Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) can interfere with daily function and quality of life, and there are no known preventive approaches. In a cohort of breast cancer patients receiving paclitaxel as part of a clinical trial (SWOG 0221), we examined the use of dietary supplements both before diagnosis and during treatment in relation to CIPN.
Methods
At registration to S0221, 1225 breast cancer patients completed questionnaires regarding the use of multivitamins and supplements before and at diagnosis. A second questionnaire at six months queried use during treatment. Supplement use was evaluated in relation to CIPN, assessed via the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v. 3.0) and the self-reported Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity (FACT/GOG-Ntx) subscale. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed with logistic regression for the CTCAE analyses and ordinal regression for the FACT/GOG-Ntx analyses.
Results
Multivitamin use before diagnosis was associated with reduced symptoms of CIPN (CTCAE-adjusted OR = 0.60, 95% CI = 0.42 to 0.87; FACT/GOG-Ntx-adjusted OR = 0.78, 95% CI = 0.61 to 1.00). Use during treatment was marginally inversely associated with CIPN (CTCAE-adjusted OR = 0.73, 95% CI = 0.49 to 1.08; FACT/GOG-Ntx-adjusted OR = 0.77, 95% CI = 0.60 to 0.99). Other supplement use, either before diagnosis or during treatment, was not statistically significantly associated with CIPN.
Conclusions
Multivitamin use may be associated with reduced risk of CIPN, although individual dietary supplement use did not appreciably affect risk. Multivitamin use could be a surrogate for other related behaviors that are the actual drivers of the association with reduced CIPN. Without prospective randomized trials of vitamin supplementation, recommendations for use or changes to clinical practice are clearly not warranted.
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent consequence of treatment with paclitaxel. Symptoms of CIPN reduce quality of life and are potentially long lasting. Additionally, CIPN may lead to dose reduction or discontinuation of chemotherapy (1,2), potentially affecting treatment efficacy and future risk of recurrence.
Few data are available to predict which patients will develop CIPN or if there are effective approaches to prevention. Dose per cycle, cumulative dose, and treatment frequency have all been shown to be positively associated with CIPN (3–6), as was smoking in one study (7). Studies of genetic polymorphisms and CIPN have not been conclusive (8–14). Small trials showed that supplementation with omega-3 fatty acids (15) and vitamin E (16,17) reduced risk of CIPN. In a randomized trial with breast cancer patients, acetyl-L-carnitine was associated with increased risk (18).
After receiving a cancer diagnosis, patients may be motivated to make lifestyle changes that they believe will improve their health, reduce their risk of recurrence, and reduce the side effects of treatment (19–22). Often, dietary supplements are used to complement traditional cancer therapies, with reports of patients’ use of dietary supplements postdiagnosis ranging from 67% to 87% (23). In the Pathways study, a prospective cohort of breast cancer survivors, 60% of women began using a dietary supplement following diagnosis (24). Given the high prevalence of supplement use by survivors, understanding the effect that supplements may have on treatment outcomes, including CIPN, is important. To investigate if supplement use prior to and during chemotherapy for breast cancer was associated with the experience of CIPN, we used data from the Diet, Exercise, Lifestyle, and Cancer Prognosis (DELCaP) study. The DELCaP study, embedded in a large cooperative group therapeutic trial of dosing regimens for treatment of high-risk breast cancer, was designed to examine lifestyle factors, particularly the use of vitamin supplements before diagnosis and during chemotherapy, in relation to treatment outcomes.
Methods
Study Population
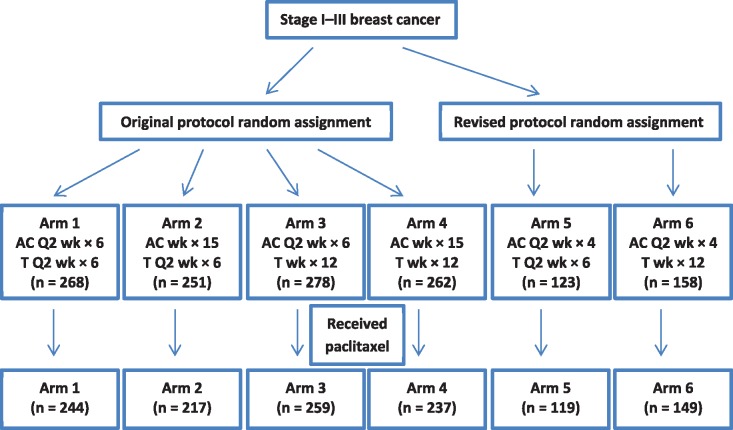
DELCaP was a questionnaire-based study ancillary to a breast cancer intergroup phase III clinical trial (SWOG 0221; NCT00070564) that compared dosing schedules of doxorubicin, cyclophosphamide, and paclitaxel (25). Figure 1 shows the design of S0221, with numbers limited to only those who participated in DELCaP.
Figure 1.
Study design schematic limited to participants with questionnaire data (n = 1340). Doxorubicin/cyclophosphamide was received according to one of the following schedules: every two weeks for six cycles, every two weeks for four cycles, or weekly for 15 cycles. Paclitaxel was received every two weeks for six cycles or weekly for 12 cycles. A = doxorubicin; C = cyclophosphamide; T = paclitaxel.
We embedded a description of the DELCaP study and permission to contact patients into the therapeutic consent for S0221, and all participants provided written informed consent. A form with patient contact information was faxed to our staff when patients were registered to the trial, which enabled us to provide them with questionnaires for completion prior to initiation of chemotherapy (26). Before sending questionnaires, study staff telephoned patients to describe the DELCaP study. If questionnaires were not returned in a timely manner, staff telephoned as a reminder and sent out replacement questionnaires, or answered questions if needed. Patients who returned incomplete questionnaires were also contacted to provide that information over the telephone, thus ensuring complete data for all questions from the majority of patients. S0221 accrual began in October 2003, with approval to conduct the DELCaP study in June 2005; thus, data on supplement use were available from patients who were enrolled during or after 2005 (n = 2014). Among these patients, 1726 consented to participation in DELCaP (85.7%), and 1482 of these patients (85.8%) completed and returned the first questionnaire. The baseline questionnaire collected data on supplement use and other lifestyle factors prior to and at diagnosis, before treatment was initiated. Approximately six months after study registration, when taxane treatment was scheduled to be completed, a second questionnaire was sent to participants, querying about lifestyle and supplement use during cancer treatment. As with the first questionnaire, patients who did not return the questionnaire or who submitted incomplete information were contacted to provide assistance in completing the questionnaire. Of the 1225 DeLCaP participants who completed the first questionnaire assessing supplement use prior to and at diagnosis and who were randomly assigned to one of the treatment arms and received paclitaxel, 1068 completed the second questionnaire (87.2%) assessing use during chemotherapy. The 157 patients who did not return the second questionnaire had lower education and were younger, less likely to be non-Hispanic white, and more likely to have smoked compared with those who completed the second questionnaire (data not shown). This study was approved by the institutional review boards at Roswell Park Cancer Institute and at all participating institutions that enrolled patients to S0221.
Toxicities Evaluated
In S0221, CIPN events were monitored and reported with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v. 3.0 (27). Grade 3 neuropathies include sensory loss or paresthesia interfering with activities of daily living. Grade 4 indicates disabling sensory loss that interferes with function. Study sites participating in S0221 were required to report grade 3 or higher neuropathy due to treatment. Therefore, we classified CIPN as a dichotomous variable (grades 3–4 vs grades 0–2).
For additional measures of patient-reported neuropathy representing a broader range than CTCAE grades 3 and 4, the second DELCaP questionnaire administered after chemotherapy also included the FACT-Taxane. In the 11-item FACT/GOG-Neurotoxicity subscale (FACT/GOG-Ntx), symptoms including numbness and discomfort in hands and feet, joint and muscle pain, and trouble feeling and walking are assessed via a five-point scale. Scores are categorized into tertiles, where tertile 1 indicates no or few symptoms, tertile 2 indicates moderate neuropathy, and tertile 3 indicates more severe neuropathy. This symptom questionnaire aspect of the DELCaP study was not initiated until after the study began; thus, 922 patients completed the FACT/GOG-Ntx.
Statistical Analyses
Baseline characteristics for participants with CTCAE grade 0 to 2 and grade 3 or 4 neuropathy, and by tertile of FACT/GOG-Ntx, were compared using chi-square tests. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using unconditional logistic regression for associations between each dietary supplement and CTCAE neuropathy, with ordinal regression for higher compared with lower neuropathy assessed via FACT/GOG-Ntx. Patients were classified as supplement users if they took a particular supplement at least once a week.
We used separate models to examine supplement use before diagnosis and during treatment in relation to neuropathy. We then considered use before and during treatment jointly. Because of low use of many individual supplements included in the questionnaires, we present data only on those with at least 10% use at any time point. Self-reported height and usual weight before diagnosis were used to compute body mass index (BMI) in kg/m2. Odds ratios were a priori–adjusted for age at completion of first questionnaire, BMI, race/ethnicity, smoking status, alcohol use, physical activity, and treatment arm. We considered menopausal status, education, and consumption of fruits and vegetables, as well as total cumulative dose and treatment modification, as covariates, but inclusion did not appreciably affect risk estimates, so they were excluded from final adjusted models. Model fit for logistic regression was assessed via Hosmer-Lemeshow tests, and the proportional odds assumption for ordinal models was assessed graphically. There was no evidence of poor fit (all P > .05). All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
The characteristics of DELCaP participants by CTCAE toxicity grade and by tertile of FACT/GOG-Ntx scores are shown in Table 1. Grade 3 and 4 CTCAE neuropathy, observed in 12.4% of patients, was more common among African Americans and among patients randomly assigned to treatment arms with a biweekly paclitaxel dose of 175 mg/m2 (arms 1, 2, and 5) for six cycles than those in arms 3, 4, and 6 (25). Using FACT/GOG-Ntx scores, neuropathy was more common in patients who were older, nonwhite, overweight and obese, former or current smokers, and those with less education than a college degree; 65.5% of patients with CTCAE-assessed grade 3 or 4 were in the highest tertile of FACT/GOG-Ntx neuropathy, compared with 30.3% of patients with CTCAE-assessed grade 0 to 2 toxicities.
Table 1.
Characteristics of breast cancer patients in relation to CTCAE neuropathy grade (n = 1225) and neuropathy score as determined by the FACT/GOG-Ntx (n = 922)
| Characteristic | CTCAE neuropathy grade score |
FACT/GOG-Ntx score tertiles |
|||||
|---|---|---|---|---|---|---|---|
| 3–4 No. (%) | 0–2 No. (%) | P* | 3 No. (%) | 2 No. (%) | 1 No. (%) | P* | |
| Total, No. (%) | 152 (12.4) | 1073 (87.6) | -- | 321 (34.8) | 320 (34.7) | 281 (30.5) | -- |
| Age, y | .05 | .001 | |||||
| <40 | 37 (9.4) | 358 (90.6) | 17 (17.2) | 39 (39.4) | 43 (43.4) | ||
| 40–49 | 60 (14.0) | 368 (86.0) | 93 (32.4) | 98 (34.1) | 96 (33.4) | ||
| 50–59 | 41 (15.7) | 220 (84.3) | 128 (38.7) | 113 (34.1) | 90 (27.2) | ||
| ≥60 | 14 (9.9) | 127 (90.1) | 83 (40.5) | 70 (34.1) | 52 (25.4) | ||
| Race/ethnicity | .007 | .12 | |||||
| Non-Hispanic white | 110 (11.2) | 874 (88.8) | 251 (33.2) | 268 (35.4) | 237 (31.3) | ||
| Hispanic | 5 (8.8) | 52 (91.2) | 18 (54.5) | 5 (15.2) | 10 (30.3) | ||
| Black/African American | 17 (20.5) | 66 (79.5) | 22 (37.3) | 22 (37.3) | 15 (25.4) | ||
| Other | 20 (19.8) | 81 (80.2) | 30 (40.5) | 25 (33.8) | 19 (25.7) | ||
| Body mass index, kg/m2 | .21 | <.001 | |||||
| Underweight (≤18.5) | 0 (0) | 11 (100) | 2 (28.6) | 3 (42.9) | 2 (28.6) | ||
| Normal (18.5–24.9) | 36 (10.5) | 308 (89.5) | 72 (26.9) | 81 (30.2) | 115 (42.9) | ||
| Overweight (25–29.9) | 68 (14.4) | 403 (85.6) | 142 (41.0) | 125 (36.1) | 79 (22.8) | ||
| Obese (≥30) | 47 (12.2) | 337 (87.8) | 105 (36.0) | 106 (36.3) | 81 (27.7) | ||
| Menopausal status, No. | .02 | ||||||
| Premenopausal | 57 (10.1) | 510 (89.9) | 124 (29.7) | 145 (34.8) | 148 (35.5) | ||
| Postmenopausal | 95 (14.4) | 563 (85.6) | 197 (39.0) | 175 (34.7) | 133 (26.3) | ||
| Smoking history | .90 | .02 | |||||
| Never smoker | 80 (12.0) | 584 (88.0) | 158 (30.9) | 184 (35.9) | 170 (33.2) | ||
| Former smoker | 51 (12.8) | 347 (87.2) | 111 (37.4) | 103 (34.7) | 83 (27.9) | ||
| Current smoker | 20 (13.2) | 132 (86.8) | 50 (46.3) | 50 (46.3) | 27 (25.0) | ||
| Education | .07 | .02 | |||||
| < High school graduate | 18 (20.5) | 70 (79.5) | 23 (41.1) | 12 (21.4) | 21 (37.5) | ||
| High school graduate or GED | 33 (12.6) | 228 (87.4) | 72 (36.5) | 74 (37.6) | 51 (25.9) | ||
| Some college | 56 (12.9) | 377 (87.1) | 130 (39.5) | 104 (31.6) | 95 (28.9) | ||
| College graduate | 23 (8.7) | 242 (91.3) | 63 (31.0) | 71 (35.0) | 69 (34.0) | ||
| Advanced degree | 22 (12.7) | 151 (87.3) | 33 (24.6) | 57 (32.8) | 44 (32.8) | ||
| Marital status | .11 | .14 | |||||
| Married or living as married | 105 (11.8) | 786 (88.2) | 234 (33.7) | 231 (33.3) | 229 (33.0) | ||
| Widowed | 12 (21.1) | 5 (78.9) | 12 (31.6) | 15 (39.5) | 11 (28.9) | ||
| Separated or divorced | 29 (14.5) | 171 (85.5) | 55 (40.7) | 53 (39.3) | 27 (20.0) | ||
| Single | 6 (8.5) | 65 (91.5) | 18 (35.3) | 19 (37.3) | 14 (27.5) | ||
| Physical activity† | .82 | .19 | |||||
| No | 28 (12.0) | 206 (88.0) | 69 (40.8) | 54 (32.0) | 46 (27.2) | ||
| Yes | 124 (12.5) | 867 (87.5) | 252 (33.5) | 266 (35.3) | 235 (31.2) | ||
| Treatment arm‡ | .003 | .25 | |||||
| 1 | 39 (16.0) | 205 (84.0) | 77 (40.1) | 66 (34.4) | 49 (25.5) | ||
| 2 | 36 (16.6) | 181 (83.4) | 58 (34.7) | 59 (35.3) | 50 (29.9) | ||
| 3 | 21 (8.1) | 238 (91.9) | 73 (36.7) | 59 (29.6) | 67 (33.7) | ||
| 4 | 19 (8.0) | 218 (92.0) | 52 (30.4) | 66 (38.6) | 53 (31.0) | ||
| 5 | 21 (17.6) | 98 (82.4) | 25 (29.1) | 38 (44.2) | 23 (26.7) | ||
| 6 | 16 (10.7) | 133 (89.3) | 36 (33.6) | 32 (29.9) | 39 (36.4) | ||
| CTCAE grade | − | − | <.001 | ||||
| 0–2 | − | − | 243 (30.3) | 290 (36.1) | 270 (33.6) | ||
| 3–4 | − | 78 (65.5) | 30 (25.2) | 11 (9.2) | |||
P values were computed using two-sided chi-square tests. CTCAE = Common Terminology Criteria for Adverse Events; FACT/GOG-Ntx = Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity.
In month before diagnosis.
Treatment arms correspond to the descriptions given in Figure 1.
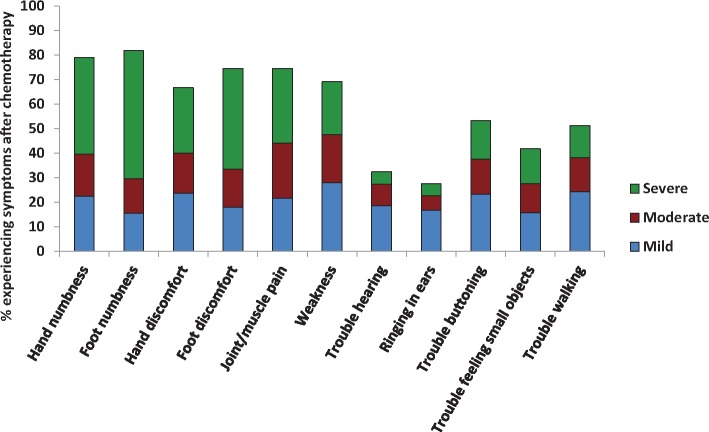
Individual components of the FACT/GOG-Ntx subscale are displayed in Figure 2. “Quite a bit” and “very much” were grouped together to represent severe neuropathy, “somewhat” was considered moderate, and “a little bit” was considered mild. Foot numbness was the most common symptom, with 81.9% of patients experiencing at least mild foot numbness and 52.3% reporting severe foot numbness (Figure 2). Hand numbness was also very high, with 79.1% reporting at least mild hand numbness; 74.5% reported foot discomfort, and 66.7% reported hand discomfort.
Figure 2.
Individual components of Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity. “Quite a bit” and “very much” were grouped together to represent severe neuropathy. “Somewhat” was considered moderate, and “a little bit” was considered mild.
As shown in Table 2, patients who used multivitamins before diagnosis were 39.7% less likely to experience CTCAE grade 3 or 4 neuropathy than nonusers (adjusted odds ratio [aOR] = 0.60, 95% CI = 0.42 to 0.87) and, according to FACT/GOG-Ntx, were 21.9% less likely to report more neuropathy than nonusers (aOR = 0.78, 95% CI = 0.61 to 1.00). Use of vitamin C, folic acid, calcium, iron, and fish oil before diagnosis was not associated with CTCAE grade 3 or 4 neurotoxicity. There were no statistically significant associations between use of any individual supplements prior to diagnosis and CIPN assessed by CTCAE or by FACT/GOG-Ntx.
Table 2.
Supplement use before diagnosis and odds of CTCAE grade 3 and 4 neuropathy (n = 1225) and neuropathy score as determined by the FACT/GOG-Ntx (n = 922)
| Use before diagnosis | CTCAE neuropathy grade |
FACT/GOG-Ntx score tertiles |
|||||
|---|---|---|---|---|---|---|---|
| 3–4 (n = 152)No. (%) | 0–2 (n = 1073)No. (%) | aOR (95% CI)* | 3 (n = 321)No. (%) | 2 (n = 320)No. (%) | 1 (n = 281) No. (%) | aOR (95% CI)* | |
| Multivitamin | |||||||
| No | 93 (14.4) | 552 (85.6) | 1.00 (ref) | 182 (38.5) | 155 (32.8) | 136 (28.8) | 1.00 (ref) |
| Yes | 58 (10.1) | 515 (89.9) | 0.60 (0.42 to 0.87) | 137 (30.8) | 164 (36.9) | 144 (32.4) | 0.78 (0.61 to 1.00) |
| Vitamin C | |||||||
| No | 121 (12.4) | 857 (87.6) | 1.00 (ref) | 248 (34.1) | 256 (35.2) | 223 (30.7) | 1.00 (ref) |
| Yes | 31 (12.8) | 212 (87.2) | 0.96 (0.62 to 1.48) | 73 (37.6) | 64 (33.0) | 57 (29.4) | 1.07 (0.79 to 1.45) |
| Vitamin D | |||||||
| No | 124 (13.0) | 829 (87.0) | 1.00 (ref) | 253 (35.7) | 237 (33.5) | 218 (30.8) | 1.00 (ref) |
| Yes | 28 (10.4) | 240 (89.6) | 0.72 (0.46 to 1.14) | 68 (31.9) | 64 (39.0) | 62 (29.1) | 0.97 (0.72 to 1.30) |
| Vitamin E | |||||||
| No | 132 (12.8) | 903 (87.2) | 1.00 (ref) | 264 (34.0) | 269 (34.7) | 243 (31.3) | 1.00 (ref) |
| Yes | 20 (10.8) | 166 (89.2) | 0.73 (0.44 to 1.23) | 56 (38.6) | 51 (35.2) | 38 (26.2) | 1.14 (0.82 to 1.61) |
| Vitamin B6 | |||||||
| No | 140 (12.6) | 968 (87.4) | 1.00 (ref) | 283 (34.1) | 291 (35.0) | 257 (30.9) | 1.00 (ref) |
| Yes | 12 (10.6) | 101 (89.4) | 0.83 (0.44 to 1.59) | 37 (41.1) | 29 (32.2) | 24 (26.7) | 1.28 (0.84 to 1.93) |
| Folic acid | |||||||
| No | 137 (12.4) | 966 (87.6) | 1.00 (ref) | 285 (34.5) | 287 (34.7) | 254 (30.8) | 1.00 (ref) |
| Yes | 15 (12.7) | 103 (87.3) | 1.04 (0.57 to 1.87) | 35 (36.8) | 33 (34.7) | 27 (28.4) | 1.05 (0.70 to 1.57) |
| Vitamin B12 | |||||||
| No | 136 (12.7) | 934 (87.3) | 1.00 (ref) | 275 (34.4) | 276 (34.5) | 248 (31.0) | 1.00 (ref) |
| Yes | 16 (10.6) | 135 (89.4) | 0.74 (0.42 to 1.31) | 46 (37.4) | 44 (35.8) | 33 (26.8) | 1.11 (0.77 to 1.59) |
| Calcium | |||||||
| No | 104 (12.7) | 715 (87.3) | 1.00 (ref) | 217 (35.7) | 202 (33.3) | 188 (31.0) | 1.00 (ref) |
| Yes | 48 (12.0) | 351 (88.0) | 0.88 (0.60 to 1.31) | 104 (33.1) | 118 (37.6) | 92 (29.3) | 0.93 (0.71 to 1.22) |
| Iron | |||||||
| No | 143 (12.5) | 1000 (87.5) | 1.00 (ref) | 298 (34.4) | 306 (35.3) | 263 (30.3) | 1.00 (ref) |
| Yes | 9 (11.5) | 69 (88.5) | 1.05 (0.50 to 2.19) | 23 (41.8) | 14 (25.5) | 18 (32.7) | 1.21 (0.72 to 2.05) |
| Omega-3 sources | |||||||
| No | 118 (12.4) | 830 (87.6) | 1.00 (ref) | 246 (35.0) | 232 (33.0) | 225 (32.0) | 1.00 (ref) |
| Yes | 31 (11.7) | 234 (88.3) | 0.86 (0.55 to 1.34) | 74 (34.9) | 85 (40.1) | 53 (25.0) | 1.21 (0.90 to 1.62) |
| Glucosamine | |||||||
| No | 136 (12.8) | 928 (87.2) | 1.00 (ref) | 274 (34.6) | 275 (34.7) | 243 (30.7) | 1.00 (ref) |
| Yes | 16 (10.4) | 138 (89.6) | 0.62 (0.35 to 1.11) | 46 (36.8) | 43 (34.4) | 36 (28.8) | 0.93 (0.64 to 1.33) |
Adjusted for age, body mass index, race/ethnicity, smoking status, physical activity, alcohol intake, and treatment arm. aOR = adjusted odds ratio; CI = confidence interval; CTCAE = Common Terminology Criteria for Adverse Events; FACT/GOG-Ntx = Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity.
Table 3 shows associations between neurotoxicity and use of supplements during chemotherapy. Odds of CTCAE grade 3 or 4 neuropathy were lower for patients who reported use of multivitamins during treatment compared with nonusers (aOR = 0.73, 95% CI = 0.49 to 1.08), as well as for users of fish oil, EPA, omega-3, flaxseed, or cod liver oil (aOR = 0.54, 95% CI = 0.28 to 1.06), although associations were not statistically significant. None of the other supplements was statistically significantly associated with likelihood of grade 3 or 4 neuropathy. Although not statistically significant, the use of iron during treatment appeared to be associated with increased odds of grade 3 or 4 neuropathy (aOR = 1.67, 95% CI = 0.92 to 3.03). Multivitamin use during treatment was also associated with lower odds of neuropathy according FACT/GOG-Ntx (aOR = 0.77, 95% CI = 0.60 to 0.99), and use of vitamins B6 and B12 with higher odds, although associations were not statistically significant.
Table 3.
Supplement use during chemotherapy treatment and odds of CTCAE grade 3 and 4 neuropathy (n = 1068) and neuropathy score as determined by the FACT/GOG-Ntx (n = 922)
| Use during treatment | CTCAE neuropathy grade |
FACT/GOG-Ntx score tertile |
|||||
|---|---|---|---|---|---|---|---|
| 3–4(n = 152) No. (%) | 0–2 (n = 1073) No. (%) | aOR (95% CI)* | 3 (n = 321) No. (%) | 2 (n = 320) No. (%) | 1 (n = 281) No. (%) | aOR (95% CI)* | |
| Multivitamin | |||||||
| No | 82 (13.9) | 509 (86.1) | 1.00 (ref) | 197 (37.3) | 183 (34.7) | 148 (28.0) | 1.00 (ref) |
| Yes | 49 (10.4) | 422 (89.6) | 0.73 (0.49 to 1.08) | 122 (31.4) | 136 (35.0) | 131 (33.7) | 0.77 (0.60 to 0.99) |
| Vitamin C | |||||||
| No | 119 (12.8) | 808 (87.2) | 1.00 (ref) | 286 (35.7) | 280 (34.9) | 236 (29.4) | 1.00 (ref) |
| Yes | 13 ( 9.8) | 120 (90.2) | 0.76 (0.41 to 1.41) | 35 (30.4) | 40 (34.8) | 40 (34.8) | 0.84 (0.58 to 1.22) |
| Vitamin D | |||||||
| No | 103 (12.9) | 695 (87.1) | 1.00 (ref) | 248 (36.1) | 238 (34.6) | 201 (29.3) | 1.00 (ref) |
| Yes | 30 (11.4) | 233 (88.6) | 0.84 (0.53 to 1.32) | 73 (31.6) | 82 (35.5) | 76 (32.9) | 0.84 (0.63 to 1.12) |
| Vitamin E | |||||||
| No | 125 (12.7) | 862 (87.3) | 1.00 (ref) | 296 (34.7) | 298 (35.0) | 258 (30.3) | 1.00 (ref) |
| Yes | 8 (11.0) | 65 (89.0) | 0.92 (0.42 to 2.03) | 25 (37.9) | 20 (30.3) | 21 (31.8) | 1.05 (0.65 to 1.69) |
| Vitamin B6 | |||||||
| No | 105 (12.4) | 742 (87.6) | 1.00 (ref) | 248 (33.8) | 255 (34.8) | 230 (31.4) | 1.00 (ref) |
| Yes | 28 (13.1) | 185 (86.9) | 1.13 (0.71 to 1.80) | 72 (38.7) | 65 (34.9) | 49 (26.3) | 1.29 (0.95 to 1.75) |
| Folic acid | |||||||
| No | 122 (12.5) | 852 (87.5) | 1.00 (ref) | 288 (34.2) | 296 (35.2) | 257 (30.6) | 1.00 (ref) |
| Yes | 11 (12.5) | 77 (87.5) | 1.02 (0.52 to 2.03) | 33 (41.8) | 24 (30.4) | 22 (27.8) | 1.31 (0.84 to 2.03) |
| Vitamin B12 | |||||||
| No | 115 (12.4) | 814 (87.6) | 1.00 (ref) | 277 (34.5) | 270 (33.6) | 256 (31.9) | 1.00 (ref) |
| Yes | 18 (13.5) | 115 (86.5) | 1.09 (0.62 to 1.91) | 44 (37.9) | 50 (43.1) | 22 (19.0) | 1.43 (0.98 to 2.09) |
| Calcium | |||||||
| No | 95 (12.5) | 666 (87.5) | 1.00 (ref) | 224 (34.0) | 235 (35.7) | 199 (30.2) | 1.00 (ref) |
| Yes | 38 (12.60) | 263 (87.4) | 1.04 (0. 68 to 1.60) | 96 (36.8) | 85 (32.6) | 80 (30.7) | 1.10 (0.83 to 1.45) |
| Iron | |||||||
| No | 117 (12.1) | 846 (87.9) | 1.00 (ref) | 294 (35.3) | 293 (35.2) | 246 (29.5) | 1.00 (ref) |
| Yes | 16 (16.0) | 84 (84.0) | 1.67 (0.92-3.03) | 27 (31.0) | 27 (31.0) | 33 (37.9) | 0.88 (0.58 to 1.35) |
| Omega-3 sources | |||||||
| No | 122 (13.2) | 802 (86.8) | 1.00 (ref) | 279 (35.1) | 278 (35.0) | 237 (29.8) | 1.00 (ref) |
| Yes | 11 ( 8.0) | 127 (92.0) | 0.54 (0.28 to 1.06) | 41 (33.3) | 42 (34.1) | 40 (32.5) | 0.94 (0.65 to 1.35) |
| Glucosamine | |||||||
| No | 123 (12.5) | 864 (87.5) | 1.00 (ref) | 289 (33.9) | 300 (35.2) | 263 (30.9) | 1.00 (ref) |
| Yes | 9 (12.0) | 66 (88.0) | 0.82 (0.47 to 1.97) | 29 (45.3) | 20 (31.3) | 15 (23.4) | 1.39 (0.85 to 2.27) |
Adjusted for age, body mass index, race/ethnicity, smoking status, physical activity, alcohol intake, and treatment arm. aOR = adjusted odds ratio; CI = confidence interval; CTCAE = Common Terminology Criteria for Adverse Events; FACT/GOG-Ntx = Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity.
Assessing use both before and during treatment (Supplementary Table 1, available online), compared with nonusers, patients who used multivitamins before diagnosis as well as those who used both before and during treatment were at reduced odds of grade 3 and 4 neuropathy. None of the other supplements was statistically significantly associated with CTCAE neuropathy. Results were somewhat similar when classifying neuropathy according to the FACT-GOG/NTX (Supplementary Table 2, available online).
Discussion
In this study of breast cancer patients enrolled in a large cooperative group clinical trial comparing dosing regimens of doxorubicin, cyclophosphamide, and paclitaxel, we observed associations between multivitamin use both before and during treatment and lower odds of neuropathy assessed by CTCAE and FACT/GOG-Ntx. Associations were strongest in patients using multivitamins both before diagnosis and during chemotherapy. There was a statistically nonsignificant association between iron use during treatment and increased neuropathy. Use of iron may have been initiated during chemotherapy to relieve treatment-induced anemia. We did not observe any statistically significant associations with use of other supplements.
To our knowledge, this is the first study embedded in a therapeutic trial to evaluate the association between dietary supplement use and taxane-induced CIPN. There are several possible explanations to account for the fact that we observed statistically significant associations with multivitamin use but no associations with individual supplements. First, multivitamin use was much higher than use of individual supplements, providing more statistical power to detect a statistically significant association. Therefore, while the association with multivitamin use could be driven by one or more individual components of multivitamins, we would have been less likely to observe a statistically significant association with use of single vitamins. Although few studies have evaluated the relationships between individual supplements and CIPN, some data exist to support this explanation. For example, small trials of omega-3 fatty acid supplementation (15) and of vitamin E (28) found associations with reduced CIPN symptoms, although no association with vitamin E was observed in a larger placebo-controlled, multicenter phase III trial (29). Conversely, however, a well-designed randomized clinical trial of acetyl-L-carnitine was unexpectedly associated with increased CIPN (18), and a recent observational follow-up study of a cohort of breast cancer survivors found that initiation of antioxidant supplements during treatment was associated with increased odds of CIPN (24). However, it is unknown if this association reflects a causal relationship or if patients began use of antioxidant supplements in an effort to reduce symptoms. Disparate results across studies and trials highlight the complexity of relationships between neuropathy and individual supplements, possibly indicating that one supplement alone may not be efficacious in treating symptoms of neuropathy. Such synergy could account for our findings; two or more individual multivitamin components, effective only in combination, could be driving the statistically significant association with multivitamin use. We were unable to evaluate this possibility, however, because of the small numbers of participants who reported individual supplement use. To our knowledge, only one study, of diabetic neuropathy, has evaluated the effect of multiple supplement use. In that placebo-controlled randomized trial, participants received either zinc, magnesium, and vitamins C and E or these supplements plus vitamins B1, B2, and B6, biotin, and folic acid (30). Compared with a placebo-only group, the two supplement groups experienced reduced symptoms of neuropathy, supporting the hypothesis that supplements may have synergistic effects.
Confounding due to lifestyle factors is another possible explanation for our findings. Participants taking multivitamins may have also engaged in other behaviors that may reduce the odds of developing CIPN. There are limited epidemiologic data regarding risk factors for CIPN, but we adjusted for the potential confounding effects of age, BMI, race, smoking status, physical activity, alcohol intake, and treatment arm in multivariable models. A recent study of cancer patients receiving taxane treatment noted that diabetes was associated with a twofold increase in odds of CIPN (31). It is possible that multivitamin users may have been more health conscious, with a lower prevalence of diabetes. While we did not have information regarding diabetes, we did control for BMI, which may have reduced some of the potential confounding; however, our results could nonetheless have been affected by uncontrolled confounding.
A limitation of this study is that it relies on the use of self-report to assess supplement use, which could introduce measurement error. Use of dietary supplements was queried with self-administered questionnaires, and thus we do not have objective data, such as blood markers, with which to conduct sensitivity analyses or statistically correct for potential measurement error. However, patients who participated were highly motivated and were asked to collect their vitamins for recording ingredients and dosages, particularly those taken during chemotherapy. Thus, we have some degree of confidence that self-reports of usage in this study are fairly accurate. Furthermore, we did not collect data regarding the actual form of certain supplements, such as vitamin E, that participants regularly consumed. This could mask the effect of a more active form of a supplement, should one truly exist. Another limitation is that, while supplement use questions referred to use during treatment, we are unable to determine if patients began supplement use before paclitaxel administration or after. It is possible that some participants initiated supplement use after symptoms arose in an attempt to alleviate those symptoms. This would attenuate a true positive association and strengthen a true inverse association. Additionally, for some supplements, use was very low, increasing the possibility of false negatives. Another potential limitation is the assessment of CIPN via CTCAE, which could be prone to poor interobserver agreement (32) and underestimation of CIPN (33). Nevertheless, because neuropathy is physician-assessed, any misclassification is unlikely to vary by supplement use and would bias results toward the null. Because sites were not required to report grade 1 or 2 neuropathies, we were unable to examine the association between supplement use and mild or intermediate neuropathies with the CTCAE, but use of the self-reported FACT/GOG-Ntx allowed us to capture a broader range of neuropathies. We noted similarities in associations between neuropathies and supplements using both measures. Multivitamin use before diagnosis and use during treatment were each associated with decreased CIPN, regardless of which measure was used.
We were also unable to assess nutritional status of patients at baseline. It is possible that multivitamin nonusers could have been deficient in some nutrients compared with users; in that case, multivitamin use could be a proxy for vitamin sufficiency and could account for the observed association. Finally, as in any observational study, our findings could be due to chance, particularly due to the fact that associations with multiple factors were tested. Only a randomized clinical trial of vitamin supplements could assess the direct effects of supplement use on cancer treatment outcomes.
A notable strength of this study, compared with epidemiological population studies of cancer patients, is that it was conducted in the context of a clinical trial, wherein patients with similar tumor characteristics were treated with the same agents, with entry into the DELCaP study at the time of registration to the trial. This enabled us to collect and record use prior to and at the time of diagnosis before patients began treatment, reducing the likelihood of recall bias if behaviors changed with chemotherapy. Furthermore, neurotoxicities were reported by each site prospectively according to CTCAE criteria, again standardizing measurement of this phenotype. However, because of the nature of cooperative group clinical trials, we were unable to obtain detailed information regarding dose modifications that individual patients may have received because of taxane-related side effects. Clinical sites were only required to report information critical to the major objectives of the parent trial, and thus only the most severe grade of toxicity and occurrence of dose reduction were captured. Because we do not know the reason for any potential dose modification or the amount by which doses may have been reduced, it is not possible to link modifications to the timing or existence of CIPN. However, when we included cumulative paclitaxel dose and treatment modification in multivariable models of supplement use and neurotoxicity, risk estimates were not appreciably changed. Prospective studies incorporating detailed information on timing of dose modifications as well as supplement use are warranted to understand the potential relationships between supplement use and the experience of CIPN.
Future studies are needed to replicate this finding as well as to better understand which components of multivitamins could be primarily responsible for the observed reduction in risk of CIPN, or if the associations are affected by potential confounding. Importantly, the potential associations between use of supplements and observed treatment side effects need to be balanced against what, if any, influence vitamin use, particularly during chemotherapy, may have on recurrence and survival in breast cancer patients. With longer follow-up and additional events in S0221, it will be of interest to examine these associations. If results indicate that there are no adverse effects of dietary supplements on cancer outcomes, randomized trials could be justified to determine if specific supplements can be used to reduce treatment-related side effects. Without these types of rigorous prospective studies, changes to clinical practice or recommendations for use of vitamin supplements are clearly not warranted.
Funding
This work was supported by R01 CA116395 (CBA), R01 CA139426 (CBA), the Breast Cancer Research Foundation (CBA), and National Cancer Institute grant P30CA016056 to Roswell Park Cancer Institute. S0221 is supported, in part, by National Cancer Institute (NCI)/Division of Cancer Prevention SWOG NCORP Research Base grant 5UG1CA189974-02; by NCI and National Clinical Trials Network grants CA180888, CA180819, CA180863, CA180858, CA180828, CA180801, CA68183, CA04919, CA13612, CA46282; and by Amgen, Inc.
Note
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
References
- 1. Speck RM, Sammel MD, Farrar JT, et al. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract. 2013;95:e234–e240. [DOI] [PubMed] [Google Scholar]
- 2. Bhatnagar B, Gilmore S, Goloubeva O, et al. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: A single-center experience. Springerplus. 2014;3:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nabholtz JM, Gelmon K, Bontenbal M, et al. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996;146:1858–1867. [DOI] [PubMed] [Google Scholar]
- 4. Winer EP, Berry DA, Woolf S, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: Cancer and Leukemia Group B Trial 9342. J Clin Oncol. 2004;2211:2061–2068. [DOI] [PubMed] [Google Scholar]
- 5. Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: Final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;2610:1642–1649. [DOI] [PubMed] [Google Scholar]
- 6. Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;2324:5542–5551. [DOI] [PubMed] [Google Scholar]
- 7. Kawakami K, Tunoda T, Takiguchi T, et al. Factors exacerbating peripheral neuropathy induced by paclitaxel plus carboplatin in non-small cell lung cancer. Oncol Res. 2012;204:179–185. [DOI] [PubMed] [Google Scholar]
- 8. Baldwin RM, Owzar K, Zembutsu H, et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res. 2012;1818:5099–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leandro-Garcia LJ, Leskela S, Jara C, et al. Regulatory polymorphisms in beta-tubulin IIa are associated with paclitaxel-induced peripheral neuropathy. Clin Cancer Res. 2012;1816:4441–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sucheston LE, Zhao H, Yao S, et al. Genetic predictors of taxane-induced neurotoxicity in a SWOG phase III intergroup adjuvant breast cancer treatment trial (S0221). Breast Cancer Res Treat. 2011;1303:993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abraham JE, Guo Q, Dorling L, et al. Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with Paclitaxel. Clin Cancer Res. 2014;209:2466–2475. [DOI] [PubMed] [Google Scholar]
- 12. Hertz DL, Roy S, Motsinger-Reif AA, et al. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann Oncol. 2013;246:1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hertz DL, Motsinger-Reif AA, Drobish A, et al. CYP2C8*3 predicts benefit/risk profile in breast cancer patients receiving neoadjuvant paclitaxel. Breast Cancer Res Treat. 2012;1341:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rizzo R, Spaggiari F, Indelli M, et al. Association of CYP1B1 with hypersensitivity induced by taxane therapy in breast cancer patients. Breast Cancer Res Treat. 2010;1242:593–598. [DOI] [PubMed] [Google Scholar]
- 15. Ghoreishi Z, Esfahani A, Djazayeri A, et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: A randomized double-blind placebo controlled trial. BMC Cancer. 2012;12:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Argyriou AA, Chroni E, Koutras A, et al. Vitamin E for prophylaxis against chemotherapy-induced neuropathy: A randomized controlled trial. Neurology. 2005;641:26–31. [DOI] [PubMed] [Google Scholar]
- 17. Argyriou AA, Chroni E, Koutras A, et al. Preventing paclitaxel-induced peripheral neuropathy: A phase II trial of vitamin E supplementation. J Pain Symptom Manage. 2006;323:237–244. [DOI] [PubMed] [Google Scholar]
- 18. Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol. 2013;3120:2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demark-Wahnefried W, Aziz NM, Rowland JH, et al. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;2324:5814–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Humpel N, Magee C, Jones SC.. The impact of a cancer diagnosis on the health behaviors of cancer survivors and their family and friends. Support Care Cancer. 2007;156:621–630. [DOI] [PubMed] [Google Scholar]
- 21. Gritz ER, Toll BA, Warren GW.. Tobacco use in the oncology setting: Advancing clinical practice and research. Cancer Epidemiol Biomarkers Prev. 2014;231:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salminen E, Bishop M, Poussa T, et al. Dietary attitudes and changes as well as use of supplements and complementary therapies by Australian and Finnish women following the diagnosis of breast cancer. Eur J Clin Nutr. 2004;581:137–144. [DOI] [PubMed] [Google Scholar]
- 23. Velicer CM, Ulrich CM.. Vitamin and mineral supplement use among US adults after cancer diagnosis: A systematic review. J Clin Oncol. 2008;264:665–673. [DOI] [PubMed] [Google Scholar]
- 24. Greenlee H, Hershman DL, Shi Z, et al. BMI, lifestyle factors, and taxane-induced neuropathy in breast cancer patients: The Pathways Study. J Natl Cancer Inst. 2017;1092: djw206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Budd GT, Barlow WE, Moore HC, et al. SWOG S0221: A phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. J Clin Oncol. 2015;331:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zirpoli GR, Brennan PM, Hong CC, et al. Supplement use during an intergroup clinical trial for breast cancer (S0221). Breast Cancer Res Treat. 2013;1373:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events v. 3.0 (CTCAE). Washington, DC: 2006:72 http://ctep.cancer.gov. Accessed March 31, 2003. [Google Scholar]
- 28. Argyriou AA, Chroni E, Koutras A, et al. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: Final results. Support Care Cancer. 2006;1411:1134–1140. [DOI] [PubMed] [Google Scholar]
- 29. Kottschade LA, Sloan JA, Mazurczak MA, et al. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: Results of a randomized phase III clinical trial. Support Care Cancer. 2011;1911:1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farvid MS, Homayouni F, Amiri Z, et al. Improving neuropathy scores in type 2 diabetic patients using micronutrients supplementation. Diabetes Res Clin Pract. 2011;931:86–94. [DOI] [PubMed] [Google Scholar]
- 31. Hershman DL, Till C, Wright JD, et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group clinical trials. J Clin Oncol. 2016;3425:3014–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Postma TJ, Heimans JJ, Muller MJ, et al. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol. 1998;97:739–744. [DOI] [PubMed] [Google Scholar]
- 33. Shimozuma K, Ohashi Y, Takeuchi A, et al. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer. 2009;1712:1483–1491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.