Abstract
Polyglutamine (polyQ) repeat expansion in the deubiquitinase ataxin-3 causes neurodegeneration in Spinocerebellar Ataxia Type 3 (SCA3), one of nine inherited, incurable diseases caused by similar mutations. Ataxin-3’s degradation is inhibited by its binding to the proteasome shuttle Rad23 through ubiquitin-binding site 2 (UbS2). Disrupting this interaction decreases levels of ataxin-3. Since reducing levels of polyQ proteins can decrease their toxicity, we tested whether genetically modulating the ataxin-3-Rad23 interaction regulates its toxicity in Drosophila. We found that exogenous Rad23 increases the toxicity of pathogenic ataxin-3, coincident with increased levels of the disease protein. Conversely, reducing Rad23 levels alleviates toxicity in this SCA3 model. Unexpectedly, pathogenic ataxin-3 with a mutated Rad23-binding site at UbS2, despite being present at markedly lower levels, proved to be more pathogenic than a disease-causing counterpart with intact UbS2. Additional studies established that the increased toxicity upon mutating UbS2 stems from disrupting the autoprotective role that pathogenic ataxin-3 has against itself, which depends on the co-chaperone, DnaJ-1. Our data reveal a previously unrecognized balance between pathogenic and potentially therapeutic properties of the ataxin-3-Rad23 interaction; they highlight this interaction as critical for the toxicity of the SCA3 protein, and emphasize the importance of considering protein context when pursuing suppressive avenues.
Introduction
Polyglutamine (polyQ)-dependent diseases comprise a class of neurodegenerative disorders characterized by abnormal CAG nucleotide repeat expansions in the disease gene (1). These repeats translate into an abnormally long polyQ tract, resulting in subsequent misfolding and aggregation of the disease protein in neurons and, ultimately, neuronal dysfunction and death. Among these disorders, Spinocerebellar Ataxia Type 3 (SCA3, or Machado-Joseph Disease), is the most common dominantly inherited ataxia in the world (1,2). Onset occurs in mid to late life, with degeneration observed in cerebellar pathways and nuclei, dentate nuclei, pontine nuclei, and basal ganglia. Symptoms include ataxia, dysarthria, dystonia, ophthalmoplegia, spasticity, and neuropathy (2). There is no cure for SCA3.
SCA3 is caused by expansion of a CAG tract in ATXN3 from 22–42 repeats to a disease range of 52–84, leading to the translation of a pathogenic form of the ataxin-3 protein with expanded polyQ (1–3). Ataxin-3, a deubiquitinating enzyme, edits ubiquitin chains on substrates (4,5); it has been reported to function in protein quality control and transcriptional regulation (2,5–8), and is neuroprotective in Drosophila melanogaster, where it suppresses degeneration from various polyQ proteins (9–12). Ataxin-3 also protects against various stressors in mammalian cell culture (7,13–15).
The N-terminal catalytic domain of ataxin-3 contains two ubiquitin-binding sites that can bind mono-ubiquitin (16,17). Ubiquitin-binding site 2 (UbS2) also binds to the proteasomal shuttle proteins, Rad23/hHR23A and B (16–19). According to our earlier published work, proteasomal degradation of ataxin-3 is regulated by the direct interaction between UbS2 and Rad23 (20). When Rad23 is bound to UbS2, ataxin-3 is protected from degradation (Fig. 1A). Disrupting this interaction decreases the steady state levels of ataxin-3 in the cell. While this previous work showcased the importance of the ataxin-3-Rad23 interaction for the SCA3 protein’s turnover, it did not directly test whether this interaction is critical for ataxin-3-dependent toxicity. In other words, does UbS2 of ataxin-3 directly modulate SCA3 pathogenicity? More importantly, should UbS2 be targeted to protect against SCA3, since it regulates overall cellular levels of ataxin-3?
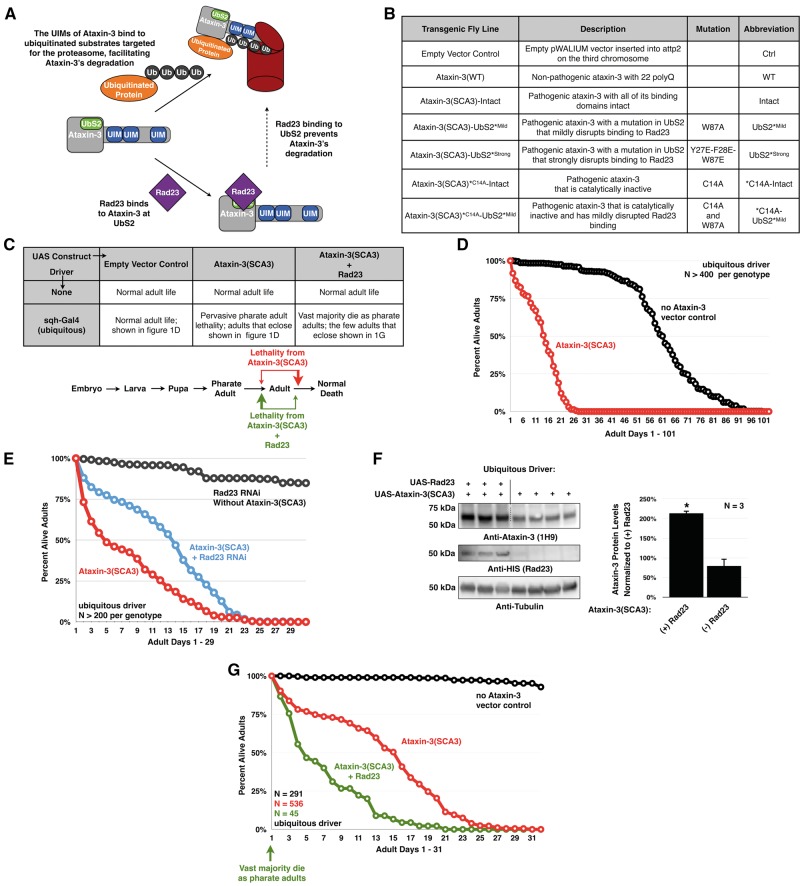
Figure 1.
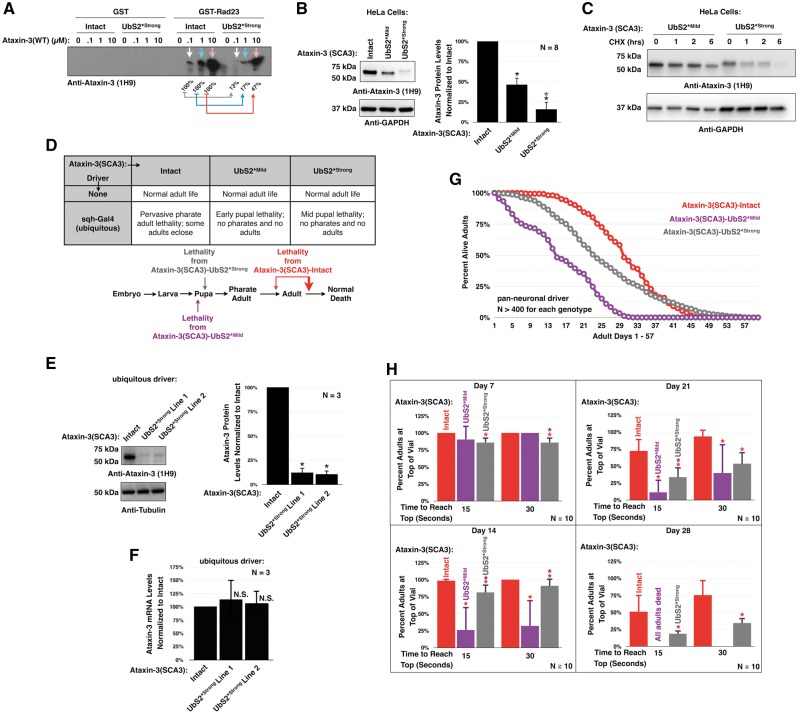
Rad23 influences ataxin-3(SCA3)-dependent toxicity. (A) Model depicting how Rad23 influences ataxin-3’s degradation, based on studies from Blount et al, 2014 (20). The ubiquitin-interacting motifs (UIMs) of ataxin-3 bind ubiquitin chains on proteasome substrates, facilitating the interaction of ataxin-3 with this machinery. Once at the proteasome, in the absence of interaction with Rad23, ataxin-3 is degraded. If ataxin-3 interacts with Rad23 through UbS2, it is rescued. (B) Summary of the transgenic Drosophila lines used. (C) Summary of observations with ubiquitous expression of pathogenic ataxin-3 with or without Rad23 overexpression. In this panel and rest of the figures, unless otherwise noted, flies were heterozygous for all transgenes. (D) Longevity of flies ubiquitously expressing pathogenic ataxin-3 or the empty host vector inserted into attP2. (E) Longevity of flies ubiquitously expressing pathogenic ataxin-3 without or with UAS-RNAi targeting Rad23. A control line was included that expresses Rad23 RNAi in the absence of ataxin-3. Flies were siblings from the same crosses and on a different background (y-w-) than flies in the rest of this figure (w1118). (F) Western blots from independent lysates of whole flies ubiquitously expressing the specified transgenes. Means ± SD. Asterisks: P< 0.05 from Student’s t-tests compared to UAS-ataxin-3(SCA3) without exogenous Rad23. When quantifying ataxin-3 protein levels for (-)Rad23 flies, lane 4 was set to 100%. Lane 5 was not quantified, due to the bubble disrupting the ataxin-3 band. (G) Longevity of flies ubiquitously expressing pathogenic ataxin-3 with or without Rad23 overexpression.
Here, we show that disrupting UbS2 of disease-causing ataxin-3 decreases the levels of the pathogenic ataxin-3 protein, but greatly enhances its toxicity in vivo. Further investigations indicate that this increase in toxicity is due to a reduction in ataxin-3’s neuroprotective function against itself, which is mediated by the UbS2-Rad23 interaction and depends on the chaperone partner, DnaJ-1. Our data demonstrate a critical role for the interaction of ataxin-3 with Rad23 in the regulation of this polyQ protein’s abundance, function, and pathogenicity.
Results
Modulating levels of Rad23 influences ataxin-3’s toxicity in Drosophila
To examine the function of the interaction between UbS2 of ataxin-3 and Rad23, we used a transgenic fly model of SCA3 that we recently generated by using the phiC31-targeted integration system (21). The phiC31 system yields a similar expression of transgenes among different fly lines, due to site-specific integration (22), in our case into attP2 on the third chromosome of the fly. This method allows for direct comparisons among the different transgenic lines we generated (Fig. 1B). Our Drosophila model of SCA3 expresses untagged, full-length, human pathogenic ataxin-3 under the control of the Gal4/UAS system. We also generated a control line that contains the empty host vector inserted into attP2 (23) and another line that expresses wild-type, human ataxin-3, inserted at a different site than attP2 (10).
When pathogenic ataxin-3 (ataxin-3(SCA3)) is expressed throughout the fly, it leads to some lethality at late pharate adult stages and during eclosion from the pupal case (Fig. 1C). Those flies that mature into adulthood only live up to ∼30 days, as opposed to the control flies that live up to ∼100 days (Fig. 1D).
Previously, we reported that reducing the levels of endogenous Rad23 through RNAi by approximately 50%, as determined through qRT-PCR, or by removing one copy of its gene decreased ataxin-3 protein levels and noticeably improved degeneration in Drosophila eyes expressing pathogenic ataxin-3 with an intact UbS2 (20; other supporting data not shown). We recapitulated this effect by reducing endogenous Rad23 levels in all the tissues of the fly through RNAi. We observed improved adult longevity compared to sibling SCA3 flies without Rad23 knockdown (Fig. 1E). Based on our earlier work with various Gal4 drivers and different polyQ and non-polyQ constructs (10,12,20,23,24), improved longevity from pathogenic ataxin-3 when Rad23 is knocked down through UAS-RNAi is not due to a dilution effect of the Gal4 driver co-expressing ataxin-3 and the knockdown construct.
Conversely to Rad23 knockdown, exogenous Rad23 noticeably increases ataxin-3(SCA3) protein levels (Fig. 1F and Supplementary Material, Fig. S1). This increase in protein levels coincides with higher lethality (Fig. 1C). Almost all of the SCA3 flies that co-express Rad23 die as pharate adults, before they eclose from the pupal case. Very few adult flies co-expressing Rad23 and ataxin-3(SCA3) eclose successfully (Fig. 1C). Those flies that survive to adulthood exhibit reduced longevity compared to the SCA3 flies that are not expressing exogenous Rad23 (Fig. 1G).
Flies expressing exogenous Rad23 or expressing RNAi targeting Rad23 in the absence of ataxin-3 were kept as healthy stocks for over a year without clear signs of reduced fecundity or of toxicity (Fig. 1E and data not shown). These collective data from the perturbation of Rad23 levels support the idea that this proteasome-associated protein regulates pathogenic ataxin-3 levels and subsequent toxicity. These results led us to next investigate if disturbing the binding site of this protein on ataxin-3 is beneficial in vivo.
Mutating UbS2 reduces ataxin-3 protein levels, but greatly enhances its toxicity
Based on the results from our experiments with altered Rad23 levels, we hypothesized that reducing binding of UbS2 to Rad23 would lower ataxin-3(SCA3) protein levels and subsequently alleviate its toxicity. We generated additional transgenic flies that express ataxin-3(SCA3) with a mutation in UbS2 that was previously shown by us and others to impair Rad23 binding (16,17,20), in this case by approximately 50 percent (20). This mutation (referred to as UbS2*Mild) replaces a critical tryptophan residue on UbS2 with an alanine. It does not alter ataxin-3’s cellular distribution, or the overall structure of the isolated catalytic domain; it does not negate the ability of the full-length protein to become ubiquitinated and catalytically activated; it does not abrogate the catalytic activity of full-length ataxin-3 ((6,17,20) and data not shown); and, in the context of ataxin-3 with a normal polyQ repeat, this mutation does not impact fly longevity (Supplementary Material, Fig. S2). This mutation also does not perturb the ability of ataxin-3 to bind another of its partners, VCP/p97 (Supplementary Material, Fig. S3). Together, these findings led us to conclude that the conformation of the overall catalytic domain of ataxin-3 is not detrimentally impacted by the UbS2*Mild mutation.
Surprisingly, we found that expression of UbS2*Mild throughout the fly causes lethality at early pupal stages (Fig. 2A). Western blots from larval lysates demonstrated that mutating UbS2 reduces ataxin-3(SCA3) protein levels (Fig. 2B), confirming our earlier data that UbS2 is critical for ataxin-3 protein levels. Nevertheless, the lethality phenotype is markedly worse than what we observe in the flies expressing ataxin-3(SCA3) with UbS2 intact, which reach pharate adult and adult stages (Fig. 2A).
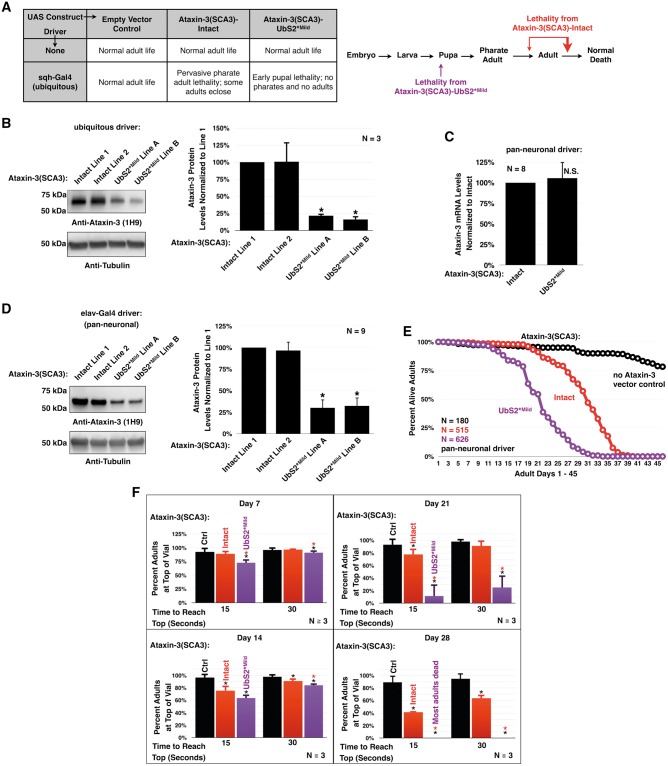
Figure 2.
Mutating UbS2 of pathogenic ataxin-3 increases its toxicity. (A) Lethality observed when pathogenic ataxin-3 with or without a mild UbS2 mutation is ubiquitously expressed. (B) Western blots from larvae ubiquitously expressing pathogenic ataxin-3 with or without a UbS2 mutation. Expression of constructs in (A) and (B) was driven by the sqh-Gal4 driver. Means ± SD. Asterisks: P < 0.05 from Student’s t-tests compared to line 1. Lines 1, 2, and A, B are from different founders, which had similar phenotypic outcomes and were combined in later studies. (C) qRT-PCR data from one day old flies pan-neuronally expressing pathogenic ataxin-3 with or without a mild UbS2 mutation. Endogenous control: rp49. Means ± SD. N.S: non statistically significant. (D) Western blots from adult flies pan-neuronally expressing pathogenic ataxin-3 with or without a UbS2 mutation. Means ± SD. Asterisks: P < 0.05 from Student’s t-tests compared to line 1. Lines 1, 2, and A, B are from different founders, which had similar phenotypic outcomes and were combined in later studies. (E) Longevity of flies pan-neuronally expressing pathogenic ataxin-3 with or without a mild UbS2 mutation. (F) Results from negative geotaxis motility assays, which were repeated at least three times, totaling at least 100 flies per genotype. Means ± SD. Asterisks: P < 0.05 from Student’s t-tests compared to Ctrl (black) or Intact (red) for each time point. Ctrl: empty vector inserted into site attP2. Expression of constructs in (C)–(F) was driven by the elav-Gal4 driver.
Since these results failed to support our above hypothesis, we pan-neuronally expressed the ataxin-3(SCA3) constructs to more thoroughly characterize the phenotype. Western blotting revealed that mutating UbS2 leads to lower ataxin-3(SCA3) protein levels, similar to what was observed with ubiquitous expression in larvae (Fig. 2D). qRT-PCR indicates that this is not due to differences at the mRNA level (Fig. 2C). To examine if mutating UbS2 alters the aggregation propensity of ataxin-3(SCA3) before the onset of pathology, we performed differential centrifugation of one-day-old flies pan-neuronally expressing ataxin-3(SCA3)-Intact or UbS2*Mild. We did not observe a noticeable difference in soluble/insoluble fractions of ataxin-3(SCA3)-Intact or UbS2*Mild in flies, or in mammalian cell culture (Supplementary Material, Fig. S4). When ataxin-3(SCA3)-Intact is expressed pan-neuronally, flies exhibit reduced longevity and motility (Fig. 2E and F). However, expression of UbS2*Mild triggers greater impairment in longevity and motility than ataxin-3(SCA3)-Intact, supporting our results from ubiquitous expression of these two proteins (Fig. 2A).
Drosophila eyes are a popular tool for investigating neurodegenerative diseases, as degeneration can be reliably visualized at the external level or internally through histological sectioning. Using the GMR-Gal4 driver, we drove the expression of pathogenic ataxin-3 transgenes in Drosophila eyes to characterize the subsequent degeneration. Once again, we witnessed a significant reduction in ataxin-3 protein in dissected fly heads expressing UbS2*Mild, as compared to ones expressing the intact variant (Fig. 3A). Expressing ataxin-3(SCA3)-Intact in fly eyes leads to a slight depigmentation of the retina, which is visible by day 14 and increases with age (Fig. 3B). On day 14, the dark, central pseudo-pupil is also lost, signifying a compromise of the underlying photoreceptor cells. In the fly eyes expressing UbS2*Mild, depigmentation and loss of the pseudo-pupil is observed as early as day 7 and progresses more markedly than with ataxin-3(SCA3)-Intact (Fig. 3B).
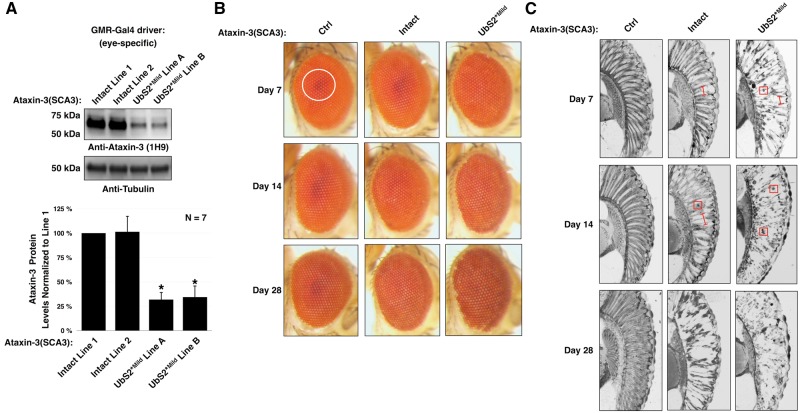
Figure 3.
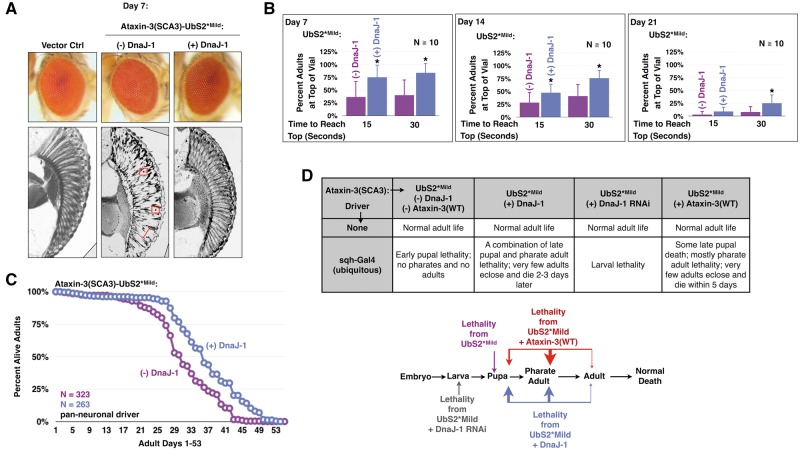
Mutating UbS2 increases pathogenicity of ataxin-3 in fly eyes. (A) Western blots from fly heads expressing pathogenic ataxin-3 without or with a UbS2 mutation. Means ± SD. Asterisks: P < 0.05 from Student’s t-tests compared to line 1. Lines 1, 2, and A, B are from different founders, which had similar phenotypic outcomes and were combined in studies in the other panels of this figure. (B) External photos and (C) histological sections of fly eyes expressing pathogenic ataxin-3 without or with a mild UbS2 mutation. Ctrl: empty vector inserted into attP2. White circle in (B): example of the pseudo-pupil. Red boxes in (C): examples of densely staining aggregates. The precise subcellular localization of the aggregates is not clear from this assay. Red bracketed lines in (C): examples of disruption of the ommatidial boundaries. Expression of constructs was driven by the GMR-Gal4 driver.
Next, we performed histology to obtain a more detailed look at the structure of the internal eye. A healthy Drosophila eye is characterized by an organized ommatidial array with distinct boundaries between the ommatidia, the functional unit of the eye. With ataxin-3(SCA3)-Intact, a slight disruption of the ommatidial array is visible at day 7 (Fig. 3C). By day 14, darkly staining structures, which were shown previously to contain the pathogenic ataxin-3 protein (9), are visible. At 28 days, the ommatidial array is significantly degenerated. Compared to the effect of ataxin-3(SCA3)-Intact, UbS2*Mild causes markedly increased disorganization of the ommatidial array and the presence of densely-staining structures as early as day 7. By day 28, the ommatidial boundaries are no longer distinguishable. The data from Figs 2 and 3 demonstrate that pathogenic ataxin-3 with mutated UbS2 causes significantly more degeneration than intact ataxin-3(SCA3) in various fly tissues.
Our working model predicted that reducing Rad23 binding to ataxin-3(SCA3) by mutating UbS2 would decrease ataxin-3(SCA3) protein levels and lower its toxicity. Characterization of flies expressing ataxin-3(SCA3)-Intact or ataxin-3(SCA3)-UbS2*Mild under the control of different drivers revealed that mutating UbS2 reduces ataxin-3(SCA3) protein levels, but greatly enhances the toxic phenotype. In the fly eyes, this toxicity is concomitant with the presence of densely-staining aggregates on an earlier time scale with UbS2*Mild than with ataxin-3(SCA3)-Intact. The exact type of aggregated species formed by pathogenic ataxin-3 in vivo is likely to be dependent on cellular context and type of tissue. These results led us to next probe how disrupting the interaction between ataxin-3(SCA3) and Rad23 increases the pathogenicity of the ataxin-3(SCA3) protein.
Mutating UbS2 increases ataxin-3 toxicity by compromising its autoprotective function
Wild-type ataxin-3 is protective in Drosophila models of polyQ diseases (9–12). These findings were supported by studies in mammalian cell culture where ataxin-3 also protects against various stressors (7,13–15). Work published by the Bonini lab (9) and ours (12) showed that wild-type ataxin-3 requires both its catalytic activity and its binding to Rad23 to protect against polyQ-dependent toxicity in Drosophila. Collectively, these earlier results from flies presented the possibility that pathogenic ataxin-3 retains the protective function against itself (Fig. 4A summarizes the model). We posited that when we mutate UbS2 to reduce Rad23 binding, we also compromise ataxin-3’s autoprotective function. Without its autoprotection, the UbS2*Mild protein is more toxic than ataxin-3(SCA3)-Intact, even though there is less protein present (Fig. 4A; also see Fig. 7).
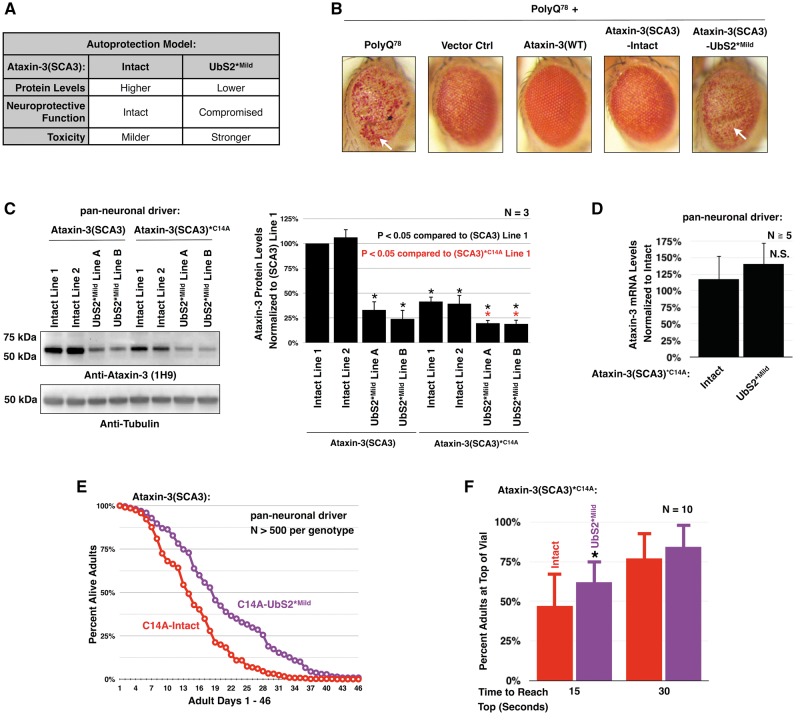
Figure 4.
Mutating UbS2 of catalytically inactive, pathogenic ataxin-3 reduces its protein levels and toxicity. (A) Table summarizing the working model for the role of ataxin-3’s UbS2 in toxicity. (B) Photos of external eyes expressing a truncated polyQ fragment of ataxin-3 with 78 repeats and co-expressing the indicated lines. Flies were heterozygous for all transgenes, except for flies homozygous for polyQ78 (far left). Constructs were expressed using the GMR-gal4 driver. White arrows: examples of indentation. (C) Western blots from adult flies pan-neuronally expressing pathogenic ataxin-3 that is catalytically active or inactive. Means ± SD. Black asterisks: P < 0.05 from Student’s t-tests compared to catalytically active ataxin-3 line 1. Red asterisks: P < 0.05 from Student’s t-tests compared to catalytically inactive ataxin-3 line 1. Lines 1, 2, and A, B are from different founders, which had similar phenotypic outcomes and were combined in later studies in this figure. (D) qRT-PCR data from one day old flies expressing pathogenic, catalytically inactive ataxin-3 with or without a mild UbS2 mutation. Endogenous control: rp49. Means ± SD. N.S: non statistically significant. (E) Longevity data from flies expressing pathogenic, catalytically inactive ataxin-3 without or with a mild UbS2 mutation. (F) Motility of flies expressing pathogenic, catalytically inactive ataxin-3 without or with a mild UbS2 mutation. Flies were 7 days old. N = 10, totaling at least 100 flies per genotype. Asterisk: P < 0.05 from Student’s t-tests compared to C14A-Intact.
Figure 7.
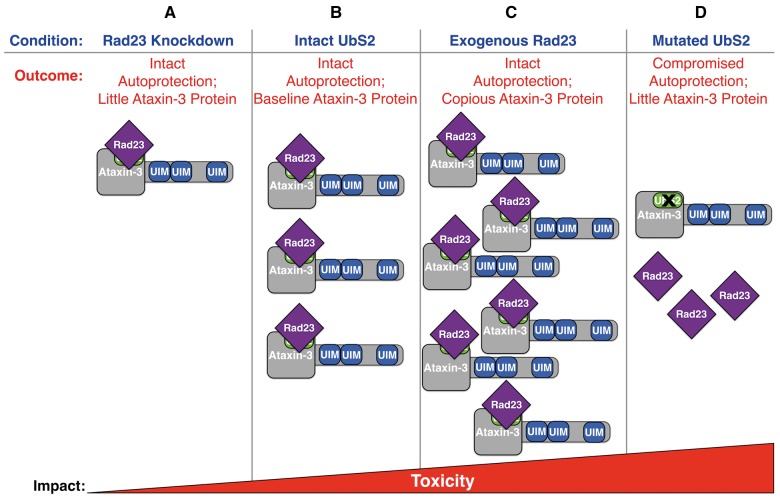
Model of Rad23 and UbS2 regulating ataxin-3’s levels and toxicity. (A) Knocking down Rad23 reduces its availability to bind ataxin-3 at UbS2, increasing ataxin-3’s degradation. This reduces ataxin-3 protein levels while leaving its autoprotection intact, resulting in lowered ataxin-3 toxicity. (B) Ataxin-3 with intact UbS2 is present at baseline levels; its pathogenicity is regulated by its autoprotection, which requires Rad23 binding to UbS2. (C) Exogenous Rad23 increases its availability to bind ataxin-3, inhibiting ataxin-3’s proteasomal degradation and increasing its toxicity. (D) Mutating UbS2 reduces Rad23 binding, resulting in lower ataxin-3 levels. However, mutating this site compromises ataxin-3’s protection against itself, thus increasing its toxicity.
To first test whether mutating UbS2 impairs the protective function of ataxin-3(SCA3), we co-expressed a polyQ peptide with 78 glutamine repeats (polyQ78) alongside full-length, pathogenic ataxin-3 transgenes in Drosophila eyes. PolyQ78 is the isolated C terminus of the ataxin-3 protein without UIMs or the N terminus (25). Fly eyes expressing two copies of polyQ78 exhibit severe depigmentation of the retina (Fig. 4B). Indentations are also visible on the external eye, indicative of the collapse of internal structures. Flies heterozygous for polyQ78 and the empty host vector for ataxin-3(SCA3) transgenes display milder, but clearly visible external depigmentation. Expression of wild-type ataxin-3 ameliorates this phenotype, as also shown previously (9–12). When ataxin-3(SCA3)-Intact is co-expressed with polyQ78, the level of degeneration is significantly less than what is observed in flies expressing two polyQ78 alleles. However, UbS2*Mild is unable to suppress polyQ78 toxicity, supporting the notion that ataxin-3(SCA3) retains some protective function, and that mutating UbS2 results in at least some loss of this role.
To further examine the importance of an intact UbS2 on the pathogenicity of the SCA3 protein, we generated additional transgenic fly lines that express catalytically inactive, pathogenic ataxin-3 either with all of its domains intact (ataxin-3(SCA3)*C14A -Intact) or with UbS2 mutated (ataxin-3(SCA3)*C14A -UbS2*Mild) (Fig. 1B). To render ataxin-3(SCA3) catalytically inactive, we mutated the critical cysteine in its protease domain to an alanine, which compromises the protein’s ability to protect against polyQ toxicity (4,6,10,26). These transgenic lines would help us to determine if mutating UbS2 mitigates ataxin-3-dependent toxicity when its autoprotective role is no longer a factor.
When these catalytically inactive transgenes are pan-neuronally expressed in Drosophila, the C14A-UbS2*Mild mutation reduces ataxin-3 protein levels, independently of transcriptional levels (Fig. 4C and D). The protein levels of catalytically inactive ataxin-3(SCA3) are also significantly lower than catalytically active ataxin-3(SCA3), indicating that ataxin-3’s catalytic activity regulates its steady state levels in vivo. Importantly, flies expressing C14A-UbS2*Mild exhibit improved adult longevity compared to the flies with C14A-Intact (Fig. 4E). Motility is also significantly improved in C14A-UbS2*Mild flies on day 7 (Fig. 4F).
Overall, these results indicate that reducing ataxin-3 protein levels can indeed decrease the toxicity of pathogenic ataxin-3, as long as its protective function is not a factor. We conclude that UbS2*Mild increased the toxicity of ataxin-3(SCA3) in our earlier experiments because the mutation impaired its autoprotective function.
Strong disruption of pathogenic ataxin-3’s UbS2 is less toxic than mild disruption
Mild disruption of Rad23 binding to UbS2, which decreases binding of ataxin-3 to Rad23 in vitro by about 50% (20), yielded a more toxic protein. We next reasoned that a stronger disruption of Rad23 binding to UbS2 might reduce the protein’s toxicity by nearly eliminating the ataxin-3 protein. To test this possibility, we mutated three amino acid residues of UbS2 to glutamic acid (ataxin-3(SCA3)-UbS2*Strong;Fig. 1B). In vitro binding assays indicate that UbS2*Strong greatly reduces binding to Rad23 (Fig. 5A). When we express pathogenic ataxin-3 with the UbS2*Strong mutation in HeLa cells, we notice a reduction in ataxin-3(SCA3) levels more profound than with UbS2* Mild (Fig. 5B). The turnover rate of UbS2*Strong is also higher than that of UbS2*Mild (Fig. 5C).
Figure 5.
A stronger mutation of UbS2 reduces pathogenic ataxin-3 protein levels and toxicity compared to UbS2*Mild. (A) Western blot from in vitro binding assays of ataxin-3(WT) and full-length, GST-tagged Rad23A. GST or GST-Rad23 were pulled down on glutathione agarose beads, washed thrice with NETN and incubated with ataxin-3(WT)-Intact or ataxin-3(WT)-UbS2*Strong for 1 h on ice. Colored arrows denote the different amounts of ataxin-3. Percentages compare UbS2-mutated ataxin-3 pulled down by GST-Rad23A to its non-mutated counterpart of the same amount. (B) Western blots from whole cell lysates. HeLa cells were transfected with the indicated constructs and harvested 24 h later. Means ± SD. Asterisks: P < 0.05 from Student’s t-tests when compared to Intact (black) or UbS2*Mild (purple). (C) Western blots from HeLa cells transfected with the indicated constructs and treated 24 h later with cycloheximide (CHX) for the indicated amounts of time. Transfection and loading were done such that the protein level of the two different versions of ataxin-3 were comparable at 0h. Example shown is from experiment repeated independently at least 4 times with similar results. (D) Lethality observed when ubiquitously expressing pathogenic ataxin-3 without or with a UbS2 mutation. (E) Western blots from larvae ubiquitously expressing pathogenic ataxin-3 without or with a strong UbS2 mutation. Means ± SD. Asterisks: P < 0.05 from Student’s t-test compared to Intact. Lines 1 and 2 are from different founders, which had similar phenotypic outcomes and were combined in phenotypic studies. (F) qRT-PCR data from larvae ubiquitously expressing pathogenic ataxin-3 without or with a strong UbS2 mutation. Endogenous control: rp49. N.S: non statistically significant. (G) Longevity of flies pan-neuronally expressing pathogenic ataxin-3 without or with a UbS2 mutation. (H) Results from motility assays of flies expressing pathogenic ataxin-3 without or with a UbS2 mutation. Means ± SD. Asterisks: P < 0.05 from Student’s t-tests compared to Intact (red) or UbS2*Mild (purple). N = 10, totaling at least 100 flies per genotype.
We generated additional fly lines that express pathogenic ataxin-3 with the UbS2*Strong mutation. Ubiquitous expression of UbS2*Strong elicits developmental lethality primarily in the mid pupal stage, which represents a slight improvement from what was seen earlier with the UbS2*Mild flies (Fig. 5D). Western blotting of larvae expressing UbS2*Strong reveals that ataxin-3 protein levels are reduced by approximately 90% (Fig. 5E). qRT-PCR indicates that the decrease in the UbS2*Strong version is not due to transcriptional changes (Fig. 5F).
We continued our analyses of the SCA3 phenotype by pan-neuronally expressing UbS2*Strong. When expressed in neurons, the protein levels of this version of ataxin-3 are also markedly lower than those of the intact counterpart (data not shown), similar to what was observed with ubiquitous expression in larval lysates (Fig. 5E). These adults live longer and perform better in the motility assay than the flies expressing UbS2*Mild (Fig. 5G and H). Still, in general they do not perform as well as flies expressing ataxin-3(SCA3)-Intact. Collectively, these data further support the role of UbS2 in the pathogenicity of ataxin-3, and stress the importance of this site on both the toxicity and protein levels of the SCA3 protein.
DnaJ-1 suppresses toxicity from ataxin-3(SCA3) with mutated UbS2
Previously, we reported that wild-type ataxin-3 (denoted here as ataxin-3(WT)) suppresses polyQ toxicity in Drosophila by binding Rad23 and leading to an increase in endogenous DnaJ-1 at mRNA and protein levels (12). A version of ataxin-3 with the UbS2*Mild mutation did not have the same effect on DnaJ-1 (12). DnaJ-1 is a member of the J/HSP40 family of co-chaperone proteins that maintain the substrate specificity and stimulate the ATPase activity of HSP70 chaperones (27,28). Since our data are consistent with the idea that ataxin-3(SCA3) with mutated UbS2 is more pathogenic due to impaired autoprotection, we tested whether DnaJ-1 can suppress this toxicity.
When we co-express DnaJ-1 with UbS2*Mild in fly eyes, we observe great improvement in the internal retinal structure: the boundaries and the organization of the ommatidia are restored (Fig. 6A). External photos of DnaJ-1 eyes display the re-appearance of the pseudo-pupil, consistent with a healthy underlying structure (Fig. 6A). Pan-neuronal expression of DnaJ-1 alongside UbS2*Mild also improves adult longevity and motility (Fig. 6B and C). Moreover, exogenous DnaJ-1 reduces the rate of developmental lethality in UbS2*Mild flies when the toxic protein is expressed everywhere (Fig. 6D). Instead of dying in the early pupal stage, these flies exhibit late pupal and pharate adult lethality. A small number of adults even successfully eclose and survive for two or three days. Importantly, RNAi-dependent knockdown of DnaJ-1 causes the UbS2*Mild flies to die much earlier (Fig. 6D).
Figure 6.
Exogenous DnaJ-1 suppresses toxicity from pathogenic ataxin-3 with mutated UbS2. (A) External eye photos and histological sections of fly eyes expressing pathogenic ataxin-3 with a mild UbS2 mutation in the absence or presence of exogenous DnaJ-1. Constructs were expressed using the GMR-Gal4 driver. Vector Ctrl: empty vector inserted into attP2. Red boxes: examples of densely staining structures. Red bracketed line: an example of disruption of the ommatidial boundaries. (B) Motility of flies expressing pathogenic ataxin-3 with a mild UbS2 mutation in the presence or absence of exogenous DnaJ-1. Here, elav-Gal4 was on the third chromosome, expressing at lower levels than in previous figures, where elav-Gal4 was on the X chromosome (46). Means ± SD. Asterisks: P < 0.05 from Student’s t-test, compared to UbS2*Mild without exogenous DnaJ-1. N = 10, totaling at least 100 flies per genotype. (C) Longevity of flies expressing pathogenic ataxin-3 with a mild UbS2 mutation in the presence or absence of exogenous DnaJ-1. (D) Lethality observed when ubiquitously expressing pathogenic ataxin-3 with a mild UbS2 mutation in the presence or absence of exogenous DnaJ-1, or wild type ataxin-3, or knockdown of DnaJ-1 through UAS-RNAi.
Lastly, we examined the protective role of ataxin-3(WT) against its pathogenic variant with UbS2 mutated. As summarized in Fig. 6D, expression of non-pathogenic ataxin-3 has a suppressive effect towards UbS2*Mild that closely mirrors the protective effect of exogenous DnaJ-1. Ataxin-3(WT) delays lethality from pathogenic ataxin-3 with a mutated Rad23-binding site from early pupal to late pupal and pharate adult stages. A handful of adults eclose and survive for a few days. Altogether, the results in Fig. 6 lend additional support to the hypothesis that pathogenic ataxin-3 with mutated UbS2 is more toxic due to a perturbation of its autoprotective role.
Wild-type ataxin-3 has a protective effect against various polyQ proteins in Drosophila (9–12). This role of ataxin-3 in the fly depends on its catalytic activity, on an intact UbS2, and on the presence of Rad23 (9,12). According to those findings, ataxin-3, in a manner dependent on Rad23, leads to an increase in the transcription of DnaJ-1 and a subsequent rise in its protein levels. This co-chaperone then functions with inducible heat shock proteins to reduce the aggregation and toxicity of polyQ species in flies. The data in Fig. 6, together with the results from prior figures where we perturbed Rad23 levels, mutated UbS2 of ataxin-3, and incapacitated the DUB activity of pathogenic ataxin-3, collectively indicate a similar mechanism at work on autoprotection from pathogenic ataxin-3.
Discussion
Our prior work showed a critical role for the SCA3 protein interaction with the proteasome-associated protein, Rad23 in the proteasomal turnover of ataxin-3 (20). Whether the site of this interaction was directly important for the toxicity of the SCA3 protein, or if targeting this interaction could be beneficial against SCA3, was unclear. We found that mutating UbS2 on ataxin-3 to disrupt this interaction substantially reduces steady state levels of the SCA3 protein in vivo, but greatly enhances its toxicity. According to our work, ataxin-3’s binding to Rad23 regulates this polyQ protein's levels, autoprotective function, and, consequently, its pathogenicity. The data presented here showcase a previously unreported role for the interaction between the SCA3 protein and Rad23 as a regulator of ataxin-3’s own pathogenicity in an intact, model organism (Fig. 7). Our results bring together a new understanding of the role of ataxin-3’s domains and its interactions with partners in its toxicity. They also highlight the importance of protein context in the biology of this polyQ disease, and the need to carefully consider it when devising strategies for suppressive avenues.
Ataxin-3 can also bind ubiquitin through UbS2 (16,17). One might surmise that binding of ubiquitin, or other similar proteins, could potentially impact ataxin-3 levels and toxicity through their interaction with UbS2. In earlier work, we found that knockdown of ubiquitin-like proteins did not impact ataxin-3 levels in Drosophila (20). Our genetic manipulations of Rad23 levels in this study and in other, previously published work (20) make a strong case for a specific and significant role from the binding of ataxin-3 to Rad23 in the toxicity and levels of the SCA3 protein.
Ataxin-3’s interaction with Rad23 might have a cellular role by assisting with the degradation of select poly-ubiquitinated substrates destined for the proteasome (29,30). Binding of ataxin-3 to Rad23 hinders the degradation of the SCA3 protein (20). This interaction between Rad23 and ataxin-3, however, need not impact the ability of these two ubiquitin-binding proteins to deliver cargo to the degradative machinery, which depends on the transfer of poly-ubiquitinated substrates from "shuttles" to the 19S component of the proteasome (31).
Our findings raise the question why we observe decreased ataxin-3-dependent toxicity when we reduce Rad23 levels through RNAi, but not when we mutate UbS2 to reduce Rad23 binding to ataxin-3. Rad23 knockdown reduces its levels and thus ataxin-3 protein levels as a result of its increased degradation (Fig. 1A;20), but the remaining Rad23 and ataxin-3 proteins can still interact since UbS2 is intact. In this scenario, ataxin-3 is likely less toxic because some of the pathogenic protein is eliminated, but the remaining SCA3 protein can still bind Rad23 and exert its unperturbed autoprotective role. When we mutate UbS2, we reduce the protective ability of all of the ataxin-3 protein present as a result of compromised binding to Rad23 (Fig. 7). Ataxin-3 with mutated UbS2 is unable to elevate expression of the co-chaperone DnaJ-1 (12). Thus, it is not surprising that when we increase the levels of DnaJ-1, we observe a reduction in the pathogenicity of ataxin-3 with mutated UbS2.
Various studies have reported that reducing levels of disease proteins improves the pathology in SCA3 and other polyQ disorders (32–39). Our work demonstrates that the means by which pathogenic protein levels are reduced can influence its toxicity. Since UbS2 is important for both ataxin-3's protein levels and autoprotective function, genetically mutating this site to prevent Rad23 binding proved inadequate for simultaneously reducing ataxin-3 levels and its toxicity. Thus, pathogenic protein levels cannot be reduced indiscriminately; the functionality of the protein binding domains and its interactions must be considered before targeting it therapeutically.
Our results show that mutating UbS2 exacerbates, rather than alleviates, ataxin-3-dependent toxicity in Drosophila. The question remains whether UbS2 could be a potential therapeutic target for SCA3 in mammalian systems. The toxicity from mutating UbS2 in flies results from disruption of ataxin-3’s protective role. While a protective role for ataxin-3 in flies and mammalian cells is clear (7,9–15), there is conflicting evidence for a similar role for ataxin-3 in mice. In one study, SCA3 transgenic mice that were heterozygous for pathogenic ataxin-3 and possessed one copy of wild-type ataxin-3 exhibited a milder phenotype than mice expressing pathogenic ataxin-3 alone (40). Alternatively, co-expression of wild-type ataxin-3 in a different SCA3 mouse model did not ameliorate the phenotype (41). Yet another study reported that complete loss of endogenous, wild-type ataxin-3 in a mouse model of Huntington's disease exacerbated a motor phenotype, but it did not affect other aspects of the pathology (42). If ataxin-3 does not play a significant protective role in intact mammals, mutating UbS2 in situ could be a potential therapeutic approach for SCA3, as this should reduce ataxin-3 protein levels without causing much additional toxicity. Since ataxin-3’s neuroprotective role in mammals remains to be fully clarified, our work suggests that the ideal approach for SCA3 therapy should aim to reduce ataxin-3 levels while leaving its autoprotection intact.
In conclusion, the interaction of ataxin-3 with Rad23 through the UbS2 domain appears to serve dual roles on the SCA3 protein by regulating its levels and inherent toxicity. Our findings provide novel clues towards the understanding of the biology of disease in SCA3, and caution against arbitrarily reducing the levels of a toxic protein for the purposes of therapy.
Materials and Methods
Antibodies
Rabbit, polyclonal anti-ataxin-3 (1:15,000; 43); mouse, monoclonal anti-ataxin-3 (clone 1H9; 1:500–1:1,000; Millipore); mouse, monoclonal anti-tubulin (clone T5168; 1:10,000; Sigma-Aldrich); rabbit, monoclonal anti-HIS (1:1,000; Cell Signaling); mouse, monoclonal anti-GAPDH (clone MAB374, 1:500; Millipore); peroxidase-conjugated secondary antibodies (goat anti-rabbit and goat anti-mouse, 1:5,000; Jackson Immunoresearch).
Drosophila lines
Husbandry was conducted at 25°C and ∼40%-60% humidity in a regulated diurnal environment. SCA3 flies were generated by using ataxin-3 with a polyglutamine repeat of 77–80 residues cloned into the pWalium10-moe vector (Perrimon lab). Mutations were generated on the ataxin-3 backbone using the QuikChange mutagenesis kit (Agilent). Rad23-overexpressing flies were generated by using HIS6-tagged Drosophila Rad23 cloned into the pWalium10-moe vector. Injections (Duke University Model Systems) were into y, w; +; attP2. Stocks from Bloomington: isogenic host strain for attP2 (#36303), DnaJ-1 RNAi (#32899), UAS-DnaJ-1 (#30553), and UAS-polyQ78 (#8141). Rad23 RNAi (#30498) and its isogenic line (#60000) were from the Vienna RNAi Center.
Longevity assay
Upon eclosion, male and female adults were collected and aged in conventional cornmeal fly media at 25°C. The total number of flies per vial was limited to ∼20. Flies were transferred to new vials every 2–3 days, until all dead. Longevity comparisons were restricted to fly lines in the same genetic background, since flies from different backgrounds can have different lifespans (44,45).
Negative geotaxis/motility assay
Approximately 10 adult flies per vial were forced to the bottom by tapping twice in rapid succession on the bench. The number of flies that reached the top at 15 and 30 s was recorded. The assay was conducted once a week for 4 weeks. Flies were transferred to new vials every 2–3 days, including a transfer 1 h before the assay was conducted. Both males and females were used.
Histology
Wings and proboscises were removed from male, adult flies and bodies were fixed overnight in 2% glutaraldehyde/2% paraformaldehyde in Tris-buffered saline with 0.1% Triton X-100. Fixed adults were then dehydrated in a series of 30, 50, 75, and 100% ethanol/propylene oxide. Dehydrated flies were embedded in Poly/Bed812 (Polysciences) and sectioned at 5 µm. Sections were stained with toluidine blue.
Western blotting
Three to five whole flies, ten dissected fly heads, or 10 larvae per group were homogenized in hot lysis buffer (50 mM Tris pH 6.8, 2% SDS, 10% glycerol, 100mM dithiothreitol), sonicated, boiled for 10 min, and centrifuged at top speed at room temperature for 10 min. Western blots were developed and quantified using a CCD-equipped VersaDoc 5000MP system and Quantity One software (Bio-Rad).
Quantitative RT-PCR
Total RNA was extracted from larvae or adult flies using TRIzol (Life Technologies). Extracted RNA was treated with TURBO DNAse (Ambion) to eliminate contaminating DNA. Reverse transcription was carried out with the high-capacity cDNA reverse transcription kit (ABI). Messenger RNA levels were quantified by using the StepOnePlus Real-Time PCR System with Fast SYBR Green Master Mix (ABI). rp49 was used as an internal control. Primers:
SCA3 - F: 5’ GAATGGCAGAAGGAGGAGTTACTA - 3’
SCA3 - R: 5’ GACCCGTCAAGAGAGAATTCAAGT - 3’
rp49-F: 5’ -AGATCGTGAAGAAGCGCACCAAG- 3’
rp49-R: 5’ -CACCAGGAACTTCTTGAATCCGG- 3’
Cell lines, plasmids, transfection & chemicals
HeLa cells were from ATCC and grown in DMEM with 10% FBS and 5% Penicillin-Streptomycin under conventional conditions. Cells were transfected using Lipofectamine LTX (Invitrogen) as directed by the manufacturer. Ataxin-3 constructs were in pcDNA3.1-HA. Mutations were generated on the wild-type ataxin-3 backbone. Twenty-four hours after transfection, cells were harvested in boiling SDS lysis buffer. Cycloheximide (A.G. Scientific) dissolved in ultra-pure water, was used at 50 µg/ml.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
We thank Ms. Holland Galante for her help with in vitro binding assays.
Conflict of Interest statement. None declared.
Funding
This work was funded by an F31 fellowship to JRS from NINDS (F31NS095575), by a grant from the Research and Education Program, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and by R00NS073936 to KMS from NINDS, by R01NS038712 to HLP from NINDS, and by R01NS086778 to SVT from NINDS.
References
- 1. Todi S.V., Williams A., Paulson H. (2007) Waxman S.G. (ed.), In Molecular Neurology. Academic Press, London, in press., pp. 257–276. [Google Scholar]
- 2. Costa Mdo C., Paulson H.L. (2012) Toward understanding Machado-Joseph disease. Prog. Neurobiol., 97, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matos C.A., de Macedo-Ribeiro S., Carvalho A.L. (2011) Polyglutamine diseases: The special case of ataxin-3 and Machado-Joseph disease. Prog. Neurobiol., 95, 26–48. [DOI] [PubMed] [Google Scholar]
- 4. Winborn B.J., Travis S.M., Todi S.V., Scaglione K.M., Xu P., Williams A.J., Cohen R.E., Peng J., Paulson H.L. (2008) The deubiquitinating enzyme ataxin-3, a polyglutamine disease protein, edits Lys63 linkages in mixed linkage ubiquitin chains. J. Biol. Chem., 283, 26436–26443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scaglione K.M., Zavodszky E., Todi S.V., Patury S., Xu P., Rodriguez-Lebron E., Fischer S., Konen J., Djarmati A., Peng J., et al. (2011) Ube2w and Ataxin-3 Coordinately Regulate the Ubiquitin Ligase CHIP. Mol. Cell, 43, 599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Todi S.V., Scaglione K.M., Blount J.R., Basrur V., Conlon K.P., Pastore A., Elenitoba-Johnson K., Paulson H.L. (2010) Activity and cellular functions of the deubiquitinating enzyme and polyglutamine disease protein ataxin-3 are regulated by ubiquitination at lysine 117. J. Biol. Chem., 285, 39303–39313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reina C.P., Nabet B.Y., Young P.D., Pittman R.N. (2012) Basal and stress-induced Hsp70 are modulated by ataxin-3. Cell Stress Chaperones, 17, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sacco J.J., Yau T.Y., Darling S., Patel V., Liu H., Urbe S., Clague M.J., Coulson J.M. (2014) The deubiquitylase Ataxin-3 restricts PTEN transcription in lung cancer cells. Oncogene, 33, 4265–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warrick J.M., Morabito L.M., Bilen J., Gordesky-Gold B., Faust L.Z., Paulson H.L., Bonini N.M. (2005) Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol. Cell, 18, 37–48. [DOI] [PubMed] [Google Scholar]
- 10. Tsou W.L., Burr A.A., Ouyang M., Blount J.R., Scaglione K.M., Todi S.V. (2013) Ubiquitination regulates the neuroprotective function of the deubiquitinase ataxin-3 in vivo. J. Biol. Chem., 288, 34460–34469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burr A.A., Tsou W.L., Ristic G., Todi S.V. (2014) Using Membrane-Targeted Green Fluorescent Protein To Monitor Neurotoxic Protein-Dependent Degeneration of Drosophila Eyes. J. Neurosci. Res., 92, 1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsou W.L., Ouyang M., Hosking R.R., Sutton J.R., Blount J.R., Burr A.A., Todi S.V. (2015) The deubiquitinase ataxin-3 requires Rad23 and DnaJ-1 for its neuroprotective role in Drosophila melanogaster. Neurobiol. Dis., 82, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reina C.P., Zhong X., Pittman R.N. (2010) Proteotoxic stress increases nuclear localization of ataxin-3. Hum. Mol. Genet., 19, 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou L., Wang H., Wang P., Ren H., Chen D., Ying Z., Wang G. (2013) Ataxin-3 protects cells against H2O2-induced oxidative stress by enhancing the interaction between Bcl-X(L) and Bax. Neuroscience, 243, 14–21. [DOI] [PubMed] [Google Scholar]
- 15. Chatterjee A., Saha S., Chakraborty A., Silva-Fernandes A., Mandal S.M., Neves-Carvalho A., Liu Y., Pandita R.K., Hegde M.L., Hegde P.M., et al. (2015) The Role of the Mammalian DNA End-processing Enzyme Polynucleotide Kinase 3'-Phosphatase in Spinocerebellar Ataxia Type 3 Pathogenesis. PLoS Genet., 11, e1004749.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicastro G., Masino L., Esposito V., Menon R.P., De Simone A., Fraternali F., Pastore A. (2009) Josephin domain of ataxin-3 contains two distinct ubiquitin-binding sites. Biopolymers, 91, 1203–1214. [DOI] [PubMed] [Google Scholar]
- 17. Nicastro G., Todi S.V., Karaca E., Bonvin A.M., Paulson H.L., Pastore A. (2010) Understanding the role of the Josephin domain in the PolyUb binding and cleavage properties of ataxin-3. PLoS One, 5, e12430.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang G., Sawai N., Kotliarova S., Kanazawa I., Nukina N. (2000) Ataxin-3, the MJD1 gene product, interacts with the two human homologs of yeast DNA repair protein RAD23, HHR23A and HHR23B. Hum. Mol. Genet., 9, 1795–1803. [DOI] [PubMed] [Google Scholar]
- 19. Nicastro G., Menon R.P., Masino L., Knowles P.P., McDonald N.Q., Pastore A. (2005) The solution structure of the Josephin domain of ataxin-3: structural determinants for molecular recognition. Proc. Natl Acad. Sci. U S A, 102, 10493–10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blount J.R., Tsou W.L., Ristic G., Burr A.A., Ouyang M., Galante H., Scaglione K.M., Todi S.V. (2014) Ubiquitin-binding site 2 of ataxin-3 prevents its proteasomal degradation by interacting with Rad23. Nat. Commun., 5, 4638.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Costa M.D., Ashraf N.S., Fischer S., Yang Y., Schapka E., Joshi G., McQuade T.J., Dharia R.M., Dulchavsky M., Ouyang M., et al. (2016) Unbiased screen identifies aripiprazole as a modulator of abundance of the polyglutamine disease protein, ataxin-3. Brain, pii: aww228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groth A.C., Fish M., Nusse R., Calos M.P. (2004) Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics, 166, 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsou W.L., Hosking R.R., Burr A.A., Sutton J.R., Ouyang M., Du X., Gomez C.M., Todi S.V. (2015) DnaJ-1 and karyopherin alpha3 suppress degeneration in a new Drosophila model of Spinocerebellar Ataxia Type 6. Hum. Mol. Genet., 24, 4385–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ristic G., Tsou W.L., Guzi E., Kanack A.J., Scaglione K.M., Todi S.V. (2016) USP5 Is Dispensable for Monoubiquitin Maintenance in Drosophila. J. Biol. Chem., 291, 9161–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warrick J.M., Paulson H.L., Gray-Board G.L., Bui Q.T., Fischbeck K.H., Pittman R.N., Bonini N.M. (1998) Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell, 93, 939–949. [DOI] [PubMed] [Google Scholar]
- 26. Todi S.V., Laco M.N., Winborn B.J., Travis S.M., Wen H.M., Paulson H.L. (2007) Cellular turnover of the polyglutamine disease protein ataxin-3 is regulated by its catalytic activity. J. Biol. Chem., 282, 29348–29358. [DOI] [PubMed] [Google Scholar]
- 27. Kampinga H.H., Craig E.A. (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol., 11, 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koutras C., Braun J.E. (2014) J protein mutations and resulting proteostasis collapse. Front. Cell Neurosci., 8, 191.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dantuma N.P., Heinen C., Hoogstraten D. (2009) The ubiquitin receptor Rad23: at the crossroads of nucleotide excision repair and proteasomal degradation. DNA Repair (Amst), 8, 449–460. [DOI] [PubMed] [Google Scholar]
- 30. Buchberger A., Bukau B., Sommer T. (2010) Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol. Cell, 40, 238–252. [DOI] [PubMed] [Google Scholar]
- 31. G R., W-L T., SV T. (2014) An optimal ubiquitin-proteasome pathway in the nervous system: the role of deubiquitinating enzymes. Front. Mol. Neurosci., 7, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia H., Mao Q., Eliason S.L., Harper S.Q., Martins I.H., Orr H.T., Paulson H.L., Yang L., Kotin R.M., Davidson B.L. (2004) RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat. Med., 10, 816–820. [DOI] [PubMed] [Google Scholar]
- 33. Harper S.Q., Staber P.D., He X., Eliason S.L., Martins I.H., Mao Q., Yang L., Kotin R.M., Paulson H.L., Davidson B.L. (2005) RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc. Natl Acad. Sci. U S A, 102, 5820–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williams A.J., Paulson H.L. (2008) Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci., 31, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodriguez-Lebron E., Gouvion C.M., Moore S.A., Davidson B.L., Paulson H.L. (2009) Allele-specific RNAi mitigates phenotypic progression in a transgenic model of Alzheimer's disease. Mol. Ther., 17, 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams A.J., Knutson T.M., Colomer Gould V.F., Paulson H.L. (2009) In vivo suppression of polyglutamine neurotoxicity by C-terminus of Hsp70-interacting protein (CHIP) supports an aggregation model of pathogenesis. Neurobiol. Dis., 33, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alves S., Nascimento-Ferreira I., Dufour N., Hassig R., Auregan G., Nobrega C., Brouillet E., Hantraye P., Pedroso de Lima M.C., Deglon N., et al. (2010) Silencing ataxin-3 mitigates degeneration in a rat model of Machado-Joseph disease: no role for wild-type ataxin-3?. Hum. Mol. Genet., 19, 2380–2394. [DOI] [PubMed] [Google Scholar]
- 38. Tsou W.L., Soong B.W., Paulson H.L., Rodriguez-Lebron E. (2011) Splice isoform-specific suppression of the Cav2.1 variant underlying spinocerebellar ataxia type 6. Neurobiol. Dis., 43, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pedersen J.T., Heegaard N.H. (2013) Analysis of protein aggregation in neurodegenerative disease. Anal. Chem., 85, 4215–4227. [DOI] [PubMed] [Google Scholar]
- 40. Cemal C.K., Carroll C.J., Lawrence L., Lowrie M.B., Ruddle P., Al-Mahdawi S., King R.H., Pook M.A., Huxley C., Chamberlain S. (2002) YAC transgenic mice carrying pathological alleles of the MJD1 locus exhibit a mild and slowly progressive cerebellar deficit. Hum. Mol. Genet., 11, 1075–1094. [DOI] [PubMed] [Google Scholar]
- 41. Hubener J., Riess O. (2010) Polyglutamine-induced neurodegeneration in SCA3 is not mitigated by non-expanded ataxin-3: conclusions from double-transgenic mouse models. Neurobiol. Dis., 38, 116–124. [DOI] [PubMed] [Google Scholar]
- 42. Zeng L., Tallaksen-Greene S.J., Wang B., Albin R.L., Paulson H.L. (2013) The de-ubiquitinating enzyme ataxin-3 does not modulate disease progression in a knock-in mouse model of Huntington disease. J. Huntingtons Dis., 2, 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paulson H.L., Perez M.K., Trottier Y., Trojanowski J.Q., Subramony S.H., Das S.S., Vig P., Mandel J.L., Fischbeck K.H., Pittman R.N. (1997) Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron, 19, 333–344. [DOI] [PubMed] [Google Scholar]
- 44. Spencer C.C., Howell C.E., Wright A.R., Promislow D.E. (2003) Testing an ′aging gene′ in long-lived drosophila strains: increased longevity depends on sex and genetic background. Aging Cell, 2, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paaby A.B., Schmidt P.S. (2009) Dissecting the genetics of longevity in Drosophila melanogaster. Fly (Austin), 3, 29–38. [DOI] [PubMed] [Google Scholar]
- 46. Tsou W.L., Sheedlo M.J., Morrow M.E., Blount J.R., McGregor K.M., Das C., Todi S.V. (2012) Systematic Analysis of the Physiological Importance of Deubiquitinating Enzymes. PLoS One, 7, e43112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.