Abstract
The mesolimbic dopamine system contributes to a remarkable variety of behaviors at multiple timescales. Midbrain neurons have fast and slow signaling components, and specific afferent systems, such as the hippocampus (HPC) and prefrontal cortex (PFC), have been demonstrated to drive these components in anesthetized animals. Whether these interactions exist during behavior, however, is unknown. To address this question, we developed a novel analysis of human functional magnetic resonance imaging data that fits models of network excitation and inhibition on ventral tegmental area (VTA) activation. We show that specific afferent systems predict distinct temporal components of midbrain VTA signal. We found that PFC, but not HPC, positively predicted transient, event-evoked VTA activation. In contrast, HPC, but not PFC, positively predicted slow shifts in VTA baseline variability. Thus, unique functional contributions of afferent systems to VTA physiology are detectable at the network level in behaving humans. The findings support models of dopamine function in which dissociable neural circuits support different aspects of motivated behavior via active regulation of tonic and phasic signals.
Keywords: baseline, hippocampus, network connectivity, prefrontal cortex, VTA
Introduction
The mesolimbic dopamine system, originating from dopamine neurons in the ventral tegmental area (VTA), contributes to a variety of behaviors including motivation, learning and memory, behavioral activation, and salience detection (Wise 2004; Redgrave and Gurney 2006; Berridge et al. 2009; Schultz 2010; Shohamy and Adcock 2010; Lisman et al. 2011). Although these behavioral relationships are well established, much remains to be understood about how a single class of neurons could be involved in such a broad array of behaviors. One important feature of the mesolimbic system is that its projection neurons have physiological properties and connectivity that allow them to influence downstream targets at multiple timescales (Hyman et al. 2006; Goto et al. 2007; Niv et al. 2007; Roeper 2013), a complexity that arises in part from the properties of afferent inputs and reciprocal efferents. The goal of the current study is to investigate how network interactions contribute to temporally distinct components of VTA activation in behaving humans.
The physiology of dopamine neurons in the VTA is modulated by at least 2 mechanisms: Direct excitation, which has mainly been demonstrated to contribute to phasic burst firing (Schultz and Romo 1990; Karreman and Moghaddam 1996; Kulagina et al. 2001), and disinhibition, which results in a prolonged increase in spontaneous firing along with an increased probability of burst firing (Floresco et al. 2003; Goto et al. 2007; Grace et al. 2007). These activity modes appear well suited to support different cognitive functions (Niv et al. 2007; Shohamy and Adcock 2010): Phasic release of dopamine has been implicated in incrementally refining goal-relevant responses in instrumental learning tasks (Wanat et al. 2009; Schultz 2010), and also been proposed to contribute to memory formation (Lisman et al. 2011). Slow changes in dopamine levels have been implicated in working memory (Cohen et al. 2002; Zweifel et al. 2009), avoidance learning (Frank et al. 2004; Dombrowski et al. 2013), motor and behavioral control (Niv et al. 2007), and long-term memory formation (Shohamy and Adcock 2010).
While there has been a great deal of research characterizing phasic firing within the VTA on the one hand and phasic and tonic fluctuations in dopamine in target structures on the other, little is known about the interplay of these timescales in the VTA during awake behavior. The prefrontal cortex (PFC), which is implicated in goal-directed behavior (Levy and Goldman-Rakic 2000; Wager and Smith 2003; Wallis and Miller 2003; Savine and Braver 2010), has both indirect and direct glutamatergic (Sesack and Pickel 1992; Sesack and Carr 2002; Frankle et al. 2006) projections to dopamine neurons in the VTA. These prefrontal afferents have mainly been implicated in phasic signaling. The PFC was first shown to regulate phasic burst firing in the VTA in anesthetized rodents (Gariano and Groves 1988; Svensson and Tung 1989; Gao and Goldman-Rakic 2003; Grace et al. 2007; Jo et al. 2013; Patton et al. 2013). More recent work has shown PFC modulation over event-evoked VTA activation consistent with phasic signals, both in human functional magnetic resonance imaging (fMRI; Ballard et al. 2011) and in awake behaving animals (Parker et al. 2011; Takahashi et al. 2011; Jo et al. 2013).
In contrast, tonic VTA activity is thought to be regulated by at least 2 sources: Excitatory inputs (Karreman and Moghaddam 1996; Kulagina et al. 2001) and strong GABAergic inhibitory inputs (Grace et al. 2007). Both PFC lesions and disruption of glutamatergic inputs to the VTA disrupt tonic levels of dopamine in striatal targets, presumably via excitatory inputs (Karreman and Moghaddam 1996; Kulagina et al. 2001). Most studies investigating tonic VTA dopamine release have studied disinhibition, showing that GABAergic inhibition increases the proportion of silent dopamine neurons, which do not fire in bursts and have low spontaneous firing rates. Similarly, in vivo evidence in rodents suggests that hippocampal activity reduces inhibition of VTA dopamine neurons via polysynaptic disinhibitory relays involving the nucleus accumbens and globus pallidus or the lateral septum (Floresco et al. 2003; Lisman et al. 2011; Luo et al. 2011). The hippocampus (HPC) has no known direct excitatory connections to dopamine neurons in the VTA, suggesting that the primary mode of communication between these 2 regions is tonic modulation. Thus, the functional properties of these circuits suggest that the PFC directly regulates both phasic and tonic firing, while the HPC directly regulates only tonic firing.
While extant data from animal models have provided important insights into the architecture of this system, they have several important limitations. First, much of the evidence comes from work done in anesthetized animals, which implies, but does not demonstrate, links to behavior (Parker et al. 2011; Takahashi et al. 2011; Jennings and Stuber 2014). Very little is known about how VTA afferent systems exert their influence during behavior. Second, tonic dopamine (DA) activity is typically inferred from measuring dopamine release in efferent target regions. However, because DA release can be regulated at the level of DA terminals [reviewed in Cachope and Cheer (2014)], DA efflux does not directly index changes in VTA activation; thus, the existence of slow changes in VTA activity during behavior has been controversial (Fiorillo et al. 2003; Niv et al. 2005; Howe et al. 2013; Totah et al. 2013). Third, physiological studies that record from a single region can provide only piecemeal pictures of the overall network. Although human neuroimaging cannot directly detect dopaminergic signals, it permits examination of networks of multiple brain regions during awake behavior. Here, we developed a novel analysis technique, in which we leveraged the strengths of human neuroimaging to simultaneously characterize how the dorsolateral prefrontal cortex (dlPFC) and HPC interact with the VTA at timescales that correspond to phasic and tonic effects.
We hypothesized that goal-directed behavior would engage the dlPFC, resulting in phasic VTA activation. Conversely, exposure to novelty would engage the HPC, resulting in slow shifts in baseline VTA activation. We developed analyses that directly tested the hypothesized network connectivity, reflecting the in vitro physiology of these afferents (excitation and disinhibition) and their downstream consequences on VTA activity at distinct timescales (transient vs. sustained). That is, we modeled transient VTA activation consistent with phasic bursts in response to isolated goal-relevant events versus sustained shifts in baseline in response to accumulated history with novelty. While undergoing MRI, participants performed a target-detection task in which they made button presses to a target scene image (i.e., goal-directed behavior) while withholding responses to nontarget novel and familiar scene images (Fig. 1). Although fMRI is not able to measure dopaminergic activation directly, we were able to test novel hypothesis about the network properties of neural systems underlying the mesolimbic system. Thus, our novel analysis approach modeled the effects of excitatory and disinhibitory pathways across networks to predict distinct temporal dynamics of VTA blood oxygen level-dependent (BOLD) signal (Fig. 2); thus, we aimed to provide evidence for dissociable contributions of dlPFC and HPC to VTA signaling during behavior.
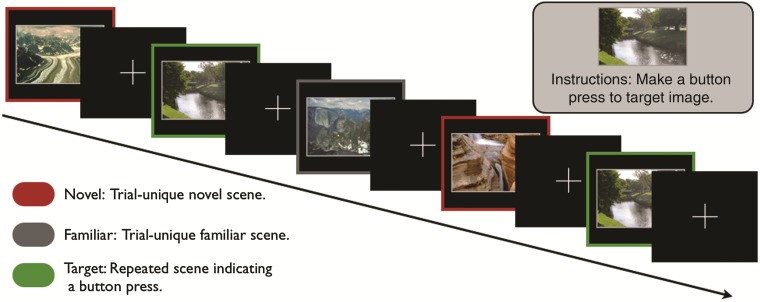
Figure 1.
Experimental task. Participants performed a target-detection task during the collection of fMRI data. During this task, participants made button presses to a single task-relevant target image, while incidentally viewing 80 trial-unique, novel images and 80 trial-unique images familiarized prior to scanning.
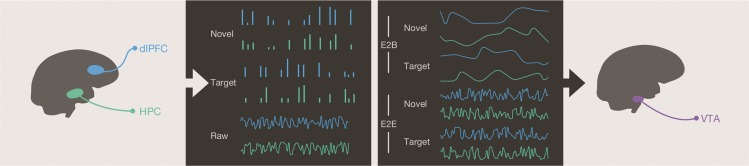
Figure 2.
Schematic of analysis method. Averaging across voxels within our dlPFC and HPC ROIs, we extracted (1) time-series of single-trial beta-weights for each novel event, (2) time-series of single-trial beta-weights for each target event, and (3) the raw time-series from these regions. In the left panel, we depict schematic, representative data of these 3 measurements for a single subject. For the event-to-baseline analyses (E2B), all 4 beta-weight time-series were smoothed with a 10-trial sliding window and convolved with an hemodynamic response function to generate a predicted baseline signal. For the event-to-event analyses (E2E), the raw signals were centered and convolved with the onset regressors for both the target and novel conditions, resulting in 4 time-series that model event-locked correlations with target regions. Representative schematics of these regressors are depicted in the right panel. Finally, regressors were combined into 2 separate GLM models, separately for novel and target events, to predict the VTA signal (not shown). We removed confounding variables (task events, nuisance regressors, and raw ROI signals) from the VTA time-series before using it as the dependent variable.
Materials and Methods
Participants
Twenty-eight healthy, right-handed participants were paid $40 to participate, plus any monetary bonuses earned during other tasks performed during the experimental session. All participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board. Four participants were excluded: 1 for excessive head motion (>1.5 mm), 1 for poor performance (chance performance), and 2 for computer malfunction. The final analyses included 24 participants (13 female, age range: 19–35; median age = 25). Data from these participants on this task have previously been reported (Murty et al. 2013).
Procedures
One hour prior to scanning, participants performed a task intended to familiarize them with 80 outdoor scene images (Fig. 1). During this task, participants viewed one outdoor scene image at a time (duration: 2 s) followed by a screen asking, “Have you seen this picture before?” Participants viewed each of the 80 outdoor scene images 6 times (familiar images) and 40 outdoor scene images once (foils) in a randomized order. This familiarization task lasted approximately 20 min. We instructed participants to respond “yes/recognize” by pressing the “1” button and to respond “no/new” by pressing the “2” button. During this pre-study familiarization task, participants demonstrated significant corrected-recognition rates (hits − false alarms) to scene images on their sixth presentation [mean ± SEM: 69.6 ± 2.9%, t(23) = 24.0, P < 0.001]. These significant corrected-recognition rates indicate that prior to scanning, participants were familiar with these images.
Immediately prior to scanning, participants were instructed on the target-detection task (Fig. 1). We informed participants that during the task, they would be presented with a series of outdoor scene images. We then showed them a single target scene image and instructed them to respond with a button press every time the target was presented. In the scanner prior to the target-detection task, we presented the target scene image a second time and repeated the instructions. During each trial of the target-detection task (Fig. 1), participants viewed an outdoor scene image (2 s) followed by a fixation cross (0.5–7 s). Over the course of the task (duration: 12 min, 2 s), participants viewed 80 nonrepeated, novel scene images (images participants never viewed before), 80 trial-unique, familiar scene images, and 40 presentations of the same target scene image. Importantly, individual novel and familiar scene images were not repeated during the target-detection task. Target detection was nearly perfect across subjects (mean ± SEM: 99.4 ± 0.3%). For both the familiarization and target-detection task, scene images were all outdoor images of landscapes with a resolution of 100 pixels/inch. We randomly divided scene images into 2 sets and counterbalanced the sets between novel and familiar conditions across participants. We optimized trial order and onsets using the Opt-seq software (Dale 1999). We counterbalanced a trial order of novel and familiar conditions across participants. Furthermore, participants' reaction times did not show significant variation over the course of the fMRI task (F3 = 1.539, P = 0.21, linear trend: P = 0.23, quadratic trend: P = 0.16). These RT results suggest that participants remained vigilant throughout the entire task.
MRI Data Acquisition and Preprocessing
fMRI data were collected on a 3.0-T GE Signa MRI scanner using a standard echo-planar imaging sequence [time echo = 27 ms, flip = 77°, time repetition (TR) = 1 s, 17 contiguous slices, size = 3.75 × 3.75 × 3.8 mm]. Partial brain data encompassing VTA, HPC, and lateral PFC were acquired with a short TR in order to maximize the data-sampling rate in regions of interest (ROIs). Data for the target-detection task consisted of one run of 722 volumes. Prior to the functional run, we collected a high-resolution anatomical image (voxel size = 1 mm, isotropic) for spatial normalization and a whole-brain echo-planar imaging (EPI) image to assist with co-registration.
fMRI preprocessing was performed using the fMRI Expert Analysis Tool (FEAT) Version 5.92 as implemented in FSL 4.1.5 (www.fmrib.ox.ac.uk/fsl). BOLD images were skull stripped, realigned, intensity normalized by a single multiplicative factor, spatially smoothed with a full-width at half-maximum of 4.0 mm, and subjected to a 100-s, high-pass temporal filter. Spatial normalization was performed using a three-step procedure. We first aligned the partial-volume EPI to the whole-brain EPI using the fMRIb Linear Registration Tool (FLIRT). We then normalized the high-resolution EPI to a standard MNI template using the fMRI Non-Linear Registration Tool (FNIRT). Finally, we applied the normalization to the aligned partial-volume EPI.
fMRI Data Analysis
fMRI data were analyzed using FEAT Version 5.92 as implemented in FSL 4.1.5. Time-series statistical analyses used FILM with local autocorrelation correction (Smith et al. 2004).
Overview of Analysis
The analysis stream proceeded in the following steps. First, we defined 3 ROIs the HPC, PFC (specifically dlPFC), and VTA. Within the PFC, we focused our analysis on the dlPFC as previous work from our laboratory has demonstrated that the dlPFC drives activation of the VTA in goal-directed behavior (Ballard et al. 2011). We constructed 2 separate general linear models (GLMs): One GLM modeling VTA activation in response to novel events and another GLM modeling VTA activation in response to target events. Within each model, we used responses from HPC and dlPFC in response to either novel or target events to generate regressors for GLMs (Fig. 2). The regressors used these regions to model temporally distinct components of the VTA BOLD signal as function of their network connectivity. The first 2 regressors model how fluctuations in HPC and dlPFC responsivity influence baseline VTA signal (event-to-baseline). The last 2 regressors model how fluctuations in HPC and dlPFC responsivity influence the event-related VTA response (event-to-event). Thus, each GLM consisted of 4 regressors modeling event-to-baseline and event-to-event signals derived from HPC and dlPFC signals. The only difference in these GLMs was whether HPC and dlPFC signals were in response to target events or novel events. These GLMs modeled a preprocessed VTA time-series in which we filtered out confounding factors using hierarchical regression.
ROI Selection
Ventral Tegmental Area
The VTA ROI was derived from a published probabilistic atlas of the dopaminergic midbrain (Murty et al. 2014). In brief, the atlas was constructed by hand drawing ROIs of the VTA in 50 participants using individually localized anatomical landmarks.
Hippocampus
The HPC is a relatively large and functionally heterogeneous structure (Poppenk et al. 2013). Accordingly, we sought to identify a smaller portion that was maximally involved in task-relevant novelty processing. We identified an ROI in the posterior HPC as the intersection of the HPC ROI available from the Harvard Oxford Subcortical Structural Atlas and an 8-mm sphere centered on the peak of activation in the contrast of linearly declining novel images > linearly declining familiar images [(x, y, z) = (24, −32, −3)]. Analyses detailed in another report on this data indicate that this contrast most robustly identifies novelty-related hippocampal responses (Murty et al. 2013). This group-level ROI was back-transformed into native subject space for further analyses.
Prefrontal Cortex
As with the HPC, we sought to identify a subregion of the dlPFC that was maximally involved in the goal-directed behaviors during our task. We defined the dlPFC by the intersection of BA46 and the middle frontal gyrus, which we acquired from the Talairach Daemon database using WFU pickatlas. We took the intersection of this anatomical region with an 8-mm sphere centered on the peak of activation in the contrast of target > baseline. This group-level ROI was back-transformed into native subject space for further analyses.
Hierarchical Regression
As opposed to using a simple preprocessed time-series in the GLM models described below, we wished to first remove unrelated variability (i.e., nuisance variability). In order to this, we used a hierarchical regression in which we first applied a GLM to the preprocessed time-series that had regressors for all of our events of no interest (detailed below). We then used the residual timecourse from this GLM in our GLMs investigating VTA interactions with HPC and PFC.
The first-stage GLM included separate regressors for all event-related responses to novel, familiar, and target events, thus removing any variance from the VTA time-series that could be accounted for by VTA-evoked activation in isolation. Task events were convolved with a double-gamma hemodynamic response (HDR) function (duration = 2 s, intensity = 1). This GLM also included regressors modeling the physiological timecourses of the HPC and PFC ROIs (modeled as the first eigenvariate), thus removing variability in the VTA time-series that could be accounted for by HPC and PFC in a task-independent manner (i.e., baseline co-variation and physiological noise). The resulting residual time-series comprised the dependent variables in our GLM models of interest investigating the PFC and HPC influences on VTA activation.
Event-to-Baseline Regressors
We were interested in generating a regressor that captures neural variability related to tonic dopamine activation. We modeled fluctuations in baseline activity in the VTA using regressors generated from the PFC and HPC ROIs based on responsivity to either target or novel events, depending on the GLM. We reasoned that regions whose activity influenced the VTA via disinhibition would have a sustained influence on the measured VTA activation. In turn, we measured trial-by-trial responsivity to trial-evoked events in both the dlPFC and the HPC using an iterative GLM procedure which gives more robust estimates of single-trial responses (Mumford et al. 2012). Given the temporal dynamics of novelty signals on VTA signaling (i.e., slow, tonic disinhibition), we smoothed novelty signals using a 10-trial, retroactive sliding average. We used 10 events because this number balanced predicted variance in peak signaling (Huettel and McCarthy 2001) while still capturing within-task variance; similar results were obtained using windows of 8 or 12 events. The sliding average was chosen to be retroactive, so that responsivity to events in the future would not influence momentary responsivity. Finally, the 2 series of smoothed estimates were transformed into a modeled time-series using linear interpolation with the actual experimental timing of trials and convolved with an hemodynamic response function in order to account for hemodynamic delay.
This regressor behaves as an estimate of the fluctuating sensitivity to novelty in the target region. Thus, if the HPC has particularly large responses to novelty for several consecutive trials, VTA activation should be relatively increased during this entire subsequent period (not just at the time points immediately following the trials). We used the same procedure was used to define the target event-to-baseline regressors. Finally, due to the sluggishness of the BOLD response, it is possible that these baseline regressors may capture fluctuations in VTA event-related responses, rather than sustained changes in baseline activation. However, we control for this by 2 means. First, the data entered in the GLM have already removed any variance accounted for by presentations of events (see first-stage GLM above). Second, we additionally control for this using an event-to-event regressor within the same model, described below.
Event-to-Event Analysis
We modeled fluctuations in VTA event-related responses to targets and to novelty using the same ROIs as for the baseline analysis. This procedure served 2 purposes. First, including this regressor in the same model as the event-to-baseline regressor ensured that the event-to-baseline regressor was capturing sustained, rather than event-locked, fluctuations in VTA activation. Second, this regressor allowed us to test the hypothesis that the PFC, rather than the HPC, is directly related to event-related activity in the VTA during goal-directed behavior. We constructed this regressor by multiplying the first eigenvariate of the dlPFC or HPC time-series with the target regressor. This procedure for generating these regressors is conceptually similar to a psycho-physiological interaction analysis, with the notable difference that we are trying to predict signals in the residuals from the first-stage GLM (see the Hierarchical Regression section), as opposed to a preprocessed time-series. Of note, this event-to-event regressor, which is often used as an indirect measure of connectivity, is a direct measure of how well fluctuations in event-related activation in one region predict event-related activations in a target region.
General Linear Model: Main Analysis
To test our main question of whether there are dissociable components of the hippocampal and prefrontal time-series that correlate with VTA activity, we constructed 2 sets of fixed-effects GLMs: One for novel events and another for target events. Each model contained 4 regressors of interest to predict VTA responses: (1) HPC-mediated event-to-event, (2) HPC-mediated event-to-baseline, (3) PFC-mediated event-to-event, and (4) PFC-mediated event-to-baseline. Each regressor was convolved with a double-gamma HDR function. We separated GLMs by condition because (1) a GLM with all 8 regressors was determined to be less reliable based on model estimations and (2) we were primarily interested in comparing different signals between the HPC and PFC. Nonetheless, the reported results remain significant when we included all regressors in a single GLM. Finally, a weighted average of parameter estimates across a probabilistic VTA ROI were extracted for all regressors.
Results
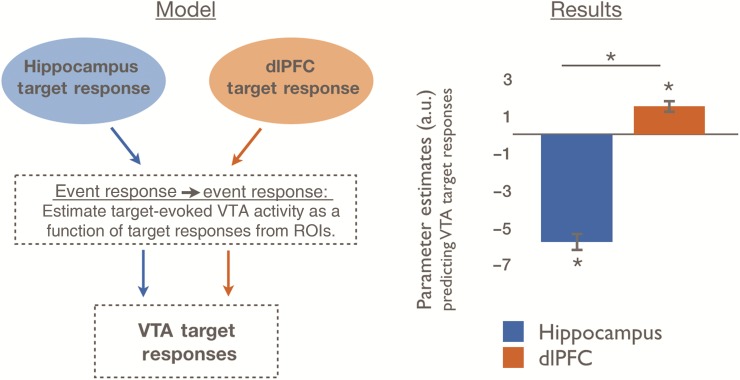
To model how network connectivity contributes to phasic VTA responses, we investigated whether activation in the dlPFC or HPC predicted event-evoked responses in VTA during goal-directed behavior (Fig. 2). We hypothesized that during the presentation of target scenes, which required a goal-directed response, event-evoked VTA activation, our proxy for phasic firing, would be better predicted by dlPFC than HPC. Confirming our predictions, we found that event-evoked VTA activations following target scenes were better predicted by dlPFC than HPC responses [dlPFC > HPC: t(24) = 9.66, P = 1.46 × 10−9, Fig. 3]. Specifically, we found that dlPFC activation predicted target-evoked VTA activation [t(24) = 4.28, P = 2.80 × 10−4], which parallels the rodent literatures demonstrating VTA phasic activity in response to dlPFC activation. Surprisingly, we found that HPC activations inversely predicted target-evoked VTA activation [t(24) = −11.39, P = 6.25 × 10−11], which may reflect that the targets were overlearned; these highly familiar scenes would thus not elicit novelty responses (see Discussion).
Figure 3.
Trial-by-trial VTA responses are distinctly predicted by trial-by-trial responses to task-relevant targets in dlPFC versus HPC. We estimated dlPFC and HPC contributions to VTA responses to target images. Specifically, we modeled fluctuations in transient VTA target responses as arising from fluctuations in transient dlPFC and HPC responses. The dlPFC showed a positive relationship with VTA target responses, whereas the HPC showed a negative relationship. dlPFC, dorsolateral prefrontal cortex; HPC, hippocampus; VTA, ventral tegmental area; ROI, regions of interest. Data are represented as means with SEM. *P < 0.001. See also Supplementary Figure 1.
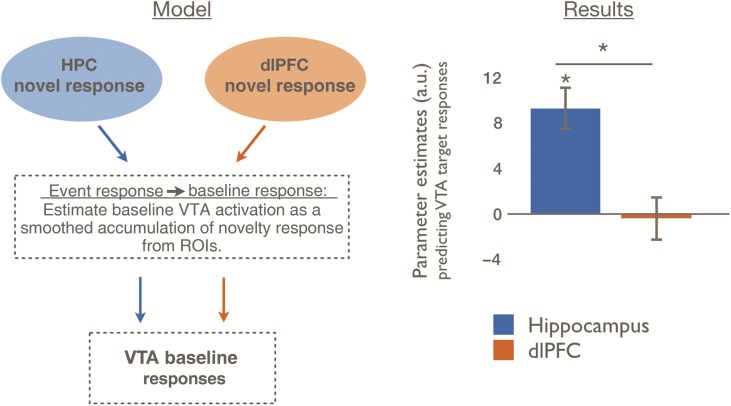
To model how network connectivity contributes to sustained changes in VTA activation (Fig. 2), we investigated whether activation in dlPFC or HPC predicted subsequent baseline variability in VTA. We hypothesized that baseline VTA variability, our proxy for tonic activity, would be better predicted by the integration of recent novelty responses in the HPC than in the dlPFC. Confirming our predictions, we found that variability in VTA baseline was better predicted by a moving average of recent HPC novelty responses than by corresponding dlPFC novelty responses [HPC > PFC: t(24) = 3.07, P = 0.006, Fig. 4]. Specifically, we found that HPC novelty responses predicted subsequent baseline variability in the VTA [t(24) = 4.82, P = 7.22 × 10−5], while there was no relationship between dlPFC novelty responses and VTA baseline variability [t(24) = −0.23, P = 0.82].
Figure 4.
Baseline VTA variability is distinctly predicted by trial-by-trial responses to novel events in the HPC, but not in the dlPFC. We estimated dlPFC and HPC contributions to VTA baseline variability by modeling the VTA BOLD signal as a smoothed accumulation of recent trial-by-trial novelty responses in each source region. For example, this model encodes the assumption that if the seed region has relatively larger trial-evoked responses to novelty for several consecutive trials, baseline VTA activation should be relatively increased during this entire period. The model derived from the HPC strongly predicted VTA activation, whereas the model derived from the dlPFC did not significantly predict VTA activation. Data are represented as means with SEM. *P< 0.001. See also Supplementary Figure 1.
These findings demonstrate a double dissociation between the timescale of VTA activation (event-evoked or baseline variability) and network interactions (dlPFC or HPC; F24 = 36.37, P = 3.37 × 10−8). Taken together, these findings support models in which dlPFC signals contribute more to phasic, event-evoked VTA signals, whereas the HPC contributes more to tonic, baseline variability in the VTA signals. We next tested whether these phasic and tonic proxy responses we identified during the hypothesized conditions of goal-directed behavior or novel stimuli were specific to those conditions; this triple interaction was not significant (F24 = 0.55, P = 0.46; see Supplementary Fig. 1). Thus, the dynamics of the relationships we demonstrate are more consistent with general physiological properties of the mesolimbic dopamine system.
Discussion
The current findings demonstrate unique contributions of the dlPFC and HPC to temporally distinct components of VTA signaling in behaving humans. These findings emerged from a modeling approach that leveraged physiological understanding of how afferents with different properties (namely, excitation and disinhibition) would influence VTA signal on different timescales. Consistent with its role in goal-directed behavior, the dlPFC positively predicted transient, event-evoked VTA activation, while the HPC did not. Consistent with its role in detection of contextual novelty, the HPC predicted tonic, baseline variability in the VTA, while the dlPFC did not. These results confirm predictions from rodent neurophysiology and offer a novel demonstration of their relevance to awake behavior for the first time in any species. Furthermore, they provide evidence of unique contributions of VTA afferents to behaviorally relevant aspects of a single behavioral task to show how a single neuromodulatory nucleus could simultaneously influence distinct components of behavior.
Our findings show that changes in VTA baseline activity are predicted by integrating prior transient hippocampal activations to novelty. In rodents, this relationship has only been observed in anesthetized animals, during evoked stimulation (Legault et al. 2000; Floresco et al. 2001, 2003; Legault and Wise 2001). Other work has established a role of hippocampal influence on accumbens dopamine release during novelty processing (Legault et al. 2000; Legault and Wise 2001; Goto and Grace 2005) and in the dopamine-dependent enhancement of long-term potentiation after exposure to novelty (Li et al. 2003). However, the circuit mechanisms and temporal dynamics underlying these effects have not been shown. Our findings extend prior research by demonstrating that baseline tone in the VTA is directly predicted by transient activation of the HPC during behavior.
Interestingly, we did not observe a relationship between dlPFC activation and baseline VTA variability for either of our behavioral conditions (i.e., target detection or novelty). Previous studies have demonstrated a relationship to sustained dopamine release only in anesthetized rodents (Karreman and Moghaddam 1996; Kulagina et al. 2001), leaving open the question of whether these mechanisms are evident during awake behavior. Dual-electrode recordings have shown that VTA responses are sensitive to the specific anatomical location and pattern of activity in the PFC (Lodge 2011). Although negative findings must be interpreted with caution, our observation may provide initial bounds on the behavioral contexts in which dlPFC activation regulates tonic VTA activation. Future studies are needed to investigate the dynamics of this system in behavioral contexts more likely to evoke prolonged regulation of the VTA by the PFC, such as cue-evoked target detection (Fiorillo et al. 2003; Totah et al. 2013) or the anticipation of distant rewards (Howe et al. 2013).
In contrast to baseline variability, transient signaling in the VTA did show an association with dlPFC during goal-directed behavior. Specifically, we found that on target trials, event-evoked dlPFC signals predicted event-evoked VTA responses. Prior research has demonstrated relationships between PFC activation and transient responses in the VTA during reward motivation in humans (Ballard et al. 2011), during goal-directed behaviors in awake rodents (Parker et al. 2011; Takahashi et al. 2011), and in anesthetized animals (Gariano and Groves 1988; Svensson and Tung 1989; Gao et al. 2007). Our current findings concord with and extend previous research by demonstrating that dlPFC activation is predictive of VTA event-evoked activity during goal-motivated behavior, even in the absence of reward. Importantly, our findings that novelty signals from the dlPFC were not predictive of baseline tone in the VTA also dovetail with the rodent literature that PFC lesions do not disrupt HPC-dependent tonic dopamine signaling (Floresco et al. 2001). Thus, our findings confirm predictions from prior physiological evidence that goal-directed phasic signaling in the VTA is driven by regions like the PFC that supply excitatory inputs, while novelty-evoked tonic signaling is driven by structures like the HPC which modulate VTA via inhibitory inputs. Given the spatial and temporal resolution of human fMRI, however, we could not disambiguate whether the documented VTA–PFC interactions were mediated by direct excitation by the PFC or indirectly via PFC afferents into pedunculopontine tegmental nucleus, which also has excitatory projections into the VTA [reviewed in Sesack and Carr (2002)].
Our current findings contrast with prior reports showing event-related HPC–VTA interactions during presentation of novel stimuli, where novelty signaled behavioral responses to gain potential reward (Adcock et al. 2006; Wittmann et al. 2007; Krebs et al. 2009, 2011). However, since novelty and goal relevance were confounded in all of these studies, it is impossible to rule out an intervening role of the dlPFC. Indeed, all of the above-discussed human studies also report dlPFC activation. The current study, which dissociates novelty and goal relevance, does not find an event-to-event relationship between HPC and VTA. Based on this, as well as evidence discussed previously that dlPFC mediates goal-relevant VTA phasic firing, we hypothesize that the HPC–VTA relationship observed in previous studies is mediated by the dlPFC, rather than by disinhibitory relays from the HPC to the VTA through the accumbens and pallidum. Specifically, when novelty co-occurs with goal-directed behavior, the dlPFC may initiate functional coupling between the VTA and HPC in response to salient events. Although further work is needed to fully test this hypothesis, prior work from our laboratory has shown that interactions between the PFC and VTA in response to goal-relevant reward cues predict HPC responses to novel expectancy violations later in that trial (Murty and Adcock 2014). Thus, although we did not find evidence that slow timescale HPC–VTA coupling depends on behavioral context, we propose that event-related HPC–VTA coupling may emerge in the context of during goal-directed behavior.
The relationship between task goals and novelty also offers an account of an unpredicted result in our finding of a negative, rather than null, relationship between event-related hippocampal and VTA responses. In the current task, participants were required to withhold responses to nontarget stimuli and only respond to a highly familiar repeated stimulus. Thus, during the presentation of target stimuli, we would predict low HPC novelty responses but high goal-related VTA responses. In instrumental learning tasks, cues indicating the omission of reward and the need to withhold responses elicit a short-latency inhibition of dopamine neurons (Tobler et al. 2003), which here would occur during all nontarget trial-unique stimuli. Either or both of these disjunctions would result in the negative relationship we demonstrate. Future research manipulating the relation between novelty and other salient contexts with behavioral response requirements will be necessary to fully understand this relationship.
Our methodological innovation of modeling how transient events predict signaling at different timescales based on the specific physiology of connections allowed us to detect effects on slow, drifting changes in VTA activation. Although fMRI does not measure dopamine, we indirectly tested mesolimbic function by investigating neurophysiological properties of the neural regions underlying this system. The results are consistent with physiological findings of dissociable influences on tonic and phasic dopamine neuron activity. Several limitations of our methods should be noted. First, fMRI does not permit us to attribute these activations to specific neuronal populations in the VTA. Similar patterns of activation could arise from nondopaminergic (e.g., GABAergic) populations within the VTA. Second, our methodology did not allow us to establish the directionality of relationships between regions for the transient activations (relevant to the PFC–VTA relationship); however, the baseline VTA activation was predicted by prior event history in the HPC, which requires HPC to VTA directionality. Finally, dopamine neurons are functionally heterogeneous and exhibit multiple firing profiles beyond tonic and phasic signaling, including gradual ramping (Fiorillo et al. 2003; Howe et al. 2013; Totah et al. 2013). Future studies using pharmacological manipulations in humans, multisite recordings and/or voltammetry in behaving animals, and more detailed physiological models will be needed to dissociate dopamine neuron firing patterns from each other and to firmly link dopamine neuron firing patterns to their afferent inputs and behavioral state.
Our findings provide an important bridge between the wealth of rodent studies detailing physiological properties of this network and human cognitive neuroscience studies identifying roles for the dlPFC and HPC in behavior. As reviewed above, phasic VTA responses are critical for initiating, refining, and executing specific goal-relevant behaviors in response to current environmental demands. The dlPFC is well suited to mediate phasic responses in these contexts, given its proposed role in the dynamic maintenance of current task demands in service of goal-directed behavior (Cohen et al. 2002; Wallis and Miller 2003; Cole et al. 2013; Sreenivasan et al. 2014). Conversely, tonic VTA responses are critical for modulating resource allocation and neural sensitivity over extended periods of time, for example when animals explore and learn about new environments. The HPC is well suited to initiate and maintain these types of state-dependent responses, given its proposed role in detecting contextual shifts (Ranganath and Rainer 2003; Kumaran and Maguire 2009), bridging events over time (Staresina and Davachi 2009; Hales and Brewer 2010; DuBrow and Davachi 2014), and maintaining temporal contexts (Ezzyat and Davachi 2014; Hsieh et al. 2014). Thus, the functional neuroanatomical framework supporting VTA signaling may have evolved to reflect the influence of—and to optimize support for—the computations being performed in these regions. The current results, however, only describe how engagement of unique afferent systems influences VTA activation. Interestingly, prior work in animals has demonstrated that sustained and transient engagement of VTA can differentially influence firing properties and plasticity in VTA efferent systems (i.e., outputs), including dlPFC and ventral striatum (Dreher and Burnod 2002; Goto and Grace 2005; Goto et al. 2007; Hauber 2010). Our work provides a foundation for future investigations of how dlPFC versus HPC modulation of VTA differentially impacts neurophysiology in downstream regions, and thus behavior.
The selective regulation of different components of VTA signaling, and of baseline variability in particular, invites further consideration of the nature of dopamine signaling and modulatory effects. Computational models have suggested that accumulations from prior phasic dopamine events were sufficient to explain tonic dopamine's proposed role in behavior (Niv et al. 2007). However, here, as in prior in vivo anesthetized rodent stimulation (Floresco et al. 2001, 2003), baseline activity in the VTA appears to be actively and independently modulated. Our observation that event-evoked and baseline VTA signaling arise from distinct sources provides additional indirect evidence that tonic dopamine is not just residue from phasic events. Our findings support a model in which tonic signaling in the VTA emerges from context-specific, hippocampal-dependent mechanisms (Grace et al. 2007). We view this context-dependent modulation of tonic dopamine activation as an especially adaptive arrangement for hippocampal function. Although dopamine from prior phasic events could enhance memory for isolated events via acute changes in consolidation-related mechanisms (Frey and Frey 2008; Takeuchi et al. 2014), we propose that actively regulated, slow modulations of VTA signal may also directly influence memory formation [see also Shohamy and Adcock (2010)].
In conclusion, the present study demonstrates separable information streams in human VTA signaling during behavior. Critically, these results were evident only when knowledge of midbrain dopamine responses to afferent networks was incorporated into the analytical models and predictions. The findings reveal an active, behaviorally responsive modulation of mesolimbic circuits over multiple timescales, providing a richer methodological and conceptual framework for understanding how signaling in the VTA and other neuromodulatory nuclei simultaneously supports manifold aspects of adaptive behavior.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This project was supported by the National Institutes of Health (grants R01 DA027802 and R01 MH094743), the Alfred P Sloan Foundation, and the Dana Foundation.
Supplementary Material
Notes
We thank R.M. Carter and A. Tompary for helpful discussion. Conflict of Interest: None declared.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. 2006. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 50:507–517. [DOI] [PubMed] [Google Scholar]
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. 2011. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 31:10340–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. 2009. Dissecting components of reward: “liking”, “wanting”, and learning. Curr Opin Pharmacol. 9:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Cheer JF. 2014. Local control of striatal dopamine release. Front Behav Neurosci. 8:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. 2002. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 12:223–229. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. 2013. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. 1999. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski PA, Maia TV, Boschen SL, Bortolanza M, Wendler E, Schwarting RKW, Brandão ML, Winn P, Blaha CD, Da Cunha C. 2013. Evidence that conditioned avoidance responses are reinforced by positive prediction errors signaled by tonic striatal dopamine. Behav Brain Res. 241:112–119. [DOI] [PubMed] [Google Scholar]
- Dreher J-C, Burnod Y. 2002. An integrative theory of the phasic and tonic modes of dopamine modulation in the prefrontal cortex. Neural Netw Off J Int Neural Netw Soc. 15:583–602. [DOI] [PubMed] [Google Scholar]
- DuBrow S, Davachi L. 2014. Temporal memory is shaped by encoding stability and intervening item reactivation. J Neurosci Off J Soc Neurosci. 34:13998–14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. 2014. Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 81:1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. 2003. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 299:1898–1902. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. 2001. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci Off J Soc Neurosci. 21:4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. 2003. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 6:968–973. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'reilly RC. 2004. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 306:1940–1943. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Laruelle M, Haber SN. 2006. Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 31:1627–1636. [DOI] [PubMed] [Google Scholar]
- Frey S, Frey JU. 2008. “Synaptic tagging” and “cross-tagging” and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog Brain Res. 169:117–143. [DOI] [PubMed] [Google Scholar]
- Gao M, Liu C-L, Yang S, Jin G-Z, Bunney BS, Shi W-X. 2007. Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J Neurosci Off J Soc Neurosci. 27:5414–5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W-J, Goldman-Rakic PS. 2003. Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc Natl Acad Sci USA. 100:2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF, Groves PM. 1988. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 462:194–198. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. 2005. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 47:255–266. [DOI] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. 2007. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 53:583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. 2007. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30:220–227. [DOI] [PubMed] [Google Scholar]
- Hales JB, Brewer JB. 2010. Activity in the hippocampus and neocortical working memory regions predicts successful associative memory for temporally discontiguous events. Neuropsychologia. 48:3351–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W. 2010. Dopamine release in the prefrontal cortex and striatum: temporal and behavioural aspects. Pharmacopsychiatry. 43(Suppl 1):S32–S41. [DOI] [PubMed] [Google Scholar]
- Howe MW, Tierney PL, Sandberg SG, Phillips PEM, Graybiel AM. 2013. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 500:575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L-T, Gruber MJ, Jenkins LJ, Ranganath C. 2014. Hippocampal activity patterns carry information about objects in temporal context. Neuron. 81:1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, McCarthy G. 2001. The effects of single-trial averaging upon the spatial extent of fMRI activation. Neuroreport. 12:2411–2416. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 29:565–598. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Stuber GD. 2014. Tools for resolving functional activity and connectivity within intact neural circuits. Curr Biol. 24:R41–R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Lee J, Mizumori SJY. 2013. Effects of prefrontal cortical inactivation on neural activity in the ventral tegmental area. J Neurosci Off J Soc Neurosci. 33:8159–8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. 1996. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 66:589–598. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Heipertz D, Schuetze H, Duzel E. 2011. Novelty increases the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: evidence from high-resolution fMRI. NeuroImage. 58:647–655. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Schütze H, Düzel E. 2009. The novelty exploration bonus and its attentional modulation. Neuropsychologia. 47:2272–2281. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Zigmond MJ, Michael AC. 2001. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 102:121–128. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. 2009. Novelty signals: a window into hippocampal information processing. Trends Cogn Sci. 13:47–54. [DOI] [PubMed] [Google Scholar]
- Legault M, Rompré PP, Wise RA. 2000. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci Off J Soc Neurosci. 20:1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Wise RA. 2001. Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur J Neurosci. 13:819–828. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. 2000. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 133:23–32. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. 2003. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 6:526–531. [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E. 2011. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 34:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ. 2011. The medial prefrontal and orbitofrontal cortices differentially regulate dopamine system function. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 36:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. 2011. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 333:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. 2012. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. NeuroImage. 59:2636–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Adcock RA. 2014. Enriched encoding: reward motivation organizes cortical networks for hippocampal detection of unexpected events. Cereb Cortex. 24:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Ballard IC, Macduffie KE, Krebs RM, Adcock RA. 2013. Hippocampal networks habituate as novelty accumulates. Learn Mem. 20:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA. 2014. Resting state networks distinguish human ventral tegmental area from substantia nigra. NeuroImage. 100:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. 2007. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl). 191:507–520. [DOI] [PubMed] [Google Scholar]
- Niv Y, Duff MO, Dayan P. 2005. Dopamine, uncertainty and TD learning. Behav Brain Funct. 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Beutler LR, Palmiter RD. 2011. The contribution of NMDA receptor signaling in the corticobasal ganglia reward network to appetitive Pavlovian learning. J Neurosci Off J Soc Neurosci. 31:11362–11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MH, Bizup BT, Grace AA. 2013. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci Off J Soc Neurosci. 33:16865–16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. 2013. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 17:230–240. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. 2003. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 4:193–202. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. 2006. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 7:967–975. [DOI] [PubMed] [Google Scholar]
- Roeper J. 2013. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 36:336–342. [DOI] [PubMed] [Google Scholar]
- Savine AC, Braver TS. 2010. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J Neurosci Off J Soc Neurosci. 30:10294–10305. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schultz W. 2010. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Romo R. 1990. Dopamine neurons of the monkey midbrain: contingencies of responses to stimuli eliciting immediate behavioral reactions. J Neurophysiol. 63:607–624. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. 2002. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 77:513–517. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. 1992. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 320:145–160. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. 2010. Dopamine and adaptive memory. Trends Cogn Sci. 14:464–472. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Sreenivasan KK, Curtis CE, D'Esposito M. 2014. Revisiting the role of persistent neural activity during working memory. Trends Cogn Sci. 18:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. 2009. Mind the gap: binding experiences across space and time in the human hippocampus. Neuron. 63:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson TH, Tung CS. 1989. Local cooling of pre-frontal cortex induces pacemaker-like firing of dopamine neurons in rat ventral tegmental area in vivo. Acta Physiol Scand. 136:135–136. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Wilson RC, Toreson K, O'Donnell P, Niv Y, Schoenbaum G. 2011. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nat Neurosci. 14:1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Morris RGM. 2014. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos Trans R Soc Lond B Biol Sci. 369:20130288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. 2003. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci Off J Soc Neurosci. 23:10402–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NKB, Kim Y, Moghaddam B. 2013. Distinct prestimulus and poststimulus activation of VTA neurons correlates with stimulus detection. J Neurophysiol. 110:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. 2003. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 3:255–274. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. 2003. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 18:2069–2081. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PEM. 2009. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2:195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. 2004. Dopamine, learning and motivation. Nat Rev Neurosci. 5:483–494. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan RJ, Düzel E. 2007. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. NeuroImage. 38:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA et al. 2009. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA. 106:7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.