Summary
We developed an HIV risk assessment tool for pregnant women to identify women who would most benefit from preexposure prophylaxis (PrEP) in pregnancy while minimizing unnecessary PrEP use among women at low risk.
Keywords: HIV, preexposure prophylaxis, risk score, PrEP, pregnancy, postpartum.
Abstract
Background.
A human immunodeficiency virus (HIV) risk assessment tool for pregnant women could identify women who would most benefit from preexposure prophylaxis (PrEP) while minimizing unnecessary PrEP exposure.
Methods.
Data from a prospective study of incident HIV among pregnant/postpartum women in Kenya were randomly divided into derivation (n = 654) and validation (n = 650) cohorts. A risk score was derived using multivariate Cox proportional hazards models and standard clinical prediction rules. Ability of the tool to predict maternal HIV acquisition was assessed using the area under the curve (AUC) and Brier score.
Results.
The final risk score included the following predictors: having a male partner with unknown HIV status, number of lifetime sexual partners, syphilis, bacterial vaginosis (BV), and vaginal candidiasis. In the derivation cohort, AUC was 0.84 (95% confidence interval [CI], .72–.95) and each point increment in score was associated with a 52% (hazard ratio [HR], 1.52 [95% CI, 1.32–1.76]; P < .001) increase in HIV risk; the Brier score was 0.11. In the validation cohort, the score had similar AUC, Brier score, and estimated HRs. A simplified score that excluded BV and candidiasis yielded an AUC of 0.76 (95% CI, .67–.85); HIV incidence was higher among women with risk scores >6 than with scores ≤6 (7.3 vs 1.1 per 100 person-years, respectively; P < .001). Women with simplified scores >6 accounted for 16% of the population but 56% of HIV acquisitions.
Conclusions.
A combination of indicators routinely assessed in antenatal clinics was predictive of HIV risk and could be used to prioritize pregnant women for PrEP.
Nearly 80% of young women living with human immunodeficiency virus (HIV) worldwide reside in sub-Saharan Africa, and new infections among young women are double that of young men in the region [1, 2]. Pregnancy is a time of biological changes that may increase HIV susceptibility [3, 4] and a period of potential sexual behavioral changes in women or their male partners that may alter risk of HIV exposure [5]. In a systematic review, including 19 studies and 22803 African women, pooled HIV incidence was 3.8 per 100 person-years (PY) during pregnancy and 4.7 per 100 PY postpartum [6]. These maternal HIV incidence rates are similar to high-risk groups, including female sex workers and HIV-serodiscordant couples [2, 6–11]. Risk of HIV acquisition during pregnancy and postpartum translates to a substantial cumulative period of risk over the course of women’s lives in sub-Saharan African regions where both fertility and HIV prevalence are high [6]. Furthermore, acute maternal HIV infection during pregnancy and breastfeeding contributes to pediatric HIV infections, especially when maternal infection is not detected and antiretrovirals are not initiated [12, 13]. Therefore, HIV prevention during pregnancy and postpartum is important for prevention of HIV in women and infants.
Current guidelines recommend preexposure prophylaxis (PrEP) for individuals at high risk of HIV acquisition, including women of reproductive age, and support continuation of PrEP in pregnancy for women already on PrEP [14, 15]. Few studies have evaluated PrEP use during pregnancy and breastfeeding, but available data suggest that PrEP is safe during this time period [16–18]. However, implementation approaches that efficiently use resources to optimize the benefit of PrEP while balancing potential concerns regarding PrEP use during pregnancy and breastfeeding have not been defined. Identification of pregnant women at the highest risk of HIV acquisition would focus PrEP counseling and provision to these women and avoid unnecessary PrEP exposure for those with no or low risk.
Clinical prediction tools have been used to identify subgroups most at risk for HIV acquisition such as men who have sex with men in the United States [19, 20], HIV-serodiscordant African couples [21], and young (non-pregnant) African women [22]. Despite recognition of the importance of primary HIV prevention for pregnant and breastfeeding women, no tool has been developed to identify women most likely to acquire HIV during this critical period. A tool for assessing HIV risk during routine antenatal care could guide prioritization of women most likely to benefit from PrEP and other prevention strategies. The objective of this analysis was to develop a tool to identify women at highest risk for HIV acquisition during pregnancy and postpartum that could be easily implemented in maternal and child health (MCH) clinic settings.
METHODS
Study Participants
Data from 1304 HIV-uninfected women in a longitudinal study of HIV incidence during and after pregnancy (the Mama Salama Study) were used to derive and internally validate a risk score for maternal HIV acquisition. The Mama Salama Study was conducted between May 2011 and July 2014 at 2 antenatal care (ANC) clinics in western Kenya. Recruitment, enrollment, and follow-up procedures for the parent study have been previously described [23]. In brief, eligible pregnant women were ≥14 years old, had a negative rapid HIV test at enrollment or within the 3 months prior, planned to remain in the study area through 9 months postpartum, were willing to have a home visit, and were not enrolled in another study. Informed consent was received from all participants and the study was approved by the Kenyatta National Hospital Ethics and Research Committee and the University of Washington Institutional Review Board.
Study Procedures
After enrollment, women attended follow-up visits during pregnancy (20, 24, 32, and 36 weeks’ gestation) and postpartum (2, 6, 10, and 14 weeks; 6 and 9 months). Questionnaires were administered on sociodemographic factors, reproductive history, sexual behavior, contraception and condom use, medical history, and genital symptoms. Male partner characteristics were reported by women [23]. Serial HIV nucleic acid amplification tests (NAATs) were conducted at every visit. Chlamydia trachomatis and Neisseria gonorrhoeae were assessed at enrollment using endocervical samples for NAAT with the APTIMA Combo 2 Assay (Hologic/Gen-Probe, San Diego, California). Syphilis serology was based on rapid plasma reagin (RPR) tests conducted at enrollment as part of routine antenatal care and abstracted from MCH booklets, or conducted by study staff if the test was not performed. All women were assessed for candidiasis, bacterial vaginosis (BV), and T. vaginalis at enrollment and follow-up. Candidiasis was detected by identification of budding yeast, pseudohyphae, or both on direct microscopy of a vaginal saline wet mount and potassium hydroxide preparation. Vaginal Gram-stained slides were evaluated using Nugent criteria, with BV defined as a score of 7–10. Trichomonas vaginalis was diagnosed based on the presence of motile trichomonads on vaginal saline wet mount microscopy.
Risk Score Variables
The primary study outcome was incident HIV infection. All acute HIV infections detected during the study were considered incident infections [23]. To simulate data routinely collected during antenatal care, only enrollment variables were assessed as potential predictors. We identified potential predictors of incident maternal HIV infection based on characteristics previously assessed in the cohort [23]: demographic information (age, education, marital status, relationship duration), male partner (age, circumcision status), behavior (age of sexual debut, number of lifetime sexual partners, trading sex, condomless sex, vaginal washing and/or drying), and clinical characteristics (self-reported history of sexually transmitted infections [STIs], laboratory-confirmed C. trachomatis, N. gonorrhoeae, T. vaginalis, syphilis, BV, and candidiasis). In the parent study, no HIV seroconversions occurred among women with a known HIV-infected male partner [23]; thus, knowledge of partner HIV status was dichotomized as known vs unknown. Because laboratory testing for STIs is uncommon in most MCH settings, a separate simplified risk score was assessed that excluded STIs and genital infections except for syphilis (which is routinely assessed in antenatal care).
Statistical Analysis
We used derivation and validation processes similar to previously developed risk scores for predicting HIV acquisition in other populations [20–22] that utilize methods for clinical prediction rule development and evaluation techniques described by McGinn et al [24], Moons et al [25], and Laupacis et al [26].
Data were randomly divided into derivation (n = 654) and validation (n = 650) cohorts [27, 28]. Baseline differences between the derivation dataset and the validation dataset were tested using χ2 tests for proportions and Kruskal-Wallis tests for continuous measures. In the derivation cohort, potential predictors that were associated (P < .10) with incident HIV infection in univariate Cox proportional hazards models were evaluated in a multivariate model. Continuous variables were dichotomized using optimal cut-points identified through signal detection receiver operating characteristic (ROC) analyses [29]. Dichotomized variables were evaluated as predictors if measure predictability was higher using this parameterization instead of the continuous parameterization. To determine the combination of variables that best predicted incident HIV, all potential predictors from the multivariate model were assessed in a fully stepwise Cox proportional hazards model and predictors were evaluated at each step for inclusion or exclusion. We used the lowest Akaike information criterion (AIC) of all possible combinations of predictors from the stepwise analysis to determine the best fit model for the risk score [30]. The score values for individual risk factors were calculated by dividing the coefficients from the stepwise model for each predictor from the final stepwise model by the lowest coefficient among all predictors and rounding to the nearest integer [21, 22, 31]. The sum of individual parameter score values for each predictor determined the final risk score for each woman. ROC analysis was used to calculate the area under the curve (AUC) with the risk score as the sole predictor of incident HIV to assess discriminative ability [32]. We also determined the Brier score to quantify how close predictions of HIV acquisition using the risk score were to the actual outcome and assess overall risk score performance [32]. Useful prediction tools have a Brier scores <0.25 [32].
The risk score was applied to the validation cohort, and AUC performance and Brier score were compared with the derivation cohort. For further internal validation, the average AUC of 10 different models from randomly partitioned subsets of the entire cohort was determined using a 10-fold cross-validation, a standard method of internal validation for clinical prediction tools [24, 26]. Risk scores were dichotomized into the most predictive categories using the optimal cut-point identified through ROC curves, and HIV incidence was calculated by dichotomizing risk score groups in all cohorts. Analyses were repeated using the simplified risk score that excluded STIs and genital infections other than syphilis.
RESULTS
Overall, 1304 women contributed 1235.1 PY of follow-up and 25 incident HIV infections were detected (incidence rate, 2.31 per 100 PY) [23]. The median age was 22 years and the median gestational age at enrollment was 26 weeks. Most women were married (78%) for a median of 4 years and the median years of completed education was 8. Few women reported a history of previous STIs (7%), about half (55%) reported condomless sex in the past month, and 29% had a male partner of unknown HIV status. We did not detect differences in sociodemographic, male partner, behavioral, or clinical characteristics between the derivation and validation cohorts (Table 1).
Table 1.
Baseline Characteristics
| Characteristic | Derivation Cohort (n = 654) |
Validation Cohort (n = 650) |
P Valuea |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y, median (IQR) | 22 (19–26) | 22 (19–27) | .24 |
| Education, y, median (IQR) | 8 (8–10) | 8 (7–11) | .92 |
| Married | 507 (79) | 516 (78) | .41 |
| Polygamous relationshipb | 70 (11) | 88 (14) | .12 |
| Relationship durationc, y, median (IQR) | 4 (1–7) | 4 (1–9) | .63 |
| Partner characteristics | |||
| Partner age differencec (years older), median (IQR) | 5 (3–8) | 5 (4–9) | .51 |
| Partner uncircumcisedc,d | 405 (69) | 403 (69) | .76 |
| Partner HIV statusc,d | |||
| Negative | 429 (72) | 413 (69) | .51 |
| Positive | 10 (1) | 8 (1) | .73 |
| Unknowne | 161 (27) | 180 (30) | .58 |
| Sexual behavior and practices | |||
| Age at sexual debut, y | 16 (15–18) | 16 (15–18) | .30 |
| Lifetime No. of sexual partners, median (IQR) | 2 (1–3) | 2 (2–3) | .12 |
| History of trading sex | 62 (9) | 72 (11) | .35 |
| Any condomless sex in the past month | 350 (55) | 359 (56) | .61 |
| Any vaginal washing in the past week | 389 (59) | 392 (60) | .76 |
| Any vaginal drying in the past week | 126 (19) | 107 (16) | .19 |
| STIs and genital tract infections | |||
| History of STIsd | 42 (6) | 45 (7) | .80 |
| Chlamydia trachomatis | 44 (7) | 28 (4) | .05 |
| Neisseria gonorrhoeae | 14 (2) | 19 (3) | .37 |
| Trichomonas vaginalis | 39 (6) | 43 (7) | .62 |
| Syphilis | 7 (1) | 6 (1) | .79 |
| Bacterial vaginosis | 158 (24) | 143 (22) | .36 |
| Candidiasis | 160 (24) | 167 (26) | .60 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; STI, sexually transmitted infection; y, years.
aχ2 test for proportions or Kruskal-Wallis for continuous measures.
bAmong married women.
cAmong women with a current male partner.
dSelf-reported by women.
eIncludes male partners who have not been tested for HIV.
Risk Score Derivation
In the derivation cohort, 14 incident infections occurred in 621.8 PY of follow-up (incidence rate 2.3 per 100 PY). Increased risk of HIV acquisition was associated with having a relationship duration <1 year, having a male partner of unknown HIV status, lifetime number of sexual partners, syphilis, BV, and candidiasis in univariate Cox proportional hazards models (Table 2). The stepwise prediction model with the lowest AIC included having a male partner of unknown HIV status, lifetime number of sexual partners, syphilis, BV, and candidiasis. Characteristics with the highest risk scores were syphilis infection and having a male partner of unknown HIV status (Figure 1).
Table 2.
Analysis of Predictors and Calculation of Risk Score in the Derivation Cohort
| Univariate | Multivariatea | Stepwiseb | Risk Scorec | |||||
|---|---|---|---|---|---|---|---|---|
| Enrollment Characteristic | Crude HR (95% CI) | P Value | Adjusted HRb (95% CI) | P Value | Adjusted HRb (95% CI) | P Value | Regression Coefficient | |
| Demographic characteristics | ||||||||
| Age <21 yd | 1.22 (.42–3.52) | .710 | ||||||
| Education <8 yd | 0.83 (.23–2.98) | .778 | ||||||
| Married | 0.74 (.23–2.36) | .610 | ||||||
| Polygamous relationship | 2.42 (.67–8.67) | .176 | ||||||
| Marriage duration <1 yd | 3.00 (.94–9.59) | .063 | 2.60 (.74–9.17) | .136 | ||||
| Partner characteristics | ||||||||
| Partner age difference (years older)d | 1.06 (.98–1.16) | .158 | ||||||
| Partner uncircumcisede | 1.31 (.35–4.84) | .686 | ||||||
| Partner HIV status unknown | 5.84 (2.05–16.65) | .001 | 10.75 (3.13–36.94) | <.001 | 11.53 (3.39–39.26) | <.001 | 2.45 | 6 |
| Sexual behavior and practices | ||||||||
| Age at sexual debut <17 yd | 2.06 (.69–6.14) | .196 | ||||||
| Lifetime No. of sexual partnersd | 1.40 (1.18–1.67) | <.001 | 1.44 (1.20–1.75) | <.001 | 1.50 (1.23–1.82) | <.001 | 0.41 | 1 |
| History of trading sex | 1.57 (.35–7.02) | .554 | ||||||
| Any condomless sex in the past month | 0.82 (.29–2.34) | .714 | ||||||
| Any vaginal washing in the past week | 1.22 (.41–3.64) | .722 | ||||||
| Any vaginal drying in the past week | 1.64 (.52–5.24) | .401 | ||||||
| STIs and genital tract infections | ||||||||
| History of STIse | 2.38 (.53–10.65) | .255 | ||||||
| Chlamydia trachomatis | 1.15 (.15–8.83) | .895 | ||||||
| Syphilis | 6.72 (.87–51.78) | .067 | 10.80 (1.29–90.50) | .028 | 9.05 (1.11–73.95) | .040 | 2.20 | 5 |
| Bacterial vaginosis | 4.29 (1.49–12.36) | .007 | 2.59 (.82–8.21) | .104 | 2.61 (.83–8.17) | .099 | 0.96 | 2 |
| Candidiasis | 3.11 (1.09–8.87) | .034 | 3.54 (1.09–11.51) | .036 | 3.51 (1.08–11.37) | .037 | 1.25 | 3 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; STI, sexually transmitted infection; y, years.
aCovariates selected for multivariate analysis were based on factors associated with HIV infection (P < .10) in univariate analysis.
bCovariates selected for the stepwise multivariate model based on lowest Akaike information criterion score from stepwise procedure.
cPoints were assigned to each risk factor by dividing each coefficient from the stepwise proportional hazard model by 0.41 (the lowest coefficient value, corresponding to lifetime number of sexual partners) and rounding to the nearest integer.
dContinuous variables were dichotomized using optimal cut-points identified through signal detection receiver operating characteristic analyses. Dichotomized variables were evaluated as predictors if measure predictability was higher using this parameterization instead of the continuous parameterization.
eSelf-reported by women.
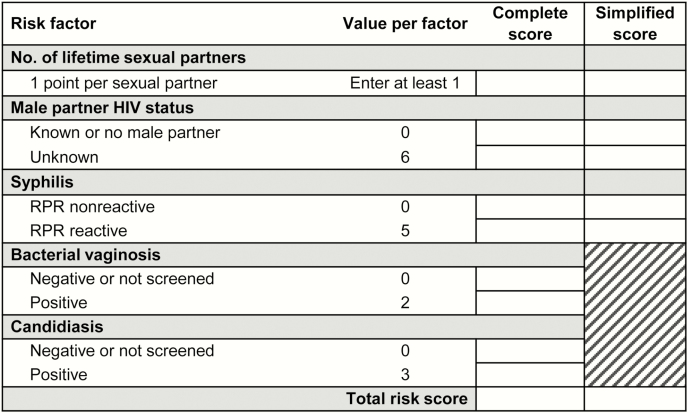
Figure 1.
Risk assessment tool score card for antenatal care clients. Abbreviation: RPR, rapid plasma reagin.
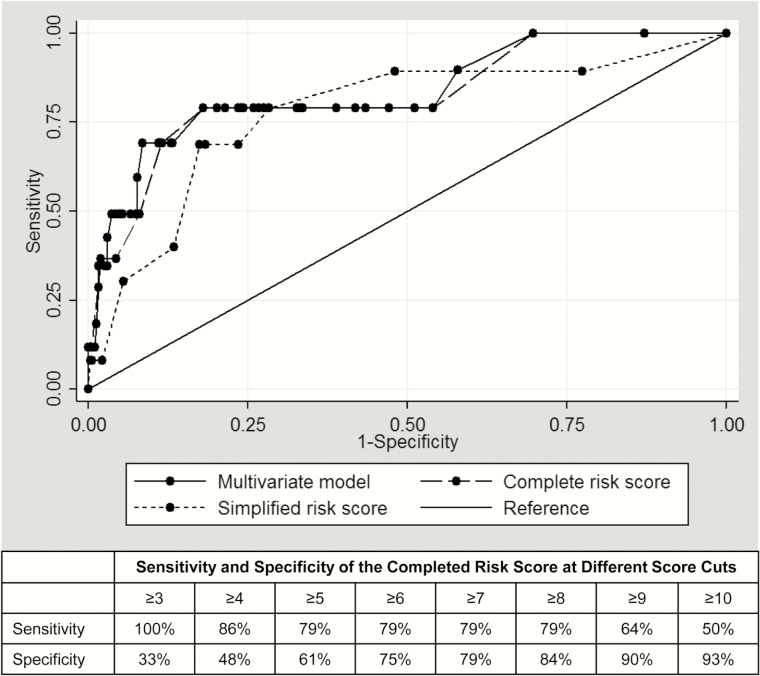
When we applied the risk score to each woman and calculated individual scores, the median risk score was 4 (interquartile range, 2–6; range, 1–19). The complete risk score captured the majority of the predictive ability of the multivariate model, demonstrated by nearly overlapping ROC curves (Figure 2). The ROC curve for the simplified risk score that excluded BV and candidiasis performed similarly but had less overlap with the multivariate model.
Figure 2.
Receiver operating characteristic curve and cut-points of risk score in derivation cohort.
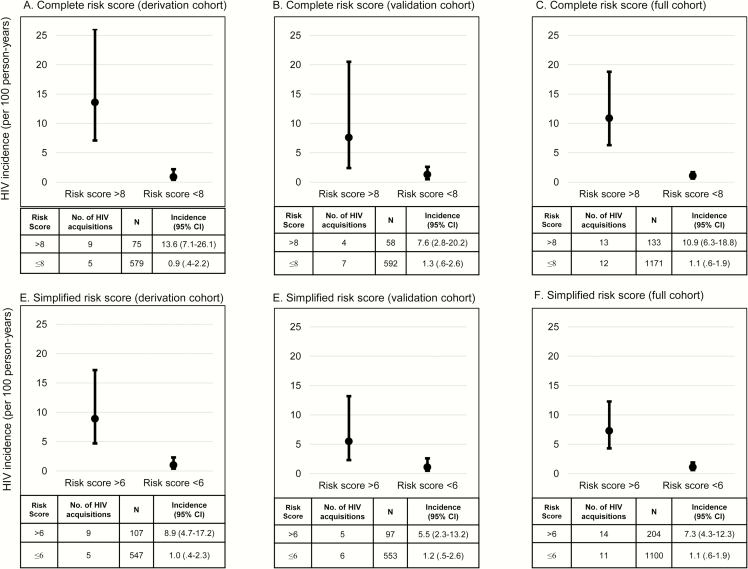
The AUC for the complete risk score correctly predicting HIV acquisition was 0.84 (95% confidence interval [CI], .72–.95; Table 3). Each point increment was associated with a 1.5-fold (hazard ratio [HR], 1.52 [95% CI, 1.32–1.76]; P < .001) increase in HIV risk. The optimal cut-point for most predictive categories was 7.5 for the complete risk score; HIV incidence was 13.6 per 100 PY among women with risk scores >8 compared to 0.9 per 100 PY among women with score ≤8 (P < .001; Figure 3A). A complete risk score >8 had a Brier score of 0.11 and correctly identified 64% of women in the derivation cohort who acquired HIV; 11% of women had a score >8. A simplified risk score using parameters routinely available in ANC clinics, excluding BV and candidiasis, had an AUC of 0.78 (95% CI, .65–.92), similar to the complete risk score, and the optimal cutpoint was 6.5. There was a significant difference in HIV incidence between women with simplified scores >6 compared to those with scores ≤6 (8.9 per 100 PY vs 1.0 per 100 PY; P < .001; Figure 3D). A simplified risk score >6 correctly identified 64% of women who acquired HIV in the derivation cohort; 16% of all women had a score >6. The Brier score was 0.16 when the simplified risk score was used.
Table 3.
Discrimination Performance of Complete and Simplified Risk Score
| Complete Risk Scorea | Simplified Risk Scoreb | |||||
|---|---|---|---|---|---|---|
| Cohort | Derivation | Validation | Overallc | Derivation | Validation | Overallc |
| Proportion of women with high risk scored | ||||||
| All women | 11% | 9% | 10% | 16% | 15% | 16% |
| HIV seroconverters | 64% | 36% | 52% | 64% | 45% | 56% |
| Discrimination performance | ||||||
| AUC (95% CI) | 0.84 (.72–.95) | 0.73 (.57–.90) | 0.74 (.62–.87) | 0.78 (.65–.92) | 0.72 (.58–.87) | 0.76 (.67–.85) |
| Brier score | 0.11 | 0.09 | 0.10 | 0.16 | 0.15 | 0.15 |
Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval; HIV, human immunodeficiency virus.
aComplete risk score includes having a male partner with unknown HIV status, lifetime number of sexual partners, syphilis, bacterial vaginosis (BV), and vaginal candidiasis.
bSimplified risk score excludes bacterial vaginosis and vaginal candidiasis.
cAUC for the overall cohort was calculated as the average AUC of 10 subsets of data randomly selected from the overall cohort.
dHigh risk scores defined as score >8 for the complete risk score and >6 for the simplified risk score excluding BV and candidiasis based on optimal cut-points in receiver operating characteristic analysis.
Figure 3.
A–F, Incidence of HIV (per 100 person-years) by risk score category. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Risk Score Validation
The risk score was applied to the validation cohort (characteristics in Table 1) in which 11 incident HIV infections were detected during 611.9 PY (incidence rate, 1.8 per 100 PY). The AUC and Brier scores for both the complete and simplified risk scores in the validation cohort were similar to the derivation cohort (Table 3). Each point increment of the complete risk score was associated with a 1.2-fold (HR, 1.21 [95% CI, 1.10–1.34]; P < .001) increase in HIV risk. HIV incidence was significantly higher among women with complete risk scores >8 (Figure 3B) and women with simplified risk scores >5 (Figure 3E).
When applied to the overall cohort for cross-validation, the average AUC of the complete risk score for 10 subsets of data randomly selected was 0.74 (95% CI, .62–.87), indicating robust generalizability. The simplified score performed slightly better with cross-validation (AUC, 0.76 [95% CI, .67–.85]). The Brier scores for both risk scores were similar (Table 3).
The risk score predicted HIV acquisition better than any single risk factor: lifetime number of sexual partners (AUC, 0.60 [95% CI .48–.71]), male partner HIV status unknown (AUC, 0.63 [95% CI .54–.73]), syphilis (AUC, 0.54 [95% CI .48–.60]), BV (AUC, 0.63 [95% CI .53–.73]), and candidiasis (AUC, 0.60 [95% CI .50–.70]).
In the overall cohort, women with complete risk scores >8 had a 6-fold increased risk of HIV (HR, 6.19 [95% CI, 2.78–13.78]; P < .001) and each point increment was associated with a 1.3-fold increased risk of HIV (HR, 1.28 [95% CI, 1.20–1.36]; P < .001). Risk of HIV associated with simplified risk scores >6 (HR, 5.12 [95% CI, 2.33–11.21]; P < .001) and each per point increment of simplified risk scores (HR, 1.31 [95% CI, 1.20–1.42]; P < .001) were similar to the complete risk score. HIV incidence was significantly higher among women with complete risk scores >8 (P < .001; Figure 3C) and among women with simplified risk scores >6 (P < .001; Figure 3F) than women with lower scores. A complete risk score >8 was observed in only 10% of women, and predicted 52% of all HIV acquisitions. Simplified risk scores >6 predicted 56% of HIV acquisitions and 16% of women had scores >6.
DISCUSSION
We derived and internally validated a risk score for HIV acquisition during pregnancy and the postpartum period using data from a large, longitudinal study designed to detect incident maternal HIV infections. We found that a composite risk score including male partner, behavioral, and clinical characteristics had good predictive ability to identify women most likely to acquire HIV. Using data that could be easily collected in standard MCH clinic settings without additional laboratory diagnostics, our simplified risk score identified 56% of pregnant women who acquired HIV while only identifying 16% of women as high risk. This risk score could identify women who would most benefit from PrEP in pregnancy and postpartum while minimizing unnecessary PrEP use among women at low risk. Our analysis contributes a unique risk assessment tool for an important subpopulation and is the first, to our knowledge, that assesses HIV risk during pregnancy and postpartum, a period of high HIV incidence with unique PrEP implementation opportunities.
Risk scores derived from serodiscordant couples have limited utility in pregnant women because 30%–80% of pregnant women in sub-Saharan Africa do not know the HIV status of their partner [21, 33–39]. Risk scores derived from nonpregnant “high risk” women, such as those in the Vaginal and Oral Interventions to Control the Epidemic (VOICE) study, also have limited relevance to pregnant women because these are predominantly unmarried women counseled to not become pregnant who frequently (>90%) used hormonal contraception [40]. The discriminatory ability of a risk score in pregnancy is also of importance to limit unnecessary fetal drug exposure for women at no or low risk of HIV.
We found that syphilis infection and having a male partner with unknown HIV status were highly predictive of HIV acquisition and were sufficiently predictive for inclusion in the simple risk score. This is consistent with previous studies that have found knowledge of male partners’ herpes simplex virus type 2 (HSV-2) and HIV status are associated with lower acquisition risk for women due to adoption of preventive behaviors following disclosure [41, 42]. Syphilis infection is a known risk factor for HIV [43]. Importantly, these 2 variables can be rapidly assessed in routine MCH settings to discriminate women who could benefit from PrEP.
Pregnancy and the postpartum period are attractive for PrEP implementation. Attendance at MCH visits for antenatal care and infant immunizations is high in sub-Saharan Africa. Women are universally offered counseling and testing for HIV in antenatal clinics, which can serve as a platform for identifying women who would benefit from PrEP. The combination of substantial maternal HIV incidence, increased mother-to-child HIV transmission associated with acute maternal HIV infection, and HIV testing infrastructure makes this a critical and feasible period for PrEP implementation. Previous studies suggest that targeting PrEP to individuals most at risk for HIV acquisition yields high impact for reducing HIV incidence [44–49]. In our study, simplified risk scores >5 were associated with a nearly 5-fold increased risk of HIV acquisition. A simple risk assessment tool would allow clinicians to rapidly identify women most likely to benefit from targeted HIV prevention, including PrEP. Prior to widespread PrEP implementation for pregnant and postpartum women, adherence and safety of PrEP in this population should be considered [40, 50–53].
Our study has limitations. Data from other settings are needed to externally validate the risk score [25, 54, 55]. Some known cofactors for HIV acquisition, such as HSV-2, were not included in our models because the parent study did not evaluate them [56]. In addition, the number of incident maternal HIV infections was smaller than in other derivation cohorts [20–22]. However, other risk prediction tools [57, 58] have successfully used a similar number of incident events, and statistical modeling demonstrations have shown that AUC estimations are minimally affected by sample size [59]. Despite the relatively small number of incident HIV infections, our risk assessment tool predicted HIV acquisition well with an AUC of 0.74, similar to HIV risk scores developed with larger sample sizes (AUC range, 0.67–0.74) [19–22].
CONCLUSIONS
We found that a combination of characteristics routinely assessed during ANC yielded high predictive utility for HIV risk in pregnant and postpartum women. Targeting PrEP for women at high risk of HIV acquisition could have substantial impact on maternal and infant HIV incidence, limit unnecessary PrEP exposure to women at low risk, and use resources efficiently.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institutes of Health (P01 HD 064915; T32 T32AI07140 to J. P; K01 AI116298 to A. L. D; K24 HD054314 to G. J. S.) the University of Washington Center for AIDS Research (P30 AI27757). The Mama Salama Study Team was supported by the University of Washington Global Center for Integrated Health of Women Adolescents and Children.
Potential conflicts of interest. R. S. M. receives research funding, paid to the University of Washington, from Hologic Corporation. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science 2005; 308:1582–3. [DOI] [PubMed] [Google Scholar]
- 2. United Nations Joint Programme on HIV/AIDS. The gap report. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 3. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol 2015; 73:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Racicot K, Kwon JY, Aldo P, Silasi M, Mor Gw. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol 2014; 72:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crawford-Williams F, Fielder A, Mikocka-Walus A, Esterman A. A critical review of public health interventions aimed at reducing alcohol consumption and/or increasing knowledge among pregnant women. Drug Alcohol Rev 2015; 34:154–61. [DOI] [PubMed] [Google Scholar]
- 6. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 2005; 366:1182–8. [DOI] [PubMed] [Google Scholar]
- 8. Humphrey JH, Hargrove JW, Malaba LC, et al. ; ZVITAMBO Study Group HIV incidence among post-partum women in Zimbabwe: risk factors and the effect of vitamin A supplementation. AIDS 2006; 20:1437–46. [DOI] [PubMed] [Google Scholar]
- 9. Leroy V, Van de Perre P, Lepage P, et al. Seroincidence of HIV-1 infection in African women of reproductive age: a prospective cohort study in Kigali, Rwanda, 1988–1992. AIDS 1994; 8:983–6. [DOI] [PubMed] [Google Scholar]
- 10. Taha TE, Dallabetta GA, Hoover DR, et al. Trends of HIV-1 and sexually transmitted diseases among pregnant and postpartum women in urban Malawi. AIDS 1998; 12:197–203. [DOI] [PubMed] [Google Scholar]
- 11. Kaul R, Kimani J, Nagelkerke NJ, et al. ; Kibera HIV Study Group Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA 2004; 291:2555–62. [DOI] [PubMed] [Google Scholar]
- 12. Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr 2012; 59:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dinh TH, Delaney KP, Goga A, et al. Impact of maternal HIV seroconversion during pregnancy on early mother to child transmission of HIV (MTCT) measured at 4–8 weeks postpartum in South Africa 2011–2012: a national population-based evaluation. PLoS One 2015; 10:e0125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep 2012; 61:586–9. [PubMed] [Google Scholar]
- 15. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 16. Mugo NR, Hong T, Celum C, et al. ; Partners PrEP Study Team Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA 2014; 312:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bunge K, et al. Pregnancy incidence and outcomes in women receiving tenofovir-based PrEP in the VOICE trial [abstract MOPEC480]. In: 8th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2015), Vancouver, Canada, 2015. [Google Scholar]
- 18. Callahan R, Nanda K, Kapiga S, et al. ; FEM-PrEP Study Group Pregnancy and contraceptive use among women participating in the FEM-PrEP trial. J Acquir Immune Defic Syndr 2015; 68:196–203. [DOI] [PubMed] [Google Scholar]
- 19. Smith DK, Pals SL, Herbst JH, Shinde S, Carey JW. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2012; 60:421–7. [DOI] [PubMed] [Google Scholar]
- 20. Menza TW, Hughes JP, Celum CL, Golden MR. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis 2009; 36:547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kahle EM, Hughes JP, Lingappa JR, et al. ; Partners in Prevention HSV/HIV Transmission Study and the Partners PrEP Study Teams An empiric risk scoring tool for identifying high-risk heterosexual HIV-1-serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr 2013; 62:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balkus JE, Brown E, Palanee T, et al. An empiric HIV risk scoring tool to predict HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 2016; 72:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kinuthia J, Drake AL, Matemo D, et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS 2015; 29:2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-based medicine working group. JAMA 2000; 284:79–84. [DOI] [PubMed] [Google Scholar]
- 25. Moons KG, Kengne AP, Woodward M, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012; 98:683–90. [DOI] [PubMed] [Google Scholar]
- 26. Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA 1997; 277:488–94. [PubMed] [Google Scholar]
- 27. Zhao C, Luo Q, Xia X, et al. Risk score to predict mortality in continuous ambulatory peritoneal dialysis patients. Eur J Clin Invest 2014; 44:1095–103. [DOI] [PubMed] [Google Scholar]
- 28. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med 2010; 25:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiernan M, Kraemer HC, Winkleby MA, King AC, Taylor CB. Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychol Methods 2001; 6:35–48. [DOI] [PubMed] [Google Scholar]
- 30. Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 2012; 17:228–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heffron R, Cohen CR, Ngure K, et al. ; Partners PrEP Study, the Partners in Prevention HSV/HIV Transmission Study, and the Partners Demonstration Project Teams A scoring tool to identify east African HIV-1 serodiscordant partnerships with a high likelihood of pregnancy. PLoS One 2015; 10:e0145515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010; 21:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baeten J, Heffron R, Kidoguchi L, et al. Near elimination of HIV transmission in a demonstration project of PrEP and ART [abstract 24]. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2015. [Google Scholar]
- 34. Osoti AO, John-Stewart G, Kiarie JN, et al. Home-based HIV testing for men preferred over clinic-based testing by pregnant women and their male partners, a nested cross-sectional study. BMC Infect Dis 2015; 15:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Msuya SE, Mbizvo EM, Hussain A, Uriyo J, Sam NE, Stray-Pedersen B. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care 2008; 20:700–9. [DOI] [PubMed] [Google Scholar]
- 36. Orne-Gliemann J, Balestre E, Tchendjou P, et al. ; Prenahtest ANRS 12127 Study Group Increasing HIV testing among male partners. AIDS 2013; 27:1167–77. [DOI] [PubMed] [Google Scholar]
- 37. Bachanas P, Medley A, Pals S, et al. ; PwP Study Group Disclosure, knowledge of partner status, and condom use among HIV-positive patients attending clinical care in Tanzania, Kenya, and Namibia. AIDS Patient Care STDS 2013; 27:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peltzer K, Jones D, Weiss SM, Villar-Loubet O, Shikwane E. Sexual risk, serostatus and intimate partner violence among couples during pregnancy in rural South Africa. AIDS Behav 2013; 17:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Schacht C, Hoffman HJ, Mabunda N, et al. High rates of HIV seroconversion in pregnant women and low reported levels of HIV testing among male partners in southern Mozambique: results from a mixed methods study. PLoS One 2014; 9:e115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marrazzo JM, Ramjee G, Richardson BA, et al. ; VOICE Study Team Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wald A, Krantz E, Selke S, Lairson E, Morrow RA, Zeh J. Knowledge of partners’ genital herpes protects against herpes simplex virus type 2 acquisition. J Infect Dis 2006; 194:42–52. [DOI] [PubMed] [Google Scholar]
- 42. Simbayi LC, Kalichman SC, Strebel A, Cloete A, Henda N, Mqeketo A. Disclosure of HIV status to sex partners and sexual risk behaviours among HIV-positive men and women, Cape Town, South Africa. Sex Transm Infect 2007; 83:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kenyon CR, Osbak K, Tsoumanis A. The global epidemiology of syphilis in the past century—a systematic review based on antenatal syphilis prevalence. PLoS Negl Trop Dis 2016; 10:e0004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med 2011; 8:e1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verguet S, Stalcup M, Walsh JA. Where to deploy pre-exposure prophylaxis (PrEP) in sub-Saharan Africa? Sex Transm Infect 2013; 89:628–34. [DOI] [PubMed] [Google Scholar]
- 46. Walensky RP, Park JE, Wood R, et al. The cost-effectiveness of pre-exposure prophylaxis for HIV infection in South African women. Clin Infect Dis 2012; 54:1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nichols BE, Boucher CA, van Dijk JH, et al. Cost-effectiveness of pre-exposure prophylaxis (PrEP) in preventing HIV-1 infections in rural Zambia: a modeling study. PLoS One 2013; 8:e59549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pretorius C, Stover J, Bollinger L, Bacaër N, Williams B. Evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS One 2010; 5:e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ying R, Sharma M, Heffron R, et al. Cost-effectiveness of pre-exposure prophylaxis targeted to high-risk serodiscordant couples as a bridge to sustained ART use in Kampala, Uganda. J Int AIDS Soc 2015; 18:20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Damme L, Corneli A, Ahmed K, et al. ; FEM-PrEP Study Group Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cowan FM, Delany-Moretlwe S, Sanders EJ, et al. PrEP implementation research in Africa: what is new? J Int AIDS Soc 2016; 19:21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hanscom B, Janes HE, Guarino PD, et al. Brief report: preventing HIV-1 infection in women using oral preexposure prophylaxis: a meta-analysis of current evidence. J Acquir Immune Defic Syndr 2016; 73:606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Corneli A, Perry B, McKenna K, et al. Participants’ explanations for nonadherence in the FEM-PrEP clinical trial. J Acquir Immune Defic Syndr 2016; 71:452–61. [DOI] [PubMed] [Google Scholar]
- 54. Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet 1999; 353:89–92. [DOI] [PubMed] [Google Scholar]
- 55. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–421. [PubMed] [Google Scholar]
- 56. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20:73–83. [DOI] [PubMed] [Google Scholar]
- 57. Chan PC, Shinn-Forng Peng S, Chiou MY, et al. Risk for tuberculosis in child contacts. Development and validation of a predictive score. Am J Respir Crit Care Med 2014; 189:203–13. [DOI] [PubMed] [Google Scholar]
- 58. Anderson ST, Kaforou M, Brent AJ, et al. ; ILULU Consortium; KIDS TB Study Group Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med 2014; 370:1712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tong L, Erdmann C, Daldalian M, Li J, Esposito T. Comparison of predictive modeling approaches for 30-day all-cause non-elective readmission risk. BMC Med Res Methodol 2016; 16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]