Abstract
Elevated level of homocysteine (Hcy) is considered a risk factor for neurodegenerative diseases, but the mechanisms remain to be established. Because high Hcy is associated with an up-regulation of the ALOX5 gene product, the 5Lipoxygenase (5LO), herein we investigated whether this activation is responsible for the Hcy effect on neurodegeneration or is a secondary event. To reach this goal, wild type mice and mice genetically deficient for 5LO were assessed after being exposed to a diet known to significantly increase brain levels of Hcy. Confirming compliance with the dietary regimen, we found that by the end of the study brain levels of Hcy were significantly increase in both groups. However, diet-induced high Hcy resulted in a significant increase in Aβ, tau phosphorylation, neuroinflammation, synaptic pathology and memory impairment in control mice, but not in mice lacking ALOX5.
Taken together our findings demonstrate that the up-regulation of the ALOX5 gene pathway is responsible for the development of the biochemical and behavioral sequelae of high Hcy brain levels in the context of a neurodegenerative phenotype. They provide critical support that this gene and its expressed protein are viable therapeutic targets to prevent the onset, or delay neurodegenerative events in subjects exposed to this risk factor.
Introduction
Homocysteine (Hcy) is a non-essential, sulfur-containing amino acid and an intermediate product of the methionine cycle, whose normal levels in the body are maintained by its re-methylation to methionine in a reaction that requires the availability of dietary folate, vitamin B6 and B12 (1). A diet with excessive methionine, a diet low in folic acid and B vitamins, or mutations in genes encoding Hcy-metabolizing enzymes are known conditions all resulting in a significant elevation of circulating and tissue levels of Hcy (2). Even a mild Hcy level increase is a risk factor for cardiovascular diseases and stroke in humans and also a risk factor for neurodegenerative disorders, such as dementia, or Alzheimer’s disease (AD) (3). For instance, despite some conflicting data, most of the available epidemiological and clinical studies have revealed that elevated Hcy doubles the risk for developing AD independently of several other major factors (4–6). However, it is not yet clear whether Hcy is a marker, or a causative agent responsible for cellular and molecular events ultimately resulting in neurodegeneration, and the mechanisms involved (7).
To this end, several mechanisms have been proposed to explain the development of AD-like neuropathological changes associated with high Hcy in vivo. These include oxidative stress, excitotoxicity, cerebrovascular damage, endoplasmic reticulum stress (8–11). However, the exact biochemical basis by which Hcy modulates neurodegeneration remains unknown. Because high Hcy occurs more frequently in old age, it has been proposed as a potential metabolic link for the frequent coexistence of aging and neurodegenerative diseases (12–14).
Previous studies showed that genetic and diet-induced high Hcy results in significant increase in Aβ levels and deposition in APP transgenic mice (15–17). More recently, we demonstrated that diet-induced elevated Hcy in the triple transgenic mice (3xTg-AD) results in an exacerbation of behavioral deficits, brain amyloidosis and tau neuropathology (18). Interestingly, we observed that under this experimental condition mice had a significant elevation of the ALOX5 gene product, the 5Lipoxygenase (5LO) protein enzyme (19).
However, it remains to be investigated whether this up-regulation associated with high Hcy is directly involved and essential in modulating the development of a neurodegenerative phenotype secondary to this condition, or it is simply a secondary event.
To address this biological question, we tested the hypothesis that genetic absence of the ALOX5 gene would protect mice from the high Hcy-dependent negative effects on memory and neuropathologies, both of which are considered antecedent of neurodegeneration and AD pathophysiology development.
Results
Hcy-dependent memory impairment is rescued by genetic absence of ALOX5
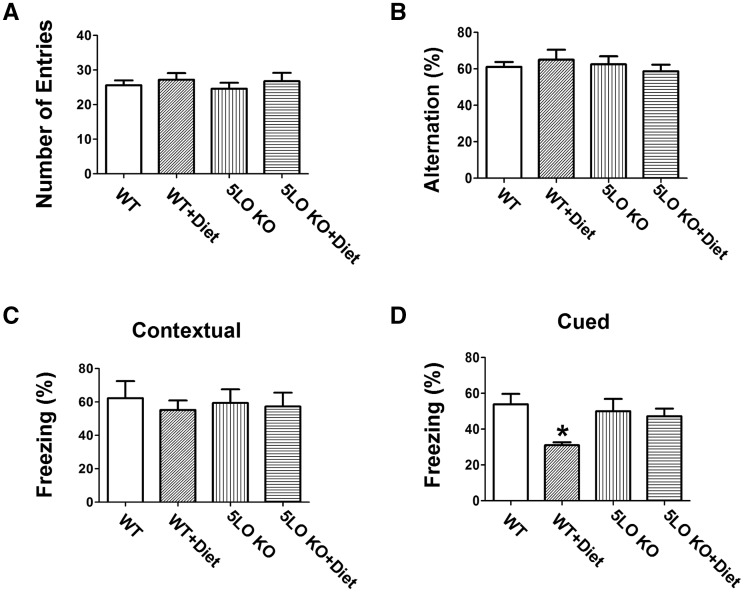
To investigate the effect of a folate and B vitamins-deficient diet (Diet) on cognition in the presence or absence of 5LO, wild type (WT) mice were assessed in two different paradigms. In the Y-maze test, we found that all mice in the different groups had a similar total number of arm entries and percentage of alternation (Fig. 1A, B).
Figure 1.
Genetic absence of ALOX5 rescues Hcy-dependent behavioral deficits. (A) Number of total arm entries for wild type mice receiving regular diet (WT); wild type mice receiving folate and B vitamin deficient diet (WT + Diet); 5LOKO mice receiving regular diet (5LOKO); and 5LOKO mice receiving the Diet (5LOKO+Diet). (B) Percentage of alternations of the four groups of mice described in panel A. (C) Contextual fear memory responses in the four groups of mice described in panel A. (D) Cued fear memory response in the same mice (*P < 0.05). Values represent mean ± S.E.M. (n = 5 WT-control, n = 5 WT + Diet, n = 5 5LOKO-control, n = 5 5LOKO + Diet).
In the fear conditioning test, compared with WT controls, mice receiving the Diet had a significant lower freezing time in the cued recall paradigm, which was absent in mice treated with the same diet but genetically deficient for 5LO (Fig. 1D). No significant differences were observed among the different groups in the contextual recall phase of the fear conditioning paradigm (Fig. 1C).
Lack of ALOX5 gene prevents Hcy-dependent increase in Aβ and tau phosphorylation
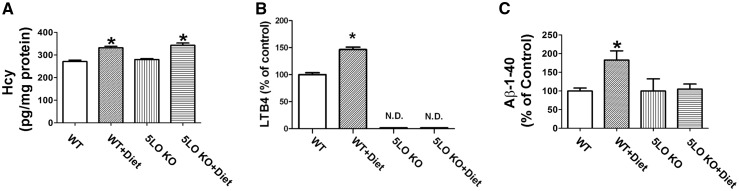
Confirming compliance of the animals with the chronic folate and B vitamins deficient dietary regimen, brain Hcy levels in these mice were significant higher than the control group, but unaffected by the genetic absence of 5LO (Fig. 2A).
Figure 2.
Effect of diet-induced high Hcy on 5LO pathway and endogenous Aβ levels. (A) Levels of homocysteine in brain cortex from wild type mice receiving regular diet (WT); wild type mice receiving folate and B vitamin deficient diet (WT + Diet); 5LOKO mice receiving regular diet (5LOKO); and 5LOKO mice receiving the Diet (5LOKO + Diet) (*P < 0.05). (B) Levels of LTB4 measured by a specific and sensitive ELISA assay in brain cortex homogenates from the same four groups of mice (*P < 0.05) (N.D.: not dosable). (C) Levels of total endogenous Aβ 1–40 peptides measured by a specific and sensitive ELISA assay in brain cortex extracts from the same four groups of mice (*P < 0.05). Values represent mean ± S.E.M. (n = 5 WT-control, n = 5 WT + Diet, n = 5 5LOKO-control, n = 5 5LOKO + Diet).
Compared with controls, brain cortex homogenates from WT mice receiving the Diet had a significant increase in the 5LO enzymatic activity as shown by the significant elevation of its main metabolic product, the leukotriene (LT)B4 (Fig. 2B), whose levels, as predicted, were undetectable in tissues from 5LO KO mice (Fig. 2B).
To investigate whether brain Aβ levels were influenced under our experimental conditions, we assayed total levels of endogenous murine Aβ1-40 in cortices of the four groups of mice. As shown in Figure 2C, we found that compared with controls, diet-induced high brain Hcy levels resulted in a significant increase in Aβ1-40 levels in cortices of WT mice. In contrast, no effect on Aβ1-40 levels was observed in mice receiving the same diet but lacking the 5LO, 5LO KO mice (Fig. 2C).
In addition, since in transgenic mouse models of AD high Hcy levels has been shown to modulate tau phosphorylation, next we wanted to see if this was also the case in WT mice.
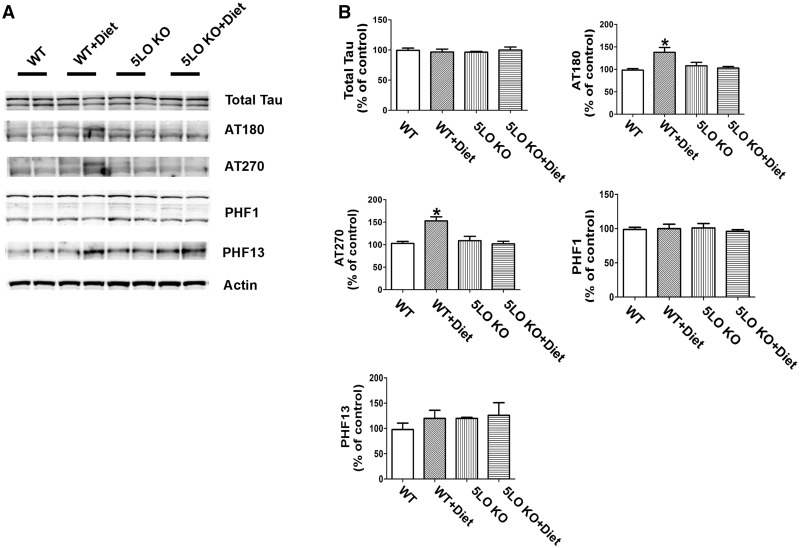
Compared with controls, diet-treated mice had a significant increase in the phosphorylation of tau at specific epitopes: at T231/S235 as recognized by the antibody AT180, and at T181 as recognized by the antibody AT270, but not changes were observed at S396/404 as recognized by the antibody PHF-1, and at S396 as recognized by the antibody PHF-13 (Fig. 3A, B). In contrast, mice receiving the same diet and having high Hcy but genetically deficient for 5LO (5LO KO) did not show any significant changes in tau phosphorylation (Fig. 3A, B). No effect of the diet or genotype was observed when the steady state levels of soluble tau protein were assayed in the four groups of mice (Fig. 3A, B).
Figure 3.
Diet-induced high Hcy-dependent increase in tau phosphorylation is prevented by genetic absence of ALOX5. (A) Representative western blots of soluble total tau, phosphorylated tau at residues T231/S235 (AT180), at T181 (AT270), S396/S404 (PHF1) and S396 (PHF13) in brain cortex homogenates from wild type mice receiving regular diet (WT); wild type mice receiving folate and B vitamin deficient diet (WT + Diet); 5LOKO mice receiving regular diet (5LOKO); and 5LOKO mice receiving the Diet (5LOKO + Diet). (B) Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (*P < 0.05). Values represent mean ± S.E.M. (n = 5 WT-control, n = 5 WT + Diet, n = 5 5LOKO-control, n = 5 5LOKO + Diet).
Synaptic pathology and neuroinflammation secondary to high brain Hcy levels are modulated by ALOX5
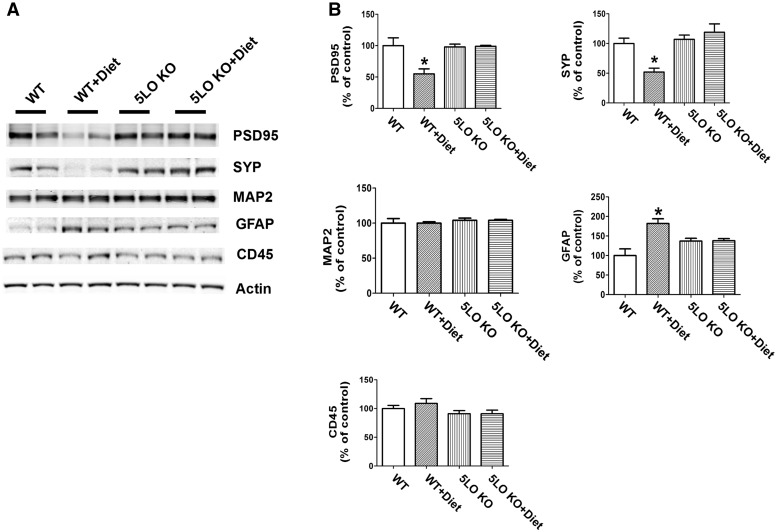
Next, we investigated the effect of the Diet in the presence or absence of the ALOX5 gene product, 5LO, on markers of synaptic integrity. Compared with WT controls, steady state levels of two distinct synaptic proteins, synaptophysin (SYP) and post-synaptic protein-95 (PDS-95), but not MAP-2, were significantly decreased in the mice administered the Diet (Fig. 4A, B). In contrast, 5LO-deficient WT mice on the same Diet had levels of all three synaptic proteins no different from controls (Fig. 4A, B). Genetic absence of ALOX5 per se did not influence the steady state levels of any of the considered synaptic proteins (Fig. 4A, B).
Figure 4.
Diet-induced high Hcy brain level effects on synaptic integrity and neuroinflammation are rescued by genetic absence of ALOX5. (A) Representative western blot analyses of post-synaptic density protein 95 (PSD-95), synaptophysin (SYP), microtubule-associated protein 2 (MAP2), GFAP and CD45 in brain cortex homogenates from wild type mice receiving regular diet (WT); wild type mice receiving folate and B vitamin deficient diet (WT + Diet); 5LOKO mice receiving regular diet (5LOKO); and 5LOKO mice receiving the Diet (5LOKO + Diet). (B) Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (*P < 0.05). Values represent mean ± S.E.M. (n = 5 WT-control; n = 5 WT + Diet; n = 5 5LOKO-control; n = 5 5LOKO + Diet).
Finally, we observed that compared with controls, Diet-treated WT mice had a significant increase in GFAP, a marker of astrocytes activation, which was blunted in 5LO KO mice receiving the same Diet (Fig. 4A, B). No significant differences between Diet-treated mice with and without 5LO were observed for CD45, a marker of microglia activation (Fig. 4A, B). The genetic absence of ALOX5 alone did not alter the expression levels of both neuroinflammation markers in WT mice (Fig. 4A, B).
Discussion
In the current study, we show that WT mice chronically exposed to a diet low in folate and B vitamins, which results in elevated levels of Hcy in the central nervous system, manifest significant memory impairments, higher Aβ levels, increased tau phosphorylation and synaptic pathology and that genetic absence of 5LO protects them for these pathological effects.
Taken together these results highlight the novel functional role that ALOX5 gene plays in the development of the biochemical, neuropathological and behavioral sequelae of an increased Hcy levels in vivo.
Despite some conflicting data, most of the available studies have revealed that high circulating levels of Hcy doubles the risk for developing AD independent of several other major risks (20,21). Experimental studies using transgenic models of AD have shown that high Hcy can influence the development of the AD-like phenotype. However, because all these models represent the genetic and rare variant of the disease, their translational value to a real life scenario in which the sporadic form is more common remains to be determined.
Since the initial report on this association, there has been an enormous effort to unravel the molecular and cellular mechanisms that could be responsible for the negative effects that high Hcy may have within the central nervous system including oxidative stress, inflammation and nucleic acids damage (22). This is a very important matter since the elucidation of these mechanisms and the relationship with neurodegenerative events and AD pathogenesis could provide valuable new insights for the treatment and or prevention of the disease onset in subjects with this risk factor.
Recently, it has been shown that high Hcy by influencing methyl-transferase reactions within the cells can modulate the methylation state of promoters which ultimately would regulate expression of genes relevant to AD pathogenesis (23,24). Among the different genes, we focused on the ALOX5 gene whose expression is tightly regulated by DNA methylation of its promoter (25), and showed that indeed high Hcy results in an up-regulation of its main product, the 5LO enzyme (19).
The 5LO is abundantly expressed in the hippocampus and cerebral cortex, with its level and activity rising as a function of aging (26). Because aging is an unavoidable risk factor in the development of AD, and 5LO is expressed in regions of the brain that are affected by AD, it supported the original hypothesis that this pathway is involved in its progression. Interestingly, a previous study has hinted that polymorphisms in the ALOX5 may be associated with AD risk (27), whereas another reported a significant up-regulation in ALOX5 gene expression and enzyme activation in peripheral blood mononuclear cells of AD patients compared with controls (28).
Herein to prove a functional role of 5LO up-regulation in modulating the Hcy effect on the functional and pathological phenotype we used WT animals and implemented a genetic approach by using mice that are deficient for this gene. At the end of the study, we observed that WT mice receiving the folate and B vitamin deficient diet had a significant increase in their brain Hcy levels, and this was associated with an impairment of their short term memory and learning ability as assessed by the significant reduction in the cued phase of the fear conditioning paradigm. In contrast, despite the elevation in brain Hcy levels, mice genetically deficient for the 5LO did not manifest this deficits and their responses were undistinguishable from control mice. No effect of the diet was observed in the Y-maze test, which measure working memory and the contextual phase of the fear conditioning, suggesting a specific target of the elevated levels of Hcy within the central nervous system.
We can rule out that the diet-induced elevated Hcy levels in the central nervous system had any effect on the motor phenotype of the animals since no differences were observed in the Y-maze test, and in particular when the number of total arm entries, which is known to assess motor function, were considered.
In accordance with the behavioral data, we found that brains from diet-treated mice had a significant elevation in the amount of endogenous Aβ peptide levels, which was not observed in the animals that received the same diet but did not have the ALOX5 gene available.
Compared with controls, whereas no significant differences were noted for levels of total soluble tau protein, WT mice receiving the Diet had a significant increase in tau phosphorylation at specific epitopes. In further support of the hypothesis that also these changes were mediate by the up-regulation of the 5LO enzymatic pathway, we observed that 5LO KO mice did not manifest them.
Several studies have shown that aberrant accumulation of hyper-phosphorylated tau protein can induce synaptic loss, typically represented by a decrease in the steady state levels of pre- and post-synaptic protein markers of synaptic integrity (29). Confirming this aspect of the tau neurobiology we found that compared with controls, diet-treated mice had a significant reduction in the levels of two of these proteins: SYP and post-synaptic density protein 95 (PSD-95), indices of pre- and post-synaptic integrity respectively. In contrast, the same proteins were not affected in brains from mice with high Hcy but genetically deficient for the 5LO. Finally, we confirmed that as result of the condition of diet-induced high Hcy these mice had cell biochemical signature of neuroinflammation, as shown by the elevation of GFAP level, a marker of astrocytes activation. However, 5LO KO mice receiving the same diet and for this reason having similar high Hcy in their central nervous system were undistinguishable from the control group.
Because data in the literature suggest that blockade or genetic absence of one LO may divert its main substrate (i.e. arachidonic acid) toward another LO such as the 12/15LO, it is possible that this is the case under our experimental conditions. However, based on our published work where we showed that 5LO KO mice do not differ from WT mice in the amount of other non-5LO derived eicosanoids we can easily exclude this possibility (30).
Additionally, while we cannot completely rule out a peripheral effect of ALOX5 gene ablation in our model, considering that this enzyme is up-regulated in the brain of the mice receiving the diet and that its metabolites act only locally, we believe that the observed biological effect is primarily dependent on the genetic absence of ALOX5 in the central nervous system.
Taken together our findings have important biological implication and value considering that, to the best of our knowledge, most of the studies trying to establish a link between Hcy and AD pathogenesis have implemented transgenic mouse models of the disease which by expressing human mutant AD-related genes (i.e. APP, tau) represent a genetic version of the disease. In contrast, our study is the first one to establish this mechanistic link in a more biologically relevant in vivo system, the WT mouse and the effect of high Hcy in the central nervous system on endogenous Aβ, tau metabolism and behavioral changes.
In summary, our work reports experimental evidence of the essential and functional role that the ALOX5 gene pathway plays in directly influencing cellular and molecular mechanisms of relevance for the development of neurodegeneration and AD pathogenesis. It provides pre-clinical rationale for targeting this pathway in individuals bearing this environmental risk factor as a viable therapeutic strategy for preventing or halting dementia and AD onset.
Materials and Methods
Animal and treatments
Animal procedures were approved by the Temple University Institutional Animal Care and Usage Committee and in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institute of Health.
C57/Bl6 WT mice and WT mice genetically deficient for ALOX5 gene (WT-5LO KO) were used in this study (31). All animals were kept in a pathogen-free environment, on a 12-h light/dark cycle and had access to food and water ad libitum. The mice were randomized to four groups. Group1 [n = 5 (two males and three females)] WT mice were given the standard rodent chow (Control); Group 2 [n = 5 (two males and three females)] WT mice were given a standard rodent chow deficient in folate (<0.2 mg/kg), vitamin B6 (<0.1 mg/kg) and B12 (<0.001 mg/kg), which is known to induce HHcy in mice (WT + Diet) (18); Group 3 [n = 5 (three males and two females)] WT-5LO KO were given the standard rodent chow (5LO KO); and Group 4 [n = 5 (three males and two females)] 5LO KO mice were given the folate and B vitamins deficient diet (5LO KO + Diet). Starting at 5 months of age, mice were randomized to the four groups above described, and followed for 7 months until they were 12-month-old. At this age time-point, they underwent behavioral testing and then euthanized. During the study, mice in all groups gained weight regularly, and no significant differences in weight were detected among them by the end of the study (data not shown). No macroscopic effect on the overall general health was observed in any of the four groups. No macroscopic differences among them were observed when major organs such as liver, spleen, heart and kidneys were compared at post-mortem examination. After euthanasia animals were perfused, brains removed and dissected in two halves by mid-sagittal dissection, and immediately stored at −80°C for biochemistry assays.
Behavioral tests
All animals were pre-handled for 3 days prior testing, they were tested in a randomized order, and all tests conducted by an experimenter blinded to the treatment and genotype.
Y-maze
The Y-maze apparatus consisted of three arms 32 cm (long) 610 cm (wide) with 26-cm walls (San Diego Instruments, San Diego, CA). Testing was always performed in the same room and at the same time to ensure environmental consistency as previously described (32,33). Briefly, each mouse was placed in the center of the Y-maze and allowed to explore freely through the maze during a 5-min session. The sequence and total number of arms entered were video recorded. An entry into an arm was considered valid if all four paws entered the arm. An alternation was defined as three consecutive entries in three different arms (i.e. 1, 2, 3 or 2, 3, 1, etc.). The percentage alternation score was calculated using the following formula: Total alternation number/(total number of entries-2) × 100.
Fear-conditioning
The fear conditioning test paradigm was performed following methods previously described (32,33). Briefly, the test was conducted in a conditioning chamber (19 cm × 25 cm × 19 cm) equipped with black methacrylate walls, transparent front door, a speaker and grid floor (Start Fear System; Harvard Apparatus). On day one, mice were placed into the conditioning chamber and allowed free exploration for 2 min in the white noise (65 Db) before the delivery of the conditioned stimulus (CS) tone (30 s, 90 Db, 2000 Hz) paired with a foot-shock unconditioned stimulus (US; 2 s, 0.6 mA) through a grid floor at the end of the tone. A total of three pairs of CS-US pairing with a 30 s inter trial interval (ITI) were presented to each animal in the training stage. The mouse was removed from the chamber 1 min after the last foot-shock and placed back in its home cage. The contextual fear conditioning stage started 24 h after the training phase when the animal was put back inside the conditioning chamber for 5 min with white noise only (65 dB). The animal’s freezing responses to the environmental context were recorded. The tone fear conditioning stage started 2 h after the contextual stage. The animal was placed back to the same chamber with different contextual cues, including white wall, smooth metal floor, lemon extract drops and red light condition. After 3 min of free exploration, the mouse was exposed to the exactly same three CS tones with 30 s ITI in the training stage without the foot-shock and its freezing responses to the tones were recorded.
Biochemical analyses
Brain cortices were homogenized and extracted in DEA buffer (0.2% diethylamine (DEA) and 50 mm NaCl), then centrifuged for 1 h at 100 000g at 4°C. The extracts were assayed for endogenous murine Aβ40 level by a sensitive sandwich ELISA kit following the manufacturer’s instructions (Invitrogen, Novex-life Technologies). Analyses were always performed in duplicate and in a coded fashion.
For the analysis of Hcy levels in the brain, tissue aliquots were frozen in liquid nitrogen and reduced into a fine powder using mortar and pestle. The powder was re-suspended in 100 μl of NKPD buffer (2.68 mm KCl, 1.47 mm KH2PO4, 51.10 mm Na2HPO4, 7.43 mm NaH2PO4, 62 mm NaCl, 1 mm EDTA and 1 mm dithiothreitol) and sonicated at 40 W and 70% duty cycle for about 2 min, then clarified by centrifugation at 10 000g for 15 min. Measurements were performed by HPLC analysis using Waters AccQ.Fluor derivitizing reagents (Waters Corp., MA), as previously described (34,35). The final concentration of Hcy in the samples was always normalized per mg protein. Analyses were always performed in triplicate and in a coded manner.
Western blot analyses
Brain cortex extracts were used for western blot analyses as previously described (32,33). Briefly, samples were electrophoresed on 10% Bis–Tris gels or 3–8% Tris–acetate gel (Bio-Rad, Richmond, CA), transferred onto nitrocellulose membranes (Bio-Rad), and then incubated overnight with the appropriate primary antibodies as indicated in Table 1. After three washings with T-TBS (pH7.4), membranes were incubated with IRDye 800CW-labeled secondary antibodies (LI-COR Bioscience, Lincoln, NE) at RT for 1 h. Signals were developed with Odyssey Infrared Imaging Systems (LI-COR Bioscience). β-Actin was always used as internal loading control.
Table 1.
Antibodies used in the study
| Antibody | Immunogen | Host | Dilution | Source | Catalog number |
|---|---|---|---|---|---|
| Tau-1 | Bovine microtubule associated protein | Mouse | 1:200 | Cell signaling | 4019 |
| AT-180 | Peptide containing phospho-T231/S235 | Mouse | 1:200 | Thermo | P10636 |
| AT-270 | Peptide containing phospho-T181 | Mouse | 1:300 | Thermo | MN1050 |
| PHF-13 | Peptide containing phospho-Ser396 | Mouse | 1:300 | Cell signaling | 9632 |
| PHF-1 | Peptide containing phosphor-Ser396/S404 | Mouse | 1:200 | Dr Davies P. | N/A |
| PSD95 | Purified recombinant rat PSD-95 | Mouse | 1:200 | Thermo | MA1-045 |
| SYP | aa 221–313 of SYP of human origin | Mouse | 1:400 | Santa Cruz | sc-55507 |
| MAP2 | Purified microtubule-associated protein from rat brain | Rabbit | 1:300 | Millipore | AB5622 |
| GFAP | Spinal cord homogenate of bovine origin | Mouse | 1:300 | Santa Cruz | sc-33673 |
| CD45 | aa 1075–1304 of CD45 of human origin | Rabbit | 1:200 | Santa Cruz | sc-25590 |
| Actin | Gizzard Actin of avian origin | Mouse | 1:1000 | Santa Cruz | sc-47778 |
Data analysis
Data analyses were performed using SigmaStat for Windows version 3.00. Statistical comparisons were performed by Unpaired Student’s t-test or the Mann–Whitney rank sum test when a normal distribution could not be assumed. Values in all figures and table represent mean ± S.E.M. Significance was set at P < 0.05.
Conflict of Interest statement. None declared.
Funding
The research presented in this paper was in part supported by grant from the National Institute of Health (HL112966, AG51684); the Wanda Simone Endowment for Neuroscience.
References
- 1. Selhub J., Troen A.M. (2016) Sulfur amino acids and atherosclerosis: a role for excess dietary methionine. Ann. N. Y. Acad. Sci., 1363, 18–25. [DOI] [PubMed] [Google Scholar]
- 2. Selhub J., Troen A., Rosenberg I.H. (2010) B vitamins and the aging brain. Nutr. Rev., 68 (Suppl. 2), S112–S118. [DOI] [PubMed] [Google Scholar]
- 3. Mizrahi E.H., Jacobsen D.W., Debanne S.M., Traore F., Lerner A.J., Friedland R.P., Petot G.J. (2003) Plasma total homocysteine levels, dietary vitamin B6 and folate intake in AD and healthy aging. J. Nutr. Health Aging, 7, 160–165. [PubMed] [Google Scholar]
- 4. Beydoun M.A., Beydoun H.A., Gamaldo A.A., Teel A., Zonderman A.B., Wang Y. (2014) Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health, 14, 643.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen L., Ji H.F. (2015) Association between homocysteine, folic acid, vitamin B12 and Alzheimer's disease: insights from meta-analysis. J. Alzheimer's Dis., 46, 777–790. [DOI] [PubMed] [Google Scholar]
- 6. Aisen P., Schneiser L.S., Sano M., Diaz-Arrastia R., van Dyck C.H., Weiner M.F., Bottiglieri T., Jin S., Stokes K.T., Thomas R.G., Thal L.J.. Alzheimer Disease Cooperative Study, (2008) High-dose B vitamin supplementation and cognitive decline in Alzheimer’s disease: a randomized controlled trial. JAMA, 300, 1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhuo J.M., Wang H., Praticò D. (2011) Is hyperhomocysteinemia an Alzheimer's disease risk factor, an AD marker, or neither? Trends Pharmacol. Sci., 32, 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perna A.F., Ingrosso D., De Santo N.G. (2003) Homocysteine and oxidative stress. Amino Acids, 25, 409–417. [DOI] [PubMed] [Google Scholar]
- 9. Kuszczyk M., Gordon-Krajcer W., Lazarewicz J.W. (2009) Homocysteine-induced acute excitotoxicity in cerebellar granule cells in vitro is accompanied by PP2A-mediated dephosphorylation of tau. Neurochem. Int., 55, 174–180. [DOI] [PubMed] [Google Scholar]
- 10. Boldyrev A.A., Johnson P. (2007) Homocysteine and its derivatives as possible modulators of neuronal and non-neuronal cell glutamate receptors in Alzheimer's disease. J. Alzheimer's Dis., 11, 219–228. [DOI] [PubMed] [Google Scholar]
- 11. Kim H.J., Cho H.K., Kwon Y.H. (2008) Synergistic induction of ER stress by homocysteine and beta-amyloid in SH-SY5Y cells. J. Nutr. Biochem., 19, 754–761. [DOI] [PubMed] [Google Scholar]
- 12. Ravaglia G., Forti P., Maioli F., Muscari A., Sacchetti L., Arnone G., Nativio V., Talerico T., Mariani E. (2003) Homocysteine and cognitive function in healthy elderly dwellers in Italy. Am. J. Clin. Nutr., 77, 668–673. [DOI] [PubMed] [Google Scholar]
- 13. Ravaglia G., Forti P., Maioli F., Servadei L., Martelli M., Arnone G., Talerico T., Zoli M., Mariani E. (2004) Plasma homocysteine and inflammation in elderly patients with cardiovascular disease and dementia. Exp. Gerontol., 39, 443–450. [DOI] [PubMed] [Google Scholar]
- 14. Parnetti L., Bottiglieri T., Lowenthal D. (1997) Role of homocysteine in age-related vascular and non-vascular diseases. Aging Clin. Exp. Res., 9, 241–257. [DOI] [PubMed] [Google Scholar]
- 15. Zhuo J.M., Portugal G.S., Kruger W.D., Wang H., Gould T.J., Pratico D. (2010) Diet-induced hyperhomocysteinemia increases amyloid-beta formation and deposition in a mouse model of Alzheimer’s disease. Curr. Alzheimer Res., 7, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pacheco-Quinto J., Rodrihguez de Turco E.B., DeRosa S., Howard A., Crzi-Sanchez F., Sambamurti K., Refolo L., Petanceska S., Pappolla M.A. (2006) Hyperhomo cysteinemic Alzheimer’s mouse model of amyloidosis shows increased brain amyloid beta peptide levels. Neurobiol. Dis., 22, 651–656. [DOI] [PubMed] [Google Scholar]
- 17. Zhuo J., Praticò D. (2010) Normalization of hyperhomocysteinemia improves cognitive deficits and ameliorates brain amyloidosis of a transgenic mouse model of Alzheimer’s disease. FASEB J., 24, 3895–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J.-G., Chu J., Barrero C., Merali S., Praticò D. (2014) Homocysteine exacerbates Aβ, tau pathology and cognitive deficit in a mouse model of Alzheimer's with plaques and tangles. Ann. Neurol., 75, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J.G., Barrero C., Gupta S., Kruger W.D., Merali S., Praticò D. (2017) Homocysteine modulates 5Lipoxygenase expression level via DNA methylation. Aging Cell, 16(2), 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCully K.S. (2015) Homocysteine metabolism, atherosclerosis, and diseases of aging. Compr. Physiol., 6, 471–505. [DOI] [PubMed] [Google Scholar]
- 21. Clarke R., Bennet D., Parish S., Lewington S., Skeaff M., Eussen S.J., Lewerin C., Stott D.J., Armitage J., Hankey G.J.. et al. (2014) Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr., 100, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith A.D., Refsum H. (2016) Homocysteine, B vitamins, and cognitive impairment. Annu. Rev. Nutr., 36, 211–239. [DOI] [PubMed] [Google Scholar]
- 23. Caudill M.A., Wang J.C., Melnyk S., Wang J.C., Melnyk S., Pogribny I.P., Jernigan S., Collins M.D., Santos-Guzman J., Swendseid M.E.. et al. (2001) Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J. Nutr., 131, 2811–2818. [DOI] [PubMed] [Google Scholar]
- 24. Castro R., Rivera I., Martins C., Struys E.A., Jansen E.E., Clode N., Graça L.M., Blom H.J., Jakobs C., de Almeida I.T. (2005) Intracellular S-adenosylhomocysteine increased levels are associated with DNA hypomethylation in HUVEC. J. Mol. Med., 83, 831–836. [DOI] [PubMed] [Google Scholar]
- 25. Uhl J., Klan N., Rose M., Entian K.D., Werz O., Steinhilber D. (2002) The 5-lipoxygenase promoter is regulated by DNA methylation. J. Biol. Chem., 277, 4374–4379. [DOI] [PubMed] [Google Scholar]
- 26. Chinnici C., Yao Y., Pratico D. (2007) The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol. Aging, 28, 457–462. [DOI] [PubMed] [Google Scholar]
- 27. Qu T., Manv R., Manev H. (2001) 5-Lipoxygenase (5-LOX) promoter polymorphism in patients with early-onset and late-onset Alzheimer’s disease. J. Neuropsychiatr. Clin. Neurosci., 13, 304–305. [DOI] [PubMed] [Google Scholar]
- 28. Di Francesco A., Arosio B., Gussago C., Dainese E., Mari D., D’Addario C., Maccarrone M. (2013) Involvement of 5-lipoxygenase in Alzheimer’s disease: a role for DNA methylation. J. Alzheim. Dis., 37, 3–8. [DOI] [PubMed] [Google Scholar]
- 29. Lasagna-Reeves C.A., Castillo-Carranza D.L., Sengupta U., Clos A.L., Jackson G.R., Kayed R. (2011) Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegen., 6, 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giannopoulos P.F., Chu J., Sperow M., Li J.G., Yu W.H., Kirby L.G., Abood M., Praticò D. (2015) Pharmacologic inhibition of 5-lipoxygenase improves memory, rescues synaptic dysfunction, and ameliorates tau pathology in a transgenic model of tauopathy. Biol. Psychiatry, 78, 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Chu J., Praticò D. (2011) 5-Lipoxygenase as an endogenous modulator of amyloid beta formation in vivo. Ann. Neurol., 69, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Meco A., Lauretti E., Vagnozzi A., Praticò D. (2014) Zileuton restores memory impairments and reverses amyloid and tau pathology in aged AD mice. Neurobiol. Aging, 35, 2458–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giannopoulos P.F., Chu J., Joshi Y.B., Sperow M., Li J.G., Kirby L.G., Praticò D. (2014) Gene knockout of 5-lipoxygenase rescues synaptic dysfunction and improves memory in the triple-transgenic model of Alzheimer’s disease. Mol. Psychiatry, 19, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Moncada C.A., Clarkson A., Perez-Leal O., Merali S. (2008) Mechanism and tissue specificity of nicotine-mediated lung d-adenosylmethionine reduction. J. Biol. Chem., 283, 7690–7696. [DOI] [PubMed] [Google Scholar]
- 35. Skelly M., Hoffman J., Fabbri M., Holzman R.S., Clarkson A.B., Merali S. (2003) S-adenosylmethionine concentrations in diagnosis of Pneumocystis carini pneumonia. Lancet, 361, 1267–1268. [DOI] [PubMed] [Google Scholar]