Abstract
Objectives: The macrolide antibiotic roxithromycin has seen widespread clinical use for several decades; however, no population pharmacokinetic analysis has been published. Early studies indicated saturation of protein binding and absorption at doses within the approved range, which may impact pharmacodynamic target attainment since regimens of 150 mg twice daily and 300 mg once daily are used interchangeably in clinical practice. This study aimed to develop a population-based meta-analysis of roxithromycin pharmacokinetics, and utilize this model to inform optimal dosing regimens.
Methods: Following an extensive search, roxithromycin pharmacokinetic data were collected or digitized from literature publications. Population pharmacokinetic modelling was undertaken with ADAPT. Dosing simulations were performed to investigate differences in exposure and pharmacodynamic target attainment between dosing regimens.
Results: A two-compartment model with saturable absorption described the data (n = 63); changes in free drug exposure were simulated using a saturable protein binding model. Simulations indicated that a 300 mg daily regimen achieves a 37% and 53% lower total or free AUC (fAUC), respectively, compared with 150 mg twice daily. These pharmacokinetic differences translated to significantly lower target attainment (fAUC/MIC ratio >20) with a 300 mg daily regimen at MICs of 0.5 and 1 mg/L (51% and 7%) compared with patients receiving 150 mg twice daily (82% and 54%).
Conclusions: Roxithromycin displays saturable absorption and protein binding leading to lower exposure and lower target attainment at MICs ≥0.5 mg/L with widely used once-daily dosing regimens, indicating that twice-daily regimens may be preferable for pathogens less susceptible to roxithromycin.
Introduction
The macrolide antibiotic roxithromycin is a well-established agent in the treatment of respiratory tract infections, community-acquired pneumonia, skin and skin structure infections and non-gonococcal urethritis.1,2 Originally approved in the late 1980s, roxithromycin is a second-generation macrolide derived from erythromycin, offering significant pharmacokinetic advantages, such as improved bioavailability, an extended terminal elimination half-life and a similar spectrum of antibacterial activity.3–6 Approved dosing regimens of roxithromycin include 150 mg twice daily or 300 mg once daily taken orally,7,8 with no intravenous formulation currently available.1,2
Despite widespread clinical use for several decades, no population pharmacokinetic analysis of roxithromycin has been published and, moreover, few studies have investigated pharmacodynamic target attainment with this agent. Furthermore, pharmacokinetic studies of roxithromycin indicated saturation of oral absorption at doses within the approved range,5,9,10 as well as saturation of protein binding to α-1-acid glycoprotein within the normal exposure range.11,12 While the approved dosing regimens of 150 mg twice daily and 300 mg once daily would be expected to produce similar total and free drug exposure at steady state in the absence of these factors, it is likely that saturation of absorption and protein binding leads to significant differences in total and free roxithromycin exposure between these dosing regimens. As pharmacodynamic activity is linked to free drug exposure,13 changes in free roxithromycin exposure may lead to therapeutically significant differences in antibacterial killing. These important clinical questions have not been addressed for roxithromycin.
Population-based meta-analyses to investigate drug pharmacokinetics have been previously reported in the literature.14,15 This approach involves the collection and collation of individual-level and aggregate (mean) pharmacokinetic data from literature publications, and the subsequent development of a population pharmacokinetic model.14–16 Using this approach, the study reported herein aimed to characterize the pharmacokinetics of roxithromycin, investigate differences in total and free roxithromycin exposure and quantify changes in pharmacodynamic target attainment, with the overall goal of informing optimal dose selection with roxithromycin.
Materials and methods
Pharmacokinetic data collection
An extensive literature search was undertaken via PubMed to identify published roxithromycin pharmacokinetic data in humans up to June 2014. All identified roxithromycin pharmacokinetic data with complete dosing information and defined concentration sampling times were included in the analysis; data with unclear or absent dosing or concentration sampling schedules were excluded. Reference lists of identified publications were hand searched to identify further relevant publications. Individual and aggregate pharmacokinetic data and dosing were collected or digitized from identified publications using DigitizeIt (version 1.5.8). All digitized data were manually inspected during the digitization process to ensure precision and accuracy. Information on pharmacokinetic data included in the analysis, study population and dose range is included in Table 1.
Table 1.
Roxithromycin pharmacokinetic data included in the final model
| Reference | Study population | Dose range | Included pharmacokinetic profiles |
|
|---|---|---|---|---|
| aggregate | individual | |||
| Wise et al., 198724 | healthy (n = 6) | 150 mg | 0 | 6 |
| Acar et al., 198825 | healthy (n = 6) | 150 mg (300 mg loading) | 1 | 0 |
| Concia et al., 198826 | healthy (n = 6) | 150 mg | 1 | 0 |
| Kees et al., 198827 | healthy (n = 10) | 150 mg | 1 | 0 |
| Koyama et al., 19889 | healthy (n = 88; 8 studies) | 100–600 mg | 10 | 0 |
| Lassman et al., 19885 | healthy (n = 133; 5 studies) | 150–450 mg | 12 | 0 |
| Paulsen et al., 198828 | healthy (n = 21) | 150 mg | 2 | 0 |
| Rimoldi et al., 198829 | patients (n = 11) | 150 mg | 0 | 11 |
| Saito et al., 198830 | healthy (n = 6) | 150 mg | 0 | 6 |
| Segre et al., 198831 | healthy (n = 8) | 300 mg | 2 | 0 |
| Tremblay et al., 198810 | healthy (n = 12) | 150–450 mg | 3 | 0 |
| Halstenson et al., 199032 | healthy (n = 10) | 300 mg | 1 | 0 |
| Moravek et al., 199033 | healthy (n = 6) | 300 mg | 1 | 0 |
| Boccazzi et al., 199134 | ICU patients (n = 7) | 150 mg | 1 | 0 |
| Nilsen et al., 199535 | healthy (n = 12) | 300 mg | 1 | 0 |
| Macek et al., 199936 | healthy (n = 26) | 300 mg | 1 | 0 |
| Motta et al., 199937 | healthy (n = 24) | 300 mg | 1 | 0 |
| Hang et al., 200738 | healthy (n = 12) | 150 mg | 1 | 0 |
| Kousoulos et al., 200839 | healthy (n = 28) | 300 mg | 1 | 0 |
Population pharmacokinetic modelling
Hierarchical modelling of the pharmacokinetic data was conducted using the maximum likelihood, expectation maximization algorithm (MLEM) in ADAPT (version 5).17 Model parameters were assumed to be log-normally distributed, while proportional, additive and combined additive and proportional residual error models were considered. Based on a previously described approach,14–16 a weighting factor was applied to aggregate pharmacokinetic data included in the model, whereby the proportional residual error was modelled as the inverse of the square root of the number of individuals that contributed data to the pharmacokinetic profile. Model validation was performed with NONMEM 7.3 (Globomax LLC, Hanover, MD, USA), Perl-Speaks-NONMEM (version 4.2.0), Pirana (version 2.8), R (version 3.0.3) and Xpose (version 4.5.0); data visualization was performed with ADAPT, GraphPad Prism (version 6) and Microsoft Excel 2013.
Model selection and validation
Pharmacokinetic models incorporating either one compartment or two compartments with linear elimination were investigated. Due to the lack of an intravenous formulation, saturation of oral absorption was investigated as changes in relative bioavailability. Model development and selection were guided by goodness-of-fit criteria, including significant decreases in the negative log likelihood (NLL) between nested models, goodness-of-fit plots and precision of parameter estimates. A decrease of 3.84 in NLL (P < 0.05) for one degree of freedom was considered statistically significant.18 Prediction-corrected visual predictive checks (pcVPCs) were used for model validation.19 One thousand simulated datasets of individuals from the original dataset were compared with prediction- and variability-corrected observed concentrations.
Dosing simulations and pharmacodynamic target attainment
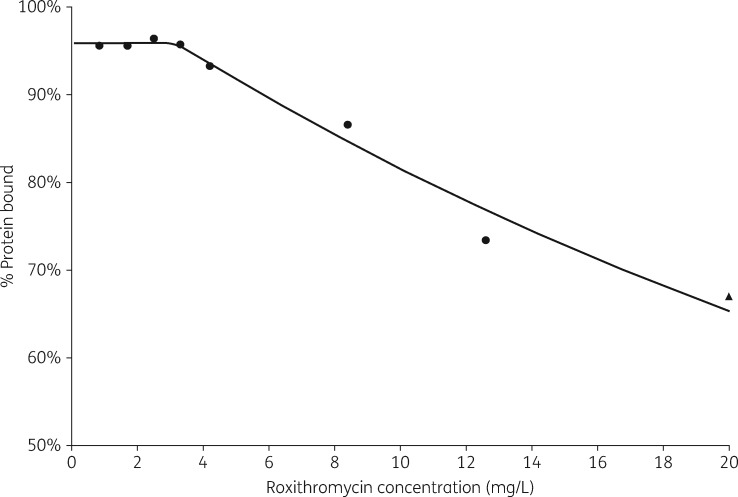
To allow the prediction of free drug roxithromycin exposure given total concentrations, a non-linear expression was constructed using published data on roxithromycin protein binding (Figure 1):11,12
Figure 1.
Observed and predicted roxithromycin protein binding data. Observed data from Zini et al.11 (circles) and Andrews et al.12 (triangle).
In the above equation, Pbound represents percentage protein bound roxithromycin, while C is the total roxithromycin concentration. When C < 3.2 mg/L, then Pbound = 95.9%.
The equation describing roxithromycin protein binding was constructed using data reported by Zini et al.11 and Andrews et al.;12 to the authors’ knowledge, these publications are the only published sources addressing roxithromycin protein binding. Zini et al.11 reported consistent roxithromycin protein binding results (95.6%–96.4% bound) at low concentrations (<3–4 mg/L), whereas Andrews et al.12 reported significant variability in protein binding at low concentrations (82%–92% bound); this difference may reflect the more reliable methodology used by Zini et al.11 (equilibrium dialysis) compared with Andrews et al.12 (ultracentrifugation). Due to this, the prediction of roxithromycin protein binding is primarily informed by the data from Zini et al.,11 with supporting data from Andrews et al.12 at higher roxithromycin concentrations as Zini et al.11 did not report protein binding results at concentrations >12.6 mg/L (Figure 1).
To quantify exposure differences between roxithromycin dosing regimens, total and free roxithromycin exposures at the approved dosing regimens of 150 mg twice daily and 300 mg once daily were simulated using Monte Carlo simulations in ADAPT for 1000 individuals. To investigate total and free roxithromycin at higher doses, 450 mg daily and 300 mg twice daily regimens were simulated. Using these simulations, pharmacodynamic target attainment on day 7 of therapy was predicted as the proportion of individuals achieving a pharmacodynamic target of an fAUC/MIC ratio >20, associated with optimal antibacterial activity.20 Proportions were evaluated with decreasing pathogen susceptibility (increasing MIC), and against MIC distributions for pathogens commonly treated with roxithromycin obtained from EUCAST.21
Results
Model development and validation
A total of 40 aggregate and 23 individual roxithromycin concentration–time profiles across a 6-fold dose range were included in the analysis (Table 1). A two-compartment model with dose-dependent first-order absorption and first-order elimination adequately described the dataset; other absorption and elimination models, such as zero-order absorption, mixed-order absorption or elimination did not improve goodness of fit.
Parameter estimates and associated standard errors for the structural model, interindividual variability and residual variability from the final model are shown in Table 2. Based on data inspection, roxithromycin oral absorption was found to be dose-proportional at doses up to 150 mg;9 hence saturation of absorption was characterized by the equation:
where F50 is the roxithromycin dose associated with a half-maximal reduction in relative bioavailability and FImax is the maximal reduction in relative bioavailability. Based on the observed less than dose-proportional increases in roxithromycin AUC, FImax was fixed to 0.5, and consequently F50 is interpreted as the roxithromycin dose associated with a 25% reduction in relative bioavailability. The population estimate of F50 in the final model was 250 mg, with significant interindividual variability [coefficient of variation (CV) 65.7%]. High interindividual variability was also observed in distributional clearance (CV 79.9%) and peripheral volume (CV 62.6%).
Table 2.
Population parameter estimates from the final model
| Parameter | Population mean (%RSE) | Interindividual variability as CV% (%RSE) |
|---|---|---|
| CL (L/h) | 1.94 (8.54) | 38.2 (20.6) |
| Vc (L) | 18.3 (11.9) | 20.2 (44.0) |
| Ka (h−1) | 1.30 (15.5) | 48.9 (39.0) |
| F50 (mg) | 250 (53.4) | 65.7 (67.4) |
| FImax | 0.5 FIX | NE |
| Vp (L) | 10.4 (20.9) | 62.6 (33.1) |
| CLD (L/h) | 1.70 (28.6) | 79.9 (54.6) |
| SDslope_AGG | 0.476 (2.39) | – |
| SDslope_IND | 0.260 (4.38) | – |
NE, not estimated; %RSE, percentage relative standard error; SDslope_AGG, proportional residual error estimated from aggregate pharmacokinetic data; SDslope_IND, proportional residual error estimated from individual pharmacokinetic data.
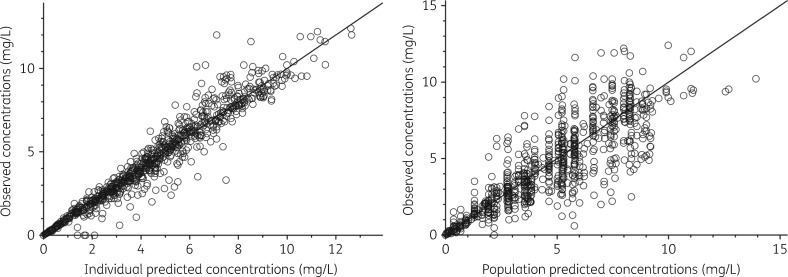
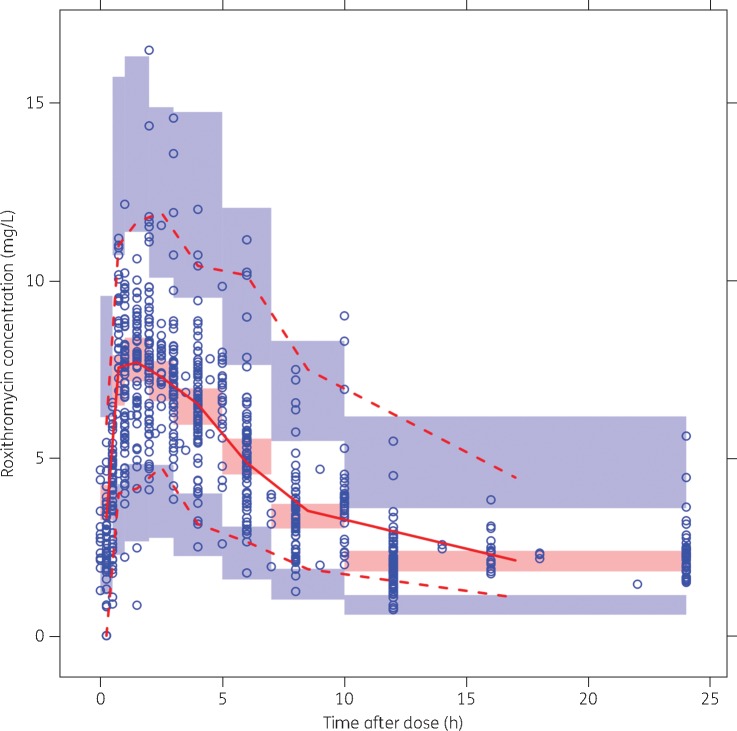
Goodness-of-fit plots and pcVPCs used throughout model development indicated satisfactory model fit (Figure 2). The pcVPC of the final model (Figure 3) indicated good predictive performance, with suitable agreement between prediction- and variability-corrected observed data and model-simulated CIs for the median and the 2.5th and 97.5th percentiles.
Figure 2.
Model goodness-of-fit plots: individual predicted versus observed and population predicted versus observed concentrations.
Figure 3.
pcVPC of the final model. Prediction-corrected observed concentrations are shown as open circles, with the continuous and lower and upper broken lines showing the median and the 2.5th and 97.5th percentiles of the observed data, respectively. The shaded areas represent 95% inclusion intervals for the model-predicted median and 2.5th and 97.5th percentiles constructed from 1000 simulated datasets of individuals from the original dataset. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Due to the inclusion of aggregate-level pharmacokinetic data, low frequency of potential covariate effects and study heterogeneity, it was not feasible to perform a covariate analysis.
Dosing simulations and target attainment
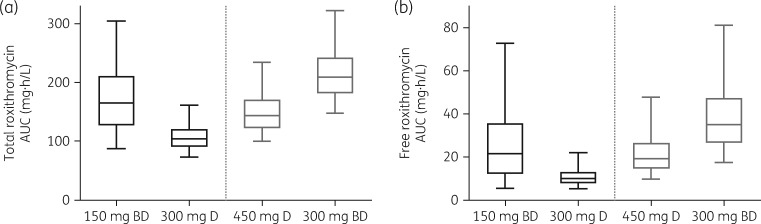
Total and free roxithromycin exposure on day 7 with approved and higher dose regimens is shown in Figure 4 (n = 5000 simulated individuals). Between approved roxithromycin dosing regimens, total roxithromycin exposure was 37% lower with 300 mg daily dosing (median 104, IQR 30.7 mg·h/L) than with 150 mg twice daily dosing (median 165, IQR 84.9 mg·h/L). A larger difference was observed with free roxithromycin exposure, which was found to be 53% lower with 300 mg daily dosing (median 10.0, IQR 5.4 mg·h/L) than with 150 mg twice daily dosing (median 21.6, IQR 23.5 mg·h/L).
Figure 4.
Simulated roxithromycin total and free exposure on day 7 of treatment with approved dosing regimens (150 mg twice daily or 300 mg daily; black) or higher dose regimens (450 mg daily or 300 mg twice daily; grey) (n = 1000 individuals). The central box line represents the median, with the box ends representing the 25th and 75th percentiles. The bars extend to the 5th and 95th percentiles. BD, twice daily; D, daily.
With higher dose regimens, 450 mg daily dosing led to total roxithromycin exposure between the exposure ranges observed for the approved dosing regimens (median 143, IQR 49.2 mg·h/L), but similar free drug exposure to the 150 mg twice daily regimen (median 19.3, IQR 12.1 mg·h/L). The 300 mg twice daily regimen led to the highest total and free roxithromycin exposures (median 209, IQR 61.5 mg·h/L and median 35.1, IQR 20.8 mg·h/L, respectively).
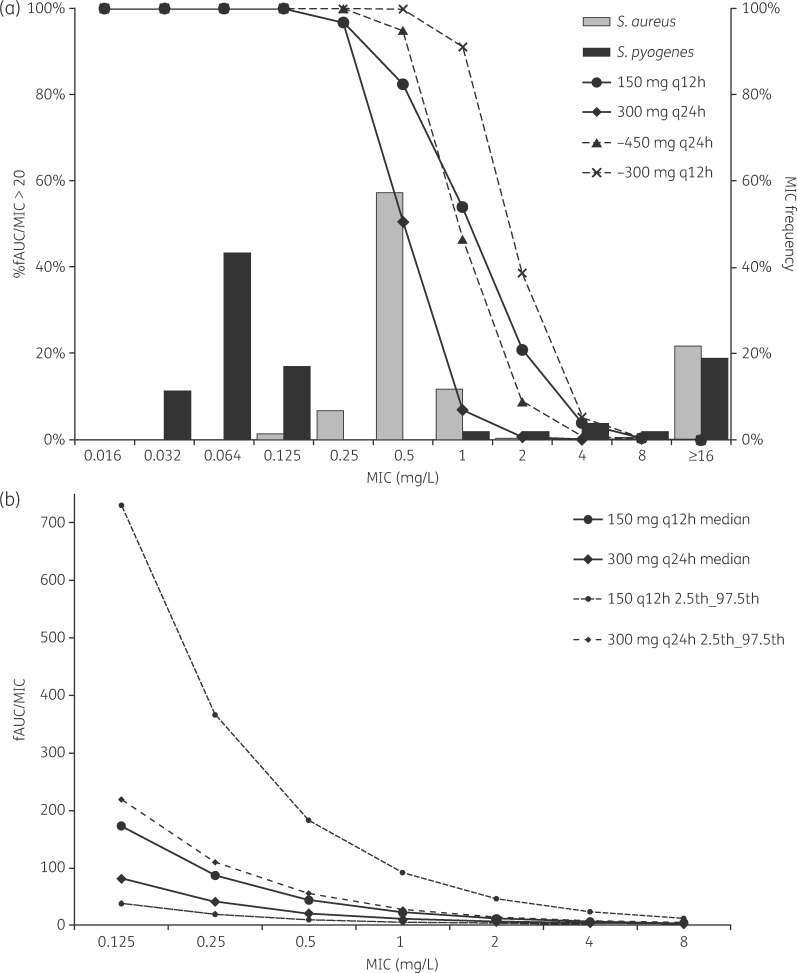
Pharmacodynamic target attainment on day 7 of therapy with approved and higher-dose roxithromycin regimens is shown in Figure 5(a), and predicted median and 2.5th and 97.5th percentiles of fAUC/MIC for approved dosing regimens are shown in Figure 5(b). Among the approved roxithromycin dosing regimens, target attainment was similar up to an MIC of 0.25 mg/L, with >95% of individuals achieving an fAUC/MIC >20. At MICs of 0.5 and 1 mg/L, 51% and 7% of patients receiving 300 mg once daily achieved an adequate fAUC/MIC, respectively, compared with 82% and 54% of patients receiving 150 mg twice daily. In comparison with pathogens commonly treated with roxithromycin, these differences are unlikely to be clinically relevant for Streptococcus pyogenes, with a distribution centred on an MIC of 0.064 mg/L, but may be clinically important for Staphylococcus aureus, which has an MIC distribution centred on an MIC of 0.5 mg/L.21 Among higher dose regimens, 450 mg daily led to target attainment broadly similar to the approved 150 mg twice daily regimen, whereas 300 mg twice daily resulted in higher target attainment at MICs of 0.5, 1 and 2 mg/L.
Figure 5.
(a) Predicted pharmacodynamic target attainment on day 7 of treatment with approved dosing regimens (150 mg twice daily or 300 mg daily; continuous lines) or higher dose regimens (450 mg daily or 300 mg twice daily; broken lines), shown as the proportion of individuals achieving an fAUC/MIC >20 (n = 1000). The MIC distributions of S. aureus (n = 4001 isolates) and S. pyogenes (n = 53 isolates) for roxithromycin are also shown.21 (b) Predicted median and 2.5th and 97.5th percentiles of fAUC/MIC ratios with approved dosing regimens (150 mg twice daily or 300 mg daily). q12h, every 12 h; q24h, every 24 h.
Discussion
Using a population-based meta-analysis approach, this study represents the first population pharmacokinetic analysis of roxithromycin, and highlights significant differences in roxithromycin exposure and pharmacodynamic target attainment between approved dosing regimens for this medicine.
Due to the effect of saturable oral absorption, significantly lower total roxithromycin exposure was observed with 300 mg daily dosing compared with the 150 mg twice daily regimen (Figure 4). Further, this reduction in exposure was larger when considering free roxithromycin exposure, with the 300 mg once daily regimen leading to less than half the free roxithromycin exposure resulting from the 150 mg twice daily regimen. This larger impact on free drug exposure, as opposed to total exposure, indicates that saturation of roxithromycin protein binding exacerbates the reduction in exposure that can be attributed to saturable absorption. These results translated to a significantly higher proportion of patients achieving the pharmacodynamic target for pathogens at MIC values of 0.5 and 1 mg/L among patients receiving 150 mg twice daily compared with 300 mg daily. While the potential clinical relevance of these findings is difficult to predict, it is noteworthy that these differences occur within the typical range of MIC values for some commonly treated pathogens for roxithromycin, such as S. aureus. Accordingly, the observed pharmacokinetic differences between the approved dosing regimens for roxithromycin might lead to differences in clinical efficacy for pathogens within this MIC range.
To investigate the effectiveness of higher-dose roxithromycin regimens in increasing exposure, roxithromycin dosing regimens of 450 or 300 mg twice daily were investigated. Between these regimens, 450 mg daily does not improve free drug exposure beyond the level observed with the 150 mg twice daily regimen, whereas 300 mg twice daily leads to a 63% increase in free roxithromycin concentrations. This increase in free roxithromycin exposure translates to a higher proportion of individuals achieving the pharmacodynamic target with pathogen MICs of 0.5, 1 and 2 mg/L. While clinical experience with roxithromycin daily doses exceeding 300 mg appears to be very limited,5,22 these results provide guidance that a twice-daily dosing regimen is preferred if higher roxithromycin exposure is required in a particular clinical situation.
A population-based meta-analysis approach was used to develop the roxithromycin population pharmacokinetic model in this study, utilizing literature publications to collate and combine individual-level and aggregate pharmacokinetic data. While the necessary pharmacokinetic data to support this model could alternatively have been generated through a dose-ranging healthy volunteer study, the meta-analysis approach utilizes existing, publicly available information and, when appropriate, may be a viable strategy to inform clinical practice at a substantially lower cost. This approach has been utilized with several other medicines,14,15,23 and is likely to have greater utility with established medicines, where a wealth of published pharmacokinetic information is often available. This technique may be particularly useful in the pharmacokinetic interrogation of medicines approved before the modern era of drug development, integrating existing individual and aggregate pharmacokinetic data to inform pressing clinical questions without the need for additional clinical studies.
In conclusion, roxithromycin displays saturable absorption and protein binding, leading to significantly lower exposure following once-daily dosing compared with twice-daily regimens. This pharmacokinetic difference translates to important differences in target attainment for pathogens with MICs ≥0.5 mg/L, indicating that twice-daily roxithromycin regimens may be preferable for pathogens less susceptible to roxithromycin.
Funding
Supported in part by the National Institute of Biomedical Imaging and Bioengineering of the NIH grant P41-EB001978 (D. Z. D.). M. J. D. was supported by a postdoctoral fellowship funded by the Alfred E. Mann Institute at the University of Southern California.
Transparency declarations
None to declare.
References
- 1. Sanofi. Product Information: Rulide & Rulide D Revised July 2012. http://www.sanofi.com.au/products/aus_pi_rulide.pdf.
- 2. Actavis Ltd. Arrow - Roxithromycin Revised July 2013. http://www.medsafe.govt.nz/profs/datasheet/a/ArrowRoxithromycintab.pdf.
- 3. Nilsen OG. Comparative pharmacokinetics of macrolides. J Antimicrob Chemother 1987; 20: 81–8. [DOI] [PubMed] [Google Scholar]
- 4. Jain R, Danziger LH.. The macrolide antibiotics: a pharmacokinetic and pharmacodynamic overview. Curr Pharm Des 2004; 10: 3045–53. [DOI] [PubMed] [Google Scholar]
- 5. Lassman HB, Puri SK, Ho I. et al. Pharmacokinetics of roxithromycin (RU 965). J Clin Pharmacol 1988; 28: 141–52. [DOI] [PubMed] [Google Scholar]
- 6. Bryskier A. Roxithromycin: review of its antimicrobial activity. J Antimicrob Chemother 1998; 41 Suppl B: 1–21. [DOI] [PubMed] [Google Scholar]
- 7. Pechère J-C. Clinical evaluation of roxithromycin 300 mg once daily as an alternative to 150 mg twice daily. Diagn Microbiol Infect Dis 1992; 15: 111–7. [DOI] [PubMed] [Google Scholar]
- 8. Markham A, Faulds D.. Roxithromycin. Drugs 1994; 48: 297–326. [DOI] [PubMed] [Google Scholar]
- 9. Koyama M, Tateno M, Shirotsuka M. et al. Absorption, metabolism and excretion of RU 28965 in humans. Chemotherapy 1988; 36: 164–83. [Google Scholar]
- 10. Tremblay D, Jaeger H, Fourtillan JB. et al. Pharmacokinetics of three single doses (150, 300, 450 mg) of roxithromycin in young volunteers. Br J Clin Pract 1988; 42: 49–50.3052558 [Google Scholar]
- 11. Zini R, Fournet MP, Barre J. et al. In vitro study of roxithromycin binding to serum proteins and erythrocytes in humans. Br J Clin Pract 1988; 42: 54. [Google Scholar]
- 12. Andrews JM, Ashby JP, Wise R.. Factors affecting the in-vitro activity of roxithromycin. J Antimicrob Chemother 1987; 20: 31–7. [DOI] [PubMed] [Google Scholar]
- 13. Smith DA, Di L, Kerns EH.. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov 2010; 9: 929–39. [DOI] [PubMed] [Google Scholar]
- 14. Van Wart SA, Shoaf SE, Mallikaarjun S. et al. Population-based meta-analysis of furosemide pharmacokinetics. Biopharm Drug Dispos 2014; 35: 119–33. [DOI] [PubMed] [Google Scholar]
- 15. Van Wart SA, Shoaf SE, Mallikaarjun S. et al. Population-based meta-analysis of hydrochlorothiazide pharmacokinetics. Biopharm Drug Dispos 2013; 34: 527–39. [DOI] [PubMed] [Google Scholar]
- 16. Ahn JE, French JL.. Longitudinal aggregate data model-based meta-analysis with NONMEM: approaches to handling within treatment arm correlation. J Pharmacokinet Pharmacodyn 2010; 37: 179–201. [DOI] [PubMed] [Google Scholar]
- 17. D’Argenio DZ, Schumitzky A, Wang X.. ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles, CA: Biomedical Simulations Resource, 2009. [Google Scholar]
- 18. Bonate PL. Pharmacokinetic-Pharmacodynamic Modeling and Simulation, 2nd edn . New York, NY: Springer, 2011. [Google Scholar]
- 19. Bergstrand M, Hooker A, Wallin J. et al. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 2011; 13: 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bambeke FV. Chapter 11: Macrolides and ketolides. In:Fundamentals of Antimicrobial Pharmacokinetics and Pharmacodynamics. New York, NY: Springer Science+Business Media, 2014, pp 257–78. [Google Scholar]
- 21. EUCAST. EUCAST MIC Distributions and ECOFFs http://www.eucast.org/mic_distributions_and_ecoffs/.
- 22. Puri SK, Lassman HB.. Roxithromycin: a pharmacokinetic review of a macrolide. J Antimicrob Chemother 1987; 20: 89–100. [DOI] [PubMed] [Google Scholar]
- 23. Ait-Oudhia S, Straubinger R, Mager D.. Meta-analysis of nanoparticulate paclitaxel delivery system pharmacokinetics and model prediction of associated neutropenia. Pharm Res 2012; 29: 2833–44. [DOI] [PubMed] [Google Scholar]
- 24. Wise R, Kirkpatrick B, Ashby J. et al. Pharmacokinetics and tissue penetration of roxithromycin after multiple dosing. Antimicrob Agents Chemother 1987; 31: 1051–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Acar JF, Saint-Salvi B, Blanc F.. Concentrations of roxithromycin in tear fluid and saliva after repeat dosing. Br J Clin Pract 1988; 42: 82.3179178 [Google Scholar]
- 26. Concia E, Cruciana M, Barzaghi N. et al. Diffusion of roxithromycin into suction skin blister fluid. Br J Clin Pract 1988; 42: 86–7. [Google Scholar]
- 27. Kees F, Grobecker H, Fourtillan JB. et al. Comparative pharmacokinetics of single dose roxithromycin (150 mg) versus erythromycin stearate (500 mg) in healthy volunteers. Br J Clin Pract 1988; 42: 51.3140864 [Google Scholar]
- 28. Paulsen O, Nilsson LG, Saint-Salvi B. et al. No effect of roxithromycin on pharmacokinetic or pharmacodynamic properties of warfarin and its enantiomers. Pharmacol Toxicol 1988; 63: 215–20. [DOI] [PubMed] [Google Scholar]
- 29. Rimoldi R, Mangiarotti P, De Rose V. et al. Penetration of roxithromycin into bronchial secretions. Br J Clin Pract 1988; 42: 74–7. [PubMed] [Google Scholar]
- 30. Saito A, Kato Y, Odagaki E. et al. Pharmacokinetics and clinical results of RU 28965. Chemotherapy 1988; 36: 216–23. [Google Scholar]
- 31. Segre G, Bianchi E, Zanolo G. et al. Influence of food on the bioavailability of roxithromycin versus erythromycin stearate. Br J Clin Pract 1988; 42: 55–7. [Google Scholar]
- 32. Halstenson CE, Opsahl JA, Schwenk MH. et al. Disposition of roxithromycin in patients with normal and severely impaired renal function. Antimicrob Agents Chemother 1990; 34: 385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moravek J, Matousovic K, Prat V. et al. Pharmacokinetics of roxithromycin in kidney grafted patients under cyclosporin A or azathioprine immunosuppression and in healthy volunteers. Int J Clin Pharmacol Ther Toxicol 1990; 28: 262–7. [PubMed] [Google Scholar]
- 34. Boccazzi A, Langer M.. Penetration of roxithromycin into bronchial secretions. Chemotherapy 1991; 37: 303–9. [DOI] [PubMed] [Google Scholar]
- 35. Nilsen OG. Pharmacokinetics of macrolides. Comparison of plasma, tissue and free concentrations with special reference to roxithromycin. Infection 1995; 23: S5–9. [DOI] [PubMed] [Google Scholar]
- 36. Macek J, Ptáček P, Klı´ma J.. Determination of roxithromycin in human plasma by high-performance liquid chromatography with spectrophotometric detection. J Chromatogr B Biomed Sci Appl 1999; 723: 233–8. [DOI] [PubMed] [Google Scholar]
- 37. Motta M, Ribeiro W, Ifa DR. et al. Bioequivalence evaluation of two roxithromycin formulations in healthy human volunteers by high performance liquid chromatography coupled to tandem mass spectrometry. Acta Physiol Pharmacol Ther Latinoam 1999; 49: 233–41. [PubMed] [Google Scholar]
- 38. Hang T-j, Zhang M, Song M. et al. Simultaneous determination and pharmacokinetic study of roxithromycin and ambroxol hydrochloride in human plasma by LC-MS/MS. Clin Chim Acta 2007; 382: 20–4. [DOI] [PubMed] [Google Scholar]
- 39. Kousoulos C, Tsatsou G, Dotsikas Y. et al. Validation of a fully automated high throughput liquid chromatographic/tandem mass spectrometric method for roxithromycin quantification in human plasma. Application to a bioequivalence study. Biomed Chromatogr 2008; 22: 494–501. [DOI] [PubMed] [Google Scholar]