Abstract
Aims
The majority of sudden cardiac arrests (SCAs) occur in patients with left-ventricular (LV) ejection fraction (LVEF) >35%, yet there are no methods for effective risk stratification in this sub-group. Since abnormalities of LV geometry can be identified even with preserved LVEF, we investigated the potential impact of LV geometry as a novel risk marker for this patient population.
Methods and results
In the ongoing Oregon Sudden Unexpected Death Study, SCA cases with archived echocardiographic data available were prospectively identified during 2002–15, and compared with geographical controls. Analysis was restricted to subjects with LVEF >35%. Based on established measures of LV mass and relative wall thickness (ratio of wall thickness to cavity diameter), four different LV geometric patterns were identified: normal geometry, concentric remodelling, concentric hypertrophy, and eccentric hypertrophy. Sudden cardiac arrest cases (n = 307) and controls (n = 280) did not differ in age, sex, or LVEF, but increased LV mass was more common in cases. Twenty-nine percent of SCA cases presented with normal LV geometry, 35% had concentric remodelling, 25% concentric hypertrophy, and 11% eccentric hypertrophy. In multivariate model, concentric remodelling (OR 1.76; 95%CI 1.18–2.63; P = 0.005), concentric hypertrophy (OR 3.20; 95%CI 1.90–5.39; P < 0.001), and eccentric hypertrophy (OR 2.47; 95%CI 1.30–4.66; P = 0.006) were associated with increased risk of SCA.
Conclusion
Concentric and eccentric LV hypertrophy, but also concentric remodelling without hypertrophy, are associated with increased risk of SCA. These novel findings suggest the potential utility of evaluating LV geometry as a potential risk stratification tool in patients with preserved or moderately reduced LVEF.
Keywords: Sudden cardiac arrest, Echocardiography, Left ventricular geometry, Left-ventricular hypertrophy, Concentric remodelling
What's new?
Any form of adverse left-ventricular (LV) remodelling is associated with the risk of SCA among subjects with preserved or moderately reduced LV systolic function.
Concentric and eccentric hypertrophy carry the highest risk of SCA, but even concentric remodelling without increased LV mass is associated with over 70% increased SCA risk.
Assessment of LV geometry by echocardiography warrants further evaluation as a tool for SCA risk stratification among patients with preserved or moderately reduced LV systolic function.
Introduction
Structural remodelling of the left ventricle occurs in response to chronic haemodynamic overload, as in systemic arterial hypertension, or following myocardial injury.1 In the short term, these adaptive changes may be beneficial by reducing left-ventricular (LV) wall stress and haemodynamic compromise, but in the long-term, LV hypertrophy (LVH) and increased LV mass are maladaptive processes that have been associated with heart failure, atrial and ventricular arrhythmias, mortality, and increased risk of sudden cardiac death.2–4 Depending on whether structural remodelling occurs in response to increase in preload or afterload, different types of adaptive changes in the LV geometry may occur.5,6 Based on the LV mass and relative wall thickness (RWT, the ratio of wall thickness to cavity diameter), four different LV geometric patterns can be distinguished: normal geometry (normal LV mass and normal RWT), concentric remodelling (normal LV mass but increased RWT), concentric LVH (increased LV mass and increased RWT), and eccentric LVH (increased LV mass but normal RWT).7 Both concentric LVH and eccentric LVH are predictors of poor overall prognosis, but the more subtle type of ventricular structural adaptation, concentric remodelling without increase in LV mass, has also been associated with increased morbidity and mortality.8,9 To our knowledge, however, the impact of concentric remodelling or the geometric form of LVH on the risk of sudden cardiac arrest (SCA) has not been previously evaluated.
The current risk stratification strategy for SCA prevention relies largely on measurement of LV function, but only a minority of SCA victims will have a significantly decreased LV ejection fraction (LVEF) <35% presently needed to qualify for a prophylactic implantable cardioverter defibrillator (ICD) therapy.10,11 Consequently, there is a critical need to improve identification of patients that are at increased risk of SCA despite having preserved or only moderately reduced LVEF. In this study, we investigated the impact of LV geometry on the risk of SCA in patients with LVEF >35%, and sought to characterize factors associated with different LV geometry patterns in SCA patients.
Methods
Study population
The Oregon Sudden Unexpected Death Study (Oregon SUDS) is an ongoing community-based collaborative that investigates out-of-hospital cardiac arrest in the Portland, Oregon metro area (population approximately one million). For the present analysis, all SCA cases that occurred between 1 February 2002 and 31 January 2015 among residents of this area were prospectively identified. Detailed description of the rationale and methods of Oregon SUDS have been published previously.12,13 Briefly, cases of out-of-hospital cardiac arrest were identified through multiple sources, which included first responders (fire department and ambulance services), local hospital emergency rooms, and the county medical examiner's office. Sudden cardiac arrest was defined as a sudden, unexpected, pulseless condition of likely cardiac aetiology, occurring within 1 h of symptoms; if death was unwitnessed, subjects were to have been seen within 24 h in their usual state of health. Subjects with non-cardiac causes of death such as trauma, drug overdose, pulmonary embolism, cerebrovascular accident, or chronic terminal illness were excluded. Both survivors and non-survivors of the cardiac arrest event were included in the case-group. Adjudication of SCA was performed by a three-physician review of available medical records and autopsy reports. During the same time period, a group of controls both with and without coronary artery disease (CAD) were ascertained from the same geographical region as the cases. These subjects were required to have no history of prior ventricular arrhythmia or cardiac arrest. Because CAD is responsible for the vast majority of SCA,10 in order to identify risk factors specific to SCA we recruited controls with the majority (>80%) having a diagnosis of CAD as well. Coronary artery disease was defined as having ≥50% stenosis of a major coronary artery, or history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting. The controls were recruited from clinics of participating health systems, from individuals referred to coronary angiography, from patients transported by emergency medical services for symptoms suggesting acute coronary ischaemia, and among members of a local health maintenance organization.
Medical records for cases and controls were reviewed in detail for demographic data and clinic history. Current analysis was limited to subjects aged ≥18 years with echocardiograms available from existing medical records that included evaluation of LVEF and measurements of LV dimensions. All included cases had their echocardiography performed prior to cardiac arrest (for 74% of cases, within 2 years of arrest), and if multiple echocardiograms were available the one closest to SCA was included in the analysis. A majority of controls (80%) had echocardiograms available that were performed within 2 years of ascertainment.
This study was approved by the Institutional Review Boards of Cedars-Sinai Medical Center, Oregon Health and Science University and all participating hospitals and health systems.
Echocardiographic analysis
Echocardiograms closest prior to the arrest were reviewed for LVEF, LV end-diastolic diameter (LVEDD), interventricular septal thickness at end diastole (IVSd), LV posterior wall thickness at end diastole (PWd), left-atrial (LA) size, and presence of valvular disease. Subjects with severe aortic stenosis, hypertrophic cardiomyopathy, and LVEF ≤35% were excluded from the present analysis. The documented reason for the echocardiographic examination was assessment of LV function or heart failure in 46%, chest pain or CAD in 17%, suspected valvular diseases in 11%, syncope in 3%, and other or missing in 23% of the SCA cases.
Relative wall thickness was calculated as 2 × PWd divided by LVEDD. LV mass was calculated using the linear formula recommended by the American Society of Echocardiography, 0.8 × (1.04([LVEDD + PWd + IVSd]3− [LVEDD]3)) + 0.6 g,7 and LV mass index was determined by dividing the LV mass by the body surface area in m2. A cut-off of 134 g/m2 for males and 110 g/m2 for females was used to define an increased LV mass index,14 and increased RWT was defined as ≥0.45.8 Moderately abnormal IVSd was defined as >13 mm for males and >12 mm for females. Four types of different LV geometry patterns were defined based on LV mass index and RWT, as recommended by the American Society of Echocardiography7: normal geometry (normal LV mass index and normal RWT), concentric remodelling (normal LV mass index but increased RWT), concentric hypertrophy (increased LV mass index and increased RWT), and eccentric hypertrophy (increased LV mass index but normal RWT).
Statistical analysis
Comparison between cases and controls was performed using independent samples t-test and χ2 test for continuous and categorical variables, respectively. To determine the differences in characteristics between LV geometry types, comparison between the LV geometry types in SCA cases was performed using ANOVA and χ2 test for continuous and categorical variables, respectively. A two-tailed P-value of ≤0.05 was considered statistically significant. Multivariable logistic regression was used to determine the odds ratios (OR) and 95% confidence intervals (CI) for independent associations between LV geometry and SCA, using normal LV geometry as the reference. Adjustments were made for age, sex, race, and diabetes. Associations of IVSd and RWT with SCA were determined in a similar fashion with multivariable logistic regression analysis. All analyses were performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp; Armonk, NY).
Results
Characteristics of cases and controls
Of the 4463 subjects (3446 cases and 1017 controls) enrolled between 1 February 2002 and 31 January 2015, 535 cases and 414 controls had sufficient echocardiographic data available for assessing LV geometry and LV function. After excluding subjects with reduced LVEF ≤35% (197 cases, 127 controls), and those without information on weight and height used to calculate LV mass index (31 cases, 7 controls), 307 SCA cases and 280 controls were included in the final analysis. Demographics and clinical characteristics of these subjects are presented in Table 1. There were no differences in age or sex between cases and controls, but cases were more likely to be of African American origin (11.5 vs. 3.7%, P < 0.001) and were more likely to have diabetes (49.8 vs. 30.0%, P < 0.001) compared with the controls. No difference in the prevalence of hypertension or obesity was noted, and in this group of subjects with LVEF >35%, LV function was similar in cases and controls (LVEF 55.3 ± 10.4 vs. 56.2 ± 9.7%, respectively).
Table 1.
Baseline characteristics of the subjects
| Case (n = 307) | Control (n = 280) | P-value | |
|---|---|---|---|
| Age | 68.8 ± 14.1 | 69.1 ± 11.1 | 0.806 |
| Male gender (%) | 182 (59.3) | 172 (61.4) | 0.596 |
| Race (%) | 0.001 | ||
| Caucasian | 252 (82.6) | 251 (91.9) | |
| African American | 35 (11.5) | 10 (3.7) | |
| Other | 18 (5.9) | 12 (4.4) | |
| Diabetes (%) | 153 (49.8) | 84 (30.0) | <0.001 |
| Current/former smoker (%) | 164 (65.6) | 137 (65.9) | 0.952 |
| Obesity (%) | 128 (41.7) | 123 (43.9) | 0.585 |
| Hypertension (%) | 254 (82.7) | 221 (78.9) | 0.242 |
| Documented CAD (%) | 197 (64.2) | 245 (87.5) | <0.001 |
| Echocardiographic parameters | |||
| LV mass index (g/m2) | 114.8 ± 40.2 | 99.8 ± 30.1 | <0.001 |
| Increased LV mass index (%) | 111 (36.2) | 50 (17.9) | <0.001 |
| LV diameter (mm) | 48.9 ± 8.9 | 48.2 ± 6.9 | 0.293 |
| LVEF (%) | 55.3 ± 10.4 | 56.2 ± 9.7 | 0.273 |
| IVSd (mm) | 12.1 ± 2.7 | 11.0 ± 2.4 | <0.001 |
| PWd (mm) | 11.7 ± 2.5 | 10.8 ± 2.1 | <0.001 |
| RWT ≥0.45 (%) | 183 (59.6) | 121 (43.2) | <0.001 |
Data are presented as mean ± SD or n (%). Smoking status available for 250 cases and 208 controls.
CAD, coronary artery disease; LV, left ventricular; LVEF, LV ejection fraction; IVSd, interventricular septum in diastole; PWd, posterior wall in diastole; RWT, relative wall thickness.
Characteristics of sudden cardiac arrest cases according to left-ventricular geometry
Table 2 shows the demographic and clinical characteristics of SCA cases according to the type of LV geometry. Age did not significantly differ between the different types of LV geometry, but there were differences in gender and racial distribution between the groups, with a larger proportion of African Americans having concentric and eccentric LVH. Diabetes and hypertension were most common in subjects with concentric LVH. When compared with subjects with normal LV geometry, concentric remodelling was also associated with a higher prevalence of hypertension.
Table 2.
Characteristics of sudden cardiac arrest cases with different types of left-ventricular geometry
| Normal (n = 90) | Concentric remodelling (n = 106) | Concentric hypertrophy (n = 77) | Eccentric hypertrophy (n = 34) | P-value | |
|---|---|---|---|---|---|
| Age | 68.5 ± 14.8 | 67.6 ± 14.1 | 71.3 ± 13.3 | 68.1 ± 13.5 | 0.350 |
| Male gender (%) | 62 (68.9) | 62 (58.5) | 37 (48.1) | 21 (61.8) | 0.056 |
| Race (%) | 0.066 | ||||
| Caucasian | 79 (88.8) | 91 (86.7) | 57 (74.0) | 25 (73.5) | |
| African American | 7 (7.9) | 7 (6.7) | 15 (19.5) | 6 (17.6) | |
| Other | 3 (3.4) | 7 (6.7) | 5 (6.5) | 3 (8.8) | |
| Diabetes (%) | 44 (48.9) | 44 (41.5) | 48 (62.3) | 17 (50.0) | 0.051 |
| Current/former smoker (%) | 43 (57.3) | 59 (65.6) | 39 (67.2) | 23 (85.2) | 0.074 |
| Obesity (%) | 42 (46.7) | 42 (39.6) | 27 (35.1) | 17 (50.0) | 0.326 |
| Hypertension (%) | 66 (73.3) | 89 (84.0) | 70 (90.9) | 29 (85.3) | 0.024 |
| Documented CAD (%) | 59 (65.6) | 62 (58.5) | 52 (67.5) | 24 (70.6) | 0.466 |
| Echocardiographic parameters | |||||
| LV mass index (g/m2) | 92.8 ± 19.2 | 90.7 ± 22.7 | 155.3 ± 33.3 | 156.8 ± 31.3 | <0.001 |
| LV diameter (mm) | 52.6 ± 6.5 | 41.7 ± 6.7 | 49.3 ± 7.2 | 60.1 ± 5.7 | <0.001 |
| LVEF (%) | 53.5 ± 8.7 | 58.1 ± 10.4 | 55.6 ± 11.3 | 50.9 ± 10.3 | <0.001 |
| IVSd (mm) | 10.0 ± 1.7 | 12.1 ± 2.1 | 14.5 ± 2.5 | 12.5 ± 2.0 | <0.001 |
| PWd (mm) | 9.4 ± 1.5 | 12.0 ± 1.8 | 14.2 ± 1.9 | 11.1 ± 1.7 | <0.001 |
| RWT | 0.36 ± 0.07 | 0.59 ± 0.12 | 0.59 ± 0.13 | 0.37 ± 0.05 | <0.001 |
| LA size (mm) | 42.9 ± 9.0 | 41.3 ± 10.6 | 46.1 ± 9.3 | 49.1 ± 11.8 | 0.023 |
Data are presented as mean ± SD or n (%).
CAD, coronary artery disease; LV, left ventricular; LVEF, LV ejection fraction; IVSd, interventricular septum in diastole; PWd, posterior wall in diastole; RWT, relative wall thickness; LA, left atrium.
Left-ventricular mass and risk of sudden cardiac arrest
Sudden cardiac arrest cases had significantly higher LV mass index (114.8 ± 40.2 vs. 99.8 ± 30.1 g/m2, P < 0.001) compared with controls. Overall, 36.2% of cases and 17.9% controls had increased LV mass index (P < 0.001). In multivariate analysis, increased LV mass was associated significantly with SCA (OR 2.22; 95%CI 1.49–3.33; P < 0.001). When introduced to the model as a continuous variable, every 10 g/m2 increase in LV mass was associated with 12% increase in risk of SCA (OR 1.12; 95%CI 1.06–1.18; P < 0.001).
Risk of sudden cardiac arrest according to left-ventricular geometry
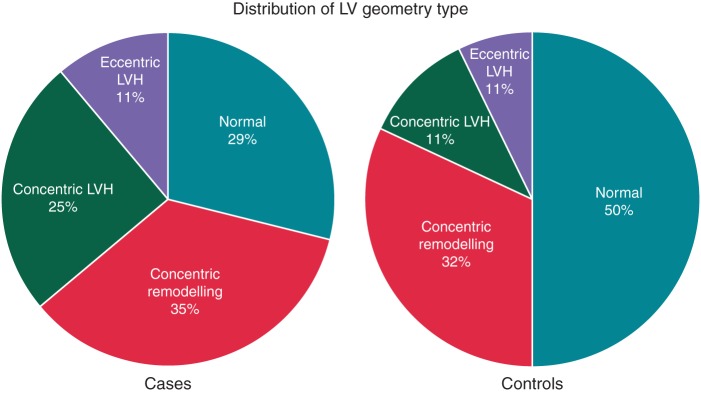
The distribution of different types of LV geometry between cases and controls are presented in Figure 1. Overall, 29.3% of cases and 50.0% of controls displayed normal LV geometry, 34.5% of cases and 32.1% of controls had concentric remodelling, 25.1% of cases and 11.1% of controls had concentric LVH, and 11.1% of cases and 6.8% of controls had eccentric LVH.
Figure 1.
Distribution of different left-ventricular geometry patterns in sudden cardiac arrest cases and controls.
There was a strong correlation between concentric remodelling, concentric and eccentric LVH, increased RWT and IVS thickness, and the risk of SCA, as presented in Figure 2. In a multivariate model including age, sex, race, and diabetes, concentric remodelling was associated with significantly increased risk of SCA compared with normal LV geometry (OR 1.76; 95%CI 1.18–2.63; P = 0.005). The increase in odds of SCA was even more pronounced among subjects with increased LV mass, with OR 3.20 for concentric LVH (95%CI 1.90–5.39; P < 0.001) and OR 2.47 for eccentric LVH (95%CI 1.30–4.66; P = 0.006) (Figure 3). When additional adjustments were made for CAD, hypertension, and cardiac medications (ACE inhibitors, angiotensin-receptor blockers, beta-blockers, and diuretics), the risk of SCA did not change markedly (OR 1.74 in concentric remodelling; 95%CI 1.12–2.71; P = 0.013; OR 4.07 in concentric LVH; 95%CI 2.29–7.25; P < 0.001; OR 2.70 in eccentric LVH; 95%CI 1.36–5.37; P = 0.005).
Figure 2.
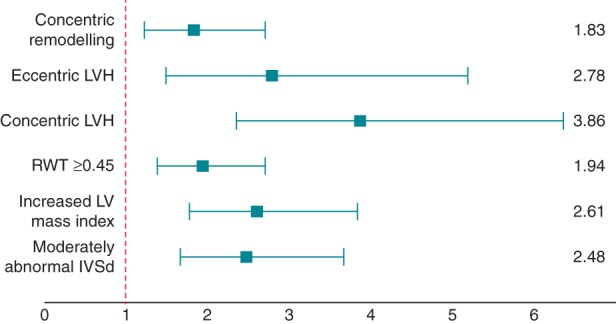
Unadjusted odds ratios and 95% confidence intervals for sudden cardiac arrest according to left-ventricular geometry. Left ventricular geometrical patterns vs. normal geometry; RWT ≥0.45 vs. RWT <0.45; increased vs. normal LV index; abnormal vs. normal IVSd. LVH, left-ventricular hypertrophy; RWT, relative wall thickness; IVSd, interventricular septum in diastole.
Figure 3.
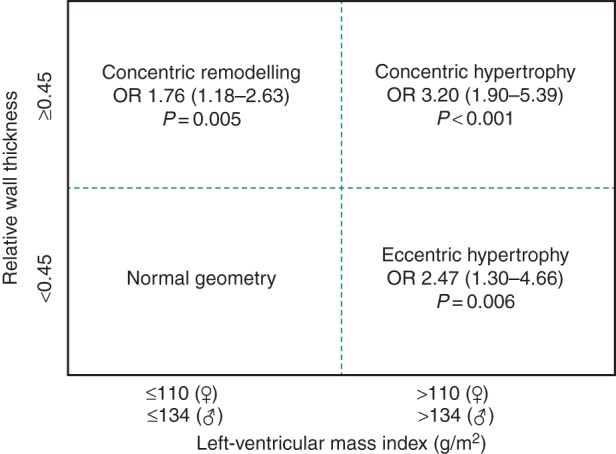
Multivariate odds ratio estimates (95% confidence intervals) for sudden cardiac arrest according to left-ventricular geometry. Adjusted for age, sex, race, and diabetes.
We conducted also a sub-group analysis restricted to subjects with LVEF ≥50% with similar results. In this sub-group, both concentric LVH and eccentric LVH remained significantly associated with SCA, but due to smaller numbers concentric remodelling was only of borderline statistical significance (OR for concentric LVH 2.85; 95%CI 1.59–5.11; P < 0.001; OR for eccentric LVH 2.63; 95%CI 1.10–6.26; P = 0.029; OR for concentric remodelling 1.51; 95%CI 0.99–2.33; P = 0.058).
Separate analyses were performed to study the association between septal thickness, RWT, and SCA. sudden cardiac arrest cases had thicker septum (12.1 ± 2.7 vs. 11.0 ± 2.4 mm, P < 0.001) and posterior wall (11.7 ± 2.5 vs. 10.8 ± 2.1 mm, P < 0.001), and a higher prevalence of increased RWT ≥0.45 (59.6 vs. 43.2%, P < 0.001) compared with controls. In a multivariate analysis, increased IVS thickness (defined as >12 mm in women and >13 mm in men) was associated with increased risk of SCA (OR 2.34; 95%CI 1.56–3.50; P < 0.001). In the similar model, abnormal RWT ≥0.45 was also associated with SCA (OR 1.78; 95%CI 1.27–2.51; P < 0.001). When septal thickness was introduced as a continuous variable in the multivariate model, each 1 mm increase in septal thickness was associated with 19% increase in risk of SCA (OR 1.19; 95%CI 1.11–1.28; P < 0.001).
Discussion
To the best of our knowledge, this case–control study is the first to provide data on the risk of SCA associated with different LV geometry patterns in the community. A new and notable finding from the study is the increased risk of SCA associated with concentric remodelling even without overt hypertrophy or LV dysfunction. We also report that both eccentric and concentric forms of hypertrophy are associated with SCA, although concentric LVH seems to carry a somewhat higher risk.
Since the early reports from the Framingham study that described increased cardiovascular mortality and sudden death associated with electrocardiographic LVH,15 more evidence has accumulated on the association between sudden cardiac death and increased LV mass.4,16,17 Koren et al.8 were the first to report that classification by LV geometry could have prognostic significance beyond just increased LV mass. In their study on patients with uncomplicated hypertension, the unadjusted 10-year mortality rates were 1, 6, 10, and 24% for patients with normal geometry, concentric remodelling, eccentric LVH, and concentric LVH, respectively. Since then, several studies conducted among different patient populations have suggested that, among patients with LVH, concentric hypertrophy might be associated with worse prognosis than eccentric hypertrophy.18–21
Abnormal LV geometry in the absence of increased LV mass has not always been appreciated as an important cardiovascular risk factor. However, since the initial report by Koren et al.8 on the adverse prognostic significance of concentric remodelling, several studies have reported increased mortality associated with concentric remodelling in the absence of LVH.19,20,22,23 This increased risk has not been apparent in all of the studies,9,18 probably partly due to limited sample size and relatively few endpoint events. The largest study to date on the prognostic significance of LV geometry was reported by Milani et al.24 from a large clinical echocardiographic database of 35 602 patients with normal LV systolic function. They identified abnormal LV geometry in 46% of these patients, with concentric remodelling present in 35% and LVH (either concentric or eccentric) in 11%. There was a strong relationship between abnormal LV geometry and mortality, and subjects with concentric remodelling exhibited a two-fold higher risk of all-cause mortality compared with those with normal LV geometry.
The present study extends these findings in the context of SCA. In the Oregon SUDS, only 29% of SCA victims with preserved LV ejection fraction had normal LV geometry identified on their archived echocardiogram. Thirty-five percent of the cases had concentric remodelling without increased LV mass, 25% demonstrated concentric LVH, and 11% eccentric LVH. Concentric LVH was associated with the highest risk of SCA, followed by eccentric LVH and concentric remodelling. After multivariate adjustments, the OR for SCA was 3.2-fold with concentric LVH and 2.5-fold with eccentric LVH. Furthermore, when only concentric remodelling without hypertrophy was present, the OR for SCA remained still nearly two-fold compared with subjects with normal LV geometry. These findings suggest that abnormal LV geometry can affect the risk of SCA among subjects without severe LV dysfunction, even without increased LV mass.
Ventricular arrhythmogenesis and increased risk of SCA associated with hypertrophy and changes in LV geometry are complex processes involving myocytes and interstitium, as well as coronary flow reserve and neurohumoral activation,5 and animal models have identified several distinct pathways leading to ventricular arrhythmias in the presence of LVH.25 It is possible that loading conditions of the LV are an important determinant of alterations in chamber geometry. If increased afterload is the dominating haemodynamic feature, as occurs in arterial hypertension or aortic stenosis, the prominent adaptive feature is the growth in cardiomyocyte thickness leading to concentric remodelling or hypertrophy. In contrast, increased preload and chronic volume increase causes cardiomyocytes to lengthen producing chamber dilatation seen in eccentric hypertrophy.1 The underlying molecular and cellular mechanisms appear to differ fundamentally in these different pathophysiological conditions. Concentric remodelling is often accompanied by increased fibrosis, inflammation, and apoptosis, which may directly impair myocardial function, and may partly explain the increased risk of SCA observed with concentric LV geometry. Conversely, volume overload may be less commonly associated with myocardial fibrosis.6,26 Eventually, however, all types of LV remodelling can progress to heart failure and ventricular dilatation with dysfunctional myocytes as well as interstitium.1
Concentric remodelling is likely to constitute an early phase in ventricular remodelling that culminates in LVH, already exhibiting some maladaptive features characteristic of increased LV mass.27 Whether concentric remodelling eventually progresses to hypertrophy likely depends on multiple clinical factors. Higher blood pressure and greater body-mass index together with older age and male sex correlate with adverse changes in LV geometry, which in turn are associated with increased risk of cardiovascular disease.28 On the other hand, several studies have also demonstrated that normalization of high blood pressure by pharmacological therapy leads to regression of LVH, as well as normalization of abnormal LV geometry in patients with concentric remodelling. In a substudy from the Losartan Intervention For Endpoint Reduction (LIFE) trial, the proportion of participants with eccentric LVH, concentric LVH and concentric remodelling decreased substantially over 2 years of treatment (44 to 30%; 24 to 2%; and 10 to 4%, respectively), and the proportion of subjects with normal LV geometry increased from 22 to 64%.29 Reduction of LV mass during treatment is a favourable marker that predicts a lesser risk for subsequent adverse cardiac events, independent of blood pressure lowering.30 Moreover, normalization of LV geometry in patients initially presenting with concentric remodelling has been also associated with improved survival.24 Although not directly assessed in the present study, it is conceivable that intensive therapy directed towards normalizing abnormal LV geometry may lead to decreased risk of SCA as well.
The present study was restricted to patients with relatively preserved LV function in order to define risk of SCA associated with established LV geometry patterns in a group of patients traditionally considered to be at lower risk of fatal arrhythmias. Given the relatively infrequent occurrence of SCA in the community compared with the size of the general population, a population-based case–control design is needed to provide feasible numbers of patients for analyses. Nevertheless, there are some inherent limitations of this study design. Since SCA can present as the first manifestation of the cardiac disease in many patients, specific clinical information such as echocardiography was available for only a smaller sub-group of the cases with potential for bias in patient selection. However, in the Oregon SUDS, control subjects have a similar distribution of coronary artery disease as SCA cases, and they are selected from the same geographical area increasing the likelihood that any differences observed between the groups would be specific for SCA. Furthermore, due to the nature of the study, we had to rely on echocardiographic measurements made as part of routine clinical practice, so in a significant minority of patients echocardiogram had been conducted over 2 years prior to SCA. Consequently, reproducibility and reliability of the measurements could not be assessed. Similarly, we could not employ more accurate and sophisticated techniques such as cardiac magnetic resonance imaging for the determination of the type of LV geometry. However, echocardiography is the most common modality used for assessment of LV geometry in the community, and the results are likely to represent the real-world utilization of echocardiography for the assessment of LV function and dimensions. Finally, although it would have been of great interest to study development of LV geometry over time, the number of subjects with repeated echocardiographic evaluation was too small for this kind of analysis.
Conclusions
In conclusion, both concentric and eccentric LVH are independently associated with SCA in the general population with preserved or only moderately reduced LV function, and even the more subtle type of abnormal LV geometry, concentric remodelling, is associated with significantly increased risk of SCA. These findings suggest the potential utility of evaluating LV geometry to assess the risk of ventricular arrhythmogenesis and SCA in these patients. However, further prospective investigation is needed to determine whether LV geometry will prove to be a useful and viable parameter for SCA risk stratification.
Funding
Funded in part by National Heart Lung and Blood Institute grants R01HL122492 and R01HL122492 to S.S.C. S.S.C. holds the Pauline and Harold Price Chair in Cardiac Electrophysiology at Cedars-Sinai, Los Angeles. A.L.A. is funded by grants from the Finnish Cultural Foundation and the Finnish Foundation for Cardiovascular Research.
Conflict of interest: none declared.
References
- 1. Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet 2006;367:356–67. [DOI] [PubMed] [Google Scholar]
- 2. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med 1990;322:1561–6. [DOI] [PubMed] [Google Scholar]
- 3. Chatterjee S, Bavishi C, Sardar P, Agarwal V, Krishnamoorthy P, Grodzicki T et al. Meta-analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol 2014;114:1049–52. [DOI] [PubMed] [Google Scholar]
- 4. Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998;32:1454–9. [DOI] [PubMed] [Google Scholar]
- 5. Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis 2009;52:153–67. [DOI] [PubMed] [Google Scholar]
- 6. Toischer K, Rokita AG, Unsold B, Zhu W, Kararigas G, Sossalla S et al. Differential cardiac remodeling in preload versus afterload. Circulation 2010;122:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 8. Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991;114:345–52. [DOI] [PubMed] [Google Scholar]
- 9. Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham heart study. J Am Coll Cardiol 1995;25:879–84. [DOI] [PubMed] [Google Scholar]
- 10. Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 2008;51:213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 12. Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. Community. J Am Coll Cardiol 2004;44:1268–75. [DOI] [PubMed] [Google Scholar]
- 13. Reinier K, Nichols GA, Huertas-Vazquez A, Uy-Evanado A, Teodorescu C, Stecker EC et al. Distinctive clinical profile of blacks versus whites presenting with sudden cardiac arrest. Circulation 2015;132:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW et al. Standardization of m-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol 1984;4:1222–30. [DOI] [PubMed] [Google Scholar]
- 15. Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med 1970;72:813–22. [DOI] [PubMed] [Google Scholar]
- 16. Reinier K, Dervan C, Singh T, Uy-Evanado A, Lai S, Gunson K et al. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm 2011;8:1177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Chugh H, Gunson K et al. Electrocardiographic versus echocardiographic left ventricular hypertrophy and sudden cardiac arrest in the community. Heart Rhythm 2014;11:1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghali JK, Liao Y, Cooper RS. Influence of left ventricular geometric patterns on prognosis in patients with or without coronary artery disease. J Am Coll Cardiol 1998;31:1635–40. [DOI] [PubMed] [Google Scholar]
- 19. Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S et al. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension 2004;43:731–8. [DOI] [PubMed] [Google Scholar]
- 20. Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the valiant (valsartan in acute myocardial infarction) echocardiographic study. JACC Cardiovasc Imaging 2008;1:582–91. [DOI] [PubMed] [Google Scholar]
- 21. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Left ventricular geometry and outcomes in patients with atrial fibrillation: the Affirm Trial. Int J Cardiol 2014;170:303–8. [DOI] [PubMed] [Google Scholar]
- 22. Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community-dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail 2014;2:512–22. [DOI] [PubMed] [Google Scholar]
- 23. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol 1995;25:871–8. [DOI] [PubMed] [Google Scholar]
- 24. Milani RV, Lavie CJ, Mehra MR, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol 2006;97:959–63. [DOI] [PubMed] [Google Scholar]
- 25. Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace 2000;2:216–23. [DOI] [PubMed] [Google Scholar]
- 26. Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 2010;122:2727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 2011;58:1733–40. [DOI] [PubMed] [Google Scholar]
- 28. Lieb W, Gona P, Larson MG, Aragam J, Zile MR, Cheng S et al. The natural history of left ventricular geometry in the community: clinical correlates and prognostic significance of change in LV geometric pattern. JACC Cardiovasc Imaging 2014;7:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Devereux RB, Palmieri V, Liu JE, Wachtell K, Bella JN, Boman K et al. Progressive hypertrophy regression with sustained pressure reduction in hypertension: the losartan intervention for endpoint reduction study. J Hypertens 2002;20:1445–50. [DOI] [PubMed] [Google Scholar]
- 30. Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004;292:2350–6. [DOI] [PubMed] [Google Scholar]