Abstract
The heterodimeric T1R1 + T1R3 receptor is considered critical for normal signaling of L-glutamate and 5′-ribonucleotides in the oral cavity. However, some taste-guided responsiveness remains in mice lacking one subunit of the receptor, suggesting that other receptors are sufficient to support some behaviors. Here, mice lacking both receptor subunits (KO) and wild-type (WT, both n = 13) mice were tested in a battery of behavioral tests. Mice were trained and tested in gustometers with a concentration series of Maltrin-580, a maltodextrin, in a brief-access test (10-s trials) as a positive control. Similar tests followed with monosodium glutamate (MSG) with and without the ribonucleotide inosine 5′-monophosphate (IMP), but always in the presence of the epithelial sodium channel blocker amiloride (A). Brief-access tests were repeated following short-term (30-min) and long-term (48-h) exposures to MSG + A + IMP and were also conducted with sodium gluconate replacing MSG. Finally, progressive ratio tests were conducted with Maltrin-580 or MSG + A + IMP, to assess appetitive behavior while minimizing satiation. Overall, MSG generated little concentration-dependent responding in either food-restricted WT or KO mice, even in combination with IMP. However, KO mice licked less to the amino acid stimuli, a measure of consummatory behavior in the brief-access tests. In contrast, both groups initiated a similar number of trials and had a similar breakpoint in the progressive ratio task, both measures of appetitive (approach) behavior. Collectively, these results suggest that while the T1R1 + T1R3 receptor is necessary for consummatory responding to MSG (+IMP), other receptors are sufficient to maintain appetitive responding to this “umami” stimulus complex in food-restricted mice.
Keywords: amino acid, knockout mice, MSG, taste hedonics, umami
Introduction
Monosodium glutamate (MSG), the L-glutamate sodium salt of L-glutamic acid, a nonessential amino acid, is considered the prototypical stimulus for eliciting the “savory” taste sensation in humans referred to as “umami”. It is commonly used as a food additive and is thought to promote feeding by stimulating oral taste receptors to signal the presence of protein-rich food.
Indeed, multiple receptors that are activated by MSG and monopotassium glutamate (MPG), specifically the metabotropic glutamate receptor subtypes 1 and 4 (mGluR1 and mGluR4) as well as the heterodimer T1R1 + T1R3, have been identified in the oral cavity (e.g., Chaudhari et al. 2000; Li et al. 2002; Nelson et al. 2002; San Gabriel et al. 2009). These L-glutamate receptor types appear to be differentially expressed across taste bud fields and display differences in their response properties with respect to the ability of 5′-ribonucleotides to amplify the effect of L-glutamate when presented in combination, a hallmark characteristic of “umami” sensation (see Yasuo et al. 2008, for review). Signals arising from activation of the T1R1 + T1R3 heterodimer by MSG are synergistically augmented by the addition of certain 5′-ribonucleotides, such as inosine 5′-monophosphate (IMP) and may be related to the binding site configuration of the receptor (Zhang et al. 2008). The chorda tympani nerve (CT), which innervates the taste buds of the anterior tongue where the T1R1 + T1R3 heterodimer is primarily expressed, typically shows the hallmark synergism between MSG and IMP (Yamamoto et al. 1991). Genetic silencing of either the T1R1 or T1R3 subunit dramatically attenuates, if not abolishes, CT responses to L-glutamate and the synergistic amplification of the signal by IMP (e.g., Nelson et al. 2002; Damak et al. 2003; Kitagawa et al. 2007; Kusuhara et al. 2013). In contrast, the glossopharyngeal nerve (GL), which innervates taste buds in the circumvallate and foliate papillae of the posterior tongue, where the taste mGluRs are primarily expressed, does not show a synergistic response when an L-glutamate salt is combined with a 5′-ribonucleotide, but it does respond to both types of compounds (e.g., Chaudhari et al. 2000; Damak et al. 2003; Kusuhara et al. 2013; but see Ninomiya and Funakoshi 1987). Although the mGluRs do not seem to demonstrate the characteristic synergism between L-glutamate and 5′-ribonucleotides, mice lacking mGluR4 do show reduced CT and GL responses to MPG, with and without IMP (Yasumatsu et al. 2015). Thus, there is growing evidence that multiple receptor types in the oral cavity signal the presence of L-glutamate in the gustatory system.
What remains unclear, however, is whether the signals arising from these different receptors play differential roles in taste function. Most behavioral work has focused on the T1R1 + T1R3 heterodimer due, in part, to the availability of knock-out mice for each receptor subunit. When amiloride, an epithelial sodium channel blocker that is tasteless to rodents and reduces the contribution of the sodium component in the taste of MSG (Eylam et al. 2003; Murata et al. 2009), is included in the stimulus, mice lacking the T1R1 receptor subunit show only a slightly reduced conditioned aversion to MSG (Kusuhara et al. 2013). In contrast, T1R1- and T1R3-KO mice show no unconditioned preference for MSG + IMP (in amiloride) when presented for the animal to freely lick in a brief-access taste test that minimizes the impact of postingestive influences on responses (Zhao et al. 2003). In a task designed to test the detectability of a taste stimulus independent of its hedonic properties, T1R1- and T1R3-KO mice also showed impaired sensitivity for MSG + IMP mixed in amiloride, relative to WT mice (Smith and Spector 2014; but see Delay et al. 2006), but they were able to detect the highest concentrations tested. Overall, behavioral studies in mice lacking a functional T1R1 + T1R3 suggest that the input from remaining receptors is capable of supporting some degree of function for some taste-guided behaviors.
Here, we incorporated a battery of behavioral tests that focus on the orosensory properties of the stimulus in mice lacking both subunits of the receptor to examine the necessity of signals arising from the T1R1 + T1R3 heterodimer in the maintenance of taste-based motivational potency of L-glutamate. To our knowledge, this is the first characterization of the taste phenotype of T1R1 + T1R3 double-knockout mice. Further, the contribution of IMP to the effectiveness of T1R1 + T1R3-independent signals to maintain hedonic responding to L-glutamate was investigated. We also probed whether ingestive experience with the amino acid can impact subsequently tested taste-guided behaviors. In all of the experiments, amiloride was added to the MSG solutions to minimize the contribution of the sodium cation in influencing behavioral responses. While not the focus of this study, the maltodextrin Maltrin-580 was used as a positive control. Maltrin is similar to Polycose, the latter having been found to be detectable and having positive hedonic qualities to mice missing T1R subunits (Treesukosol et al. 2009; 2012; Treesukosol and Spector 2012).
Materials and methods
Subjects
Male and female breeding pairs of mice that were homozygous null for the Tas1r1 or Tas1r3 gene (initially derived from 129X1/SvJ and backcrossed for at least 3 generations with C57BL/6 mice) were generously provided by Dr. Charles Zuker (University of California San Diego; now at Columbia University). Homozygous null mice and wild-type C57BL/6J mice (B6, Jackson Laboratory) were paired to generate mice that were heterozygous for Tas1r1 or Tas1r3. These mice were in turn crossed to generate heterozygous, homozygous null, and wild-type mice. From these mice, homozygous null mice for the Tas1r1 or Tas1r3 and wild-type mice for either gene were separately bred to generate the T1R1 + T1R3 double knock-out (KO) and wild-type (WT) strains used in the experiment. For the wild-type group, wild-type mice from heterozygous pairings of both single KO genotypes were paired to generate a combined set of wild-type mice. To generate mice lacking both receptor subunits, null mice for the Tas1r1 or Tas1r3 were paired with null mice for the other gene, and the subsequent generation (heterozygous for both Tas1r1 and Tas1r3) was in turn paired to eventually generate mice heterozygous, homozygous null, and wild-type for Tas1r1 and/or Tas1r3. Mice that were homozygous null for both Tas1r1 and Tas1r3 were paired to generate more animals that were homozygous null for both genes. These animals were assigned as subjects in the behavioral tests (T1R1 + T1R3 KO: 7 males, 6 females; WT: 6 males, 7 females). Genotypes were independently confirmed both before and after the experiment by Transnetyx, Inc. The groups were matched by body weight and age at the start of the experiment.
Behavioral tests began when the mice were 8–18 weeks of age. Prior to the experiment, a vivarium-wide pinworm eradication protocol was followed that used fenbendazole-treated chow, and all experimental mice were fed with the treated chow for at least 1 week. This protocol was completed 6 weeks before the start of the experiment. Animals were single-housed throughout the experiment in polycarbonate shoebox cages in a room with computer-controlled temperature, humidity, and a 12h:12h light–dark cycle. A cotton-fiber nestlet (Ancare Corp.) was provided for environmental enrichment. Testing occurred during the light phase. Mice were provided ad libitum chow (Rodent Laboratory Chow 5001; Nestlé Purina Petcare, St. Louis, MO) and deionized reverse-osmosis water, except where noted.
Body mass was measured when food- or water-restriction occurred and on all training and test days. During phases with consecutive days of restriction, any animal falling below 85% of its ad libitum body mass was provided 1 mL supplemental water 1 h after the end of the daily session. When repletion occurred, water bottles or food were returned to the cage 30 min after the last daily session. All procedures followed institutional and national guidelines for the care and use of laboratory animals, and were approved by the Animal Care and Use Committee at Florida State University.
Taste stimuli
All solutions were prepared daily with deionized reverse-osmosis water and presented at room temperature. Amiloride hydrochloride (A; Sigma-Aldrich, St. Louis, MO) was prepared the day before use and left to stir overnight in a flask covered with aluminum foil to prevent degradation by exposure to light. Test stimuli consisted of the maltodextrin Maltrin-580 (Grain Processing Corporation, Muscatine, IA), monosodium glutamate (MSG; Sigma-Aldrich), inosine 5′-monophosphate disodium (IMP; Sigma-Aldrich), and sodium gluconate (NaGlu; Sigma-Aldrich). IMP was held constant at 2.5 mM, a concentration effective for allowing the detection of L-glutamate in mice lacking a single subunit of the T1R1 + T1R3 receptor (Smith and Spector, 2014), and enhancing the response of T1R1 + T1R3 receptors to L-amino acids (Nelson et al., 2002). MSG and IMP were always presented in 100 µM amiloride, a concentration found to be effective at attenuating the detectability of NaCl for mice without itself being detectable (Eylam and Spector 2002; Eylam et al. 2003).
Apparatus
Except where noted, training and testing took place in a gustometer, as described in detail elsewhere (Spector et al. 2015). A mouse was placed in the rectangular testing chamber that consisted of 3 Plexiglas sides and a stainless steel front panel. During brief-access training and testing, a centrally positioned access slot was available through which the mouse could access fluid deposited upon a borosilicate sample ball which spins around a horizontal axis. Licks were registered by a force transducer connected to the sample ball; fluid was deposited onto the sample ball via Teflon tubing connected to a syringe that was mounted to a stepper motor. Each stimulus presented in a session was delivered by a different syringe through isolated tubing that was threaded through a circular turret that rotated to position the appropriate tubing before the start of a trial. A trial began after 2 dry licks within 250 ms were registered, whereupon ~10 µL of solution was deposited on the ball as a preload. This design element reduces the likelihood of stimulus cues being present (e.g., olfactory stimuli) before licking commenced. Each subsequent lick in a trial resulted in ~1 µL of fluid being deposited on the ball. At the end of each trial, an intertrial interval (~6 s) began during which the sample ball was retracted into a washing well, rinsed with distilled water, and dried with pressurized air before returning to its position behind the access slot in preparation for a new trial. During that time, the sample tubing turret rotated to align the tubing with the sample ball for the next trial. Two additional access slots were blocked with stainless steel shutters during brief-access testing. For the progressive ratio task, the center access slot was covered with a stainless steel shutter and the left access slot was available. Behind this slot, a fixed polyoxymethylene response ball was connected to a force transducer that could register licks as described for the sample ball. Fluid was delivered by a pump via PTFE tubing connected to stainless steel tubing threaded through the ball. No preload was necessary for the response ball.
The testing chamber was housed within a sound attenuation chamber and masking noise was presented during all sessions to reduce extraneous auditory cues. Air was drawn away from the sample ball via ductwork connected to an exhaust fan to reduce olfactory cues.
Brief-access taste testing
To measure hedonic responses to the stimuli, we employed the brief-access taste test. This task allows the animal to consume a stimulus freely for a very short period of time (here, 10 s) in randomized trials with other concentrations, thus reducing the impact of postingestive signals on behavior during any particular trial (see Spector 2003, for review).
During the training phase for the brief-access test, mice were water-restricted for 23.5 h with fluid being provided during 30-min sessions. Table 1 provides a summary of all training and testing phases. For 3 days, a single 30-min trial was presented wherein the animal could lick freely for water from a stationary water line. During days 4 and 5, the mice were given water trials, each with a 10-s duration. During these sessions, 7 tubing lines filled with water were presented to the animal in a randomized block schedule without replacement. Animals could initiate as many trials as possible during the session. On day 6, one tubing line was filled with water (the vehicle for Maltrin) and 6 were filled with one of a concentration series of Maltrin: 1%, 2%, 8%, 16%, 24% and 32%. While stimuli were presented in randomized blocks without replacement, the first block of the session always began with 16% Maltrin. Following the last training session, water bottles were returned.
Table 1.
Experimental phases
| Phase | # | Access | Vehiclea | Stimulus |
|---|---|---|---|---|
| Brief-Access Trainingb | ||||
| Free-Access | 3 | Constant | N/A | 1 tube water |
| Water Trials | 2 | 10-s trials | N/A | 7 tubes water |
| Maltrin | 1 | 10-s trials | Water | Maltrin-580 series |
| Brief-Access Test Series 1b: Started with either 16% Maltrin or 0.2 M MSG | ||||
| Maltrin | 1 | 10-s trials | Water | Maltrin-580 series |
| MSG + A + I | 1 | 10-s trials | A + I | monosodium glutamate series |
| MSG + A | 1 | 10-s trials | A | monosodium glutamate series |
| Short-Term Accessb: 30-min access to a single stimulus | ||||
| MSG + A | 1 | Constant | N/A | 0.2 M monosodium glutamate + A |
| MSG + A + I | 5 | Constant | N/A | 0.2 M monosodium glutamate + A + I |
| Brief-Access Test Series 2b: Started with either 16% Maltrin or 0.2 M MSG/NaGlu | ||||
| Maltrin | 1 | 10-s trials | Water | Maltrin-580 series |
| MSG + A + I | 1 | 10-s trials | A + I | monosodium glutamate series |
| MSG + A | 1 | 10-s trials | A | monosodium glutamate series |
| NaGlu + A + I | 1 | 10-s trials | A + I | sodium gluconate series |
| NaGlu + A | 1 | 10-s trials | A | sodium gluconate series |
| Maltrin | 1 | 10-s trials | Water | Maltrin-580 series |
| Two-Bottle Preference Testing: 48-h, 0.2 M MAI and water | ||||
| Brief-Access Test Series 3b: Started with 0.2 M MSG | ||||
| MSG + A + I | 1 | 10-s trials | A + I | monosodium glutamate series |
| MSG + A | 1 | 10-s trials | A | monosodium glutamate series |
| Progressive Ratiob: 3-min inactivity limit. Reward: 15 licks of stimulus | ||||
| Training | ||||
| Free-access | 1 | Constant | N/A | 1 tube water |
| Water | 2 | Fixed ratio: 3 | N/A | Water |
| Maltrin | 1 | Progressive ratio: 3 | N/A | 8% Maltrin-580 |
| Testing | ||||
| Maltrin | 2 | Progressive ratio: 3 | N/A | 8% Maltrin-580 |
| MSG + A + I | 3 | Progressive ratio: 3 | N/A | 0.4 M monosodium glutamate + A + I |
aA: 100 µM amiloride hydrochloride. I: 2.5 mM inosine 5′-monophosphate disodium.
bTraining sessions were conducted while animals were water-restricted. Testing sessions were conducted while animals were food-restricted, with 24–48 hours of repletion between each session.
During all brief-access testing, food was removed from home cages the afternoon prior to a test day and returned 1-h after the last daily session. Restriction did not occur again for 24–48 h in an attempt to maintain an equal level of motivation across test days.
A series of brief-access tests were conducted across 3 test days (Brief-Access Test Series 1, see Table 1). On the first test day, animals were presented with the Maltrin concentration series used during training. On the second test day, animals were presented with the MSG concentrations (0.025, 0.05, 0.1, 0.2, 0.4 and 0.8 M), all in a mixture with 100 µM amiloride and 2.5 mM IMP (MSG + A + I). On the third test day, animals were presented with MSG concentrations isomolar with those used in the second test but dissolved only in 100 µM amiloride without IMP (MSG + A). During these and all subsequent tests using MSG, the first block always began with 0.2 M MSG (with amiloride and with/without IMP) and all stimuli were presented in randomized blocks without replacement.
Following short-term ingestive experience with L-glutamate solutions (see below, Table 1), the test series was repeated in Brief-Access Test Series 2. Additionally, following the MSG + A test there were 2 tests with sodium gluconate at concentrations isomolar with those used in the MSG tests and vehicle presented in randomized blocks of trials; one test was NaGlu + A + I, and the second was NaGlu + A. The first randomized block began with 0.2 M NaGlu (with amiloride and with/without IMP). After NaGlu + A, another Maltrin test was conducted.
Following long-term ingestive experience with L-glutamate solutions (see below, Table 1), MSG + A + I and MSG + A tests were conducted again in Brief-Access Test Series 3, in a manner consistent with the Brief-Access Test Series 1.
Ingestive experience with L-glutamate solutions
To assess whether experience with L-glutamate would affect later taste-guided behavioral testing, perhaps through flavor preference learning, we gave mice short- and long-term exposure to the amino acid in drinking tests administered between the Brief-Access Test series (see Table 1). Following Brief-Access Test Series 1, all mice were repleted for 48 h. Food was removed from the home cage 18–23 h prior to the first short-term drinking test wherein mice were given a single 30-min session with free access to 0.2 M MSG + A in a manner similar to that described for stationary brief-access training but with the left response ball as the manipulandum. With so few licks being taken by most mice (see Results), 5 subsequent sessions were conducted using 0.2 M MSG + A + I. Each session was separated by 24–48 h of repletion.
Following Brief-Access Test Series 2, a long-term drinking test was conducted in the home cage. All mice were given ad libitum food and water access for 7 days prior to the start of the long-term drinking test. Water bottles were removed and replaced with 2 graduated 25 mL tubes connected to a customized drinking spout with an aperture diameter of ~1.5 mm. One bottle contained deionized water and the other contained 0.2 M MSG in 2.5 mM IMP and 100 µM amiloride. Both drinking tubes were covered in aluminum foil while on the cage to reduce degradation of amiloride, and positioned to be elevated so that the foil was not accessible to the animal. After 24 h each tube was removed, amount consumed (mL) was recorded, and all tubes were rinsed, refilled, and placed back on the cage with positions switched. Following another 24-h period, volume consumed was recorded and water bottles were returned ahead of Brief-Access Test Series 3 (see Table 1).
Progressive Ratio
The progressive ratio task is a method of assessing the appetitive qualities of a taste stimulus while minimizing postingestive cues and satiation (Hodos 1961; see Spector 2003 for review). Water bottles were removed from the home cages 3 days after the last brief-access testing session in preparation for training in the progressive ratio task. On consecutive training days, mice were first given 25 min of free access to deionized water via the left response ball. In 2 subsequent training sessions, mice earned 15 licks of water after completion of a fixed ratio of 3 dry licks. These, and all subsequent, sessions ended after a 3-min period of inactivity. During the fourth training session, mice were trained in the progressive ratio task. The first ratio was set to 1 dry lick to provide a warm-up for the session. Following the first reward (15 licks of 8% Maltrin), the animal was required to take 3 dry licks to receive the Maltrin reinforcer, and each subsequent contingency increased by 3 dry licks (i.e., step size = 3) until a 3-min period of inactivity.
Following 2 days of ad libitum food and water access, food was removed from the home cages and the animals were tested in the progressive ratio task (step size = 3) for 2 sessions using 8% Maltrin and then for 3 sessions using 0.4 M MSG + A + I. These sessions were conducted using the same parameters as those for the final training session, and were separated by 24–48 h of repletion.
Data analysis
Licks taken per trial were recorded for all brief-access sessions. During sessions involving a tastant, the average number of licks to the vehicle in which the stimulus was dissolved in each session was subtracted from the average licks for each concentration for each animal (Taste Licks – Vehicle Licks). This adjusts for individual differences in baseline lick rates. Training data, trials initiated, and vehicle licks during testing were compared using 2-sample t-tests. Taste Licks – Vehicle Licks for each concentration and stimulus were compared across genotype and stimulus in ANOVAs. For these and all statistical analyses, a P ≤ 0.05 was considered statistically significant. Only mice that initiated at least 1 trial per concentration for all Brief-access tests using a particular stimulus were included in analyses for that stimulus. Training and NaGlu data were analyzed using the same groups as for MSG analyses. Final group sizes can be found in figure captions. Due to the general lack of concentration-dependent responding, psychometric functions could not be derived from the MSG data and thus are also not included for Maltrin.
For short-term drinking test sessions, lick onset times were recorded. However, due to the low number of licks taken by KO mice, further analyses were not conducted, and only total licks were compared across genotype. For long-term drinking test sessions, the volume consumed for MSG + A + I and water were each combined across the 2 days. A preference ratio for MSG + A + I was derived by dividing the consumption of MSG + A + I by total volume consumed across the 48-h period. A ratio of 1.0 indicates that all fluid consumed during the test was MSG + A + I, whereas a ratio of 0.5 indicates equal consumption of MSG + A + I and water. This ratio and intakes were compared across genotype in t-tests.
For progressive ratio tests, the number of operant licks in the final completed ratio, referred to as the breakpoint, was determined for each test. The median breakpoint (±SIQR) for a stimulus was derived across all test sessions using that stimulus. These medians were compared across genotype using Kruskal–Wallis one-way analyses of variance.
Results
Brief-access testing
In general, KO mice licked more than WT mice for water while water-restricted during training (Table 2). There was no significant difference between genotypes for licks taken to vehicle during any brief-access test, with the single exception of NaGlu + A; KO mice took fewer licks to vehicle during that test (Table 2).
Table 2.
Training and vehicle lick data
| KO | WT | Between-subjects comparison | ||
|---|---|---|---|---|
| T-statistic | P value | |||
| Brief-access training dataa (water-restricted sessions) | ||||
| Stationary licks (2nd day) | 1169 (169) | 758 (84) | 2.18 | 0.05 |
| Interlick interval (ms) | 128.6 (1.8) | 131.7 (4.7) | −0.597 | 0.56 |
| Water training | ||||
| Trials initiated | 25.0 (3.3) | 16.7 (1.7) | 2.21 | 0.05 |
| Average licks | 59.4 (2.3) | 54.4 (4.3) | 1.05 | 0.31 |
| Maltrin Training | ||||
| Trials Initiated | 33.9 (2.9) | 29.0 (3.2) | 1.13 | 0.27 |
| Brief-Access Vehicle Lick Dataa (food-restricted tests) | ||||
| Vehicle: Water | ||||
| Maltrin-1 | 13.9 (1.4) | 15.2 (2.6) | 0.22 | 0.64 |
| Maltrin-2 | 12.6 (1.9) | 11.5 (3.2) | 0.1 | 0.76 |
| Maltrin-3 | 11.2 (1.0) | 10.2 (1.3) | 0.34 | 0.56 |
| Vehicle: amiloride + inosine monophosphate (A + I) | ||||
| MSG + A + I-1 | 15.8 (2.4) | 17.9 (1.7) | 1.33 | 0.26 |
| MSG + A + I-2 | 10.2 (1.4) | 16.4 (2.4) | 3.56 | 0.07 |
| MSG + A + I-3 | 13.7 (3.3) | 16.6 (1.9) | 1.31 | 0.27 |
| NAGLU + A + I | 10.3 (2.5) | 13.2 (2.8) | 0.51 | 0.49 |
| Vehicle: amiloride (A) | ||||
| MSG + A-1 | 13.5 (2.6) | 14.0 (2.8) | 0.16 | 0.70 |
| MSG + A-2 | 14.1 (1.8) | 15.1 (2.9) | 0.39 | 0.54 |
| MSG + A-3 | 23.0 (8.5) | 15 (2.0) | 0.65 | 0.43 |
| NAGLU + A | 8.2 (2.9) | 18.7 (3.0) | 5.83 | 0.03 |
| Progressive ratio trainingb (water-restricted sessions) | U-statistic | P-value | ||
| Water Responses (FR-3) | 94 (14) | 66 (9) | 121 | 0.06 |
| Maltrin Breakpoint (progressive ratio-3) | 79 (9) | 68 (6) | 127.5 | 0.03 |
Bolded values represent statistical significance (P ≤ 0.05).
aData reported: Mean (SE) for each genotype. Between-Subjects comparison: 2-sample t-tests.
bData reported: Median (SIQR) for each genotype. Between-subjects comparison: Kruskal–Wallis 1-way analysis of variance.
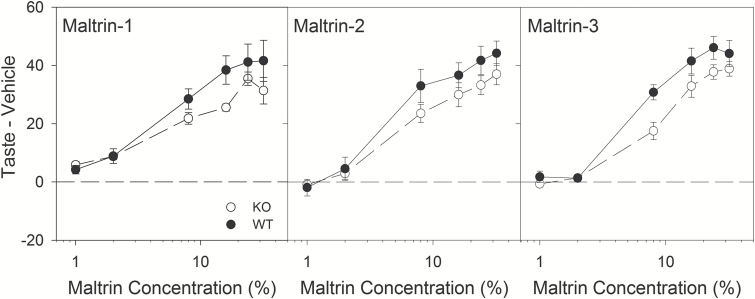
Maltrin was included as a positive hedonic stimulus to demonstrate that the mice could display concentration-dependent responding and that they were competent in the behavioral task, given the possibility that KO mice would not respond to the umami stimuli. In the first test of Maltrin while under food-restricted conditions, there were no significant differences in responsiveness between the genotypes (Figure 1; Table 3) although the KO mice tended to lick less to the higher concentrations than did WT mice. Repeated testing following L-glutamate exposure resulted in little change overall to licking responses to Maltrin (Figure 1), albeit with a significant Phase × Concentration interaction (Table 4). Across all tests, there was a significant effect of Genotype and a Genotype × Concentration interaction, with KO mice licking less to higher concentrations consistently across the Maltrin tests. However, both genotypes showed concentration-dependent responding to Maltrin, with licks increasing as a function of concentration.
Figure 1.
Brief-Access Lick Data: Maltrin. Mean (±SE) Taste – Vehicle scores for wild-type (filled circles, n = 12) and T1R1 + T1R3 KO mice (open circles, n = 13) for all concentrations in each of the 3 brief-access tests for Maltrin. The vehicle for Maltrin was distilled water.
Table 3.
Two-way ANOVAs: lick data from Brief-Access Test Series 1 stimuli
| Maltrin | MSG + A + I | MSG + A | |
|---|---|---|---|
| Genotype | F(1,23) = 3.0; 0.10 | F(1,20) = 23.64; 0.01 | F(1,20) = 3.17; 0.09 |
| Concentration | F(5,115) = 42.70; 0.01 | F(5,100) = 8.90; 0.01 | F(5,100) = 2.76; 0.02 |
| Genotype × Concentration | F(5,115) = 1.67; 0.15 | F(5,100) = 2.14; 0.07 | F(5,100) = 0.97; 0.44 |
Table 4.
Three-way ANOVAs comparing effects of experience on brief-access lick data (across all tests for each stimulus)
| Maltrin | MSG + A + I | MSG + A | |
|---|---|---|---|
| Genotype | F(1,23) = 5.40; 0.03 | F(1,20) = 27.14; 0.01 | F(1,20) = 6.68; 0.02 |
| Phase | F(2,46) = 0.14; 0.87 | F(2,40) = 2.91; 0.07 | F(2,40) = 0.90; 0.41 |
| Concentration | F(5,115) = 143.29; 0.01 | F(5,100) = 10.15; 0.01 | F(5,100) = 3.41; 0.01 |
| Genotype × phase | F(2,46) = 0.04; 0.96 | F(2,40) = 1.62; 0.21 | F(2,40) = 0.41; 0.67 |
| Genotype × concentration | F(5,115) = 2.369; 0.04 | F(5,100) = 2.54; 0.03 | F(5,100) = 1.35; 0.25 |
| Phase × Concentration | F(10,230) = 2.88; 0.01 | F(10,200) = 0.95; 0.49 | F(10,200) = 1.03; 0.42 |
| Geno × phase × Conc | F(10,230) = 0.51; 0.86 | F(10,200) = 0.59; 0.82 | F(10,200) = 1.36; 0.20 |
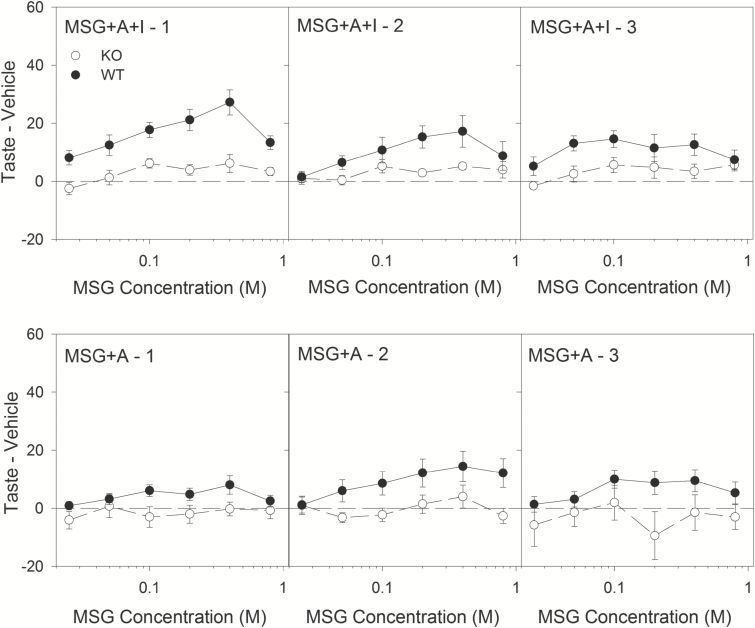
In the first test using MSG + A + I, in comparison, KO mice displayed very little concentration-dependent responding, with almost no licks above vehicle at any MSG concentration (Figure 2; Table 3). Although WT mice displayed some significant influence of concentration on licking, it is obvious in Figure 2 that it was modest and nonmonotonic. These 2 different lick profiles resulted in a significant effect of Genotype and Concentration (Table 3). In the first test of MSG + A, neither genotype licked a meaningful amount to any concentration relative to vehicle licks, although there was a significant effect of Concentration (Figure 2; Table 3). Repeated brief-access tests of MSG following additional L-glutamate exposures did not significantly impact licking behavior to MSG + A + I or MSG + A, with no significant Phase effects detected (Table 4). There was, however, a consistent effect of Genotype, with WT mice licking more for both MSG + A + I and MSG + A than KO mice (Figure 2; Tables 4 and 5).
Figure 2.
Brief-Access Lick Data: MSG. Top row: Mean (±SE) Taste – Vehicle scores for wild-type (filled circles, n = 12) and T1R1 + T1R3 KO mice (open circles, n = 10) for all concentrations in each of the 3 brief-access tests for MSG + A + I. The vehicle for MSG + A + I was A + I (100 µM amiloride + 2.5 mM IMP). Bottom row: Mean (±SE) Taste – A (amiloride, vehicle) scores for wild-type (filled circles) and T1R1 + T1R3 KO mice (open circles) for all concentrations in each of the 3 brief-access tests for MSG + A. The vehicle for MSG + A was A (100 µM amiloride).
Table 5.
Three-way ANOVAs testing the contribution of IMP in brief-access lick data (MSG + A + I vs. MSG + A; NaGlu + A + I vs. NaGlu)
| MSG (Series 1) | MSG (Series 2) | NaGlu | MSG (Series 3) | |
|---|---|---|---|---|
| Genotype | F(1,20)=23.48; 0.01 | F(1,20)=11.31; 0.01 | F(1,18)=0.17; 0.68 | F(1,20)=7.86; 0.01 |
| Vehicle | F(2,40)=17.65; 0.01 | F(2,40)=0.55; 0.47 | F(1,18)=0.16; 0.70 | F(2,40)=2.78; 0.11 |
| Concentration | F(5,100)=9.24; 0.01 | F(5,100)=3.86; 0.01 | F(5,90)=0.40; 0.85 | F(5,100)=3.07; 0.01 |
| Genotype x Vehicle | F(2,40)=4.32; 0.05 | F(2,40)=0.11; 0.74 | F(1,18)=8.49; 0.01 | F(2,40)=0.01; 0.98 |
| Genotype x Concentration | F(5,100)=2.23; 0.06 | F(5,100)=3.86; 0.01 | F(5,90)=0.67; 0.65 | F(5,100)=0.93; 0.48 |
| Vehicle x Concentration | F(10,200)=2.87; 0.02 | F(10,200)=0.34; 0.89 | F(5,90)=0.96; 0.45 | F(10,200)=0.38; 0.87 |
| Genotype x Vehicle x Concentration | F(10,200)=0.96; 0.45 | F(10,200)=0.70; 0.63 | F(5,90)=0.44; 0.82 | F(10,200)=1.53; 0.19 |
The role of IMP on responsiveness to L-glutamate can be inferred by comparing licks to MSG + A + I with licks to MSG + A across concentrations. Although a 3-way ANOVA (Genotype × Vehicle × Concentration; Table 5) of licking during Brief-Access Test Series 1 found significant effects of Vehicle (i.e., amiloride with or without IMP), this was primarily driven by the effect of IMP on licking in the WT compared to the KO mice as is evident in Figure 2 and supported by the significant interaction of Genotype × Vehicle (Table 5). In contrast, the presence of IMP no longer significantly affected licking behavior to L-glutamate in the final 2 Brief-Access Test Series, with no significant main or interaction effects involving Vehicle (Figure 2; Table 5). This is reflected in the data by a general decrease in licking behavior from WT mice across the 3 MSG + A + I tests (Figure 2). Overall, these results suggest that while the addition of IMP can boost consummatory responsiveness (i.e., licking) to the taste properties of L-glutamate, it does so primarily for mice with an intact T1R1 + T1R3 receptor during early exposures.
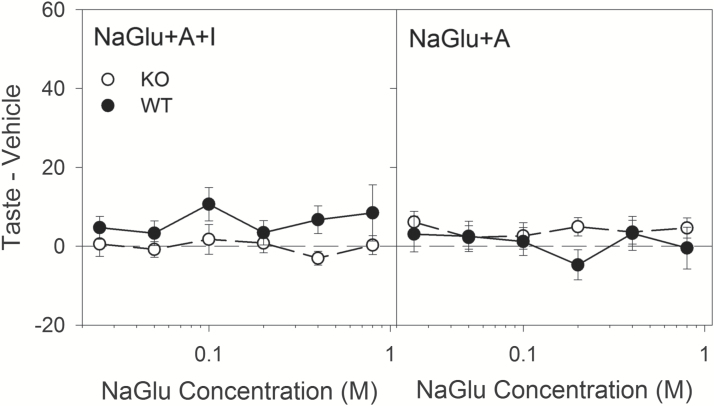
In NaGlu tests, neither genotype showed concentration-dependent responding (Figure 3; Table 5), and there seemed to be little impact of IMP on responding despite a significant Genotype × Vehicle interaction when NaGlu + A + I was compared with NaGlu + A (Table 5). This stimulus was included as a tool to infer the contribution of L-glutamate during MSG tests, as gluconate is not thought to stimulate taste receptors (Ye et al. 1991) but is a similarly sized organic anion and thus could control for the potential amiloride-insensitive component of sodium taste, which is known to be suppressed by larger anions (e.g., Beidler 1953; Formaker and Hill 1988; Ye et al. 1991; Geran and Spector 2000). When IMP was included and NaGlu tests were compared to MSG tests from Brief-Access Test Series 2, a significant effect of Genotype was found, with WT mice licking slightly more than KO mice (Figures 2 and 3; Table 6). Without IMP, only a Genotype x Anion interaction was significant (Table 6). Thus, in general, L-glutamate (e.g., MSG + A) drives little consummatory behavior in this task regardless of whether T1R1 + T1R3 signaling is intact.
Figure 3.
Brief-access Lick Data: Sodium Gluconate. Left: Mean (±SE) Taste – A + I (amiloride + IMP, vehicle) scores for wild-type (filled circles, n = 12) and T1R1 + T1R3 KO mice (open circles, n=10) for all concentrations in the Brief-access test for NaGlu + A+ I. The vehicle for NaGlu + A + I was A + I (100 µM amiloride + 2.5 mM IMP). Right: Mean (±SE) Taste – A (amiloride, vehicle) scores scores for wild-type (filled circles) and T1R1 + T1R3 KO mice (open circles) for all concentrations in the brief-access test for NaGlu + A. The vehicle for NaGlu + A was A (100 µM amiloride).
Table 6.
Three-way ANOVAs testing the contribution of L-glutamate in brief-access lick data (MSG+A+I-2 vs. NaGlu + A + I; MSG+A-2 vs. NaGlu + A)
| MSG + A + I vs. NaGlu + A + I | MSG + A vs. NaGlu + A | |
|---|---|---|
| Genotype | F(1,18) = 4.61; 0.04 | F(1,18) = 0.95; 0.34 |
| Anion | F(1,18) = 3.36; 0.08 | F(1,18) = 0.56; 0.47 |
| Concentration | F(5,90) = 2.18; 0.06 | F(5,90) = 0.93; 0.47 |
| Genotype × Anion | F(1,18) = 0.03; 0.87 | F(1,18) = 5.68; 0.03 |
| Genotype × concentration | F(5,90) = 0.93; 0.47 | F(5,90) = 0.93; 0.47 |
| Anion × Concentration | F(5,90) = 1.34; 0.25 | F(5,90) = 1.55; 0.18 |
| Genotype × Anion × Concentration | F(5,90) = 0.6; 0.7 | F(5,90) = 1.10; 0.37 |
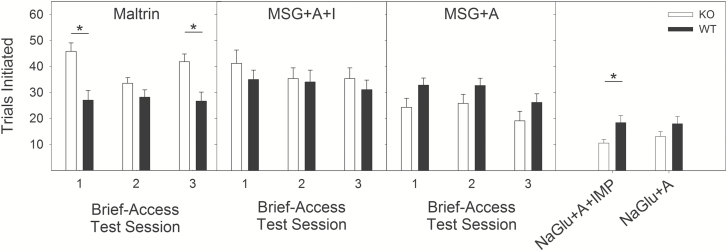
While licks taken to a stimulus is a consummatory behavior, the trials initiated in a brief-access test, as used here in which trials do not begin until an animal is actively licking, measures appetitive (i.e., approach) behavior driven by a stimulus. The analysis of trials initiated during brief-access tests yielded a slightly different profile of results than what was found for licking. First, KO mice initiated more trials for Maltrin (Figure 4; Table 7). On the other hand, there was no difference between genotypes in trials initiated for MSG + A + I, MSG + A, or NaGlu + A. KO mice initiated fewer trials to NaGlu + A + I. Of note, however, is the fact that while WT mice initiated similar numbers of trials to MSG + A + I as to MSG + A, KO mice initiated fewer trials when IMP was not included for MSG tests. This result was consistent across all brief-access test series. That is, while IMP did not impact consummatory (licking) behavior for KO mice, its presence increased appetitive behavior in the form of trials initiated when mixed with MSG. Additional exposure to L-glutamate had little impact on trial-taking behavior for either genotype, although both WT and KO mice did significantly decrease this behavior following the long-term 2-bottle test (Figure 4; Table 7).
Figure 4.
Trials Initiated in brief-access tests. Top: Mean (±SE) number of trials initiated by wild-type (black bars, n = 13) and T1R1 + T1R3 KO mice (open bars, n = 13) for all brief-access sessions with Maltrin. Middle: Mean (±SE) number of trials initiated by wild-type (black bars) and T1R1 + T1R3 KO mice (open bars) for all brief-access sessions with MSG + A + I and MSG. Bottom: Mean (±SE) number of trials initiated by wild-type (black bars) and T1R1 + T1R3 KO mice (open bars) for brief-access sessions with NaGlu + A + I and NaGlu + A. *Significant difference between genotypes.
Table 7.
t-tests comparing trials initiated during brief-access tests
| Test | Statistic | |||
|---|---|---|---|---|
| Two-Sample t-tests | ||||
| Contribution of T1R1 + T1R3 receptor | T-statistic | P value | ||
| Maltrin-1 | 14.20 | 0.01 | ||
| Maltrin-2 | 2.22 | 0.15 | ||
| Maltrin-3 | 11.40 | 0.01 | ||
| MSG + A + I-1 | 1.00 | 0.33 | ||
| MSG + A-1 | 3.70 | 0.07 | ||
| MSG + A + I-2 | 0.05 | 0.83 | ||
| MSG + A-2 | 2.43 | 0.13 | ||
| MSG + A + I-3 | 0.61 | 0.44 | ||
| MSG + A-3 | 1.99 | 0.17 | ||
| NaGlu + A + I | −2.54 | 0.02 | ||
| NaGlu + A | −1.50 | 0.15 | ||
| Paired t-tests | ||||
| WT | KO | |||
| T | P | T | P | |
| Contribution of IMP | ||||
| MSG + A + I-1 vs. MSG + A-1 | 0.71 | 0.49 | 4.67 | 0.01 |
| MSG + A + I-2 vs. MSG + A-2 | 0.38 | 0.71 | 2.05 | 0.05 |
| MSG + A + I-3 vs. MSG + A-3 | 2.30 | 0.04 | 3.17 | 0.01 |
| NaGlu + A + I vs. NaGlu + A | 0.18 | 0.86 | −1.28 | 0.23 |
| Contribution of L-glutamate | ||||
| MSG + A + I-2 vs. NaGlu + A + I | 3.99 | 0.01 | 6.89 | 0.01 |
| MSG + A-2 vs. NaGlu + A | 8.50 | 0.01 | 2.87 | 0.01 |
| Contribution of experience with L-glutamate | WT | KO | ||
| MSG + A + I | ||||
| 1 vs. 2 | 0.34 | 0.74 | 0.91 | 0.38 |
| 2 vs. 3 | 1.09 | 0.30 | 0.001 | 1.00 |
| 1 vs. 3 | 1.05 | 0.32 | 1.21 | 0.25 |
| MSG + A | ||||
| 1 vs. 2 | 0.08 | 0.93 | -0.38 | 0.71 |
| 2 vs. 3 | 2.42 | 0.03 | 1.99 | 0.07 |
| 1 vs. 3 | 2.64 | 0.02 | 2.43 | 0.03 |
Bolded values indicate statistical significance (P ≤ 0.05).
Data for these analyses can be found in Figure 4.
Progressive ratio
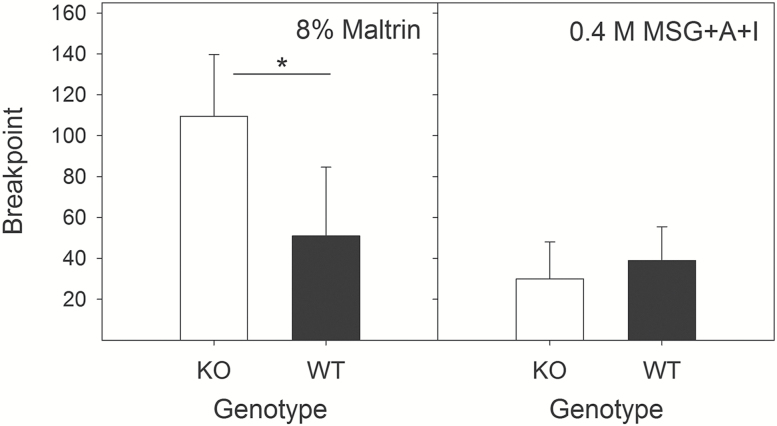
The progressive ratio task focuses on the effectiveness of a stimulus to drive appetitive behavior while minimizing the impact of postingestive signals and satiation. Here, KO mice were willing to work more for 8% Maltrin, with a significantly higher breakpoint relative to WT mice (U =138; P < 0.01; Figure 5), although they also worked more for water while water-restricted (Table 2). By contrast, there was no genotype difference for 0.4 M MSG + A + I (U = 69; P > 0.43).
Figure 5.
Progressive Ratio Breakpoints. Left: Median (±SIQR) breakpoint for wild-type (black bars, n = 13) and T1R1 + T1R3 KO mice (open bars, n = 13) for 8% Maltrin. Right: Median (±SIQR) breakpoint for wild-type (black bars) and T1R1 + T1R3 KO mice (open bars) for 0.4 M MSG + A + I. Breakpoint is defined as the number of licks in the last completed ratio before 3 min of inactivity. *Significant difference between genotypes.
L-glutamate exposure tests
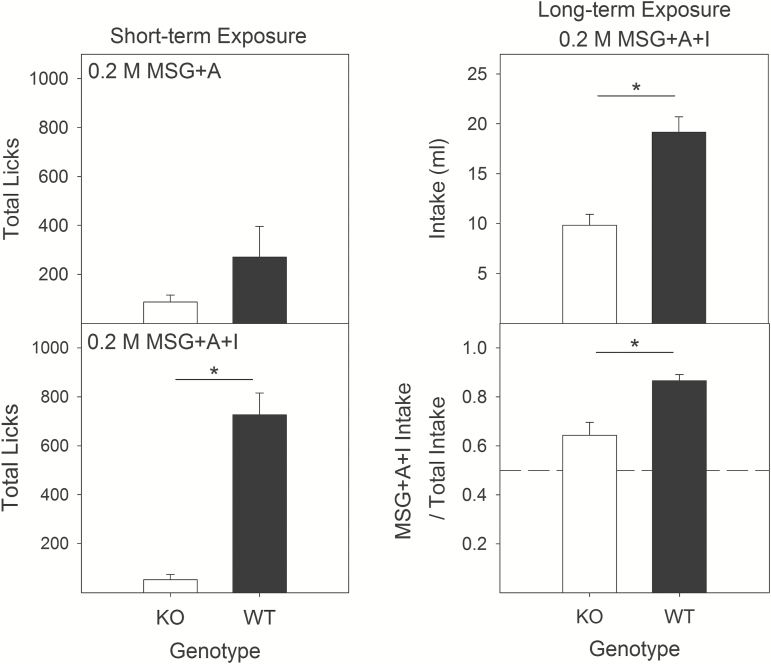
In the short-term exposure tests during which animals had access to a single stimulus for 30-min sessions, KO mice took significantly fewer licks for 0.2 M MSG + A + I (t = −6.2; P < 0.01) but not MSG + A (t = −1.03; P > 0.31 relative to WT mice; Figure 6, left). The addition of IMP did not significantly increase total licks for KO mice (t = −1.138; P > 0.29, but did so for WT mice (t = 5.02; P < 0.01; Figure 6, left). These results are similar to the lick data for the brief-access tests, in that the synergism between L-glutamate and IMP was dependent on the signal from the T1R1 + T1R3 receptor.
Figure 6.
Short- and long-term L-glutamate exposures. Left: Mean (±SE) number of licks by wild-type (black bars, n = 13) and T1R1 + T1R3 KO mice (open bars, n = 13) during short-term (30 min) drinking tests with 0.2 M MSG + A and 0.2 M MSG + A + I. Right: Mean (±SE) intake, in mls, and preference for wild-type (black bars) and T1R1 + T1R3 KO mice (open bars) for long-term drinking test (48 hr) with 0.2 M MSG + A + I. Preference scores are calculated by dividing the intake of MSG + A + I by total fluid intake across the 48-h test. *Significant difference between genotypes.
In the long-term preference test, WT mice showed a clear preference for 0.2 M MSG + A + I (Figure 6, bottom right; t = 14.83, P < 0.01). KO mice, by comparison, showed a significantly lower preference for (t = −3.79; P < 0.01) and intake of the stimulus (t = −4.86; P < 0.01), albeit with a preference slightly above indifference (t = 2.69, P < 0.02; Figure 6).
Discussion
In general, the prototypical umami stimulus MSG + A + I promoted only modest behavioral responsiveness, at best, in the motivationally oriented taste tasks used here. The slight concentration-dependent increase in licking in the brief-access test suggests that while the MSG + A + I has some positive consummatory properties, it is not a potent stimulus for unconditionally driving taste-guided responsiveness. Nevertheless, what little consummatory behavior is generated by this umami stimulus, here in the form of licks, requires T1R1 + T1R3 signaling; KO mice showed no relevant concentration-dependent responding above vehicle and no difference between MSG + A + I and MSG + A (Figure 2). These results are consistent with previous work (Zhao et al. 2003) and extend the concentrations tested to those clearly detectable by mice lacking only one subunit of the receptor (see Smith and Spector 2014). As such, the remaining T1R1 + T1R3-independent signals are not sufficient to generate unconditioned consummatory behavior for the umami stimulus mixture, at least when mice are food-restricted as in this study.
In contrast, there were no differences between WT and KO mice for appetitive behavior to the same stimuli, as trials initiated in the brief-access tests were similar between groups (Figure 4). There were also no genotype differences in median breakpoints during the progressive ratio tests for 0.4 M MSG + A + I (Figure 5). Thus, 2 separate behavioral measures suggest that the T1R1 + T1R3-independent signals are sufficient to support appetitive taste-guided behavior to MSG + A + I under fasting conditions.
Contribution of inosine 5′-monophosphate
The synergistic gustatory responses to L-glutamate when combined with 5′-ribonucleotides is considered the hallmark characteristic of an “umami” stimulus (see Yasuo et al. 2008). Here, IMP increased lick rates to L-glutamate for only WT mice (Figure 2; Table 3), adding evidence that the T1R1 + T1R3 receptor is crucial for the enhanced sensory effectiveness of the stimulus mixture.
WT mice also licked significantly more to stimuli mixed with IMP (in amiloride) than amiloride alone, even in the absence of L-glutamate. That is, WT mice licked more to MSG + A + I than to MSG + A (Figure 2; Table 5), and also licked slightly more to NaGlu + A + I than to NaGlu + A (Figure 3; Table 5). Similarly, WT mice licked slightly more than KO mice to both MSG + A + I and NaGlu + A + I (Figures 2 and 3; Table 6). Thus, despite generating electrophysiological responses in mice lacking T1R1 (Kusuhara et al. 2013), IMP does not seem to stimulate taste-guided consummatory behavior without an intact T1R1 + T1R3 receptor, at least at the concentration used here. This result may be related to the finding that mice lacking either subunit of the receptor do not detect 2.5 mM IMP, unlike WT mice (Smith and Spector 2014). Interestingly, the effect on consummatory behavior in the WT mice did not impact licks to IMP as a vehicle in MSG + A + I tests when compared to KO mice (Table 2). That is, in the absence of any other chemical, the WT and KO mice licked IMP (with amiloride) at similar rates. It may be, then, that IMP and the isomolar sodium concentrations present in both the MSG and NaGlu tests were interacting, even with the inclusion of amiloride. Indeed, there is some evidence from canines that NaCl can influence CT responses to guanosine monophosphate, a 5′-ribonucleotide similar to IMP (Ugawa and Kurihara 1994), though the molecular basis for the phenomenon, or whether it replicates in a rodent model, is not clear.
While the addition of IMP increased consummatory behavior only with an intact T1R1 + T1R3 receptor, its presence increased appetitive behavior in the brief-access test for KO mice. That is, KO mice initiated more trials during MSG + A + I tests than when MSG + A was the stimulus (Figure 4; Table 7). The same increase for KO mice was not found for sodium gluconate, however, as KO mice initiated a similar number of trials between those tests (Figure 4; Table 7). As such, it suggests that while the T1R-independent signal is being influenced by IMP, it does so primarily in combination with L-glutamate and thus implies some degree of chemospecificity for the behavior. In contrast, WT mice generated a similar number of trials for both MSG + A + I as MSG + A. It may be, then, that the T1R1 + T1R3 receptor is providing some signal triggering trial initiation for stimuli containing L-glutamate, but in the absence of the heterodimer the T1R-independent mechanisms are sufficient to generate this appetitive behavior especially when L-glutamate is combined with IMP. Thus, although IMP had a minimum impact in consummatory responsiveness to L-glutamate in KO mice, it nonetheless augmented trial initiation in the brief-access test. Because trial initiation is subject to the influence of the accumulating postingestive load, we cannot rule out that the relevant effects of these stimuli to generate this form of appetitive behavior is of a postoral origin. That said, it is clear that the T1R1 + T1R3 heterodimer is unnecessary to maintain appetitive behavior to 0.4 M MSG + A + I in the progressive ratio task in which postingestive influences are minimized.
Contribution of L-glutamate
Separately from IMP, the contribution of L-glutamate to these behavioral results can be inferred by comparing the outcomes of the MSG and NaGlu tests. Sodium gluconate is thought to be a poor stimulant of taste receptors (e.g., Ye et al. 1991) and therefore unlikely to contribute meaningfully to these tests. Interestingly, neither WT nor KO mice displayed much consummatory behavior to L-glutamate compared to NaGlu, even in the presence of IMP (Figures 2 and 3; Table 6). While MSG + A + I generated relatively weak consummatory behavior overall in WT mice and almost none in the KO group, in the absence of IMP both genotypes had lick rates barely above vehicle for L-glutamate (i.e., MSG + A; Figure 2). The latter result is generally consistent with another brief-access study showing that MSG + A elicited only modest enhanced licking responses above vehicle in gustducin-KO mice and heterozygous controls at low to mid-range concentrations (Glendinning et al. 2005). In that study, at 1.0 M, MSG + A caused significant avoidance of licking by the control mice. While not avoiding the stimulus here, WT mice did decrease lick rates to 0.8 M MSG + A + I (Figure 2) compared with mid-range concentrations of the amino acid. In general, the pattern of results suggests that L-glutamate alone (i.e., MSG + A) is a weak stimulus for generating consummatory taste-guided behavior.
On the other hand, both WT and KO mice initiated significantly more trials for MSG + A + I and MSG + A than their respective NaGlu comparisons (Figure 4; Table 7). As such, L-glutamate (with and without IMP) appears to be able to generate appetitive behavior, despite the relative lack of consummatory behavior seen during the same tests. This also increases confidence that the appetitive trial-taking behavior seen in KO mice is based on the presence of L-glutamate (and IMP), and not simply a general motivational state driven by the food-restricted protocol used during testing. These results also further suggest that the T1R1 + T1R3 heterodimer is unnecessary for appetitive behavior in response to L-glutamate to be expressed. That said, as noted above, the effect of L-glutamate on trial initiation in the brief-access test may be of a postoral origin.
Maltrin
While not the focus of this study, the results with Maltrin are consistent with those found previously for Polycose, another maltodextrin. Maltrin-580 is similar to Polycose in its ratios of glucose moieties, and was included as a stimulus as a positive control because of its expected hedonic characteristics even in T1R KO mice (Treesukosol et al. 2009, 2011). KO mice showed clear concentration-dependence for the stimulus, albeit with blunted lick rates relative to WT mice at the highest concentrations across all tests (Figure 1). Overall, these results buttress the implication of an unidentified T1R-independent taste receptor(s) that signals the presence of polysaccharides (see Sclafani 1987, for review). It is unclear why KO mice tended to initiate more trials for Maltrin in the brief-access test, as well as display more willingness to work for 8% Maltrin in the progressive ratio task. It may be that the food restriction led the animals to initiate more trials in the brief-access test to compensate for obtaining fewer calories that follow from having blunted responses at the higher concentrations. Then, given that the progressive ratio tests followed multiple exposures to Maltrin, the progressive ratio breakpoints in KO mice may reflect the mice having had experience with approaching the maltodextrin stimulus often during the brief-access test.
Effect of experience with L-glutamate stimuli
Overall, the results of the short- and long-term exposure sessions were not unexpected. WT mice licked more for MSG + A + I than they did for MSG + A, and more than the KO mice (Figure 6) in the short-term tests. In the long-term preference testing, WT mice showed a clear preference that was higher than that seen for the KO mice (Figure 6). The KO mice did, however, show a slight preference for the stimulus. In general, this is consistent with other preference studies showing reduced avidity for MSG in mice lacking one T1R-receptor subunit (Damak et al. 2003), though amiloride was not included as part of the stimulus in that study.
The short- and long-term licking tests were included to provide additional exposure to L-glutamate and the umami stimulus complex of L-glutamate + IMP. It has been shown that while C57BL/6J mice do not display unconditioned preferences to MSG, they will learn to associate its taste with positive postingestive signals and display a preference for it (without amiloride; Ackroff et al. 2012), as well as learn to prefer a hedonically neutral flavor paired with intragastric infusions of the stimulus (Ackroff and Sclafani 2013a). Conditioned preferences formed through pairings of a flavor with oral MSG appear to be independent of the T1R3 receptor subunit (Ackroff and Sclafani 2013b). The conditioning does not seem to be related to the postingestive signals from NaCl, at least in rats (Uematsu et al. 2009). Overall, here, experience with L-glutamate and IMP had little impact on further taste-guided behavioral testing, with minimal changes to brief-access consummatory and appetitive behaviors for either WT or KO mice (Figures 2 and 4). The lack of difference between Brief-Access Test Series 1 and 2 could somewhat be explained by the low levels of consumption during the short-term tests (Figure 6). However, while both groups of mice consumed little of the stimuli in the short-term exposure sessions, they did consume more MSG + A + I in the long-term tests (Figure 6). Yet, these animals did not display any increases in taste-guided behavior in the brief-access tests (Figure 2), in contrast to what would be expected if the animals were learning to pair the taste of MSG + A + I with positive postingestive feedback. If anything, behavior decreased across testing (Figures 2 and 4). We cannot, however, entirely rule out that enhancements in licking in the brief-access tests may have eventually appeared with either further experience with the 2-bottle test or a higher stimulus concentration.
It is difficult to compare our findings with other conditioning studies that did not use amiloride as a vehicle because reduction of the sodium component of MSG has been shown to significantly decrease consumption in rats (Murata et al. 2009). However, the lack of unconditioned preference reported in the background strains for the KO mice used here (Ackroff and Sclafani 2012, 2013b) suggests that the clear avidity shown here by WT mice in these drinking tests (Figure 6) could be the result of previous exposures in the brief-access and short-term tests having been sufficient for learning to have influenced preference behavior in the long-term tests. The comparative lack of preference in KO mice, despite conditioned preferences being T1R3-independent (Ackroff and Sclafani 2013b), could be due to the low consumption by KO mice in the short-term exposure sessions resulting in insufficient experience with the stimulus to generate a preference. Another possibility is that the T1R1 receptor subunit, intact in the previous study (Ackroff and Sclafani 2013b), formed a homodimer that provided a sufficient signal for the T1R3 KO mice that would not have been available to the double-KO mice used in this study. Alternatively, it could be that the T1R3 KO mice were using the sodium component of MSG as part of the conditioned stimulus. Regardless of the differences in preference, however, neither KO nor WT mice in this study increased consummatory or appetitive behavior towards MSG + A + I in the subsequent brief-access test, a task that focuses on the orosensory properties of the stimulus (Figures 2 and 4).
Indeed, the lick rates for WT mice in all brief-access tests of MSG (with/without IMP) suggest that, when used in a behavioral task that focuses on its orosensory properties, the amino acid is not a motivationally potent taste stimulus. While difficult to compare directly, the highest lick rate by WT mice for MSG + A + I is less than that for Maltrin (Figures 1 and 2). Other work has shown similarly low lick rates, or neutral or aversive responding depending on the conditions of testing (e.g., Nelson et al. 2002; Glendinning et al. 2005). Collectively, the results strongly suggest that the signals arising from the peripheral gustatory system in response to this prototypical umami stimulus only weakly activate neural circuits promoting unconditioned consummatory licking behavior. That activation, as weak as it may be, is enhanced by IMP and depends on the presence of a functional T1R1 + T1R3 heterodimer. That said, L-glutamate (i.e., MSG + A) is capable of activating neural circuits promoting appetitive behavior and this does not appear to depend on the T1R1 + T1R3 heterodimer. As such, the results of this study add to the growing literature suggesting that multiple receptor types signaling the presence of L-glutamate in the oral cavity are independently capable of supporting some taste-guided behavior to the amino acid. However, these receptors do not equally contribute to all such behaviors, requiring careful experimental design to illuminate their roles.
Funding
Funded by NIDCD R01DC004574 (ACS).
Acknowledgements
The authors would like to thank Stefan Grabhofer and Fabienne Schmid for their assistance in collecting portions of the data presented here.
References
- Ackroff K, Weintraub R, Sclafani A. 2012. MSG intake and preference in mice are influenced by prior testing experience. Physiol Behav. 107(2):207–217. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. 2013a. Flavor preferences conditioned by intragastric monosodium glutamate in mice. Chem Senses. 38(9):759–767. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. 2013b. Flavor preferences conditioned by oral monosodium glutamate in mice. Chem Senses. 38(9):745–758. [DOI] [PubMed] [Google Scholar]
- Beidler LM. 1953. Properties of chemoreceptors of tongue of rat. J Neurophysiol. 16(6):595–607. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. 2000. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 3(2):113–119. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. 2003. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 301(5634):850–853. [DOI] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. 2006. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 31(4):351–357. [DOI] [PubMed] [Google Scholar]
- Eylam S, Spector AC. 2002. The effect of amiloride on operantly conditioned performance in an NaCl taste detection task and NaCl preference in C57BL/6J mice. Behav Neurosci. 116(1):149–159. [PubMed] [Google Scholar]
- Eylam S, Tracy T, Garcea M, Spector AC. 2003. Amiloride is an ineffective conditioned stimulus in taste aversion learning in C57BL/6J and DBA/2J mice. Chem Senses. 28(8):681–689. [DOI] [PubMed] [Google Scholar]
- Formaker BK, Hill DL. 1988. An analysis of residual NaCl taste response after amiloride. Am J Physiol. 255(6 Pt 2):R1002–R1007. [DOI] [PubMed] [Google Scholar]
- Geran LC, Spector AC. 2000. Sodium taste detectability in rats is independent of anion size: the psychophysical characteristics of the transcellular sodium taste transduction pathway. Behav Neurosci. 114(6):1229–1238. [PubMed] [Google Scholar]
- Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. 2005. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 30(4):299–316. [DOI] [PubMed] [Google Scholar]
- Hodos W. 1961. Progressive ratio as a measure of reward strength. Science. 134(3483):943–944. [DOI] [PubMed] [Google Scholar]
- Kitagawa J, Takahashi Y, Matsumoto S, Shingai T. 2007. Response properties of the pharyngeal branch of the glossopharyngeal nerve for umami taste in mice and rats. Neurosci Lett. 417(1):42–45. [DOI] [PubMed] [Google Scholar]
- Kusuhara Y, Yoshida R, Ohkuri T, Yasumatsu K, Voigt A, Hübner S, Maeda K, Boehm U, Meyerhof W, Ninomiya Y. 2013. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol. 591(7):1967–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. 2002. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 99(7):4692–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Beauchamp GK, Bachmanov AA. 2009. Taste perception of monosodium glutamate and inosine monophosphate by 129P3/J and C57BL/6ByJ mice. Physiol Behav. 98(4):481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. 2002. An amino-acid taste receptor. Nature. 416(6877):199–202. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. (1987) Qualitative discrimination among “umami” and the four basic taste substances in mice. In: Kawamura Y, Kare MR, editors. Umami: a basic taste (pp. 365–386). [Google Scholar]
- San Gabriel A, Maekawa T, Uneyama H, Torii K. 2009. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr. 90(3):743S–746S. [DOI] [PubMed] [Google Scholar]
- Sclafani A. 1987. Carbohydrate taste, appetite, and obesity: an overview. Neurosci Biobehav Rev. 11(2):131–153. [PubMed] [Google Scholar]
- Smith KR, Spector AC. 2014. The importance of the presence of a 5’-ribonucleotide and the contribution of the T1R1 + T1R3 heterodimer and an additional low-affinity receptor in the taste detection of L-glutamate as assessed psychophysically. J Neurosci. 34(39):13234–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC. (2003) Psychophysical evaluation of taste function in nonhuman mammals. In: Doty RL, editor. Handbook of olfaction and gustation. Marcel Dekker, Inc, New York: p. 861–879. [Google Scholar]
- Spector AC, Blonde GD, Henderson RP, Treesukosol Y, Hendrick P, Newsome R, Fletcher FH, Tang T, Donaldson JA. 2015. A new gustometer for taste testing in rodents. Chem Senses. 40(3):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde GD, Spector AC. 2009. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 296(4):R855–R865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Smith KR, Spector AC. 2011. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci. 31(38):13527–13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Spector AC. 2012. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol. 303(2):R218–R235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Tsurugizawa T, Kondoh T, Torii K. 2009. Conditioned flavor preference learning by intragastric administration of L-glutamate in rats. Neurosci Lett. 451(3):190–193. [DOI] [PubMed] [Google Scholar]
- Ugawa T, Kurihara K. 1994. Enhancement of canine taste responses to umami substances by salts. Am J Physiol. 266(3 Pt 2):R944–R949. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Fujimoto Y, Fukunaga I, Miyasaka A, Imoto T. 1991. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav. 49(5):919–925. [DOI] [PubMed] [Google Scholar]
- Yasumatsu K, Manabe T, Yoshida R, Iwatsuki K, Uneyama H, Takahashi I, Ninomiya Y. 2015. Involvement of multiple taste receptors in umami taste: analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice. J Physiol. 593(4):1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo T, Kusuhara Y, Yasumatsu K, Ninomiya Y. 2008. Multiple receptor systems for glutamate detection in the taste organ. Biol Pharm Bull. 31(10):1833–1837. [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. 1991. The anion paradox in sodium taste reception: resolution by voltage-clamp studies. Science. 254(5032):724–726. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. 2003. The receptors for mammalian sweet and umami taste. Cell. 115(3):255–266. [DOI] [PubMed] [Google Scholar]
- Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. 2008. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci U S A. 105(52):20930–20934. [DOI] [PMC free article] [PubMed] [Google Scholar]