Abstract
Ssbs of Saccharomyces cerevisiae are ribosome-associated molecular chaperones, which can be cross-linked to nascent polypeptide chains. Because Ssbs are members of a divergent subclass of Hsp70s found thus far only in fungi, we asked if the structural requirements for in vivo function were similar to those of “classic” Hsp70s. An intact peptide-binding domain is essential and an alteration of a conserved residue in the peptide-binding cleft (V442) affects function. However, Ssb tolerates a number of alterations in the peptide-binding cleft, revealing a high degree of flexibility in its functional requirements. Because binding of Ssb to peptide substrates in vitro was undetectable, we assessed the importance of substrate binding using the chimera BAB, in which the peptide binding domain of Ssb is exchanged for the analogous domain of the more “classical” Hsp70, Ssa. BAB, which binds peptide substrates in vitro, can substitute for Ssb in vivo. Alteration of a residue in the peptide-binding cleft of BAB creates a protein with a reduced affinity for peptide and altered ribosome binding that is unable to substitute for Ssb in vivo. These results indicate that Ssb's ability to bind unfolded polypeptides is likely critical for its function. This binding accounts, in part, for its stable interaction with translating ribosomes, even although it has a low affinity for peptides that detectably bind to other Hsp70s in vitro. These unusual properties may allow Ssb to function efficiently as a chaperone for ribosome-bound nascent chains.
INTRODUCTION
At the onset of translation of a polypeptide chain, the first 30–40 amino acids are protected within the exit channel of the ribosome (Malkin and Rich, 1967). Once the polypeptide progresses beyond this length, amino acids begin to be exposed to the cytosol. Until an entire domain is synthesized, the translating protein likely remains unfolded, thereby exposing hydrophobic patches to the crowded environment outside the ribosome. Chaperones are thought to protect newly synthesized chains from aggregation by binding to these exposed hydrophobic patches until the protein is able to fold correctly (Horwich et al., 1993; Hartl, 1996; Deuerling et al., 1999; Teter et al., 1999).
The Hsp70, Ssb, from Saccharomyces cerevisiae has been implicated as a chaperone for nascent polypeptides. Ssb is found associated with ribosomes and can be cross-linked to ribosome-bound nascent polypeptide chains (Pfund et al., 1998). Previous data support a model in which Ssb is localized to the ribosome before the emergence of the nascent chain. Once translation begins, Ssb may form a stable interaction with the translating ribosome, allowing it to function as a chaperone for translating polypeptides. The interaction of Ssb with nontranslating ribosomes is salt sensitive, whereas interaction of Ssb with ribosome-nascent chain complexes (RNCs) is resistant to 1.5 M KCl (Pfund et al., 1998), consistent with the idea that Ssb binds nascent chains.
The Ssb family is composed of two proteins that share 99% sequence identity, Ssb1 and Ssb2. These two proteins are referred to collectively as the Ssbs. Strains carrying deletions of both SSB1 and SSB2, are cold sensitive, osmosensitive, and sensitive to translation-inhibiting drugs such as verrucarin A and the aminoglycoside, paromomycin (Nelson et al., 1992). In particular, this sensitivity to translation-inhibiting drugs provides in vivo evidence for a function of Ssb on the ribosome. Interestingly, SSB genes are regulated like ribosomal protein genes, suggesting a coordinate mode of regulation between this molecular chaperone and the protein synthetic machinery (Lopez et al., 1999).
Like all other Hsp70s, Ssb is structurally composed of three domains: a highly conserved 44-kDa N-terminal ATPase domain (amino acids 1–400), followed by a polypeptide-binding domain that is less conserved (amino acids 401–507) and a variable extreme C-terminal domain (amino acids 507–613). The structures of the ATPase domains from several Hsp70s have been solved (Flaherty et al., 1990; Harrison et al., 1997; Sriram et al., 1997). These structures resemble that of actin and hexokinase, consisting of two lobes with a deep cleft between them in which nucleotide binds. More recently, the structure of the 18-kDa peptide-binding domain from an Escherichia coli Hsp70 was determined (Zhu et al., 1996; Wang et al., 1998; Pellecchia et al., 2000). This domain consists of eight antiparallel β-strands, which form a hydrophobic pocket in which peptide substrate can bind. This domain has been shown to be sufficient to bind substrate in vitro (Wang et al., 1993; Pellecchia et al., 2000). The structure of the complete C-terminal 10-kDa variable region has not been determined; however, the first part of this domain appears to form an alpha helical “lid” for the peptide-binding cleft (Zhu et al., 1996; Wang et al., 1998).
Much of the structure-function analysis of Hsp70s has been focused on DnaK of E. coli. However, the generality of the conclusions drawn from these data is unclear, especially for members of the Hsp70 family that have diverged from those studied most extensively. Because Ssb has evolved to function as a ribosome-bound chaperone, we set out to determine if its structural requirements for function were similar to those of “classic” Hsp70s. Our data indicate that binding of polypeptide substrates is essential for Ssb function in vivo. This binding accounts, in part, for its stable interaction with translating ribosomes, even although it has a low affinity for at least some peptides that bind to other Hsp70s in vitro.
MATERIALS AND METHODS
Yeast Strains, Plasmids, and Media
The yeast strains used in this study are NL164: MATa his3-11,15 leu2-3112 lys1 lys2 Δtrp1 ura3-52 ssb1::HIS3 ssb2::LEU2 (Lopez-Hoyo, unpublished results); JN298: MATa his3-11,15 leu2-3112 ura3-52 trp1–1 ssb1::HIS3 ssb2::LEU2 pep4::HIS3; WY12: MATα his3-11,15 leu2-3112 ura3-52 trp1-1 lys2 SSA1 ssa2-1 ssa3-1 ssa4-2 pep4::HIS3 ssa2::LEU2 ssa3::TRP1 ssa4::LYS2; MW123: MATα his3-11,15 leu2-3112 lys2 Δtrp1 ura3 ssa1::HIS3 ssa2::LEU2; DS10: MATa GAL2 his3-11,15 leu2-3112 lys1 lys2 Δtrp1 ura3-52. Strains were grown in YPD (1% yeast extract, 2% peptone, 2% dextrose) or minimal media lacking uracil (0.67% yeast nitrogen base without amino acids, 2% dextrose, supplemented with all required amino acids). Paromomycin (Sigma Chemical Co., St. Louis, MO) was added at 150 μg/ml unless otherwise noted.
Wild-type SSB1, SSB1 carrying the peptide-binding cleft mutations, were each carried on a pRS316 plasmid (Sikorski and Hieter, 1989) in which the KpnI and in some cases, the XhoI site in the polylinker has been destroyed (pRS316ΔKX). These genes contain three engineered restriction sites (KpnI, NdeI, XhoI) as previously described (James et al., 1997). The His-tagged C-terminal truncations were each carried on a p416-TEF plasmid (Mumberg et al., 1995) except for 488, which was carried on a 2 micron version of the same plasmid. To create the mutations in the peptide-binding clefts of both SSB1 and SSA1 and the stop codons after the indicated β strands, we used sequential rounds of PCR with two complementary degenerate oligonucleotide primers containing mutations at the designated positions (Cormack, 1994). Regions subjected to PCR either were replaced by wild-type gene or were sequenced to verify that no additional mutations were created.
The V435F mutation in SSA1 was cloned as a 0.5-kb NdeI-XhoI fragment into the same sites of pRS316ΔKX-ABA (James et al., 1997) to generate pRS316ΔKX-SSA1-V435F. The V435F mutation in BAB was cloned as a 0.55-kb MunI-XhoI fragment into the same sites of pRS316ΔKX-BAB to generate pRS316ΔKX-BAB-V435F.
To generate the His-tagged version of SSB, a BamHI site was engineered just upstream of the ATG by PCR. This site was then used to clone a 3.2-kb fragment containing SSB into the BamHI site of pRSETB (Invitrogen, Carlsbad, CA), which contains six histidines and an anti-express antibody (Invitrogen) epitope. The gene encoding His-Ssb1 was moved as a 3.4-kb XbaI-Ecl136II fragment from pRSETB into the XbaI-SmaI sites of p416-TEF (Mumberg et al., 1995) to allow the N-terminal His-tagged fusion protein to be expressed under control of the TEF promoter in yeast. The His-tag is not thought to disrupt function, because wild-type Ssb1 carrying this tag can rescue the phenotypes of a ssb deletion strain. The His-tagged BAB gene under the TEF promoter was generated by a triple ligation cloning strategy with the use of NcoI/AatII/Ecl136II from pRS316ΔKX-BAB into pRS416-TEF-HIS-SSB1. The His-tagged BAB can substantially rescue the phenotypes of a strain disrupted for the Ssbs. His-BAB-V435F, also under the TEF promoter was generated by replacing the 1.17-kb MunI-SacII fragment of pRS416-TEF-HIS-BAB with one containing the V435F mutation. The PCR-generated fragments containing premature stop codons in SSB1 were cloned as a 1.3-kb BglII-SacII fragments into the same sites of p416-TEF-HIS-SSB1. His-tagged SSA1 was generated by engineering a BamHI site upstream of the ATG by PCR. This site was used to clone a 3.0-kb fragment containing SSA1 into the BamHI site of pRSETB (see above). The gene encoding His-Ssa1 was moved as a 3.1-kb XbaI-HindIII fragment into the same sites of p416-TEF (Mumberg et al., 1995). His-TEF-SSA1-V435F was created by cloning a 2.0-kb BlpI-SphI fragment containing the V435F mutation from SSA1 into the same sites of p416-TEF-HIS-SSA1.
Purification of His-tagged Proteins
His-tagged Ssb, BAB, and BAB-V435F were purified from JN298 yeast cells as follows. Six liters of cells were grown overnight at 30°C to an OD600 of ∼3. Pelleted cells were resuspended in 60 ml of binding buffer (5 mM imidazole, 0.5 M NaCl, 20 mM Tris, pH 8.0, 10% glycerol, 1 mM PMSF, 5 mM pepstatin A, 100 μM TPCK, 1 mM leupeptin) and disrupted by French press. Cleared and filtered lysate was loaded onto a 5-ml column of His-Bind Resin (Novagen, Madison, WI) at a flow-rate of 0.4 ml/min. The column was washed with 50 ml binding buffer, and the protein was eluted with a 40-ml imidazole gradient from 5 to 500 mM at a flow rate of 0.2 ml/min. Fractions containing the protein of interest were diluted fivefold in ATP-agarose binding buffer (25 mM HEPES, pH 7.5, 20 mM NaCl, 5 mM MgCl2, 0.1 mM EGTA, 15 mM 2-mercaptoethanol, 10% glycerol, 100 μM TPCK, 1 mM PMSF) and loaded onto a 2.0-ml ATP agarose column (Sigma Chemical Co.) at a flow rate of 0.2 ml/min. The column was washed with 30 ml ATP agarose binding buffer and then 30mls of the same buffer containing 500 mM NaCl. Proteins of interest were eluted with a 20mls of ATP agarose binding buffer containing 5 mM ATP at a flow-rate of 0.1 ml/min. Fractions containing the protein of interest were dialyzed against a standard Tris buffer (20 mM Tris, pH 7.5, 20 mM NaCl,10 mM MgCl2, 5 mM 2-mercapthoethanol, 10% glycerol). His-Ssa1 and His-Ssa1-V435F were purified in a similar manner to that described for His-Ssb from WY12 cells.
Fluorescence Anisotropy Peptide-Binding Assay
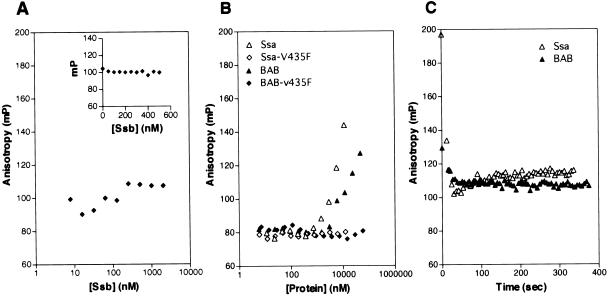
Peptide APPY (CALLQSRLLLSAPRRAAATARY) was labeled with fluorescein (F-APPY) as described (Harrison et al., 1997). F-APPY (10 nM) was incubated with His-Ssa1 or His-Ssa1-V435F ranging in concentration from ∼6 nM to >10 μM overnight at 4°C in the dark to reach equilibrium. F-APPY (35 nM) was incubated in the same manner with His-Ssb1, His-BAB, or His-BAB-V435F ranging in concentrations from ∼4 nM to >10 μM. Measurements were made with the Beacon 2000 Fluorescence Polarization system (Panvera, Madison, WI) at 25°C with excitation at 490 nm and emission at 535 nm. Data were fitted to a single binding site equation to obtain Kd values with the use of Prism2 (GraphPad, San Diego, CA).
Preparation and Treatment of Yeast Extracts
Yeast lysates were prepared in CSB buffer as previously described (Nelson et al., 1992). Polysome analysis was performed as previously described (Yan et al., 1998). Sucrose cushion analysis was performed as described (Pfund et al., 1998). In the case of the ATP addition experiment, ATP was added to lysates at a final concentration of 1 mM in the presence of an ATP-regenerating system (200 mM creatine phosphate and 350 U creatine kinase). These samples were incubated for 20 min at 30°C before centrifugation. All samples used for immunoblot analysis were subjected to SDS-PAGE, transferred to nitrocellulose (Hybond-C; Amersham Corp., Arlington Heights, IL), and immunoblotted for Ssb or L3 with the use of ECL detection system (Amersham). All mutant proteins unable to fully rescue ssb1 ssb2 cells were found to be soluble; thus, phenotypes were not due to aggregation (our unpublished results). In addition, lysates from cells expressing wild-type Ssb or mutant proteins were used for immunoblot analysis with anti-Ssa antibody 60591 that recognizes the SSA3 and SSA4 proteins. No change in protein level was observed in any mutant lysate compared with wild-type (our unpublished results).
RESULTS
Ssb Does Not Require the 10-kDa C-terminal Domain nor β Strand 8 for In Vivo Function
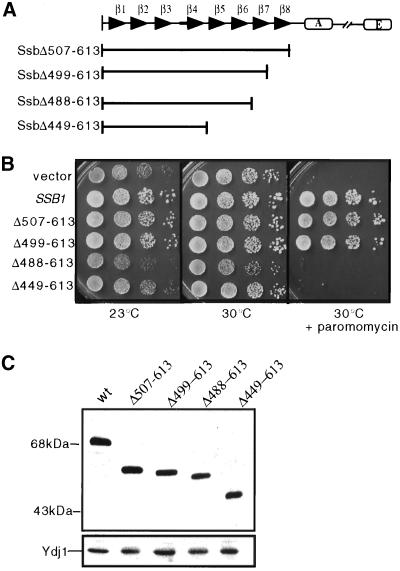
To determine the minimal sequences of Ssb1 required for its function, a series of C-terminal truncation mutants of Ssb1 were generated (Figure 1A). Truncation clones were transformed into a Δssb strain and tested for their ability to rescue the phenotypes associated with the lack of Ssb function: cold-sensitivity and hypersensitivity to translation-inhibiting drugs such as paromomycin. As shown in Figure 1B, Δssb strains expressing truncations lacking the 10-kDa C terminus (Δamino acids 507–613) or the 10-kDa C terminus plus β-strand 8 (Δ499–613) grew well at the semipermissive temperature of 23°C as well as in the presence of 150 μg/ml paromomycin. However, progressive deletion of putative β-strand 7 (Δamino acids 488–613) or strands 4 through 8 (Δamino acids 449–613) resulted in proteins unable to substitute for wild-type Ssb as Δssb strains expressing these truncations failed to grow in the presence of 150 μg/ml paromomycin and grew poorly at low temperature.
Figure 1.
Ssb does not require putative β-strand 8 or the 10-kDa variable domain for function. (A) Schematic diagram of Ssb1 truncations used in this study. The composition of each β-strand and the alpha helicies were predicted based on sequence alignment with the E. coli Hsp70, DnaK and its secondary structure (Zhu et al., 1996; Wang et al., 1998). β-Strands are labeled 1–8, and α-helices comprising the distal C-terminal domain are labeled A–E. (B) Cells from Δssb strains carrying the indicated His-tagged gene were grown overnight, and an equal number of cells were spotted as a 10-fold serial dilution series on SD-uracil plates containing the indicated additions and incubated at the indicated temperatures for 2 d. (C) Lysates were prepared from the strains described above and separated by SDS-PAGE. Immunoblot analysis was performed with the use of antibody against the Express epitope. The blot was also probed with antibody against the cytosolic Hsp40, Ydj1, to assess loading.
The inability of the Δ488–613 and Δ449–613 constructs to substitute for wild-type Ssb1 was not due to decreased levels of expression compared with wild type. All proteins tested were N-terminally His-tagged, and their expression was monitored by immunoblotting with the use of antibodies against an epitope encoded in the tag. Truncations were expressed in Δssb at comparable levels (Figure 1C). These observations suggest that the 10-kDa domain and putative β-strand 8 are not required for Ssb function.
Ssb Differs from DnaK in Its Putative Peptide-binding Domains
The above results indicate that Ssb does not require the C-terminal 10-kDa domain nor β-strand 8 for function. However, Ssb does require most of the predicted peptide-binding domain. To test the contribution of peptide binding to Ssb's function in vivo, we wanted to generate mutations in the peptide-binding cleft of Ssb that would disrupt its interaction with peptide, and thus presumably the nascent chain. Residues thought to interact directly with peptide were predicted based on the 2.0-Å resolution structure of DnaK crystallized in complex with a seven amino acid peptide (NRLLLTG) (Zhu et al., 1996). In DnaK, the side chains of residues F426, V436, I401, T403, S427, and I438 interact with the core leucine residue of the bound peptide and are thought to be critical for peptide binding. These data are supported by results demonstrating the DnaK mutant proteins F426S, S427P, and V436F show a lower affinity for peptide and are unable to substitute for DnaK in vivo (Montgomery et al., 1999; Mayer et al., 2000).
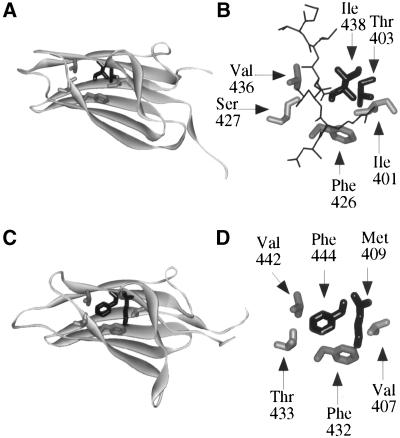
A modeled structure of the peptide-binding domain of Ssb1, with the use of the DnaK coordinates as a template, was generated to identify the residues that were most likely to interact with bound peptide (Figure 2). Comparison of the peptide-binding domain structure of DnaK and a modeled structure of Ssb1 (Figure 2) indicated that the overall structures of the peptide-binding domains are very similar. Not surprisingly, some of the above mentioned residues in DnaK that are thought to participate in peptide binding are conserved both in sequence and position in the putative peptide-binding cleft of Ssb (F432, V442; Figure 2). Others are very similar (T433 and V407 in Ssb compared with S427 and I401 in DnaK; Figure 2). However, structural comparisons also indicated two striking differences between Ssb1 and DnaK at the top of their peptide-binding clefts. Whereas DnaK has an Ile (amino acid 438) and Thr (amino acid 403) at the top of its cleft, Ssb1 has a Phe (amino acid 444) and Met (amino acid 409; Figure 2, shown in black). Based on comparison to DnaK, it is likely that these two residues would contact a peptide bound in the cleft.
Figure 2.
Modeled structure of the peptide-binding domain of Ssb1 compared with the crystal structure of DnaK. (A) The structure of the peptide-binding domain of DnaK (Flaherty et al., 1990; Zhu et al., 1996) as obtained from the Protein Data Bank (PDB) of the Research Collaboratory for Structural Bioinformatics (RCSB; http://www.rcsb.org/pdb/; PDB file 1 DKZ). Structure was prepared with the use of WebLab Viewer Pro (Molecular Simulations Inc., San Diego, CA). The peptide with which this domain was crystallized has been removed for clarity. The side chains of some of the residues that interact with this peptide, which are conserved or similar between Ssb and DnaK, are shown in gray. The side chains of the residues that are not conserved in Ssb are shown in black. (B) Close-up of the side chains that interact with the peptide (black) in DnaK. (C) Modeled structure of the peptide-binding domain of Ssb1 with residues predicted to interact with peptide shown in gray and black as above. The structure of the peptide-binding domain of Ssb was modeled with the use of the coordinates for the peptide-binding domain of DnaK from PDB file 1 DKZ. The model was generated with the use of Sybyl Version 6.4 (Tripos, Inc., St. Louis, MO). (D) Close-up of the side chains that are thought to interact with nascent chain in Ssb. In all structures the hydrogens of the amino acid side chains have been removed for clarity.
Hsp70s Most Closely Related to Ssb Are Found in Fungi
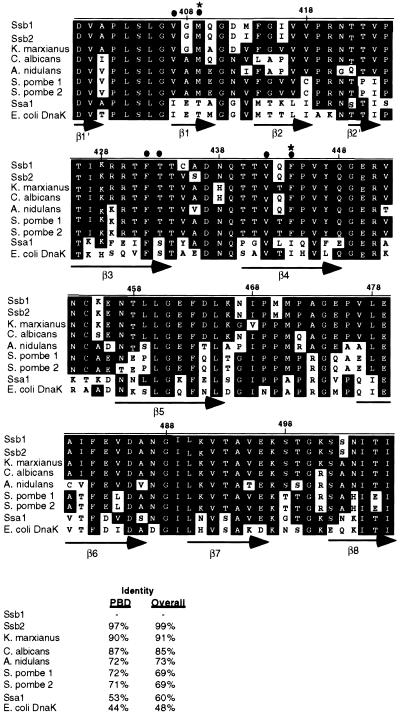
Because these two amino acids were not conserved in the E. coli Hsp70, DnaK, we sought to determine whether they were conserved among any Hsp70s. We examined sequences from 75 Hsp70s from a wide range of species extending from bacteria to humans. This analysis revealed that full-length Ssb shares between 69 and 91% similarity with Hsp70s from four other fungi, whereas it shares only 60% similarity with the other cytosolic Hsp70 from S. cerevisiae, Ssa1. Comparison of only the peptide-binding domains revealed that Ssb is between 90 and 71% similar to other fungal Hsp70s compared with 53% similarity with Ssa1 and 44% similarity with DnaK (Figure 3).
Figure 3.
Alignment of the peptide-binding domains of Ssb1 and its fungal homologues compared with Ssa1 and DnaK. Alignment of Ssb1-like sequences from several fungi as well as Ssa1 from S. cerevisiae and DnaK from E. coli. Amino acid numbers correspond to the SSB1 gene. Amino acid numbers corresponding to the DnaK gene are offset by −6 (e.g., V444 in Ssb1 is V436 in DnaK). β-Strands of DnaK indicated below by arrows. Sequences were aligned with the use of MegAlign (DnaStar, Madison, WI). Sequences were obtained from various sources with the use of a BLAST search through the NIH genebank database (http://www.ncbi.nlm.nih.gov). The accession numbers of the sequences shown are as follows: Ssb1: NP_010052.1, Ssb2: NP_014190.1; K.m.: P41770; C.a.: P87222; E.n.: CAA67431.1; S.p.1: T39633 and Q10284; S.p.2: BAA20093.1; Ssa1: NP_009396; DnaK: AAC73125. *Residues conserved in other Hsp70s that differ in Ssb1 and its fungal homologues; ●, Residues altered in Ssb1
Residues predicted to be on the inside of the peptide-binding cleft (indicated in Figure 3) are conserved between Ssb1 and its fungal homologues but differ from other Hps70s including the other cytosolic Hsp70 from S. cerevisiae, Ssa1. Although all 75 Hsp70s have an Ile (I438 in DnaK) at the top of the peptide-binding cleft, Ssb1 and the other fungal Ssbs have a Phe (444 in Ssb) in this position (Figure 3). In addition, almost every Hsp70 has Thr on the right side of the cleft, whereas this residue is a Met in Ssb1 (Figure 3). There are only two exceptions: an Hsp70 from Rhizopus stolonifer (accession no. pir JC7132), which has a Val at that position and an Hsp70 from Caenorhabditis elegans (accession no. F11F.1), which has a Cys at the same position. These data suggest that Ssb is a fungal-specific Hsp70 that differs in amino acid composition, particularly in its peptide-binding cleft, from other Hsp70s. These genes will be referred to as the fungal Ssbs.
Effects of Alteration of Several Residues in the Peptide-binding Cleft of Ssb
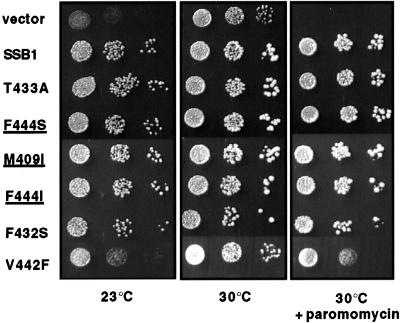
To determine which residues within the peptide-binding cleft of Ssb1 are important for function, we altered three residues in Ssb that are analogous to residues important for the ability of DnaK to bind peptide (F432, V442, T433) as well as the two residues unique to Ssb1's peptide-binding cleft (F444, M409). F432 and V442 were changed to Ser and Phe, respectively, to mimic the mutations that were previously shown to have significant affects on DnaK both in vitro and in vivo (Montgomery et al., 1999; Mayer et al., 2000). Other bulky residues were replaced with small aliphatic ones to disrupt potential contact sites between the binding cleft and peptide. To determine if Phe is absolutely required in position 444, we also changed F444 to Ile, which is the identity of the residue at this position in all other Hsp70s.
The five mutants were assessed for their ability to rescue the phenotypes of a strain lacking Ssb. As shown in Figure 4, Ssb1-V442F demonstrated a reduced ability to substitute for wild-type Ssb, because strains expressing this mutant protein as the only Ssb remained cold-sensitive and mildly sensitive to 650 μg/ml paromomycin, a concentration four times the amount that inhibits Δssb strains from forming single colonies. Ssb1-V442F was expressed at levels equivalent to wild type, indicating that a reduced level of Ssb was not the cause of defects in this mutant (our unpublished results). Therefore, a single amino acid change in the putative binding cleft disrupts function. However, all of the other SSB mutant genes were able to rescue the phenotypes of a Δssb strain in that they grew as well as the Δssb strain carrying a wild-type copy of SSB1 at 23°C and in the presence of 650 μg/ml paromomycin. Therefore, Ssb does not require its unique Phe in the peptide binding cleft for function, nor is its function disrupted by some of the alterations known to disrupt DnaK's function.
Figure 4.
Mutation of several residues in the peptide-binding cleft of Ssb have little effect in vivo. Cells from Δssb strains carrying the indicated mutant on a centromeric plasmid were grown overnight, and an equal number of cells were spotted as a 10-fold serial dilution series on minimal media lacking uracil at the indicated temperature and containing 650 μg/ml paromomycin where indicated. Plates were incubated for 2 d. Residues unique to Ssbs compared with other Hsp70s are underlined.
There Is No Detectable Interaction of Ssb and Peptide In Vitro
The reduced function of Ssb1-V442F suggests that binding to unfolded polypeptides is important for Ssb function. However, the ability of several of the Ssb1 proteins with peptide-binding cleft alterations to rescue the phenotypes of a strain deleted for Ssb did raise the question of whether or not Ssb1 actually interacts with peptide or unfolded polypeptide substrates. Therefore, to directly test the interaction of Ssb1 with peptide, we assessed its ability to interact with a fluorescein-labeled peptide by a fluorescence anisotropy–based assay. The peptide APPY (CALLQSRLLLSAPRRAAATARY) was selected based on its ability to bind DnaK with a Kd of ∼0.1 μM (Montgomery et al., 1999). No change in anisotropy was detected with increasing concentrations of Ssb1 (Figure 5A), indicating that if Ssb1 binds this peptide in vitro, it does so with an extremely low affinity. A second peptide also failed to show an increase in anisotropy (our unpublished results). In addition, we were unable to find a suitable peptide substrate for binding experiments by other screening methods. Therefore, we turned to another approach to test whether peptide binding is important for Ssb function, as described below.
Figure 5.
Fluorescence polarization assays of peptide-binding for Ssa1, Ssb1, BAB, and BAB-V435F. Fluorescence-labeled peptide F-APPY (10 nM) was incubated with the indicated concentrations of His-Ssb1 (A), or His-Ssa1, His-BAB, or His-Ssa1 and His-BAB carrying the V435F mutation (B) until equilibrium was reached at 4°C in the dark. Data were fitted to a single-site binding equation with the use of Prism (GraphPad, San Diego, CA) to determine the Kd of the indicated proteins for peptide. Data are expressed as the observed change in anisotropy over an increasing range of protein concentrations. The inset box in A shows anisotropy of F-APPY and Ssb over a small range of Ssb concentrations. (C) ATP (1 mM) was added to a reaction having a high level of Hsp70 after equilibrium was reached. The change in anisotropy was immediately measured as above. Data are expressed as change in anisotropy over time.
BAB Binds to Peptide In Vitro
A possible reason for the lack of detectable binding between Ssb and peptides could be the differences in its peptide-binding cleft compared with other Hsp70s such as DnaK. Unlike Ssb1, the residues in the peptide-binding cleft of another cytosolic Hsp70 from S. cerevisiae, Ssa1, are conserved with those of DnaK. Therefore, we tested the ability of Ssa1 to interact with fluorescein-labeled APPY peptide (F-APPY). With increasing concentrations of Ssa1, we saw an increase in anisotropy indicating binding of Ssa1 to F-APPY (Figure 5B). Although we were unable to reach saturable binding of the peptide because of our inability to obtain higher concentrations of purified Ssa1, we estimate an apparent Kd of Ssa1 for F-APPY of 5 μM. The increase in anisotropy for Ssa1 was abolished in the presence of ATP as would be predicted for a typical Hsp70: peptide interaction (Figure 5C). In addition, the increase in anisotropy was competed by unlabeled peptide (our unpublished results).
Because we were able to detect an interaction of Ssa1 and F-APPY, we rationalized that a fusion protein, BAB, which contains the ATPase domain of Ssb, the peptide-binding domain of Ssa1, and the 10-kDa domain of Ssb, might demonstrate a detectable interaction with the F-APPY peptide. This fusion protein functionally substitutes for Ssb in vivo, as it substantially rescues the phenotypes of strains lacking Ssb and comigrates with translating ribosomes (James et al., 1997).
BAB was purified from yeast as described in MATERIAL AND METHODS and tested for its ability to bind F-APPY with the use of fluorescence anisotropy. As shown in Figure 5B, an increase in anisotropy was observed with increasing concentrations of BAB. Again, we were unable to reach saturable binding because of limitations in the concentration of BAB that we were able to obtain. Binding of BAB to F-APPY was sensitive to addition of ATP, because the increase in anisotropy observed in the absence of ATP was abolished in the presence of ATP (Figure 5C). In addition, anisotropy levels decreased when competed with unlabeled peptide (our unpublished results). The binding curve for BAB is more shallow than the curve observed for Ssa1, perhaps reflecting a slightly altered interaction of BAB with the F-APPY peptide, in which the peptide has some flexibility inside the peptide-binding pocket of BAB that does not occur with Ssa1. In summary, because BAB can substitute for Ssb and shows detectable interaction with peptide in an in vitro assay, we used BAB to address the importance of nascent-chain binding for the function of Ssb in vivo and the contribution of nascent chain binding to ribosome association.
Alteration of the Peptide-binding Cleft of BAB Leads to an Increase in Kd for Peptide
In attempts to disrupt the ability of BAB (and Ssa1) to bind peptide, a mutation causing a V435F alteration in both Ssa1 and BAB was generated. This alteration is analogous to V442F in Ssb and V436F in DnaK. In Ssb, this mutation disrupts Ssb function as shown in Figure 4. In DnaK, this mutation causes a general decrease in peptide binding to a variety of substrates and renders DnaK null in vivo (Montgomery et al., 1999; Mayer et al., 2000; Rudiger et al., 2000). Ssa1 and BAB proteins carrying this alteration were purified and tested for their ability to bind to the F-APPY peptide with the use of fluorescence anisotropy. As shown in Figure 5B, we were unable to detect any increase in anisotropy with increasing concentrations of either Ssa1 or BAB carrying the V435F mutation. These data suggest that V435 is a residue involved in the peptide-binding ability of Ssa1 (and BAB) and that BAB-V435F can be used to address the importance of peptide binding in BAB's ability to functionally substitute for Ssb in vivo.
A Functional Peptide-binding Cleft Is Needed for BAB Function
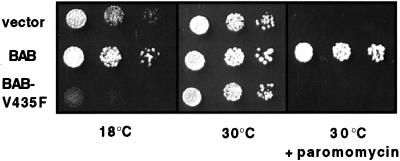
If peptide binding is necessary for BAB function, then BAB-V435F, which is decreased in its ability to bind peptide should be unable to rescue the phenotypes of a Δssb strain. To test this prediction, BAB-V435F was introduced into a Δssb strain and its ability to rescue the cold-sensitivity and hypersensitivity to translation-inhibiting drugs was determined. As shown in Figure 6, BAB-V435F was unable to substitute for the wild-type BAB protein suggesting that peptide binding is important for the function of BAB and therefore, Ssb, in vivo.
Figure 6.
BAB-V435F is unable to substitute for BAB in vivo. Cells from Δssb strains carrying the indicated mutant on a centromeric plasmid were grown overnight, and an equal number of cells were spotted as a 10-fold serial dilution series on minimal media lacking uracil at the indicated temperature and containing the indicated additions. Plates were incubated for 2 d except for the 18°C plate, which was incubated for 5 d.
Ssa1-V435F was also tested for its ability to function in vivo. We assessed whether Ssa1-V435F could rescue the temperature-sensitivity of a strain deleted for SSA1 and SSA2 (MW123). MW123 carrying plasmid-born Ssa1-V435F grew much more poorly at 23°C than the same strain carrying a control vector and was inviable at temperatures >30°C. Therefore, Ssa1-V435F is not functional in vivo and in fact has deleterious effects.
BAB-V435F and Ssb1-V442F Demonstrate a Salt-sensitive Interaction with Translating Ribosomes
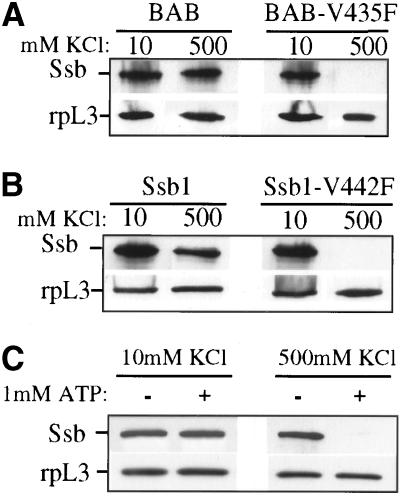
The interaction of Ssb with translating ribosomes is stable in the presence of 1.5 M KCl, whereas the interaction of Ssb with ribosomes lacking nascent chain is no longer detectable after treatment with 0.5 M KCl, bringing up the idea that interaction with the nascent chain may contribute to the chaperone's salt-resistant interaction with the ribosome complex. To assess the contribution of peptide binding to the interaction of Ssb with ribosomes, the association of BAB-V435F and Ssb1-V442F with translating ribosomes was assayed. Crude lysates were centrifuged through sucrose cushions containing 10 mM KCl (low salt), and the pelleted ribosomes were resuspended in a low-salt buffer. These isolated ribosomes were then incubated in either 10 mM or 0.5 M KCl and then centrifuged through cushions containing the same concentration of salt. Isolated ribosomes were analyzed by immunoblot for the presence of BAB or Ssb and the ribosomal protein L3. As shown in Figure 7, A and B, the interaction of both Ssb1-V442F and BAB-V435F with translating ribosomes is sensitive to 0.5 M KCl (high salt). Because the peptide-binding ability of BAB-V435F is severely compromised, this result supports the idea that nascent-chain binding is important for generating the salt-resistant complex. The sensitivity of the interaction of Ssb1-V442F with translating ribosomes is also consistent with the idea that Ssb binds nascent chains and that this ability is partially disrupted in the mutant.
Figure 7.
Interaction of peptide-binding mutants with translating ribosomes is salt sensitive. Extracts were prepared from Δssb cells carrying (A) BAB or BAB-V435F; (B) SSB or SSB-V442F; or (C) SSB, on a centromeric plasmid. Extracts for A and B were run through sucrose cushions, and ribosome pellets were resuspended in buffer containing the indicated amount of KCl. Pellets were incubated for 25 min on ice and then run through sucrose cushions of the indicated concentration of KCl. (C) Lysates were incubated for 20 min at 30°C in the presence of 1 mM ATP plus a regenerating system and the indicated amount of KCl, followed by centrifugation through sucrose cushions of the same KCl concentration. All pellets were resuspended, separated by SDS-PAGE, and immunoblotted with antibodies against Ssb and the ribosomal protein L3 (RpL3).
To further test this idea, we asked whether the interaction of Ssb1 with translating ribosomes became sensitive to high salt in the presence of ATP, because ATP would be expected to destabilize the interaction of Ssb with nascent chains. Indeed, Ssb is no longer associated with ribosomes in 0.5 M KCl after incubation in the presence of ATP (and a regenerating system; Figure 7C). However, ATP had no affect on ribosome association under low-salt conditions. These results further suggest that the ability to bind the nascent chain is necessary for formation of the salt-resistant complex of Ssb with translating ribosomes and its function in vivo.
DISCUSSION
Our aim in this study was to determine the requirements for the in vivo function of Ssb, a specialized ribosome-associated Hsp70. We found that the C-terminal domain is not essential. The peptide-binding domain is required, although the cleft may be able to tolerate more alterations than other Hsp70s. Ssb appears to be a member of a subclass of Hsp70s found only in fungi to date. This Ssb subclass has likely diverged from “classic” Hsp70s to fulfill a role as a chaperone for nascent polypeptide chains.
The C-terminal Domain of Ssb
Despite considerable effort, the general importance of the C-terminal domain for Hsp70 function is unclear. Both structural and biochemical data indicate a role for this domain in the regulation of peptide binding (Zhu et al., 1996; Misselwitz et al., 1998; Pellecchia et al., 2000). Furthermore, there is evidence supporting a role for the 10-kDa domain in communication between the peptide-binding domain and the ATPase domain as well as in facilitation of the interaction of Hsp70 with cohort proteins such as Hsp40 and Hop (Freeman et al., 1995; Demand et al., 1998). Yet, our results indicate that Ssb does not require its C-terminal ∼12 kDa for function in vivo (Figure 1). In good agreement with our results, the C terminus of DnaK of E. coli and Kar2 of the endoplasmic reticulum of S. cerevisiae have recently been shown not to be absolutely required for biological function in vivo (Tokunaga et al., 1998; Mayer et al., 2000; Pellecchia et al., 2000), because proteins lacking various amounts of the C-terminal α helices can partially rescue the phenotypes of null mutants. However, in no case is the result as extreme as we found in the case of Ssb: a deletion that removes not only all the α helices but also the terminal β-strand of the peptide-binding domain functions as well as wild type.
In contrast to the results mentioned above, some data from mammalian systems suggests that the C terminus of Hsc70 is not important for the coupling of the ATPase and peptide-binding domains (Hu and Wang, 1996; Boise and Hightower, 1997; Leng et al., 1998). However, these results in combination with our own, do not necessarily imply the C-terminal domain has no role, even though it is the least conserved of the three domains. In fact, previous results indicate a role for the C terminus of Ssb in cellular localization, as the C terminus of Ssb has a nuclear export signal (NES). Although nearly all wild-type Ssb is found in the cytoplasm, Ssb lacking either the NES or the entire C terminus is found distributed throughout both the nucleus and cytoplasm (Shulga et al., 1999). However, because Ssb lacking the C terminus is functional, active export of Ssb from the nucleus is not required. Furthermore, the levels of Ssb that exist in the cytosol under such circumstances must be adequate for function.
The Peptide-Binding Domain of Ssb
We conclude that peptide-binding activity is required. This conclusion is partially based on the fact that the fusion BAB having an alteration of a valine to phenyalanine in the peptide-binding cleft has a decreased affinity for peptide substrate and is unable to functionally substitute for Ssb1 in vivo. In addition, the analogous Ssb mutant protein (V442F) is reduced in function in vivo. Perhaps, this conclusion is not surprising, considering Ssb's substantial sequence similarity with other Hsp70s. However, several differences in the biochemical properties of Ssb compared with other Hsp70s, including the inability of a number of peptides to stimulate its ATPase activity (Lopez-Buesa et al., 1998) and our inability to detect peptide binding in vitro, made the possibility that Ssb may not bind peptide a concern.
Why have we been unable to detect interaction of Ssb and peptide in vitro thus far? Ssb may have a different substrate binding specificity or a general lowered affinity for substrates compared with other Hsp70s. This possibility is particularly attractive as Ssb, and its fungal homologues have two residues in their peptide-binding clefts that differ from all other Hps70s. Perhaps the presence of these residues generates a peptide-binding cleft with a very low affinity for most peptides substrates that bind well to other Hsp70s, such that interaction of Ssb with peptide in vitro is undetectable.
If Ssb has a decreased affinity for many peptide sequences, then how can it effectively interact with nascent chain? We speculate that by positioning Ssb on the ribosome near the exit site of the nascent chain, the effective concentration of peptide for Ssb is high enough to promote binding. This scenario has some similarities to Bip, which has been hypothesized to bind a wide range of peptides with little substrate specificity by a nonspecific trapping mechanism (Misselwitz et al., 1998). This hypothesis is consistent with our data, which demonstrate that a number of amino acid changes expected to disrupted peptide binding based on their effects on DnaK, do not affect Ssb in vivo (Montgomery et al., 1999; Mayer et al., 2000). According to this scenario, small changes in Kd would have little effect in vivo, as the effective concentration of nascent chain would be higher than the elevated Kd for peptide. Clearly, the peptide binding requirement for Ssb function is somewhat flexible because the Ssa peptide-binding domain can substitute for the binding domain of Ssb. Only changes like the valine to phenylalanine change, which places a large bulky residue in the cleft may result in large changes in Kd that would affect the ability of Ssb (and BAB) to interact with nascent chain and to substitute for wild-type Ssb in vivo. The alteration in DnaK (V436F), which is analogous to the valine to phenylalanine alteration studied here, has been shown to have a general negative effect on the binding of a variety of peptides but does not appear to influence peptide specificity (Rudiger et al., 2000).
It is also possible that Ssb only interacts with peptide (i.e., nascent chain) in the context of the ribosome or in the presence of a regulatory protein(s). Expression of Ssb has evolved to be similar to that of ribosomal proteins genes (Lopez et al., 1999). Therefore, such a regulation in biochemical activity would not be surprising. The peptide-binding cleft of Ssb may be closed when Ssb is free in the cytosol, unattached to the ribosomes. On binding of the ribosome, Ssb may undergo a conformational change that results in opening of the peptide-binding cleft, thereby making it accessible to peptide. If, in fact, Ssb has a broader substrate specificity compared with other Hsp70s, then it may be problematic to have Ssb capable of binding unfolded proteins in the cytosol. Regulating Ssb's ability to interact with peptide would eliminate such a problem.
In both the chimeric BAB peptide domain mutant and Ssb-V442F, the decreased affinity for peptide was accompanied by a decrease in binding to RNC complexes in the presence of high salt. These data suggest that the salt-resistant interaction that is characteristic of Ssb's interaction with translating ribosomes is caused by interaction with the nascent polypeptide chain, as would be expected of the hydrophobic interaction of an Hsp70 with a peptide substrate. Previously, we reported that the interaction of Ssb with RNC complexes was not disrupted by ATP (Pfund et al., 1998). However, these experiments were done at salt concentrations far below 0.5 M. Our results are consistent with Ssb having an interaction with the ribosome in the absence of nascent chains, but the high-salt resistant interaction requires a stable interaction with the nascent chain and is thus destabilized by a weakening of the interaction of Ssb with the nascent chain (i.e., an amino acid alteration in the peptide-binding cleft or the presence of ATP).
Ssb, A Fungal-specific Hsp70
We conclude that Ssb is a fungal-specific chaperone, based on the fact that Ssb is more closely related to other “fungal Ssbs” than to any of the other Hsp70s examined. As discussed above, two residues in the putative peptide-binding cleft of Ssb differ from all other Hsp70s (Figure 3). Interestingly, these residues are conserved among the fungal Ssbs, suggesting functional significance. The contribution of these residues to function is still unclear, yet we hypothesize that they play a role in broadening substrate specificity. One important question is why fungi require a specific ribosome-bound chaperone with broad substrate affinity for nascent chains emerging from the ribosome. At this time, we have no answer. It is tempting to speculate that all organisms require some form of chaperone assistance for nascent chains. This function may not necessarily be carried out by a “classic” Hsp70. For example, the ribosome-bound peptidyl-cis-trans isomerase, trigger factor is a prime candidate for a nascent chain chaperone in E. coli, which like Ssb can be cross-linked to short nascent chains (Lill et al., 1988; Deuerling et al., 1999; Teter et al., 1999).
It has been proposed that trigger factor interacts with nascent polypeptides, first on the translating ribosome and subsequently hands these newly synthesized proteins off to DnaK (Bukau et al., 2000). In yeast, a similar folding pathway for newly synthesized proteins may exist, with Ssb being the first chaperone to interact with nascent chains as they emerge from the ribosome. It is unclear whether Ssb functions as a chaperone for a large population of nascent chains or has specific substrates; however, there are enough molecules of Ssb for there to be two on every ribosome (Pfund et al., 1998). Ssb may remain stationary on the ribosome, binding to different sites on the emerging polypeptide chain or detach from the ribosome, remaining bound to these polypeptides until translation is complete. In any case, Ssb is probably not the last chaperone to bind to the newly synthesized polypeptide. It likely binds to other cytosolic chaperones such as the soluble cytosolic Hsp70 Ssa that act to complete their folding, assist them in translocation across organellar membranes, or hand them off to other chaperone systems (Chirico et al., 1988; Deshaies et al., 1988; Kim et al., 1998)
In mammalian cells Hsp70 has been shown to bind newly synthesized proteins (Beckmann et al., 1990, 1992; Hansen et al., 1994; Eggers et al., 1997; Thulasiraman et al., 1999), but there is no evidence that Hsp70 is ribosome bound. Some have suggested that nascent chain-associated complex (NAC) may function as a chaperone early in protein biogenesis (Wiedmann et al., 1994; Wang et al., 1995); however, yeast have both NAC and Ssb, in addition to another ribosome-associated Hsp70, Pdr13/Ssz1 (Gautschi et al., 2001), increasing questions of functional specificity and/or overlap.
In summary, chaperone assistance during translation is likely a general requirement in all organisms. We propose that in fungi, Ssb has diverged from “classic” Hsp70s to fulfill this role. Some nascent polypeptides may require Ssb for their proper folding, whereas others may require alternative chaperone systems. Much investigation still needs to be done to identify all of the players in folding pathways and the means by which they facilitate efficient protein biogenesis.
ACKNOWLEDGMENTS
We thank John Warner for the L3 antibody, William Walter for technical assistance, Jarek Marszalek for help in the protein purifications, Qinglian Liu for helpful suggestions concerning the anisotropy experiments, and Catherine Fox for critical reading of the manuscript. This work was funded by a grant from National Institutes of Health (NIH) grant 5RO1 GM31107. N.L.H. was supported by NIH fellowship 5F31GM-18507.
REFERENCES
- Beckmann RP, Lovett M, Welch WJ. Examining the function and regulation of hsp70 in cells subjected to metabolic stress. J Cell Biol. 1992;117:1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann RP, Mizzen L, Welch W. Interaction of Hsp70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–856. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Boise JA, Hightower LE. A mutational study of the peptide-binding domain of Hsc70 guided by secondary structure predictions. J Biol Chem. 1997;272:24825–24831. doi: 10.1074/jbc.272.40.24825. [DOI] [PubMed] [Google Scholar]
- Bukau B, Deuerling E, Pfund C, Craig EA. Getting newly synthesized proteins into shape. Cell. 2000;101:119–122. doi: 10.1016/S0092-8674(00)80806-5. [DOI] [PubMed] [Google Scholar]
- Chirico W, Waters MG, Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988;332:805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Cormack B. Introduction of a point mutation by sequential PCR steps. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Vol. 1. New York: John Wiley & Sons; 1994. pp. 8.5.7–8.5.9. [Google Scholar]
- Demand J, Luders J, Hohfeld J. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol Cell Biol. 1998;18:2023–2028. doi: 10.1128/mcb.18.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R, Kock B, Werner-Waxhburne M, Craig E, Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988;332:800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Deuerling E, Schulze-Specking A, Tomoyasu T, Mogk A, Bukau B. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature. 1999;400:693–696. doi: 10.1038/23301. [DOI] [PubMed] [Google Scholar]
- Eggers DK, Welch WJ, Hansen WJ. Complexes between nascent polypeptides and their molecular chaperones in the cytosol of mammalian cells. Mol Biol Cell. 1997;8:1559–1573. doi: 10.1091/mbc.8.8.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KM, DeLuca-Flaherty C, McKay DB. Three dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990;346:623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Freeman B, Myers M, Schumacher R, Morimoto R. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi M, Lilie H, Funfschilling U, Mun A, Ross S, Lithgow T, Rucknagel P, Rospert S. RAC, a stable ribosome-associated complex in yeast formed by the DnaK-DnaJ homologs Ssz1p, and zoutin. Proc Natl Acad Sci USA. 2001;98:3762–3767. doi: 10.1073/pnas.071057198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen WJ, Lingappa VR, Welch WJ. Complex environment of Nascent polypeptide chains. J Biol Chem. 1994;269:26610–16613. [PubMed] [Google Scholar]
- Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl F, Kuriyan J. Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science. 1997;276:431–435. doi: 10.1126/science.276.5311.431. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Horwich AL, Low KB, Fenton WA, Hirshfield IN, Furtak K. Folding in vivo of bacterial cytoplasmic proteins role of GroEL. Cell. 1993;74:909–917. doi: 10.1016/0092-8674(93)90470-b. [DOI] [PubMed] [Google Scholar]
- Hu SM, Wang C. Involvement of the 10-kDa C-terminal fragment of hsc70 in complexing with unfolded protein. Arch Biochem Biophys. 1996;332:163–169. doi: 10.1006/abbi.1996.0328. [DOI] [PubMed] [Google Scholar]
- James P, Pfund C, Craig E. Functional specificity among Hsp70 molecular chaperones. Science. 1997;275:387–389. doi: 10.1126/science.275.5298.387. [DOI] [PubMed] [Google Scholar]
- Kim S, Schilke B, Craig EA, Horwich AL. Folding in vivo of a newly translated yeast cytosolic enzyme is mediated by the SSA class of cytosolic yeast Hsp70 proteins. Proc Natl Acad Sci USA. 1998;95:12860–12865. doi: 10.1073/pnas.95.22.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng C-H, Brodsky JL, Wang C. Isolation and characterization of a DnaJ- like protein in rats: the C-terminal 10-kDa domain of hsc70 is not essential for stimulating the ATP-hydrolytic activity of hsc70 by a DnaJ-like protein. Prot Sci. 1998;7:1186–1194. doi: 10.1002/pro.5560070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R, Crooke E, Guthrie B, Wickner W. The “Trigger Factor Cycle” includes ribosomes, presecretory proteins, and the plasma membrane. Cell. 1988;54:1013–1018. doi: 10.1016/0092-8674(88)90116-x. [DOI] [PubMed] [Google Scholar]
- Lopez N, Halladay J, Walter W, Craig EA. SSB, encoding a ribosome-associated chaperone, is coordinately regulated with ribosomal protein genes. J Bacteriol. 1999;181:3136–3143. doi: 10.1128/jb.181.10.3136-3143.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Buesa P, Pfund C, Craig EA. The biochemical properties of the ATPase activity of a 70-kDa heat shock protein (Hsp70) are governed by the C-terminal domains. Proc Natl Acad Sci USA. 1998;95:15253–15258. doi: 10.1073/pnas.95.26.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin LI, Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967;26:329–346. doi: 10.1016/0022-2836(67)90301-4. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Schroder H, Rudiger S, Paal K, Laufen T, Bukau B. Multistep mechanism of substrate binding determines chaperone activity of Hsp70. Nat Struct Biol. 2000;7:586–593. doi: 10.1038/76819. [DOI] [PubMed] [Google Scholar]
- Misselwitz B, Staeck O, Rapoport T. J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences. Mol Cell. 1998;2:593–603. doi: 10.1016/s1097-2765(00)80158-6. [DOI] [PubMed] [Google Scholar]
- Montgomery D, Morimoto R, Gierasch L. Mutations in the substrate binding domain of the Escherichia coli70 kD molecular chaperone, DnaK, which alter substrate affinity or interdomain coupling. J Mol Biol. 1999;286:915–932. doi: 10.1006/jmbi.1998.2514. [DOI] [PubMed] [Google Scholar]
- Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig EA. The translation machinery and seventy kilodalton heat shock protein cooperate in protein synthesis. Cell. 1992;71:97–105. doi: 10.1016/0092-8674(92)90269-i. [DOI] [PubMed] [Google Scholar]
- Pellecchia M, Montgomery DL, Stevens SY, Kooi CWV, Feng H-p, Gierasch LM, Zuiderweg ERP. Structural insights into substrate binding by the molecular chaperone DnaK. Nat Struct Biol. 2000;7:298–303. doi: 10.1038/74062. [DOI] [PubMed] [Google Scholar]
- Pfund C, Lopez-Hoyo N, Ziegelhoffer T, Schilke BA, Lopez-Buesa P, Walter WA, Wiedmann M, Craig EA. The molecular chaperone SSB from S. cerevisiaeis a component of the ribosome-nascent chain complex. EMBO J. 1998;17:3981–3989. doi: 10.1093/emboj/17.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger S, Mayer MP, Schneider-Mergener J, Bukua B. Modulation of substrate specificity of the DnaK chaperone by alteration of a hydrophobic arch. J Mol Biol. 2000;304:245–251. doi: 10.1006/jmbi.2000.4193. [DOI] [PubMed] [Google Scholar]
- Shulga N, James P, Craig EA, Goldfarb DS. A nuclear export signal prevents Sacchoromyces cerevisaeHsp70 Ssb1p from stimulating nuclear localization signal-directed nuclear transport. J Biol Chem. 1999;274:16501–16507. doi: 10.1074/jbc.274.23.16501. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram M, Osipiuk J, Freeman B, Morimoto R, Joachimiak A. Human Hsp70 molecular chaperone binds two calcium ions within the ATPase domain. Structure. 1997;5:403–414. doi: 10.1016/s0969-2126(97)00197-4. [DOI] [PubMed] [Google Scholar]
- Teter SA, Houry WA, Ang D, Tradler T, Rockabrand D, Fischer G, Blum P, Georgopoulus C, Hartl FU. Polypeptide flux through bacterial Hsp70: DnaK cooperates with trigger factor in chaperoning nascent chains. Cell. 1999;97:755–765. doi: 10.1016/s0092-8674(00)80787-4. [DOI] [PubMed] [Google Scholar]
- Thulasiraman V, Yang C-F, Frydman J. In vivonewly translated polypeptides are sequestered in a protected folding environment. EMBO J. 1999;18:85–95. doi: 10.1093/emboj/18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M, Kato S, Kawamura-Watabe A, Tanaka R, Tokunaga H. Characterization of deletion mutation in the carboxy-terminal peptide-binding domain of the Kar2 protein in Saccharomyces cerevisiae. Yeast. 1998;14:1285–1295. doi: 10.1002/(SICI)1097-0061(1998100)14:14<1285::AID-YEA329>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Kurochkin AV, Pang Y, Hu W, Flynn GC, Zuiderweg ER. NMR solution structure of the 21 kDa chaperone protein DnaK substrate binding domain: a preview of chaperone-protein interaction. Biochemistry. 1998;37:7929–7940. doi: 10.1021/bi9800855. [DOI] [PubMed] [Google Scholar]
- Wang S, Sakai H, Wiedmann M. NAC covers ribosome-associated nascent chains thereby forming a protective environment for regions of nascent chains just emerging from the peptidyl transferase center. J Cell Biol. 1995;130:519–528. doi: 10.1083/jcb.130.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T-F, Chang J-h, Wang C. Identification of the peptide binding domain of hsc70. J Biol Chem. 1993;268:26049–26051. [PubMed] [Google Scholar]
- Wiedmann B, Sakai H, Davis T, Wiedmann M. A protein complex required for signal-sequence-specific sorting and translation. Nature. 1994;370:434–440. doi: 10.1038/370434a0. [DOI] [PubMed] [Google Scholar]
- Yan W, Schilke B, Pfund C, Walter W, Kim S, Craig EA. Zuotin, a ribosome-associated DnaJ molecular chaperone. EMBO J. 1998;17:4809–4817. doi: 10.1093/emboj/17.16.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder W, Gragerov A, Ogata C, Gottesman M, Hendrickson W. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]