Abstract
Background:
Motor slowing is associated with risk of Alzheimer’s disease. Whether β-amyloid (Aβ) burden is associated with motor decline, independent of cognitive decline, is unknown.
Methods:
About 59 cognitively unimpaired older participants had baseline PET-PiB scans and repeated measures of lower (usual gait speed, 400-m time, Health ABC Physical Performance Battery (HABCPPB) score, total standing balance time) and upper (mean tapping time) extremity performance during a mean follow-up of 4.7 years. Linear mixed effect models examined the relationship between baseline Aβ burden and motor decline, adjusting for age, sex, body mass index, cardiovascular risk, APOE ɛ4 status, memory decline, depressive symptoms, ankle–arm index, processing speed, executive function, and cerebrovascular disease.
Results:
Higher mean cortical Aβ burden was associated with greater declines in gait speed and HABCPPB score and a greater increase in 400-m time. Higher Aβ of putamen was associated with declines in all lower extremity measures, including balance. Higher Aβ of dorsolateral prefrontal cortex and lateral temporal lobe was associated with declines of gait speed and 400-m time, and of precuneus with a greater increase in 400-m time. Associations remained similar after further adjustment.
Conclusions:
In cognitively unimpaired older adults, Aβ burden overall and in specific brain regions are risk factors for lower extremity motor decline, independent of memory function. These findings provide the first empirical evidence that Aβ burden is a risk factor for mobility decline in older adults.
Keywords: Neuroimaging, Gait, Epidemiology
Age-related declines in mobility and motor function contribute to late-life disability and have many causes, including cardiopulmonary, musculoskeletal, and neurological impairments (1,2). Other than neurological diagnoses such as stroke and Parkinson’s disease, neurological contributors to age-related motor decline are not well established. Periventricular white matter lesions and gray matter atrophy, particularly in prefrontal and temporal areas, appear to lead to poor mobility (3,4). Although emerging evidence suggests that small vessel ischemic brain disease affects motor performance, β-amyloid (Aβ) burden might also play an independent role in age-related motor decline.
Aβ deposition is closely associated with subsequent Alzheimer’s disease (AD), and motor slowing appears to precede the onset of AD and mild cognitive impairment (MCI) (5). Aβ deposition is known to occur in older persons without cognitive impairment; about 40% of older adults in their 70s known to be without dementia have detectable brain Aβ deposition (6). Compared to controls, individuals who subsequently developed MCI show accelerated motor slowing as early as 12 years prior to the diagnosis (7). With motor decline appearing to develop prior to cognitive decline, and with the known accumulation of Aβ years prior to the development of dementia, the question arises, “Does Aβ accumulation predict motor decline in addition to, and independent of cognitive decline and dementia?” A relationship between global and regional Aβ burden with motor slowing may help explain the observed early motor signs in cognitive decline and AD. In addition, Aβ accumulation could be a potentially modifiable contributor to age-related motor slowing and mobility disability.
There is limited evidence about the relationship between Aβ deposition and motor function. To date, a single cross-sectional study in older adults who had memory complaints suggested that higher amyloid burden was associated with slower gait (8). It remains unclear whether the amyloid burden develops before motor slowing, whether motor slowing is evident beyond gait alone, or whether the relationship is independent of cognitive impairment or vascular risk.
The current study followed older adults over time with extensive assessments of cognition and movement to evaluate whether mean cortical and regional fibrillary Aβ burden is associated with longitudinal change in lower and upper extremity motor function. It assesses whether the effects are independent of vascular risk and memory decline. We predicted that higher baseline cortical Aβ burden was associated with greater motor decline, and the relationship localized to motor planning-related regions.
Methods
Study Population
Participants were drawn from the Baltimore Longitudinal Study of Aging neuroimaging (BLSA-NI) substudy (9). Eligibility for the BLSA-NI substudy includes age 55 years or older without baseline diagnoses involving central nervous system disease (dementia, stroke, bipolar illness, epilepsy), severe cardiac disease (myocardial infraction, coronary artery disease requiring angioplasty, or coronary artery bypass surgery), severe pulmonary disease, or metastatic cancer.
A total of 144 participants who met the inclusion criteria above had in vivo [11C] Pittsburgh compound B (PiB) positron emission tomography (PET) scans of cerebral fibrillar Aβ deposition between 2006 and 2014; 62 of 144 had repeated motor performance testing concurrent with and subsequent to [11C]PiB images. Motor performance was assessed every 4 years in those aged less than 60 years, every 2 years in those 60–79 years, and annually in those aged ≥80 years. At every visit, participants were screened with the Blessed Information Memory Concentration (BIMC) test, the Clinical Dementia Rating (CDR) Scale, or the dementia questionnaire. If the BIMC was ≥4, the CDR was ≥0.5, or if the questionnaire revealed concerns about cognitive status, the participant was reviewed at a consensus conference. The conference adjudicated diagnoses of MCI, AD, and dementia according to published standards (10–13). We excluded data points at and after the date of onset of cognitive impairment or dementia. This study includes 59 cognitively unimpaired participants who had repeated motor performance concurrent with and subsequent to [11C]PiB images. Participants had an average of 3.5 (SD = 1.5) visits during a mean follow-up of 4.7 (SD = 2.2) years. No participants used walking aids while walking 6 or 400 m at baseline.
All participants provided written informed consent at each assessment. This study was approved by the Institutional Review Board of the NIA Intramural Research Program and Johns Hopkins Medical Institutions.
[11C]PiB PET Studies
Dynamic [11C]PiB PET studies (33 time frames over 70 minutes) were performed in 3D mode on a GE Advance scanner at the Johns Hopkins Hospital and University PET facility. Participants were fitted with a thermoplastic mask for PET imaging to minimize motion during scanning. The PET scanning started immediately after intravenous bolus injection of a mean (SD) 14.9 (0.7) mCi of [11C]PiB. PET data were acquired in time frames of 4×0.25, 8×0.5, 9×1, 2×3, and 10×5 minutes. Dynamic images were reconstructed using filtered back projection with a ramp filter, yielding a spatial resolution of about 4.5-mm full width at half maximum at the center of the field of view (image matrix = 128×128, 35 slices, pixel size = 2×2mm, slice thickness = 4.25mm).
Region of Interest Definition
Magnetization-prepared rapid gradient echo (MPRAGE) images acquired on a 3 T scanner at the NIA (Philips Achieva; repetition time = 6.8ms, echo time = 3.2ms, flip angle = 8°, image matrix = 256×256, 170 slices, pixel size = 1×1mm, slice thickness = 1.2mm) were used as the magnetic resonance imaging for regional definitions for 58 participants. One underwent a spoiled gradient-recalled acquisition sequence on a 1.5 T scanner (GE Signa; repetition time = 35ms, echo time = 5ms, flip angle = 45°, image matrix = 256×256, 124 slices, pixel size = 0.94×0.94mm, slice thickness = 1.5mm).
Imaging Processing
Details are described elsewhere (14). Briefly, time frames of each dynamic PET scan were aligned to the average of the first 2 minutes to correct for motion (15). For registration purposes, we obtained static images by averaging the first 20 minutes of each dynamic PET scan. These 20-minute averages were used to align PET scans longitudinally as well as to perform coregistration with corresponding baseline magnetic resonance imagings. A study-specific template was nonlinearly registered onto baseline magnetic resonance imagings. Anatomical labels were then mapped onto the PiB-PET scans. White matter hyperintensities (WMH) were quantified using MPRAGE, T2, and fluid-attenuated inversion recovery images based on a validated support vector machine classifier approach (16,17).
Quantification of Distribution Volume Ratio
Distribution volume ratio (DVR) images were calculated in the native space of each PET image using the simplified reference tissue model with the cerebellar gray matter as the reference tissue (18). Mean cortical DVR (cDVR) was calculated as the average of DVR values from cingulate, frontal, parietal, lateral temporal, and lateral occipital cortices, excluding the sensorimotor strip. We explored the spatial distribution by examining mean DVRs in regions of interest, including early deposition regions (precuneus, putamen, caudate, dorsolateral prefrontal cortex, and lateral temporal lobe) and regions that exhibited PiB signal increases in later stages (primary motor cortex and hippocampus). These regions were selected based on their known relationship with motor function. Primary motor cortex primarily involves movement control of voluntary motor activity. Putamen and caudate are important for sensorimotor control and cognitive information, respectively (for review, see ref. (19)). Dorsolateral prefrontal cortex, lateral temporal lobe, hippocampus, and precuneus are critical for motor planning and spatial navigation (20).
Motor Assessment
Usual gait speed was measured on a 6-m course in an uncarpeted corridor using two trials. Time to complete the course at a usual pace was recorded, and the faster trial was used. The Health ABC Physical Performance Battery (HABCPPB) included timed performance of five repeated chair stands, timed standing balance, timed 6-m walk at a usual pace, and timed narrow 6-m walk test (21). The 400-m walk test was performed at the fastest possible pace and time to complete was recorded (22). Balance was assessed as total standing balance time using a subscale of the HABCPPB, including semitandem, full-tandem, and single leg standing trials.
Upper extremity motor function was measured using a finger-tapping task. The simple task, performed three times, required the participant to tap on a keyboard with a single index finger as quickly as possible for 10 seconds. The complex task, performed three times, required the participant to tap for 10 seconds as quickly as possible on a key board using the left and right index finger alternately. The average tap time for simple and complex tapping tasks, converted from the number of taps, was used for analyses.
Other Measures of Interest
Body mass index was calculated as body weight in kilograms divided by the square of the height in meters. A cardiovascular risk score was calculated as the sum of seven cardiovascular conditions, including smoking history, the presence of high cholesterol (total cholesterol levels ≥240mg/dL), diabetes, hypertension, myocardial infarction, transient ischemic attack, and angina. APOE ε4 status was determined using standard procedures (23). Verbal memory was assessed using the California Verbal Learning Test (CVLT) immediate recall. Processing speed was assessed using the Digit Symbol Substitution Test (DSST). Executive function was assessed using the Trails Making Test Part B (TMT-B). Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D). Cerebrovascular disease was assessed as a ratio of WMH to the intracranial volume (ICV). Peripheral arterial disease was assessed using ankle–arm index (24). Of 59, five had a CDR of 0.5 at the baseline visit but none met consensus criteria for MCI.
Statistical Analysis
Associations of cDVR and regional DVRs with motor change were examined using linear mixed effects models (SAS v. 9.3; SAS Institute, Cary, NC). Each motor measure served as a separate dependent variable. The linearity assumption was confirmed because the quadratic interval term (ie, number of years between baseline and each follow-up visit) was not significant for all motor measures (p > .05). DVR, interval, and DVR × interval were modeled as fixed effects. The intercept and interval were allowed to vary between individuals and modeled as random effects. For lower extremity motor function, models first adjusted for baseline age, sex, and body mass index, and for upper extremity function adjusted for baseline age and sex as fixed effects. Baseline age was centered to the mean. DVR values were centered to the mean and multiplied by 10 for interpretation purposes.
To examine whether cardiovascular risk, APOE ε4 status, memory, depressive symptoms, peripheral arterial disease, processing speed, executive function, or cerebrovascular disease would affect the relationship, models further adjusted for each. Models adjusted for baseline cardiovascular risk score, APOE ε4 status, ankle–arm index, DSST, TMT-B, and WMH/ICV, while models adjusted for repeated measures of CVLT and CES-D. Covariates and their interactions with interval were fixed effects. Analyses were repeated by excluding five with a baseline CDR of 0.5. Analyses were repeated in 62 participants regardless of cognitive status. To evaluate the influence of interval, we stratified the analyses in those aged less than 80 years who had evaluations every 2 years (n = 40) and those aged 80 and older (n = 19) who had evaluations annually. Effects persisted for the younger strata, but sample size was too small to evaluate the older strata (not shown). For cDVR associations, significance was reported at p < .05. For regional associations, we corrected for multiple comparisons using a Bonferroni correction (p ≤ .007; 0.05/7 = 0.007). For graphical illustration, participants were grouped into PiB+ (n = 18) and PiB− (n = 41), using a cDVR threshold of 1.066 (14).
Results
The 59 participants are described in Table 1. They are diverse in age, sex, race, and magnitude of Aβ burden. During follow-up, the group as a whole declined 0.011 m/s/y in gait speed (p = .014), increased 9.352s/y in 400-m time (p < .001), decreased 0.048 per year in HABCPPB score (p = .007), and decreased 1.8s/y in balance time (p < .001). There was no significant change in either tapping time (p > .05).
Table 1.
Baseline Sample Characteristics (n = 59)
| Mean (SD) or N (%) | Range | |
|---|---|---|
| Characteristics | ||
| Age, y | 74.8 (7.8) | 56.0–89.0 |
| Female sex | 30 (50.8) | — |
| White | 49 (83.1) | — |
| Education, y | 16.9 (2.1) | — |
| Height, cm | 169.1 (8.5) | 149.5–184.5 |
| Weight, kg | 77.7 (13.6) | 41.0–108.2 |
| Body mass index, kg/m2 | 27.1 (4.3) | 18.3–42.1 |
| MMSE (range 0–30) (n = 53) | 29.0 (1.2) | 25–30 |
| CES-D (range 0–60) | 4.5 (5.6) | 0–22 |
| CVD risk score | 1.2 (1.0) | 0–4 |
| Ankle–arm index (n = 55) | 1.17 (0.10) | 0.83–1.40 |
| WMH/ICV (n = 56) | 0.003 (0.004) | 0–0.023 |
| Cognitive measures | ||
| CDR of 0.5 at baseline | 5 (0.08) | — |
| CVLT immediate recall (n = 56) | 59.0 (10.7) | 39.0–80.0 |
| DSST (n = 57) | 45.0 (11.1) | 26.0–71.0 |
| TMT-B (n = 51), s | 68.0 (26.0) | 32.0–135.0 |
| APOE ɛ4+ (n = 53) | 14 (23.7) | — |
| Lower extremity motor function | ||
| Usual gait speed, m/s | 1.14 (0.20) | 0.48–1.55 |
| 400-m time, s | 277.9 (64.0) | 209.6–574.9 |
| HABCPPB score | 2.7 (0.7) | 0.3–3.3 |
| Total standing balance time, s | 75.5 (22.2) | 3.1–90.0 |
| Upper extremity motor function | ||
| Simple tapping (average per tap, s) | 0.19 (0.03) | 0.13–0.32 |
| Complex tapping (average per tap, s) | 0.17 (0.06) | 0.11–0.39 |
| Mean cortical DVR | 1.079 (0.156) | 0.942–1.622 |
| Regional DVR | ||
| Precuneus | 1.169 (0.199) | 0.979–1.824 |
| Putamen | 1.309 (0.146) | 1.077–1.755 |
| Caudate | 1.096 (0.136) | 0.922–1.416 |
| Dorsolateral prefrontal cortex | 1.082 (0.179) | 0.915–1.676 |
| Lateral temporal lobe | 1.046 (0.134) | 0.911–1.531 |
| Primary motor cortex | 1.023 (0.096) | 0.916–1.402 |
| Hippocampus | 1.021 (0.049) | 0.920–1.179 |
Note: CDR = Clinical Dementia Rating; CES-D = Center for Epidemiologic Studies Depression Scale; CVD = cardiovascular; CVLT = California Verbal Learning Test; DSST = Digit Symbol Substitution Test; DVR = distribution volume ratio; HABCPPB = Health ABC Physical Performance Battery ICV = intracranial volume; MMSE = Mini Mental State Exam TMT-B = Trails Making Test, part B; WMH = white matter hyperintensities.
cDVR and Motor Change
In cross-sectional unadjusted models, higher cDVR was associated with poorer HABCPPB score and shorter balance time, but not with gait speed or 400-m time (Table 2, Model 1). After adjustment for age, sex, and body mass index, cross-sectional associations with HABCPPB score and balance time were attenuated (Table 2, Model 2). Further adjustment did not change the cross-sectional associations (Table 2, Models 3–5).
Table 2.
Associations of Mean Cortical DVR With Lower Extremity Motor Change (n = 59)
| Model | Usual Gait Speed, m/s | 400-m Time, s | HABCPPB Score | Balance Time, s | ||||
|---|---|---|---|---|---|---|---|---|
| Cross-sectional | Longitudinal | Cross-sectional | Longitudinal | Cross-sectional | Longitudinal | Cross-sectional | Longitudinal | |
| β (95% CI), p Value | ||||||||
| 1 | −0.004 (−0.040, 0.032), .84 | −0.009 (−0.016, −0.003), .006 | 2.851 (−10.379, 16.080), .67 | 3.638 (1.636, 5.640), <.001 | −0.094 (−0.183, −0.005), .04 | −0.028 (−0.053, −0.003), .03 | −4.705 (−7.969, −1.441), .006 | −0.552 (−1.289, 0.184), .15 |
| 2 | 0.024 (−0.009, 0.057), .15 | −0.009 (−0.016, −0.003), .004 | −5.586 (−16.788, 5.615), .32 | 3.566 (1.591, 5.541), <.001 | −0.024 (−0.108, 0.059), .56 | −0.028 (−0.052, −0.003), .03 | −2.434 (−5.569, 0.702), .13 | −0.571 (−1.307, 0.164), .13 |
| 3 | 0.023 (−0.010, 0.056), .17 | −0.009 (−0.016, −0.001), .02 | −4.640 (−16.120, 6.840), .42 | 3.176 (1.088, 5.263), .003 | −0.031 (−0.118, 0.055), .47 | −0.023 (−0.050, 0.003), .08 | −3.138 (−6.236, −0.040), .05 | −0.356 (−1.142, 0.430), .37 |
| 4 | 0.031 (−0.007, 0.068), .10 | −0.010 (−0.017, −0.003), .008 | −5.380 (−19.332, 8.572), .44 | 4.108 (2.059, 6.157), <.001 | −0.055 (−0.177, 0.067), .37 | −0.030 (−0.053, −0.008), .009 | −2.689 (−6.649, 1.270), .18 | −0.458 (−1.209, 0.294), .23 |
| 5 | 0.018 (−0.015, 0.052), .28 | −0.011 (−0.018, −0.003), .005 | −4.713 (−15.956, 6.530), .40 | 3.212 (1.086, 5.338), .003 | 0.003 (−0.087, 0.092), .95 | −0.034 (−0.058, −0.009), .008 | −1.103 (−4.138, 1.931), .47 | −0.840 (−1.621, −0.059), .03 |
| 6 | 0.025 (−0.008, 0.057), .13 | −0.010 (−0.016, −0.003), .005 | −5.643 (−16.998, 5.712), .32 | 3.561 (1.576, 5.545), <.001 | −0.023 (−0.107, 0.061), .59 | −0.028 (−0.052, −0.003), .03 | −2.308 (−5.597, 0.981), .17 | −0.637 (−1.316, 0.042), .07 |
| 7 | 0.035 (0.000, 0.070), .05 | −0.008 (−0.016, −0.000), .04 | −6.569 (−20.070, 6.932), .33 | 3.392 (−24.504, 16.084), .007 | −0.010 (−0.102, 0.083), .83 | −0.012 (−0.041, 0.017), .41 | −2.660 (−6.024, 0.705), .12 | −0.068 (−0.927, 0.791), .88 |
| 8 | 0.032 (−0.005, 0.069), .09 | −0.009 (−0.017, −0.002), .01 | −6.375 (−19.272, 6.522), .33 | 4.146 (1.968, 6.324), <.001 | −0.054 (−0.149, 0.042), .27 | −0.023 (−0.050, 0.003), .08 | −3.163 (−6.830, 0.504), .09 | −0.561 (−1.367, 0.246), .17 |
| 9 | 0.023 (−0.010, 0.057), .17 | −0.007 (−0.014, −0.001), .04 | −5.471 (−17.055, 6.114), .35 | 2.927 (0.925, 4.928), .005 | −0.019 (−0.107, 0.068), .67 | −0.022 (−0.048, 0.004), .10 | −2.269 (−5.536, 0.999), .17 | −0.471 (−1.265, 0.323), .24 |
| 10 | 0.022 (−0.020, 0.064), .30 | −0.006 (−0.014, 0.001), .10 | −6.077 (−21.216, 9.062), .42 | 2.803 (0.524, 5.081), .02 | −0.079 (−0.191, 0.033), .16 | −0.017 (−0.044, 0.009), .19 | −3.763 (−7.694, 0.168), .06 | −0.210 (−1.038, 0.617), .62 |
| 11 | 0.024 (−0.010, 0.058), .16 | −0.009 (−0.016, −0.002), .010 | −4.531 (−16.289, 7.226), .443 | 2.916 (0.819, 5.014), .007 | −0.016 (−0.102, 0.071), .72 | −0.024 (−0.049, 0.001), .06 | −2.118 (−5.429, 1.192), .20 | −0.545 (−1.303, 0.212), .16 |
Note: BMI = body mass index; CDR = Clinical Dementia Rating; CES-D = Center for Epidemiologic Studies Depression Scale; CVD = cardiovascular; CVLT = California Verbal Learning Test; DSST = Digit symbol substitution test; DVR = distribution volume ratio; HABCPPB = Health ABC Physical Performance Battery; ICV = intracranial volume; TMT-B = Trails Making Test; WMH = white matter hyperintensities. Model 1: unadjusted; Model 2: age, sex, BMI; Model 3: Model 2 + CVD score; Model 4: Model 2 + ApoE ε4 (n = 53); Model 5: Model 2 + ΔCVLT + baseline CVLT; Model 6: Model 2 + time-dependent CES-D score; Model 7: Model 3 excluding those with a baseline CDR of 0.5 (n = 54). Model 8: Model 2 + ankle–arm index (n = 55). Model 9: Model 2 + DSST (n = 57). Model 10: Model 2 + TMT-B (n = 51). Model 11: Model 2 + WMH/ICV (n = 56). Bold numbers reflected associations of p < .05 (*with Bonferroni corrections).
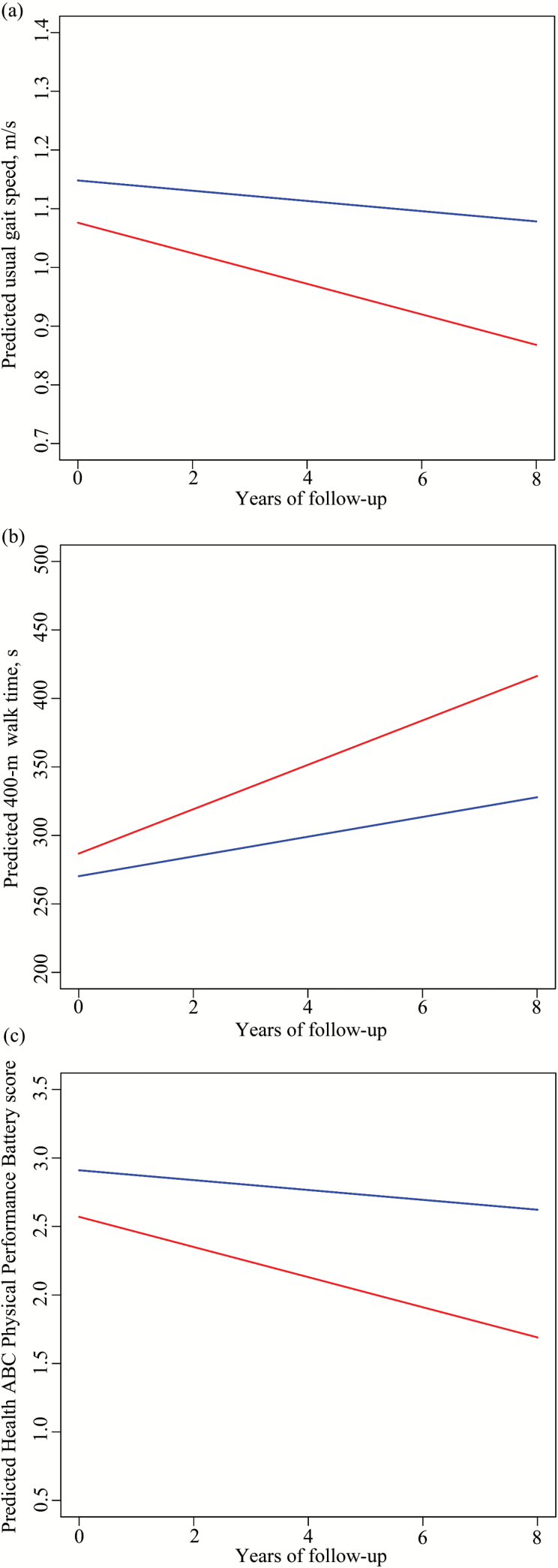
Longitudinally, higher cDVR was associated with greater declines in gait speed and HABCPPB score and with a greater increase in 400-m time (Figure 1), and not with change in balance time (Table 2, Model 1). Associations with change in 400-m time remained significant in all adjusted models (Table 2, Models 1–10). Most associations with changes in gait speed and HABCPPB remained significant after adjustment. Adjustment for TMT-B attenuated the association with gait decline (Table 2, Model 10). Adjustment for cardiovascular risk, ankle–arm index, DSST, TMT-B, or WMH/ICV attenuated the association with change in HABCPPB score (Table 2, Models 3, 8–11). The analysis which excluded five with a baseline CDR of 0.5 attenuated the association with change in HABCPPB score (Table 2, Model 7).
Figure 1.
Predicted longitudinal change in usual gait speed (a), 400-m walk time (b), and Health ABC Physical Performance Battery score (c) in PiB+ (red: cortical distribution volume ratio [cDVR] ≥ 1.066) and PiB− (blue: cDVR < 1.066) groups.
There were no significant cross-sectional or longitudinal associations of cDVR with either tapping time, with or without covariate adjustment (Supplementary Table 1).
Regional DVR and Motor Change
The cross-sectional associations between regional DVRs and motor performance measures were not significant after covariate adjustment (p > .05).
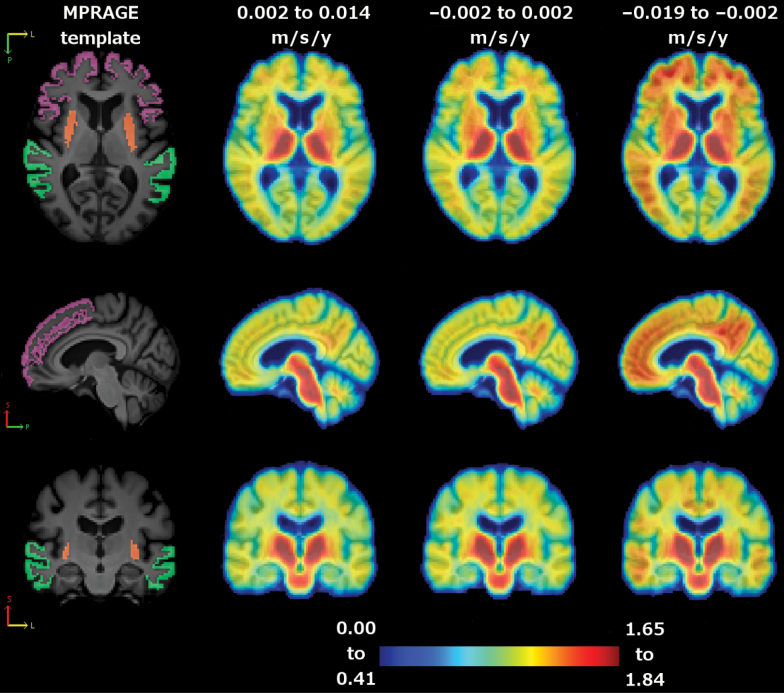
In analyses adjusted for age, sex, and body mass index, higher DVR in putamen was associated with greater declines in all lower extremity motor performances, including balance (Table 3). Higher DVR in precuneus was associated with a greater increase in 400-m time. Higher DVRs in dorsolateral prefrontal cortex and lateral temporal lobe were associated with a greater gait decline and a greater increase in 400-m time (Table 3). These associations were robust with further adjustment for APOE ε4 status, change in CVLT, or time-dependent CES-D score (Supplementary Table 2A–C). When regional analyses were adjusted for cardiovascular risk score, ankle–arm index, DSST, TMT-B, or WMH/ICV, some findings were diluted but others remained robust (Supplementary Table 2D–H). For example, higher DVRs in dorsolateral prefrontal lobe and lateral temporal lobe remained significantly associated with change in 400-m time. In Figure 2, regional DVRs significantly associated with gait decline are illustrated for each tertile of rate of change in gait speed. Some longitudinal associations with an uncorrected p value of <.05 did not survive Bonferroni corrections, including associations of DVR in precuneus and caudate with gait decline, associations of DVR in primary motor cortex with 400-m time change, and associations of DVR in precuneus and dorsolateral prefrontal cortex with change in HABCPPB score.
Table 3.
Longitudinal Associations of Regional DVR With Lower Extremity Motor Change Adjusted for Age, Sex, and Body Mass Index (n = 59)
| Regions of Interest | Usual Gait Speed, m/s | 400-m Time, s | HABCPPB Score | Balance Time, s |
|---|---|---|---|---|
| β (95% CI), p Value | ||||
| Precuneus | −0.006 (−0.011, −0.001), .01 | 1.944 (0.592, 3.296), .005* | −0.023 (−0.041, −0.006), .009 | −0.364 (−0.895, 0.167), .18 |
| Putamen | −0.011 (−0.017, −0.004), .001* | 3.198 (0.946, 5.449), .006* | −0.040 (−0.064, −0.016), .001* | −0.975 (−1.651, −0.299), .005* |
| Caudate | −0.007 (−0.013, −0.000), .04 | 1.796 (−0.183, 3.776), .08 | −0.019 (−0.044, 0.005), .12 | −0.466 (−1.169, 0.237), .19 |
| Dorsolateral prefrontal lobe | −0.008 (−0.014, −0.002), .006* | 3.508 (1.724, 5.291), <.001* | −0.023 (−0.045, −0.001), .04 | −0.520 (−1.173, 0.132), .12 |
| Lateral temporal | −0.012 (−0.020, −0.004), .004* | 4.347 (2.038, 6.655), <.001* | −0.026 (−0.057, 0.004), .09 | −0.646 (−1.548, 0.256), .16 |
| Primary motor cortex | −0.011 (−0.022, 0.000), .06 | 3.556 (0.462, 6.649), .03 | −0.040 (−0.082, 0.002), .06 | −0.381 (−1.657, 0.895), .56 |
| Hippocampus | −0.008 (−0.026, 0.010), .36 | −3.114 (−8.342, 2.114), .24 | 0.025 (−0.044, 0.094), .47 | −0.860 (−2.833, 1.114), .39 |
Notes: DVR = distribution volume ratio; HABCPPB = Health ABC Physical Performance Battery. Bold numbers reflected associations of p < .05 (*with Bonferroni corrections).
Figure 2.
Average cortical distribution volume ratio (cDVR) images by tertile of gait decline. Left to right: magnetization-prepared rapid gradient echo (MPRAGE) template (orange: putamen; purple: dorsolateral prefrontal cortex; and green: lateral temporal lobe), average cDVR image for lower tertile, middle tertile, and upper tertile. Top to bottom: axial, sagittal, and coronal slice.
There were no cross-sectional or longitudinal associations with either tapping time in any regions of interest, with or without adjustment (p > .05).
Results remained similar in 62 participants regardless of cognitive status (Supplementary Table 3).
Discussion
Among cognitively unimpaired older adults, total and regional Aβ burden predicted decline in multiple aspects of lower extremity performance. Results were robust with adjustment for cardiovascular risk or APOE ε4 status and were independent of memory decline. Decline in lower extremity performance over time was associated with Aβ burden localized to early deposition regions important for motor planning, such as the putamen, dorsolateral prefrontal cortex, lateral temporal lobe, and precuneus, but not in regions of later deposition, such as the primary motor cortex or hippocampus. Our findings show for the first time that amyloid deposition may affect the rate of decline in mobility in older adults who do not meet criteria for cognitive impairment.
Our findings regarding Aβ burden in the putamen are consistent with previous cross-sectional results showing the significant association of Aβ burden in the putamen, but not caudate, with slower gait (8). We also did not find significant associations in the caudate, despite evidence of caudate amyloid deposition. This is difficult to explain because the caudate plays important roles in cognitive and motor function, including spatial navigation and goal-directed activity (19). The motor tasks we tested may not have been sensitive to complex motor functions. We did not find strong associations of Aβ in the primary motor cortex or hippocampus. Studies in healthy older adults may not be able to detect effects in these regions where there is little early Aβ deposition and low interindividual variation.
We did not find a cross-sectional association between Aβ burden and gait speed, although we observed unadjusted cross-sectional associations with HABCPPB score and balance time. The recent cross-sectional study found an association between Aβ burden and gait speed in an older population, in whom 99% had memory complaints (8). In contrast, our study population was healthier, free of cognitive and mobility impairment, and walked faster at baseline. Thus, the relative homogeneity of our sample at baseline may limit our ability to detect cross-sectional effects. Because lower extremity motor function is affected by many factors, including cardiovascular and musculoskeletal deficits, the cross-sectional association between Aβ burden and gait may be confounded by individual differences in these other factors. Contrary to the multiple significant associations with lower extremity motor decline, we did not find any associations with finger tapping. Perhaps these upper extremity tasks reflect speed of movement more purely and did not require the kind of motor planning and integration that was demanded by our lower extremity tasks. Alternatively, our upper extremity tasks may not be very demanding because we also did not find an expected effect of aging over time.
This study has several strengths. Our cohort was followed longitudinally with multiple measures of physical performance repeated over time, allowing us to test whether Aβ burden is followed by performance declines. All BLSA participants are evaluated with respect to MCI and dementia, allowing us to exclude participants with cognitive impairment. Our findings remained robust despite the challenges of a Bonferroni correction and adjustment for covariates, including cardiovascular risk score and APOE ε4 status. The associations remained after adjustment for memory decline, suggesting that motor decline may be independent of memory decline with age. We explored the spatial distribution of Aβ burden in relation to motor changes. The pattern of involved regions generally supports a model of adverse effects of localized amyloid burden on motor planning and sensorimotor integration.
This study has limitations. Our study sample was in good health with very good physical function at enrollment into the BLSA-NI substudy, potentially limiting generalizability. Although our sample size could be considered modest for a longitudinal study, it is quite sizable for a PET study that includes longitudinal follow-up of both multiple aspects of motor function and careful assessment of cognition. Although age-stratified results are difficult to interpret due to the small sample size in the older age stratum, most of longitudinal associations persisted in the younger age stratum which was somewhat larger. Future studies or multisite collaborations could overcome these limitations. We selected brain regions of interest a priori, based on known relationships with motor planning and integration. By ignoring other regions, we might have missed potentially important associations that were not expected given current knowledge. In this exploratory analysis, a Bonferroni correction is conservative. We did not adjust for brain atrophy. Future studies in larger samples with longitudinal assessment of amyloid burden, brain atrophy, and gait could investigate the contribution of brain atrophy to the relationship between amyloid burden and gait decline.
Early amyloid deposition in motor planning-related brain regions might play a key role in age-related decline in physical performance and mobility. These findings may help to explain why motor changes seem to precede the cognitive changes that can lead to cognitive impairment and AD. Because cognitive and mobility-related deficits are major contributors to late-life disability, early detection of amyloid burden may provide a novel strategy to prevent or delay these twin evils of aging.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research was supported in part by the Intramural Research Program of the NIA (03-AG-0325).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Material
References
- 1. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. [DOI] [PubMed] [Google Scholar]
- 2. Brach J, Rosano C, Studenski S. Mobility. In: Halter J, ed. Textbook of Geriatrics and Gerontology. 7th ed. New York, NY: McGraw-Hill; 2009. [Google Scholar]
- 3. Annweiler C, Beauchet O, Celle S, et al. ; WALK Team (Working Group Angers-London for Knowledge) Contribution of brain imaging to the understanding of gait disorders in Alzheimer’s disease: a systematic review. Am J Alzheimers Dis Other Demen. 2012;27:371–380. doi:10.1177/1533317512454710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi:10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 2015;11:70–98. doi:10.1016/j.jalz.2014.04.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13:997–1005. doi:10.1016/S1474-4422(14)70194-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980–986. doi:10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Del Campo N, Payoux P, Djilali A, et al. Relationship of regional brain beta-amyloid to gait speed. Neurology. 2016; 86: 36–43. doi:10.1212/WNL.0000000000002235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Resnick SM, Goldszal AF, Davatzikos C, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. [DOI] [PubMed] [Google Scholar]
- 10. Zonderman AB, Giambra LM, Arenberg D, Resnick SM, Costa PT, Jr, Kawas CH. Changes in immediate visual memory predict cognitive impairment. Arch Clin Neuropsychol. 1995;10:111–123. [PubMed] [Google Scholar]
- 11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. 4th ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 12. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 13. Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. [DOI] [PubMed] [Google Scholar]
- 14. Bilgel M, An Y, Zhou Y, et al. Individual estimates of age at detectable amyloid onset for risk factor assessment. Alzheimer’s & Dementia 2016;12:373–379. doi:10.1016/j.jalz.2015.08.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 16. Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–313. doi:10.1016/j.acra.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv. 2008;11(Pt 1):620–627. [DOI] [PubMed] [Google Scholar]
- 18. Zhou Y, Resnick SM, Ye W, et al. Using a reference tissue model with spatial constraint to quantify [11C]Pittsburgh compound B PET for early diagnosis of Alzheimer’s disease. Neuroimage. 2007;36:298–312. doi:10.1016/j.neuroimage.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi:10.1016/j.pneurobio.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 20. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi:10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 22. Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. [DOI] [PubMed] [Google Scholar]
- 23. Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 24. Aboyans V, Criqui MH, Abraham P, et al. ; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi:10.1161/CIR.0b013e318276fbcb [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.