastectomy. For the SEER-Medicare cohort, tumor characteristics were extracted from SEER data. For the MarketScan cohort, adjuvant endocrine therapy within one year of diagnosis was determined using National Drug Codes (Supplementary Table 3, available online).
Statistical Analysis
Complications within two years of diagnosis were determined using claims and included wound complication, infection, hematoma/seroma, breast pain, fat necrosis, radiation pneumonitis, rib fracture, graft/implant complication, implant removal, and other postoperative complications (Supplementary Table 4, available online) (17–19). Risks of complications separately and in aggregate by treatment were compared using the chi-square test. The relative risk of complications was determined using multivariable logistic regression with candidate covariables selected based on a priori clinical significance and/or univariate statistical significance (P < .25). The model was iteratively refined to minimize collinearity. Goodness of fit was assessed using the Hosmer-Lemeshow method. Odds ratios were converted to relative risks (20).
Cumulative net payer cost within two years of diagnosis was calculated using all inpatient, outpatient, and carrier claims from within two years of diagnosis. All costs were adjusted to 2014 dollars using the Prospective Payment System for inpatient claims and the Medicare Economic Index for outpatient claims. Complication cost was determined by summing all costs occurring on days when a diagnosis or procedure code indicating a complication occurred. Noncomplication cost was the difference between total cost and complication cost and thus approximates the cost of treatment in the absence of complications. To account for highly skewed distribution of medical costs, generalized linear regression (log link function, gamma distribution) models were created for each cost outcome. Each cost model began with the candidate variables listed in Table 1 and was iteratively refined to optimize fit and minimize collinearity.
Table 1.
Baseline characteristics by local treatment strategy for each cohort*
| MarketScan cohort* |
SEER-Medicare cohort* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lump+WBI | Lump+ Brachy | Lump alone | Mast alone | Mast+Recon | Lump+WBI | Lump+ Brachy | Lump alone | Mast alone | Mast+Recon | |
| (n = 16 700) | (n = 2313) | (n = 2513) | (n = 11 432) | (n = 11 386) | (n = 29 764) | (n = 2733) | (n = 7502) | (n = 19 096) | (n = 1772) | |
| Variable | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) |
| Age, y | ||||||||||
| <40 | 592 (3.5) | 5 (0.2) | 259 (10.3) | 697 (6.1) | 1221 (10.7) | |||||
| 40–49 | 4480 (26.8) | 381 (16.5) | 911 (36.3) | 2519 (22.0) | 4320 (37.9) | |||||
| 50–59 | 8657 (51.8) | 1354 (58.5) | 1135 (45.2) | 5861 (51.3) | 4753 (41.7) | |||||
| 60–64 | 2971 (17.8) | 573 (24.8) | 208 (8.3) | 2355 (20.6) | 1092 (9.6) | |||||
| 66–69 | 7368 (24.8) | 661 (24.2) | 644 (8.6) | 3051 (16.0) | 758 (42.8) | |||||
| 70–74 | 8981 (30.2) | 823 (30.1) | 963 (12.8) | 4692 (24.6) | 614 (34.7) | |||||
| 75–79 | 7376 (24.8) | 661 (24.2) | 1379 (18.4) | 4746 (24.9) | 293 (16.5) | |||||
| 80–84 | 4431 (14.9) | 429 (15.7) | 1892 (25.2) | 3968 (20.8) | 81 (4.6) | |||||
| 85+ | 1608 (5.4) | 159 (5.8) | 2624 (35.0) | 2639 (13.8) | 26 (1.5) | |||||
| Race | ||||||||||
| White | 26 508 (89.1) | 2467 (90.3) | 6643 (88.6) | 16 363 (85.7) | 1642 (92.7) | |||||
| Black | 1678 (5.6) | 151 (5.5) | 498 (6.6) | 1475 (7.7) | 82 (4.6) | |||||
| Other | 1578 (5.3) | 115 (4.2) | 361 (4.8) | 1258 (6.6) | 48 (2.7) | |||||
| Type of coverage | ||||||||||
| non-HMO | 13 713 (82.1) | 1989 (86.0) | 2035 (81.0) | 9304 (81.4) | 9200 (80.8) | |||||
| HMO | 2987 (17.9) | 324 (14.0) | 478 (19.0) | 2128 (18.6) | 2186 (19.2) | |||||
| Covered individual | ||||||||||
| Employ | 10 304 (61.7) | 1461 (63.2) | 1439 (57.3) | 6651 (58.2) | 6641 (58.3) | |||||
| Depen | 6396 (38.3) | 852 (36.8) | 1074 (42.7) | 4781 (41.8) | 4745 (41.7) | |||||
| Comorbidity | ||||||||||
| 0 | 15 145 (90.7) | 2065 (89.3) | 2289 (91.1) | 9676 (84.6) | 10 564 (92.8) | 19 971 (67.1) | 1810 (66.2) | 4216 (56.2) | 11 098 (58.1) | 1270 (71.7) |
| 1 | 1355 (8.1) | 212 (9.2) | 183 (7.3) | 1396 (12.2) | 721 (6.3) | 6723 (22.6) | 627 (22.9) | 1948 (26.0) | 5038 (26.4) | 378 (21.3) |
| ≥2 | 200 (1.2) | 36 (1.6) | 41 (1.6) | 360 (3.2) | 101 (0.9) | 3070 (10.3) | 296 (10.8) | 1338 (17.8) | 2960 (15.5) | 124 (7.0) |
| Year | ||||||||||
| 2000 | 270 (1.6) | 4 (0.2) | 126 (5.0) | 275 (2.4) | 221 (1.9) | 2015 (6.77) | 30 (1.1) | 481 (6.4) | 1755 (9.2) | 96 (5.4) |
| 2001 | 300 (1.8) | 1 (0.0) | 116 (4.6) | 282 (2.5) | 238 (2.1) | 2288 (7.69) | 14 (0.5) | 524 (7.0) | 1938 (10.2) | 114 (6.4) |
| 2002 | 336 (2.0) | 9 (0.4) | 182 (7.2) | 462 (4.0) | 326 (2.9) | 2528 (8.49) | 63 (2.3) | 597 (8.0) | 1977 (10.4) | 117 (6.6) |
| 2003 | 635 (3.8) | 51 (2.2) | 240 (9.6) | 658 (5.8) | 529 (4.7) | 2581 (8.67) | 110 (4.0) | 519 (6.9) | 1781 (9.3) | 122 (6.9) |
| 2004 | 824 (4.9) | 65 (2.8) | 230 (9.2) | 649 (5.7) | 594 (5.2) | 2550 (8.57) | 142 (5.2) | 616 (8.2) | 1708 (8.9) | 122 (6.9) |
| 2005 | 1125 (6.7) | 92 (4.0) | 272 (10.8) | 903 (7.9) | 750 (6.6) | 2406 (8.08) | 191 (7.0) | 649 (8.7) | 1574 (8.2) | 119 (6.7) |
| 2006 | 1212 (7.3) | 176 (7.6) | 239 (9.5) | 885 (7.7) | 804 (7.1) | 2479 (8.33) | 256 (9.4) | 680 (9.1) | 1483 (7.8) | 113 (6.4) |
| 2007 | 1921 (11.5) | 321 (13.9) | 267 (10.6) | 1172 (10.3) | 1252 (11.0) | 2502 (8.41) | 333 (12.2) | 616 (8.2) | 1505 (7.9) | 162 (9.1) |
| 2008 | 1870 (11.2) | 382 (16.5) | 214 (8.5) | 1288 (11.3) | 1307 (11.5) | 2476 (8.32) | 371 (13.6) | 626 (8.3) | 1469 (7.7) | 170 (9.6) |
| 2009 | 2748 (16.5) | 419 (18.1) | 288 (11.5) | 1721 (15.1) | 1795 (15.8) | 2618 (8.8) | 390 (14.3) | 641 (8.5) | 1389 (7.3) | 219 (12.4) |
| 2010 | 2915 (17.5) | 424 (18.3) | 209 (8.3) | 1727 (15.1) | 1848 (16.2) | 2619 (8.8) | 418 (15.3) | 745 (9.9) | 1294 (6.8) | 213 (12.0) |
| 2011 | 2544 (15.2) | 369 (16.0) | 130 (5.2) | 1410 (12.3) | 1722 (15.1) | 2702 (9.08) | 415 (15.2) | 808 (10.8) | 1223 (6.4) | 205 (11.6) |
| Chemotherapy | ||||||||||
| No | 10 263 (61.5) | 1876 (81.1) | 2200 (87.5) | 8554 (74.8) | 7248 (63.7 | 24 658 (82.9) | 2484 (90.9) | 7124 (95.0) | 15 710 (82.3) | 1263 (71.28) |
| Yes | 6437 (38.5) | 437 (18.9) | 313 (12.5) | 2878 (25.2) | 4138 (36.3) | 5106 (17.2) | 249 (9.1) | 378 (5.0) | 3386 (17.7) | 509 (28.72) |
| Trastuzumab | ||||||||||
| No | 15 672 (93.8) | 2233 (96.5) | 2482 (98.77) | 11 005 (96.26) | 10 643 (93.5) | 28 998 (97.4) | 2688 (98.4) | 7439 (99.2) | 18 591 (97.4) | 1665 (94.0) |
| Yes | 1028 (6.2) | 80 (3.5) | 31 (1.23) | 427 (3.74) | 743 (6.5) | 766 (2.57) | 45 (1.7) | 63 (0.8) | 505 (2.6) | 107 (6.0) |
| Axillary surgery | ||||||||||
| No | 3456 (20.7) | 514 (22.2) | 1788 (71.2) | 5830 (51.0) | 2009 (17.6) | 2042 (6.86) | 106 (3.9) | 3347 (44.6) | 943 (4.9) | 32 (1.8) |
| Yes | 13 244 (79.3) | 1799 (77.8) | 725 (28.9) | 5602 (49.0) | 9377 (82.4) | 27 722 (93.1) | 2627 (96.1) | 4155 (55.4) | 18 153 (95.1) | 1740 (98.19) |
| Bilateral mastectomy | ||||||||||
| No | 16 700 (100) | 2313 (100) | 2513 (100) | 10 801 (94.5) | 8015 (70.4) | 29 764 (100) | 2733 (100) | 7502 (100) | 18 542 (97.1) | 1485 (83.8) |
| Yes | 0 (0) | 0 (0) | 0 (0) | 631 (5.5) | 3371 (29.6) | 0 (0) | 0 (0) | 0 (0) | 554 (2.9) | 287 (16.2) |
| Node positive | ||||||||||
| No | 14 520 (87.0) | 2259 (97.7) | 2381 (94.8) | 10 239 (89.6) | 9986 (87.7) | 25 307 (85.0) | 2604 (95.3) | 7038 (93.8) | 15 462 (81.0) | 1464 (82.62) |
| Yes | 2180 (13.1) | 54 (2.3) | 132 (5.3) | 1193 (10.4) | 1400 (12.3) | 4457 (15.0) | 129 (4.7) | 464 (6.2) | 3634 (19.0) | 308 (17.38) |
| Endocrine therapy | ||||||||||
| Yes | 9930 (59.5) | 1436 (62.1) | 585 (23.3) | 3574 (31.3) | 5107 (44.9) | |||||
| No | 6770 (40.5) | 877 (37.9) | 1928 (76.7) | 7858 (68.7) | 6279 (55.2) | |||||
| ER positive | ||||||||||
| Yes | 24 414 (82.0) | 2387 (87.3) | 6102 (81.3) | 14060 (73.6) | 1389 (78.39) | |||||
| No | 3441 (11.6) | 208 (7.6) | 553 (7.4) | 2684 (14.1) | 247 (13.94) | |||||
| Unknown | 1909 (6.41) | 138 (5.1) | 847 (11.3) | 2352 (12.3) | 136 (7.67) | |||||
P value < .001 (chi-square) for all comparisons of treatment strategy by patient and treatment characteristics for each cohort. Brachy = brachytherapy; Depen = dependent; Employ = employee; HMO = Health Maintenance Organization; Lump = lumpectomy; Mast = mastectomy; SEER = Surveillance, Epidemiology, and End Results; WBI = whole breast irradiation.
Exploratory analyses evaluated the impact of WBI fractionation (hypofractionation: 15–22 treatments; conventional fractionation: ≥23 treatments), unilateral vs bilateral mastectomy, and type of breast reconstruction on complications and cost.
All statistical tests were two-sided, with a P value of .05 or less indicating statistical significance. Analyses were conducted using SAS v. 9.3 (Cary, NC) and STATA/MP 13.1 (College Station, TX). Lump+WBI served as the referent category for all analyses. The SEER-Medicare data set and the MarketScan data set are assembled with different selection methodology. Therefore no direct statistical comparisons or inferences between these two groups were conducted. Our institutional review board granted this study exempt status.
Results
Patient Characteristics and Extent of Complications
We identified 105 211 patients (44 344 MarketScan, 60 867 SEER-Medicare) diagnosed with early-stage breast cancer between 2000 and 2011. Baseline features are enumerated in Table 1. Lump+WBI was the most common treatment in both cohorts.
Unadjusted and adjusted total complication rates are provided in Figure 1 and Table 2, respectively. Rates of individual complications for each treatment are enumerated in Supplementary Table 5 (available online). Mast+Recon was associated with the highest complication risk in all years studied for both cohorts (Figure 1). In adjusted models, Mast+Recon was associated with a statistically significantly higher complication risk than Lump+WBI (MarketScan: 54.3% vs 29.6%, relative risk [RR] = 1.87, 95% confidence interval [CI] = 1.82 to 1.91, P < .001; SEER-Medicare: 66.1% vs 37.6%, RR = 1.75, 95% CI = 1.69 to 1.82, P < .001). Common complications after Mast+Recon included infection (24.5% for MarketScan, 30.9% for SEER-Medicare), hematoma/seroma (16.3%, 20.3%), implant removal (15.0%, 19.9%), wound complication (12.8%, 11.7%, respectively), and graft/implant complications (9.5%, 13.6%) (Supplementary Table 5, available online). Unlike Mast+Recon, Mast alone was not associated with increased complication risk compared with Lump+WBI (MarketScan: RR = 0.88, 95% CI = 0.85 to 0.91; SEER-Medicare: RR = 0.98, 95% CI = 0.96 to 1.00).
Figure 1.
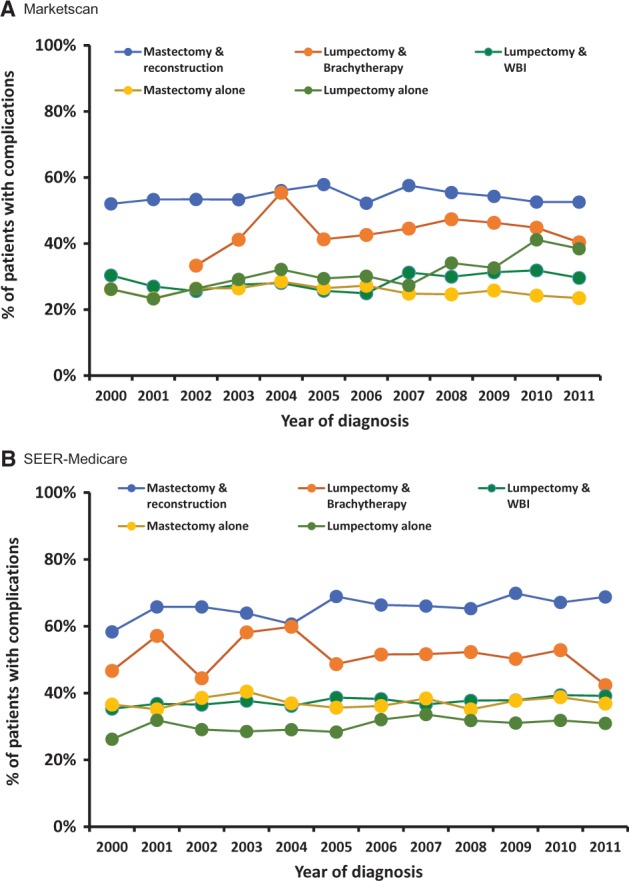
Complications by year of diagnosis and local therapy (unadjusted). Unadjusted time trends in risk of any complication by type of local therapy. Data for lumpectomy plus brachytherapy in 2000 and 2001 are not shown for the MarketScan cohort because of small numbers (n = 5). SEER = Surveillance, Epidemiology, and End Results; WBI = whole breast irradiation.
Table 2.
Logistic regression models for any local treatment complication*
| Variable | MarketScan cohort* |
SEER-Medicare cohort |
||
|---|---|---|---|---|
| RR (95% CI) | P† | RR (95% CI) | P† | |
| Local therapy | ||||
| Lump+WBI | 1 (Ref) | 1 (Ref) | ||
| Lump+Brachy | 1.47 (1.40 to 1.53) | <.001 | 1.36 (1.31 to 1.41) | <.001 |
| Lump alone | 1.10 (1.04 to 1.16) | .001 | 0.87 (0.83 to 0.90) | <.001 |
| Mast alone | 0.88 (0.85 to 0.91) | <.001 | 0.98 (0.96 to 1.00 | .08 |
| Mast+Recon | 1.87 (1.82 to 1.91) | <.001 | 1.75 (1.69 to 1.82) | <.001 |
| Age, y | ||||
| <40 | 1 (Ref) | |||
| 40–49 | 1.04 (0.99 to 1.10) | .14 | ||
| 50–59 | 1.05 (1.00 to 1.11) | .06 | ||
| 60–64 | 1.08 (1.01 to 1.14) | .01 | ||
| 66–69 | 1 (Ref) | |||
| 70–74 | 1.00 (0.97 to 1.03) | 1.00 | ||
| 75–79 | 0.97 (0.94 to 1.00) | .05 | ||
| 80–84 | 0.94 (0.91 to 0.97) | <.001 | ||
| 85+ | 0.94 (0.90 to 0.98) | .003 | ||
| Race | ||||
| White | 1 (Ref) | |||
| Black | 0.95 (0.90 to 0.99) | .02 | ||
| Other/Unknown | 0.88 (0.84 to 0.93) | <.001 | ||
| Comorbidity | ||||
| 0 | 1 (Ref) | 1 (Ref) | ||
| 1 | 1.24 (1.19 to 1.29) | <.001 | 1.10 (1.08 to 1.13) | <.001 |
| 2 or higher | 1.37 (1.26 to 1.48) | <.001 | 1.26 (1.22 to 1.29) | <.001 |
| Chemotherapy | ||||
| None | 1 (Ref) | 1 (Ref) | ||
| Without trastuzumab | 1.14 (1.11 to 1.18) | <.001 | 1.05 (1.01 to 1.08) | .01 |
| With trastuzumab | 1.20 (1.14 to 1.27) | <.001 | 1.03 (0.97 to 1.10) | .32 |
| Axillary surgery | ||||
| No | 1 (Ref) | |||
| Yes | 1.14 (1.09 to 1.19) | <.001 | ||
| Node positive | ||||
| No | 1 (Ref) | |||
| Yes | 1.11 (1.08 to 1.15) | <.001 | ||
No patients were excluded from either model. Brachy = brachytherapy; Lump = lumpectomy; Mast = mastectomy; Recon = reconstruction; SEER = Surveillance, Epidemiology, and End Results; WBI = whole breast irradiation.
All P values were two-sided and calculated in the logistic regression models.
Alternative breast conserving strategies were also compared with Lump+WBI. In both cohorts, Lump+Brachy was associated with higher complication risk than Lump+WBI (Marketscan: 44.5% vs 29.6%, RR = 1.47, 95% CI = 1.40 to 1.53; SEER-Medicare: 50.6% vs 37.6%, RR = 1.36, 95% CI = 1.31 to 1.41). In the SEER-Medicare population, where Lump alone is a guideline-concordant treatment option for selected patients (6), Lump alone was associated with lower complication risk than Lump+WBI (30.5% vs 37.6%, RR = 0.87, 95% CI = 0.83 to 0.90).
Counterintuitively, Lump alone had modestly higher complications than Lump+WBI among the younger women in the MarketScan cohort (31.0% vs 29.6%, RR = 1.10, 95% CI = 1.04 to 1.16). This result is likely because of the finding that 13.3% (335/2513) of patients who underwent Lump alone (defined as within the first year of diagnosis) subsequently underwent mastectomy and reconstruction in the second year after diagnosis. Furthermore, use of mastectomy and reconstruction in year 2, after initial management with Lump alone in year 1, increased substantially over time, rising to 36.2% (47/130) of patients initially managed with Lump alone in 2011 in the MarketScan cohort.
We next performed exploratory analyses on treatment subgroups including hypofractionated WBI, bilateral mastectomy, and subtypes of reconstruction (Supplementary Table 6, available online). Hypofractionated WBI was associated with a similar rate of complications as conventional WBI (Marketscan: RR = 0.99, 95% CI = 0.91 to 1.07; SEER-Medicare: RR = 1.01, 95% CI = 0.96 to 1.07; referent hypofractionated WBI). In comparison with Lump+WBI, bilateral Mast alone was associated with higher complication risk in the Marketscan cohort (RR = 1.21, 95% CI = 1.10 to 1.33) but not in the SEER-Medicare cohort (RR = 1.07, 95% CI = 0.96 to 1.18). In contrast, unilateral Mast alone was associated with lower complication risk than Lump+WBI in the MarketScan cohort (RR = 0.86, 95% CI = 0.82 to 0.89) but not in the SEER-Medicare cohort (RR = 0.98, 95% CI = 0.95 to 1.00).
For patients undergoing Mast+Recon, both bilateral Mast+Recon (MarketScan: RR = 1.80, 95% CI = 1.75 to 1.86; SEER-Medicare: RR = 1.83, 95% CI = 1.68 to 1.96) and unilateral Mast+Recon (MarketScan: RR = 1.80, 95% CI = 1.75 to 1.84; SEER-Medicare: RR = 1.73, 95% CI = 1.67 to 1.80) resulted in similar complication risk relative to Lump+WBI. All reconstruction techniques (implant, autologous tissue transfer, tissue expanders, or a combination) were associated with statistically significantly higher complication risk than Lump+WBI (RR range = 1.47–2.20).
Cost
Trends in adjusted cost for each treatment are provided in Supplementary Figure 1 (available online). All interventions experienced cost growth exceeding inflation among both patient cohorts. For comparing costs, we included only patients diagnosed in the final two years of the study period so that costs accrued during the subsequent 24 months would reflect contemporary expenditures. Table 3 stratifies the findings by treatment strategy and highlights the portion attributable to complications. Cost differentials relative to Lump+WBI are graphically summarized in Figure 2.
Table 3.
Unadjusted and Adjusted 2-year Costs by Local Therapy Strategy, Diagnosis years 2010–2011*
| Treatment | Unadjusted costs, $ |
Adjusted costs, $ |
||||||
|---|---|---|---|---|---|---|---|---|
| Total |
Complication | Noncomplication |
Total | Complication | Noncomplication | |||
| Mean (95% CI) | Median (IQR) | Mean (95% CI) | Mean (95% CI) | Median (IQR) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| MarketScan | ||||||||
| Lump+WBI | 77 309 (75 768 to 78 850) | 60 258 (41 228–93 905) | 1431 (1270 to 1592) | 75 878 (74 361 to 77 395) | 59 209 (40 374–91 950) | 65 608 (64 067 to 67 149) | 1385 (1210 to 1560) | 63 834 (62 348 to 65 320) |
| Lump+Brachy | 72 184 (68 637 to 75 732) | 60 339 (39 424–89 279) | 1718 (1308 to 2128) | 70 467 (66 970 to 73 963) | 58 805 (38 340–87 453) | 77 130 (72 423 to 81 837) | 1802 (1206 to 2399) | 75 058 (70 536 to 79 581) |
| Lump alone | 60 741 (53 872 to 67 611) | 45 714 (16 972–79 532) | 3331 (2294 to 4367) | 57 411 (50 636 to 64 185) | 38 684 (15 763–75 064) | 70 520 (63 964 to 77 077) | 3620 (1789 to 5452) | 67 169 (61 018 to 73 321) |
| Mast alone | 49 214 (46 608 to 51 821) | 26 244 (11584–59 058) | 2134 (1551 to 2717) | 47 080 (44 675 to 49 486) | 25 231 (11 254–56 834) | 48 258 (46 707 to 49 811) | 1897 (1575 to 2219) | 46 798 (45 305 to 48 292) |
| Mast+Recon | 95 922 (93 527 to 98 316) | 75 848 (49 229–117 680) | 10 065 (9250 to 10 881) | 85 857 (83 630 to 88 083) | 66 620 (43 008–105 407) | 88 089 (85 509 to 90 670) | 10 402 (8772 to 12 032) | 77 316 (75 076 to 79 556) |
| SEER-Medicare | ||||||||
| Lump+WBI | 36 816 (36 251 to 37382) | 30 790 (23 842–42 198) | 584 (529 to 640) | 36 231 (35 672 to 36 791) | 30 195 (23 463–41 252) | 34 087 (33 633 to 34 541) | 549 (497 to 602) | 33 539 (33 087 to 33 992) |
| Lump+Brachy | 36 128 (34 983 to 37 272) | 31 545 (26 503–40 967) | 701 (560 to 841) | 35 427 (34 283 to 36 570) | 30 881 (25 739–40 251) | 37 617 (36 356 to 38 877) | 708 (536 to 879) | 36 904 (35 651 to 38 157) |
| Lump alone | 19 461 (18 662 to 20 260) | 14 645 (9919–23 432) | 490 (390 to 590) | 18 971 (18 179 to 19 762) | 14 251 (9655–22 740) | 21 005 (20 452 to 21 557) | 530 (434 to 626) | 20 442 (19 897 to 20 987) |
| Mast alone | 25 367 (24 551 to 26 183) | 19 191 (13 208–29 228) | 677 (569 to 785) | 24 690 (23 884 to 25 496) | 18 558 (12 723–28 623) | 22 182 (21 751 to 22 612) | 656 (564 to 748) | 21 517 (21 094 to 21 941) |
| Mast+Recon | 43 154 (40 813 to 45 496) | 37 288 (26 861–53 043) | 2962 (2463 to 3462) | 40 192 (37 882 to 42 502) | 33 562 (24 549–49 157) | 35 835 (34 140 to 37 531) | 2641 (1734 to 3548) | 33 035 (31 452 to 34 619) |
Adjusted costs are adjusted for the variables listed in Table 1. Each cost model was iteratively refined to remove covariables that did not statistically significantly contribute to the model. No patients were excluded from any calculation. All figures are reported in 2014 dollars. Brachy = brachytherapy; CI = confidence interval; Lump = lumpectomy; Mast = mastectomy; Recon = reconstruction; SEER = Surveillance, Epidemiology, and End Results; WBI = whole breast irradiation.
Figure 2.
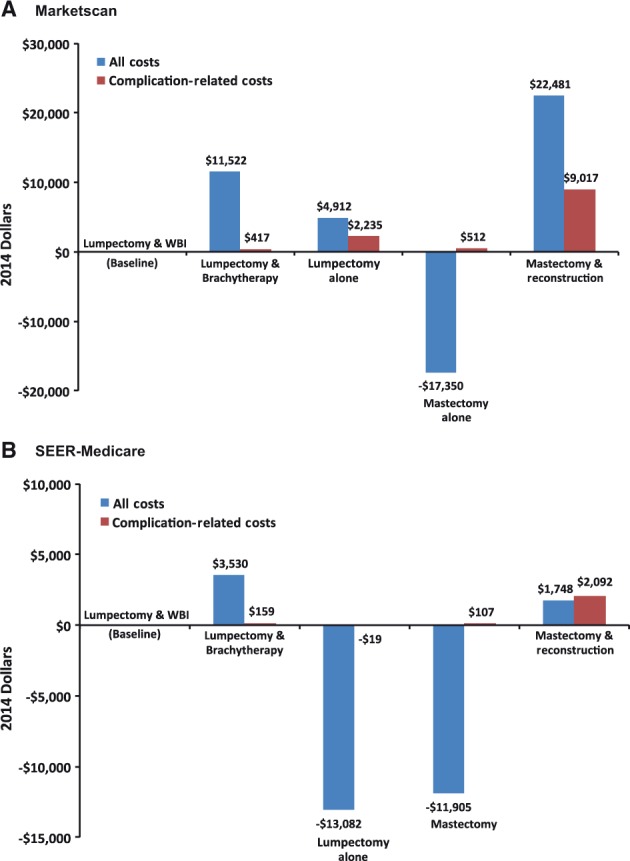
Adjusted total and complication-related cost relative to lumpectomy and whole breast irradiation for patients diagnosed in 2010 and 2011. Adjusted difference in total cost and complication-related cost of local therapy options, relative to lumpectomy plus whole breast irradiation, for patients diagnosed in 2010 and 2011. SEER = Surveillance, Epidemiology, and End Results; WBI = whole breast irradiation.
In the MarketScan cohort, Lump+WBI was associated with the lowest adjusted mean cumulative cost ($65 608) among options that provided a breast mound (ie, all treatments except for Mast alone). Mast+Recon was associated with a 34% higher adjusted cost per patient ($88 089) because of both cost of intervention ($77 316 vs $63 834) and cost of complications ($10 402 vs $1385). In the SEER-Medicare cohort, Mast+Recon was also associated with higher adjusted mean cost than lumpectomy plus WBI ($35 835 vs $34 087), which was attributable solely to cost of complications ($2641 vs $549). Subgroup analysis showed that bilateral Mast+Recon and Mast+Recon involving a combination of autologous transfer and implant were associated with higher cost for both cohorts (Supplementary Table 7, available online). Mast alone was associated with lower cost than Lump+WBI among both MarketScan ($48 258) and SEER-Medicare ($22 182) patients.
With regard to lumpectomy-based strategies, Lump+Brachy was associated with $11 522 excess cost over Lump+WBI among MarketScan patients and $3530 excess cost among SEER-Medicare patients. In contrast, hypofractionated WBI was associated with $2467 and $4462 in savings per patient in Marketscan and SEER-Medicare, respectively, compared with conventional WBI (Supplementary Table 7, available online). Among MarketScan patients, Lump alone ($70 520) was more costly than Lump+WBI because of the cost associated with frequent secondary surgeries performed in the second year after diagnosis (Table 3). Among SEER-Medicare patients, Lump alone was associated with the lowest cumulative costs ($21 005) of all interventions.
Discussion
Utilizing two large databases comprised of younger women with private insurance and older women with public insurance, we characterized costs and complications during the first 24 months after diagnosis of early breast cancer treated with one of five common local therapy strategies. Despite the considerable clinical and sociodemographic differences between the two cohorts, we observed a striking concordance in the results of our analysis. In both populations, Mast+Recon was associated with nearly two-fold increased complication risk compared with Lump+WBI, a finding that persisted throughout the study interval of 2000 to 2011. Additionally, compared with Lump+WBI, Mast+Recon yielded $22 481 higher total cost and $9017 higher complication-related cost in the MarketScan cohort, and $1748 higher total cost and $2092 higher complication-related cost in the SEER-Medicare cohort.
The direct comparison of complication risk and cost outcomes between Mast+Recon and Lump+WBI, in addition to the three other treatment strategies included in this analysis, is a novel addition to the literature, which informs ongoing public health discussions aimed at identifying high-value treatment options for common cancers. Prior literature has typically focused on comparing complications and cost within only one or two treatment options (17,19,21–26) and thus lacks a comprehensive comparative assessment of all the common treatment options typically presented to a patient with early breast cancer. For example, in 2001, a seminal paper by Barlow et al. concluded that Lump+WBI was more expensive than mastectomy within the first six months of diagnosis, but that mastectomy was more expensive when including costs within five years. This study was limited, though, as less than 5% of the sample underwent breast reconstruction (23). As another example, our group has previously reported excess complications with Lump+Brachy compared with Lump+WBI (17,19,21), but these studies did not contextualize complications of brachytherapy relative to mastectomy with or without reconstruction, which is frequently the alternative to Lump+Brachy, the treatment contemplated by patients seeking to avoid a protracted course of WBI.
In recent years, there has been a marked increase in the use of bilateral mastectomy and reconstruction to treat early breast cancer (27–32). While some of these procedures are clearly medically indicated, the choice for mastectomy is often driven by nonmedical factors such as patient preferences for more “complete” cancer treatment by extirpating the entirety of the affected organ, patient fears of in-breast recurrence following Lump+WBI, or patient anxiety regarding the need for ongoing mammographic surveillance of the conserved breast (8). Our finding of substantially higher cost of Mast+Recon compared with Lump+WBI highlights an important conflict that will be increasingly confronted in an era focused on “value” in health care. Specifically, patients may prefer a more expensive treatment such as Mast+Recon for nonmedical reasons when a less expensive treatment such as Lump+WBI may be equally effective from a purely medical perspective. If such a patient is receiving care from a health care entity with a financial stake in promoting “high-value” care, the entity may profit financially if the patient receives the lower-cost intervention and, conversely, may experience a financial loss if the patient receives the higher-cost intervention. Developing a framework to guide health care entities, physicians, and patients through such conflicted decisions will become increasingly important as innovative reimbursement models employing episodic payments or value-based insurance designs begin to permeate the marketplace (3).
Aside from the marked differences between Mast+Recon and Lump+WBI, our study findings also reveal important differences in cost and complications across the different lumpectomy-based options. Specifically, Lump+Brachy yielded an approximately 40% higher relative risk of complications and higher costs than Lump+WBI. The differences in cost were mainly attributable to the procedure itself, as differences in cost because of complications were small: $417 greater for brachytherapy in the MarketScan cohort and $159 greater in the SEER-Medicare cohort. Notably, the shorter course of brachytherapy may decrease indirect patient costs because of travel and lost work and thereby offset a portion of its excess cost. Regarding Lump+WBI, our findings confirm other randomized and population-based studies, which have shown that hypofractionated treatment reduces cost without increasing complications (33–35). The opportunity to encourage this form of WBI should be examined closely in light of recent evidence concluding that only one-third of eligible American women receive hypofractionated WBI (36).
Another key result is that Lump alone was a high-value intervention in older patients, in whom it is considered guideline-concordant (6). However, in the younger cohort, Lump alone was associated with higher costs and complications because of the high prevalence of mastectomy occurring in the second year after diagnosis. The underlying cause of mastectomy—for example, because of true local relapse vs fear of recurrence vs initial pathological findings requiring mastectomy—cannot be determined. However, these procedures and underlying causes were not considered “complications” in our study design and thus do not influence our finding of excess complications and complication-related cost in this group. Finally, our study illustrates that mastectomy alone is a high-value intervention for women who do not prioritize retaining a native or reconstructed breast mound.
In interpreting results of this study, we note that not all individual patients would have been eligible for every treatment strategy. For example, a patient with early breast cancer may have extensive premalignant changes in the breast or a hereditary breast cancer syndrome, thus prompting treatment with Mast alone or Mast+Recon. Nevertheless, our population findings remain broadly representative of actual aggregate outcomes for American patients, the majority of whom are candidates for multiple therapeutic approaches. Another limitation is that analyses relied on the perspective of the payer and were unable to evaluate outcomes such as patient satisfaction or quality of life following one treatment compared with another. Complementary patient-level data are needed to inform physician-patient discussions on the entirety of the risks and benefits of their treatment options. Thirdly, this study is limited by the nature of claims data, which rely on the accuracy of coders and are not comprehensive of all provider and patient characteristics or all potential complications. Future studies should also explore long-term costs, for example, the costs of surveillance mammography and treating second cancers, which are also likely to differ by chosen initial treatment. Finally, although we adjusted for important clinical variables in all analyses, residual confounding may exist in this retrospective study design.
In conclusion, there are major differences in cost and complication profile within two years of diagnosis among guideline-concordant local treatment strategies for breast cancer. Mast alone is a high-value treatment for patients who do not wish to retain a breast mound. Mast+Recon is associated with considerably higher cost and complications. Lump alone can be a high-value intervention for older patients but is not high value for younger patients. Lumpectomy plus hypofractionated WBI combines high oncologic effectiveness, breast preservation, and a favorable complication profile. These findings should be helpful to patients contemplating their treatment options for early breast cancer and will inform ongoing public health discussions aimed at identifying high-value treatment options for common cancers.
Funding
This work was supported by a research grant from Varian Medical Systems to Dr. Smith and by a grant from the Cancer Prevention & Research Institute of Texas (Grant RP140020) to Drs. Smith and Giordano. This work was also supported by the Department of Health and Human Services National Cancer Institute (Grant P30CA016672), the Duncan Family Institute, the Center for Radiation Oncology Research of the MD Anderson Cancer Center, and a philanthropic gift from Ann and Clarence Cazalot. Dr. Smith is supported by the Andrew Sabin Family Fellowship.
Notes
The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Dr. Shaitelman reports research funding from Elekta Incorporated that is unrelated to the current project. She is also a consultant to the MD Anderson Physician’s Network.
The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Supplementary Material
References
- 1. Young RC. Value-based cancer care. N Engl J Med. 2015;373(27):2593–2595. [DOI] [PubMed] [Google Scholar]
- 2. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. [DOI] [PubMed] [Google Scholar]
- 3. de Souza JA, Ratain MJ, Fendrick AM. Value-based insurance design: aligning incentives, benefits, and evidence in oncology. J Natl Compr Canc Netw. 2012;10(1):18–23. [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 5.SEER Stat Fact Sheet: Breast Cancer. http://seer.cancer.gov/statfacts/html/breast.html. Accessed January 3, 2016.
- 6. Gradishar WJ, Anderson BO, Balassanian R, et al. Breast Cancer Version 2.2015. J Natl Compr Canc Netw. 2015;13(4):448–475. [DOI] [PubMed] [Google Scholar]
- 7. Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32(9):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fisher CS, Martin-Dunlap T, Ruppel MB, et al. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol. 2012;19(10):3246–3250. [DOI] [PubMed] [Google Scholar]
- 9. Lee CN, Chang Y, Adimorah N, et al. Decision making about surgery for early-stage breast cancer. J Am Coll Surg. 2012;214(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albornoz CR, Matros E, Lee CN, et al. Bilateral mastectomy versus breast-conserving surgery for early-stage breast cancer: The role of breast reconstruction. Plast Reconstr Surg. 2015;135(6):1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Litiere S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13(4):412–419. [DOI] [PubMed] [Google Scholar]
- 12. Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567–575. [DOI] [PubMed] [Google Scholar]
- 13. Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 14. Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39(6 Pt 1):1733–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gold HT, Do HT. Evaluation of three algorithms to identify incident breast cancer in Medicare claims data. Health Serv Res. 2007;42(5):2056–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 17. Smith GL, Xu Y, Buchholz TA, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307(17):1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: A claims-based analysis. Ann Surg. 2016;263(2):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith GL, Jiang J, Buchholz TA, et al. Benefit of adjuvant brachytherapy versus external beam radiation for early breast cancer: Impact of patient stratification on breast preservation. Int J Radiat Oncol Biol Phys. 2014;88(2):274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. [DOI] [PubMed] [Google Scholar]
- 21. Huo J, Giordano SH, Smith BD, et al. Contemporary toxicity profile of breast brachytherapy versus external beam radiation after lumpectomy for breast cancer. Int J Radiat Oncol Biol Phys. 2016;94(4):709–718. [DOI] [PubMed] [Google Scholar]
- 22. Warren JL, Brown ML, Fay MP, et al. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002;20(1):307–316. [DOI] [PubMed] [Google Scholar]
- 23. Barlow WE, Taplin SH, Yoshida CK, et al. Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. J Natl Cancer Inst. 2001;93(6):447–455. [DOI] [PubMed] [Google Scholar]
- 24. Fischer JP, Fox JP, Nelson JA, et al. A longitudinal assessment of outcomes and healthcare resource utilization after immediate breast reconstruction-comparing implant- and autologous-based breast reconstruction. Ann Surg. 2015;262(4):692–699. [DOI] [PubMed] [Google Scholar]
- 25. Israeli R, Funk S, Reaven NL. Comparative analysis of 18-month outcomes and costs of breast reconstruction flap procedures. Plast Reconstr Surg. 2014;133(3):471–479. [DOI] [PubMed] [Google Scholar]
- 26. Deshmukh AA, Cantor SB, Crosby MA, et al. Cost of contralateral prophylactic mastectomy. Ann Surg Oncol. 2014;21(9):2823–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol. 2013;20(5):1436–1443. [DOI] [PubMed] [Google Scholar]
- 28. Dragun AE, Huang B, Tucker TC, et al. Increasing mastectomy rates among all age groups for early stage breast cancer: A 10-year study of surgical choice. Breast J. 2012;18(4):318–325. [DOI] [PubMed] [Google Scholar]
- 29. Dragun AE, Pan J, Riley EC, et al. Increasing use of elective mastectomy and contralateral prophylactic surgery among breast conservation candidates: A 14-year report from a comprehensive cancer center. Am J Clin Oncol. 2013;36(4):375–380. [DOI] [PubMed] [Google Scholar]
- 30. Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27(25):4082–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16(10):2682–2690. [DOI] [PubMed] [Google Scholar]
- 32. Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9–16. [DOI] [PubMed] [Google Scholar]
- 33. Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. [DOI] [PubMed] [Google Scholar]
- 34. Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. [DOI] [PubMed] [Google Scholar]
- 35. Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol. 2015;1(7):931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008-2013. JAMA. 2014;312(23):2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


