Abstract
Background/Aims: Previous results on the association between MTR gene A2756G polymorphism and PCa risk are inconclusive.
Methods: We used odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs) to evaluate the correlation between MTR A2756G polymorphism and risk of PCa in meta-analysis. Serum expression of MTR was detected by ELISA and in-silico tools were utilized to assess this variant.
Results: Our study included 2,921 PCa patients and 3,095 control subjects. The results indicated that the MTR A2756G polymorphism is linked with an increased risk of PCa using three genetic models (G-allele vs. A-allele: OR = 1.16, 95%CI = 1.04 - 1.30; GA vs. AA: OR = 1.17, 95%CI = 1.02 - 1.33; GG+GA vs. AA: OR = 1.18, 95%CI = 1.04 - 1.34). Stratified analysis produced similar results. A significant association was also indicated in advanced PCa from the meta-analysis. Finally, our experiments showed evidence that serum MTR levels in PCa patients with AA genotypes were statistically higher than in those with GG/GA genotypes.
Conclusions: Our present study suggests that the MTR A2756G polymorphism may contribute to the risk of developing PCa, particularly in Asian and hospital-based studies. Moreover, serum MTR might be utilized in diagnosis of PCa.
Keywords: methionine synthase, genetic variation, prostate cancer, polymorphism, ELISA
Introduction
Prostate cancer (PCa) remains the most common carcinoma in males and the sixth leading cause of tumor-associated deaths in Western countries [1]. Although the occurrence of PCa in Asia is much lower than that in developed countries, the incidence and mortality rate of the cancer has been increasing remarkably probably due toimproved diet, westernized lifestyles and development of screening technique [2–4]. To date, numerous factors including family history, prostate specific antigen (PSA) test, and age have been identified as strong predictors for PCa susceptibility; nevertheless, the underlying etiology remains unclear [5,6]. Recently, more and more research has indicated that genetic background, such as genetic variants, may play a vital role in prostate carcinogenesis [7,8].
Previous studies have indicated that folate-metabolizing genes play a significant role in carcinogenesis through DNA methylation [9–11]. Aberrant DNA methylation has been thought to be associated with PCa initiation by inactivating tumor suppressor genes (such as P53) or stimulating proto-oncogenes (such as Ras) [12,13]. Human methionine synthase (MTR), located on chromosome 1 (1q43), is one of the core factors in the folate pathway [14,15]. The MTR gene encodes a protein containing 1265 amino acids (140.5 kDa), which acts as a key enzyme in DNA repair, synthesis, and methylation [16]. Published epidemiological studies have been performed to test whether the MTR A2756G (rs1805087) variant is associated with PCa risk; however, the results remain inconsistent rather than conclusive [17,18]. There were several inconsistent results on the association between MTR A2756G polymorphism and PCa from various case-control studies. Therefore, we performed an updated meta-analysis with accumulated data from all eligible researches to enhance the statistical powers of previous studies and ascertain precise correlation between the MTR variant and PCa risk [18–24]. Furthermore, we enrolled 200 newly diagnosed PCa patients in our centers and investigated the serum expression of MTR by ELISA and utilized in-silico tools to confirm the results of our meta-analysis.
RESULTS
Study characteristics
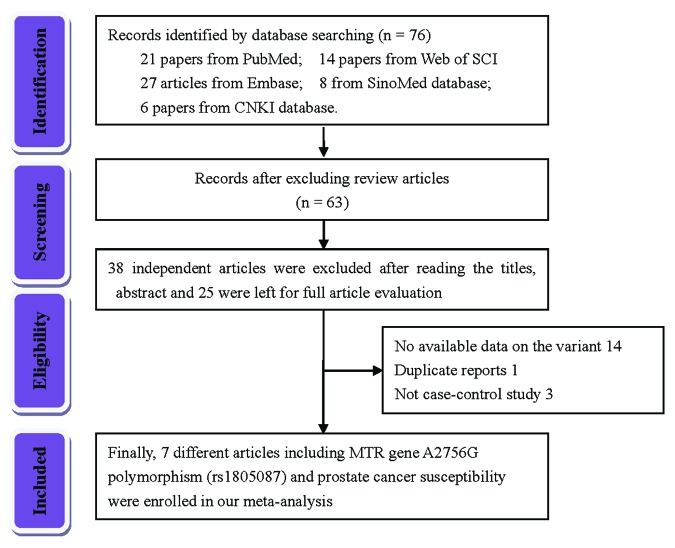
Initially, 76 articles were retrieved according to the selection strategy (Fig. 1). Among them, 13 articles were excluded because they were review articles. Then, 38 articles were removed after screening of titles and abstracts. Another 14 articles were excluded because they did not provide available data on the MTR A2756G variant. Among the remaining 11 articles, one article was a duplicate report and 3 articles contained no information about a control group. As a result, a total of 7 articles containing 2,921 PCa patients and 3,095 control subjects concerning the MTR A2756G polymorphism were enrolled in our meta-analysis. Relevant parameters for the selected studies are summarized in Table 1. For the selected seven studies, three were performed on a Caucasian population, three were carried out on an Asian population and only one study was performed concerning South-Americans. In stratified analysis by source of control, HB controls were investigated in five of these studies and the rest were performed on PB controls. For the genotyping methods, three studies used the classical RFLP while the rest utilized real-time PCR and TaqMan. Furthermore, we checked the Minor Allele Frequency (MAF) of MTR A2756G reported for the main worldwide populations. For American (AMR) population: A=0.823, G=0.177; for African (AFR) population: A=0.716, G=0.284; for East Asian (EAS) population: A=0.895, G=0.105; for South Asian (SAS) population: A=0.679, G=0.321; and European (EUR) population: A=0.827, G=0.173 (Fig. 2).
Figure 1.
Flow chart of the strategy of literature search among the related studies.
Table 1. Study characteristics of MTR rs1805087 A2756G polymorphism included in this meta-analysis.
| First author | Year | Country | Ethnicity | Source | Genotyping | Sample size of case | Sample size of control | Average age | PHWE | NOS | |||||||||
| methods | GG | GA | AA | MAF | Total | GG | GA | AA | MAF | Total | Case | Control | |||||||
| Ebrahimi | 2017 | Iran | Asian | HB | PCR-RFLP | 13 | 53 | 34 | 0.395 | 100 | 6 | 37 | 57 | 0.245 | 100 | NA | NA | 0.999 | 7 |
| Qu | 2016 | China | Asian | HB | real-time PCR | 20 | 316 | 1481 | 0.098 | 1817 | 15 | 319 | 1692 | 0.086 | 2026 | 66.7 ± 7.2 | 66.9 ± 6.8 | 0.993 | 9 |
| López-Cortés | 2013 | Ecuador | SA | PB | PCR-RFLP | 3 | 9 | 92 | 0.072 | 104 | 1 | 4 | 105 | 0.027 | 110 | NA | NA | 0.001 | 9 |
| Weiner | 2012 | Russia | Caucasian | PB | real-time PCR | 15 | 134 | 221 | 0.222 | 370 | 16 | 96 | 173 | 0.225 | 285 | 69 ± 8.0 | 59 ± 17 | 0.580 | 7 |
| Cai | 2010 | China | Asian | HB | PCR-RFLP | 5 | 27 | 185 | 0.085 | 217 | 3 | 29 | 188 | 0.080 | 220 | 72.36 ± 12.16 | 72.83± 12.27 | 0.139 | 7 |
| Marchal | 2008 | Spain | Caucasian | HB | Taqman | 9 | 54 | 118 | 0.199 | 181 | 11 | 55 | 138 | 0.189 | 204 | 70.7 ± 7.29 | 70.3 ± 7.82 | 0.088 | 7 |
| Kimura | 2000 | Germany | Caucasian | HB | PCR-RFLP | 4 | 41 | 87 | 0.186 | 132 | 4 | 44 | 102 | 0.173 | 150 | 65.6 ± 6.0 | 62.0 ± 11.4 | 0.773 | 9 |
SA: South America; HWE: Hardy-Weinberg equilibrium of controls, HB: Hospital-based; PB: Population-based; MAF: Minor allele frequency; NOS: Newcastle-Ottawa Scale; RFLP: restriction fragment length polymorphism; NA: not available.
Figure 2.
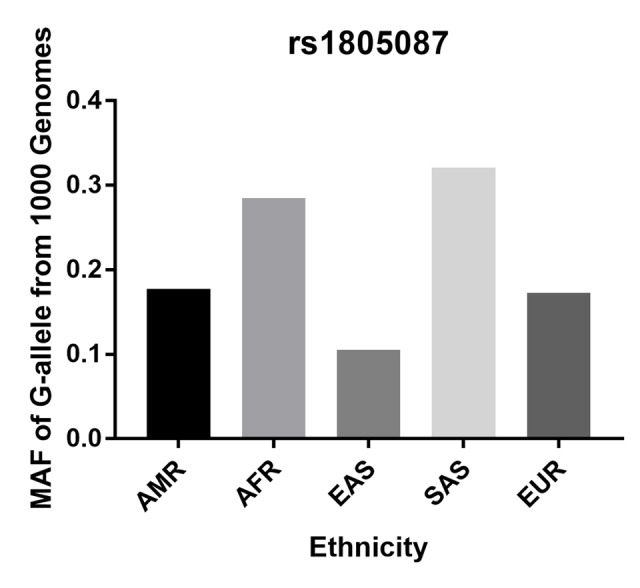
Minor allele frequencies (MAF) for MTR rs1805087 A2756G polymorphism in control stratified by ethnicity. Vertical line, minor allele frequency of G-allele from 1,000 Genomes; Horizontal line, ethnicity type; American (AMR), African (AFR), East Asian (EAS), South Asian (SAS), and European (EUR).
Meta-analysis
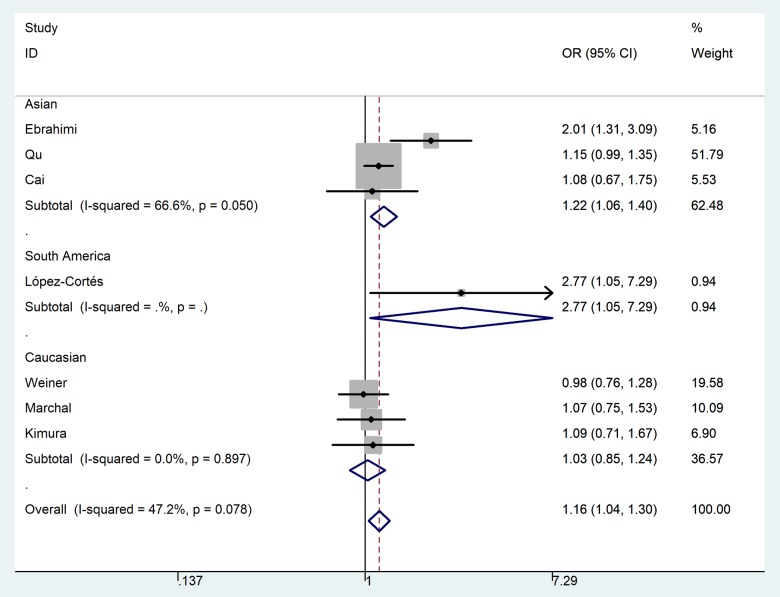
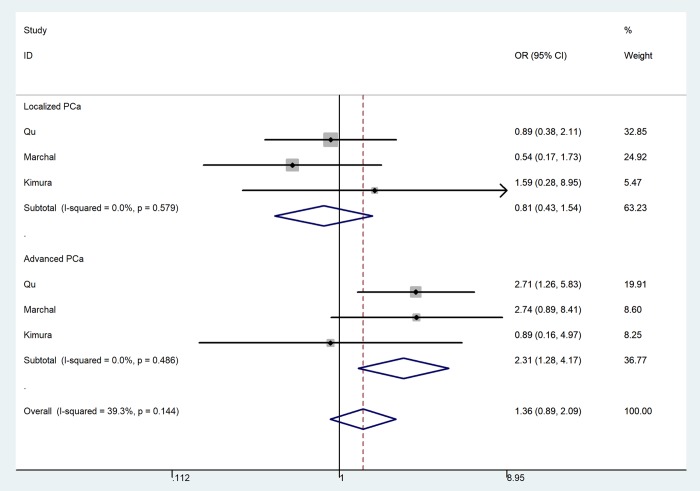
In the pooled analysis of all selected studies, an increased risk of PCa was associated with the MTR A2756G polymorphism (Table 2) in three genetic models. For G-allele vs. A-allele: OR = 1.16, 95%CI = 1.04 - 1.30, Pheterogeneity = 0.078, P = 0.008, I2 = 47.2 (Fig. 3). GA vs. AA: OR = 1.17, 95%CI = 1.02 - 1.33, Pheterogeneity = 0.229, P = 0.020, I2 = 26.1. For GG+GA vs. AA: OR = 1.18, 95%CI = 1.04 - 1.34, Pheterogeneity = 0.102, P = 0.010, I2 = 43.4. In subgroup analysis by race, we observed increased risk between MTR A2756G polymorphism and PCa risk in Asian population in three comparisons. For G-allele vs. A-allele: OR = 1.22, 95%CI = 1.06 - 1.40, Pheterogeneity = 0.050, P = 0.006, I2 = 66.6. Similar results were also indicated in a homozygote comparison (OR = 1.93, 95%CI = 1.14 – 3.26, Pheterogeneity = 0.390, P = 0.014, I2 = 0) and recessive genetic model (OR = 1.72, 95%CI = 1.02 – 2.89, Pheterogeneity = 0.767, P = 0.041, I2 = 0). In stratified analysis by tumor stage, we indicated a positive correlation between the MTR A2756G variant and advanced PCa (GG vs. GA+AA: OR = 2.31, 95%CI = 1.28 - 4.17, Pheterogeneity = 0.486, P = 0.006, I2 = 0), but not in localized PCa (OR = 0.81, 95%CI = 0.43 – 1.54, Pheterogeneity = 0.579, P = 0.252, I2 = 0) (Fig. 4). Furthermore, we observed positive correlations between MTR A2756G variant and PCa susceptibility in hospital-based studies while applying four genetic models (allelic contrast: OR = 1.19, 95%CI = 1.05 - 1.34, Pheterogeneity = 0.159, P = 0.007, I2 = 39.4; homozygote comparison: OR = 1.58, 95%CI = 1.03 – 2.42, Pheterogeneity = 0.444, P = 0.038, I2 = 0; heterozygote comparison: OR = 1.17, 95%CI = 1.01 - 1.35, Pheterogeneity = 0.175, P = 0.033, I2 = 36.9; dominant genetic model: OR = 1.19, 95%CI = 1.04 - 1.36, Pheterogeneity = 0.105, P = 0.014, I2 = 47.8).
Table 2. Stratified analyses of the MTR gene rs1805087 A2756G polymorphism on prostate cancer risk.
| Variables | N | Cases/ | G-allele vs. A-allele | GG vs. AA | GA vs. AA | GG+GA vs. AA | GG vs. GA+AA | ||||
| Controls | OR(95%CI) Phetera P I2 | OR(95%CI) Phetera P I2 | OR(95%CI) Phetera P I2 | OR(95%CI) Phetera P I2 | OR(95%CI) Phetera P I2 | ||||||
| Total | 7 | 2921/3095 | 1.16(1.04-1.30) 0.078 0.008 47.2 | 1.33(0.93-1.92) 0.280 0.119 19.6 | 1.17(1.02-1.33) 0.229 0.020 26.1 | 1.18(1.04-1.34) 0.102 0.010 43.4 | 1.24(0.86-1.77) 0.493 0.246 0 | ||||
| Ethnicity | |||||||||||
| Caucasian | 3 | 683/639 | 1.03(0.85-1.24)0.897 0.792 0 | 0.86(0.50-1.46) 0.812 0.569 0 | 1.11(0.88-1.40) 0.983 0.392 0 | 1.07(0.86-1.35) 0.962 0.535 0 | 0.83(0.49-1.40) 0.810 0.478 0 | ||||
| Asian | 3 | 2134/2346 | 1.22(1.06-1.40) 0.050 0.006 66.6 | 1.93(1.14-3.26) 0.390 0.014 0 | 1.31(0.84-2.05) 0.044 0.233 68.0 | 1.38(0.87-2.20) 0.024 0.174 73.2 | 1.72(1.02-2.89) 0.767 0.041 0 | ||||
| South America | 1 | 104/110 | 2.77(1.05-7.29) - 0.039 - | 3.42(0.35-33.49) - 0.290 - | 2.57(0.77-8.02) - 0.127 - | 2.74(0.93-8.07) - 0.067 - | 3.24(0.33-31.63) - 0.312 - | ||||
| Source of control | |||||||||||
| Population-based | 2 | 474/395 | 1.48(0.55-4.00) 0.042 0.439 75.7 | 0.87(0.44-1.73) 0.206 0.699 37.4 | 1.16(0.85-1.60) 0.182 0.344 43.9 | 1.13(0.84-1.53) 0.092 0.417 64.8 | 0.84(0.43-1.65) 0.213 0.615 35.6 | ||||
| Hospital-based | 5 | 2447/2700 | 1.19(1.05-1.34) 0.159 0.007 39.4 | 1.58(1.03-2.42) 0.444 0.038 0 | 1.17(1.01-1.35) 0.175 0.033 36.9 | 1.19(1.04-1.36) 0.105 0.014 47.8 | 1.44(0.94-2.20) 0.734 0.093 0 | ||||
a P value of Q-test for heterogeneity test(Pheter).
Figure 3.
Forest plot of PCa risk associated with the MTR A2756G polymorphism (allelic contrast of G-allele vs. A-allele, fixed-effects) in the stratified analyses by ethnicity. The squares and horizontal lines represent the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond corresponds to the summary OR and 95% CI. Separate details were summarized in Table 2.
Figure 4.
Forest plot of prostate cancer risk associated with the MTR A2756G polymorphism (recessive model of GG vs. GA+AA, fixed-effects) in the stratified analyses by tumor stage. The squares and horizontal lines represent the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond corresponds to the summary OR and 95% CI.
In-silico analysis of MTR expression
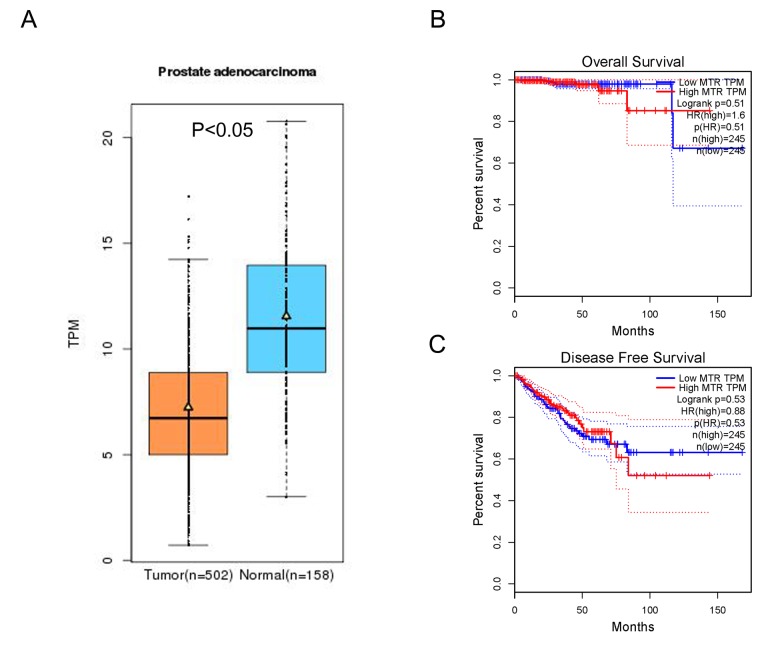
In-silico analysis showed evidence that MTR expression in PCa tissue was lower than that in normal tissue (including 502 PCa and 158 normal samples, P < 0.05, Fig. 5 A). Furthermore, the association between MTR expression and PCa overall survival time or disease free survival time was also studied. Kaplan-Meier estimate demonstrated no significant difference for either overall survival time or disease free survival time (P > 0.05, Fig. 5 B). Unfortunately, we have not indicated a positive association between the MTR polymorphism and different stage (OR = 1.07, 95%CI = 0.83 - 1.40, P = 0.591) and grade (OR = 1.11, 95%CI = 0.85 - 1.44, P = 0.445) of PCa in our PCa patient study. String online server indicated that MTR gene interacts with numerous genes. The network of gene-gene interaction has been illustrated in figure 6 (Fig. 6).
Figure 5.
In-silico analysis of MTR expression. (A) The relative expression of MTR in PCa tissue and paracancerous tissue (Normal) using TCGA database. TPM (Transcripts Per Kilobase Million) stands for the expression of MTR in each tissue. MTR expression in PCa tissue was lower than that in normal tissue (data from 502 PCa and 158 normal samples, P < 0.05). The correlation between MTR expression and overall survival time (B) or disease free survival time (C) of prostate cancer patients (P > 0.05).
Figure 6.
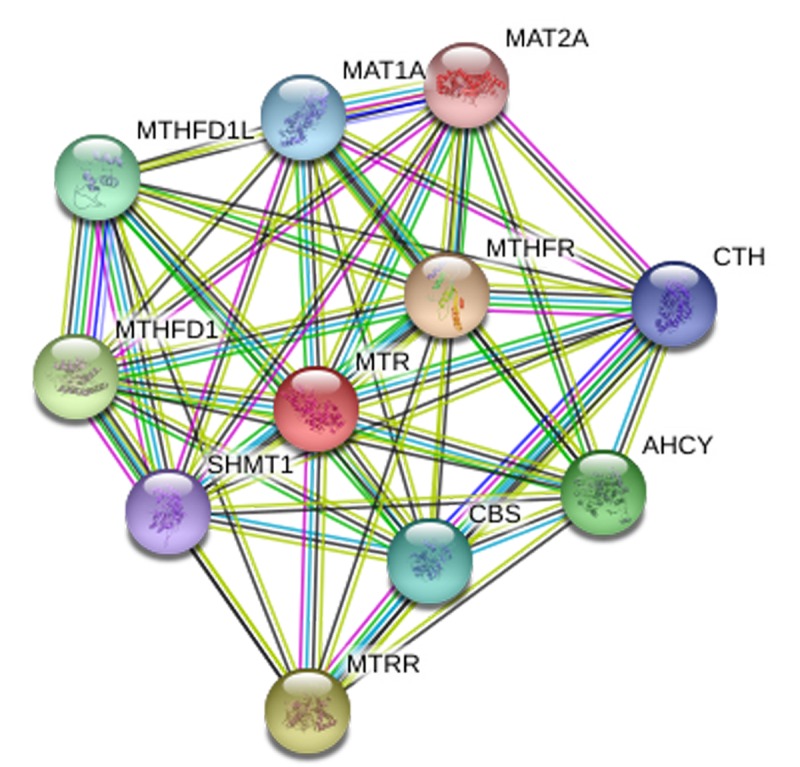
Human MTR interactions network with other genes obtained from String server. At least 10 genes have been indicated to correlate with MTR gene. CTH: Cystathionase; AHCY: adenosylhomocysteinase; MTRR: 5-methyltetrahydrofolate-homocysteine methyltransferase reductase; SHMT1: Serine hydroxymethyltransferase 1; MTHFD1: methylenetetrahydrofolate dehydrogenase 1; MTHFD1L: methylenetetrahydrofolate dehydrogenase 1-like;CBS: cystathionine beta-synthase; MAT1A: methionine adenosyltransferase I, alpha. MAT2A: methionine adenosyltransferase II, alpha; MTHFR: methylenetetrahydrofolate reductase.
Serum expression of MTR with different genotypes in PCa patients
Two-hundred serum samples of PCa patients were collected in our study with different genotypes of the MTR A2756G variant. We screened the MTR gene frequency of PCa patients recruited in our hospital. The distribution for GG, GA, and AA was 27 (13.5%), 65 (32.5%), 108 (54.0%), respectively. MAF of MTR A2756G in our study participants was 0.298, which is a bit higher than the one reported for AFR population (0.284) and lower than the one reported for EAS population (0.321). P value for HWE in our PCa cases is 0.002. Furthermore, we investigated the serum expression of MTR by ELISA. Serum MTR levels in PCa patients with GG/GA genotypes were statistically lower than those with AA genotypes (P < 0.01, Fig. 7).
Figure 7.
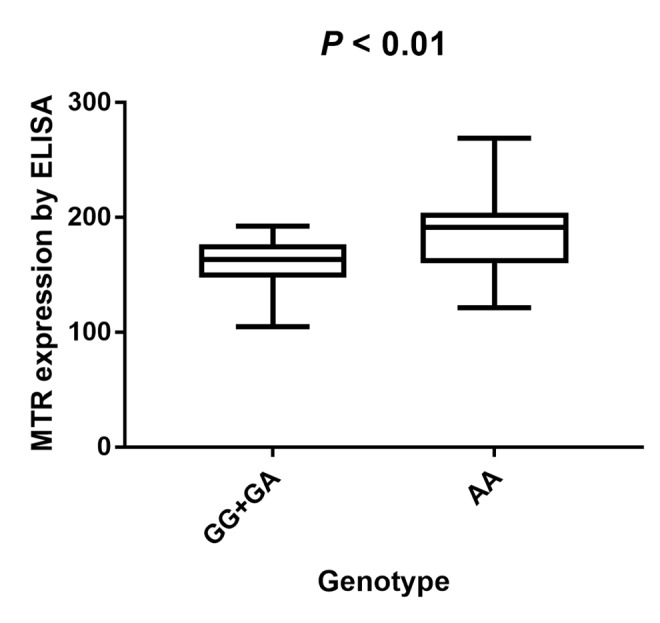
Analysis of serum MTR levels in rs1805087 A/G genotype of PCa cases with mean values (horizontal lines, mean values). Serum MTR levels in PCa patients carrying GG/GA genotypes were relatively lower than that carrying AA genotypes. P < 0.01 compared with the GG/GA and AA genotypes.
Publication bias
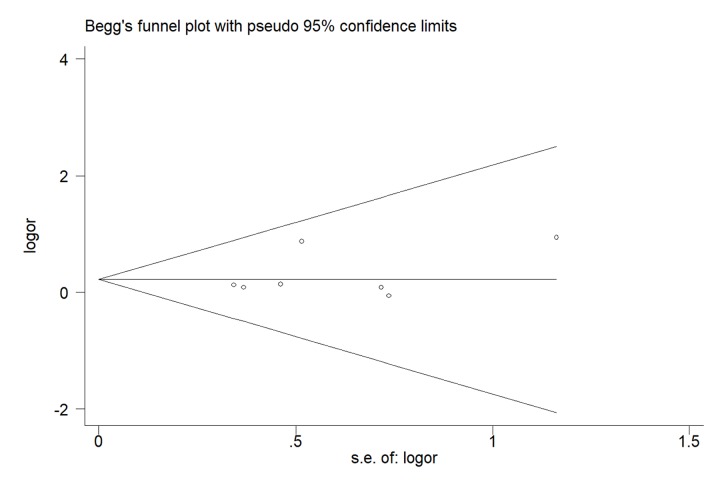
We used Begg’s and Egger’s test to assess publication bias of our meta-analysis. No evidence of bias was indicated in all the genetic models. For G-allele vs. A-allele: t = 1.14, P = 0.305; GG vs. AA: t = 0.92, P = 0.399; GA vs. AA: t = 1.24, P = 0.270; GG+GA vs. AA: t = 1.28, P = 0.257; GG vs. GA+AA: t = 0.83, P = 0.445 (Fig. 8)
Figure 8.
Begg's funnel plots to examine publication bias for recessive genetic model of MTR A2756G polymorphism. The funnel plot did not indicate any substantial asymmetry, which means no evidence of publication bias was found. t value = 0.83, P value = 0.445 for GG vs. GA+AA.
DISCUSSION
PCa remains one of the most prevalent malignancies among men globally, frequently occurring in Western countries [25,26]. Previous research has shown evidence that some risk factors, including ethnicity, toxins, lifestyle, single nucleotide polymorphism (SNP), and family history may contribute to the process and development of PCa [27–29]. Accumulating data have indicated that MTR, a folate-metabolizing gene, may be involved in the process of prostate carcinogenesis [30–32]. However, the association between MTR gene A2756G polymorphism and PCa risk remains unclear. Marchal and his group indicated that MTR 2756G allele acts as a factor of tumor aggressiveness in prostate carcinoma [33], nevertheless, some other research suggested no correlation between MTR A2756G polymorphism and PCa susceptibility [22]. Previous meta-analysis has assessed the association between MTR A2756G polymorphism and PCa in 2009 [17], however, no statistically significant correlation of this variant on the susceptibility to PCa was detected. After that, more articles have been published and provided new information to correlate MTR A2756G polymorphism and PCa susceptibility, such as Qu et al., who investigated the effect of the MTR polymorphism in the Han Chinese population and suggested that this variant was independently related to prostatic carcinogenesis by decreasing methylation potential [18]. In our present study, we selected some novel and larger case-control studies to draw a convincing conclusion. Thus, an updated meta-analysis with 2,921 PCa patients and 3,095 control subjects was undertaken to study this SNP.
In our meta-analysis, seven studies for MTR A2756G polymorphism were investigated. The overall results implicated MTR A2756G polymorphism in an increased risk of PCa. We observed that GA genotype presented 1.17-fold higher risk of PCa than the AA genotype in the overall analysis. Ethnicity is a vital biological factor; we uncovered a positive association between MTR A2756G polymorphism and PCa risk in Asian population, but not in Caucasian and other populations. Similar positive results were also observed in HB studies when stratified by source of control. To our knowledge, tumor stage is an independent prognostic factor for PCa; significant association between MTR A2756G variant and advanced PCa was indicated. In addition, we investigated the serum expression of MTR by ELISA and found that individuals who were GG/GA carriers of MTR A2756G polymorphism had a lower serum expression level than AA carriers. This result suggests that mutation from A to G at this locus (Asp to Gly substitution at codon 919 in MTR protein) may cause low expression of MTR, which means that serum concentration of MTR may be utilized as a detector in the diagnosis of PCa. To further ascertain the effect of MTR A2756G variant in PCa susceptibility, an online gene expression mini-database was utilized to evaluate the expression of MTR in PCa tissues. The Cancer Genome Atlas (TCGA) projects produced large-scale RNA sequencing data and may provide a chance for integrated expression analysis for all genes in large quantities of tumor and normal tissue specimens. It showed that MTR expression in PCa tissue was lower than that in normal tissue, which was consistent with the result from our meta-analysis. In addition, interactions of MTR with other gene might play a role in the current genetic polymorphism. As was shown in Figure 6, ten other genes may be involved in the gene-gene interaction. Further research is warrant to confirm these interactions in more details.
Although this is the first study that assesses the association between MTR A2756G polymorphism and PCa risk up to now, some limitations of the present analysis should be noted. First, all enrolled articles were the type of retrospective case-control studies, so selection bias may be existed in the present meta-analysis. Second, it is possible that gene-gene interactions influence the function of gene product and predominate in the development of cancer, which may lead to different ORs. We tried to evaluate the potential interactions, however, raw information from enrolled articles was insufficient. Some more precise researches need to be carried out if other covariates including PSA level, smoking exposure, and so on becomes available in the future case-control studies. Third, no enrolled article was carried out in African population, which should be considered in the future studies. Furthermore, age has been indicated to be associated with PCa risk, we tried to further assess the possible linkage between aging and MTR A2756G polymorphism, nevertheless, the raw data was not available either. Our PCa patients study indicated that serum MTR levels in PCa patients with AA genotypes were statistically higher than in those with GG/GA genotypes. Then, results from meta-analysis indicated a significant association between this variant and advanced PCa. Unfortunately, we have not indicated a positive association between the MTR polymorphism and different stage or grade of PCa in our case study. The possible reasons include: (a), the sample size of our experiment was not large enough; (b), there may be selection bias. In addition, MAF of MTR A2756G in our study participants was a bit higher than the one reported for AFR population (0.284) and lower than the one reported for EAS population (0.321). P value for HWE in our PCa cases is 0.002, which indicated that the departure of frequencies of MTR gene might exist.
Meta-analysis is considered to be an effective method for combining previously published results to draw more comprehensive conclusions. In spite of this, some other limitations should be addressed. To start with, only 7 studies based on MTR A2756G polymorphism and PCa were included, which means that the total participant count remains relatively low for comprehensive analysis. Second, only articles written in Chinese or English language were screened; hence, research published in other languages may be neglected. Third, some other risk factors, including family history, lifestyle, and environmental factors were not assessed due to insufficient data. Therefore, future research ought to collect and evaluate this information in more detail. Despite these limitations, some advantages in the present study should be acknowledged. First, heterogeneity is of great significance in meta-analysis. No obvious heterogeneity was detected in the current analysis for MTR A2756G polymorphism. Second, the quality of the selected studies completely satisfied the selection criteria and the statistical efficiency was enhanced. Third, most of the included studies, except one, are consistent with HWE [20]. Fourth, no evidence of publication bias was identified when we assessed the impact of the MTR variant in PCa risk.
In summary, our current study indicated that the MTR A2756G polymorphism was not only associated with increased PCa risk, particularly in people of Asian descent and in HB studies, but also related to PCa prognosis. Furthermore, studies with larger sample size should be continued to fully elucidate this issue.
MATERIALS AND METHODS
Search strategy and eligibility of relevant studies
PubMed, Embase, CNKI, and WanFang databases searches were conducted using the following keywords: ' methionine synthase ' or ' MTR ', 'prostate cancer' or 'prostate carcinoma' and 'variant' or 'polymorphism' (last search was carried out on Feb 10, 2018). References cited in the retrieved articles were also screened by a manual search. The inclusion criteria were: (a) study of association between MTR A2756G polymorphism and PCa susceptibility or prognosis; (b) available data for all genotypes (GG, GA, and AA); (c) case–control study with no duplicated design.
Inclusion criteria, exclusion criteria, data extraction and quality assessment
The following criteria were used to screen the eligible studies: (a) evaluation of MTR A2756G polymorphism and PCa susceptibility; (b) containing available data for genotype frequencies; (c) sufficient information for evaluating ORs; (d) case-control studies. Major exclusion criteria were: (a) no available genotype frequency; (b) studies used a case-case or case-only type; (c) If authors published two or more articles using the same population, the article with most complete information was enrolled. Two investigators independently screened all the searched data from studies according to the selection criteria. Any discrepancies were fully discussed by all authors unto consensus. The following parameters from the selected studies were extracted: the first author's surname, publication year, country, race, methods of genotyping, source of control (hospital-based, HB or population-based, PB), sample size of the participants, Minor Allele Frequency (MAF) in case and control, Average age for case group and control group. Hardy-Weinberg equilibrium (HWE). In addition, the selected studies contained data on clinical stage of PCa, which were separated into two sub-groups: localized (Gleason score =< 7 or tumor stage T1-T2c) and advanced PCa (Gleason score > 7 or tumor stage T3-T4). Moreover, we used the Newcastle-Ottawa Scale (NOS) score, ranging from zero stars (worst) to nine stars (best), to assess the quality of each study. The NOS score was calculated by comparability of participants and ascertainment of exposure to risks [34]. An article with 7 stars or more can be known as a high quality one.
Enzyme Linked immunosorbent Assay (ELISA)
We collected the blood in standard cubes without anticoagulant, then utilized serum separator tube (SST) and allow samples to clot for two hours at room temperature or overnight at 4°C before centrifugation for 15 minutes at 1000 ×g. Removed serum and assay immediately or aliquot and stored samples at -20°C or -80°C. Repeated freeze-thaw cycles should be avoided. Serum expression of MTR levels were measured by ELISA kit (CUSABIO Co. ltd.). Micro plate reader capable of measuring absorbance at 450 nm, with the correction wavelength set at 540 nm or 570 nm. The absorbance is related to the standard curve.
In-silico analysis of MTR expression
The online gene expression mini database was utilized to evaluate the expression of MTR in prostate tissues (http://gemini.cancer-pku.cn/) [35]. This database contains RNA expression profiles of 502 clinically confirmed PCa samples and 158 normal parts, which were isolated from the corresponding tissues. String online server was utilized to study the network of gene-gene interaction for MTR gene (http://string-db.org/).
Statistical analysis
We utilized odds ratios (ORs) with 95% confidence intervals (CIs) to investigate the strength of association between MTR A2756G polymorphism and PCa susceptibility. Z-test was used to determine the statistical significance of ORs. Chi-square-based Q-test was utilized to evaluate the heterogeneity assumption. A random effects model (DerSimonian-Laird method) was used when the P-value for the Q-test was less than 0.05 [36], which demonstrated a lack of heterogeneity among the studies. Otherwise, a fixed effects model (Mantel-Haenszel method) was applied [37]. I2 was also utilized to measure the heterogeneity. If I2 > 50%, this would suggest statistically significant heterogeneity among the studies. For the MTR A2756G genetic variant, the association with PCa risk was evaluated according to the models as follows: allelic comparison (G-allele vs. A-allele), homozygote comparison (GG vs. AA), heterozygote comparison (GA vs. AA), dominant model (GG+GA vs. AA) and recessive model (GG vs. GA+AA). Begg's [38] and Egger's [39] tests were chosen to calculate the funnel plot asymmetry and publication bias. P values less than 0.05 were considered statistically significant. We calculated the departure of frequencies of MTR gene variant from expectation under HWE by utilizing the Pearson chi-square test. Stata software (version 11.0, College Station, TX) was selected to investigate all statistical tests.
Genotyping methods
Genotyping of MTR A2756G variant was performed utilizing various techniques in different studies, including quantitative real-time PCR, restriction fragment length polymorphism PCR (PCR-RFLP), and allele-specific Taqman probes and primers as mentioned by Ulvik [40]. In our study, MTR A2756G polymorphism was evaluated using a TaqMan assay as conducted by Li et al [41].
Study population
Overall, 200 newly diagnosed PCa patients from February 2013 to July 2017 in the third Affiliated Hospital of Nantong University and Affiliated Changzhou No.2 People's Hospital of Nanjing Medical University were involved in our study (Table 3). The age range of the recruited PCa patients was from 52 to 85 years old. They were diagnosed with this cancer by transurethral resection of prostate (TURP) or needle biopsy within the preceding one year. Laparoscopic radical prostatectomy (LRP) was carried out on all patients and the pathological examination was performed. Every study participant should provide 2 milliliter peripheral blood sample. This study was approved by the Institution Review Board of the third Affiliated Hospital of Nantong University and Affiliated Changzhou No.2 People's Hospital of Nanjing Medical University. The written informed consent of each patient was obtained before all samples were collected.
Table 3. Clinicopathological and demographic characteristics of PCa patients included in this study.
| Features | PCa patients |
| n | 200 |
| Age, n(%) | |
| <60 | 101(50.5) |
| ≥60 | 99(49.5) |
| Sex, n(%) | |
| Male | 102(51) |
| Female | 98(49) |
| Smoking, n (%) | |
| Ever | 89(44.5) |
| Never | 111(55.5) |
| Alcohol drinking, n (%) | |
| Ever | 121(60.5) |
| Never | 79(39.5) |
| PSA, n (%) | |
| 4-10 | 123(61.5) |
| 10-20 | 62(31) |
| >20 | 15(7.5) |
| Gleason score(%) | |
| <7 | 91(45.4) |
| =7 | 62(31) |
| >7 | 47(23.5) |
| TNM stage(%) | |
| ≤T2c | 141(70.5) |
| =T3a | 38(19) |
| ≥T3b | 21(10.5) |
| Recurrent(%) | |
| Yes | 17(8.5) |
| No | 183(91.5) |
Footnotes
AUTHOR CONTRIBUTIONS: L-FZ and LZ designed this study, H-BS analyzed and acquired the data, K-WR and S-LG collected full-text papers, Y-YM and J-GZ drafted the manuscript. L-FZ and Y-YM prepared all figures and tables. LZ provided funding support. AO and TY supervised the study. All of the authors approved the final manuscript.
CONFLICTS OF INTEREST: The authors declare that they have no competing interests.
FUNDING: This work was supported by grants from the High-Level Medical Talents Training Project (Number: 2016CZBJ035), Changzhou 23rd Science and Technology Project (International Science and Technology Collaboration, Number: CZ20160017), Jiangsu '333 Project' Scientific Research Grant (No.: BRA 2016118), Jiangsu Post-Doc Scientific Research Grant (No.:1701184C), Young talents of ‘Ke Jiao Qiang Wei’ Project in Wuxi Commission of Health and Family Planning (Number: QNRC043), Youth projects of Wuxi Commission of Health and Family Planning (Number: Q201746, jzyx03, Z201712), the Science and Technology Development Fund of Wuxi (Number: CSE31N1605) funded project.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. 2005; 41:834–45. 10.1016/j.ejca.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Yang BX, Zhang HT, Wang JG, Wang HL, Zhao XJ. Prostate cancer: an emerging threat to the health of aging men in Asia. Asian J Androl. 2011; 13:574–78. 10.1038/aja.2010.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K. Prostate cancer in Asian men. Nat Rev Urol. 2014; 11:197–212. 10.1038/nrurol.2014.42 [DOI] [PubMed] [Google Scholar]
- 6.Kimura T. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer. 2012; 31:421–29. 10.5732/cjc.011.10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knipe DW, Evans DM, Kemp JP, Eeles R, Easton DF, Kote-Jarai Z, Al Olama AA, Benlloch S, Donovan JL, Hamdy FC, Neal DE, Smith GD, Lathrop M, Martin RM. Genetic variation in prostate-specific antigen-detected prostate cancer and the effect of control selection on genetic association studies. Cancer Epidemiol Biomarkers Prev. 2014; 23:1356–65. 10.1158/1055-9965.EPI-13-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sissung TM, Price DK, Del Re M, Ley AM, Giovannetti E, Figg WD, Danesi R. Genetic variation: effect on prostate cancer. Biochim Biophys Acta. 2014; 1846:446–56. 10.1016/j.bbcan.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim KC, Friso S, Choi SW. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. J Nutr Biochem. 2009; 20:917–26. 10.1016/j.jnutbio.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamid A, Wani NA, Kaur J. New perspectives on folate transport in relation to alcoholism-induced folate malabsorption--association with epigenome stability and cancer development. FEBS J. 2009; 276:2175–91. 10.1111/j.1742-4658.2009.06959.x [DOI] [PubMed] [Google Scholar]
- 11.Liu JJ, Ward RL. Folate and one-carbon metabolism and its impact on aberrant DNA methylation in cancer. Adv Genet. 2010; 71:79–121. 10.1016/B978-0-12-380864-6.00004-3 [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Dhanasekaran SM, Prensner JR, Cao X, Robinson D, Kalyana-Sundaram S, Huang C, Shankar S, Jing X, Iyer M, Hu M, Sam L, Grasso C, et al. Deep sequencing reveals distinct patterns of DNA methylation in prostate cancer. Genome Res. 2011; 21:1028–41. 10.1101/gr.119347.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003; 300:455. 10.1126/science.1083557 [DOI] [PubMed] [Google Scholar]
- 14.Karimian M, Hosseinzadeh Colagar A. Methionine synthase A2756G transition might be a risk factor for male infertility: evidences from seven case-control studies. Mol Cell Endocrinol. 2016; 425:1–10. 10.1016/j.mce.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 15.Chen LH, Liu ML, Hwang HY, Chen LS, Korenberg J, Shane B. Human methionine synthase. cDNA cloning, gene localization, and expression. J Biol Chem. 1997; 272:3628–34. 10.1074/jbc.272.6.3628 [DOI] [PubMed] [Google Scholar]
- 16.Goulding CW, Postigo D, Matthews RG. Cobalamin-dependent methionine synthase is a modular protein with distinct regions for binding homocysteine, methyltetrahydrofolate, cobalamin, and adenosylmethionine. Biochemistry. 1997; 36:8082–91. 10.1021/bi9705164 [DOI] [PubMed] [Google Scholar]
- 17.Collin SM, Metcalfe C, Zuccolo L, Lewis SJ, Chen L, Cox A, Davis M, Lane JA, Donovan J, Smith GD, Neal DE, Hamdy FC, Gudmundsson J, et al. , and UK Genetic Prostate Cancer Study Group. Association of folate-pathway gene polymorphisms with the risk of prostate cancer: a population-based nested case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009; 18:2528–39. 10.1158/1055-9965.EPI-09-0223 [DOI] [PubMed] [Google Scholar]
- 18.Qu YY, Zhou SX, Zhang X, Zhao R, Gu CY, Chang K, Yang XQ, Gan HL, Dai B, Zhang HL, Shi GH, Zhu Y, Ye DW, Zhao JY. Functional variants of the 5-methyltetrahydrofolate-homocysteine methyltransferase gene significantly increase susceptibility to prostate cancer: results from an ethnic Han Chinese population. Sci Rep. 2016; 6:36264. 10.1038/srep36264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebrahimi A, Hosseinzadeh Colagar A, Karimian M. Association of Human Methionine Synthase-A2756G Transition With Prostate Cancer: A Case-Control Study and in Silico Analysis. Acta Med Iran. 2017; 55:297–303. [PubMed] [Google Scholar]
- 20.López-Cortés A, Jaramillo-Koupermann G, Muñoz MJ, Cabrera A, Echeverría C, Rosales F, Vivar N, Paz-y-Miño C. Genetic polymorphisms in MTHFR (C677T, A1298C), MTR (A2756G) and MTRR (A66G) genes associated with pathological characteristics of prostate cancer in the Ecuadorian population. Am J Med Sci. 2013; 346:447–54. 10.1097/MAJ.0b013e3182882578 [DOI] [PubMed] [Google Scholar]
- 21.Weiner AS, Oskina NA, Lacarev AF, Petrova VD, Ganov DI, Boyarskih UA, Tonacheva OG, Voronina EN, Filipenko ML. Role of polymorphic variants of MTR gene A2756G and SHMT1 gene C1420T in the development of prostatic cancer in residents of the Western Siberian Region of Russia. Bull Exp Biol Med. 2012; 152:466–69. 10.1007/s10517-012-1554-6 [DOI] [PubMed] [Google Scholar]
- 22.Cai D, Ning L, Pan C, Liu X, Bu R, Chen X, Wang K, Cheng Y, Wu B. Association of polymorphisms in folate metabolic genes and prostate cancer risk: a case-control study in a Chinese population. J Genet. 2010; 89:263–67. 10.1007/s12041-010-0037-7 [DOI] [PubMed] [Google Scholar]
- 23.Marchal C, Redondo M, Reyes-Engel A, Perea-Milla E, Gaitan MJ, Machuca J, Diaz F, Caballero J, Carnero J. Association between polymorphisms of folate-metabolizing enzymes and risk of prostate cancer. Eur J Surg Oncol. 2008; 34:805–10. 10.1016/j.ejso.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 24.Kimura F, Franke KH, Steinhoff C, Golka K, Roemer HC, Anastasiadis AG, Schulz WA. Methyl group metabolism gene polymorphisms and susceptibility to prostatic carcinoma. Prostate. 2000; 45:225–31. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Zheng Y, Huang JY, Zhang AQ, Zhou YH, Wang JN. Folate intake, serum folate levels, and prostate cancer risk: a meta-analysis of prospective studies. BMC Public Health. 2014; 14:1326. 10.1186/1471-2458-14-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Key TJ. Nutrition, hormones and prostate cancer risk: results from the European prospective investigation into cancer and nutrition. Recent Results Cancer Res. 2014; 202:39–46. 10.1007/978-3-642-45195-9_4 [DOI] [PubMed] [Google Scholar]
- 27.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012; 61:1079–92. 10.1016/j.eururo.2012.02.054 [DOI] [PubMed] [Google Scholar]
- 28.Kabir A, Zendehdel R, Tayefeh-Rahimian R. Dioxin Exposure in the Manufacture of Pesticide Production as a Risk Factor for Death from Prostate Cancer: A Meta-analysis. Iran J Public Health. 2018; 47:148–55. [PMC free article] [PubMed] [Google Scholar]
- 29.Gathirua-Mwangi WG, Zhang J. Dietary factors and risk for advanced prostate cancer. Eur J Cancer Prev. 2014; 23:96–109. 10.1097/CEJ.0b013e3283647394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collin SM. Folate and B12 in prostate cancer. Adv Clin Chem. 2013; 60:1–63. 10.1016/B978-0-12-407681-5.00001-5 [DOI] [PubMed] [Google Scholar]
- 31.Jackson MD, Tulloch-Reid MK, McFarlane-Anderson N, Watson A, Seers V, Bennett FI, Egleston B, Ragin C. Complex interaction between serum folate levels and genetic polymorphisms in folate pathway genes: biomarkers of prostate cancer aggressiveness. Genes Nutr. 2013; 8:199–207. 10.1007/s12263-012-0321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collin SM, Metcalfe C, Refsum H, Lewis SJ, Smith GD, Cox A, Davis M, Marsden G, Johnston C, Lane JA, Donovan JL, Neal DE, Hamdy FC, et al. Associations of folate, vitamin B12, homocysteine, and folate-pathway polymorphisms with prostate-specific antigen velocity in men with localized prostate cancer. Cancer Epidemiol Biomarkers Prev. 2010; 19:2833–38. 10.1158/1055-9965.EPI-10-0582 [DOI] [PubMed] [Google Scholar]
- 33.Marchal C, Redondo M, Reyes-Engel A, Perea-Milla E, Gaitan MJ, Machuca J, Diaz F, Caballero J, Carnero J. Association between polymorphisms of folate-metabolizing enzymes and risk of prostate cancer. Eur J Surg Oncol. 2008; 34:805–10. 10.1016/j.ejso.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 34.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed Oct 20, 2011).
- 35.Tang Z, Li C, Zhang K, Yang M, Hu X. GE-mini: a mobile APP for large-scale gene expression visualization. Bioinformatics. 2017; 33:941–43. 10.1093/bioinformatics/btw775 [DOI] [PubMed] [Google Scholar]
- 36.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959; 22:719–48. [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 38.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 39.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulvik A, Ueland PM. Single nucleotide polymorphism (SNP) genotyping in unprocessed whole blood and serum by real-time PCR: application to SNPs affecting homocysteine and folate metabolism. Clin Chem. 2001; 47:2050–53. [PubMed] [Google Scholar]
- 41.Li M, Huang L, Qiu H, Fu Q, Li W, Yu Q, Sun L, Zhang L, Hu G, Hu J, Yuan X. Helicobacter pylori infection synergizes with three inflammation-related genetic variants in the GWASs to increase risk of gastric cancer in a Chinese population. PLoS One. 2013; 8:e74976. 10.1371/journal.pone.0074976 [DOI] [PMC free article] [PubMed] [Google Scholar]