Abstract
Background
Existing studies of the 1944–45 Dutch famine found little evidence of the association between early life malnutrition and midlife cognition.
Methods
Among 2446 rural participants born between 1958 and 1963 in the China Health and Retirement Longitudinal Study, we examined effects of exposure to China’s 1959–61 Great Leap Forward famine during prenatal and early postnatal life, on four cognitive measures in 2011 (baseline) and changes in cognition between 2011 and 2013 (first follow-up). We obtained difference-in-differences (DID) estimates of the famine effects by exploiting temporal variation in the timing and duration of famine exposure across six birth cohorts born between 1958 and 1963, together with geographical variation in famine severity at the prefecture level.
Results
After adjusting for gender, marital status and provincial fixed effects, we found that the 1961 cohort who experienced full-term prenatal and partial-term postnatal exposures to famine had lower scores on the Telephone Interview of Cognitive Status (TICS), a test of drawing pentagons, and general cognition at age 50 years compared with the unexposed 1963 cohort. Adjusting for education, the famine effects on drawing pentagons and general cognition were fully attenuated, but the effect on TICS persisted. We also found a robust negative famine effect on the longitudinal change in general cognition during the 2-year follow-up in the 1959 cohort.
Conclusions
Severe nutritional deprivation during prenatal and postnatal periods has a lasting impact on cognitive performance in Chinese adults in their early 50s.
Keywords: China, cognition, famine, Great Leap Forward
Key Messages
Early life exposure to famine is negatively associated with baseline cognition in midlife and change in cognition over a 2-year follow-up period.
The estimation of famine effect is sensitive to measures of cognition.
We also found evidence of mortality selection among the famine survivors at work.
Introduction
Cognition involves the mental capacity to acquire and process information, to learn and reason, and to apply knowledge and make use of experiences to solve problems.1 For the elderly, cognitive decline implies reduced ability to plan and manage the demands of many daily tasks and ultimately to live independently and care for oneself.2 Understanding the causes of cognitive decline has important policy implications for successful ageing in high-income countries3 and in low- and middle-income countries like China, where population ageing is accelerating.4
From a life course perspective, malnutrition experienced in utero has been hypothesized to have a lasting impact on cognitive function in later life through both biological and socioeconomic pathways.5–7 Prenatal nutritional deprivation may have a lifelong impact on cognition by damaging normal biological conditions such as disrupting fetal brain development and increasing the risk of neurodevelopmental anomalies.8 Prenatal malnutrition may also harm human capital development and lead to poor educational achievement,9–11 which in turn increase the risk of cognitive problems in old age.12–14 Nevertheless, empirical evidence for the long-term effect of prenatal malnutrition on later life cognition remains scarce.
Using the 1944–45 Dutch famine as a natural experiment, an earlier study reported no differences in average IQ (measured by scores on the Raven Progressive Matrices test) or prevalence of mental retardation at about age 19 years between those exposed to famine in utero and those unexposed.5 A recent study of the Dutch famine administered a well-established battery of cognitive tests to a non-representative sample of 946 famine survivors when they were 59 years old.7 After comparing them against 890 men and women born immediately before or after the famine (from 1943 to 1947), no effect of prenatal malnutrition was found for the majority of the cognitive measures except a reduction in a measure of general cognition with famine exposure in early gestation (weeks 1–10) and an unexpected increase in a measure of memory encoding (immediate words recall) with famine exposure in later gestation (weeks 31 to delivery). In a separate but comparable Dutch famine study, a selective sample of 737 participants born from 1943 to 1947 were administered a battery of cognitive tests when they were 56–59 years old.6 Similarly, no association was found between prenatal exposure to famine and all but one cognitive measure—those exposed to famine during the first and second trimesters performed worse on the selective attention task compared with the unexposed cohorts (i.e. those born before or conceived after the famine).
This study seeks to advance the literature by examining the effects of prenatal and early postnatal exposure to the 1959–61 Chinese famine on cognitive functioning at 48–53 years of age and on cognitive decline over a 2-year follow-up period. The Chinese famine was jointly caused by natural disaster and policy mistakes made during the Great Leap Forward (GLF) campaign launched in 1958,15 and thus is also known as the GLF famine. The GLF famine is characterized by its greater magnitude relative to other famines in terms of duration (3 years), geographical scope (pandemic as opposed to endemic) and level of damage (16.5–30 million excess deaths, with a mortality rate of over 3.0%).16, 17 One recent study found that prenatal exposure to the GLF famine was associated with a decreased score of the Mini-Mental State Examination (MMSE) but not associated with scores of the Montreal Cognitive Assessment, logic memory test or Stroop colour-word test.18 Another study reported that prenatal exposure to the GLF famine was negatively associated with scores of the Stroop colour-word test and the trailing-making test, but not associated with general intelligence, short-term or long-term memory or visuospatial skill.19 These two studies rely on either a household sample recruited in a single county or a clinical sample recruited from tertiary hospitals in multiple regions, restricting the generalizability of their findings.
We drew on data from a nationally representative survey of middle- and older-aged Chinese adults. We compared cognitive scores across multiple birth cohorts born between 1958 and 1963, who varied in both the timing and the duration of the famine exposure. Some of these cohorts experienced prolonged malnutrition during both the prenatal and postnatal periods. Such cumulative exposure to an adverse early life environment could further exacerbate cognitive deficits.8 Within each cohort we exploited, additionally, geographical variation in the intensity of famine exposure by comparing those born and living in areas hit harder by the famine with those in less affected areas. We expect that early life exposure to the GLF famine is detrimental to midlife cognitive function. We also expect the negative famine effect to be stronger for longer duration of exposure and higher level of famine severity.
Methods
Study participants
We used individual-level data from the 2011 baseline and 2013 follow-up surveys of the China Health and Retirement Longitudinal Study (CHARLS), a nationally representative longitudinal survey of adults aged 45 years and older and their spouses, if available. CHARLS sampled 17 708 residents from 150 counties across 28 provinces in China, with a response rate of 80.5%.20 We focus on six birth cohorts born between 1958 and 1963. The 1958 cohort experienced famine-induced malnutrition during the first 3 years of life. The 1959 cohort experienced prenatal exposure to famine either partial (conceived in 1958 and born in early 1959) or full term, as well as early postnatal exposure from birth to age 2 years. The 1960 cohort was fully exposed to famine in utero and during the first year of life. The 1961 cohort experienced prenatal exposure to famine full term and postnatal exposure before age 1 year. The 1962 cohort only experienced partial prenatal exposure to famine (conceived in 1961 and born in early 1962) when the GLF campaign approached its end. The 1963 cohort served as the reference group, since they were not exposed to famine at all.
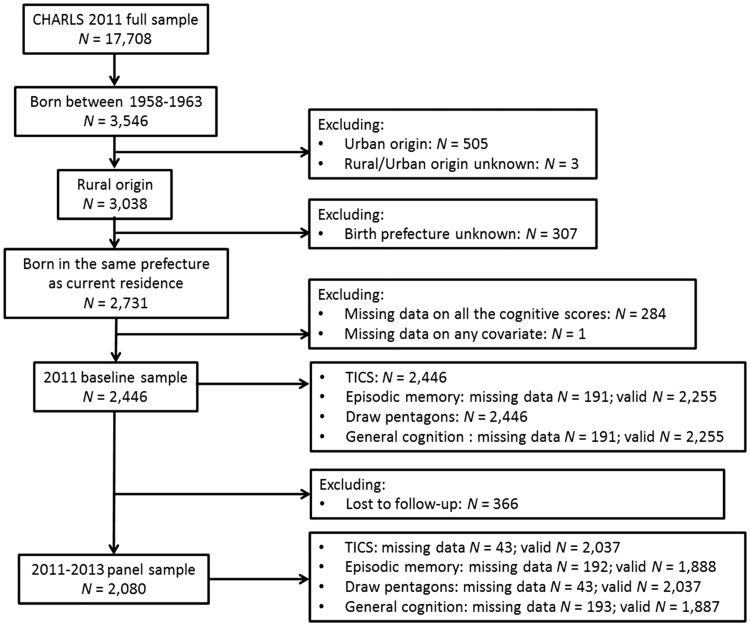
Figure 1 illustrates the derivation of our analytical sample. In the CHARLS baseline survey, 3546 participants were born between 1958 and 1963. Among them, 3038 participants were born and lived in rural mainland China during childhood and hence were hit harder by the famine than their urban peers, who were protected through state-controlled food rationing. We dropped 307 participants whose birth prefecture was unknown because they had moved since birth. For the main analysis, we dropped 285 participants who had missing data on all the cognitive measures or any covariate. To maximize statistical power, we allowed the analytical sample size to vary depending on the number of valid responses for each cognitive measure. As a result, the sample sizes ranged from 2255 to 2446 for analysing baseline cognition. In the analysis of cognitive change over the 2-year follow-up, we further excluded 366 baseline participants who were not tracked in 2013. Attribution was adjusted by using the CHARLS longitudinal weights. The longitudinal sample sizes ranged from 1887 to 2037, depending on the cognitive measure of interest.
Figure 1.
Flow chart of study participants.
Measures
Cognition
We constructed four cognitive scores in similar ways as other CHARLS studies of cognition.21,22 The measure of Telephone Interview of Cognition Status (TICS) captures intact mental status and consists of five items on time orientation (awareness of the year, month and day of today’s date, the day of the week, and the season of the year) and five items from the serial-7s test. The value of the summed TICS score across these individual items ranges from 0 to 10 (Cronbach’s alpha = 0.8). Episodic memory was measured by the average score of immediate and delayed recalls of 10 words. The measure of visuospatial skills is dichotomous, indicating whether participants were able to redraw a picture of two overlapped pentagons shown to them. The measure of general cognition was calculated as the summary score of the three measures above, ranging from 0 to 21 and reflecting participants’ overall cognitive status.
Famine severity
To measure famine severity, we derived prefecture-level cohort size shrinkage indices (CSSI) from the publicly available 1% sample of the 1990 China Population Census.23,24 Let denote the average cohort size of those born during the 3 years preceding the famine (1956–58) and the 3 years after the famine (1962–64) in the jth prefecture, and denote the average cohort size of those born during the 3 famine years (1959–61). The CSSI for the jth prefecture is calculated as a ratio:
| (1) |
where a larger value indicates a greater reduction in cohort size due to reduced fertility and increased infant mortality, both presumably induced by the GLF famine.23–25 We matched prefecture-level CSSIs to the CHARLS respondents based on their self-reported birth prefecture. Measures of cohort loss such as CSSIs can more or less capture famine severity under the assumptions of accurate census data on fertility and mortality, stable secular trends in fertility and mortality in the counterfactual absence of the famine, and strictly restricted migration. Previous demographic research on the GLF famine suggests that these assumptions are reasonably met for the cohort-loss measure calculated from the 1% sample of the 1990 census.26,27
Control variables
Both gender (male = 1 and female = 0) and marital status (= 1 if married and living with spouse, and 0 otherwise) were coded as dichotomous variables. Educational attainment was divided into three groups: no formal schooling, middle school or less, and high school or beyond. Provincial fixed effects were captured by dummy variables for all the provinces but one as the reference group.
Statistical analyses
We employed a difference-in-differences (DID) method that exploited both cohort and geographical variations in famine exposure to estimate the effects of the GLF famine. Let denote a cognitive outcome for ith participant born in prefecture j and year k (kth birth cohort), denote the control variables and be a random error; a regression-based DID estimator can be obtained from:
| (2) |
where , the coefficients of the interaction between birth cohorts and CSSI, is the DID estimate of the famine effect on later life cognition. When we entered mean-centred CSSI in the model, represents the average famine effect across prefectures on cognition corresponding to a one unit change above or below the average CSSI.
To estimate the famine effects on cognitive decline during 2011–13, we fitted change score models:28
| (3) |
where the dependent variable is the change in cognition for the same person from the baseline () to the follow-up survey ().
We estimated two models for each outcome variable. The first model only controlled for gender, marital status and provincial fixed effects. The second model additionally controlled for educational attainment, as a potential mediator of the relationship between famine exposure and cognitive ability. Estimation of the famine effect in Model 2 is subject to endogenous selection bias if education is a collider. Nevertheless, the famine-induced economic hardship and social disorder had a detrimental impact on education supply, in the form of a substantial decline in teacher attendance and a sharp increase in school closures, particularly in rural areas.21,29 The loss of educational opportunities and the resulting lower educational attainment for those who were born and grew up in the famine period have been linked to lower cognitive abilities at older ages. Model 2 may help inform whether part of the negative famine impact on cognition is channelled through reduced educational attainment. However, Model 1 would be more correct than Model 2 if education is a collider that should not be controlled for. In this study, we cannot ascertain which model is more accurate if the results differ between Models 1 and 2. We chose to fit both models as a sensitivity analysis.
We calculated P-values based on robust standard errors that adjust for the potential correlation of observations clustered within the same prefectures. We accounted for sample selection and missing data by applying the survey weights provided by the CHARLS team. Specifically, we used the baseline individual-level weights with household and individual non-response adjustment for modelling baseline cognition. These weights were constructed by the CHARLS research team and calculated as the product of the household sample selection weight, an inverse probability weighting factor for household non-response, and an inverse probability weighting factor for individual non-response conditional on household participation.30 We used the individual-level longitudinal weights for modelling cognitive change over the 2-year follow-up period. These weights were also constructed by the CHARLS research team. The longitudinal weights were calculated as the baseline weights multiplied by an inverse probability weighting factor which was constructed from a logit regression of whether a respondent participates in the second wave conditional on the participation in the baseline.31 These weights were designed to adjust for individual non-response and longitudinal attrition.
Results
Descriptive statistics
Table 1 reports cohort-specific descriptive statistics of the cognitive measures in 2011 and 2013, as well as those of the control variables. Without further stratifying each cohort by degree of prefecture famine severity, between-cohort differences in all the cognitive measures appeared to be modest at best. All six cohorts also resembled each other in terms of gender composition (roughly 44–47% men) and marital status (about 86–90% married and living with spouse) except that the 1958 cohort consisted of more men (57.28%) than women. The majority of our analytical sample (over 80%) did not attend high school. The reference cohort (born in 1963 without any famine exposure) had the lowest proportion of illiterate members (12.94%) but also the lowest proportion of high school graduates or beyond (13.28%).
Table 1.
Cohort-stratified distributions of cognitive test scores among rural Chinese
| Pre-famine |
Born during the famine period |
Post-famine |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1958 cohort |
1959 cohort |
1960 cohort |
1961 cohort |
1962 cohort |
1963 cohort |
|||||||
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | |
| 2011 baseline | ||||||||||||
| TICS (range: 0–10) | 6.89 | 404 | 7.06 | 327 | 7.14 | 307 | 7.22 | 291 | 7.44 | 522 | 7.30 | 595 |
| (2.80) | (2.63) | (2.74) | (2.57) | (2.59) | (2.50) | |||||||
| Episodic memory (range: 0–10) | 3.55 | 377 | 3.65 | 293 | 3.82 | 277 | 3.52 | 269 | 3.98 | 493 | 3.90 | 546 |
| (1.60) | (1.60) | (1.71) | (1.52) | (1.66) | (1.74) | |||||||
| Draw pentagons (binary: 0/1) | 0.69 | 404 | 0.67 | 327 | 0.72 | 307 | 0.70 | 291 | 0.74 | 522 | 0.72 | 595 |
| (0.46) | (0.47) | (0.45) | (0.46) | (0.44) | (0.45) | |||||||
| General cognition (range: 0–21) | 11.37 | 377 | 11.40 | 293 | 11.81 | 277 | 11.55 | 269 | 12.18 | 493 | 12.06 | 546 |
| (3.76) | (4.00) | (3.55) | (3.74) | (3.50) | ||||||||
| (3.79) | ||||||||||||
| 2013 follow-up | ||||||||||||
| TICS (range: 0–10) | 6.67 | 338 | 7.09 | 281 | 6.93 | 258 | 7.04 | 246 | 7.15 | 436 | 7.36 | 478 |
| (2.98) | (2.82) | (2.73) | (2.70) | (2.73) | (2.63) | |||||||
| Episodic memory (range: 0–10) | 3.59 | 338 | 3.73 | 280 | 3.87 | 258 | 3.85 | 246 | 4.04 | 436 | 3.99 | 479 |
| (1.68) | (1.85) | (1.88) | (1.69) | (1.76) | (1.78) | |||||||
| Draw pentagons (binary: 0/1) | 0.61 | 338 | 0.65 | 281 | 0.71 | 258 | 0.68 | 246 | 0.73 | 436 | 0.75 | 478 |
| (0.49) | (0.48) | (0.46) | (0.47) | (0.45) | (0.43) | |||||||
| General cognition (range: 0–21) | 10.86 | 338 | 11.48 | 280 | 11.50 | 258 | 11.58 | 246 | 11.91 | 436 | 12.11 | 478 |
| (4.23) | (4.05) | (3.96) | (4.01) | (3.92) | ||||||||
| (4.11) | ||||||||||||
| Baseline control variables | % | % | % | % | % | % | ||||||
| Male | 57.18 | 44.34 | 46.58 | 45.70 | 46.36 | 44.71 | ||||||
| Living with spouse | 87.38 | 87.77 | 89.25 | 86.25 | 88.70 | 89.75 | ||||||
| Education | ||||||||||||
| No formal education | 23.02 | 26.30 | 20.52 | 16.49 | 15.90 | 12.94 | ||||||
| < = Middle school | 60.64 | 54.74 | 55.05 | 66.67 | 66.67 | 73.78 | ||||||
| > = High school | 16.34 | 18.96 | 24.43 | 16.84 | 17.43 | 13.28 | ||||||
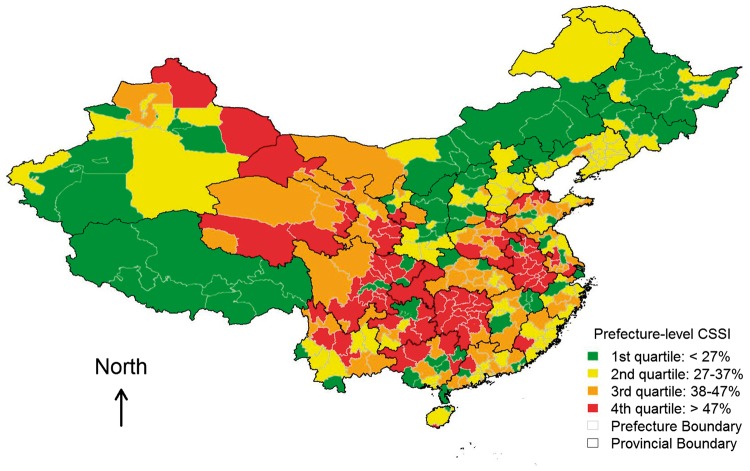
Among the 114 prefectures in our analytical sample, the value of CSSI ranged from 8.6 to 79.8%, with a mean of 40.9% and standard deviation (SD) of 14.9%. Figure 2 depicts prefecture-level spatial variation in CSSI. Regional clusterings of high CSSI, corresponding to severe famine both within and across provincial boundaries, are visually notable. Mixed colours in any given province also suggest that within-province variation in CSSI was quite common even in the least (green) or hardest (red) hit provinces.
Figure 2.
Geographical distribution of cohort size shrinkage index at the prefecture level.
Regression estimates
Tables 2–3 show the main coefficient estimates of interest, as specified in equations (2) and (3), which represent the DID estimates of the famine effects on cognitive measures. With respect to the baseline cognition (Table 2), we found negative effects of full-term prenatal exposure to famine and postnatal exposure before age 1 year on TICS, drawing pentagons and general cognition in the 1961 cohort (Model 1). The negative effects on drawing pentagons and general cognition were fully attenuated after controlling for education in Model 2, but the effect on TICS remained significant (, ). Given that the SD of TICS was 2.57 in the 1961 cohort, the average TICS score would be reduced by about 0.17 SD () for every one SD increase in CSSI () across all prefectures. Full-term prenatal and postnatal exposure to famine also negatively affected TICS in the 1960 cohort (Model 1, , ), but the effect was fully attenuated after controlling for education.
Table 2.
OLS regression estimates of famine effects on later-life cognition in 2011
| Cognition in 2011 | TICS |
Episodic memory |
Draw pentagons |
General cognition |
||||
|---|---|---|---|---|---|---|---|---|
| Coef. | P-value | Coef. | P-value | Coef. | P-value | Coef. | P-value | |
| Model 1 (reference cohort: 1963)a | ||||||||
| −0.010 | 0.417 | 0.004 | 0.643 | 0.000 | 0.890 | −0.003 | 0.862 | |
| (−0.035, 0.014) | (−0.013, 0.021) | (−0.004, 0.004) | (−0.038, 0.032) | |||||
| 0.010 | 0.415 | 0.014 | 0.159 | 0.003 | 0.304 | 0.033 | 0.076 | |
| (−0.015, 0.035) | (−0.006, 0.034) | (−0.002, 0.008) | (−0.004, 0.070) | |||||
| −0.027 | 0.076 | 0.008 | 0.477 | −0.003 | 0.266 | −0.019 | 0.443 | |
| (−0.058, 0.003) | (−0.014, 0.029) | (−0.007, 0.002) | (−0.069, 0.030) | |||||
| −0.038 | 0.000 | 0.000 | 0.987 | −0.004 | 0.034 | −0.037 | 0.043 | |
| (−0.058, −0.018) | (−0.023, 0.023) | (−0.007, 0.000) | (−0.073, −0.001) | |||||
| −0.007 | 0.505 | 0.005 | 0.598 | 0.001 | 0.557 | 0.002 | 0.912 | |
| (−0.026, 0.013) | (−0.014, 0.024) | (−0.003, 0.005) | (−0.032, 0.035) | |||||
| Model 2 (reference cohort: 1963)b | ||||||||
| −0.003 | 0.774 | 0.007 | 0.414 | 0.001 | 0.785 | 0.009 | 0.545 | |
| (−0.025, 0.018) | (−0.010, 0.024) | (−0.004, 0.005) | (−0.021, 0.040) | |||||
| 0.016 | 0.121 | 0.015 | 0.123 | 0.003 | 0.145 | 0.039 | 0.009 | |
| (−0.004, 0.036) | (−0.004, 0.034) | (−0.001, 0.008) | (0.010, 0.068) | |||||
| −0.018 | 0.175 | 0.011 | 0.263 | −0.001 | 0.520 | −0.006 | 0.766 | |
| (−0.044, 0.008) | (−0.008, 0.031) | (−0.005, 0.003) | (−0.045, 0.033) | |||||
| −0.030 | 0.002 | 0.002 | 0.838 | −0.003 | 0.116 | −0.023 | 0.197 | |
| (−0.048, −0.011) | (−0.021, 0.026) | (−0.006, 0.001) | (−0.059, 0.012) | |||||
| −0.007 | 0.436 | 0.003 | 0.735 | 0.001 | 0.506 | −0.001 | 0.951 | |
| (−0.026, 0.011) | (−0.015, 0.022) | (−0.002, 0.005) | (−0.033, 0.031) | |||||
Numbers in the parentheses are 95% confidence intervals.
Coef, coefficient; CSSI, mean-centred cohort size shrinkage index at the prefecture level.
aAdjusted for cohort, CSSI, gender, marital status, provincial fixed effects.
bModel 1 + additionally adjusted for education.
Table 3.
OLS regression estimates of famine effects on later-life cognitive decline during 2011–2013
| Cognitive change during 2011–13 | TICS |
Episodic memory |
Draw pentagons |
General cognition |
||||
|---|---|---|---|---|---|---|---|---|
| Coef. | P-value | Coef. | P-value | Coef. | P-value | Coef. | P-value | |
| Model 1 (reference cohort: 1963)a | ||||||||
| 0.007 | 0.679 | 0.002 | 0.874 | 0.002 | 0.565 | 0.012 | 0.591 | |
| (−0.025, 0.038) | (−0.022, 0.025) | (−0.005, 0.008) | (−0.033, 0.058) | |||||
| −0.026 | 0.126 | −0.019 | 0.170 | −0.005 | 0.222 | −0.044 | 0.042 | |
| (−0.059, 0.007) | (−0.046, 0.008) | (−0.013, 0.003) | (−0.087, −0.002) | |||||
| −0.001 | 0.910 | 0.007 | 0.601 | −0.002 | 0.488 | 0.001 | 0.947 | |
| (−0.027, 0.024) | (−0.020, 0.035) | (−0.008, 0.004) | (−0.035, 0.038) | |||||
| 0.013 | 0.346 | −0.005 | 0.719 | 0.000 | 0.949 | 0.004 | 0.863 | |
| (−0.015, 0.041) | (−0.033, 0.023) | (−0.008, 0.008) | (−0.041, 0.049) | |||||
| −0.012 | 0.275 | 0.005 | 0.615 | −0.001 | 0.663 | −0.008 | 0.620 | |
| (−0.035, 0.010) | (−0.015, 0.026) | (−0.007, 0.005) | (−0.041, 0.024) | |||||
| Model 2 (reference cohort: 1963)b | ||||||||
| 0.008 | 0.610 | 0.003 | 0.823 | 0.002 | 0.537 | 0.015 | 0.519 | |
| (−0.023, 0.039) | (−0.021, 0.026) | (−0.004, 0.009) | (−0.031, 0.060) | |||||
| −0.025 | 0.141 | −0.018 | 0.189 | −0.005 | 0.210 | −0.043 | 0.052 | |
| (−0.059, 0.009) | (−0.045, 0.009) | (−0.013, 0.003) | (−0.087, 0.000) | |||||
| 0.000 | 0.991 | 0.008 | 0.563 | −0.002 | 0.525 | 0.004 | 0.840 | |
| (−0.026, 0.026) | (−0.019, 0.035) | (−0.008, 0.004) | (−0.034, 0.041) | |||||
| 0.015 | 0.288 | −0.004 | 0.792 | 0.000 | 0.947 | 0.007 | 0.755 | |
| (−0.013, 0.042) | (−0.032, 0.024) | (−0.008, 0.008) | (−0.038, 0.052) | |||||
| −0.013 | 0.243 | 0.006 | 0.592 | −0.002 | 0.600 | −0.009 | 0.583 | |
| (−0.035, 0.009) | (−0.015, 0.026) | (−0.008, 0.005) | (−0.042, 0.024) | |||||
Numbers in the parentheses are 95% confidence intervals.
Coef, coefficient; CSSI, mean-centred cohort size shrinkage index at the prefecture level.
aAdjusted for cohort, CSSI, gender, marital status, provincial fixed effects.
bModel 1 + additionally adjusted for education.
By contrast, the 1959 cohort showed a higher score of general cognition compared with the reference cohort both before (Model 1, , ) and after controlling for education (Model 2, , ). Given that the SD of general cognition was 3.76 in the 1959 cohort, this estimate amounted to a 0.15 SD () difference in general cognition per one SD difference in CSSI.
Turning to the longitudinal analysis (Table 3), we found little effect of early life exposure to famine on changes in cognitive measures between 2011 and 2013, with one exception. In the 1959 cohort, partial-term prenatal exposure to famine and postnatal exposure from birth to age 2 years would reduce general cognition by 0.17 SD () on average per one SD increase in CSSI. This impact was partially mitigated after controlling for education (Model 2, , ).
Sensitivity checks
We first assessed the impact of missing data by repeating the same analyses as shown in Tables 2–3 after performing multiple imputations. We generated 10 replicated datasets for the missing data on cognitive measures from which we combined the estimates of coefficients and standard errors according to Rubin’s rule.32 The results on the baseline cognition, reported in Table 4, presented the same pattern as that shown in Table 2. Before adjusting for education, we found similar negative effects of full-term prenatal exposure to famine and postnatal exposure from birth to age 1 year in the 1961 cohort on TICS, drawing pentagons and general cognition as that shown in Table 2. After adjusting for education, only the effect on TICS persisted (, ). The 1959 cohort showed a higher score of general cognition after controlling for education (, ).
Table 4.
OLS regression estimates of famine effects on later-life baseline (2011) cognition after multiple imputations for missing data
| Cognition in 2011 | TICS |
Episodic memory |
Draw pentagons |
General cognition |
||||
|---|---|---|---|---|---|---|---|---|
| Coef. | P-value | Coef. | P-value | Coef. | P-value | Coef. | P-value | |
| Model 1 (reference cohort: 1963)a | ||||||||
| −0.007 | 0.571 | 0.004 | 0.624 | 0.000 | 0.937 | 0.000 | 0.997 | |
| (−0.030, 0.017) | (−0.013, 0.022) | (−0.004, 0.004) | (−0.035, 0.035) | |||||
| 0.010 | 0.464 | 0.013 | 0.203 | 0.002 | 0.334 | 0.032 | 0.108 | |
| (−0.017, 0.036) | (−0.007, 0.034) | (−0.003, 0.008) | (−0.007, 0.071) | |||||
| −0.026 | 0.090 | 0.007 | 0.529 | −0.002 | 0.320 | −0.019 | 0.444 | |
| (−0.056, 0.004) | (−0.015, 0.029) | (−0.007, 0.002) | (−0.067, 0.030) | |||||
| −0.036 | 0.002 | 0.000 | 0.971 | −0.003 | 0.072 | −0.033 | 0.084 | |
| (−0.058, −0.014) | (−0.022, 0.023) | (−0.007, 0.000) | (−0.071, 0.005) | |||||
| −0.006 | 0.580 | 0.005 | 0.596 | 0.001 | 0.507 | 0.002 | 0.888 | |
| (−0.026, 0.014) | (−0.014, 0.024) | (−0.002, 0.005) | (−0.032, 0.037) | |||||
| Model 2 (reference cohort: 1963)b | ||||||||
| −0.001 | 0.936 | 0.006 | 0.507 | 0.001 | 0.670 | 0.008 | 0.586 | |
| (−0.022, 0.020) | (−0.011, 0.022) | (−0.003, 0.005) | (−0.021, 0.037) | |||||
| 0.018 | 0.102 | 0.013 | 0.200 | 0.004 | 0.115 | 0.034 | 0.038 | |
| (−0.004, 0.039) | (−0.007, 0.032) | (−0.001, 0.009) | (0.002, 0.065) | |||||
| −0.017 | 0.208 | 0.011 | 0.287 | −0.001 | 0.584 | −0.005 | 0.804 | |
| (−0.043, 0.009) | (−0.009, 0.030) | (−0.005, 0.003) | (−0.044, 0.034) | |||||
| −0.027 | 0.017 | 0.001 | 0.948 | −0.002 | 0.293 | −0.025 | 0.178 | |
| (−0.049, −0.005) | (−0.022, 0.024) | (−0.006, 0.002) | (−0.062, 0.012) | |||||
| −0.007 | 0.457 | 0.001 | 0.898 | 0.001 | 0.500 | −0.005 | 0.779 | |
| (−0.026, 0.012) | (−0.018, 0.020) | (−0.002, 0.005) | (−0.039, 0.029) | |||||
Numbers in the parentheses are 95% confidence intervals.
Coef, coefficient; CSSI, mean-centred cohort size shrinkage index at the prefecture level.
aAdjusted for cohort, CSSI, gender, marital status, provincial fixed effects.
bModel 1 + additionally adjusted for education.
Similar to the pattern shown in Table 3, we found no firm evidence of any long-term famine effect on cognitive change over the 2-year follow-up after multiple imputations, except for the 1959 cohort (Appendix Table A1, available as Supplementary data at IJE online). In the 1959 cohort, partial-term prenatal exposure to famine and postnatal exposure from birth to age 2 years would reduce general cognition by 0.17 SD () on average per one SD increase in CSSI.
We then assessed the potential problem of famine-induced differential population attrition, by fitting the same models to the urban sample in CHARLS as we did to the rural sample. The rationale is that with a priori knowledge that the urban population experienced much lower mortality during the GLF famine, we may be able to infer the presence of both a negative cognitive effect of prenatal famine exposure from the urban sample (if we find negative results) and a positive mortality selection effect from the rural sample (if we find null or positive results).33 In the urban sample, the 1962 cohort showed lower baseline scores of TICS and general cognition (see Appendix Table A2, available as Supplementary data at IJE online) and both the 1960 and 1961 cohorts experienced a decline in episodic memory between the baseline and the follow-up (Table A3, available as Supplementary data at IJE online).
Discussion
The biological basis of cognition is amenable to interventions to the extent that a wide range of factors such as parenting styles, nutritional intake, exposure to environmental pollution, health behaviours and socioeconomic status have been linked to either cognitive development in early life or cognitive decline in later life, or both.34,35 These non-biological factors are modifiable, which in turn can induce cognitive change. For this study, malnutrition during the prenatal and early postnatal periods may disrupt the normal process of brain development by altering brain structure and neuronal connectivity.36–38 According to the life course model of health,39 the resulting negative impact on mental performance and cognition may have long latency periods before its manifestation in adulthood. Using the 1944–45 Dutch famine as a natural experiment, earlier studies of the life course consequences of prenatal nutritional deprivation with respect to neuropsychiatric outcomes focused on one specific disorder—schizophrenia.40–44 More recent studies have shifted attention onto subtle deficits in cognitive functioning in middle age,6,7 although they all found a lack of long-term associations for most of the cognitive measures being studied. We have contributed to the literature in several ways:
First, we turned to an alternative famine event that occurred in recent human history—China’s 1959–61 famine. As Susser and St Clair argued,17 we would be in a better position to strengthen the inference about long-term effects of famine exposure by comparing concordant or discordant results from different social and historical contexts. The longer duration of the GLF famine relative to the Dutch famine enables us to investigate famine exposure in not only the prenatal but also the postnatal periods. Second, unlike the two recent Dutch famine studies that relied on simple cohort comparisons, we additionally exploited the spatial variation in famine severity across about a third of China’s vast geography (= 118/347 prefectures) and employed a DID estimation strategy to better control for unobserved between-cohort differences due to non-famine factors.45,46 Third, unlike the two recent GLF famine studies that relied on non-representative samples,18,19 we improved the generalizability of our findings by: (i) drawing on a nationally representative sample of middle- and older-aged Chinese adults with a high response rate (80.5%), compared with military or regional samples with lower response rates (60% or below) in the Dutch famine studies; (ii) applying survey weights to adjust for individual non-response and longitudinal attrition; and (iii) conducting sensitivity checks regarding missing cognitive scores. Lastly, we examined the famine effects not only on later life cognition measured at one point of time but also on cognitive decline over a 2-year follow-up.
Using the 2011 baseline data, we found robust evidence of the scarring effects on multiple cognitive measures at about 50 years of age in the 1961 cohort, which experienced full-term prenatal and postnatal exposure to famine before age 1 year. The negative effects on two cognitive measures were no longer evident after controlling for education. This could be due to an endogenous selection bias, because recent studies have shown that prenatal exposure to the GLF famine reduced educational attainment,9,25, 47 which in turn diminished cognitive abilities at older ages.12–14 However, it is also possible that people with higher cognitive abilities are more likely to have higher levels of education. If this is the case, the estimates from Model 2 would be biased and we should refer to the estimates from Model 1 as the correct results. It is beyond the scope of the current study to pinpoint the specific mechanisms linking famine exposure to educational attainment. One study suggested that the negative impact of the GLF famine on education was driven by the loss of schooling opportunities rather than nutritional deprivation,21 whereas another study showed that the opposite was true.47 Nevertheless, the negative effect on TICS persisted for the 1961 cohort after controlling for education. This robust finding against the potential endogenous selection bias provides strong evidence of an early onset of cognitive deficits in middle age (50 years) caused by early life malnutrition.
We found no significant effects of famine exposure in the 1962 cohort. This result was reassuring, because the 1962 cohort experienced much less famine exposure during the prenatal period compared with the 1959–61 cohorts, and no exposure during the postnatal period. But how do we interpret the positive effects on certain cognitive measures in the 1959 cohort and the lack of effects in the 1960 cohort? One plausible explanation involves the possibility of mortality selection effect at work. Among the three famine cohorts studied here, the 1959 cohort had the worst famine experience, followed by the 1960 cohort. In order to be recruited into the CHARLS sample, members of the 1959 cohort had to survive severe malnutrition not only in utero but also throughout the first 2 years of their lives (1960–61), perhaps the most critical and yet vulnerable period of human development after birth.48 By contrast, members of the 1961 cohort only had to survive malnutrition in utero and during the first year of their lives for us to observe them. In other words, there might be a gradient of the overall effect of mortality selection—the 1959 cohort suffered the most and the 1961 suffered the least, with the 1960 cohort in between. As a result, the surviving 1959 cohort may consist of ‘the fittest of the fittest,’ followed by the surviving 1960 cohort. Our empirical estimate of the famine effect would reflect a mixture of a positive mortality selection effect and a negative cognition effect.45,49 We observe an overall positive effect of famine on cognition when mortality selection dominates the cognition-damaging effect (the 1959 cohort), an overall negative effect when the cognition-damaging effect outweighs mortality selection (the 1961 cohort) and lack of effect when the two forces are equivalent (the 1960 cohort). Similar dose-response patterns are also observed in other studies of the GLF famine. One study found an increased risk of developing schizophrenia in early adulthood, due to prenatal famine exposure, only in the urban population, but not in the rural population who suffered the more severe famine.16 Another study found the GLF famine impact on sex ratio at birth only evident in the urban population but not in the rural population.33
We found evidence of famine effect on decline in general cognition between 2011 and 2013 for the 1959 cohort as they grew from 52 to 54 years old. This finding is robust against controlling for education and multiple imputations for missing data. Despite having a higher general cognition at the baseline due to positive mortality selection, the 1959 cohort was subject to prolonged malnutrition during early life and likely impaired brain development. Being the oldest famine cohort, they were likely the first to experience the negative famine impact on longitudinal change in cognition as the early life deficit in cognitive reserve was aggravated by the ageing process.35 On the other hand, the lack of evidence for longitudinal change in other cognitive measures or in the other famine cohorts indicates that further research is warranted to assess the validity of the cognitive measures and the robustness of the results, as subsequent waves of CHARLS are fielded and richer longitudinal data become available over a longer period of time.
Similar to a handful of existing studies on the Dutch famine and the GLF famine, we found the estimate of the famine effect to be sensitive to the measure of cognition. Cognitive neuroscience is still young, and the exact mechanisms linking specific brain structure and its development to a particular cognitive ability remain uncertain. We can only speculate possible explanations about the measurement-dependent results. The TICS in our study captures both attention and time orientation. Previous famine studies have reported an increased risk of developing schizophrenia in adulthood for the individuals conceived during the famine period,16,41,50 implicating possible disruptive neurodevelopment in the prefrontal cortex.51 The prefrontal cortex is also involved in executive functions including, among others, selective attention. To the extent that TICS measures attentional control and it is a subset of MMSE, our finding of the negative famine effect on TICS confirmed previous findings on selective attention and MMSE from the Dutch famine and the GLF famine.6,18,19
The cognitive abilities involved in the tasks of word recall and drawing overlapped pentagons are verbal episodic memory and visuospatial skill, respectively. The medial temporal lobe, a structure composed of the hippocampus and the amygdala, is crucial in the formation of new episodic memory, and the occipital lobe is responsible for visual-cognitive processes including pattern recognition and visual mental imagery.51 Both the hippocampus and occipital lobe are known for their protracted postnatal development in childhood and adolescence, and thus are susceptible to structural alterations caused by environmental stress and stimulation until early adulthood.52 In fact, being implicated in episodic memory and spatial pattern separation, the dentate gyrus of the hippocampus is one of the few brain regions where neurogenesis occurs throughout life.53 These features imply the possibility of postnatal recovery of episodic memory and visuospatial skill from the negative influence of prenatal malnutrition, which also helps explain the consistent lack of evidence for these two types of cognitive abilities in the current and previous studies.
Unlike two famine studies that adopted a test of general intelligence designed for the general population, 6,19 our measure of general cognition is a composite index of cognitive tests designed to detect impairment. In a general population, our measure of general cognition perhaps has limited measurement sensitivity to fully capture heterogeneity in normative cognitive ageing.54 However, in famine studies where research participants suffered severe malnutrition in different stages of early life, our measure of general cognition may better discern between cognitive impairment and normal cognition. This could help explain why we found a negative famine effect on longitudinal change in general cognition, whereas others reported no effect on general intelligence.
Several study limitations are noteworthy. First, we do not have data to accurately measure individual-level prenatal exposure. Using CSSI as an ecological measure of famine severity at the prefecture level is a reasonable alternative, but may conceal important individual heterogeneity within the same region. Second, CSSI is estimated from government statistics that may be subject to falsification. Third, like other studies of China’s 1959–61 famine,9,23–25,46 we have difficulty demarcating the timing of in utero famine exposure due to the lack of reliable and accurate vital statistics for this period.17 Despite these limitations, our study has been among the first to investigate the long-term cognitive consequences of early life exposure to famine in a non-Western context. Continued efforts are required to fully understand the challenge of successful ageing among many older Chinese adults whose cognitive decline may have accelerated because of their frequent experiences of early life malnutrition during China’s troubled early 20th century.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development under a centre grant to the Population Studies Center, University of Michigan [R24 HD041028], and by the Lieberthal Rogel Center for Chinese Studies at the University of Michigan under a faculty research grant to H.X.
Conflict of interest: None declared.
Supplementary Material
References
- 1. Gottfredson LS. Mainstream science on intelligence:an editorial with 52 signatories, history, and bibliography. Intelligence 1997;24:13–23. [Google Scholar]
- 2. Blazer DG, Yaffe K, Liverman CT; Committee on the Public Health Dimensions of Cognitive Aging, Board on Health Sciences Policy, Institute of Medicine. Cognitive Aging: Progress in Understanding and Opportunities for Action. Washington, DC: National Academies Press, 2015. [PubMed] [Google Scholar]
- 3. Rowe JW, Kahn RL. Human aging:usual and successful. Science 1987;237:143–49. [DOI] [PubMed] [Google Scholar]
- 4. Bloom DE, Eggleston KN. The Economic Implications of Population Ageing in China and India: Introduction to the Special Issue. J Econ Ageing 2014;4:1–7. [Google Scholar]
- 5. Stein Z, Susser M, Saenger G, Marolla F. Prenatal exposure to the Dutch famine of 1944–1945 seems not related to mental performance at age 19. Nutr Ment Perform 1972;178:708–13. [Google Scholar]
- 6. de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci U S A 2010;107:16881–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Groot RHM, Stein AD, Jolles J, et al. Prenatal famine exposure and cognition at age 59 years. Int J Epidemiol 2011;40:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Factor-Litvak P, Susser E. A life course approach to neuropsychiatric outcomes. In: Kuh D, Ben-Shlomo Y (eds). A Life Course Approach to Chronic Disease Epidemiology. New York, NY: Oxford University Press, 2004. [Google Scholar]
- 9. Almond D, Edlund L, Li H, Zhang J. Long-term effects of early life development: evidence from the 1959 to 1961 China famine. In: Ito T, Rose A (eds). The Economic Consequences of Demographic Change in East Asia. Chicago, IL: University of Chicago Press, 2010. [Google Scholar]
- 10. Almond D. Is the 1918 influenza pandemic over? Long-term effects of in utero influenza exposure in the post-1940 U.S. population. J Polit Econ 2006;114:672–712. [Google Scholar]
- 11. Currie J. Healthy, wealthy, and wise: socioeconomic status, poor health in childhood, and human capital development. J Econ Lit 2009;47:87–122. [Google Scholar]
- 12. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–10. [PubMed] [Google Scholar]
- 13. Fors S, Lennartsson C, Lundberg O. Childhood living conditions, socioeconomic position in adulthood, and cognition in later life: exploring the associations. J Gerontol B Psychol Sci Soc Sci 2009;64B:750–57. [DOI] [PubMed] [Google Scholar]
- 14. Lei X, Smith JP, Sun X, Zhao Y. Gender differences in cognition in China and reasons for change over time: evidence from CHARLS. J Econ Ageing 2014;4:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kung JK-s, Lin Justin Y. The Causes of China’s Great Leap Famine, 1959–1961. Econ Dev Cultural Change 2003;52:51–73. [Google Scholar]
- 16. Song S, Wang W, Hu P. Famine, death, and madness: schizophrenia in early adulthood after prenatal exposure to the Chinese Great Leap Forward Famine. Soc Sci Med 2009;68:1315–21. [DOI] [PubMed] [Google Scholar]
- 17. Susser ES, St Clair D. Prenatal famine and adult mental illness: interpreting concordant and discordant results from the Dutch and Chinese Famines. Soc Sci Med 2013;97:325–30. [DOI] [PubMed] [Google Scholar]
- 18. Wang C, An Y, Yu H, et al. Association between exposure to the Chinese Famine in different stages of early life and decline in cognitive functioning in adulthood. Front Behav Neurosci 2016;10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Na L, Ma H, et al. Multigenerational effects of parental prenatal exposure to famine on adult offspring cognitive function. Sci Rep 2015;5:13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort Profile: The China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol 2014;43:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang W, Zhou Y. Effects of education on cognition at older ages: evidence from China's Great Famine. Soc Sci Med 2013;98:54–62. [DOI] [PubMed] [Google Scholar]
- 22. Lei X, Hu Y, McArdle JJ, Smith JP, Zhao Y. Gender differences in cognition among older adults in China. J Hum Human Resources 2012;47:951–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang C, Li Z, Wang M, Martorell R. Early life exposure to the 1959–1961 Chinese Famine has long-term health consequences. J Nutr 2010;140:1874–78. [DOI] [PubMed] [Google Scholar]
- 24. Huang C, Li Z, Venkat Narayan KM, Williamson DF, Martorell R. Bigger babies born to women survivors of the 1959–1961 Chinese Famine: a puzzle due to survival selection? J Dev Origins Health Dis 2010;1:412–18. [DOI] [PubMed] [Google Scholar]
- 25. Meng X, Qian N. The long term consequences of famine on survivors: evidence from a unique natural experiment using China's Great Famine. NBER Working Paper Series 2009;No. 14917. [Google Scholar]
- 26. Garnaut A. The Geography of the Great Leap Famine. Modern China 2014;40:315–48. [Google Scholar]
- 27. Cai Y, Wang F. Reproductive consequences of China's Great Leap Forward Famine. In: Kurosu S, Bengtsson T, Campbell C (eds). Demographic Responses to Economic and Environmental Crises. Kashiwa, China: Reitaku University Press, 2010. [Google Scholar]
- 28. Allison PD. Change scores as dependent variables in regression analysis. Sociol Methodol 1990;20:93–114. [Google Scholar]
- 29. Hannum E. Political change and the urban-rural gap in basic education in China, 1949–1990. Comp Educ Rev 1999;43:193–211. [Google Scholar]
- 30. Zhao Y, Strauss J, Yang G, et al. China Health and Retirement Longitudinal Study: 2011–2012 National Baseline Users' Guide. Beijing: Peking University, 2013. [Google Scholar]
- 31. China Center for Economic Research. China Health and Retirement Longitudinal Study Followup 2013 Release Note. Beijing: Peking University, 2015. [Google Scholar]
- 32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, 1987. [Google Scholar]
- 33. Song S. Malnutrition, sex ratio, and selection. Hum Nat 2014;25:580–95. [DOI] [PubMed] [Google Scholar]
- 34. Nisbett RE. Intelligence and How to Get It:Why Schools and Cultures Count. New York, NY: W.W. Norton, 2009. [Google Scholar]
- 35. Haan MN, Wallace R. Can dementia be prevented? brain aging in a population-based context. Annu Rev Public Health 2004;25:1–24. [DOI] [PubMed] [Google Scholar]
- 36. Rubin LP. Historical perspective of developmental origins of health and disease in humans. In: Rosenfeld CS. The Epigenome and Developmental Origins of Health and Disease. Boston, MA: Academic Press, 2016. [Google Scholar]
- 37. Georgieff MK. Nutrition and the developing brain:nutrient priorities and measurement. Am J Clin Nutr 2007;85:614–20S. [DOI] [PubMed] [Google Scholar]
- 38. Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev 2014;72:267–84. [DOI] [PubMed] [Google Scholar]
- 39. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 2002;31:285–93. [PubMed] [Google Scholar]
- 40. Hoek HW, Susser E, Buck KA, Lumey LH, Lin SP, Gorman JM. Schizoid personality disorder after prenatal exposure to famine. Am J Psychiatry 1996;153:1637–39. [DOI] [PubMed] [Google Scholar]
- 41. Susser ES, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: the story of the Dutch Famine Study. Am J Epidemiol 1998;147:213–16. [DOI] [PubMed] [Google Scholar]
- 42. Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Arch Gen Psychiatry 1992;49:983–88. [DOI] [PubMed] [Google Scholar]
- 43. Susser ES, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine: further evidence. Arch Gen Psychiatry 1996;53:25–31. [DOI] [PubMed] [Google Scholar]
- 44. Hoek HW, Brown AS, Susser ES. The Dutch famine and schizophrenia spectrum disorders. Soc Psychiatr Epidemiol 1998;33:373–79. [DOI] [PubMed] [Google Scholar]
- 45. Xu H, Li L, Zhang Z, Liu J. Is natural experiment a cure? Re-examining the long-term health effects of China's 1959–1961 Famine. Soc SciMed 2016;148:110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Y, Zhou L-A. The long-term health and economic consequences of the 1959–1961 famine in China. J Health Econ 2007;26:659–81. [DOI] [PubMed] [Google Scholar]
- 47. Shi X. Famine, fertility, and fortune in China. China Econ Rev 2011;22:244–59. [Google Scholar]
- 48. Lynch J, Davey Smith G. A life course approach to chronic disease epidemiology. Annu Rev Public Health 2005;26:1–35. [DOI] [PubMed] [Google Scholar]
- 49. Song S. Mortality consequences of the 1959–1961 Great Leap Forward Famine in China: debilitation, selection, and mortality crossovers. Soc Sci Med 2010;71:551–58. [DOI] [PubMed] [Google Scholar]
- 50. St Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese Famine of 1959–1961. JAMA 2005;294:557–62. [DOI] [PubMed] [Google Scholar]
- 51. Hanson J, Hackman DA. Cognitive neuroscience and disparities in socioeconomic status. In: Wolfe B, Evans W, Seeman TE (eds). The Biological Consequences of Socioeconomic Inequalities. New York, NY: Russell Sage Foundation, 2012. [Google Scholar]
- 52. Hanson J, Hair N, Chandra A, et al. Brain development and poverty: a first look. In: Wolfe B, Evans W, Seeman TE (eds). The Biological Consequences of Socioeconomic Inequalities. New York, NY: Russell Sage Foundation, 2012. [Google Scholar]
- 53. Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009;325:210–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carlson MC, Seplaki CL, Seeman TE. Reversing the impact of disparities in socioeconomic status over the life course on cognitive and brain aging. In: Wolfe B, Evans W, Seeman TE (eds). The Biological Consequences of Socioeconomic Inequalities. New York, NY: Russell Sage Foundation, 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.