Abstract
In Central Europe, the most abundant aphid infesting the leaves of small grain cereals is Metopolophium dirhodum (Walker) (Homoptera: Aphididae). Annual variation in its seasonal dynamics was evaluated using a 25-yr series of standardized weekly censuses of winter wheat plots. M. dirhodum made up >50 % of the aphids on the foliage. Date of immigration (8 May–3 July), length of period of population increase (0–9 wk), and date of attaining maximum abundance (28 May–22 July) varied greatly. For the prediction, we regressed maximum numbers/tiller on numbers recorded in the first week after heading. The regression of maximum abundance on nonzero aphid counts revealed a critical number of ≥1.50 aphids/tiller, which if exceeded resulted in a harmful maximum abundance of ≥10 aphids/tiller at the peak. Zero aphid counts resulted in 10% of cases with a harmful maximum abundance. Using this regression for prediction will result in 18% of the recorded cases being false negatives and 9% false positives. Parallel annual variation in the average maximum numbers of M. dirhodum, Sitobion avenae (Fabricius) (Homoptera: Aphididae), and Rhopalosiphum padi (Linné) (Homoptera: Aphididae) indicated the following factors that affected their abundance: temperature in winter and host plant quality. The predictions apply only in areas where M. dirhodum is holocyclic and aphids do not overwinter in wheat stands.
Keywords: aphid, Sitobion avenae, Rhopalosiphum padi, forecast, population dynamics
The aphid Metopolophium dirhodum (Walker) (Homoptera: Aphididae) along with Sitobion avenae (Fabricius) (Homoptera: Aphididae) and Rhopalosiphum padi (Linné) (Homoptera: Aphididae) regularly infest cereals in Europe. M. dirhodum is native in the Holarctic and was introduced into South America, South Africa, Australia, and New Zealand (Blackman and Eastop 1984). In central Europe, holocyclic populations of M. dirhodum overwinter on Rosaceae plants, mostly Rosa spp. (Holman 2009). Fundatrices hatch in March–April, produce a few generations on primary hosts, and then alate exules colonize stands of small grain cereals and maize in May (Bode 1980, Leather and Walters 1984), where they produce several generations and increase in abundance, until late June–early July, when it sharply declines in abundance due to alate emigration (Howard and Dixon 1992) and mortality (Honek 1991, Honek and Martinkova 2004). At that time, M. dirhodum populations decline not only on mature small grain cereals but also on maize plants, which are still suitable for aphids (Honek et al. 1998). The migrants move to alternative host plants, grasses, or cereal volunteers where they survive until autumn and then migrate to the primary hosts, in September–October (Blackman and Eastop 1984).
In the continental climate of central Europe, M. dirhodum is usually the most abundant aphid species on cereals (Honek 1987, Sengonca et al. 1994, Praslicka 1996, Basky 1996). This situation is specific to this area as further north (Debek-Jankowska and Barczak 2005) and west (Dedryver 1978, Leather et al. 1984, Oakley and Walters 1994), and M. dirhodum is usually not the dominant species of cereal aphid.
Although cereal aphids are important pests (Vickerman and Wratten 1979, Dixon 1987, Dedryver et al. 2010), M. dirhodum, in our opinion, has not aroused the attention it deserves due to its abundance. This is because M. dirhodum colonizes only leaves (Honek 1987, Honek and Martinkova 2002), and more attention is paid to aphids colonizing ears, because they are likely to cause more damage (Wetzel 2004). Nevertheless, its economic importance is well documented in countries where M. dirhodum is abundant, North America (Johnston and Bishop 1987, Schotzko and Bosque-Perez 2000, Clement et al. 2004), South America (Lopes-da-Silva and Vieira 2007, Sepulveda et al. 2017), and Central Europe (Havlickova 1997).
Despite M. dirhodum’s importance, there are no studies of its population dynamics or predictions of its maximum abundance in central Europe. In this article, using a 25-yr-long series of observations on the seasonal dynamics of M. dirhodum in stands of winter wheat, we address three topics: 1) Annual variation in seasonal population dynamics, 2) prediction of maximum abundance attained in a season, and 3) its correlation with the presence of other cereal aphid species.
Material and Methods
Aphid Counts
Aphids were counted from 1992 to 2016 in production stands of winter wheat at Praha-Ruzynĕ in the western Czech Republic growing within a 1-km2 area centered at 50.086N 14.294E and at an altitude of 320–360 m. The wheat stands were cultivated in accordance with recommended agriculture practices (Spaldon 1982, Palik et al. 2009). No insecticides were applied during this study, and fungicides and fertilizers were sprayed early in the season before aphids arrived. Every year, 5- × 10-m plots were placed in a line transect across a wheat field, 30–50 m apart, with a 30-m minimum distance from the field margin. The number of plots varied but in most years there were three replicates. The plots were split into a 5- × 5-m subplot in which the wheat crop was thinned by removing plants in mid-April (further referred to as sparse, with crop density at the time of maximum aphid abundance [MA] 40–45 tillers/m2) and a 5- × 5-m subplot where the crop stand was left not thinned (further referred to as dense, 270–290 tillers/m2). Tiller density was established at the time of aphid maxima at 10 randomly placed plots of 25 × 25 cm size in each sparse and dense subplot. Since aphid abundances in sparse and dense stands are different (Honek and Martinkova 1999), this design increased the spatial diversity in aphid abundance. Each year, weekly aphid counts were conducted on 30–300 tillers per subplot during the 6–10 wk that they were present on wheat. Data were recorded as aphids/tiller within each subplot, separately for each aphid species.
Statistical Analysis
The analysis was made in R version 3.3.3 (R Core Team 2017). Prior to the analysis, the aphid counts were log10 transformed to increase the normality of the data. As the first step we tested whether the MA differed between dense and sparse stands to ascertain whether this factor should be included in the following analyses. To do this, we used a linear mixed model with random intercept, in which the random term consisted of the year of counting, and the nlme package (Pinhero et al. 2017). This is because aphid counts might be correlated across stands within a particular year (Honěk et al. 2017b), so this possible correlation was accounted for in all the models tested. Restricted maximum likelihood (REML) was used during the selection of the random term, and maximum likelihood was used to simplify the fixed terms in the models within the final structure of the random term (Zuur et al. 2009). The final model was then refitted using REML to obtain estimates of the parameters along with their standard errors (Zuur et al. 2009). Log-likelihood ratio (L-ratio) was used to compare the models (Zuur et al. 2009).
We tested whether the abundance of M. dirhodum on leaves at the time of heading can be used to predict the probability that the loss of yield due this aphid will be economically important. For the following practical reasons, the time when 50% of the tillers are at the heading stage was chosen as the time when aphid counts could be used to predict maximum abundance: 1) the timing of the peak in population is unknown, 2) heading stage can be easily determined in practice, and 3) its use resulted in reliable predictions in the case of S. avenae (Honěk et al. 2017). For this, we set the ‘damage threshold’ (DT) at 10 aphids/tiller, which causes a 6% loss of yield according to Niehoff and Stäblein (1998). The maximum abundance was therefore regressed within the mixed effect modeling against the abundance at the time of heading. This was repeated both with and without taking the factor of crop density into account. Including plant density in the model enabled the use of specific prediction formulae for dense or sparse sites if the density factor is significant. Neglecting the plant density factor, on the other hand, provides a more general formula for situations when crop density varies greatly within the field, which is the usual situation in certain countries. To choose the best model, the two random effects models (one that included random intercept only and another that included both random intercept and random slope) were compared based on the L-ratio. Critical aphid abundance (Ncrit) at heading, i.e., the abundance that results in the aphid maximum exceeding DT, was estimated based on the regression parameters. In evaluating the quality of the predictions (predicted abundance denoted as PA) of the regressions, the experimental values were considered as a proxy of predicted data and classified as true negative (MA < DT, PA < Ncrit), true positive (MA > DT, PA > Ncrit), false negative (MA > DT, PA < Ncrit), and false positive values (MA < DT, PA > Ncrit).
The correlation of maximum abundance of M. dirhodum with that of S. avenae or R. padi on leaves in dense and sparse stands was tested in a similar way to that described above, i.e., including crop density in the fixed term, and comparing two different random effect models, to reveal whether the response of individual aphid species to plant quality and environmental conditions is similar. Relationship of the level of MA of M. dirhodum each year on the date it was reached (Julian day) was analyzed using a random effect model with density, Julian day, and their interaction as fixed terms and the year of sampling as the random term.
Results
Annual Variation in Abundance
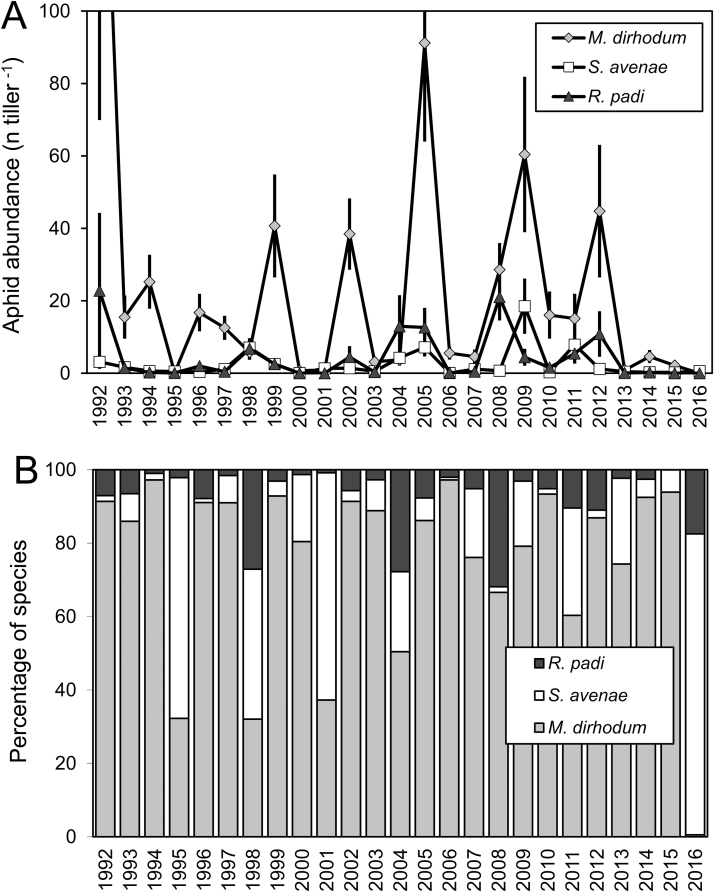
Maximum abundance of M. dirhodum across stands varied among years with peaks in 1992, 2005, and 2009, and minima in 1995, 2000, and 2016 (Fig. 1A). Overall, M. dirhodum was the most abundant species (mean maximum abundance calculated using annual average data is 25.2 ± 8.23 individuals/tiller) followed by R. padi (4.4 ± 1.32 individuals/tiller) and S. avenae (2.5 ± 0.81 individuals/tiller). The relative contribution of individual species to the total aphid population varied among years. M. dirhodum made up the highest percentage (>97% of total aphid population on leaves) in 1994 and 2006, lower percentages (32–37%) in 1995, 1998, and 2001, and in 2016, it was nearly absent (<1% of aphid population on leaves; Fig. 1B). Thus, during the period of this study, M. dirhodum accounted for 75% of the aphid population on leaves. It was the species that made up the majority of the aphid population in years when aphids were abundant and the only species that frequently reached an economically important level of abundance on leaves. Other species, although sometimes dominant in aphid populations on leaves (as S. avenae in 1995, 1998, 2001, and 2016), rarely exceeded economically important levels of abundance (S. avenae in 2009; R. padi in 2008). The prediction of maximum abundance was thus calculated only for M. dirhodum.
Fig. 1.
Annual variation in the abundance of Metopolophium dirhodum, Sitobion avenae, and Rhopalosiphum padi on leaves of winter wheat at the time of peak abundance. (A) Average (±SE) abundance (n individuals/tiller). In 1992, average abundance of M. dirhodum was 191 ± 121.4 aphids/tiller. (B) Percentage of particular species in the total aphid population at the time of maximum abundance.
Seasonal Dynamics
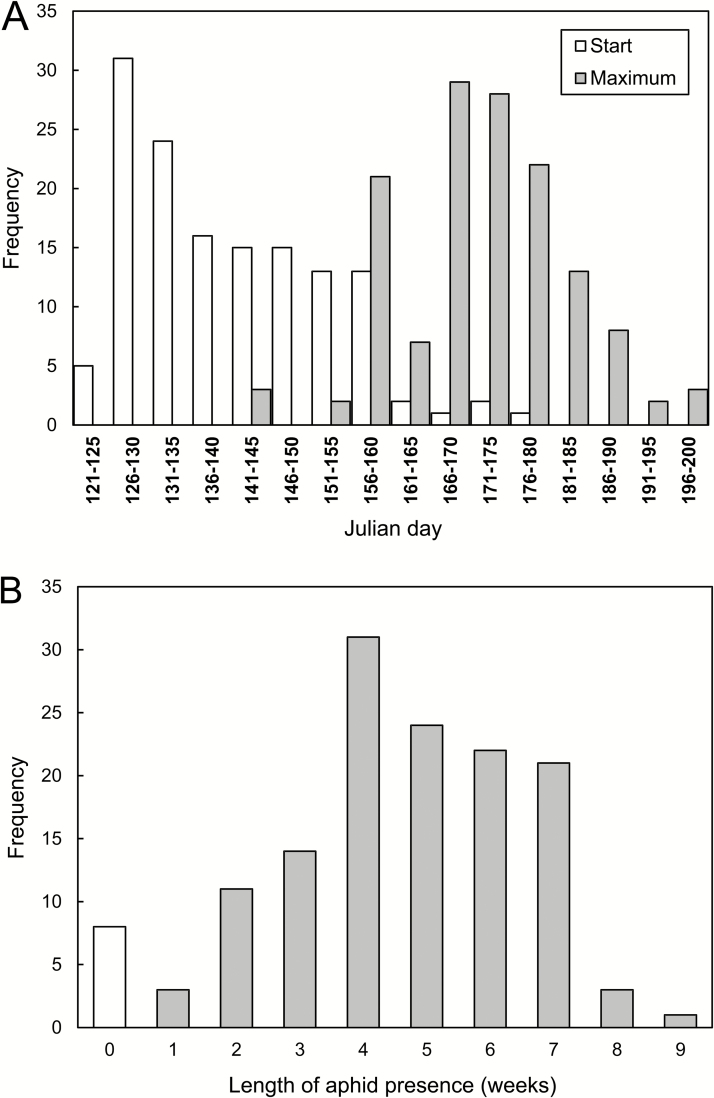
The dates of first record and maximum abundance of M. dirhodum varied among years (Fig. 2A). In particular years, M. dirhodum in the subplots was first observed at the earliest on 8 May (2007) and at the latest on 3 July (1995; Fig. 2A). The median date of first observation was 22 May. The maximum abundances were recorded between 28 May (2001) and 22 July (1996; Fig. 2A). The median date of maximum abundance was 26 June. The extremely early or late dates of the maximum abundance were recorded in years when M. dirhodum was rare. As the times for M. dirhodum of the first record and maximum abundance varied greatly, the period for which it infested the crop varied considerably, between 1 and 9 wk, with a mode of 4 wk (Fig. 2B). Mean length of the period from first detection of this aphid to the day of maximum abundance (calculated excluding eight cases when aphids were recorded only once and the length of their persistence on plants was thus zero) was 4.8 ± 0.15 wk. Across years, maximum abundance was independent of the date it was attained (LME: L-ratio = 1.528; df = 1; P = 0.216), but again the dates of maximum abundance were correlated within each year (LME: L-ratio = 165.7455; df = 1; P << 0.001; intra-class correlation, ICC = 0.500).
Fig. 2.
Seasonal variation in the presence of Metopolophium dirhodum in particular subplots, 1992–2016. (A) Frequency of the date (Julian day) of the first finding (start of population development) and date when maximum abundance was recorded (maximum). (B) Frequency of the length (weeks) of the period of aphid presence from date of first finding until date when the maximum abundance was recorded (0 wk = aphids were established only at one census in the season).
Effect of Crop Density
The maximum abundance of M. dirhodum differed between dense and sparse stands (LME: L-ratio = 127.330; df = 1; P << 0.001), with aphids being more abundant in sparse (18.5 ± 3.17 individuals/tiller) than in dense (3.6 ± 0.70 individuals/tiller) stands. The random intercept model fitted the data significantly better than the fixed effect model (LME: L-ratio = 172.546; df = 1; P << 0.001), which indicates that within years the maximum abundances were correlated (ICC = 0.83), i.e., the within-year variation in abundance was similar across plots.
Predicting Maximum Abundance
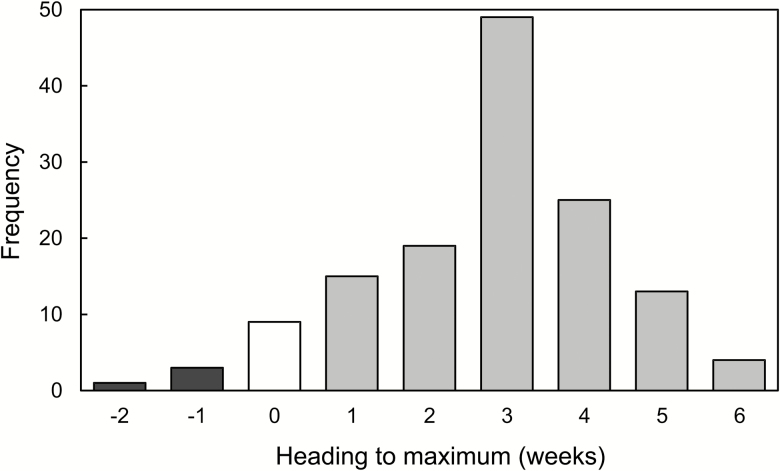
The period from heading to maximum abundance varied greatly between years. In the 138 subplots investigated over the 25 yr, there were 4 cases (2.9%) of the maximum abundance occurring before heading, 9 (6.5%) of the population peaking at heading, and 125 (90.2%) of the population peaking after heading, most frequently 3 wk after heading (Fig. 3). In the 134 plots where aphids peaked at or after heading, there were 33 cases (24.6%) when the recorded aphid abundance was zero at heading and the aphids might have arrived later in these stands. Data for the 101 plots (75.4%) with nonzero aphid counts at heading were used to calculate the regression of MA on abundance at heading.
Fig. 3.
The frequency of the length of the time interval (weeks) from heading to the date when maximum abundance of Metopolophium dirhodum was recorded in particular subplots, 1992–2016. The maxima occurred 1–2 wk before heading (black), at the time of heading (white), or 1–6 wk after heading (gray).
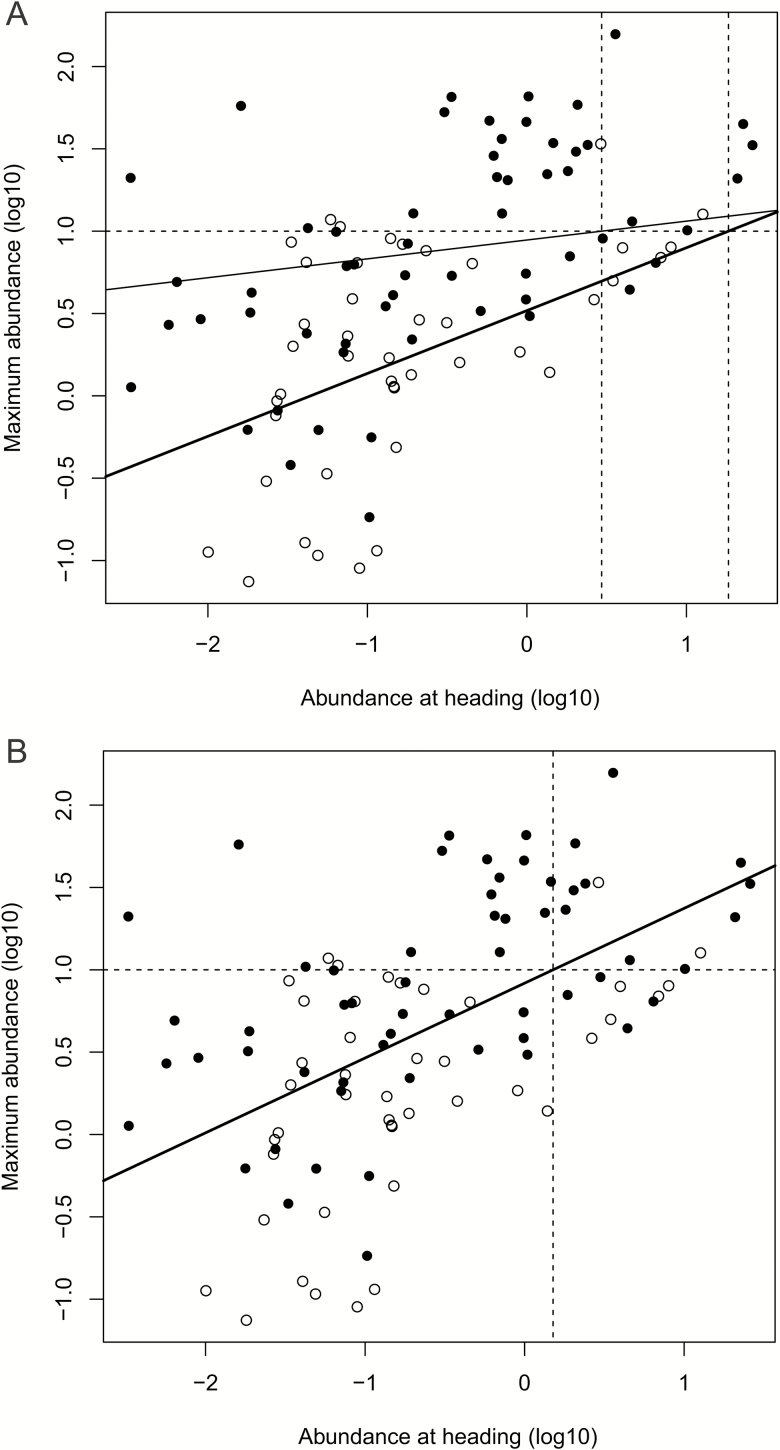
The full mixed ANCOVA model indicates that the relationship between the density at heading and maximum density differs among years as the random slope and intercept model provided a better fit to the data than the simpler random intercept model (LME: L-ratio = 13.607; df = 2; P = 0.001). The subsequent model simplification revealed that the slope of the regression was close to the level of significance (Table 1), indicating that density at heading cannot be used for predicting the maximum abundance when the density of plants is included in the model. Therefore, we tested this relationship separately for dense and sparse stands (Fig. 4A). We found that in sparse stands the maximum abundance was unrelated to abundance at heading, whereas it was in dense stands (Table 1). We also found that the random slope and intercept model provided a better fit for dense stands (LME: L-ratio = 11.191; df = 2; P = 0.004) than for sparse stands (LME: L-ratio = 0; df = 2; P = 1), which indicates that the variability in the relationship among years was important in dense but not in sparse stands.
Table 1.
The results of using mixed effect models to predict the maximum abundance of Metopolophium dirhodum on leaves based on its abundance at the time of heading (EE) assessed in dense and sparse plots of wheat
| Model terms | Slope | Intercept | L-ratio | df | P |
|---|---|---|---|---|---|
| Full modela | |||||
| Density | 82.153 | 1 | <0.001 | ||
| Heading | 3.457 | 1 | 0.063 | ||
| Density by heading | 1.518 | 1 | 0.218 | ||
| Simple modelb | |||||
| Heading | 0.454 ± 0.081 | 0.919 ± 0.121 | 27.956 | 1 | <0.001 |
| Sparse stands onlyb | |||||
| Heading | 0.115 ± 0.080 | 0.946 ± 0.148 | 1.933 | 1 | 0.164 |
| Dense stands onlya | |||||
| Heading | 0.382 ± 0.168 | 0.518 ± 0.131 | 4.550 | 1 | 0.033 |
aRandom slope and intercept model (random term: ~1 + EE|year).
bRandom intercept model (random term: ~1|year).
Fig. 4.
Regression of maximum abundance (log10[individuals/tiller]) of Metopolophium dirhodum in particular subplots on abundance at heading. (A) Separate models for sparse and dense stands, (B) model combining dense and sparse stands. Solid lines—trend lines as predicted by the LME models, thick lines indicate slopes significantly different from 0; dashed horizontal line—economic threshold of 10 aphids/tiller at maximum abundance; dashed vertical lines—critical abundance at heading as predicted by the models. Closed symbols—sparse stands; open symbols—dense stands.
As variation in crop density is usual in the field, we created a common regression for stands regardless of plant density (Fig. 4B). In this model, the maximum density was highly correlated with the density at heading (Table 1), though the within-year correlation was not high (ICC = 0.450). From this model, the critical abundance of M. dirhodum on leaves at heading was estimated to be Ncrit = 1.5 individuals/tiller. Based on the results of this regression, there were 101 cases of which 80 were negative and 21 positive predictions, of which 18 were false negative predictions (maximum abundances 10.4–65.8 aphids/tiller) and 9 false positive predictions (maximum abundances 5.2–9.0 aphids/tiller). Of the populations, whose abundance at heading was zero, only four exceeded 10 aphids/tiller at maximum abundance.
Correlations in the Abundance of the Three Species of Aphids on Wheat
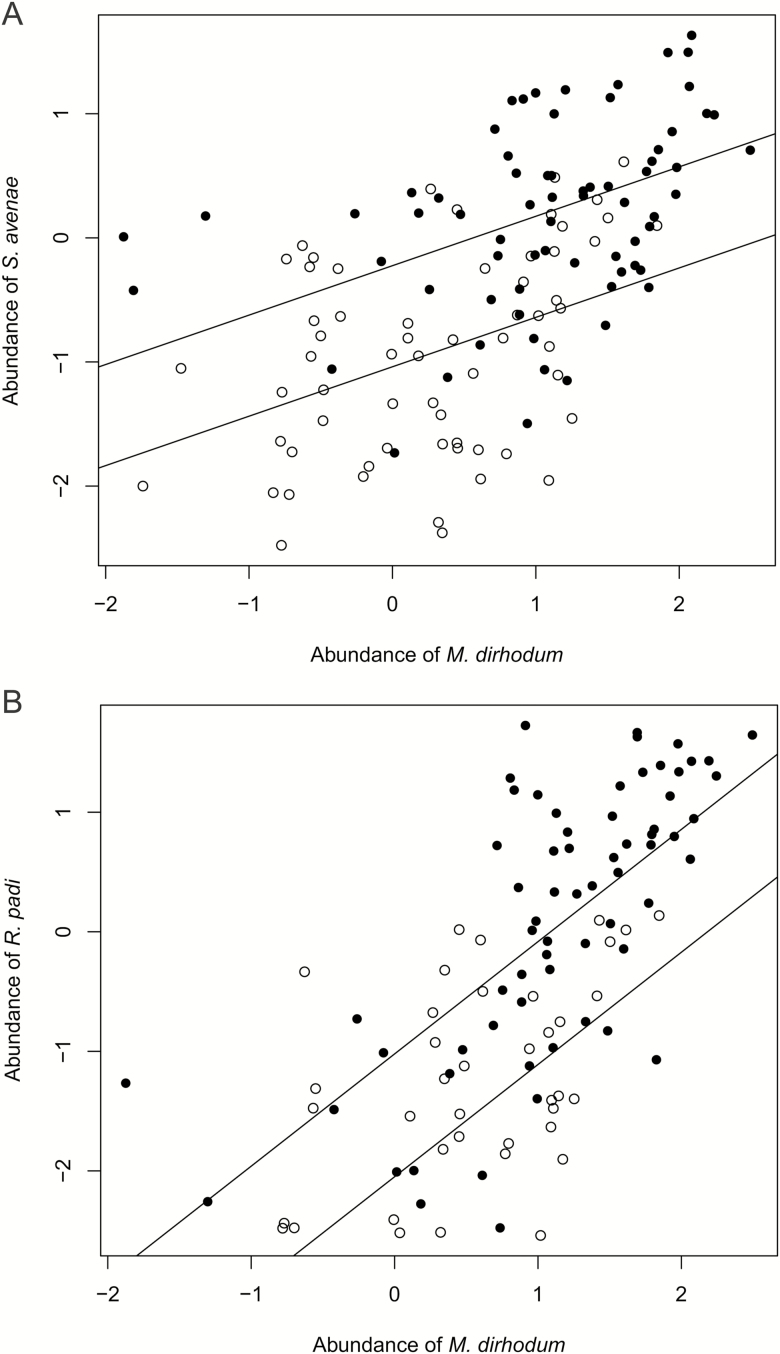
Despite the annual variation in abundance (Fig. 1), maximum numbers of the three species on leaves were correlated. Maximum abundances of S. avenae and R. padi were higher in sparse than in dense plots (Fig. 5). Maximum abundances of M. dirhodum and S. avenae were correlated in both sparse and dense stands based on the random intercept model (LME: L-ratio = 142.671; df = 2; P << 0.001; ICC = 0.681), with identical slope for both stands (LME: L-ratio = 0.422; df = 1; P = 0.516; Fig. 5A). Similarly, maximum abundances of M. dirhodum and R. padi were correlated in both sparse and dense stands based on the mixed effects model (LME: L-ratio = 68.620; df = 2; P << 0.001), with identical slopes (LME: L-ratio = 0.003; df = 1; P = 0.956; Fig. 5B); however in this case the random intercept and slope model fitted the data better (LME: L-ratio = 142.671; df = 2; P = 0.028), which indicates that the relationship between these two species was different.
Fig. 5.
Regression of maximum abundance (log10[individual/tiller]) of (A) Sitobion avenae and (B) Rhopalosiphum padi on maximum abundance of Metopolophium dirhodum in particular subplots. Solid lines—trend lines as predicted by the LME models. Closed symbols—sparse stands; open symbols—dense stands. Data for subplots where both species have nonzero abundance.
Discussion
Timing of M. dirhodum Presence in Wheat Crops
Because M. dirhodum is the only cereal aphid that feeds exclusively on the leaves, it can be present on wheat from seedling emergence until crop senescence. In central Europe, M. dirhodum may for a time colonize early sown wheat stands in late summer and autumn (Miller 1956) but do not survive overwinter. In Western Europe, populations of M. dirhodum may survive in cereal stands until spring (Dedryver and Gellé 1982), but their survival is lower than that of other cereal aphids (Alford et al. 2014). In the Czech Republic, autumn populations on wheat die out during winter because of low temperatures. The authors have not observed overwintering of anholocyclic strains in cereal stands over the last 40 yr (Honěk et al. 2017). This is consistent with the report that M. dirhodum populations overwintering in crop stands experience 60% mortality on any day when temperatures drop below −10°C (Williams 1980, Leather 1993). Since 1993, there were on average 13.1 ± 1.70 (2–32) such frost days per winter period at Praha-Ruzyně, which may decrease the size of the overwintering population to 6.7 × 10–6 of its autumn value, i.e., result in extinction. There were only four winters with less than five ‘frost days’, which could result in the survival of more than 1% of the original population. Nevertheless, even under the more favourable conditions of mild winters, no M. dirhodum have survived overwinter on cereals at the study site since 1977 when systematic observations using sweeping and/or direct counts started (A. Honek, unpublished data).
Winter wheat stands were thus populated in the spring by migrants from primary hosts. In western Europe, migration from Rosa terminates around mid-May (Leather and Walters 1984). In our study, the median date of first finding, 22 May, was slightly later. This may reflect a difference in the timing of this aphid’s life cycle in oceanic and continental climates. A difficulty in determining the exact date of aphid arrival is the low abundance of immigrant populations at that time; in our study, the average was 0.043 ± 0.0053 aphids/tiller (range 0.0031–0.4048 aphids/tiller). Therefore, the very rare early immigrants may have escaped attention. Precise determination of aphid numbers at arrival will require an enormous increase in sample size (Boeve and Weiss 2002), most likely to ≥1,500 tillers (Honek et al. 2006), which is likely to be impracticable. The catches of M. dirhodum by the national grid of Johnson-Taylor suction traps also indicate early immigration (Anonymous 2017). In 1999–2015, first catches were recorded between 27 April and 16 May, which on average is 3 May ±1.6 d (Anonymous 2017). In our study, the aphids arrived in some years on some plots as late as 25 June. The late immigrants may not originate from primary hosts but from neighboring cereal and maize stands that were colonized earlier in the year.
Factors Affecting Population Growth
Aphids that colonize the leaves of wheat have a much longer period over which to increase in abundance than those species that colonize the ears, which are only available for a short period from heading to the dough developmental stage. In fact, the time that elapsed between arrival and maximum abundance, 0–9 wk for M. dirhodum on leaves (Fig. 4), is greater than the 2–7 wk for S. avenae on the ears (Honek et al. 2017). The wider ‘temporal niche’ of M. dirhodum probably accounts for the great variation in the trajectories of the population dynamics of this species recorded in this study.
Parallel variation in the abundance of M. dirhodum and the other species colonizing leaves (Fig. 3) indicates there are effects specific for particular years, probably that of temperature on host plant phenology, which is reflected in an earlier start to the increase in aphid abundance, before the migration to wheat stands (Brabec et al. 2014). Following immigration to wheat stands, the trajectories of population development are affected by several factors. Maximum abundance of M. dirhodum increases with the length of period available for population development (Honek 1991), but this does not significantly affect annual variation in species abundance. Another factor affecting maximum abundance, population growth rate (Honek 1991), is determined by host plant quality. Although not empirically supported, we noticed that M. dirhodum thrived better in subplots with sparse plants, which have increased tiller mass, leaf size, and chlorophyll content, and an extended tillering period and greater number of tillers compared with dense stands. M. dirhodum prefers actively growing leaves (Havlickova 1987), and its abundance is positively correlated with rainfall (Davis et al. 2014). Moreover, sparse and dense stands differ in microclimate with sparse stands being warmer and drier than dense stands (Honek 1985). Temperature positively affects the development rate and fecundity of aphids, whereas high humidity has an adverse effect on population growth because it favors the development of mycoses (Feng et al. 1992, Honek and Martinkova 2004). Other determinants of host plant quality do not have an important effect. M. dirhodum thrives in well-fertilized stands (Duffield et al. 1997, Garratt et al. 2010, Winder et al. 2013). As our experimental stands were maintained using standard agricultural practices and grown in a small area with uniform soil quality, plant nutrition is unlikely to have affected the variation in maximum abundance. Over the course of this 25-yr study, stands were sown with different cultivars, most of which were used for 2–3 consecutive years. As there is only a small variation in the effect of cultivars on the performance of M. dirhodum (Dedryver and DiPietro 1986, Havlickova 1993), it is unlikely that this factor accounts for a significant proportion of the variation in maximum abundance.
Predicting Maximum Abundance
Predicting maximum abundance is difficult because there are large variations in the trajectories of the seasonal dynamics of M. dirhodum. Although maximum abundance is well correlated with M. dirhodum abundance up to 5 wk before the maximum (data not shown), this relationship cannot be used for prediction. This is because of extensive variation in the time of the maximum abundance, which is fixed neither with respect to a calendar date nor with respect to a stage in the development of the host plant. Here we attempted a prediction based on aphid abundance at heading. Unlike in S. avenae (Honek et al. 2017), peak density of M. dirhodum may not only follow but also precede heading (Fig. 3) and consequently aphid abundances at heading may be very low.
Our predictions were based on the results for both stands with high and low plant densities (number of tillers/m2). Intensively cultivated crops tend to be densely planted, and all the tillers tend to mature at the same time. In sparsely planted crops, the individual plants produce tillers over a longer period of time than plants in dense crops. As a consequence, there will be leaves suitable for M. dirhodum for longer periods in sparse than in dense crops in which the lower leaves die early and aphids occur only on the upper leaves (Honek and Martinkova 2002). Along with other factors, like microclimate, these differences account for our results. In terms of prediction, it is therefore important to know what percentage of the crop can be categorized as sparse. Intensively cultivated crops grown in small fields tend to be densely planted and the total area where the crop is sparse extremely small and can be ignored. A different situation prevails in countries with fields covering up to 4 km2 as in lowland areas in Central and Eastern Europe, formerly managed by collective farming. In such large fields, variation in soil conditions, temporary waterlogging, local failures of agrotechnics, etc. may cause the quality of the crop to vary spatially. Currently, there are no estimates of the percentage of the area of commercial crops in which the crop is sparse. In the absence of such information, a short-term prediction for crop stands that vary spatially in quality may be useful.
The mixed model based on results from sparse and dense subplots indicates that aphid abundance at heading is a useful predictor of whether aphid density will exceed the DT of 10 aphids/tiller. Indeed, this model is quite precise in predicting the maximum abundance, as only ca 26% of cases resulted in false predictions (18 false negative and 9 false positive out of a total 101 cases). The effect of crop stand density is still worth further consideration. In the mixed model, sparse stands constituted the majority of the false predictions (20 out of 27), while abundance of aphids in dense stands seemed to be predicted more accurately (only 7 false predictions). When dense and sparse stands were analyzed separately, the prediction was not significant for the sparse sites so it seems that the predictive value of our correlation model was poor for sparse stands. The rate of M. dirhodum development in spare stands is enormously variable and M. dirhodum numbers exceeded the DT in nearly half of these stands regardless of the abundance at heading. From a practical point of view, populations of this aphid in sparse stands are very likely to exceed the DT, whereas those in dense stands only did so in four cases (ca. 10 %), which indicates that, in most cases, it may be unnecessary to spray dense crops.
The regression is based on the results for 25 yr, and it is unlikely that additional data will significantly change the parameters. Greater precision of population counts may be achieved by increasing numbers of tillers sampled, but this would be labor consuming in experimental stands and even more difficult in practice. Other methods of predicting maximum numbers also provide less precise predictions at low aphid densities (Zhou and Carter 1979).
Importance of M. dirhodum
Substantial annual variation in its maximum abundance indicates that M. dirhodum may in some years become an economically significant pest. The DT density used in this study (10 individuals/tiller) may cause a 6% loss of yield compared with an aphid-free control (Niehoff and Stäblein 1998). This threshold was exceeded in 36 subplots (25%) and 14 yr (57%) of this study. A higher threshold of 20 aphids/tiller (yield loss of 12% compared with control) was exceeded in 26 (18 %) subplots and 10 (40 %) yr, a 40 aphids/tiller threshold (yield loss 17%) was exceeded in 11 (8 %) subplots and 6 (24 %) yr. Thus, in the Czech Republic, M. dirhodum was seldom abundant enough to cause significant economic loses. This situation may be far from permanent. Cereal aphids were abundant in the 1970s and 1980s and prompted intensive research (Dixon 1987). Although current long-term predictions indicate that cereal aphid abundance will decrease in the future (Newman 2005), it is still useful to have a way of providing an early warning of a harmful occurrence since there is no guarantee that there will not be a massive cereal aphid outbreak in the future. It also remains to be shown whether these results are applicable in geographical areas where M. dirhodum is an established pest, such as in North America (Schotzko and Bosque-Perez 2000, Clement et al. 2004) and South America (Lopes-da-Silva and Vieira 2007, Sepulveda et al. 2017), as well as in countries where it is still uncommon, Australia (De Barro 1991) and Eastern Asia (Chen and Feng 2004).
Of the cereal aphids infesting leaves of winter wheat, M. dirhodum, S. avenae, and R. padi, only the former species frequently reached economically significant levels of abundance. We demonstrate that M. dirhodum abundance is highly influenced by crop quality, and this species particularly endangers sparse stands of well-developed plants. Dense stands of winter wheat are usually less vulnerable than sparse stands. The relationship between crop quality and abundance of cereal aphids on leaves can be used, in particular, to target protection measures in precision farming. Other aphid species on leaves are from the point of protection of cereals of minor importance.
Acknowledgments
We thank Mrs Helena Uhlířová, Jana Kohoutová, Ludmila Kreslová, and Irena Kubečková for excellent technical assistance. The work was supported by project QJ1530373 and institutional support of The Ministry of Agriculture of the Czech Republic .
References Cited
- Alford L., T. O. Andrade R. Georges F. Burel, and van Baaren J.. 2014. Could behaviour and not physiological thermal tolerance determine winter survival of aphids in cereal fields?PLoS One 9: e114982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous 2017. http://eagri.cz/public/web/ukzuz/portal/skodlive-organismy/aphid-bulletin/aphid-bulletin/ Aphid Bulletin.
- Basky Z. 1996. Fluctuation in abundance of cereal aphids in Hungary, with special regards to Diuraphis noxia. IOBC WPRS Bulletin. 19: 9–11. [Google Scholar]
- Blackman R. L., and Eastop V. F.. 1984. Aphids on the World’s crops. John Wiley and Sons, Chichester, New York, Brisbane, Toronto and Singapore. [Google Scholar]
- Bode E. 1980. Aphids in winter wheat: abundance and limiting factors from 1976 to 1979. Bull SROP WPRS Bull. 3: 49–57. [Google Scholar]
- Boeve P. J., and Weiss M.. 2002. Spatial distribution and sampling plants with fixed levels of precision for cereal aphids (Homoptera: Aphididae) infesting spring wheat. Can. Entomol. 130: 67–77. [Google Scholar]
- Brabec M., A. Honěk S. Pekár, and Martinková Z.. 2014. Population dynamics of aphids on cereals: digging in the time-series data to reveal population regulation caused by temperature. PLoS One 9: e106228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., and Feng M. G.. 2004. Observation on the initial inoculum source and dissemination of Entomophthorales-caused epizootics in populations of cereal aphids. Sci. China C Life Sci. 47:38–43. [DOI] [PubMed] [Google Scholar]
- Clement S. L., Elberson L. R., Youssef N., Young F. L., and Evans A. A.. 2004. Cereal aphid and natural enemy populations in cereal production systems in eastern Washington. J. Kans. Entomol. Soc. 77: 165–173. [Google Scholar]
- Davis T. S., Abatzoglou J. T., Bosque-Perez N. A., Halbert S. E., Pike K., and Eigenbrode S. D.. 2014. Differing contributions of density dependence and climate to the population dynamics of three eruptive herbivores. Ecol. Entomol. 39: 566–577. [Google Scholar]
- De Barro P. J. 1991. Attractiveness of 4 colors of traps to cereal aphids (Hemiptera: Aphididae) in South Australia. J. Austral. Entomol. Soc. 30:263–264. [Google Scholar]
- Debek-Jankowska A., and Barczak T.. 2005. Parasitoid complex of cereal aphids in Poland. J. Aphidol. 19: 1–10. [Google Scholar]
- Dedryver C. A. 1978. Biologie des pucerons des céréales dans l’ouest de la France. I.—Répartition et évolution des populations de Sitobion avenae F., Metopolophium dirhodum Wlk, et Rhopalosiphum padi L., de 1974 a 1977 sur blé d’hiver dans le bassin de Rennes. Ann. Zool. Ecol. Anim. 10: 483–505. [Google Scholar]
- Dedryver C. A., and DiPietro J. P.. 1986. Biologie des pucerons des céréales dans l’Ouest de la France. VI.—Etude comparative des fluctuations au champ des populations de Sitobion avenae (F.), Metopolophium dirhodum (Wlk.) et Rhopalosiphum padi (L.) sur différentes cultivars de blé d’hiver. Agronomie 6: 75–84. [Google Scholar]
- Dedryver C. A., and Gellé A.. 1982. Biologie des pucerons des céréales dans l’ouest de la France IV.—Étude de l’hivernation de populations anholocycliques de Rhopalosiphum padi L., Metopolophium dirhodum Wlk. et Sitobion avenae F. sur repousses de céréales, dans trois stations de Bretagne et du Bassin parisien. Acta Oecol. Oecol. App. 3: 321–342. [Google Scholar]
- Dedryver C. A., A. Le Ralec, and Fabre F.. 2010. The conflicting relationships between aphids and men: a review of aphid damage and control strategies. C. R. Biol. 333: 539–553. [DOI] [PubMed] [Google Scholar]
- Dixon A. F. G. 1987. Cereal aphids as an applied problem. Agric. Zool. Rev. 2: 1–57. [Google Scholar]
- Duffield S. J., Bryson R. J., Young J. E. B., Sylvester-Bradley R., and Scott R. K.. 1997. The influence of nitrogen fertiliser on the population development of the cereal aphids Sitobion avenae (F.) and Metopolophium dirhodum (Wlk.) on field grown winter wheat. Ann. Appl. Biol. 130: 13–26. [Google Scholar]
- Feng M. G., Nowierski R. M., Johnson J. B., and Poprawski T. J.. 1992. Epizootics caused by Entomophthoralean fungi (Zygomycetes, Entomophthorales) in populations of cereal aphids (Hom., Aphididae) in irrigated small grains of southwestern Idaho, USA. J. Appl. Entomol. 113: 376–390. [Google Scholar]
- Garratt M. P. D., Wright D. J., and Leather S. R.. 2010. The effects of organic and conventional fertilizers on cereal aphids and their natural enemies. Agric. For. Entomol. 12: 307–318. [Google Scholar]
- Havlickova H. 1987. Behaviour and reproduction of cereal aphids in relation to changes in the content of water and free amino acids in wheat during the growing season. J. App. Entomol. 103: 142–147. [Google Scholar]
- Havlickova H. 1993. Level and nature of the resistance to the cereal aphid, Sitobion avenae (F.), in thirteen winter wheat cultivars. J. Agron. Crop Sci. 171: 133–137. [Google Scholar]
- Havlickova H. 1997. Character and extent of damage to winter wheat cultivars caused by cereal aphids. Rostl. Vyroba 43: 113–116. [Google Scholar]
- Holman J. 2009. Host plant catalog of aphids. Springer-Verlag, Berlin and Heidelberg, Germany. [Google Scholar]
- Honek A. 1985. Plant density and abundance of cereal aphids (Hom., Aphidina). Z. Angew.Entomol. 100: 399–409. [Google Scholar]
- Honek A. 1987. Effect of plant quality and microclimate on population growth and maximum abundances of cereal aphids, Metopolophium dirhodum (Walker) and Sitobion avenae (F.) (Hom., Aphididae). Z. Angew. Entomol. 104: 304–314. [Google Scholar]
- Honek A. 1991. Factors determining the peak abundance of Metopolophium dirhodum (Homoptera, Aphididae) on cereals. Bull. Entomol. Res. 81: 57–64. [Google Scholar]
- Honek A., and Martinkova Z.. 1999. Host-plant mediated influences on population development of Sitobion avenae (Sternorrhyncha: Aphididae). Eur. J. Entomol. 96: 135–141. [Google Scholar]
- Honek A., and Martinkova Z.. 2002. Factors of between- and within-plant distribution of Metopolophium dirhodum (Hom., Aphididae) on small grain cereals. J. Appl. Entomol. 126: 378–383. [Google Scholar]
- Honek A. and Martinkova Z.. 2004. Host plant age and population development of a cereal aphid, Metopolophium dirhodum (Hemiptera: Aphididae). Bull. Entomol. Res. 94: 19–26. [DOI] [PubMed] [Google Scholar]
- Honek A., Martinkova Z., and Lipavska H.. 1998. Distribution of Metopolophium dirhodum in maize and cereals, pp 569–578. In J. M. Nieto-Nafria, and A. F. G. Dixon (eds.), Aphids in natural and managed ecosystems. Universidad de Leon, Leon, Spain. [Google Scholar]
- Honek A., V. Jarosik, and Dixon A. F.. 2006. Comparing growth patterns among field populations of cereal aphids reveals factors limiting their maximum abundance. Bull. Entomol. Res. 96: 269–277. [DOI] [PubMed] [Google Scholar]
- Honek A., Martinkova Z., Dixon A. F. G., and Saska P.. 2017a. Annual predictions of the peak numbers of Sitobion avenae infesting winter wheat. J. App. Entomol. 141: 352–362. [Google Scholar]
- Honek A., Martinkova Z., Lukas J., Rezac M., Saska P., and Skuhrovec J.2017b. https://www.vurv.cz/sites/File/2017_certifikovana_metodika_Msice.pdf Mšice na obilninách: biologie, prognóza a regulace (Aphids on cereals: biology, forecasting and control)
- Howard M. T., and Dixon A. F. G.. 1992. The effect of plant phenology on the induction of alatae and the development of populations of Metopolophium dirhodum (Walker), the rose-grain aphid, on winter wheat. Ann. Appl. Biol. 120: 203–213. [Google Scholar]
- Johnston R. L., and Bishop G. W.. 1987. Economic injury levels and economic threshold for cereal aphids (Homoptera, Aphididae) on spring-planted wheat. J. Econ. Entomol. 80: 478–482. [Google Scholar]
- Leather S. R. 1993. Overwintering in six arable aphid pests: a review with particular relevance to pest management. J. App. Entomol. 116: 217–233. [Google Scholar]
- Leather S. R., and Walters K. F. A.. 1984. Spring migration of cereal aphids. Z. Angew. Entomol. 97: 431–437. [Google Scholar]
- Leather S. R., Carter N., Walters K. F. A., Chroston J. R., Thornback N., Gardner S. M., and Watson S. J.. 1984. Epidemiology of cereal aphids on winter wheat in Norfolk, 1979–1981. J. App. Ecol. 21: 103–114. [Google Scholar]
- Lopes-da-Silva M., and Vieira L. G. E.. 2007. Analysis of the genetic diversity in Metopolophium dirhodum (Walker) (Hemiptera, Aphididae) by RAPD markers. Rev. Bras. Entomol. 51: 54–57. [Google Scholar]
- Miller F. 1956. Zemědělská entomologie (Agricultural entomology). Nakladatelství ČSAV, Praha. [Google Scholar]
- Newman J. A. 2005. Climate change and the fate of cereal aphids in Southern Britain. Glob. Change Biol. 11: 940–944. [Google Scholar]
- Niehoff B., and Stäblein J.. 1998. Vergleichende Untersuchungen zum Schadoptential der Getreideblattlausarten Metopolophium dirhodum (Wlk.) und Sitobion avenae (F.) in Winterweizen. J. Appl. Entomol. 122: 223–229. [Google Scholar]
- Oakley J. N., and Walters K. F. A.. 1994. A field evaluation of different criteria for determining the need to treat winter wheat against the agrain aphid Sitobion avenae and the rose-grain aphid Metopolophium dirhodum. Ann. Appl. Biol. 124: 195–211. [Google Scholar]
- Palik S., Buresova I., Edler S., Sedlackova I., Tichy F., and Vanova M.2009. http://www.vukrom.cz/vyzkum/ukoncene-2009/qg50041/metodika Metodika pěstování ozimé pekárenské pšenice (Methodology of winter wheat growing)
- Pinheiro J., Bates D., DebRoy S., Sarkar D.,and R Core Team 2017. nlme: linear and nonlinear mixed effects models. R package version 3.1–131 https://CRAN.R-project.org/package=nlme
- Praslicka J. 1996. Influence of some growing factors on the occurrence of cereal aphids associated with winter wheat. Rostl. Vyroba 42: 499–502. [Google Scholar]
- R Core Team 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www. R-project.org/ [Google Scholar]
- Schotzko D. J. and Bosque-Pérez N. A.. 2000. Seasonal dynamics of cereal aphids on Russian wheat aphid (Homoptera: Aphididae) susceptible and resistant wheats. J. Econ. Entomol. 93: 975–981. [DOI] [PubMed] [Google Scholar]
- Sengonca C., Jösch H., and Kleinhenz B.. 1994. Einfluss verschiedener Wintergerste- und Winterweizensorten auf die Besiedelung und Populationsentwicklung von Getreideblattläusen. Gesunde Pflanzen. 46: 3–7. [Google Scholar]
- Sepúlveda D. A., F. Zepeda-Paulo C. C. Ramírez B. Lavandero, and Figueroa C. C.. 2017. Diversity, frequency, and geographic distribution of facultative bacterial endosymbionts in introduced aphid pests. Insect Sci. 24: 511–521. [DOI] [PubMed] [Google Scholar]
- Spaldon E. 1982. Rastlinna vyroba (Plant production). Priroda, Bratislava, Czechoslovakia. [Google Scholar]
- Vickerman G. P., and Wratten S. D.. 1979. The biology and pest status of cereal aphids in Europe: a review. Bull. Entomol. Res. 69: 1–32 [Google Scholar]
- Wetzel T. 2004. Integrierter Pflanzenschtz und Agroökosysteme. Steinbeis-Transferzentrum (STZ), Pausa/Vogtland, Germany. [Google Scholar]
- Williams C. T. 1980. Low temperature mortality of cereal aphids. IOBC WPRS Bull. 3: 63–66. [Google Scholar]
- Winder L., Alexander C. J., Wooley C., Perry J. N., and Holland J. M.. 2013. The spatial distribution of canopy-resident and ground-resident cereal aphids (Sitobion avenae and Metopolophium dirhodum) in winter wheat. Arthropod Plant Interact. 7: 21–32. [Google Scholar]
- Zhou X., and Carter N.. 1979. A simulation model describing the population dynamics and damage potential of the rose grain aphid, Metopolophium dirhodum (Walker) (Hemiptera: Aphididae), in the UK. Bull. Entomol. Res. 79: 373–380. [Google Scholar]
- Zhou X., and Carter N.. 1991. The effects of nitrogen and fungicide on cereal aphid population development and the consequences for the aphid-yield relationship in winter wheat. Ann. Appl. Biol. 119: 433–441. [Google Scholar]
- Zuur A. F., Ieno E. N., Walker N. J., Saveliev A. A., and Smith G. M.. 2009. Mixed effect models and extensions in ecology with R. Springer-Verlag, Berlin, Germany. [Google Scholar]