Abstract
Aims
Increased spatial angle between QRS complex and T wave loop orientations has repeatedly been shown to predict cardiac risk. However, there is no consensus on the methods for the calculation of the angle. This study compared the reproducibility and predictive power of three most common ways of QRS-T angle assessment.
Methods and results
Electrocardiograms of 352 healthy subjects, 941 survivors of acute myocardial infarction (MI), and 605 patients recorded prior to the implantation of automatic defibrillator [implantable cardioverter defibrillator (ICD)] were used to obtain QRS-T angle measurements by the maximum R to T (MRT), area R to T (ART), and total cosine R to T (TCRT) methods. The results were compared in terms of physiologic reproducibility and power to predict mortality in the cardiac patients during 5-year follow-up. Maximum R to T results were significantly less reproducible compared to the other two methods. Among both survivors of acute MI and ICD recipients, TCRT method was statistically significantly more powerful in predicting mortality during follow-up. Among the acute MI survivors, increased spatial QRS-T angle (TCRT assessment) was particularly powerful in predicting sudden cardiac death with the area under the receiver operator characteristic of 78% (90% confidence interval 63–90%). Among the ICD recipients, TCRT also predicted mortality significantly among patients with prolonged QRS complex duration when the spatial orientation of the QRS complex is poorly defined.
Conclusion
The TCRT method for the assessment of spatial QRS-T angle appears to offer important advantages in comparison to other methods of measurement. This approach should be included in future clinical studies of the QRS-T angle. The TCRT method might also be a reasonable candidate for the standardization of the QRS-T angle assessment.
Keywords: QRS-T angle, Total cosine R to T, Reproducibility, Mortality risk prediction, Standardization
What’s new?
There are substantial differences between the QRS-T spatial angle calculations by the maximum vector, QRS-T area, and integrative methods.
Of these calculation possibilities, the maximum vector method provides least stable results.
In cardiac patients, the integrative method provides, in comparison to the other methods, statistically superior risk predictors.
An increased QRS-T angle appears to powerfully predict sudden cardiac death in survivors of acute myocardial infarction.
Introduction
The topic of cardiac risk assessment has been the subject of numerous studies. The low accuracy of univariable patient stratification approaches, such as those using left ventricular ejection fraction (LVEF), led to the understanding that multivariable combination of different risk indicators needs to be pursued. This highlights the value of risk factors that are obtainable easily at little additional costs.
One of the simplest of such risk indicators is the angle between the QRS complex and the T wave spatial orientations. The seminal study showing the usefulness of the QRS-T angle in risk assessment after myocardial infarction (MI) was published by Zabel et al.1 in 2000. Since then, the value of the QRS-T angle has been repeatedly shown in different populations, including not only cardiac patients2 but also general population3,4 and patients with other primary diagnoses.5,6
Still direct comparisons of published studies are potentially intricate since different authors use different methods for the calculation of the angle in digital electrocardiographic (ECG) recordings. The notion that both QRS complex and T wave loops have a well-defined spatial orientation is too simplistic5 and different methods may provide different QRS-T angle results especially in abnormal ECGs.
We have therefore compared three most frequently used methods for QRS-T angle calculations in three different datasets: ECGs of normal subjects were used to assess physiologic variability of the results and the reproducibility of the measurements in normal recordings, ECGs of acute MI survivors were used to assess the predictive value of the QRS-T angle during the post-MI follow-up, and ECGs in recipients of implantable cardioverter defibrillator (ICD) were used to assess the predictive strength of the results in a high-risk population with frequently very abnormal ECGs.
Methods
Populations
As stated, three distinct populations and related ECG dataset were investigated. Clinical characteristics of the populations are described in Table 1.
Table 1.
Demographic description of the investigated populations
| Healthy subjects | MI survivors | ICD recipients | |
|---|---|---|---|
| N | 352 | 941 | 605 |
| Females (%) | 50.0 | 19.3 | 17.7 |
| Age (years) | 32.7 ± 9.1 | 59.9 ± 11.5 | 65.4 ± 11.4 |
| Ischemic HD (%) | 100 | 68.4 | |
| LVEF (%) | 52.1 ± 12.2 | 28.3 ± 9.8 | |
| Primary ICD indication (%) | 72.2 |
Numerical values shown as mean ± standard deviation, proportions of the populations shown in percent.
HD, heart disease; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MI, myocardial infarction; N, number of subjects.
Population of healthy subjects involved, as previously published,6 176 female and 176 male healthy subjects (mean age 32.7 ± 9.1 years). In each subject, 4 repeated 12-lead Holter recordings were obtained off any medication. During 10 min repeated windows within each recording, the subjects were placed in strict motionless supine positions to stabilize heart rates. The recordings were obtained using SEER MC 2 recorders by GE Healthcare (Milwaukee, WI, USA) using Mason–Likar electrode configurations.
Population of survivors of acute MI was composed of the 941 consecutive patients (19.3% females, mean age 59.9 ± 11.5 years, mean LVEF 52.1 ± 12.2%) enrolled into the Autonomic Regulation Trial conducted at the German Heart Center and the Klinikum rechts der Isar, both in Munich, Germany. As previously described,7,8 within the first 2 weeks after the index MI, all the patients underwent 30 min supine recordings that included 3-lead orthogonal ECG signal acquisition using recorders by TMS international (Enschede, The Netherlands). Following the index MI, the patients were followed and 5-year follow-up data were available for this study. During the follow-up, all deaths were classified as non-cardiac, cardiac, and sudden cardiac based on standard criteria.7,8
Finally, the population of ICD recipients included 605 patients (17.7% females, mean age 65.4 ± 11.4 years, mean LVEF 28.3 ± 9.8%, 68.4% ischemic heart disease, 72.2% primary ICD indication) of the ICD recipients registry compiled at University Medical Center Göttingen, Germany. All the patients had an ICD implanted for guideline recommended indications.9 As previously described,10 the analysed population included all the patients from the registry who had technically suitable ECG recording obtained prior to the ICD implantation. These 12-lead electronic ECG recordings were obtained using electrocardiograph by Schiller Inc. (Baar, Switzerland). Following the ICD implantation, patients were followed and 5-year follow-up data of all-cause mortality were available for this study.
QRS-T angle measurements
The vast majority of previous studies used three different methods for spatial QRS-T angle calculation.
The maximum vector R to T angle (MRT) calculation is based on the notion that QRS and T wave orientations are defined by the maxima of the vector magnitudes within the corresponding orthogonal loops.11 That is, orthogonal XYZ system of ECG leads is either directly recorded or obtained by suitable transformation from standard ECG leads. Vector magnitude of the XYZ system is calculated (i.e. the signal obtained) and within the QRS complex (between the Q onset and the J point) and within the T wave (between the J point and the T offset), time instances of maximal value of the orthogonal vector magnitude are identified. Each of these instances corresponds to X, Y, and Z lead values which are considered to represent a vector of QRS complex and T wave spatial orientation. The spatial angle is measured between these three-dimensional (3D) vectors.
The area-based R to T angle (ART) method uses the integrals of the orthogonal ECG leads. That is, as with the MRT method, orthogonal XYZ system of ECG leads is obtained. The integral of the signal in each of the three orthogonal leads is measured between the Q onset and the J point.11 These integrals define the QRS integral vector in the 3D space. Likewise, the integrals of the orthogonal signals between the J point and the T offset define the T wave integral vector. The spatial angle is measured between these 3D vectors.
The total cosine R to T (TCRT) is based on singular value decomposition providing orthogonal lead system in which the T wave vector is defined by its maximal vector magnitude. Cosines of the angles between this T wave vector and all vectors within the QRS complex exceeding a pre-defined proportion of the maximum vector magnitude are averaged. The method was designed12 to deal with the problem that while T wave can be considered to have an underlying spatial orientation, the QRS loops are much wider and spatially curved so that no single vector can represent their orientation.5 The numerical settings of the method were obtained from the original design12 (the pre-defined proportion of maximum QRS spatial vector magnitude set at 70%).
Electrocardiographic processing
All measurements were made in ECG representative median beats sampled at 1 kHz. By its design, the construction of these beats eliminated all abnormal morphologies of the QRS-T patterns (e.g. those of ventricular premature beats). The recordings of the MI survivors were initially samples at 1.6 kHz; cubic spline down-sampling to 1 kHz of these recordings was used.
In the ECGs of the healthy population, all ECG segments obtained during episodes of stable supine positions were considered and in each episode, five different 10 s segments were selected that were least noise polluted and their representative median beats were constructed. In these, Q onset, J point and T offset were obtained with careful visual verification and manual adjustment using previously described procedures.13 For the purposes of applying the MRT and ART methods for QRS-T angle assessment, the orthogonal ECG leads were obtained using the transformation proposed by Guldenring et al.14 suitable for the conversion of Mason–Likar recordings. The TCRT method was applied to the original representative beats.
In the population of acute MI survivors, a 30 s window was used starting 7.5 min after the beginning of the recordings (i.e. after one-fourth of the complete 30 min recording) in order to ensure stable recording conditions. In this window, all QRS complexes were identified and median representative beat constructed. In these, Q onset, J point, and T offset were measured under visual control with manual adjustments where necessary. Since the original recordings were composed of orthogonal leads, MRT and ART methods were applied to the representative beats without any transformation. Total cosine R to T method was also applied to these representative beats.
In the population of ICD recipients, original 10 s 12-lead recordings were used to detect all QRS complexes and to construct representative median beats as previously described.10 In these, Q onset, J point, and T offset were measured under visual control with manual adjustments where necessary. To apply MRT and ART methods, the 12-lead representative signals were transformed to orthogonal leads using the inverse Dower matrix.15 TCRT method was applied to the original representative beats.10
QRS-T angle evaluations
The QRS-T angle measurements in the healthy subjects were used to evaluate reproducibility of the measurements under physiologic conditions. Since the angle depends on heart rate16 intra-subject heart rate differences had to be eliminated. For this purpose, the median heart rate of all measured ECG segments was evaluated in each subject and all ECG segments in the same subject that differed in their heart rate by ±1 beat per minute (bpm) from the median heart rate were selected. The MRT, ART, and TCRT measurements in these ECGs were used to calculate their mean (as the characterization of the subject) and their standard deviation (as the characterization of the reproducibility in the subject). The same procedure was repeated for ECG segments that differed in their heart rate by ± 2.5 and ± 5 bpm from the median heart rate of the given subject.
The populations of acute MI survivors and of ICD recipients were used to compare the predictive value of the QRS-T angles obtained by the MRT, ART, and TCRT methods. For these purposes, deaths during the 5-year follow-ups of these populations were used. In the population of MI survivors, the sub-classification of deaths cases to cardiac mortality and to sudden cardiac deaths was also used.
Statistics and data presentation
All the QRS-T angle values are shown in degrees ranging between 0 and 180. Their and other continuous variables are shown as mean ± standard deviation. Numerical comparison of different measurements of the QRS-T angles was based on Bland–Altman-type comparisons. In addition, Spearman rank correlations between the different measurements were calculated in each of the investigated populations.
In the population of healthy subjects, the mean values of QRS-T angles between female and male subjects were compared using two-sample two-tail t-test assuming different variances. The standard deviations of intra-subject QRS-T angle measurements obtained by different methods were compared using two-tail Wilcoxon test. These paired comparisons were made for the complete population as well as for sub-populations of female and male subjects separately.
In the populations of acute MI survivors and of ICD recipients, the QRS-T angle values were compared between those who did and did not die during follow-up using two-sample two-tail t-test assuming different variances. Receiver operator characteristics (ROC) for the prediction of mortality during follow-up were constructed together with their 90% confidence bands using bootstrap with 1000 repetitions. The tertiles of each population were constructed based on the QRS-T angle values derived by different methods and the probabilities of death during follow-up in these tertiles were portrayed using the Kaplan–Meier method. The probabilities of death were compared using the long-rank test for the comparison of two cases and by the χ2 test for the comparison of three cases. Finally, in both patient populations, multivariable Cox regression model was used for the prediction of mortality during follow-up based on continuous measurements of the QRS-T angle obtained by the MRT, ART, and TCRT methods.
In the population of acute MI survivors, these tests were also repeated for the prediction of cardiac death and of sudden cardiac death.
Since the median QRS width of the population of ICD recipients was 120 ms,10 the prediction of mortality in this population was also repeated for the subpopulation of patients with QRS width above 120 ms. As the patients with prolonged QRS complex are at greater risk, the two tertiles with the largest QRS-T angles were pooled for this sub-analysis.
Statistical tests were performed using the Statistica 6.1 package by StatSoft, Inc. (Tulsa, OK, USA).
Results
Valid QRS-T angle measurements by all three methods were obtained in all healthy subjects and in all ICD recipients. In the population of acute MI survivors, the assessment failed in five patients in whom the ECG recordings were not suitable for the calculation because of missing signal in one of the orthogonal ECG leads. For this population, results derived from the remaining 936 patients are shown.
Data distribution
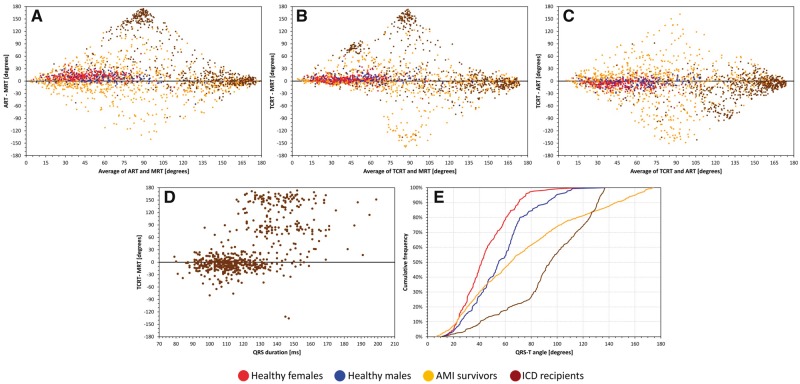
Figure 1A–C shows Bland–Altman-type comparisons between the different calculations of the QRS-T angle. Very substantial differences can be seen, particularly between the MRT and the other two methods applied to the abnormal ECGs of the cardiac patients. Even in the healthy subjects (in whom the representative per-subject values were obtained on averages of multiple measurements) the methods are not consistent with differences of up to 30°. Figure 1D uses the population of ICD recipients to show that, as expected, substantial differences between the methods appear mainly for ECGs with prolonged QRS duration in which the definition of the QRS loop orientation fails if based on a single vector.
Figure 1.
Comparisons of QRT-T angle measurements. Panels A–C show the differences between the different methods for QRS-T angle calculation obtained for individual subjects and patients (see the text for details). Panel D shows the relationship of the differences between the TCRT and MRT methods in dependency on the QRS complex duration which is used as a surrogate of QRS loop abnormality. Panel E shows the cumulative distributions of the TCRT assessment of the QRS-T angle in different populations. Note that in panels A–C, the trapezoidal shape of the graphs is determined by the fixed range of all measurements between 0 and 180° (e.g. the maximum difference of 180° between two measurements can only exist if one measurement is 0 and the other 180°). The red, blue, yellow, and brown marks and lines correspond to healthy females, healthy males, survivors of acute myocardial infarction, and recipients of implantable defibrillators (ICD), respectively. (Note that panel D shows the data for the ICD recipients.) MRT, maximum R to T; ART, area R to T; TCRT, total cosine R to T; ICD, implantable cardioverter defibrillators (see the text for explanations).
Figure 1E shows the population distributions of the TCRT results. The figure shows the previously described difference between healthy female and male subjects.16 It also demonstrates that in diseased hearts, the angle is getting wider—in particular in the ICD recipients in whom the abnormalities of cardiac electrophysiology are more pronounced.
In healthy subjects, the Spearman rank correlations between the MRT and ART, MRT and TCRT, and ART and TCRT measurements were 0.900, 0.907, and 0.897, respectively. In the population of MI survivors, the corresponding correlations were 0.711, 0.665, and 0.524, respectively. In the ICD recipients, the corresponding values were 0.119, 0.268, and 0.628, respectively.
Reproducibility of measurements in healthy subjects
The MRT, ART, and TCRT values of the QRS-T angle were systematically and statistically significantly smaller in females compared to males: MRT: 39.9 ± 19.8 vs. 51.2 ± 25.2°—P < 0.0001; ART: 52.4 ± 19.6 vs. 59.7 ± 21.9°—P < 0.002; and TCRT: 44.8 ± 18.7 vs. 56.7 ± 23.9°—P < 0.0001.
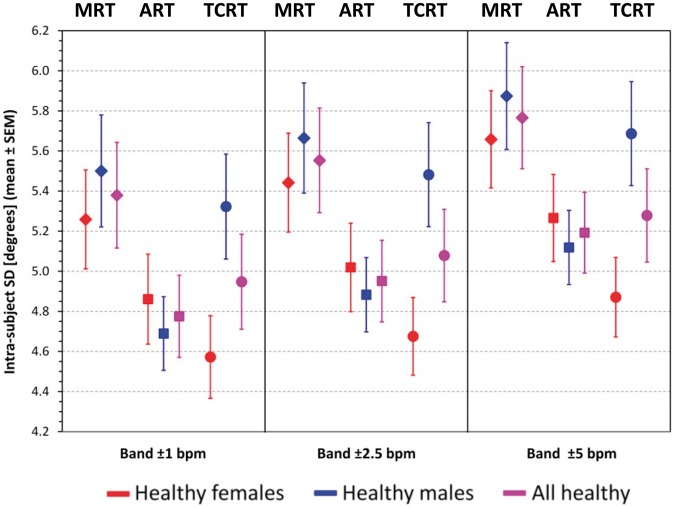
Figure 2 shows that the intra-subject standard deviations of all the methods were around 5°. In all cases, the standard deviations of MRT were larger than those of ART and TCRT (P < 0.0001 in all cases). Somewhat surprisingly, while the TCRT standard deviations were smaller than those of ART in female subjects, the opposite was the case in male subjects. In the total population, there was no statistically significant difference between the standard deviations of ART and TCRT in any of the heart rate windows.
Figure 2.
Reproducibility of QRT-T angle measurements in healthy subjects. Intra-subject standard deviations of repeated QRS-T angle measurements in healthy subjects repeated for different heart rate bands around intra-subject heart rate medians (see the text for details). The graph shows population means ± standard errors of means. The diamonds, squares, and circles correspond to maximum R to T, area R to T, and total cosine R to T measurements, respectively. The red, blue, and violet marks correspond to females, males, and the complete pooled population of healthy subjects, respectively.
Risk prediction among survivors of acute myocardial infarction
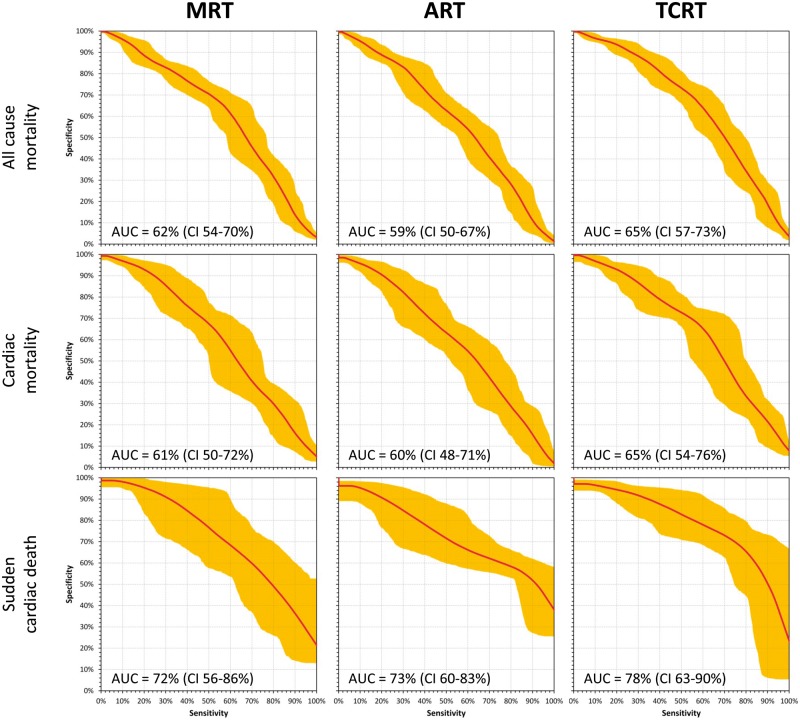
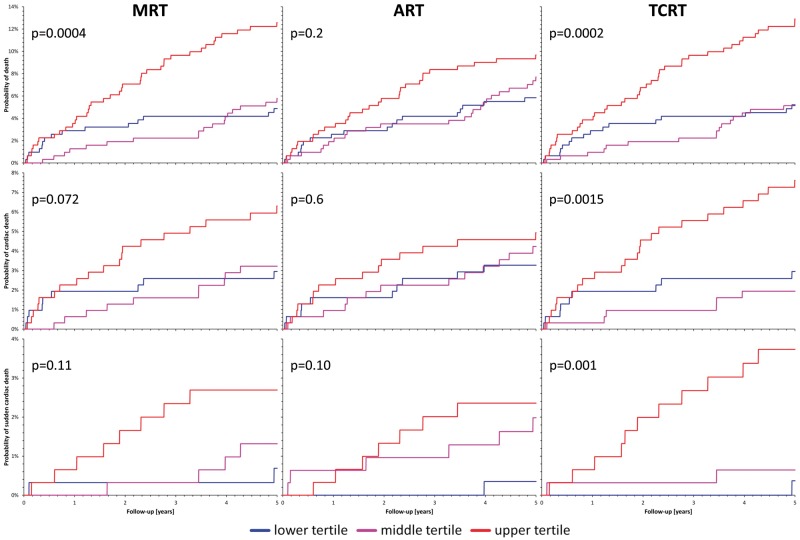
The results of the MRT, ART, and TCRT comparisons in prediction of all-cause mortality among the population of acute MI survivors are summarized in Table 2, top part. For all the QRS-T angle expressions, there were significant differences between the values in follow-up survivors and non-survivors with the statistically strongest separation of the TCRT values. Total cosine R to T values also led to the largest area under the ROC curve (Figure 3, top row) and to the statistically strongest separation of the probabilities of death between the tertiles of patients with the largest, middle, and lowest QRS-T values (Figure 4, top row). Of the three expressions of QRS-T angle, TCRT was also the only one that remained statistically significant in the Cox regression model involving all three expressions.
Table 2.
QRS-T angle measurements in MI survivors
| MRT | ART | TCRT | ||
|---|---|---|---|---|
| ACM (N = 72/864) | Event + (°) | 96.6 ± 53.2 | 89.9 ± 50.4 | 97.0 ± 50.1 |
| Event − (°) | 74.5 ± 49.6 | 75.9 ± 45.4 | 71.3 ± 43.8 | |
| Comparison P | 0.001 | 0.02 | 0.00003 | |
| ROC AUC (%) | 62.0 (53.7–70.0) | 58.7 (50.4–67.0) | 65.2 (57.2–73.1) | |
| Cox model P | 0.5 | 0.6 | 0.004 | |
| CM (N = 38/898) | Event + (°) | 95.4 ± 55.9 | 90.2 ± 50.9 | 96.6 ± 49.8 |
| Event − (°) | 75.4 ± 49.8 | 76.4 ± 45.7 | 72.3 ± 44.4 | |
| Comparison P | 0.03 | 0.08 | 0.003 | |
| ROC AUC (%) | 61.4 (50.1–72.0) | 59.6 (48.1–70.6) | 65.3 (54.3–75.5) | |
| Cox model P | 0.8 | 0.9 | 0.03 | |
| SCD (N = 14/922) | Event + (°) | 110.2 ± 53.9 | 107.4 ± 38.8 | 116.0 ± 44.0 |
| Event − (°) | 75.7 ± 50.0 | 76.5 ± 45.9 | 72.6 ± 44.6 | |
| Comparison P | 0.01 | 0.008 | 0.001 | |
| ROC AUC [%] | 72.1 (55.7–86.3) | 72.7 (60.5–83.2) | 78.3 (63.4–89.6) | |
| Cox model P | 0.7 | 0.6 | 0.03 |
For each follow-up event type (ACM, all-cause mortality; CM, cardiac mortality; SCD, sudden cardiac death), the association of different measurements of QRS-T angle is shown. N shows number of patient with follow-up events/without events. Event+ and Event− show the measurements in patients with and without events respectively (mean ± standard deviation, in degrees). Comparison P—the result of statistical comparison of the values in patients with and without events. ROC AUC—area under the receiver operator characteristics for the prediction of the follow-up events (median value, 90% confidence interval in brackets, in percent). Cox model P—statistical significance in a multivariable Cox regression model predicting the follow-up event using all three measurements of the QRS-T angle.
MRT, maximum R to T; ART, area R to T; TCRT, total cosine R to T.
Figure 3.
Prediction of outcome in survivors of acute myocardial infarction. The figure shows receiver operator characteristics for the prediction of outcome events among survivors of acute myocardial infarction based on increased values of the spatial QRS-T angle. The graphs show the median curvatures and their 90% confidence bands obtained by bootstrap (see the text for details). The top, middle, and bottom row correspond to the prediction of all-cause mortality, cardiac death, and sudden cardiac death, respectively. The left, middle, and right column correspond to the prediction based on the maximum R to T (MRT), area R to T (ART), and total cosine R to T (TCRT) calculations, respectively. Numerical values of the areas under the characteristics (AUC) and of their 90% confidence intervals (CI) are shown in each panel (see also Table 2).
Figure 4.
Probabilities of follow-up events among survivors of acute myocardial infarction. The figure shows Kaplan–Meier curves of the probabilities of follow-up events among survivors of acute myocardial infarction stratified according to the spatial QRS-T angle. For each graph, the P-value of the χ2 test comparison of the probabilities is also shown. The blue, violet, and red curves correspond to patients with QRS-T angles within the smallest, middle, and largest tertile of the measurements, respectively. The top, middle, and bottom row correspond to the prediction of all-cause mortality, cardiac death, and sudden cardiac death, respectively. The left, middle, and right column correspond to the prediction based on the maximum R to T (MRT), area R to T (ART), and total cosine R to T (TCRT) calculations, respectively.
Similar results were also obtained for the prediction of cardiac mortality (Table 2, middle part, and middle rows of Figures 3 and 4). Total cosine R to T was again more powerful than the other methods and the ART measurements did not separate the survivors and non-survivors with statistical significance. Total cosine R to T again outperformed the other methods in the multivariable Cox regression analysis.
The prediction of sudden cardiac death in this population was remarkably strong based on the QRS-T angle, in particular when measured by the TCRT method (Table 2, bottom part, and bottom rows of Figures 3and4). Although the number of sudden death cases was not large, of the 14 cases, 11 appeared among patients with the largest QRS-T angle by the TCRT method (right bottom panel of Figure 4). Note also the substantial area under the ROC curve for sudden cardiac death prediction by TCRT (Table 2, bottom part, and Figure 3, right bottom panel). Similar to all-cause and cardiac mortality prediction, TCRT outperformed the MRT and ART methods in the multivariable Cox regression analysis.
Risk prediction among recipients of implantable defibrillators
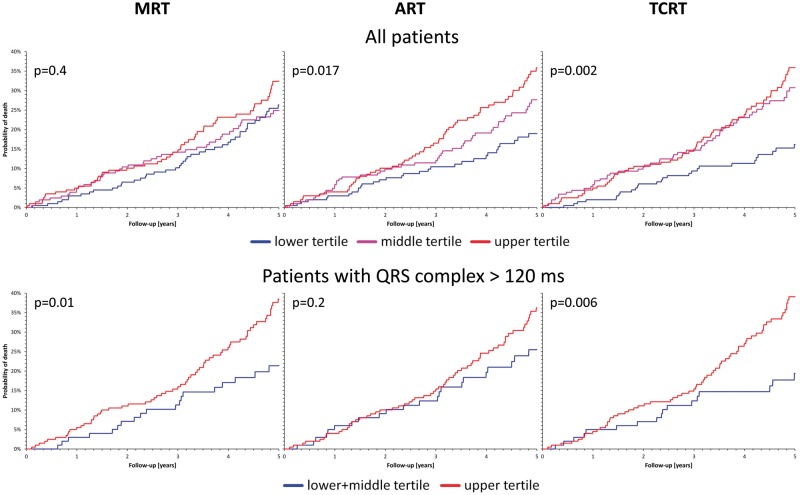
Analogous results were obtained when comparing the power of the different methods of QRS-T angle calculation to predict death among the ICD recipients (Table 3 and Figure 5). Total cosine R to T calculation led to consistent separation of patients at lower and higher mortality risk in both the total population and the sub-population of patients with QRS complex above 120 ms. In both analyses, TCRT also outperformed the MRT and ART methods in the multivariable Cox regression analysis although, as seen in the areas under the ROC curves, the distribution of the QRS-T angles (by all three methods) was bimodal among patients with prolonged QRS complex.
Table 3.
QRS-T angle measurements in implanted defibrillator recipients
| MRT | ART | TCRT | ||
|---|---|---|---|---|
| All patients (N = 137/468) | Event + (°) | 95.2 ± 64.0 | 148.7 ± 30.6 | 135.8 ± 32.4 |
| Event − (°) | 89.8 ± 62.9 | 138.7 ± 35.5 | 121.9 ± 41.5 | |
| Comparison P | 0.3 | 0.0006 | 0.003 | |
| ROC AUC (%) | 72.1 (55.7–86.3) | 72.7 (60.5–83.2) | 78.3 (63.4–89.6) | |
| Cox model P | 0.7 | 0.6 | 0.04 | |
| Patients with QRS > 120 ms (N = 82/218) | Event + (°) | 72.5 ± 66.9 | 153.1 ± 29.4 | 141.1 ± 29.4 |
| Event − (°) | 55.8 ± 61.8 | 150.0 ± 28.0 | 128.1 ± 40.0 | |
| Comparison P | 0.04 | 0.4 | 0.007 | |
| ROC AUC (%) | 59.6 (49.4–69.5) | 56.7 (46.8–66.8) | 56.7 (47.3–66.0) | |
| Cox model P | 0.07 | 0.6 | 0.02 |
The association of different measurements of QRS-T angle with follow-up all-cause mortality is shown. The top part shows the results of all patients of the population, the bottom part the results of sub-population of patients with QRS duration exceeding 120 ms. See the legend of Table2 for further explanations.
MRT, maximum R to T; ART, area R to T; TCRT, total cosine R to T.
Figure 5.
Probabilities of death during follow-up of ICD recipients. Kaplan–Meier curves show the probability of death during follow-up among the recipients of implantable defibrillators stratified according to the spatial QRS-T angle. For each graph, the P-value of the χ2 (top row) and log-rank (bottom row) test comparison of the probabilities is also shown. The top and bottom rows correspond to the complete population and to patients with QRS complex width > 120 ms. In the top row, the blue, violet, and red curves correspond to patients with QRS-T angles within the smallest, middle, and largest tertile of the measurements. In the bottom row, the blue and red curves correspond to patients with the QRS-T angles within the smallest and the two larger tertiles of the measurements, respectively. The left, middle, and right column correspond to the prediction based on the maximum R to T (MRT), area R to T (ART), and total cosine R to T (TCRT) calculations, respectively.
Discussion
The results of these analyses suggest that while the spatial QRS-T angle is a powerful and very simply obtainable indicator of cardiac risk, its future studies and clinical utility need to consider substantial differences between different methods of calculation. In physiologic recordings of healthy individuals, we have not observed very substantial differences since although the MRT method proved to be statistically significantly less reproducible compared to the other possibilities, the numerical differences of the intra-subject reproducibility were small. The comparisons of the numerical values shown in Figure 1 as well the Spearman correlation coefficients show that the more abnormal the ECGs, the larger the differences between the different calculation methods. The comparison of the correlation coefficients also shows that the TCRT method is approximately between the MRT and ART methods.
Nevertheless, in the tests of predictive power, the integrative principle of the TCRT method was found much more reliable and statistically more powerful compared to the other possibilities. This is not surprising since in ECG influenced by cardiac abnormalities, the vectorcardiographic loops are more complex and their spatial direction less clearly defined. This is particularly the case of the QRS complex loops which, even without obvious QRS complex prolongation, is highly influenced by intraventricular conduction abnormalities. Thus integrating over all possible directions of the QRS complex loop leads to improved clinical risk prediction assessment.
The analyses presented here were designed retrospectively albeit without influence of interim result. The results obtained with the two independent and substantially different populations of cardiac patients are consistent and thus support the observation of the superiority of the integrative TCRT approach. Still, similar tests are desirable in other clinical populations. Thus at present, a firm suggestion should be made that this integrative approach is included in future investigations of the phenomenon of the QRS-T angle. If the TCRT superiority is further confirmed, the method would offer an approach to standardization of future studies and of clinical use.
The study in healthy subjects also suggests that the measurement of the QRS-T angle is subject to biological variability and imprecision. The intra-subject standard deviations suggest that inaccuracies of up to 10° need to be expected. Obviously, this does not decrease the predictive value of the measurement since in the populations of cardiac patients, we have found larger differences between patients with and without follow-up events.
The very strong relationship between increased QRS-T angle and sudden cardiac death that we observed in the population of acute MI survivors deserves further investigation and validation in independent data. Among others, it needs to be seen in the context of previous report10 that showed that in the population of ICD recipients, there were no TCRT differences between patients who did and did not experience an appropriate ICD shock during follow-up (124.1 ± 44.6 vs. 125.3 ± 39.0°). There are several possible reasons for this possible paradox. Firstly, the differences between risk assessment shortly and late after index MI might be argued.17 Nevertheless, this is not very likely since as seen in Figure 4 (bottom row) most of the sudden death cases occurred at times remote from the index MI. It is more possible that in patients with frequent intracardiac conduction abnormalities (which was the case of our ICD recipient population), substantially increased QRS-T angle (compare the values in Tables 2and3) signifies considerable risk of fatal events that even the defibrillator cannot prevent. It is also likely that appropriate ICD shocks are only a very approximate surrogate of sudden cardiac death since not all terminated tachyarrhythmias would be fatal without the device intervention.
As far as the comparisons between the different calculations of QRS-T angle, there is little in the literature to which our results can be compared. Apart from that, our data are consistent with previous publications of differences between females and males16 and confirm previous repeated reports of the risk assessment value associated with increased QRS-T angles. Since the population of ICD recipients was on greater cardiac risk compared to the acute MI survivors (compare Figures 4and5), the validity of our data is also confirmed by the substantial angle differences in these populations (compare Tables 2and3).
Limitations
Several limitations of our investigation need to be considered. We have involved only three most frequently used methods for QRS-T angle calculation omitting, among others, the maximum amplitude method18 and the 12-lead approximation.19 These other methods have not been systematically used in previous risk stratification studies and do not necessarily reflect the orientation of the QRS and T wave loops accurately.20 For the purposes of evaluating the MRT and ART methods, the ECG recordings of healthy subjects and of the ICD recipients were converted into orthogonal lead systems using previously published conversion matrices. In individual cases, these conversions might reflect the true orthogonal Frank leads only approximately.20 Nevertheless, no such conversion was used with the TCRT method and compared to Frank orthogonal system, 12-lead ECGs are more likely to be used in clinical practice. Considering the number of possible conversions to reconstruct orthogonal lead systems,21,22 we have also experimented with other conversion possibilities and obtained the same principal results for the comparisons of the methods (data not shown). Consistency of the results independent of the particular conversion equations also supports the study conclusions. While the follow-up of the survivors of acute MI included classification of mortality modes, such data were not available for the population of ICD recipients. Nonetheless, classification of death in spite of active ICD protection might have only been fairly approximate since underlying heart failure might exacerbate fatal decline of non-cardiac causes. Finally, as far as risk prediction is concerned, we only used singular recordings in each patient. It has previously been reported that studying the relationship between the QRS-T angle and the underlying heart rate improves risk assessment further.23 Still, it is likely that the same advantage of the TCRT method would also be offered for such risk assessment.
Conclusion
Despite these limitations, the presented results show that the integrative approach incorporated initially into the TCRT method for the assessment of spatial QRS-T angle may offer important advantages in comparison to other methods of measurement. This approach should therefore be included (potentially in conjunction with other measurements) in future clinical studies of the QRS-T angle. The integrative approach is likely a reasonable candidate for the standardization of the QRS-T angle assessment.
Funding
The research leading to the results has received partial funding from the British Heart Foundation (New Horizons Grant NH/16/2/32499), the European Community’s Seventh Framework Programme FP7 (grant agreement n° 602299; EU-CERT-ICD), and the Ministry of Health, Czech Republic—conceptual development of research organization (FNBr, 65269705).
Conflict of interest: none declared.
References
- 1. Zabel M, Acar B, Klingenheben T, Franz MR, Hohnloser SH, Malik M.. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation 2000;102:1252–7. [DOI] [PubMed] [Google Scholar]
- 2. Huang H-C, Lin L-Y, Yu H-Y, Ho Y-L.. Risk stratification by T-wave morphology for cardiovascular mortality in patients with systolic heart failure. Europace 2009;11:1522–8. [DOI] [PubMed] [Google Scholar]
- 3. Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC.. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J 2003;24:1357–64. [DOI] [PubMed] [Google Scholar]
- 4. Aro AL, Huikuri HV, Tikkanen JT, Junttila MJ, Rissanen HA. et al. QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace 2012;14:872–6. [DOI] [PubMed] [Google Scholar]
- 5. Tereshchenko LG, Kim ED, Oehler A, Meoni LA, Ghafoori E, Rami T. et al. Electrophysiologic substrate and risk of mortality in incident hemodialysis. J Am Soc Nephrol 2016;27:3413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Bie MK, Koopman MG, Gaasbeek A, Dekker FW, Maan AC, Swenne CA. et al. Incremental prognostic value of an abnormal baseline spatial QRS-T angle in chronic dialysis patients. Europace 2013;15:290–6. [DOI] [PubMed] [Google Scholar]
- 7. Malik M. Ventricular gradient and cardiac risk. Europace 2011;13:605–7. [DOI] [PubMed] [Google Scholar]
- 8. Malik M, Hnatkova K, Kowalski D, Keirns JJ, van Gelderen EM.. QT/RR curvatures in healthy subjects: sex differences and covariates. Am J Physiol Heart Circ Physiol 2013;305:H1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barthel P, Wensel R, Bauer A, Müller A, Wolf P, Ulm K. et al. Respiratory rate predicts outcome after acute myocardial infarction: a prospective cohort study. Eur Heart J 2013;34:1644–50. [DOI] [PubMed] [Google Scholar]
- 10. Sinnecker D, Dirschinger RJ, Barthel P, Müller A, Morley-Davies A, Hapfelmeier A. et al. Postextrasystolic blood pressure potentiation predicts poor outcome of cardiac patients. J Am Heart Assoc 2014;3:e000857.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NAM 3rd. et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . J Am Coll Cardiol 2012;60:1297–313. [DOI] [PubMed] [Google Scholar]
- 12. Seegers J, Hnatkova K, Friede T, Malik M, Zabel M.. T-wave loop area from a pre-implant 12-lead ECG is associated with appropriate ICD shocks. PLoS One 2017;12:e0173868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Oosterom A. The case of the QRS-T angles versus QRST integral maps. J Electrocardiol 2014;47:144–50. [DOI] [PubMed] [Google Scholar]
- 14. Acar B, Yi G, Hnatkova K, Malik M.. Spatial, temporal and wavefront direction characteristics of 12–lead T wave morphology. Med Biol Eng Comput 1999;37:574–84. [DOI] [PubMed] [Google Scholar]
- 15. Malik M, van Gelderen EM, Lee JH, Kowalski DL, Yen M, Goldwater R. et al. Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: a randomized, double-blind, placebo-, and active-controlled thorough QT study. Clin Pharmacol Ther 2012;92:696–706. [DOI] [PubMed] [Google Scholar]
- 16. Guldenring D, Finlay DD, Strauss DG, Galeotti L, Nugent CD, Donnelly MP. et al. Transformation of the Mason-Likar 12-lead electrocardiogram to the Frank vectorcardiogram. Conf Proc IEEE Eng Med Biol Soc 2012;2012:677–80. [DOI] [PubMed] [Google Scholar]
- 17. Edenbrandt L, Pahlm O.. Vectorcardiogram synthesized from a 12-lead ECG: superiority of the inverse Dower matrix. J Electrocardiol 1988;21:361–7. [DOI] [PubMed] [Google Scholar]
- 18. Smetana P, Batchvarov VN, Hnatkova K, Camm AJ, Malik M.. Ventricular gradient and nondipolar repolarization components increase at higher heart rate. Am J Physiol Heart Circ Physiol 2004;286:H131–6. [DOI] [PubMed] [Google Scholar]
- 19. Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D. et al. Defibrillator implantation early after myocardial infarction. N Engl J Med 2009;361:1427–36. [DOI] [PubMed] [Google Scholar]
- 20. Salvi V, Clark E, Karnad DR, Macfarlane PW, Panicker GK, Hingorani P. et al. Comparison of the spatial QRS-T angle derived from digital ECGs recorded using conventional electrode placement with that derived from Mason-Likar electrode position. J Electrocardiol 2016;49:714–9. [DOI] [PubMed] [Google Scholar]
- 21. Rautaharju PM, Prineas RJ, Zhang ZM.. A simple procedure for estimation of the spatial QRS/T angle from the standard 12-lead electrocardiogram. J Electrocardiol 2007;40:300–4. [DOI] [PubMed] [Google Scholar]
- 22. Schreurs CA, Algra AM, Man SC, Cannegieter SC, van der Wall EE, Schalij MJ. et al. The spatial QRS-T angle in the Frank vectorcardiogram: accuracy of estimates derived from the 12-lead electrocardiogram. J Electrocardiol 2010;43:294–301. [DOI] [PubMed] [Google Scholar]
- 23. Kors JA, van Herpen G, Sittig AC, van Bemmel JH.. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J 1990;11:1083–92. [DOI] [PubMed] [Google Scholar]
- 24. Cortez DL, Schlegel TT.. When deriving the spatial QRS-T angle from the 12-lead electrocardiogram, which transform is more Frank: regression or inverse Dower? J Electrocardiol 2010;43:302–9. [DOI] [PubMed] [Google Scholar]
- 25. Kenttä T, Viik J, Karsikas M, Seppänen T, Nieminen T, Lehtimäki T. et al. Postexercise recovery of the spatial QRS/T angle as a predictor of sudden cardiac death. Heart Rhythm 2012;9:1083–9. [DOI] [PubMed] [Google Scholar]