Abstract
Bisphenol A (BPA, 2,2-bis(4-hydroxyphenyl) propane) is a widely used industrial chemical. The extensive distribution of BPA in the environment poses risks to humans. However, the molecular mechanisms underlying BPA toxicity as well as its effective detoxification and elimination are not well understood. We have investigated specifically for BPA the notion raised in the literature that the optimal sensing, detoxification, and elimination of xenobiotics requires retinoid (natural derivatives and synthetic analogs of vitamin A) actions. The objective of the study was to explore how retinoids, both those stored in the liver and those originating from recent oral intake, help maintain an optimal xenobiotic detoxification response, affecting mRNA expression and activities of elements of xenobiotic detoxification system upon BPA administration to mice. Wild-type and mice lacking hepatic retinoid stores (Lrat−/−) were acutely treated with BPA (50 mg/kg body weight), with or without oral supplementation with retinyl acetate. Hepatic mRNA expression levels of the genes encoding nuclear receptors and their downstream targets involved in xenobiotic biotransformation, phase I and phase II enzyme activities, and levels of oxidative damage to cellular proteins and lipids in hepatic microsomes, mitochondria and cytosol, were assessed. BPA treatment induced hepatic activities needed for its detoxification and elimination in wild-type mice. However, BPA failed to induce these activities in the livers of Lrat−/− mice. Oral supplementation with retinyl acetate restored phase I and phase II enzyme activities, but accelerated BPA-induced oxidative damage through enhancement of non-mitochondrial ROS production. Thus, the activities of the enzymes involved in the hepatic elimination of BPA require hepatic retinoid stores. The extent of hepatic damage that arises from acute BPA intoxication is directly affected by retinoid administration during the period of BPA exposure and hepatic retinoid stores that have accumulated over the lifetime of the organism.
Keywords: vitamin A, retinoic acid, retinyl esters, hepatic stellate cells, cytochrome P450, xenobiotics, oxidative stress.
INTRODUCTION
Bisphenol A (BPA, 2,2-bis(4-hydroxyphenyl) propane) is an industrial chemical widely used in the manufacture of polycarbonate plastic and epoxy resins that are applied as protective coatings on food and beverage cans, as dental sealants, and as additives to other plastics (Rubin, 2011). The broad use of BPA in food-packaging and in dentistry raises concern regarding the extensive human exposure to this xenobiotic (Rubin, 2011). Due to its structural similarity to 17β-estradiol and ability to bind estrogen receptors, BPA possesses estrogenic activity (Hiroi et al., 1999). Recent evidence indicates that BPA also influences other endocrine-related pathways acting as an endocrine disruptor (Vandenberg et al., 2009). Although BPA's xenoestrogenic activity is well characterized (Richter et al., 2007) and its hazards to humans are grave (Rochester, 2013), studies are still needed to understand fully the adverse health effects of BPA exposure and to understand the multiple pathways underlying BPA toxicity.
The liver is the major site for BPA metabolism, where the majority of this xenobiotic undergoes biotransformation leading to its subsequent excretion as inert chemical species (Inoue et al., 2001; Snyder et al., 2000). The metabolism of BPA has been well characterized in vivo in different murine models as well as in vitro in models employing human cell lines and hepatic subcellular fractions. Most hepatic BPA biotransformation is initiated in the endoplasmic reticulum and involves the formation of glucuronide conjugates (Fay et al., 2015; Hanioka et al., 2008; Pottenger et al., 2000; Pritchett et al., 2002; Yokota et al., 1999). As a glucuronide conjugate, BPA possesses negligible estrogenic activity and this enables its further excretion, primarily into the bile (Inoue et al., 2001). The presence of BPA-sulfates (Elsby et al., 2001; Pottenger et al., 2000) and BPA–glutathione conjugates (Jaeg et al., 2004; Schmidt et al., 2013) in vivo and in vitro indicates that other transferases (i.e. sulfotransferases, glutathione-S-transferases [GSTs]) are also involved in BPA conjugation. Moreover, GSTs have been shown to conjugate BPA oxidative cleavage products, strongly suggesting a role for GSTs in BPA metabolite elimination (Jaeg et al., 2004).
Unconjugated BPA can also undergo cytochrome P450 (CYP)-mediated oxidation, resulting in the formation of several stable hydroxylated species, including 5-hydroxy-BPA (5-OHBPA) (Elsby et al., 2001), 3-hydroxy-BPA (3-OHBPA), and 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP) (Okuda et al., 2011). CYP-mediated modifications of BPA may give rise to the formation of unstable deleterious reactive intermediates (Atkinson and Roy, 1995a; Atkinson and Roy, 1995b) and radical fragments (Babu et al., 2013; Okuda et al., 2011; Schmidt et al., 2013) leading to oxidative damage and cellular dysfunction. Indeed, many reports have established that BPA administration in vitro and in vivo is accompanied with an induction of reactive oxygen species (ROS) production underlying the development of oxidative stress (Babu et al., 2013; Bindhumol et al., 2003; Hassan et al., 2012; Kabuto et al., 2003; Ooe et al., 2005; Sakuma et al., 2010; Xin et al., 2014).
A significant literature supports the notion that BPA metabolism is inducible (Quesnot et al., 2014), involving transcriptional control of BPA-metabolizing enzymes following direct interaction of BPA with, and activation of ligand-dependent nuclear receptors (Delfosse et al., 2014; Li et al., 2015; Sui et al., 2012; Takeshita et al., 2001). There is a large body of published data suggesting that the optimal functioning of the xenobiotic detoxification system requires the actions of retinoids (vitamin A, its metabolites and synthetic analogs) for optimal sensing, detoxification and elimination of xenobiotics (reviewed in Shmarakov, 2015). These responses to xenobiotic exposure involve the actions of the retinoic acid nuclear receptors (both the retinoic acid receptors (RARs) and the retinoid X receptors [RXRs]) in regulating gene expression. RXRs are common heterodimerization partners for many xenobiotic receptors, including human orphan steroid and xenobiotic receptor (SXR) or its rodent ortholog pregnane X receptor (PXR), constitutive androstane receptor (CAR), and peroxisome proliferator-activated receptors (PPARs) (Aleksunes and Klaassen, 2012; Woods et al., 2007). For nuclear factor erythroid 2-related factor 2 (NRF2), a transcription factor that is activated in response to electrophiles and oxidative stress, RXRα has been shown to inhibit the transcriptional activity of this oxidative stress sensor through physical interaction between the two factors even in the absence of ligand (Wang et al., 2013). Likewise, formation of the RARα–NRF2 complex has been reported to suppress the expression of NRF2-target antioxidant genes (Wang et al., 2007). This enables the RXRs and RARs to function as central regulators of target genes encoding proteins involved in xenobiotic metabolism (Wan et al., 2000; Wu et al., 2004).
The present study was undertaken to establish the extent to which hepatic retinoids are required to allow for the induction and optimal function of the hepatic xenobiotic detoxification system upon BPA administration to mice. To address this question, we employed a genetic mouse model of hepatic retinoid-insufficiency where the gene encoding lecithin:retinol acyltransferase (Lrat) was ablated.
MATERIALS AND METHODS
Animal Husbandry and Dietary Regimens
All mice employed in our studies (males weighing 20–25 g, 10–12 weeks of age) were treated and maintained according to the NIH Guide for the Care and Use of Laboratory Animals (National Research Council Committee for the Update of the Guide for the Care et al., 2011). All experimental procedures were reviewed and approved by the Chernivtsi University Institutional Animal Care and Use Committee. The Lrat−/− mice were derived from ones originally described on a mixed genetic background through 10 backcrosses into the C57BL/6J genetic background, rendering the mice employed in our studies congenic in this genetic background. During the breeding and lactation periods, mice were maintained on breeder chow that contained 15 IU retinol/g diet. After weaning, mice were maintained on a standard chow diet that also contained 15 IU retinol/g diet.
BPA, dissolved in corn oil (used as a vehicle), was administered per os daily for 3 days at a dose of 50 mg/kg body weight, corresponding to the Lowest Observable Adverse Effect Level (LOAEL) (Shelby, 2008). Control-treated mice received the same volume of vehicle per os. Routinely, 6 mice of each genotype were studied per group.
For retinoid-supplementation studies, separate groups of age-matched mice (6 for each genotype) received 3000 IU of retinyl acetate in corn oil by gavage at 12 h intervals immediately after either BPA or vehicle administration. At the time of sacrifice, 24 h after BPA administration, mice were weighed, blood was taken from the inferior vena cava, and the liver was immediately removed. The dissected livers were rapidly weighed and either used immediately for subcellular fraction isolations or frozen in liquid N2 and stored at –80 °C for other analyses. Tissues were stored continuously without thaw at −80 °C until analysis.
RNA Preparation and Quantitative Real-Time PCR
Total RNA was extracted from minced liver employing TRIzol Reagent (Ambion) and isolated using the E.Z.N.A Total RNA Kit II (Omega Bio-tek) according to the protocol of the manufacturer. RNA was quantitated at 260 nm using a Nanodrop spectrophotometer. cDNA synthesis was performed using 2 μg of total RNA (in a final volume of 20 μl) and was carried out for 10 min at 25 °C followed by 120 min at 37 °C employing reverse transcriptase (Applied Biosystems). The reaction was stopped at 85 °C for 5 min, using a thermal cycler (Eppendorf). The primers employed for quantitative real-time PCR (qRT-PCR) analyses of target gene expression are provided in Supplementary Table 1. As the reference housekeeping gene used to normalize mRNA expression, we employed 18S RNA. This gene gave excellent reproducibility, never varying in its Ct value by more than 0.5 units. qRT-PCR was performed in a total volume of 20 µl, including 40 ng of cDNA template, forward and reverse primers (100 nM each), and LightCycler 480 SYBR Green I Master (Roche) using a LightCycler 480 instrument (Roche). After initial enzyme activation (95 °C for 10 min), 40 cycles (94 °C for 10 s, 55 °C for 30 s, 72 °C for 30 s) were performed for the annealing/extension steps, and fluorescence was measured. A dissociation curve program was performed after each cycle. Expression of target genes was calculated based on the efficiency of each reaction and the crossing point deviation of each sample versus a control and expressed as fold difference in comparison with the 18S rRNA reference gene.
Preparation of Hepatic Microsomes, Mitochondria, Cytosol
Hepatic microsomes were prepared according to a non-ultracentrifugal, calcium-precipitation procedure exactly as described in (Hamilton et al., 1999). The remaining supernatant after the last centrifugation following the calcium precipitation step was considered to be the cytosolic (post-microsomal) fraction. Functional hepatic mitochondria were isolated according to a standard procedure described by Frezza et al. (2007).
The purity of each cellular fraction was assessed using known marker enzymes for subcellular organelles, as described in Archakov et al. (1973). Specifically, we assessed succinate dehydrogenase (a marker for the inner mitochondrial membrane), glucose-6-phosphatase (a marker for the endoplasmic reticulum) and Na+/K+-ATPase (a marker for the plasma membrane) activities. For all of our studies, we only employed preparations in which contaminating marker enzyme assays did not exceed 10% of the total activity measured in the crude liver homogenate used for fraction isolation. Aliquots of mitochondria, microsomes and cytosol were stored at −80°C until use. The protein content of each subcellular fraction was determined using the Bradford method employing bovine serum albumin as a standard (Bradford, 1976).
Microsomal Monooxygenase Activities
The aniline p-hydroxylase activity of hepatic cytochrome P450s was determined by the method of Archakov et al. (1974). The reaction mixture consisted of 40 mМ Tris-НСl buffer (pH 7.3), containing 16 mМ MgCl2, 3 mМ NADPH and 2 mg of microsomal protein in a total incubation volume 1 ml. The reaction was initiated by adding aniline to a final concentration 3 mМ. In control samples, NADPH was added after the termination of the reaction. Test and control samples were incubated at 37°C for 20 min with constant shaking. The reaction was terminated through addition of 15% trichloroeacetic acid, followed by centrifugation at 3500 × g for 10 min. Following centrifugation, 1 ml of 10% (w/v) Na2CO3 and 2% (w/v) phenol in 0.2 M NaOH was added to the supernatant. The samples were incubated in a water bath at 37°C for 30 min. To assess enzymatic activity, the absorbance was determined spectrophotometrically at 630 nm using a molar extinction coefficient for p-aminophenol of 13.3 mM − 1cm − 1. This and all subsequent absorbance measurements were performed using an Agilent Cary 60 UV–Vis spectrophotometer (Agilent Technologies, Santa Clara, CA). Aniline p-hydroxylase specific activity is reported as nmol/min/mg microsomal protein.
To assess the N-demethylase activity of hepatic cytochrome P450s, a reaction mixture consisting of 40 mM Tris-HCl buffer (pH 7.6), containing 3 mM NADPH, 16 mМ MgCl2, and 1.5 mg microsomal protein in a total volume 1 ml was employed. The reaction was initiated by addition of N,N-dimethylaniline to a final concentration 6 mМ. Test and control (no NADPH added) samples were incubated at 37°C for 30 min with vigorous shaking. The reaction was terminated by addition of an equal volume of 25% (w/v) ZnSO4 in a saturated Ba(OH)2, followed by centrifugation at 3500 × g for 10 min. The formaldehyde content in the supernatant was determined employing the Nash color reaction (Nash, 1953). Color intensity was determined spectrophotometrically at 412 nm. The activity was calculated using a formaldehyde molar extinction coefficient of 1.5 mM − 1cm − 1 and expressed as nmol/min/mg microsomal protein.
Flavin-containing mono-oxygenase (FMO) activity was determined by the method of Ziegler and Pettit (Pettit et al., 1964) with some modifications. One milliliter of the reaction mixture consisted of 40 mM Tris-HCl (pH 7.6), containing 3 mM NADPH, 16 mМ MgCl2 and 1.5 mg microsomal protein. The reaction was initiated by the addition of N,N-dimethylaniline to a final concentration 6 mМ. The test and control (no NADPH added) samples were incubated at 37°C for 30 min with vigorous shaking. The reaction was terminated by addition of 0.9 M HClO4. Precipitated protein was pelleted by centrifugation at 3500 × g for 10 min. The clarified supernatants were then transferred to graduated test tubes and pHs were adjusted to 9.4 through addition of 1 M NaOH. To eliminate unoxidized dimethylaniline, the samples were extracted three-times with diethyl ether, each after vigorous shaking for 2 min. After the third extraction the samples were left open for 20 min to allow for evaporation of the diethyl ether. After the ether had evaporated, the pH was adjusted to 2.4 through the addition of several microliters of 5% trichloroeacetic acid. Subsequently, 0.2 ml of 0.1 M NaNO2 was added, followed by adjustment of the final volume to 3 ml through addition of citrate buffer (pH 2.4). For color development, the tubes were placed in a water bath at 60°C for 5 min. The absorbance was measured at 420 nm. To calculate enzyme specific activity, a molar extinction coefficient for p-nitroso-N,N-dimethylaniline of 8.2 mM − 1cm − 1 was employed.
Xanthine Oxidase Activity
Xanthine oxidoreductase (XO) activity was determined by measuring uric acid production from xanthine in the absence of NAD+ (Shmarakov and Marchenko, 2008). The reaction mixture contained 0.1 ml of the cytosolic fraction (2 mg of protein) and 0.7 ml of a buffer, containing 4 mM xanthine in 0.01 M NаОН, 0.2 ml of 0.1 M Tris-НСl (pH 8.2), 2 mM EDTA and 1 mM phenylmethylsulfonyl fluoride (PMSF). After 30 min incubation at 38°С the level of uric acid formed was measured using a kit from Felisit Diagnostics (Kiev, Ukraine), according to the instructions from the manufacturer. Xanthine oxidoreductase activity was expressed as nmol/min/mg cytosolic protein.
Transferase Activities
The cytosolic and microsomal GST activity was assessed by standard colorimetric assay using 1-chloro-2,4-dinitrobenzene (CDNB) as an electrophilic substrate (dissolved in ethanol) (Habig et al., 1974). The reaction mixture (total volume of 2.0 ml) contained 0.1 ml of cytosol or microsomes (1–2 mg of protein), 0.2 ml of 1 mM reduced glutathione, 20 µl of 0.1 M CDNB and 1.68 ml of 0.1 M phosphate buffer (pH 6.5). The absorbance of the rising product S-(2,4-dinitrophenyl) glutathione was detected at 340 nm. GST activity was expressed as nmol/min/mg either cytosolic or microsomal protein.
Microsomal UDP-glucuronosyl transferase activity was assayed by the method of Burchell and Weatherill (1981). The reaction mixture (total volume of 0.25 ml) consisted of microsomal suspension (equal to 0.4 mg microsomal protein), 1 mM UDP-glucuronic acid (UDPGA), 0.4 mM p-nitrophenol, 5 mM MgCl2, and 20 mM phosphate buffer (pH 7.5). Following 20 min incubation at 25°C, the reaction was stopped by immersing the mixture in boiling water for 2 min, followed by centrifugation at 12,000 × g for 5 min. The supernatant solution was diluted 10-times with 20 mM phosphate buffer (pH 7.5) and the absorbance was measured at 405 nm to assess the reduction in color caused by the formation of p-nitrophenol glucuronide. The microsomal UDP-glucuronosyl transferase activity was expressed as nmol/min/mg of microsomal protein.
Measurement of O2· and NO· Production
production by cellular fractions was determined by colorimetric nitroblue tetrazolium (NBT) assay (Auclair and Voisin, 1985). The reaction mixture contained 1 ml of 50 mM phosphate buffer (pH 7.4), 0.1 ml of a cellular fraction (equal to 1 mg of microsomal protein or 2 mg of mitochondrial protein), and either 0.2 mM NADPH (for microsomes) or 45 mM NADH (for mitochondria). Following pre-incubation of the reaction mixtures at 37°C for 10 min, 0.2% NBT solution (w/v) dissolved in phosphate buffer (pH 7.4) was added and the samples were incubated for an additional 5 min at 37°C. A chloroform:dimethyl sulfoxide (1:2 v/v) mixture was added to each sample, followed by vigorous shaking for 1 min. The samples were centrifuged at 1000 × g for 5 min. The supernatant was collected and the absorbance was measured at 540 nm. To calculate the intensity of formation based on the amount of NBT utilized, a calibration curve was constructed using standard NBT concentrations. Levels of production by cellular fractions were expressed as nmol/min/mg protein.
Nitric oxide (NO·) production was assessed by measuring the level of accumulation of its stable metabolite, nitrite. Nitrite was quantified colorimetrically after its reaction with Griess reagent (Green et al., 1982). The reaction mixture for the NO-synthase assay contained 0.1 M Tris-HCl (pH 7.4), 10 mM EDTA, 32 mM l-arginine and 1 mM NADPH. The reaction was initiated by addition of 2 mg of cytosolic protein. Control samples contained all the reagents, except for NADPH, substituted by doubly distilled water. The samples were incubated for 10 min at 37°C, then 2 M HClO4 was added. Samples were then centrifuged at 1000 × g for 15 min. The supernatant was mixed with an equal volume of Griess reagent (1% sulfanilamide (w/v), 0.1% naphthalene diamine dichloride (w/v), and 2% phosphoric acid (v/v)) and after 10 min at room temperature the absorbance was measured at 540 nm and compared with a standard curve generated with known concentrations of sodium nitrite. Nitric oxide production was expressed as nmol/min/mg of cytosolic protein.
Oxidative Damage Measurement
The degree of oxidative modification of cellular proteins was determined through assessment of the levels of protein carbonylation (Levine et al., 1990) and of protein sulfhydryl groups (Murphy and Kehrer, 1989). Lipid peroxidation in liver cellular fractions was determined by assessing the level of thiobarbituric acid-reactive substances (TBARS) (Ohkawa et al., 1979).
Statistical Analysis
All data are presented as means ± SD. Student’s t test was used to analyze differences between wild-type and Lrat−/− mice. Statistical comparisons involving larger groups were first analyzed by a one-way ANOVA followed by multiple comparisons employing Tukey's HSD post hoc test. P values less than 0.05 were considered to be statistically significant.
RESULTS
The Absence of Endogenous Hepatic Retinoid Stores Does Not Affect Basal mRNA Expression or the Activities of the Elements of the Hepatic Detoxification System
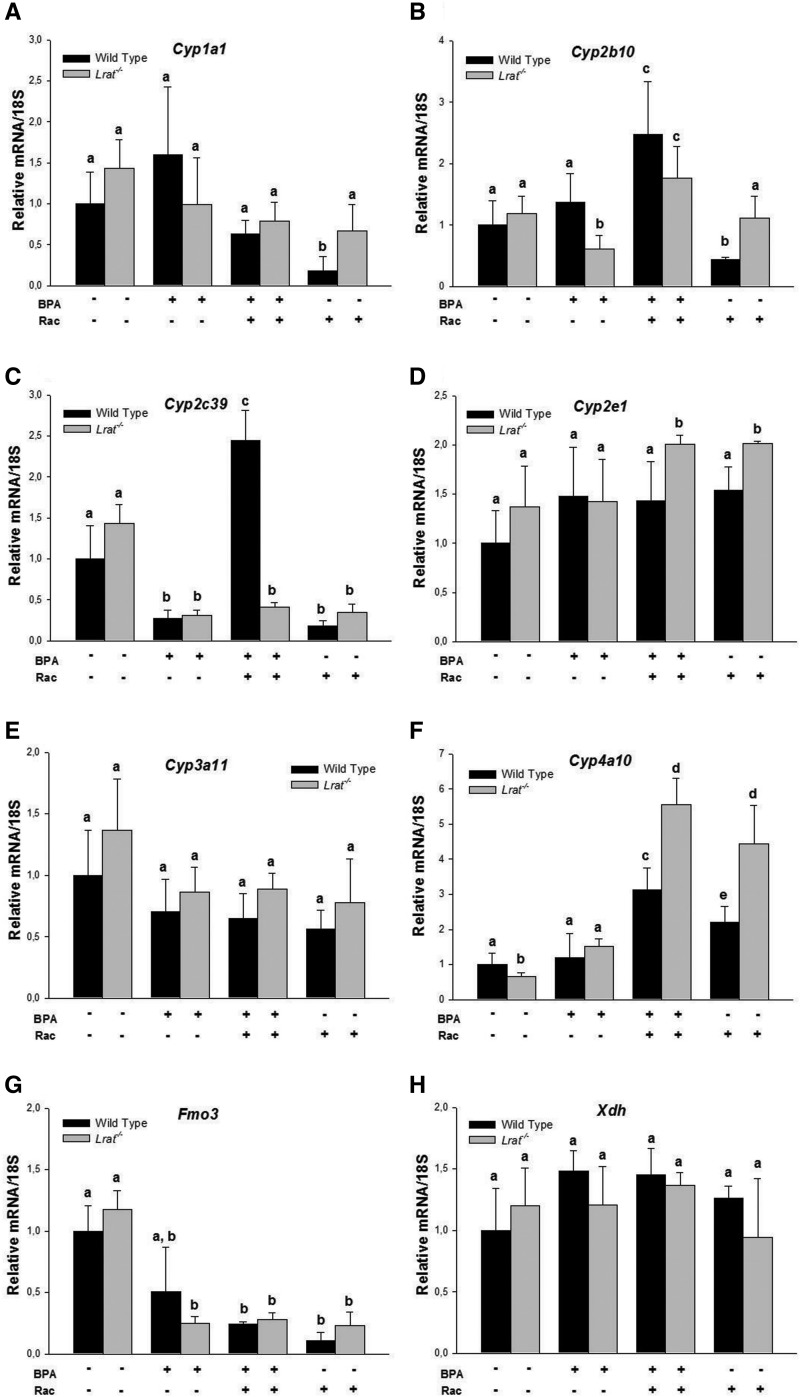
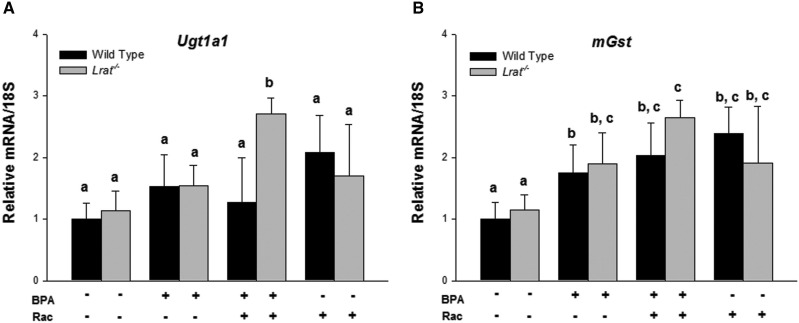
In order to establish whether the absence of endogenous retinoid stores affects the basal state of the hepatic xenobiotic detoxification system in intact animals, we first compared mRNA expression levels of key nuclear receptors important for xenobiotic sensing and their downstream targets in the livers of both wild-type and Lrat−/− mice. The level of specific transcripts for genes encoding constitutive androstane receptor (Car), pregnane X receptor (Pxr), nuclear factor erythroid 2-related factor 2 (Nrf2), PPARs (Pparα, Pparβ/δ and Pparγ), and aryl hydrocarbon receptor (AhR) was not different between untreated wild-type and Lrat−/− livers (Supplementary Fig. 1). mRNA expression of the corresponding phase I downstream targets in the livers of Lrat−/− mice also was not different from that of matched wild-type mice. Furthermore, for untreated wild-type and Lrat−/− mice, we did not observe statistically significant differences in the expression levels of mouse cytochrome P450 isoforms, including the members of 1A (Cyp1a1), 2B (Cyp2b10), 2C (Cyp2c39), 2E (Cyp2e1), and 3A (Cyp3a11) families (Fig. 1A–E) . The only statistically significant difference observed was a lower expression level of Cyp4a11 mRNA in Lrat−/− livers (Fig. 1F). Hepatic flavin-containing monooxygenase 3 (Fmo3) and xanthine dehydrogenase (Xdh) message levels were not different between the two genotypes (Fig. 1G and H). Similarly, expression levels were not different between wild-type and Lrat−/− livers for genes encoding the phase II enzymes UDP-glucuronosyl transferase 1A1 (Ugt1a1) and the microsomal isoform of GST (mGst) (Fig. 2).
FIG. 1.
mRNA expression of Cyp1a1, Cyp2b10, Cyp2c39, Cyp2e1, Cyp3a11, Cyp4a10, Fmo3, and Xdh in livers of wild-type and Lrat−/− mice after bisphenol A and/or retinyl acetate administration. Gene expression values (normalized to 18S rRNA levels) were determined by qRT-PCR for RNA obtained from livers of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 SD, n = 3 for each group.
FIG. 2.
mRNA expression of Ugt1a1 and mGst in livers of wild-type and Lrat−/− mice after bisphenol A and/or retinyl acetate administration. Gene expression values (normalized to 18S rRNA levels) were determined by qRT-PCR for RNA obtained from livers of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Values marked with different letters (a, b, c) are statistically different, P < 0.05. All values are given as the mean ± 1 SD, n = 3 for each group.
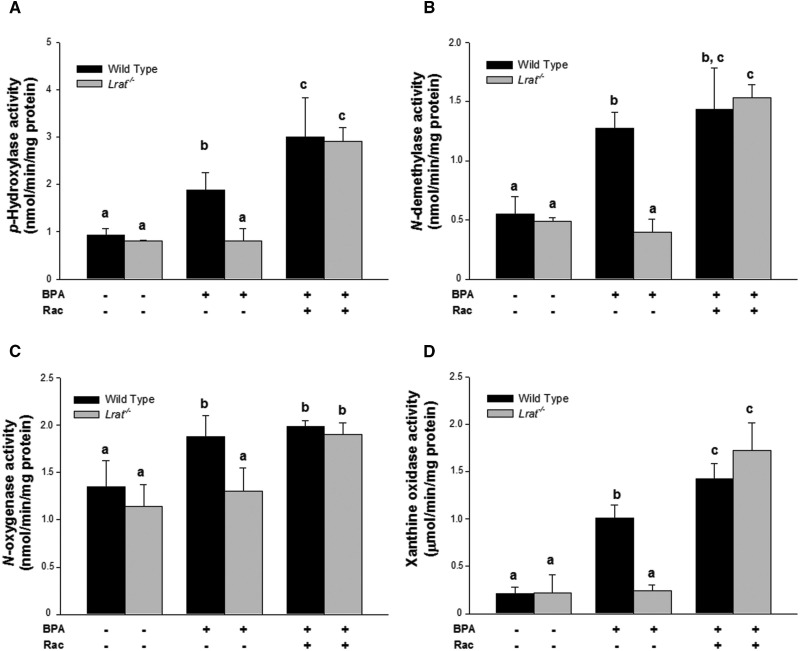
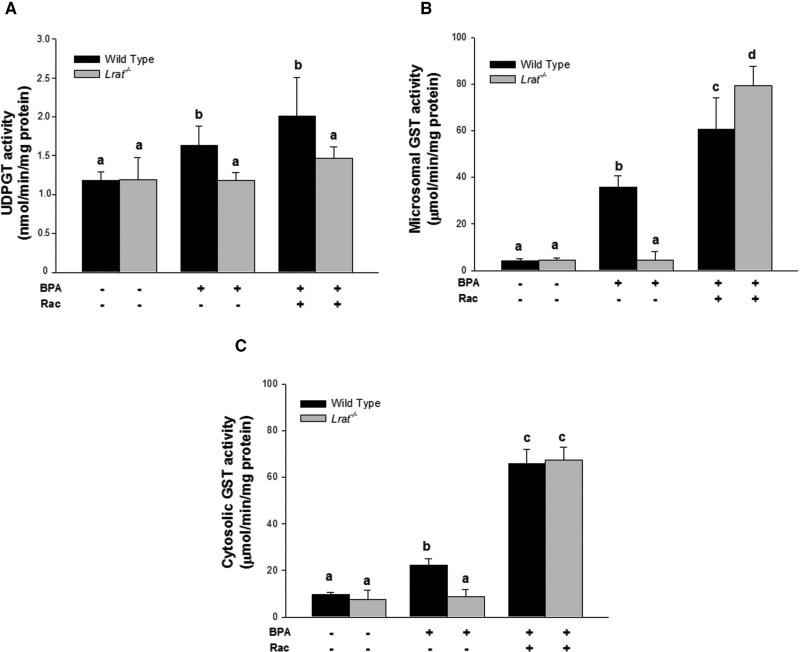
Consistent with our mRNA expression data, the enzyme-specific activities for phase I enzymes (including CYPs, FMOs, and xanthine oxidase), as well as phase II enzymes (such as GSTs and UDP-glucuronosyl transferases) were not affected by the absence of hepatic retinyl esters in Lrat−/− mice. The specific activities measured in hepatic microsomes and cytosol were similar in Lrat−/− mice to those seen in wild-type mice which have normal hepatic retinoid levels (Figs. 3 and 4).
FIG. 3.
Hepatic monooxygenase system and xanthine oxidase activities (phase I enzyme activities) for wild-type and Lrat−/− mice after bisphenol A administration. p-Hydroxylase (Panel A), N-demethylase (Panel B), and N-oxygenase (Panel C) activities were determined for microsomes isolated from livers of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3,000 IU) administration. Xanthine oxidase (Panel D) activity was determined for cytosol isolated from livers of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Values marked with different letters (a, b, c) are statistically different, P < 0.05. All values are given as the mean ± 1 SD, n = 6 for each group.
FIG. 4.
Hepatic transferase activities (phase II enzyme activities) for wild-type and Lrat−/− mice after bisphenol A administration. UDP-glucuronosyl transferase (Panel A) activity was determined for microsomes isolated from livers of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Glutathione S-transferase activities were determined for microsomes (Panel B) and cytosol (Panel C) isolated from livers of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 SD, n = 6 for each group.
BPA Administration Differentially Affects mRNA Expression of the Genes Encoding Xenosensors and Their Target Genes
To get insight into the expression of the genes encoding the nuclear receptors involved in sensing of xenobiotics as well as their downstream targets participating in xenobiotic hepatic biotransformation qRT-PCR was employed to assess mRNA levels of the specific genes following BPA administration with or without supplementation with retinyl acetate (3000 IU) given per os. BPA administration did not significantly affect mRNA levels of the xenosensors we studied, including Car, Pxr, Nrf2, Pparα, Pparβ/δ, and Pparγ (Supplementary Fig. 1B–G). Nor did retinyl acetate administration to BPA-treated mice influence hepatic mRNA levels of these nuclear receptors (Supplementary Fig. 1B–G). The level of specific transcripts for the gene-encoding aryl hydrocarbon receptor (AhR) although was elevated in the livers of mice of both genotypes receiving BPA (Supplementary Fig. 1A). Moreover, the concomitant supplementation of BPA-treated Lrat−/− mice with 3000 IU of retinyl acetate induced a significant elevation of AhR and Pparγ mRNA levels (Supplementary Fig. 1A and G). This elevation was primarily retinoid-dependent in this mouse knockout strain. Treatment of Lrat−/− mice with 3000 IU of retinyl acetate alone led to higher levels of AhR and Pparγ mRNAs compared with wild-type animals (Supplementary Fig. 1A and G).
Among the genes encoding the phase I detoxification enzymes, BPA treatment of wild-type and Lrat−/− mice did not affect mRNA expression levels of Cyp1a1, Cyp2e1, Cyp3a11, Cyp4a10, and Xdh (Fig. 1A, D–F, H). However, this treatment led to significantly lower levels of transcripts encoding Cyp2b10 and Fmo3 in the livers of Lrat−/− mice, whereas in the livers of wild-type BPA-treated mice mRNA levels of these genes remained unchanged (Fig. 1B and G). Cyp2c39 mRNA levels were significantly diminished in the livers of both wild-type and Lrat−/− mice after BPA administration (Fig. 1C).
Retinyl acetate supplementation of BPA-treated mice (both wild-type and Lrat−/−) had no influence on hepatic Cyp1a1, Cyp3a11, Fmo3, and Xdh mRNA levels compared with the message levels of mice receiving BPA alone (Fig. 1A, E, G, and H). Concomitant administration of BPA and 3000 IU of vitamin A resulted in an induction of hepatic Cyp2b10 and Cyp4a10 mRNA expression in both wild-type and Lrat−/− mice (Fig. 1B and F). This treatment was accompanied with Cyp2c39 mRNA elevation in wild type, but not in Lrat−/− livers (Fig. 1C). Conversely, Cyp2e1 mRNA elevation was observed in Lrat−/−, but not in wild-type livers (Fig. 1D).
The observed changes in hepatic Cyp2e1 and Cyp4a10 mRNA levels of BPA-treated mice supplemented with retinyl acetate (Fig. 1D and F) developed as a result of retinoid administration. Treatment of mice with 3000 IU retinyl acetate alone was accompanied by similar inductions of mRNA expression for Cyp2e1 in Lrat−/− mice and Cyp4a10 in both genotypes (Fig. 1D and F). Conversely, higher levels of Cyp2b10 transcripts in the livers of wild-type and Lrat−/− mice (Fig. 1B) as well as Cyp2c39 transcripts in wild-type livers (Fig. 1C) could not be explained as a result of induction caused by either BPA or retinyl acetate alone. These changes must rather represent a result of xenobiotic-nutrient interactions originating from concomitant BPA and retinoid administration.
Among the genes encoding phase II enzymes, a small but significant upregulation in mRNA expression was detected for the mGst gene after BPA treatment of mice of both genotypes (Fig. 2B). The level of mRNA expression of Ugt1a1 remained unchanged upon BPA administration (Fig. 2A). Retinyl acetate treatment of BPA-exposed animals resulted in an upregulation of both Ugt1a1 and mGst transcription, however, only in Lrat−/− mice (Fig. 2).
BPA Administration Induces Phase I and Phase II Detoxification Activities in the Liver in a Retinoid-Dependent Manner
The specific activities of the phase I enzymes, including CYPs, FMOs, and xanthine oxidase were assessed in microsomal and cytosolic fractions of mouse liver after BPA administration (Fig. 3A–D). For BPA-treated wild-type mice, among phase I activities microsomal aniline p-hydroxylase and dimethylaniline N-demethylase activities (corresponding to CYP catalytic activity) were significantly increased by 2-fold compared with mice receiving only vehicle (Fig. 3A and B). N-Oxygenase activity (corresponding to FMO catalytic activity) was also significantly elevated by 1.4-fold.
A similar induction of these activities was not seen for Lrat−/− mice upon BPA administration. The specific activities of the enzymes were the same for wild-type and Lrat−/− mice receiving the vehicle alone (Fig. 3A–D). We observed both lower CYP hydroxylation activity and lower FMO oxygenase activity in microsomes prepared from Lrat−/− livers compared with activities determined for microsomes prepared from wild-type livers after BPA administration (Fig. 3A and B).
To understand how BPA-induced detoxification depends on hepatic retinoid availability, we administered orally 3000 IU retinyl acetate at each of four 12 h intervals to wild-type and Lrat−/− mice after BPA administration. This is a relatively large dose of retinoid given that mice consuming a chow diet would be consuming approximately 45–50 IU per day. Like wild-type mice, supplementation of Lrat−/− mice with 3000 IU of retinyl acetate after BPA administration resulted in a significant elevation of both CYP and FMO activities. CYP-mediated aniline hydroxylation and dimethyl aniline demethylation rates increased 5.8- and 5.3-times, respectively, compared with those of Lrat−/− mice not supplemented with retinyl acetate. FMO-associated N-oxygenase activity also was significantly increased in Lrat−/− mice receiving both retinyl acetate and BPA, however, only by 1.3-fold (Fig. 3C).
For wild-type mice supplemented with 3000 IU of retinyl acetate following BPA administration, we observed a further induction of aniline p-hydroxylation activity (Fig. 3A), whereas other activities remained unchanged from those observed after only BPA administration (Fig. 3B and C). Interestingly, both wild-type and Lrat−/− livers showed the same level of specific activities for all enzyme activities studied upon simultaneous BPA and retinyl acetate administration.
Xanthine oxidase activity, a representative cytosolic phase I detoxification enzyme that displays a broad substrate specificity, was also assessed for BPA- and retinyl acetate-treated wild-type and Lrat−/− mice. The specific activity of xanthine oxidase in the livers of wild-type mice was significantly elevated by 5-fold upon BPA treatment (Fig. 3D). In the livers of BPA-treated Lrat−/− mice, xanthine oxidoreductase-specific activity remained unchanged from that observed in untreated Lrat−/− mice. However, retinyl acetate supplementation led to an elevation of XO specific activity when these mice were treated with BPA (Fig. 3D).
Phase II enzyme activities were also elevated following BPA administration to wild-type mice (Fig. 4A–C). The activity of an enzyme involved in BPA-glucuronide conjugate formation—UDP-glucuronosyl transferase (UGT), was significantly (by 28%) elevated in microsomes prepared from the livers of BPA-treated wild-type mice (Fig. 4A). GST catalytic activity, an enzyme responsible for conjugation both BPA and oxidized BPA metabolites with glutathione, was assessed in cytosolic and microsomal fractions prepared from liver homogenates by measuring its conjugation activity with the universal substrate 1-chloro-2,4-dinitrobenzene (CDNB). In wild-type mice, GST activity was induced in both microsomal and cytosolic fractions upon BPA administration. However, in an absolute sense, the level of induction of the specific activity of microsomal GST was much higher than observed for cytosolic GST-specific activity (Fig. 4B and C).
In Lrat−/− mice, BPA administration did not lead to an increase in hepatic UGT and GST-specific activities (Fig. 4A–C). However, supplementation of Lrat−/− mice with 3000 IU retinyl acetate supplementation led to an increase in phase II enzyme activity solely for the GSTs (Fig. 4B and C). UGT-specific activity, assayed using p-nitrophenol as a substrate, remained unchanged in these mutant mice even following BPA and retinyl acetate administration (Fig. 4A). In wild-type mice, retinyl acetate administration following BPA-treatment resulted in an elevation of phase II enzyme specific activities by approximately 2- to 3-folds for microsomal and cytosolic GSTs, respectively, and a 2-fold increase in UGT activity (Fig. 4A–C).
BPA-Induced Activation of Hepatic Detoxification Correlates with Oxidative Damage of Hepatic Lipids and Proteins
Because BPA detoxification results in the formation of potentially toxic intermediates, including highly reactive meta-, ortho-OH-ВРА as well as quinone forms of BPA (Jaeg et al., 2004; Schmidt et al., 2013) that are capable of inducing intracellular oxidative stress, we assessed lipid and protein oxidative damage products in hepatic subcellular fractions of treated matched wild-type and Lrat−/− mice (Figs. 5–7).
FIG. 6.
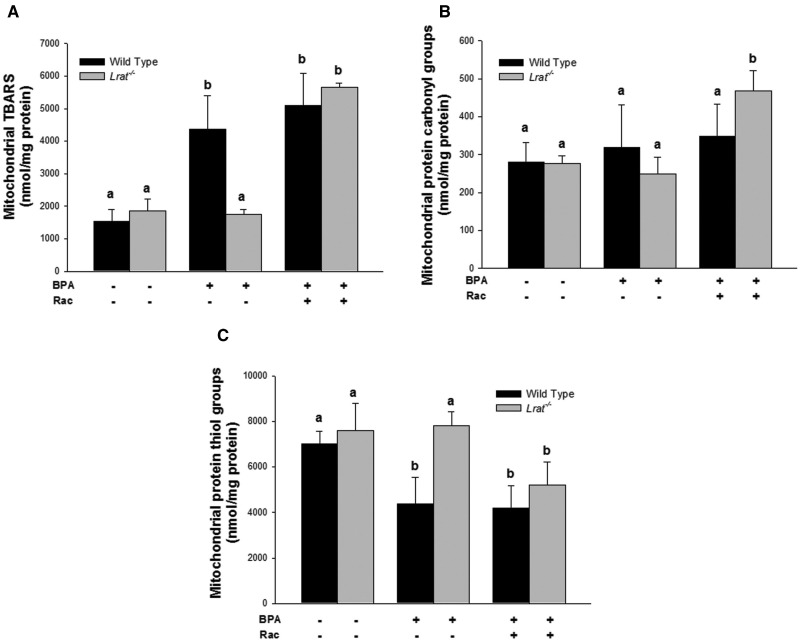
Oxidative damage products in mitochondria isolated from livers of wild-type and Lrat−/− mice following bisphenol A administration. Levels of thiobarbituric acid reactive substances (Panel A), protein carbonyl (Panel B), and thiol groups (Panel C) were determined in hepatic mitochondria of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Values marked with different letters (a, b) are statistically different, P < 0.05. All values are given as the mean ± 1 SD, n = 6 for each group.
FIG. 5.
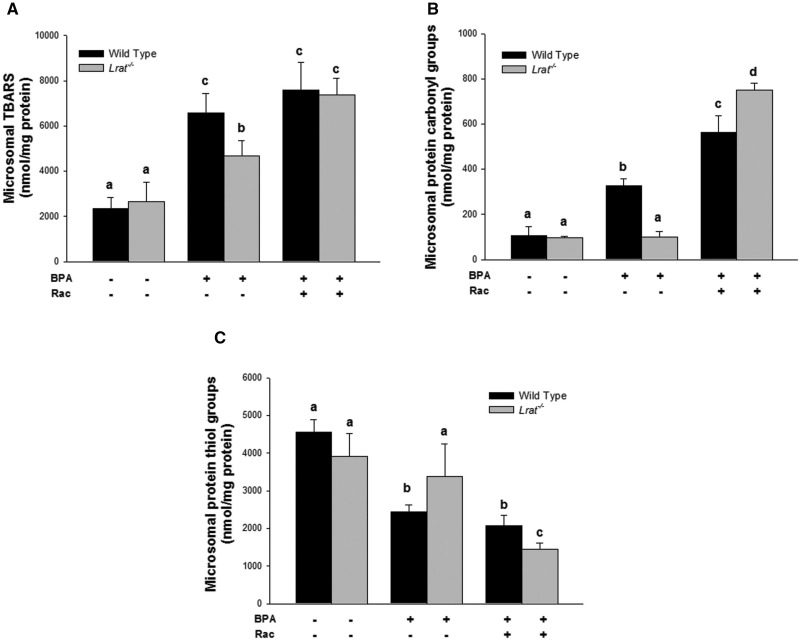
Oxidative damage products in hepatic microsomes prepared from wild-type and Lrat−/− mice following bisphenol A administration. Levels of thiobarbituric acid reactive substances (Panel A), protein carbonyl (Panel B), and thiol groups (Panel C) were determined in hepatic microsomes of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 SD, n = 6 for each group.
FIG. 7.
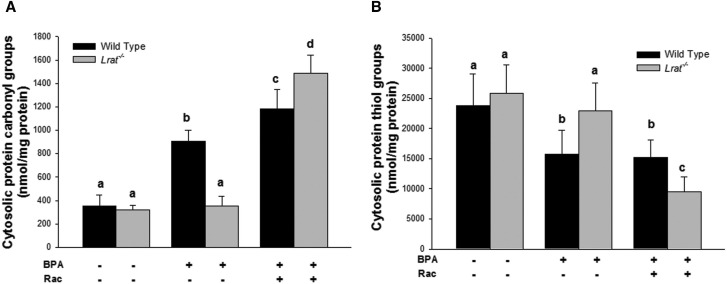
Oxidative damage products present in cytosol prepared from livers of wild-type and Lrat−/− mice following bisphenol A administration. Levels of protein carbonyl (Panel A) and thiol groups (Panel B) were determined in hepatic cytosol of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3,000 IU) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 SD, n = 6 for each group.
BPA treatment of wild-type mice resulted in significantly elevated levels of TBARS and protein carbonyl derivatives, and a significantly lower level of protein sulfhydryl groups in hepatic microsomes, mitochondria, and cytosol. Upon BPA administration to wild-type mice the highest rates of lipid and protein oxidation were observed for microsomes, whereas for the mitochondrial and cytosolic fractions, these parameters, although significantly changed, were not of the same magnitude as those observed for microsomes. BPA administration to wild-type mice resulted in a significant 2.8-fold increase on TBARS levels, a 3.1-fold increase of protein carbonyls, and a 2-fold decrease in protein thiols in the hepatic microsomal fraction compared with vehicle-treated animals (Fig. 5). This analysis of lipid and protein oxidation markers in hepatic subcellular fractions suggests that microsomal lipids and proteins are much more affected by BPA-derived radical oxidation, whereas mitochondrial and cytosolic proteins were either less affected or alternatively the rate of oxidized protein turnover in these fractions is much greater than the rate of oxidation.
For the BPA-treated Lrat−/− mice (Figs. 5–7), these parameters were not different from those measured for Lrat−/− mice treated with vehicle only. The only significant change observed in BPA-treated Lrat−/− mice was a 1.8-fold increase in microsomal TBARS level. However, this level was still significantly lower than in BPA-treated wild-type mice (Fig. 5A).
Retinyl acetate administration following BPA treatment of Lrat−/− mice resulted in an increase of the levels of lipid and protein oxidation products in all hepatic subcellular fractions isolated from the mutant mice (Figs. 5–7). The levels of lipid and protein oxidation in the microsomes, mitochondria, and cytosol of Lrat−/− mice treated both BPA and retinyl acetate reached the levels observed in wild-type ones after BPA administration alone. The most dramatic changes in the levels of TBARS, protein carbonyls and oxidized thiols were observed after combined BPA and retinyl acetate administration to Lrat−/− mice in the microsomal fraction (Fig. 5). For this subcellular component, the level of TBARS and protein carbonyls increased 1.6- and 7.6-folds, respectively, whereas protein thiol groups level decreased 2.3-fold compared with the parameters of Lrat−/− mice receiving BPA alone. Thus, retinyl acetate supplementation of Lrat−/− mice, followed by the induction of CYP hydroxylase and FMO oxygenase activities (Fig. 3), was accompanied by increased levels of markers for protein and lipid oxidation, predominantly in hepatic microsomes (Fig. 5). These changes were identical to or greater in magnitude to those observed for BPA-treated wild-type mice.
BPA Detoxification Triggers Reactive Oxygen and Nitrogen Species Generation that is of Non-Mitochondrial Origin
As a byproduct of BPA metabolism, ROS can be formed through one-electron oxygen reduction. To test whether the observed induction of the hepatic detoxification system is accompanied by the production of the superoxide anion radical (O2·) formation as a primary reactive oxygen species, we monitored subcellular fractions prepared from mouse liver homogenates. We observed that BPA administration to wild-type mice resulted in elevated O2· formation in hepatic microsomes, mitochondria and cytosol (Fig. 8). However, analysis of O2· formation by different subcellular fractions revealed that the majority of this increased formation could be attributed to the microsomal fraction (Fig. 8B). Strikingly, BPA treatment of Lrat−/− mice was not accompanied by increased superoxide production as observed for wild-type animals (Fig. 8).
FIG. 8.
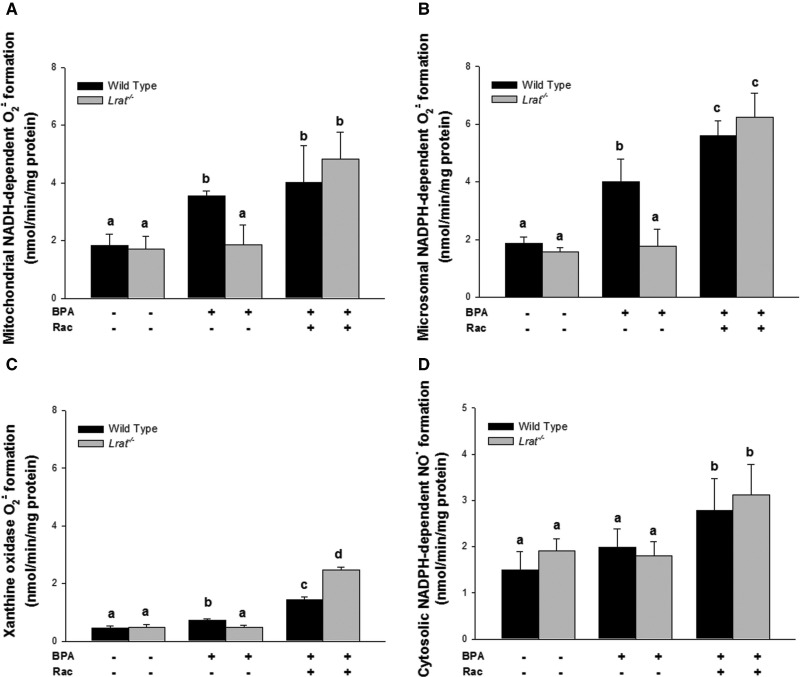
Reactive oxygen and reactive nitrogen species formed enzymatically that are present in hepatic subcellular fractions prepared from wild-type and Lrat−/− mice following bisphenol A administration. Levels of superoxide anion radical formation in hepatic mitochondria (Panel A), microsomes (Panel B), and xanthine oxidase reaction (Panel C) were determined in mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Level of nitric oxide formation (Panel D) in NO-synthase reaction was determined in hepatic cytosol of mice 72 h after per os administration of corn oil (vehicle) or bisphenol A (BPA, at a dose of 50 mg/kg body weight), either with or without oral retinyl acetate (Rac, at a dose of 3000 IU) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 SD, n = 6 for each group.
Retinyl acetate administration to BPA-treated Lrat−/− mice stimulated O2· formation for all of the subcellular fractions. However, the hepatic microsomes were quantitatively the greatest source of O2· (Fig. 8B). The rate of superoxide production by microsomes from these livers was 5.61 nmol/min/mg, which was greater than for mitochondria (4.83 nmol/min/mg) or cytosol (2.48 nmol/min/mg) (Fig. 8A and C).
The metabolic response to BPA we observed likely also involves NO-synthase-mediated nitric oxide (NO·) formation as a signaling molecule. However, under conditions when superoxide is formed, the potentially more toxic peroxynitrite (ONOO−) anion may be formed. An experiment was performed to measure the level of NO· formation in NO-synthase reaction using L-arginine as a substrate and a cytosol as a source for the enzyme. An elevated level of cytosolic NO· formation was detected upon combined retinyl acetate and BPA-treatment of wild-type as well as of Lrat−/− mice (Fig. 8D). This, together with elevated superoxide formation, could account for the increased protein oxidative damage observed in BPA-treated mice following supplementation with 3000 IU retinyl acetate.
DISCUSSION
In the present study, mRNA expression of the genes-encoding xenosensors and their target genes, catalytic activities of both phase I and phase II enzymes, and levels of oxidative damage to cellular proteins and lipids were assessed in subcellular fractions (microsomes, mitochondria, and cytosol) prepared from livers of wild-type and Lrat−/− mice upon BPA administration. The Lrat−/− (lecithin:retinol acyltransferase-deficient) mice are unable to synthesize and store retinyl esters and consequently lack hepatic retinoid (retinyl ester) stores (O'Byrne et al., 2005), but when maintained on a control retinoid-sufficient diet are otherwise physiologically normal. In the context of the current study, these genetically modified mice do not differ with regard to basal mRNA expression levels of key genes involved in xenobiotic sensing and detoxification, as well as representative phase I and phase II enzymatic activities. Thus, our studies explored how the availability of hepatic retinoid stores contributes towards maintaining an optimal xenobiotic detoxification response, influencing the expression and activities of elements of xenobiotic detoxification system. Wild-type and Lrat−/− mice were repeatedly treated per os with BPA, a xenobiotic known to induce its hepatic detoxification (Quesnot et al., 2014). BPA was administered as a repeated gavage of 50 mg BPA/kg body weight, a dose corresponding to the lowest observable adverse effect level (LOAEL) (Shelby, 2008). This treatment led to the induction of the activities of elements of the hepatic xenobiotic detoxification system within 72 h in wild-type mice that were normal with regard to their hepatic retinoid stores. This same treatment, however, failed to induce specific activities of detoxification enzymes in the livers of Lrat−/− mice which have no hepatic retinoid stores. These results convincingly establish that retinoid stores within the liver are needed to allow for the induction of xenobiotic elimination following BPA administration.
The reactions we studied represent critical bioactivations observed in response to intoxication with BPA and its analogs (Quesnot et al., 2014). These reactions are not limited to one particular CYP isozyme. Several studies employing human liver microsomes and human recombinant cytochrome P450 isoforms were performed to identify CYP specificity towards BPA intoxication. These studies suggest that members of a number of CYP subfamilies specifically, CYP1A, CYP2B, CYP2C, CYP2D, CYP2E, and CYP3A can be involved in BPA metabolism (Nakamura et al., 2011; Niwa et al., 2001; Schmidt et al., 2013). However, based on these published studies, it has been proposed that the biotransformation from BPA to hydroxylated-BPA species is predominantly catalyzed by hepatic CYP2C subfamily members (Niwa et al., 2001). This may partially explain our observations, because members of the CYP2C subfamily, including rat CYP2C22 and its human homologs CYP2C9 and CYP2C8, are reported to contain retinoic acid response (RAR-dependent) elements in their respective genes (Qian et al., 2010).
RXRs are common heterodimerization partners for many xenobiotic receptors, including human SXR, its rodent ortholog PXR, and CAR (Aleksunes and Klaassen, 2012; Woods et al., 2007). Thus, RXRs function as central regulators of target genes-encoding proteins involved in xenobiotic sensing and metabolism (Wan et al., 2000). Among xenobiotic-metabolizing CYPs, the genes-encoding CYP2A, CYP2B, CYP2C, CYP3A, CYP4A, and CYP7A have all been shown to be retinoid X receptor α (RXRα)-responsive target genes in vivo (Cai et al., 2003; Cai et al., 2002; Pascussi et al., 2003). This too provides greater molecular understanding of our findings by underscoring the need for RXRα liganded with retinoic acid as an obligate heterodimer partner for xenobiotic receptors controlling expression of genes-encoding CYPs involved in xenobiotic metabolism (Wan et al., 2000). However, this also imposes added complexity for defining the specific mechanisms underlying the induction of specific CYPs or other phase I oxidoreductases. In our study, the absence of hepatic retinoid stores in Lrat−/− mice (i.e. the absence of retinyl esters (O'Byrne et al., 2005) and diminished levels of retinol and retinoic acid (Shmarakov et al., 2013) do not affect basal mRNA expression or basal levels of specific activities of the phase I enzymes. Rather, this influences the ability of the liver to respond to BPA administration through induction of the monooxygenase activities. It is difficult to predict whether these observed BPA–retinoid interactions arise directly from retinoic acid-dependent transcriptional regulation of CYP-expression, or whether they may occur more indirectly through actions of the xenobiotic sensing nuclear receptors, or even through other mechanisms including ones at a posttranscriptional level (Al Tanoury et al., 2013).
In order to understand the molecular mechanisms of the BPA–retinoid interactions, we first assessed mRNA levels of the genes encoding nuclear receptors involved in xenobiotic sensing and the genes encoding phase I and phase II enzymes required for BPA biotransformation. In our study, BPA treatment did not give rise to significant changes of specific mRNAs encoding xenobiotic sensing nuclear receptors (Supplementary Fig. 1). Expression levels for mRNA transcribed from Car, Pxr, Nrf2 and the 3 Ppar genes were not different among untreated and BPA-treated mice, nor was expression affected by retinoids. We did not observe differences for the expression of these genes among retinoid stores deficient Lrat−/− mice as well as in retinyl acetate supplemented mice of both genotypes. Even though we did observe significant changes of AhR mRNAs in response to BPA and retinoid treatment of mice, these changes did not affect the hepatic expression of its downstream target Cyp1a1 gene (Fig. 1A).
The observed upregulation of Cyp2b10, Cyp2c39, Cyp4a10, Ugt1a1, and mGst transcription that resulted from combined BPA–retinoid interactions are consistent with the observed changes in phase I and phase II enzymes-specific activities we measured. However, our mRNA transcription data do not fully explain the observed changes in the enzymatic activities we measured. This strongly suggests an involvement of other important posttranscriptional events arising from the BPA–retinoid interactions. To allow for better delineation of the specific processes underlying our findings and resolving logical gaps for this aspect of our research, future research will be needed.
Nevertheless, it should be emphasized that our data indicate that retinoids, specifically retinoic acid, act as a permissive factor that enables proper transcriptional and posttranscriptional responses leading to an appropriate BPA biotransformation. However, when more retinoic acid that is derived from mobilized retinoids becomes available, it becomes involved in signaling that is required for BPA sensing and biotransformation. This ultimately requires retinoids to be mobilized from either endogenously stored retinoids (as retinyl esters residing in hepatic stellate cells) or exogenously acquired dietary retinoids. Moreover, these xenobiotic-mediated metabolic changes may give rise to alterations in retinoid metabolism and alter availability of different retinoid species (Shmarakov, 2015).
The retinoid-dependent changes observed in hepatic phase I and phase II detoxification activities prompted us to assess how retinoid availability may affect the oxidative damage caused by BPA toxicity, given that BPA is known to induce oxidative damage through BPA radical intermediate formation (Babu et al., 2013; Bindhumol et al., 2003). Our data show that BPA administration to wild-type mice leads to the development of oxidative stress in the hepatic subcellular fractions we examined, as evidenced by elevated levels of lipid and protein oxidation markers (Figs. 5–7). However, in Lrat−/− mice 72 h after BPA administration, no significant increases in protein and lipid oxidative damage were observed, aside from a small but significant increase in microsomal lipid oxidation. This likely can be explained by the low mono-oxygenase activities present in the livers of Lrat−/− mice following BPA administration. This would result in little or no BPA-derived radical intermediates formation, given that CYP actions are required for generating these reactive BPA metabolites via radical generation, oxidative cleavage, and dimerization (Okuda et al., 2011).
Our data establish that either endogenously derived retinoids, ones mobilized from retinyl esters stored in hepatic stellate cells, or exogenous retinoids acquired from the diet are needed for induction of CYP-mediated hydroxylation (both p-hydroxylation and N-demethylation) and FMO-mediated oxygenation (Fig. 3). Oral supplementation of Lrat−/− mice with 3000 IU of retinyl acetate restored the ability of these mutant mice to respond normally to BPA administration by increasing specific activities of CYP-catalyzed biotransformations (Fig. 3). This supplementation, which was aimed at elevating hepatic retinoid levels following BPA treatment, resulted in much more hepatic protein oxidation compared with matched wild-type mice. It should be pointed out that when retinyl acetate was administered alone, not in conjunction with BPA, we did not observe either significant induction of oxidative activities or oxidative damage in the livers of Lrat−/− mice over the short period (72 h) of supplementation employed in our studies. Nor did we observe an effect in wild-type mice upon retinyl acetate supplementation (Supplementary Table 2). The elevated accumulation of protein oxidation markers, including increased carbonylation and thiol disulfide formation (Figs. 5–7), suggests that the rapid restoration of BPA-induced oxidation upon retinyl acetate supplementation to Lrat−/− mice is more harmful than in wild-type mice. We propose that this results from a synergistic effect of simultaneous BPA and retinyl acetate administration, because in Lrat−/− mice both these compounds may be metabolized by hepatic CYPs. Because retinoid provided orally cannot be stored in the livers of Lrat−/− mice (O'Byrne et al., 2005), it is metabolized oxidatively, resulting in retinaldehyde and retinoic acid. This latter retinoid must be further catabolized through CYP-mediated oxidation and UGT-mediated glucuronidation in order to eliminate the retinoid from cells that are unable to store it (O'Byrne and Blaner, 2013; Samokyszyn et al., 2000). The need of Lrat−/− mice to eliminate excess oral retinoid simultaneously with the BPA dose likely affected the saturation and functionality of the hepatic detoxification of BPA.
As a result of BPA intoxication, the production of intracellular superoxide radical (O2·) and its derivatives, including hydroxyl radical (OH.), hydrogen peroxide (H2O2) and peroxynitrite (ONOO—), has been reported for hepatic, neuronal, and spermatogenic cells (Asahi et al., 2010; Ooe et al., 2005). We observed an elevation of NAD(P)H-dependent O2· formation by hepatic mitochondria, microsomes, and cytosol upon retinyl acetate administration in both BPA-treated Lrat−/− and wild-type mice (Fig. 8A–C). However, the majority of the increased superoxide formation could be attributed to the microsomal fraction (Fig. 8B). This elevated O2· formation correlated with enhanced oxidative damage detected in microsomes (Fig. 5). This was likely a consequence of elevated phase I activities (Fig. 3) upon retinyl acetate supplementation of BPA-treated mice.
Several important considerations regarding phase I and II enzymes in BPA intoxication grow out of our studies. The induction of CYP-mediated oxidation as well as UGT- and GST-mediated conjugation of BPA enables its biotransformation and elimination in wild-type mice that contain normal hepatic retinoid stores. However, the detoxification of BPA when hepatic retinoid stores are not available, as in Lrat−/− mice, leads either to impaired BPA biotransformation or to a lack of induction of its metabolism. Hence, we conclude that hepatic retinoid stores are required to allow for optimal BPA detoxification. Upon BPA administration to Lrat−/− mice, the diminished activities of components of the hepatic detoxification system also resulted in less harm to the liver compared with wild-type mice. We believe that the enhancement of BPA biotransformation upon retinyl acetate supplementation results in more harm to the organ because it triggers oxidative damage of cellular lipids and proteins due to enhanced ROS production of non-mitochondrial origin. The monooxygenase/oxidase reactions involved in BPA oxidation and stimulated by simultaneous retinyl acetate supplementation are likely a source for ROS formation that contributes to an increase of BPA hepatotoxicity. Thus, the results of our investigations raise a fundamental question regarding specific nutrient–xenobiotic interactions given the increased exposure of living organisms to different kinds of xenobiotics and/or environmentally persistent pollutants. From our data, it is clear that hepatic retinoid stores, ones arising from a dietary acquisition over a lifetime, may not benefit the health of the liver upon xenobiotic exposure. Retinyl ester stores accumulate through a mechanism that evolved to buffer against dietary vitamin A-insufficiency. This benefits the organism. However, this accumulation also becomes an injurious factor contributing to xenobiotic-induced hepatotoxicity. Our data also establish that overconsumption of dietary vitamin A under xenobiotic imposition poses a threat for increased xenobiotic hepatotoxicity. Collectively, these observations raise a question as to what is the optimal dietary amount of vitamin A to allow for safe xenobiotic elimination upon exposure. This issue merits investigation in future studies.
Our data, obtained from studies of BPA-treated mice, clearly establish that the activities of phase I and phase II enzymes involved in the hepatic elimination of BPA require hepatic retinoid stores. Thus, hepatic BPA biotransformation is retinoid-dependent. This conclusion is supported by the observation that large oral doses of retinoids allow for the elevation of both phase I and phase II activities upon BPA administration. However, oral supplementation with retinoid also has adverse consequences for the liver, accelerating BPA-induced oxidative damage through enhancement of non-mitochondial ROS production. Paradoxically, although hepatic retinoids are required to bring about BPA oxidation and elimination from the body, retinoid intake can enhance the adverse biological consequences of BPA intoxication. Thus, the extent of hepatic damage that arises from acute BPA intoxication is directly modulated by dietary retinoid intake during the period of BPA exposure and the hepatic retinoid stores that have accumulated over the lifetime of the organism. We suggest that BPA toxicity cannot be adequately understood unless there is appropriate consideration of these nutrient–toxicant interactions.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institutes of Health (grant nos. R01 DK068437 and R01 DK101251).
Supplementary Material
REFERENCES
- Al Tanoury Z., Piskunov A., Rochette-Egly C. (2013). Vitamin A and retinoid signaling: genomic and nongenomic effects. J. Lipid Res. 54, 1761–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes L. M., Klaassen C. D. (2012). Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab. Dispos. 40, 1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archakov A. I., Karuzina I. I., Tveritinov V. N., Kokareva I. S. (1974). Hydroxylation of aniline and aminoantipyrine (1-phenyl-2,3-dimethyl-aminopyrasolon-5) derivatives in liver endoplasmatic reticulum. Biochem. Pharmacol. 23, 1053–1063. [DOI] [PubMed] [Google Scholar]
- Archakov A. I., Panchenko L. F., Kapitanov A. B., Efron I. I., Knyazeva T. I., Zherebkova N. S. (1973). A quantitative estimation of degree of purity of preparations of subcellular structures. Anal. Biochem. 54, 223–233. [DOI] [PubMed] [Google Scholar]
- Asahi J., Kamo H., Baba R., Doi Y., Yamashita A., Murakami D., Hanada A., Hirano T. (2010). Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 87, 431–438. [DOI] [PubMed] [Google Scholar]
- Atkinson A., Roy D. (1995a). In vitro conversion of environmental estrogenic chemical bisphenol A to DNA binding metabolite(s). Biochem. Biophys. Res. Commun. 210, 424–433. [DOI] [PubMed] [Google Scholar]
- Atkinson A., Roy D. (1995b). In vivo DNA adduct formation by bisphenol A. Environ. Mol. Mutagen. 26, 60–66. [DOI] [PubMed] [Google Scholar]
- Auclair C., Voisin E. (1985). Nitroblue tetrazolium reduction.In Handbook of Methods for Oxygen Radical Research (Greenwald R., Ed.), pp. 123–132. CRC Press, Boca Raton. [Google Scholar]
- Babu S., Uppu S., Claville M. O., Uppu R. M. (2013). Prooxidant actions of bisphenol A (BPA) phenoxyl radicals: Implications to BPA-related oxidative stress and toxicity. Toxicol. Mech. Methods 23, 273–280. [DOI] [PubMed] [Google Scholar]
- Bindhumol V., Chitra K. C., Mathur P. P. (2003). Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 188, 117–124. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Burchell B., Weatherill P. (1981). 4-Nitrophenol UDPglucuronyltransferase (rat liver). Methods Enzymol. 77, 169–177. [DOI] [PubMed] [Google Scholar]
- Cai Y., Dai T., Ao Y., Konishi T., Chuang K. H., Lue Y., Chang C., Wan Y. J. (2003). Cytochrome P450 genes are differentially expressed in female and male hepatocyte retinoid X receptor alpha-deficient mice. Endocrinology 144, 2311–2318. [DOI] [PubMed] [Google Scholar]
- Cai Y., Konishi T., Han G., Campwala K. H., French S. W., Wan Y. J. (2002). The role of hepatocyte RXR alpha in xenobiotic-sensing nuclear receptor-mediated pathways. Eur. J. Pharm. Sci. 15, 89–96. [DOI] [PubMed] [Google Scholar]
- Delfosse V., Grimaldi M., le Maire A., Bourguet W., Balaguer P. (2014). Nuclear receptor profiling of bisphenol-A and its halogenated analogues. Vitam. Horm. 94, 229–251. [DOI] [PubMed] [Google Scholar]
- Elsby R., Maggs J. L., Ashby J., Park B. K. (2001). Comparison of the modulatory effects of human and rat liver microsomal metabolism on the estrogenicity of bisphenol A: implications for extrapolation to humans. J. Pharmacol. Exp. Ther. 297, 103–113. [PubMed] [Google Scholar]
- Fay M. J., Nguyen M. T., Snouwaert J. N., Dye R., Grant D. J., Bodnar W. M., Koller B. H. (2015). Xenobiotic metabolism in mice lacking the UDP-glucuronosyltransferase 2 family. Drug Metab. Dispos. 43, 1838–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C., Cipolat S., Scorrano L. (2007). Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2, 287–295. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126, 131–138. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139. [PubMed] [Google Scholar]
- Hamilton R. L., Moorehouse A., Lear S. R., Wong J. S., Erickson S. K. (1999). A rapid calcium precipitation method of recovering large amounts of highly pure hepatocyte rough endoplasmic reticulum. J. Lipid Res. 40, 1140–1147. [PubMed] [Google Scholar]
- Hanioka N., Naito T., Narimatsu S. (2008). Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere 74, 33–36. [DOI] [PubMed] [Google Scholar]
- Hassan Z. K., Elobeid M. A., Virk P., Omer S. A., El Amin M., Daghestani M. H., AlOlayan E. M. (2012). Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid. Med. Cell Longev. 2012, 194829.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi H., Tsutsumi O., Momoeda M., Takai Y., Osuga Y., Taketani Y. (1999). Differential interactions of bisphenol A and 17beta-estradiol with estrogen receptor alpha (ERalpha) and ERbeta. Endocr. J. 46, 773–778. [DOI] [PubMed] [Google Scholar]
- Inoue H., Yokota H., Makino T., Yuasa A., Kato S. (2001). Bisphenol a glucuronide, a major metabolite in rat bile after liver perfusion. Drug Metab. Dispos. 29, 1084–1087. [PubMed] [Google Scholar]
- Jaeg J. P., Perdu E., Dolo L., Debrauwer L., Cravedi J. P., Zalko D. (2004). Characterization of new bisphenol A metabolites produced by CD1 mice liver microsomes and S9 fractions. J. Agric. Food Chem. 52, 4935–4942. [DOI] [PubMed] [Google Scholar]
- Kabuto H., Hasuike S., Minagawa N., Shishibori T. (2003). Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ. Res. 93, 31–35. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A. G., Ahn B. W., Shaltiel S., Stadtman E. R. (1990). Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186, 464–478. [DOI] [PubMed] [Google Scholar]
- Li L., Wang Q., Zhang Y., Niu Y., Yao X., Liu H. (2015). The molecular mechanism of bisphenol A (BPA) as an endocrine disruptor by interacting with nuclear receptors: Insights from molecular dynamics (MD) simulations. PLoS One 10, e0120330.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. E., Kehrer J. P. (1989). Oxidation state of tissue thiol groups and content of protein carbonyl groups in chickens with inherited muscular dystrophy. Biochem. J. 260, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Tezuka Y., Ushiyama A., Kawashima C., Kitagawara Y., Takahashi K., Ohta S., Mashino T. (2011). Ipso substitution of bisphenol A catalyzed by microsomal cytochrome P450 and enhancement of estrogenic activity. Toxicol. Lett. 203, 92–95. [DOI] [PubMed] [Google Scholar]
- Nash T. (1953). The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 55, 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care, Use of Laboratory Animals, Institute for Laboratory Animal, R., and Press, N. A. (2011). Guide for the Care and Use of Laboratory Animals. National Academies Press, Washington, DC. [Google Scholar]
- Niwa T., Fujimoto M., Kishimoto K., Yabusaki Y., Ishibashi F., Katagiri M. (2001). Metabolism and interaction of bisphenol A in human hepatic cytochrome P450 and steroidogenic CYP17. Biol. Pharm. Bull. 24, 1064–1067. [DOI] [PubMed] [Google Scholar]
- O'Byrne S. M., Blaner W. S. (2013). Retinol and retinyl esters: Biochemistry and physiology. J. Biol. Chem. 54, 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne S. M., Wongsiriroj N., Libien J., Vogel S., Goldberg I. J., Baehr W., Palczewski K., Blaner W. S. (2005). Retinoid absorption and storage is impaired in mice lacking lecithin: Retinol acyltransferase (LRAT). J. Biol. Chem. 280, 35647–35657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. [DOI] [PubMed] [Google Scholar]
- Okuda K., Fukuuchi T., Takiguchi M., Yoshihara S. (2011). Novel pathway of metabolic activation of bisphenol A-related compounds for estrogenic activity. Drug Metab. Dispos. 39, 1696–1703. [DOI] [PubMed] [Google Scholar]
- Ooe H., Taira T., Iguchi-Ariga S. M., Ariga H. (2005). Induction of reactive oxygen species by bisphenol A and abrogation of bisphenol A-induced cell injury by DJ-1. Toxicol. Sci. 88, 114–126. [DOI] [PubMed] [Google Scholar]
- Pascussi J. M., Gerbal-Chaloin S., Drocourt L., Maurel P., Vilarem M. J. (2003). The expression of CYP2B6, CYP2C9 and CYP3A4 genes: A tangle of networks of nuclear and steroid receptors. Biochim. Biophys. Acta 1619, 243–253. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Orme-Johnson W., Ziegler D. M. (1964). The requirement for flavin adenine dinucleotide by a liver microsomal oxygenase catalyzing the oxidation of alkylaryl amines. Biochem. Biophys. Res. Commun. 16, 444–448. [DOI] [PubMed] [Google Scholar]
- Pottenger L. H., Domoradzki J. Y., Markham D. A., Hansen S. C., Cagen S. Z., Waechter J. M. Jr. (2000). The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol. Sci. 54, 3–18. [DOI] [PubMed] [Google Scholar]
- Pritchett J. J., Kuester R. K., Sipes I. G. (2002). Metabolism of bisphenol a in primary cultured hepatocytes from mice, rats, and humans. Drug Metab. Dispos. 30, 1180–1185. [DOI] [PubMed] [Google Scholar]
- Qian L., Zolfaghari R., Ross A. C. (2010). Liver-specific cytochrome P450 CYP2C22 is a direct target of retinoic acid and a retinoic acid-metabolizing enzyme in rat liver. J. Lipid Res. 51, 1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnot N., Bucher S., Fromenty B., Robin M. A. (2014). Modulation of metabolizing enzymes by bisphenol a in human and animal models. Chem. Res. Toxicol. 27, 1463–1473. [DOI] [PubMed] [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. (2007). In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 24, 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester J. R. (2013). Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 42, 132–155. [DOI] [PubMed] [Google Scholar]
- Rubin B. S. (2011). Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 127, 27–34. [DOI] [PubMed] [Google Scholar]
- Sakuma S., Nakanishi M., Morinaga K., Fujitake M., Wada S., Fujimoto Y. (2010). Bisphenol A 3,4-quinone induces the conversion of xanthine dehydrogenase into oxidase in vitro. Food Chem. Toxicol. 48, 2217–2222. [DOI] [PubMed] [Google Scholar]
- Samokyszyn V. M., Gall W. E., Zawada G., Freyaldenhoven M. A., Chen G., Mackenzie P. I., Tephly T. R., Radominska-Pandya A. (2000). 4-Hydroxyretinoic acid, a novel substrate for human liver microsomal UDP-glucuronosyltransferase(s) and recombinant UGT2B7. J. Biol. Chem. 275, 6908–6914. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Kotnik P., Trontelj J., Knez Z., Masic L. P. (2013). Bioactivation of bisphenol A and its analogs (BPF, BPAF, BPZ and DMBPA) in human liver microsomes. Toxicol. In Vitro 27, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Shelby M. D. (2008). NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. Ntp Cerhr Mon 22, v, vii-ix, 1-64 passim. [PubMed] [Google Scholar]
- Shmarakov I. O. (2015). Retinoid–xenobiotic interactions: The Ying and the Yang. Hepatobiliary Surg. Nutr. 4, 243–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmarakov I. O., Jiang H., Yang K. J., Goldberg I. J., Blaner W. S. (2013). Hepatic retinoid stores are required for normal liver regeneration. J. Lipid Res. 54, 893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmarakov I. O., Marchenko M. M. (2008). Xanthine oxidase activity in the rat liver tissue in the process of oncogenesis. Ukrains'kyi Biokhimichnyi Zhurnal 80, 86–91. [PubMed] [Google Scholar]
- Snyder R. W., Maness S. C., Gaido K. W., Welsch F., Sumner S. C., Fennell T. R. (2000). Metabolism and disposition of bisphenol A in female rats. Toxicol. Appl. Pharmacol. 168, 225–234. [DOI] [PubMed] [Google Scholar]
- Sui Y., Ai N., Park S. H., Rios-Pilier J., Perkins J. T., Welsh W. J., Zhou C. (2012). Bisphenol A and its analogues activate human pregnane X receptor. Environ. Health Perspect. 120, 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita A., Koibuchi N., Oka J., Taguchi M., Shishiba Y., Ozawa Y. (2001). Bisphenol-A, an environmental estrogen, activates the human orphan nuclear receptor, steroid and xenobiotic receptor-mediated transcription. Eur. J. Endocrinol. 145, 513–517. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Maffini M. V., Sonnenschein C., Rubin B. S., Soto A. M. (2009). Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 30, 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. J., An D., Cai Y., Repa J. J., Hung-Po Chen T., Flores M., Postic C., Magnuson M. A., Chen J., Chien K. R., et al. (2000). Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol. Cell Biol. 20, 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu K., Geng M., Gao P., Wu X., Hai Y., Li Y., Li Y., Luo L., Hayes J. D., et al. (2013). RXRalpha inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 73, 3097–3108. [DOI] [PubMed] [Google Scholar]
- Wang X. J., Hayes J. D., Henderson C. J., Wolf C. R. (2007). Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. (*Pnas Usa) PNAS USA 104, 19589–19594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C. G., Heuvel J. P., Rusyn I. (2007). Genomic profiling in nuclear receptor-mediated toxicity. Toxicol. Pathol. 35, 474–494. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang X., Bardag-Gorce F., Robel R. C., Aguilo J., Chen L., Zeng Y., Hwang K., French S. W., Lu S. C., et al. (2004). Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol. Pharmacol. 65, 550–557. [DOI] [PubMed] [Google Scholar]
- Xin F., Jiang L., Liu X., Geng C., Wang W., Zhong L., Yang G., Chen M. (2014). Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat. Res. 769, 29–33. [DOI] [PubMed] [Google Scholar]
- Yokota H., Iwano H., Endo M., Kobayashi T., Inoue H., Ikushiro S., Yuasa A. (1999). Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem. J. 340(Pt 2), 405–409. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.