Abstract
This scientific commentary refers to ‘Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination’, by Clark et al.. (doi:10.1093/brain/awx012).
This scientific commentary refers to ‘Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination’, by Clark et al.. (doi:10.1093/brain/awx012).
Peripheral myelination is an essential process in the development of the peripheral nervous system. Peripheral myelin permits saltatory action potential propagation, and also supports axons via an intricate system of interactions between Schwann cells and the axolemma. Both hereditary and acquired inflammatory processes can damage peripheral myelin and this is the pathological basis of demyelinating peripheral neuropathies such as Charcot-Marie-Tooth disease type 1, Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). The study of peripheral myelination and demyelination has relied heavily on animal models as systems that allow for dissecting the molecular mechanisms by which Schwann cells regulate myelin production, and for exploring the interaction between these cells and peripheral axons. Another classical approach to investigate peripheral myelination is based on in vitro co-culture systems of rodent dorsal root ganglia neurons and Schwann cells; however, the lack of an analogous human cellular system has limited the study of human peripheral myelination. In this issue of Brain, Clark and co-workers address this obstacle by using cellular reprogramming to create a system to study peripheral myelination of human axons in vitro (Clark et al., 2017).
Induced pluripotent stem cells (iPSCs) are generated by forcing expression of pluripotency factors in somatic cells such as fibroblasts, a technique termed cellular reprogramming. Human iPSCs can be differentiated into cells from all three germinal layers, including several neuronal types, and are an invaluable tool for modelling human neurological disorders at the cellular level. IPSCs have been used in the past to generate human motor neurons and to model inherited (Saporta et al., 2015) and acquired (Harschnitz et al., 2016) neuropathies. However, neurons behave quite differently in the absence of myelin. Juxtacrine signalling from Schwann cells helps organize the entire length of myelinated axons into a series of polarized domains centred around nodes of Ranvier, which are necessary for normal saltatory nerve conduction (Salzer, 2003). Axonal calibre, neurofilament phosphorylation and packing density, as well as axonal transport, are all disrupted when axons are ensheathed by abnormal myelin. This has been shown in elegant studies in rodent models (de Waegh et al., 1992; Kirkpatrick and Brady, 1994), such as Trembler (Tr) mice, a naturally occurring dysmyelinating model caused by missense mutations in the peripheral myelin protein 22 (PMP22) gene (Suter et al., 1992). Accordingly, many human disorders cannot be accurately modelled using iPSC-derived neurons owing to the absence of myelin and axon-Schwann cell interactions. Human neuronal Schwann cell co-cultures have not been possible to date because of the inability to differentiate myelinating Schwann cells from iPSCs, as will be discussed below. However, in this issue of Brain, Clark et al. (2017) establish the first myelinating co-culture systems using human iPSC-derived sensory neurons and rat Schwann cells, and use them to demonstrate the important role of the neuregulin-ErbB signalling pathway in the myelination of human sensory axons. In addition, they use their system to demonstrate the effects of anti-disialosyl antibodies in myelinated axons, and thereby establish an in vitro system to model acquired demyelinating neuropathies.
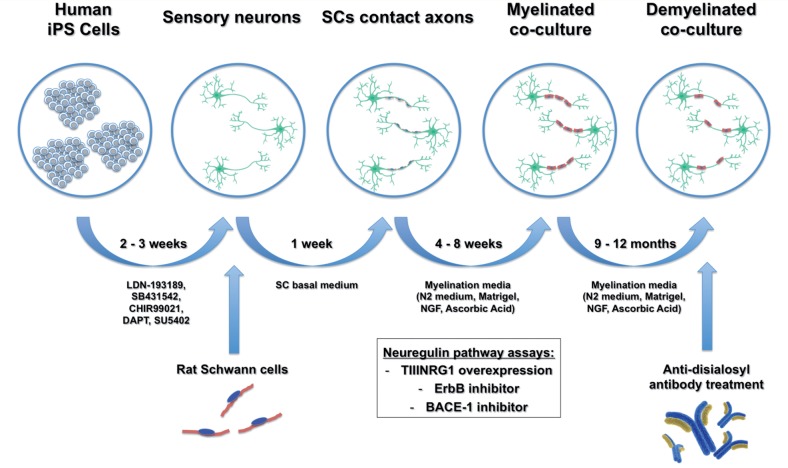
By successfully establishing co-cultures of human iPSC-derived sensory neurons and rat Schwann cells, Clark et al. showed that two different species have enough molecular signalling homology to allow for efficient Schwann cell-axonal interactions and to initiate alignment, basal lamina formation and myelination. The authors also introduced adaptations to the classical protocol for co-culture assays, including the use of a dedicated myelination medium consisting of a neuronal medium (N2) supplemented with Matrigel® and ascorbic acid (Fig. 1). This simple modification yielded cultures with enhanced neuronal health and alignment of Schwann cells to axons as well as significantly increased levels of myelination. The fact that these co-cultures could be maintained for at least 12 months without any signs of axonal or myelin deterioration is also noteworthy. The authors further demonstrated that their co-culture system beautifully reproduced the molecular organization of myelinated axons in discrete compartments, including the node of Ranvier and the paranodal and juxtaparanodal regions, as previously demonstrated in rodent peripheral nerves (Salzer, 2003) as well as in human myelinated dermal nerve fibres (Li et al., 2005).
Figure 1.
Schematic of experimental design. Human iPSC-derived sensory neurons were generated following a 14-day differentiation protocol based on small molecules. Rat Schwann cells were added and co-cultured with the human neurons in Schwann cell basal medium for 1 week to allow engagement with axons. Next, co-cultures were switched to a myelination medium consisting of N2 neuronal medium supplemented with Matrigel®, NGF and ascorbic acid, which supported the co-culture for very long periods (at least 12 months). Assays to evaluate the neuregulin-ErbB signalling pathways were performed throughout the myelination period. When added to mature myelinated co-cultures, anti-disialosyl antibodies induced demyelination demonstrating the potential of this system to model acquired demyelinating neuropathies. SC = Schwann cell.
Membrane-bound type III neuregulin 1 (TIIINRG1) is a key regulator of peripheral myelination through its interactions with ErbB2-ErbB3 receptors at the Schwann cell membrane (Taveggia et al., 2005). Clark and co-authors investigated the role of TIIINRG1-ErbB signalling pathways in their co-cultures using a number of approaches. First, they established that overexpression of TIIINRG1 by iPSC-derived sensory neurons strongly promoted myelination. Second, they demonstrated that pharmacological inhibition of ErbB receptors caused a dose-dependent reduction in myelination. Lastly, inhibition of beta secretase 1 (BACE-1), an enzyme involved in the proteolytic activation of neuregulin 1 in the peripheral nervous system, also inhibited myelination. Taken together, these results indicate that the TIIINRG1-ErbB signalling pathway is also an essential regulator of human peripheral myelination. Of note, these findings raise concern that BACE-1 inhibition, a current treatment strategy for Alzheimer’s disease, may be complicated by peripheral demyelination, limiting its use as a human therapeutic.
Finally, the authors used their co-culture system to study the effects of anti-disialosyl antibodies on myelinated axons. Anti-disialosyl antibodies are associated with several acquired inflammatory demyelinating neuropathies, including acute sensory neuropathy associated with anti-GD1b IgG antibodies and CANOMAD (chronic ataxic neuropathy with opthalmoplegia and M-protein, cold agglutinins and anti-disialosyl antibodies). Using both mouse and human anti-disialosyl monoclonal antibodies, the authors demonstrated impaired myelination of human sensory axons when antibodies were added to co-cultures at the onset of myelination. Even more strikingly, human anti-disialosyl IgM antibody caused demyelination in established co-cultures aged 9 to 12 months, quantified as a 10% increase in myelin loss compared to control cultures, as well as the presence of disintegrating myelin surrounding intact axons. These findings demonstrate the potential of this system to model demyelinating immune neuropathies such as Guillain-Barre syndrome and CIDP and to identify and validate new therapeutic agents for this group of acquired neuropathies.
Unfortunately, the ideal co-culture system composed of both Schwann cells and neurons derived from human iPSCs remains elusive. Such a system would be particularly useful in modelling inherited demyelinating neuropathies, where having Schwann cells and neurons with an identical genetic background to patients would be a valuable asset. Despite significant advancements in the development of Schwann cell differentiation protocols (Liu et al., 2012), iPSC-derived Schwann cells have failed to demonstrate robust in vitro myelination when co-cultured with iPSC-derived neurons, a limitation also observed in primary human Schwann cells. This is the major roadblock that needs to be overcome to allow further development of this field.
This work by Clark and colleagues has created a wide range of opportunities for the study of peripheral neuropathies. Their system is robust and durable and seems ideal for use in high-content platforms for drug discovery in demyelinating neuropathies and should also help expand our understanding of the basic mechanisms involved in peripheral myelination and Schwann cell-axonal interactions.
Glossary
Anti-disialosyl antibodies: Anti-disialosyl antibodies react with the disialosyl epitopes shared by gangliosides, sialic-acid containing glycosphingolipids that are abundant in the nervous system. Monoclonal IgM anti-disialosyl antibodies react with gangliosides GD1b, GQ1b, GT1c and GD3 and are typically associated with an ataxic sensory neuropathy, such as CANOMAD. IgG antibodies to disialosyl epitopes, including GD1b and GQ1b are associated with Miller-Fisher syndrome (acute ataxic neuropathy with ophthalmoplegia).
Induced pluripotent stem cells: Embryonic-like iPSCs capable of differentiating into a variety of cells in the body can be derived from somatic cells by the forced expression of defined factors, including KLF4, OCT3/4, SOX2 and C-MYC. Distinct combinations of factors, and strategies to induce their expression, have been used to generate iPSCs from a number of human tissues with varying degrees of efficiency. iPSCs can be coaxed into specific cell types by manipulating the culture environment. Growth factors, small molecules and extracellular matrix proteins can be applied in a sequential manner to emulate the normal development of the cell lineage of interest. Using this approach, investigators have been able to differentiate human pluripotent cells into lineages necessary for modelling neurological diseases, including cholinergic, glutamatergic and dopaminergic neurons, astrocytes, oligodendrocytes and Schwann cells.
Neuregulin signalling pathway: Neuregulins (NRGs) comprise a large family of EGF-like signalling molecules involved in cell-cell communication during development and disease. NRG1 plays important roles in the development of the nervous system, heart, and mammary glands and this diversity of in vivo functions is partially explained by the many different isoforms of NRG1. Neuregulins transmit their signals to target cells by interacting with transmembrane tyrosine kinase receptors of the ErbB family. In vivo, functional NRG1 receptors are heterodimers composed of ErbB2 with either an ErbB3 or ErbB4 molecule. The tissue-specific distribution of the different receptor types further contributes to the diversity and specificity of the biological functions of this signalling pathway. This pathway functions in early precursor proliferation, maturation, as well as in the myelination of Schwann cells, mainly by interactions between type III neuregulin 1 and ErbB2/B3 receptors.
Funding
Both authors receive support from the Inherited Neuropathy Consortium (INC) RDCRC (U54NS065712) supported by NINDS/ORDR, NCATS and also by funding from the Muscular Dystrophy Association (MDA) and Charcot Marie Tooth Association (CMTA).
References
- Clark AJ, Kaller MS, Galino J, Willison HJ, Rinaldi S, Bennett DLH. Co-cultures with stem cell derived human sensory neurons reveal regulators of peripheral myelination. Brain 2017; 140: 898–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 1992; 68: 451–63. [DOI] [PubMed] [Google Scholar]
- Harschnitz O, van den Berg LH, Johansen LE, Jansen MD, Kling S, Vieira de Sá R, . et al. Autoantibody pathogenicity in a multifocal motor neuropathy induced pluripotent stem cell-derived model. Ann Neurol 2016; 80: 71–88. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LL, Brady ST. Modulation of the axonal microtubule cytoskeleton by myelinating Schwann cells. J Neurosci 1994; 14: 7440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bai Y, Ghandour K, Qin P, Grandis M, Trostinskaia A, . et al. Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain 2005; 128: 1168–77. [DOI] [PubMed] [Google Scholar]
- Liu Q, Spusta SC, Mi R, Lassiter RNT, Stark MR, Höke A, . et al. Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl Med 2012; 1: 266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron 2003; 40: 297–318. [DOI] [PubMed] [Google Scholar]
- Saporta MA, Dang V, Volfson D, Zou B, Xie XS, Adebola A, . et al. Axonal Charcot-Marie-Tooth disease patient-derived motor neurons demonstrate disease-specific phenotypes including abnormal electrophysiological properties. Exp Neurol 2015; 263: 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U, Welcher AA, Ozcelik T, Snipes GJ, Kosaras B, Francke U, . et al. Trembler mouse carries a point mutation in a myelin gene. Nature 1992; 356: 241–4. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, . et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 2005; 47: 681–94. [DOI] [PMC free article] [PubMed] [Google Scholar]