Abstract
Study Objectives:
Experimental evidence suggests that restorative processes depend on synaptic plasticity changes in the brain during sleep. We used the expression of plasticity-related genes to assess synaptic plasticity changes during drug-induced sleep.
Methods:
We first characterized sleep induced by eszopiclone in mice during baseline conditions and during the recovery from sleep deprivation. We then compared the expression of 18 genes and two miRNAs critically involved in synaptic plasticity in these mice. Gene expression was assessed in the cerebral cortex and hippocampus by the TaqMan reverse transcription polymerase chain reaction and correlated with sleep parameters.
Results:
Eszopiclone reduced the latency to nonrapid eye movement (NREM) sleep and increased NREM sleep amounts. Eszopiclone had no effect on slow wave activity (SWA) during baseline conditions but reduced the SWA increase during recovery sleep (RS) after sleep deprivation. Gene expression analyses revealed three distinct patterns: (1) four genes had higher expression either in the cortex or hippocampus in the group of mice with increased amounts of wakefulness; (2) a large proportion of plasticity-related genes (7 out of 18 genes) had higher expression during RS in the cortex but not in the hippocampus; and (3) six genes and the two miRNAs showed no significant changes across conditions. Even at a relatively high dose (20 mg/kg), eszopiclone did not reduce the expression of plasticity-related genes during RS period in the cortex.
Conclusions:
These results indicate that gene expression associated with synaptic plasticity occurs in the cortex in the presence of a hypnotic medication.
Keywords: eszopiclone, sleep deprivation, plasticity, genes, cerebral cortex, hippocampus, mice.
Statement of Significance
It is thought that mechanisms of synaptic plasticity underlie motor and cognitive recovery occurring during sleep. We hypothesized that similar synaptic plasticity changes can also occur during drug-induced sleep. To test this hypothesis, we studied expression of plasticity-related genes during drug-induced sleep. The results indicate that gene expression associated with synaptic plasticity can occur in the brain in the presence of a hypnotic such as eszopiclone.
INTRODUCTION
Disturbed sleep can lead to a wide range of health problems such as cognitive impairment, depressed mood, and negative effects on cardiovascular, endocrine, and immune function.1 Sleep-inducing agents are often used to obtain sufficient amounts of sleep to avoid such problems. The restorative processes that occur during sleep have been suggested to depend, to a large extent, on synaptic plasticity.2 However, the question of whether synaptic plasticity processes can occur as efficiently during drug-induced sleep as during physiological sleep has received little attention. Sleep-dependent plasticity processes have been studied in the primary visual cortex in the ocular dominance plasticity model.3 In this study, three commonly prescribed hypnotics (trazodone, zaleplon, and eszopiclone) had strong effects on sleep electroencephalogram (EEG) activity, but only trazodone significantly interfered with sleep-dependent consolidation of cortical plasticity.3 In the present study, we selected eszopiclone as sleep-inducing agent. Since eszopiclone has strong effects on sleep EEG activity but does not interfere with sleep-dependent consolidation of cortical plasticity,3 we hypothesized that eszopiclone would not interfere with the expression of plasticity-related genes during recovery sleep (RS) following sleep deprivation.
Because synaptic plasticity changes are manifested by upregulation of a number of plasticity-related genes, the expression of these genes may provide an indicator of plasticity that occurs during sleep. In the present study, we assessed the effects of eszopiclone on the expression of 18 genes that are critically involved in synaptic plasticity mechanisms. We compared gene expression in two brain regions (cortex and hippocampus) during spontaneous sleep with gene expression during RS following sleep deprivation either in the presence or absence of eszopiclone treatment. Since expression of the majority of plasticity-related genes is reduced during sleep,4–6 expression of these genes was analyzed by quantitative real-time polymerase chain reaction (RT-PCR). We also included two relevant miRNAs as a target in our study because genes involved in synaptic plasticity are controlled at the translational level by miRNAs and the RNA-induced silencing complex (RISC).7–9
METHODS
Experimental Animals
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at SRI International. Thirty-one male C57BL/6 mice were obtained from Charles River Laboratories. All mice were maintained at an ambient temperature of 20°C–22°C under a 12-hour light-dark cycle (lights on at 07:00 hours) with food and water ad libitum.
Surgery
Mice were surgically implanted at 10 weeks of age with EEG and electromyogram (EMG) electrodes for sleep/wake recording. To record the EEG, each mouse was implanted with four stainless steel miniature screw electrodes placed bilaterally into the skull over the frontal and parietal cortices. To record the EMG, two electrodes made of Teflon-coated, stainless steel–braided wires were inserted into nuchal muscles. All electrodes were attached to a seven-pin electrical socket (Pinnacle Technology), and the entire assembly was fixed onto the skull with dental acrylic. Following surgery, each mouse was allowed a minimum of 3 weeks’ recovery followed by 1 week of adaptation to the recording cable and chamber.
Dose-Finding Study
The doses of eszopiclone (Sepracor Inc.) chosen for the dose-finding study were based on the published behavioral effects of zopiclone in mice10 and the sleep-promoting effects of zopiclone in rats.11–14 In four mice that were surgically implanted with EEG and EMG electrodes and allowed to recover as described above, we tested three doses of eszopiclone (5, 10, and 20 mg/kg) along with a vehicle control (saline). Eszopiclone was dissolved in saline by solubilization in a small amount of 1 N HCl.14 The pH of the drug solution was adjusted to approximately 7 with 1 N NaOH.14 Eszopiclone was administered intraperitoneally (i.p.) in a volume of 0.1 mL. Doses were randomized and counterbalanced between animals, for a total of four injections/animal. Mice were injected at Zeitgeber Time (ZT) 6, and EEG and EMG were recorded for the next 4–6 hours. Three days elapsed between injections. Since no adverse EEG activity was detected after any of the doses tested, we used the highest dose (20 mg/kg) for further experiments.
Experimental Treatment and Sleep Recordings
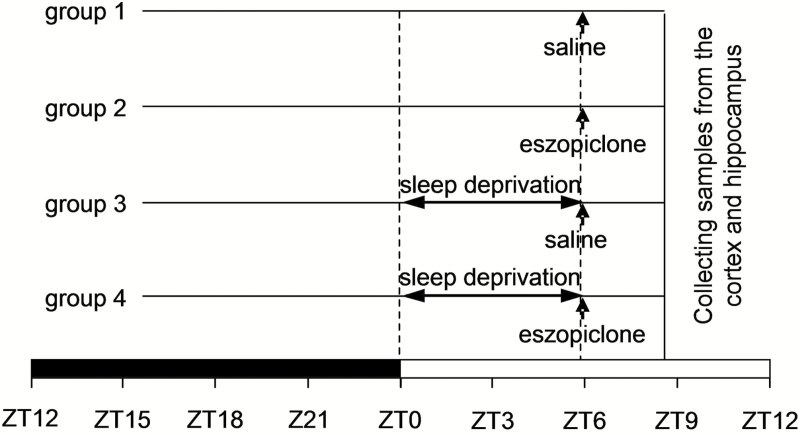
Experiments were conducted in the following four conditions: (1) physiological sleep control (saline-injected; n = 7); (2) eszopiclone treatment (n = 6); (3) RS following sleep deprivation (saline-injected; n = 6); and (4) eszopiclone treatment during RS (n = 8). Figure 1 presents the experimental design for the four groups. Eszopiclone (20 mg/kg) or vehicle (saline) was injected i.p. in a volume of 0.1 mL in all mice 6 hours after the light onset (ZT6). EEG and EMG were recorded continuously at 22°C for 24 hours of baseline, for 6 hours of sleep deprivation, and during 160 minutes after cessation of sleep deprivation to determine the response to sleep deprivation. For groups 3 and 4, sleep deprivation was initiated at light onset (ZT0) by disturbing cage bedding around the mouse, stroking the vibrissae using an artist’s brush, and toward the end of the sleep deprivation period, stroking the fur with the brush when slow waves became evident in the EEG. Digitized EEG (digitization rate 100 Hz) and EMG (digitization rate 200 Hz) were collected via the Embla 16-channel A10 hardware system and visualized and stored with the Somnologica Science software application. Unihemispheric, frontal-parietal EEG potentials were filtered at 0.3 and 35 Hz and stored in 10-second epochs on a personal computer. EMG data were high-pass filtered ( > 10 Hz) for ease of visualization. At the end of the treatment period (Figure 1), brains from all mice were rapidly removed, the cerebral cortex and hippocampus dissected, flash-frozen on dry ice, and stored at –80°C.
Figure 1.
Schematic presentation of the experimental design. Mice were injected with saline or eszopiclone 6 hours after the light onset (ZT6). In groups 3 and 4, mice were sleep deprived from ZT0 to ZT6 and then allowed a recovery sleep opportunity from ZT6 to approximately ZT9. Brain tissue samples were taken from all mice at 9 hours after light onset (ZT9). The black and white bars at the base of the schematic represent the dark and light phases of the light/dark cycle.
EEG/EMG Data Scoring
Arousal states (wakefulness, rapid eye movement [REM] sleep, nonrapid eye movement [NREM] sleep) were determined in 10-second epochs using Sleep Sign (Kissei Comtec Co.) by a person blind to the type of drug administered to the mice. Wakefulness was identified by the presence of desynchronized EEG and high EMG activity. NREM sleep consisted of high-amplitude slow waves together with a low EMG tone relative to waking. REM sleep was identified by the presence of desynchronized EEG coupled with low EMG relative to NREM sleep. The amount of time spent in wakefulness, NREM, and REM sleep was determined in 20-minute bins. After the EEG/EMG recordings were scored, the code was broken to reveal the identity of each mouse. The EEG power spectrum was distributed into four frequency bands, namely, the delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), and beta frequency bands (13–30 Hz). Relative delta power (ratio to total power) was calculated in 20-minute bins using Sleep Sign.
Gene Expression Studies
Total RNA was isolated from homogenate of the entire cerebral cortex and hippocampus using TRIZOL Reagent (Invitrogen) and TissueLyser (Qiagen). RNA was then purified using the RNeasy MiniKit (Qiagen). The RNase-free DNase Set (Qiagen) was used during RNA purification to remove any genomic DNA contamination. The RNeasy MiniKit and DNase digestion steps were omitted in case of miRNA amplification. First-strand cDNA was prepared from the cerebral cortex and hippocampus from each of the four groups (27 individual cDNA syntheses) using the TaqMan® Reverse Transcription kit (Applied Biosystems, Foster City, California). For each reaction, a target cDNA of interest and the reference cDNA (glyceraldehyde-3-phosphate dehydrogenase; G3pdh) were simultaneously PCR amplified in duplicate or triplicate in 96-well plates along with eight concentrations of a mouse cortex RNA standard.15,16 Thus, relative expression levels were determined in the cortex and hippocampus for all genes. Primers and probes for the plasticity-related genes were chosen using Primer Express v3.0 Software (Perkin-Elmer Applied Biosystems, Foster City, California) and are provided in Table 1. Primers and probes for the following genes were based on previous publications: Egr1,15Egr3,15Fra2,15G3pdh,15Egr4,17Mmp9,18Tpa,19 and Bdnf.20 TaqMan® MicroRNA Reverse Transcription kit and miRNA amplification kits were used to assess the miR-132 and miR-134 expression.
Table 1.
Primer and Probe Sequences Used for Quantitative Real-Time PCR Analyses.
| Gene Symbol | Amplicon (bp) | Forward (F) and reverse (R) primers and probe (P) sequence |
|---|---|---|
| Arc | 68 | F: GCAGGTGGGTGGCTCTGA |
| R: TCTTGGCTGGCCCATTCA | ||
| P: 6FAM5′-AATATTGGCTGTCCCAGATCCAGAACCAC-3′TAMRA | ||
| Egr1 | 66 | F: AAGACACCCCCCCATGAAC |
| R: GCGAGAAAAGCGGCGAT | ||
| P: 6FAM5′-CCCATATGCTTGCCCTGTCGAGTCC-3′TAMRA | ||
| Grp78 | 59 | F: CACGTCCAACCCCGAGAA |
| R: ATTCCAAGTGCGTCCGATG | ||
| P: 5′FAM-CGGTCTTCGATGCCAAGCGCC-3′TAMRA | ||
| Grp94 | 64 | F: CTTCCTTGTAGCAGATAAGGTCATTG |
| P: 5′FAM-CACATCGAAACACAACAATGATACCCAGCA-3′TAMRA | ||
| R: TCATTGGAGTCTGATTCCCAGA | ||
| Nr4a3 | 65 | F: CCTCCGATCTGTATGATGAATGC |
| R: GTGGGACAGTATCTGGAATAATCAAG | ||
| P: 5′FAM-CGAGCTTTAACAGATGCAACACCCAGAGA-3′TAMRA | ||
| Fra2 | 90 | F: TCTTCCTCAGCAGGGATGGA |
| R: CAGAGGCTCTTCCCCGTAGA | ||
| P: 6FAM5′-CCAGCGCTCTGTCATCAAGCCCAT-3′TAMRA | ||
| Egr3 | 68 | F: TTGCTAAATCAATTGCCTGACAA |
| R: CCGGAGAAGAGGTTGAGCG | ||
| P: 6FAM5′-CTGTACCCCGAGGAGATCCCCAGC-3′TAMRA | ||
| Egr2 | 111 | F: GGACCCAGGTCTCATTCCTATG |
| R: GGACAGGGAAACGGCTTTC | ||
| P: 6FAM5′-ATCCTGGATTTTTTCCATCTCCGTGCC-3′TAMRA | ||
| Egr4 | 79 | F: TACAGCGGCAGCTTCTTCATC |
| R: TGCCAGACATGAGGTTGAAGAG | ||
| P: 6FAM5′-TTCCCGAACACCCGCACGACC-3′TAMRA | ||
| Mmp9 | 62 | F: ACCCGAAGCGGACATTGTC |
| R: CGAAGGGATACCCGTCTCC | ||
| P: 6FAM5′-TCCAGTTTGGTGTCGCGG-3′TAMRA | ||
| Tpa | 93 | F: CAACAGCGGCCTGGTACAAT |
| R: TACAGGGCCTGCTGACACGT | ||
| P: 6FAM5′-TGCCTGTCCGAAGTTGCAGCGAAC-3′TAMRA | ||
| Camk2 | 88 | F: TCCTGCCGCATTGAAGGA |
| R: AACCAGCAGCCACATTCCA | ||
| P: 5′FAM-CTTGCTTCGCAGAGATCCGCTCTTTG-TAMRA-3′ | ||
| Homer | 120 | F: TTGACCCGAACACAAAGAAGAA |
| R: TATTGCCTTTGAGCCATCTAAACTG | ||
| P: 5′FAM-CCCACCAGCAAGCATGCAGTTACTGTATC-TAMRA-3′ | ||
| Narp | 134 | F: GGCAAGCCAACGAGATTGTG |
| R: AGTGGTCCAGGTGATGCAGAT | ||
| P: 5′FAM-CTGCTCATCAACGACAAGGTCGCACA-TAMRA-3′ | ||
| Neurogranin | 91 | F: GGCCAGAGCTGAACGTTTTAGA |
| R: GCTCACAAACACAGTAGGGAAGTC | ||
| P: 5′FAM-CGCGTCCCCTTCGCAGTGACA-TAMRA-3′ | ||
| Scg2 | 84 | F: TTTAATGCCCAATTTCCCTTCTT |
| R: CACTGTCAACAGGATAGAGATCAACA | ||
| P: 5′FAM-CCCCCAAGTAAGCCCCCTACATTTCTCT-TAMRA-3′ | ||
| Syt4 | 105 | F: GACAGAGCACGCAGAAAACATG |
| R: GTGAAGACGAGGCCAAAAGC | ||
| P: 5′FAM-CTCCTATCACCACCAGCCGCGTG-TAMRA-3′ | ||
| Bdnf | 68 | F: CCATAAGGACGCGGACTTGTAC |
| R: GAGGAGGCTCCAAAGGCACTT | ||
| P: 5′FAM-CTTCCCGGGTGATGCTCAGCAGT-TAMRA-3′ | ||
| G3pdh | 65 | F: CAACGGGAAGCCCATCAC |
| R: CGGCCTCACCCCATTTG | ||
| P: 6VIC5′-ATCTTCCAGGAGCGAGACCCCACTAACA-TAMRA-3′ | ||
| hsa-miR-132 | Applied Biosystems, Catalog Number 4373143 | |
| mmu-miR-134 | Applied Biosystems, Catalog Number 4373299 |
Statistical Analysis
Sleep data were analyzed using two-way analysis of variance (ANOVA) with day (treatment vs. baseline) as a repeated factor—to control for individual differences in sleep parameters under baseline conditions—and treatment as a grouping factor (Statview 5.0.1; SAS Institute, Cary, North Carolina, USA). One-way ANOVA with treatment as grouping factor was used to compare gene expression between groups. Significant effects identified by ANOVA were followed by t-test with Student-Newman-Keuls corrections to identify differences.
RESULTS
Sleep Physiology Studies
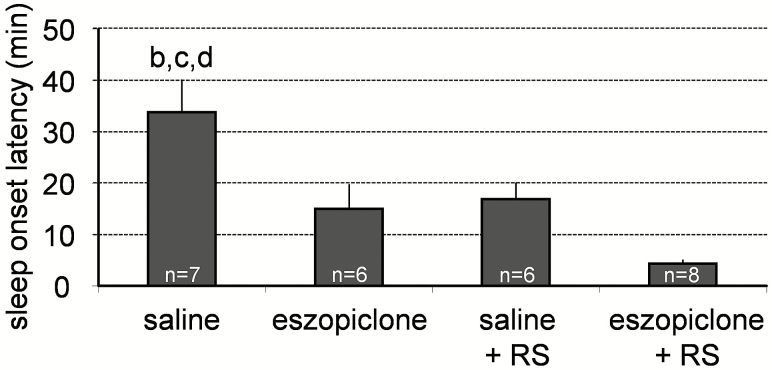
Eszopiclone (20 mg/kg, i.p.) reduced the latency to NREM sleep after injection compared to the saline injections (Figure 2). This result is in agreement with previous studies in which zopiclone reduced latency to NREM sleep following the dose of 2 or 4 mg/kg p.o. in rats11 and the dose of 10 mg/kg i.p. in mice.21 The latency to NREM sleep after eszopiclone injection was similar to that occurring after 6 hours of sleep deprivation (Figure 2).
Figure 2.
Effects of eszopiclone on the latency to sleep onset in undisturbed mice and in mice that were subjected to 6-hour sleep deprivation. Eszopiclone, sleep deprivation, or combination of eszopiclone and sleep deprivation significantly reduced the latency to sleep onset. Data represent the mean ± SEM percentage of wake, REM sleep, and NREM sleep from the total recording time. Statistical comparisons were made among all groups; letters at the top of bars indicate significant differences (p < .05) compared to the treatment groups indicated (from left to right: a–d). NREM = nonrapid eye movement; REM = rapid eye movement; RS = recovery sleep after sleep deprivation; SEM = standard error of the mean.
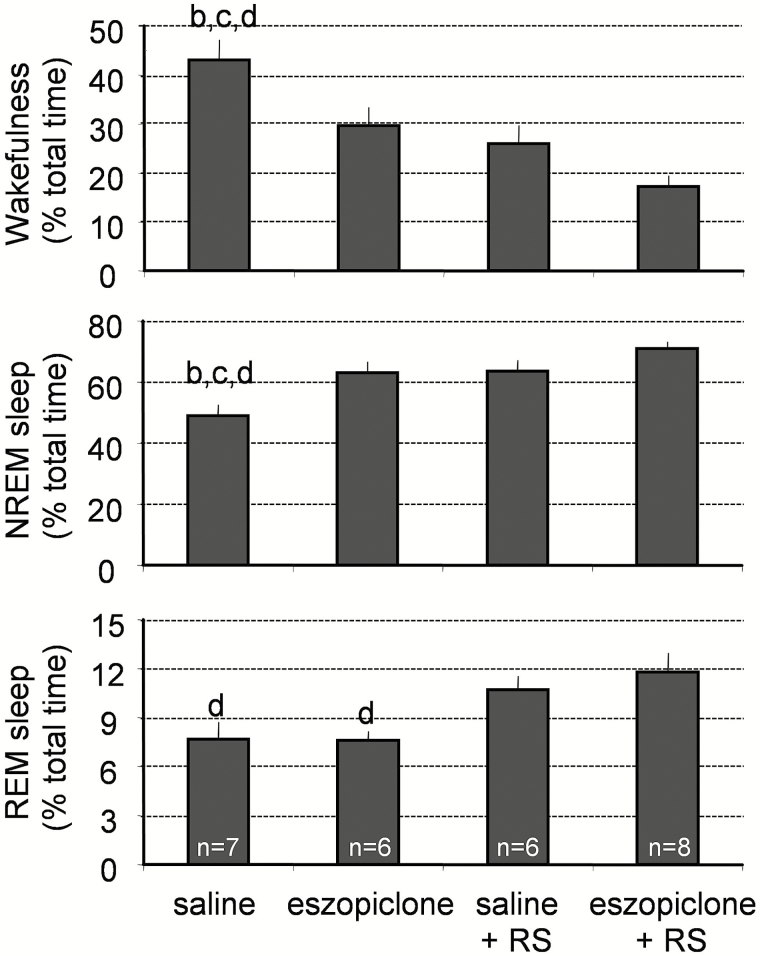
A significant decline in wakefulness and increase in NREM sleep amounts occurred during the 160 minutes after the injection of 20 mg/kg of eszopiclone without a change in REM sleep amounts (Figure 3). Both NREM sleep amounts and REM sleep amounts were greater in the RS or RS + eszopiclone groups of mice than in the saline-treated mice (Figure 3).
Figure 3.
Effects of eszopiclone on wakefulness, REM sleep, and NREM sleep in undisturbed mice and in mice that were subjected to 6-hour sleep deprivation. Eszopiclone (20 mg/kg) or saline was injected ip at ZT6, and sleep was recorded 160 minutes following the injection. Saline-treated animals had significantly higher amounts of wakefulness than eszopiclone-treated mice or mice recorded during the recovery sleep period (RS) after sleep deprivation. Data represent the mean ± SEM percentage of wake, REM sleep, and NREM sleep from the total recording time. Statistical comparisons were made among all groups; letters at the top of bars indicate significant differences (p < .05) compared to the treatment groups indicated (from left to right: a–d). NREM = nonrapid eye movement; REM = rapid eye movement; RS = recovery sleep after sleep deprivation; SEM = standard error of the mean.
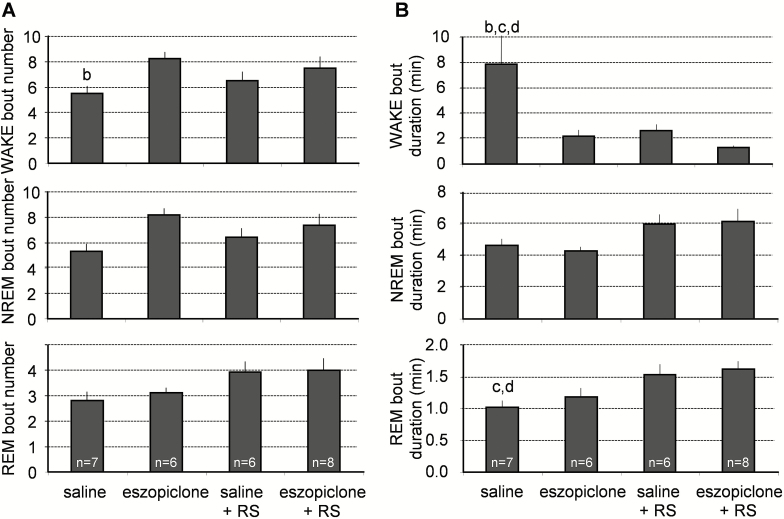
Eszopiclone did not significantly affect the frequency (Figure 4A) or duration (Figure 4B) of the NREM or REM sleep bouts. However, eszopiclone + RS reduced the duration of wakefulness bouts (Figure 4B) and shorter wake bouts were evident with eszopiclone alone as well as RS alone.
Figure 4.
Effects of eszopiclone on the number of wakefulness, REM sleep, and NREM sleep bouts and bout durations in undisturbed mice and in the mice that were subjected to 6-hour sleep deprivation. (A) Mice of the saline-treated group had significantly fewer wakefulness bouts than mice of the eszopiclone-treated group. (B) Mice of the saline-treated group had significantly longer bouts of wakefulness than the mice treated with eszopiclone during the recovery sleep period (eszopiclone + RS group). Statistical comparisons were made among all groups; letters at the top of bars indicate significant differences (p < .05) compared to the treatment groups indicated (from left to right: a–d). NREM = nonrapid eye movement; REM = rapid eye movement; RS = recovery sleep after sleep deprivation.
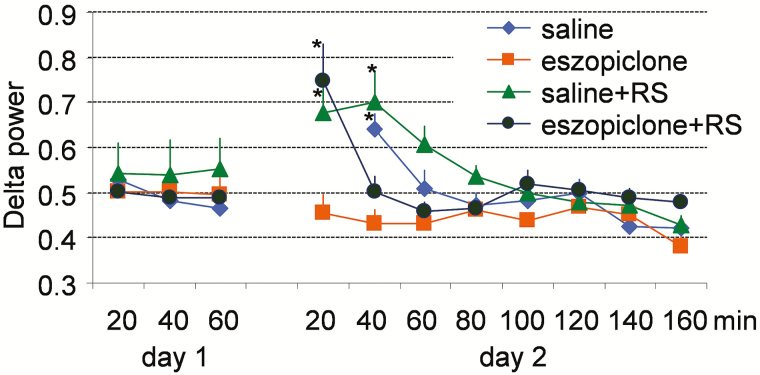
During the 6-hour period of sleep deprivation, mice in group 3 spent 95.1 ± 1.4% of the time awake and 4.9 ± 1.4% of the time in NREM sleep and mice in group 4 spent 94.5 ± 1.2% of awake and 5.5 ± 1.2% in NREM sleep. As expected, the relative power density in the EEG delta frequency band was significantly increased during RS after sleep deprivation (Figure 5). However, this increase was transient in the mice treated with eszopiclone, suggesting that eszopiclone inhibits EEG delta activity. Surprisingly, a transient increase in EEG delta power also occurred during first 20 minutes after sleep onset in mice treated with saline. It is possible that a stress associated with the saline injection resulted in a delayed sleep onset (more than 30 minutes; Figure 2), allowing homeostatic drive to accumulate during the post-injection period.
Figure 5.
NREM sleep SWA profile on the baseline day (day 1) and on the injection day (day 2) in mice. Zero minutes correspond to ZT6, the time when injections occurred on day 2. Results are shown as the mean ± SEM. *p < .05 versus the same group of mice on day 1. NREM = nonrapid eye movement; SEM = standard error of the mean; SWA = slow wave activity.
Gene Expression Studies
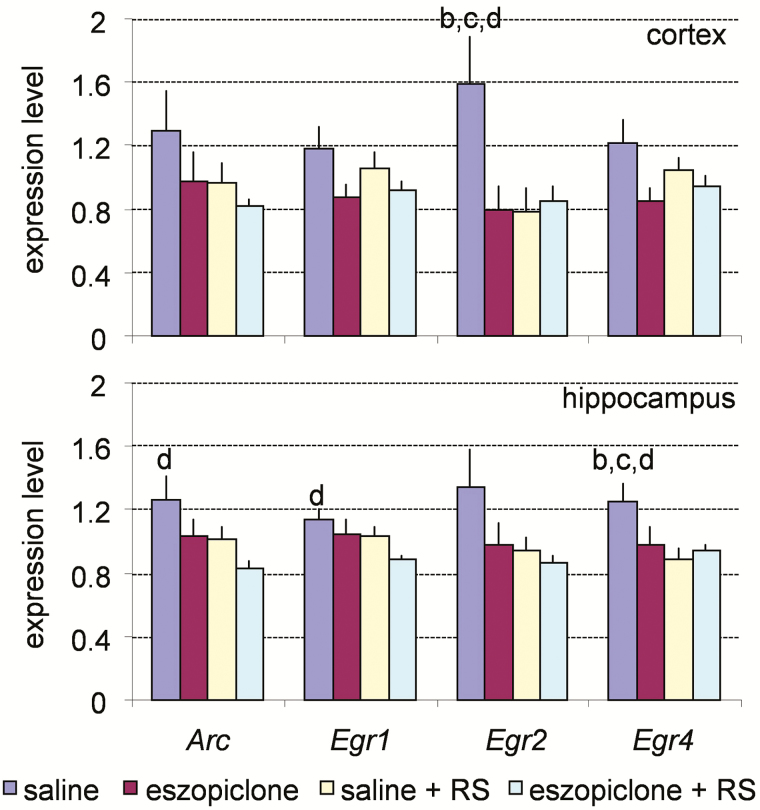
Expression of 18 plasticity-related genes was measured by TaqMan quantitative RT-PCR in both the cortex and hippocampus of mice from the four different treatment groups. The expression levels of these genes were classified as three distinct patterns: wake-associated, RS-associated, and state invariant. Genes in the wake-associated category showed the highest expression in either the cortex or hippocampus of the saline-injected mice (group 1) and included Arc, Egr1, Egr2, and Egr4 (Figure 6).
Figure 6.
Real-time polymerase chain reaction analysis of the expression of the plasticity-related genes in the cerebral cortex (upper panel) and hippocampus (lower panel) across the four experimental conditions for the wake-associated genes. G3pdh expression was used as an internal standard. Values are mean ± SEM. p values are based on ANOVA; letters at the top of bars indicate significant differences (p < .05) compared to the groups indicated (from left to right: a–d). Note the increased level of expression in the group of mice treated with saline (group 1). ANOVA = analysis of variance; RS = recovery sleep; SEM = standard error of the mean.
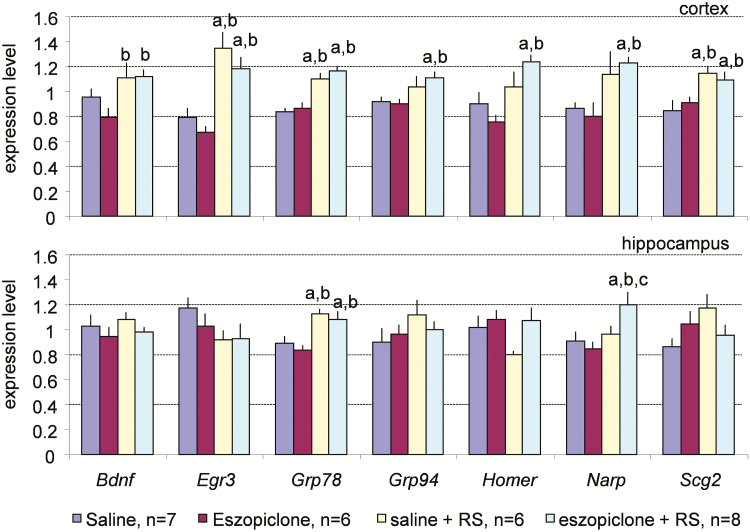
Genes in the RS-associated category had higher expression levels in group 3 or 4 than in group 1 or 2 (Figure 7) and included Bdnf, Egr3, Grp78, Grp94, Homer, Narp, and Scg2. The mice belonging to groups 3 and 4 were sacrificed during RS after sleep deprivation, whereas the mice belonging to groups 1 and 2 were not subjected to sleep deprivation. Thus, this expression pattern is characteristic of genes that have a high expression level during RS.
Figure 7.
Real-time polymerase chain reaction analysis of the expression of the plasticity-related genes in the cerebral cortex (upper panel) and hippocampus (lower panel) across the four experimental conditions for the sleep-associated genes. G3pdh expression was used as an internal standard. Values are mean ± SEM. p values are based on ANOVA; letters at the top of bars indicate significant differences (p < .05) compared to the groups indicated (from left to right: a–d). Increased levels of expression are seen during recovery sleep after sleep deprivation in the mice injected with saline (group 3) or eszopiclone (group 4). ANOVA = analysis of variance; RS = recovery sleep; SEM = standard error of the mean.
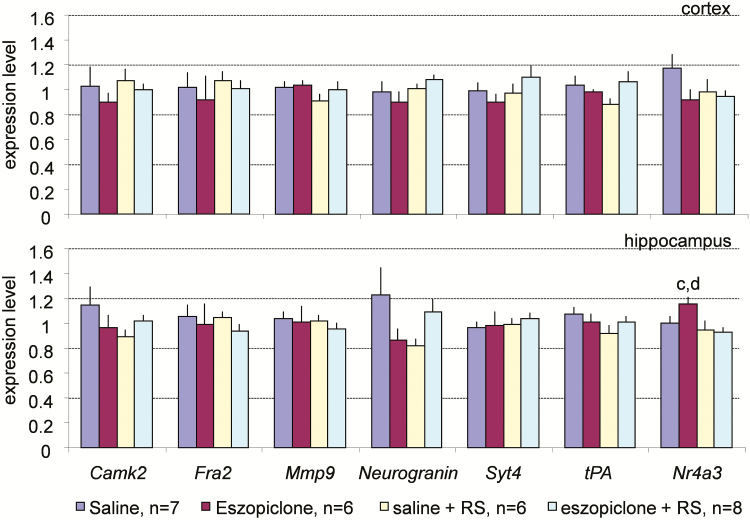
Genes designated as “state invariant” did not show any significant changes in expression between the treatment groups (Figure 8). This category included Camk2, Fra2, Mmp9, Neurogranin, Syt4, and Tpa.
Figure 8.
Real-time polymerase chain reaction analysis of the expression of the plasticity-related genes in the cerebral cortex (upper panel) and hippocampus (lower panel) across the four experimental conditions for the state-invariant genes. G3pdh expression was used as an internal standard. Values are mean ± SEM. p values are based on ANOVA; letters at the top of bars indicate significant differences (p < .05) compared to the groups indicated (from left to right: a–d). Except for Nr4a3, there are no statistically significant differences in gene expression. ANOVA = analysis of variance; RS = recovery sleep; SEM = standard error of the mean.
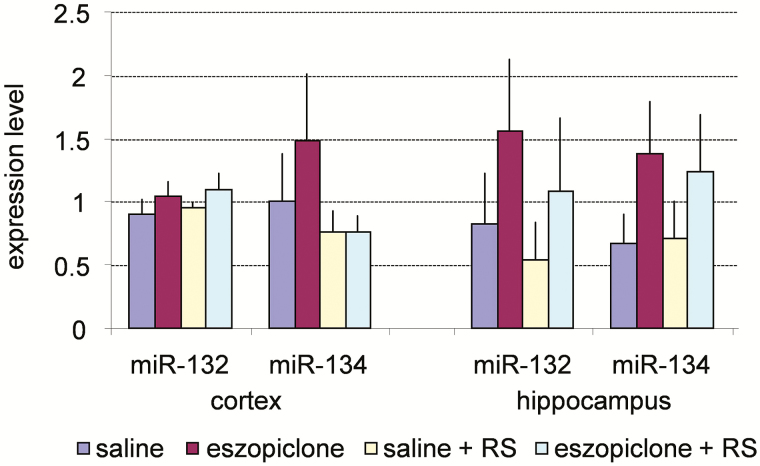
Although miRNA expression had a tendency to increase following the eszopiclone treatment, these differences did not reach statistical significance (Figure 9).
Figure 9.
Real-time polymerase chain reaction analysis of the expression of two miRNAs in the cerebral cortex and hippocampus across the four experimental conditions. Values are mean ± SEM. ANOVA did not reveal any statistically significant differences in miRNA expression between groups. ANOVA = analysis of variance; RS = recovery sleep; SEM = standard error of the mean.
DISCUSSION
The experimental design of the present study differed from previous sleep-related gene-expression studies. Whereas several previous studies focused on comparing wakefulness versus sleep4–6; comparison of spontaneous sleep versus sleep deprivation-associated RS has received relatively little attention.15,22 We chose to test gene expression during the RS period because it has been proposed that restorative processes in the brain occur most efficiently during this period. According to the “synaptic homeostasis hypothesis”, restorative processes in the brain depend on synaptic plasticity changes. This hypothesis states that plastic processes occurring during wakefulness result in a net increase in synaptic strength in many brain circuits.2,23–25 The role of sleep is to downscale synaptic strength to a baseline level that is energetically sustainable, makes efficient use of gray matter space, and is beneficial for learning and memory.2
At the molecular level, synaptic potentiation during wakefulness is reflected by an increase in the expression of a large number of plasticity genes (Arc, Camk2, Bdnf, Egr1, Homer, Narp, etc.).26,27 In the hippocampus, such molecular changes are usually associated with long-term potentiation (LTP).27,28 Although the “synaptic homeostasis” hypothesis predicts an overall reduction in synaptic strength during sleep, synaptic strength is expected to increase in at least some neuronal circuits such as those that underlie the reactivation of memory traces that were acquired during the preceding periods of wakefulness.29,30 Hypnotics may have an effect on sleep-dependent brain plasticity,3,31 but their effect on the expression of plasticity–related genes has been little studied. Therefore, we compared gene expression during spontaneous sleep with gene expression during RS following sleep deprivation either in presence or absence of eszopiclone treatment.
Effects of Eszopiclone on Sleep
Eszopiclone is the active stereoisomer of zopiclone, so the effects of eszopiclone and zopiclone on sleep are expected to be similar. Earlier studies in mice21 and rats11 demonstrated that the primary effect of zopiclone on sleep is to increase the amount of NREM sleep. In one of these studies, zopiclone at a dose of 20 mg/kg i.p. did not significantly increase absolute amounts of NREM sleep in rats but significantly increased the NREM sleep time/total recording time ratio calculated within 6 hours of post-injection period.12 The effects of eszopiclone on REM sleep are less consistent. A reduction in the amounts of REM sleep after 20 or 100 mg/kg i.p. of zopiclone was observed in one study in rats,12 but no such reduction in REM sleep amounts was seen in other studies in which doses up to 10 mg/kg were used.11,21
Consistent with other published studies,13,21,32 eszopiclone reduced the latency to NREM sleep, increased NREM sleep amounts, and did not change REM sleep amounts (Figures 2 and 3). Similar to another study in mice in which zopiclone at 2–10 mg/kg did not modify the mean duration of NREM or REM sleep bouts or their frequency,21 there was also no change in the duration or frequency of the NREM or REM sleep bouts in the present study (Figure 4, A and B). Eszopiclone had no effect on slow wave activity (SWA) when it was injected during baseline conditions, but it reduced the SWA increase during RS after sleep deprivation (Figure 5). In this regard, the eszopiclone effect on EEG is similar to the effects of most of the presently used hypnotic drugs that alter sleep EEG parameters despite effectively improving sleep quality from the patient’s self-reported point of view.33 In healthy young men, a single dose of midazolam (15 mg) or zopiclone (7.5 mg) reduced SWA and enhanced spindle frequency activity (11.25 to 15.0 Hz), but the time course of these parameters across and within sleep cycles as well as their mutual relationship were little affected.34 These investigators concluded that hypnotics acting as benzodiazepine-receptor agonists do not substantially interfere with the homeostatic aspect of sleep regulation.34
Effects of Eszopiclone on Expression of Plasticity-Related Genes
We chose 18 genes that have been critically implicated in various aspects of synaptic plasticity.35–47 The other common feature of all these genes is that their expression changes between wakefulness and sleep states.6,15,48,49 We assessed expression of these genes in the cerebral cortex and hippocampus because these brain regions exhibit significant changes in the expression of plasticity-related genes during the sleep–wake cycle.4,5,6,49,50 Analysis of the gene expression revealed three distinct patterns.
Wakefulness-Associated Genes
The first pattern observed is represented by the highest gene expression levels in the mice treated with saline (group 1). We identified four genes with such a pattern: Arc, Egr1, Egr2, and Egr4 (Figure 6). Mice of group 1 had significantly higher amounts of wakefulness during the period of 160 minutes before sacrifice than mice of other three groups, suggesting that the amounts of wakefulness is the major factor affecting expression of Arc, Egr1, Egr2, and Egr4 in the cerebral cortex and hippocampus. This conclusion is supported by a number of other studies in which expression of the Arc and Egr family of genes markedly increased after extended periods of wakefulness.6,15,27 In those studies, increases in Arc and Egr1 expression were among the most robust gene expression changes observed during wakefulness.6,15 In our study, we observed a less robust increase in the level of expression of these genes (Figure 6), perhaps because the amounts of wakefulness were lower in the present study than in other studies. The maximum amount of wakefulness in the group 1 mice in our study was 43% of the total recording time, whereas the amounts of wakefulness were close to 100% in other studies in which sleep deprivation was performed. A recent study demonstrated that Arc, Egr1, and Egr2 belong to the group of “fast response” genes that are upregulated by sleep deprivation and remain elevated during approximately 2 hours of RS before returning to the original level.22 Since we sampled brain tissue after 2.5 to 3 hours of RS, this transient increase in expression at the beginning of the RS period was not detected.
The activity-regulated cytoskeletal-associated (ARC) protein is a key protein implicated in synaptic plasticity and memory consolidation.35Arc mRNA is quickly induced and dynamically upregulated by behavioral experience.35,51,52 It is thought that ARC and CAMK2 act as plasticity partners to promote functional and/or structural synaptic modifications.36 The early growth response (EGR) family of transcription regulatory factors is implicated in orchestrating the changes in gene expression that underlie neuronal plasticity.37 The most extensively studied member of this family is EGR1. EGR1 has been linked to the induction of hippocampal LTP,53,54 neuronal morphological changes after exposure to an enriched environment,55 and other plasticity-related phenomena.56,57 The expression of other genes encoding EGR family members is also extremely sensitive to the environmental stimuli that cause plasticity.37,58
RS-Associated Genes
The second pattern of gene expression consists of a higher expression in mice subjected to sleep deprivation with subsequent RS (group 3) or mice subjected to a combination of the eszopiclone treatment and RS (group 4) than in mice of the other two groups (groups 1 and 2). Remarkably, almost 40% of plasticity-related genes assessed in the present study showed this pattern of expression (Figure 7). This result is consistent with those of a recent study in which many genes including some of those examined in the present experiment (eg, Bdnf, Homer) were found to be upregulated during RS22. These results suggest that synaptic plasticity processes actively occur during the RS periods after sleep deprivation. However, it is also possible that this expression pattern is related to other cellular processes that require transcriptional regulation during first hours of homeostatic discharge of RS. Previous studies may not have detected such expression changes because those studies were conducted during spontaneous sleep associated with low homeostatic pressure (typically, after 6–12 hours of sleep).
Seven genes had increased expression in the cortex during RS either with or without eszopiclone treatment. These genes are Bdnf, Egr3, Grp78, Grp94, Homer, Narp, and Scg2. High expression of these genes is typically found after periods of extended wakefulness.15,16,59–63 We previously reported higher expression of Egr3, Grp78, and Grp94 in the cortex during the RS period than during the period of spontaneous sleep.16,49 We now show that a large percentage of plasticity-related genes are highly expressed during the RS period. Despite such similarities in gene expression, the EEG patterns are very different between these behavioral states. High frequency (gamma or beta) predominate the EEG pattern during wakefulness.64 Large increases in SWA are seen during RS, and SWA is especially high in the cortical areas that were actively involved in the learning during preceding wakefulness.65 SWA was suppressed by eszopiclone during RS in the present study, but this suppression did not have an effect on the expression of plasticity-related genes in the cerebral cortex. Indeed, the level of expression of these genes was as high in the eszopiclone + RS group as in the RS group and even reached statistically significant difference for some genes (Grp94, Homer, and Narp) in the eszopiclone + RS group but not in the RS group (Figure 7). Thus, our results indicate that eszopiclone does not interfere with the expression of plasticity-related genes in the mouse cortex, even at the relatively high dose used in the present study (20 mg/kg).
Compared to the seven genes that had increased expression in the cortex during RS either with or without eszopiclone treatment, there were only two genes with a similar pattern of expression (Grp78 and Narp) in the hippocampus (Figure 7). This difference between the cortex and hippocampus may be related to temporal differences in the gene expression profile in these brain structures. If coordinated cortical and hippocampal replay of the multicell firing sequences evoked by awake experience29 is followed by a longer phase of gradual strengthening of cortical-cortical synapses for long-term memory storage,66 we would likely detect only the plasticity processes associated with the later phase because the gene expression was studied as long as 160 minutes after the onset of RS period in the present study.
Genes of the second expression pattern have been implicated in various aspects of synaptic function. BDNF is known to play a key role in the survival, growth, and maintenance of neurons during development67 and to modulate synaptic plasticity in the adult brain.38 Brain-derived neurotrophic factor (BDNF) affects synaptic plasticity through (1) the regulation of axonal and dendritic branching and remodeling68; (2) synaptogenesis in arborizing axon terminals69; (3) increasing the efficacy of synaptic transmission70; and (4) the functional maturation of excitatory and inhibitory synapses.71 HOMER has been implicated in various models of synaptic plasticity.72,73 The protein product of Homer is known to be targeted to the synapse where it interacts directly with other proteins in the postsynaptic density to facilitate changes in synaptic structure and function.39,74Narp is a member of the pentraxin family.75 Neuronal-activity-regulated pentraxin protein localizes specifically to excitatory synapses in primary neuronal cultures and in adult brain and is present in both the presynaptic and postsynaptic compartments.76 It is an essential synaptogenic factor that is specific for excitatory synapses.40,76Grp78 and Grp94 are frequently upregulated under conditions leading to plastic changes. They are classified as immediate early genes or molecular chaperones. For example, the chaperone function of Grp78 might serve to fold proteins and assemble protein complexes necessary for the structural changes characteristic of long-term memory.41 Chromogranins (A, B, and C) are secretory glycoproteins that coexist with peptide transmitters in large dense-core synaptic vesicles of many types of neurons.42 Chromogranin C (a product of the Scg2 gene) is released upon depolarization and is sensitive to changes in neuronal activity.42 Recent studies performed in Egr3-deficient mice suggest that Egr3 has an essential role in regulating cortical arousal, wakefulness, and sleep, presumably by its regulation of 5-HT2 receptors.77
State-Invariant Genes
The third pattern of gene expression observed was the absence of changes associated with either the eszopiclone treatment or RS. The genes with this expression pattern did not display statistically significant changes across four groups of mice by ANOVA analysis (Figure 8). Although Nr4a3 was significantly higher in the hippocampus of mice treated with eszopiclone (group 2) than in the mice of RS groups (groups 3 and 4), it was not presented here as a separate expression pattern because this pattern was unique to this gene (Figure 8).
Six genes did not show statistically significant changes in expression: Camk2, Fra2, Mmp9, Neurogranin, Syt4, and Tpa. The expression of these genes was found to differ between wakefulness and sleep in previous studies.15,48,78 This discrepancy may be due to differences in the experimental design between the previous and present studies. In the present study, gene expression levels were determined about 160 minutes after the end of sleep deprivation. If sleep deprivation induces expression of these genes, a sufficient period of time may have elapsed for these genes to return to control levels. Future work may determine the time course of gene expression to establish the effects of wakefulness on expression of these genes.
Genes in the state-invariant category have been shown to play a role in synaptic function. CAMK2 is the main protein of the postsynaptic density.43 It translocates to synapses and binds directly to the N-methyl-D-aspartate (NMDA) receptor and might act as a bistable switch for the long-term storage of synaptic memory.43 A number of knockout, transgenic overexpression, and inhibitor studies have demonstrated that CAMK2 is essential for synaptic plasticity and learning.43 Matrix metalloproteinases (MMPs) are a large family of endopeptidases, the substrates of which are proteins of the extracellular matrix as well as some nonmatrix proteins such as adhesion proteins.79 MMPs act critically in regulating functional and structural remodeling of cellular architecture.80 MMP9 plays a role for in hippocampal synaptic physiology, plasticity, and memory.44 MMP9 protein levels and proteolytic activity are rapidly increased by stimuli that induce late-phase LTP in area CA1, whereas blockade of MMP9 pharmacologically selectively prevents induction of L-LTP.44 A number of studies support the role of neurogranin in synaptic plasticity. Injection of antibodies to neurogranin into hippocampal CA1 pyramidal cells prevented induction of LTP in these neurons.81 In agreement with this finding, knockout of neurogranin resulted in a large decrease in the LTP induced by a single 100 Hz, 1s tetanus, whereas LTD was slightly enhanced.45 Inducing LTP requires calmodulin stored in spines in the form of rapidly dissociating calmodulin–neurogranin complexes.82 Synaptotagmin IV is an abundant membrane protein that is localized to synaptic vesicles.83 In cultured neurons, synaptotagmin IV is present in the Golgi apparatus and in distal parts of growing neurites, where its expression increases in response to membrane depolarization.46 Because of its activity-dependent expression and its subcellular localization, synaptotagmin IV has been considered as a marker of synaptic plasticity. tPA, a serine protease classically known for its profibrinolytic role in the vasculature, has been implicated in numerous aspects of the synaptic plasticity process.47,84 tPA has been shown to be upregulated in the cerebellum of rats during learning of a complex motor skill,85 and the absence of tPA in tPA–/– mice results in a significant reduction in the rate and extent of learning.86
Effects of Eszopiclone on Expression of miRNAs
Since genes involved in synaptic plasticity are controlled at the translational level by miRNAs and the RISC,7–9 we also evaluated expression of two relevant miRNAs in our study. Dendritic spine volume is regulated by miR-134, which appears to function through the translational control of LimK1, a regulator of actin polymerization.8 miR-132 is regulated by cyclic AMP-response element binding protein, an important regulator of different forms of plasticity.9,87 In addition, the activity of both of these miRNAs may be controlled by BDNF—another major regulator of synaptic plasticity—via direct or indirect mechanisms.8,9,88 We did not observe statistically significant differences in the levels of miR-132 and miR-134 either in the cortex or hippocampus (Figure 9).
Some limitations of the present study need to be acknowledged. Although we selected genes based on their role in plasticity, these genes are also implicated in other cellular processes. In addition, we did not measure protein concentrations. A large number of studies suggest a role for post-transcriptional, translational, and degradation regulation contribute to local protein concentrations.89 Therefore, at least some proteins may be regulated in a different temporal pattern from that observed for mRNA in the present study.
CONCLUSION
Increased expression of synaptic plasticity-related genes has been found during wakefulness in many previous studies. We observed that a large proportion of plasticity-related genes increased expression also during RS (7 out of 18 genes included in the study). The significance of this increase in gene expression is currently unknown, but we hypothesize that it is related to restorative processes in the brain that actively occur during RS after sleep deprivation. Eszopiclone did not interfere with the expression of plasticity-related genes during RS in the mouse cortex even at a relatively high dose (20 mg/kg). These results suggest that synaptic plasticity changes and restorative processes can occur in the cortex in the presence of eszopiclone.
FUNDING
Research supported by Sepracor Inc., NIH R21 NS092926 and NIH R01 HL059658.
DISCLOSURE STATEMENT
None declared.
REFERENCES
- 1. Zaharna M, Guilleminault C. Sleep, noise and health: review. Noise Health. 2010; 12(47): 64–69. [DOI] [PubMed] [Google Scholar]
- 2. Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006; 10(1): 49–62. [DOI] [PubMed] [Google Scholar]
- 3. Aton SJ, Seibt J, Dumoulin MC, Coleman T, Shiraishi M, Frank MG. The sedating antidepressant trazodone impairs sleep-dependent cortical plasticity. PLoS One. 2009; 4(7): e6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004; 41(1): 35–43. [DOI] [PubMed] [Google Scholar]
- 5. Porkka-Heiskanen T. Gene expression during sleep, wakefulness and sleep deprivation. Front Biosci. 2003; 8: S421–S437. [DOI] [PubMed] [Google Scholar]
- 6. Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000; 885(2): 303–321. [DOI] [PubMed] [Google Scholar]
- 7. Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006; 124(1): 191–205. [DOI] [PubMed] [Google Scholar]
- 8. Schratt GM, Tuebing F, Nigh EA et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006; 439(7074): 283–289. [DOI] [PubMed] [Google Scholar]
- 9. Vo N, Klein ME, Varlamova O et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA. 2005; 102(45): 16426–16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weizman R, Paz L, Peter Y, Toren P, Pick CG. Behavioral effects of agents active at the gamma-aminobutyric acid receptor complex in the staircase paradigm. Brain Res. 2001; 901(1–2): 137–142. [DOI] [PubMed] [Google Scholar]
- 11. Noguchi H, Kitazumi K, Mori M, Shiba T. Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats. J Pharmacol Sci. 2004; 94(3): 246–251. [DOI] [PubMed] [Google Scholar]
- 12. Yoshimoto M, Higuchi H, Kamata M, Yoshida K, Shimizu T, Hishikawa Y. The effects of benzodiazepine (triazolam), cyclopyrrolone (zopiclone) and imidazopyridine (zolpidem) hypnotics on the frequency of hippocampal theta activity and sleep structure in rats. Eur Neuropsychopharmacol. 1999; 9(1–2): 29–35. [DOI] [PubMed] [Google Scholar]
- 13. Gauthier P, Arnaud C, Stutzmann JM, Gottesmann C. Influence of zopiclone, a new generation hypnotic, on the intermediate stage and paradoxical sleep in the rat. Psychopharmacology (Berl.). 1997; 130(2): 139–143. [DOI] [PubMed] [Google Scholar]
- 14. Carlson JN, Haskew R, Wacker J, Maisonneuve IM, Glick SD, Jerussi TP. Sedative and anxiolytic effects of zopiclone’s enantiomers and metabolite. Eur J Pharmacol. 2001; 415(2–3): 181–189. [DOI] [PubMed] [Google Scholar]
- 15. Terao A, Greco MA, Davis RW, Heller HC, Kilduff TS. Region-specific changes in immediate early gene expression in response to sleep deprivation and recovery sleep in the mouse brain. Neuroscience. 2003; 120(4): 1115–1124. [DOI] [PubMed] [Google Scholar]
- 16. Terao A, Steininger TL, Hyder K et al. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience. 2003; 116(1): 187–200. [DOI] [PubMed] [Google Scholar]
- 17. Bonnert TP, Bilsland JG, Guest PC et al. Molecular characterization of adult mouse subventricular zone progenitor cells during the onset of differentiation. Eur J Neurosci. 2006; 24(3): 661–675. [DOI] [PubMed] [Google Scholar]
- 18. Van Valckenborgh E, Bakkus M, Munaut C et al. Upregulation of matrix metalloproteinase-9 in murine 5T33 multiple myeloma cells by interaction with bone marrow endothelial cells. Int J Cancer. 2002; 101(6): 512–518. [DOI] [PubMed] [Google Scholar]
- 19. Wygrecka M, Markart P, Ruppert C et al. Cellular origin of pro-coagulant and (anti)-fibrinolytic factors in bleomycin-injured lungs. Eur Respir J. 2007; 29(6): 1105–1114. [DOI] [PubMed] [Google Scholar]
- 20. Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J Neurosci Methods. 2000; 98(1): 9–20. [DOI] [PubMed] [Google Scholar]
- 21. Alexandre C, Dordal A, Aixendri R, Guzman A, Hamon M, Adrien J. Sleep-stabilizing effects of E-6199, compared to zopiclone, zolpidem and THIP in mice. Sleep. 2008; 31(2): 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerstner JR, Koberstein JN, Watson AJ et al. Removal of unwanted variation reveals novel patterns of gene expression linked to sleep homeostasis in murine cortex. BMC Genomics. 2016; 17(Suppl 8): 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010; 30(25): 8671–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009; 324(5923): 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olcese U, Esser SK, Tononi G. Sleep and synaptic renormalization: a computational study. J Neurophysiol. 2010; 104(6): 3476–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003; 54(1): 224–237. [DOI] [PubMed] [Google Scholar]
- 27. Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000; 20(24): 9187–9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romcy-Pereira RN, Erraji-Benchekroun L, Smyrniotopoulos P et al. Sleep-dependent gene expression in the hippocampus and prefrontal cortex following long-term potentiation. Physiol Behav. 2009; 98(1–2): 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007; 10(1): 100–107. [DOI] [PubMed] [Google Scholar]
- 30. Tononi G. Slow wave homeostasis and synaptic plasticity. J Clin Sleep Med. 2009; 5(2 Suppl): S16–S19. [PMC free article] [PubMed] [Google Scholar]
- 31. Seibt J, Aton SJ, Jha SK, Coleman T, Dumoulin MC, Frank MG. The non-benzodiazepine hypnotic zolpidem impairs sleep-dependent cortical plasticity. Sleep. 2008; 31(10): 1381–1391. [PMC free article] [PubMed] [Google Scholar]
- 32. Xi M, Chase MH. Effects of eszopiclone and zolpidem on sleep and waking states in the adult guinea pig. Sleep. 2008; 31(7): 1043–1051. [PMC free article] [PubMed] [Google Scholar]
- 33. Billiard M, Besset A, de Lustrac C, Brissaud L, Cadilhac J. [Effects of zopiclone on sleep, daytime somnolence and nocturnal and daytime performance in healthy volunteers]. Neurophysiol Clin. 1989; 19(2): 131–143. [DOI] [PubMed] [Google Scholar]
- 34. Aeschbach D, Dijk DJ, Trachsel L, Brunner DP, Borbely AA. Dynamics of slow-wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacology. 1994; 11(4): 237–244. [DOI] [PubMed] [Google Scholar]
- 35. Guzowski JF, Lyford GL, Stevenson GD et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000; 20(11): 3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vazdarjanova A, Ramirez-Amaya V, Insel N et al. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006; 498(3): 317–329. [DOI] [PubMed] [Google Scholar]
- 37. O’Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999; 22(4): 167–173. [DOI] [PubMed] [Google Scholar]
- 38. Lo DC. Neurotrophic factors and synaptic plasticity. Neuron. 1995; 15(5): 979–981. [DOI] [PubMed] [Google Scholar]
- 39. Brakeman PR, Lanahan AA, O’Brien R et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997; 386(6622): 284–288. [DOI] [PubMed] [Google Scholar]
- 40. Xu D, Hopf C, Reddy R et al. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003; 39(3): 513–528. [DOI] [PubMed] [Google Scholar]
- 41. Kuhl D, Kennedy TE, Barzilai A, Kandel ER. Long-term sensitization training in Aplysia leads to an increase in the expression of BiP, the major protein chaperon of the ER. J Cell Biol. 1992; 119(5): 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shen PJ, Gundlach AL. Differential increases in chromogranins, but not synapsin I, in cortical neurons following spreading depression: implications for functional roles and transmitter peptide release. Eur J Neurosci. 1998; 10(7): 2217–2230. [DOI] [PubMed] [Google Scholar]
- 43. Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002; 3(3): 175–190. [DOI] [PubMed] [Google Scholar]
- 44. Nagy V, Bozdagi O, Matynia A et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006; 26(7): 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang KP, Huang FL, Jager T, Li J, Reymann KG, Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J Neurosci. 2004; 24(47): 10660–10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ibata K, Fukuda M, Hamada T, Kabayama H, Mikoshiba K. Synaptotagmin IV is present at the Golgi and distal parts of neurites. J Neurochem. 2000; 74(2): 518–526. [DOI] [PubMed] [Google Scholar]
- 47. Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006; 50(5): 673–678. [DOI] [PubMed] [Google Scholar]
- 48. Taishi P, Sanchez C, Wang Y, Fang J, Harding JW, Krueger JM. Conditions that affect sleep alter the expression of molecules associated with synaptic plasticity. Am J Physiol Regul Integr Comp Physiol. 2001; 281(3): R839–R845. [DOI] [PubMed] [Google Scholar]
- 49. Terao A, Wisor JP, Peyron C et al. Gene expression in the rat brain during sleep deprivation and recovery sleep: an Affymetrix GeneChip study. Neuroscience. 2006; 137(2): 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guzman-Marin R, Ying Z, Suntsova N et al. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006; 575(Pt 3): 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999; 2(12): 1120–1124. [DOI] [PubMed] [Google Scholar]
- 52. Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002; 22(23): 10067–10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Richardson CL, Tate WP, Mason SE, Lawlor PA, Dragunow M, Abraham WC. Correlation between the induction of an immediate early gene, zif/268, and long-term potentiation in the dentate gyrus. Brain Res. 1992; 580(1–2): 147–154. [DOI] [PubMed] [Google Scholar]
- 54. Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989; 340(6233): 474–476. [DOI] [PubMed] [Google Scholar]
- 55. Wallace CS, Withers GS, Weiler IJ, George JM, Clayton DF, Greenough WT. Correspondence between sites of NGFI-A induction and sites of morphological plasticity following exposure to environmental complexity. Brain Res Mol Brain Res. 1995; 32(2): 211–220. [DOI] [PubMed] [Google Scholar]
- 56. Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993; 363(6431): 718–722. [DOI] [PubMed] [Google Scholar]
- 57. Kaplan IV, Guo Y, Mower GD. Developmental expression of the immediate early gene EGR-1 mirrors the critical period in cat visual cortex. Brain Res Dev Brain Res. 1995; 90(1–2): 174–179. [DOI] [PubMed] [Google Scholar]
- 58. Li L, Yun SH, Keblesh J et al. Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Mol Cell Neurosci. 2007; 35(1): 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fujihara H, Sei H, Morita Y, Ueta Y, Morita K. Short-term sleep disturbance enhances brain-derived neurotrophic factor gene expression in rat hippocampus by acting as internal stressor. J Mol Neurosci. 2003; 21(3): 223–232. [DOI] [PubMed] [Google Scholar]
- 60. Hairston IS, Peyron C, Denning DP et al. Sleep deprivation effects on growth factor expression in neonatal rats: a potential role for BDNF in the mediation of delta power. J Neurophysiol. 2004; 91(4): 1586–1595. [DOI] [PubMed] [Google Scholar]
- 61. Nelson SE, Duricka DL, Campbell K, Churchill L, Krueger JM. Homer1a and 1bc levels in the rat somatosensory cortex vary with the time of day and sleep loss. Neurosci Lett. 2004; 367(1): 105–108. [DOI] [PubMed] [Google Scholar]
- 62. Maret S, Dorsaz S, Gurcel L et al. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci USA. 2007; 104(50): 20090–20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005; 92(5): 1150–1157. [DOI] [PubMed] [Google Scholar]
- 64. Miller R. Theory of the normal waking EEG: from single neurones to waveforms in the alpha, beta and gamma frequency ranges. Int J Psychophysiol. 2007; 64(1): 18–23. [DOI] [PubMed] [Google Scholar]
- 65. Huber R, Ghilardi MF, Massimini M et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006; 9(9): 1169–1176. [DOI] [PubMed] [Google Scholar]
- 66. O’Reilly RC, Rudy JW. Computational principles of learning in the neocortex and hippocampus. Hippocampus. 2000; 10(4): 389–397. [DOI] [PubMed] [Google Scholar]
- 67. Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994; 390: 45–56. [PubMed] [Google Scholar]
- 68. McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996; 17(6): 1057–1064. [DOI] [PubMed] [Google Scholar]
- 69. Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001; 4(11): 1093–1101. [DOI] [PubMed] [Google Scholar]
- 70. Boulanger L, Poo MM. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999; 2(4): 346–351. [DOI] [PubMed] [Google Scholar]
- 71. Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998; 18(18): 7256–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Igaz LM, Bekinschtein P, Izquierdo I, Medina JH. One-trial aversive learning induces late changes in hippocampal CaMKIIalpha, Homer 1a, Syntaxin 1a and ERK2 protein levels. Brain Res Mol Brain Res. 2004; 132(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 73. de Bartolomeis A, Iasevoli F. The Homer family and the signal transduction system at glutamatergic postsynaptic density: potential role in behavior and pharmacotherapy. Psychopharmacol Bull. 2003; 37(3): 51–83. [PubMed] [Google Scholar]
- 74. Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998; 273(37): 23969–23975. [DOI] [PubMed] [Google Scholar]
- 75. Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996; 16(8): 2463–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999; 23(2): 309–323. [DOI] [PubMed] [Google Scholar]
- 77. Grønli J, Clegern WC, Schmidt MA et al. Sleep homeostatic and waking behavioral phenotypes in Egr3-deficient mice associated with serotonin receptor 5-HT2 deficits. Sleep. 2016; 39(12): 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Neuner-Jehle M, Rhyner TA, Borbely AA. Sleep deprivation differentially alters the mRNA and protein levels of neurogranin in rat brain. Brain Res. 1995; 685(1–2): 143–153. [DOI] [PubMed] [Google Scholar]
- 79. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001; 17: 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001; 2(7): 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fedorov NB, Pasinelli P, Oestreicher AB, DeGraan PN, Reymann KG. Antibodies to postsynaptic PKC substrate neurogranin prevent long-term potentiation in hippocampal CA1 neurons. Eur J Neurosci. 1995; 7(4): 819–822. [DOI] [PubMed] [Google Scholar]
- 82. Zhabotinsky AM, Camp RN, Epstein IR, Lisman JE. Role of the neurogranin concentrated in spines in the induction of long-term potentiation. J Neurosci. 2006; 26(28): 7337–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ferguson GD, Vician L, Herschman HR. Synaptotagmin IV: biochemistry, genetics, behavior, and possible links to human psychiatric disease. Mol Neurobiol. 2001; 23(2–3): 173–185. [DOI] [PubMed] [Google Scholar]
- 84. Yepes M, Lawrence DA. New functions for an old enzyme: nonhemostatic roles for tissue-type plasminogen activator in the central nervous system. Exp Biol Med (Maywood). 2004; 229(11): 1097–1104. [DOI] [PubMed] [Google Scholar]
- 85. Seeds NW, Basham ME, Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci. 2003; 23(19): 7368–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Siconolfi LB, Seeds NW. Mice lacking tissue plasminogen activator and urokinase plasminogen activator genes show attenuated matrix metalloproteases activity after sciatic nerve crush. J Neurosci Res. 2003; 74(3): 430–434. [DOI] [PubMed] [Google Scholar]
- 87. Josselyn SA, Nguyen PV. CREB, synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord. 2005; 4(5): 481–497. [DOI] [PubMed] [Google Scholar]
- 88. Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006; 9(5): 580–586. [PubMed] [Google Scholar]
- 89. Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012; 13(4): 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]