Summary
Chronic asymptomatic Plasmodium falciparum infection during the dry season predicts decreased clinical malaria risk during the ensuing malaria season; however, treating these infections did not alter this reduced risk, challenging the notion that chronic P. falciparum infection maintains malaria immunity.
Keywords: Plasmodium falciparum, malaria, asymptomatic, malaria/drug therapy, mass drug administration.
Abstract
Background.
Chronic asymptomatic Plasmodium falciparum infections are common in endemic areas and are thought to contribute to the maintenance of malaria immunity. Whether treatment of these infections increases the subsequent risk of clinical episodes of malaria is unclear.
Methods.
In a 3-year study in Mali, asymptomatic individuals with or without P. falciparum infection at the end of the 6-month dry season were identified by polymerase chain reaction (PCR), and clinical malaria risk was compared during the ensuing 6-month malaria transmission season. At the end of the second dry season, 3 groups of asymptomatic children were identified: (1) children infected with P. falciparum as detected by rapid diagnostic testing (RDT) who were treated with antimalarials (n = 104), (2) RDT-negative children whose untreated P. falciparum infections were detected retrospectively by PCR (n = 55), and (3) uninfected children (RDT/PCR negative) (n = 434). Clinical malaria risk during 2 subsequent malaria seasons was compared. Plasmodium falciparum–specific antibody kinetics during the dry season were compared in children who did or did not harbor asymptomatic P. falciparum infections.
Results.
Chronic asymptomatic P. falciparum infection predicted decreased clinical malaria risk during the subsequent malaria season(s); treatment of these infections did not alter this reduced risk. Plasmodium falciparum–specific antibodies declined similarly in children who did or did not harbor chronic asymptomatic P. falciparum infection during the dry season.
Conclusions.
These findings challenge the notion that chronic asymptomatic P. falciparum infection maintains malaria immunity and suggest that mass drug administration during the dry season should not increase the subsequent risk of clinical malaria.
(See the Editorial Commentary by White on pages 654–5.)
Plasmodium falciparum is responsible for approximately 200 million cases of malaria and 400000 deaths annually [1]. Encouragingly, the scale-up of mosquito control measures and artemisinin-based combination therapy has been associated with reduced malaria burden in many regions [2]. Consequently, an increasing number of endemic countries are working toward elimination and considering the interventions that will be required to achieve this objective. Importantly, a large proportion of people in endemic areas are infected with P. falciparum without symptoms [3, 4]. This clinically silent parasite reservoir, which persists for months to years [5] and contributes to ongoing malaria transmission [4, 6–9], poses a challenge for elimination efforts. The strategies of antimalarial mass drug administration (MDA) to at-risk populations or mass screening and treatment of asymptomatically infected individuals are being considered in certain settings [10–12], particularly in areas of seasonal transmission where MDA during the dry season could reduce the number of gametocyte carriers and decrease transmission to the mosquito vector as the rainy season ensues [11, 13, 14].
However, asymptomatic P. falciparum infections have long been thought to directly contribute to the maintenance of immunity to malaria, a notion referred to as “premunition” [15–17]. Consistent with this hypothesis, studies in areas of seasonal malaria have shown that asymptomatic P. falciparum infection at the end of the dry season predicts decreased risk of febrile malaria during the ensuing malaria season [18–22]. This raises the question of whether treatment of asymptomatic infections during the dry season might increase the risk of symptomatic malaria in the event of P. falciparum reinfection.
Two studies in areas of seasonal malaria assessed the impact of treating asymptomatic P. falciparum infection during the dry season on the subsequent risk of clinical malaria. A trial in The Gambia randomized villages to placebo vs 1 dose of sulfadoxine-pyrimethamine combined with 1 dose of artesunate and found no difference in malaria incidence during 20 weeks of follow-up, although there was a significant drop in the incidence of malaria during the period immediately after drug administration [23]. Similarly, a trial in Burkina Faso randomized villages to screening and treatment of asymptomatic P. falciparum infection with artemether-lumefantrine (AL) or no intervention and found no difference in the subsequent incidence of malaria [24]. In contrast, a study in Zambia—where malaria transmission is year-round—randomized health districts during the low transmission season to screening and treatment of infected individuals with AL or no intervention and found a modest decrease in malaria risk in the intervention group [25].
Importantly, these studies compared malaria risk at the community rather than individual level, which may have confounded the results due to heterogeneity in P. falciparum transmission across communities. Additionally, at the time of treatment, these studies did not distinguish chronic asymptomatic P. falciparum infection from recently transmitted infections that may have progressed to clinical malaria without treatment [26], and which may have different effects on host immunity. Together, the limitations of these studies leave open the question of whether treatment of chronic asymptomatic P. falciparum infection impacts the subsequent risk of clinical malaria at the individual level.
In this longitudinal study conducted in an area of seasonal malaria, we addressed 4 objectives: (1) determine whether asymptomatic P. falciparum parasitemia detected at the end of the 6-month dry season represents chronic infection; (2) confirm that asymptomatic P. falciparum infections during the dry season predict protection from clinical malaria during the ensuing malaria season; (3) determine the impact of treating asymptomatic P. falciparum infection during the dry season on the subsequent risk of clinical malaria; and (4) determine whether chronic asymptomatic P. falciparum infection maintains P. falciparum–specific humoral immunity.
METHODS
Ethics Statement
The Ethics Committee of the Faculty of Medicine, Pharmacy and Odontostomatology (FMPOS) at the University of Bamako, and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health Institutional Review Board approved this study. Written informed consent was obtained from all subjects and the parents/guardians of participating children. The study is registered at ClinicalTrials.gov (identifier NCT01322581).
Study Design and Participants
From May 2011 through December 2013, a cohort study was conducted in Kalifabougou, Mali, a rural village of approximately 5000 inhabitants where malaria transmission occurs from July through December. A single clinic and pharmacy provided the only access to antimalarial drugs. A detailed description of the study site and cohort design has been published elsewhere [27]. From an age-stratified, random sample of the entire village population, 695 healthy individuals aged 6 months to 25 years were enrolled. Exclusion criteria were hemoglobin concentration <7 g/dL, axillary temperature ≥37.5°C, acute systemic illness, or use of antimalarial or immunosuppressive medications in the preceding 30 days. Clinical malaria episodes were detected prospectively by active and passive surveillance and were defined by an axillary temperature of ≥37.5°C, ≥2500 asexual parasites per microliter of blood, and no other cause of fever on physical examination.
Detection of P. falciparum Infection
Thick blood smears were stained with Giemsa and Plasmodium parasites were counted against 300 leukocytes; parasite densities were recorded as the number of parasites per microliter of whole blood based on a mean leukocyte count of 7500 cells/µL. Two expert microscopists evaluated each smear separately, and a third resolved discrepancies. The First Response Combo Malaria Ag (pLDH/HRP2) card was used as a rapid diagnostic test (RDT), the sensitivity of which is approximately 100 parasites/μL [28]. Nested polymerase chain reaction (PCR) amplification of Plasmodium DNA was performed from dried blood spots as previously described [27], the sensitivity of which is approximately 0.5–1 parasites/μL [27].
Additional methods are described in the Supplementary Materials.
RESULTS
Asymptomatic P. falciparum Infection During the Dry Season Is Associated With Lower Risk of Clinical Malaria During the Ensuing Malaria Season
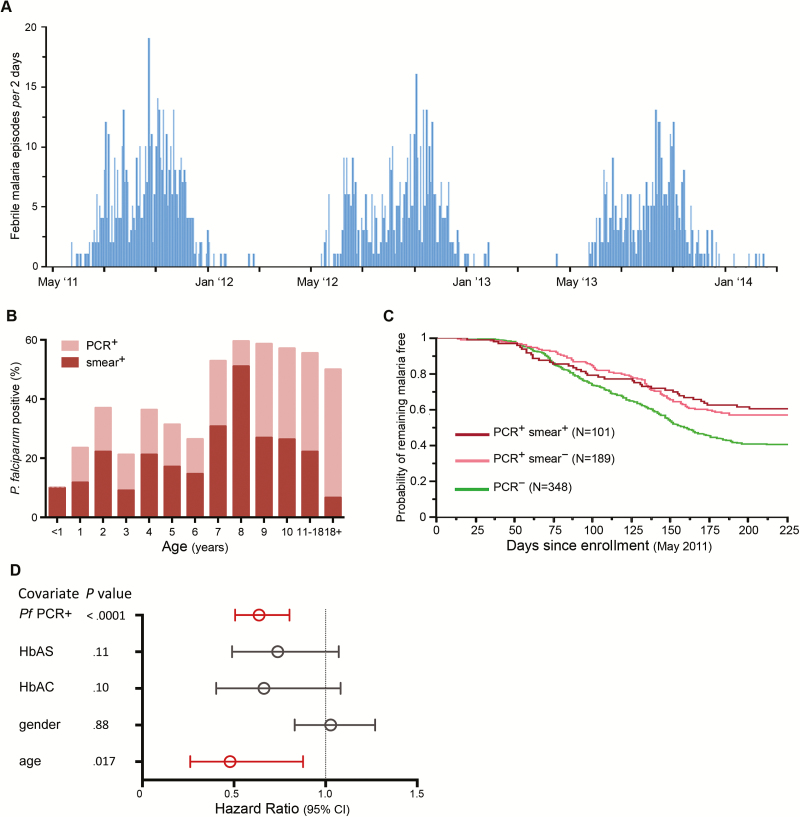
We sought to confirm prior studies that associated asymptomatic P. falciparum infection with lower risk of clinical malaria [18, 19, 21, 22]. During a 2-week period in May 2011, we enrolled 695 asymptomatic subjects just prior to the 6-month malaria season (Figure 1A). The prevalence of P. falciparum infection at enrollment was 45.6% by PCR and 26.3% by blood smear. The prevalence of infection by PCR plateaued by 8 years of age, while the prevalence by blood smear declined after 8 years of age (Figure 1B), consistent with an age-dependent decrease in parasitemia [27].
Figure 1.
Asymptomatic Plasmodium falciparum infection at the end of the dry season independently predicts decreased febrile malaria risk during the ensuing malaria season. A, Frequency of clinical malaria episodes every 2 days over 3 years in a cohort of 695 subjects aged 3 months–25 years. Clinical malaria defined as axillary temperature ≥37.5°C, ≥2500 asexual parasites/μL of blood, and no other cause of fever discernible on physical examination. B, Age-stratified point prevalence of asymptomatic P. falciparum infection detected by polymerase chain reaction (PCR) or blood smear at the end of the dry season in May 2011. C, Kaplan-Meier analysis of time to first febrile malaria episode during the 2011 malaria season stratified by P. falciparum infection status in May 2011. Pairwise comparisons by log-rank test: PCR+smear+ vs PCR–smear–(P < .0001); PCR+smear– vs PCR–smear– (P < .0001); PCR+smear+ vs PCR+smear– (P = .51). D, Cox model showing the effect of P. falciparum infection status in May 2011 on the risk of febrile malaria during the ensuing 2011 malaria season, adjusted for covariates. Hazard ratios and 95% confidence intervals are represented by open circles and horizontal bars, respectively. Abbreviations: CI, confidence interval; HbAC, hemoglobin type AC; HbAS, hemoglobin type AS; PCR, polymerase chain reaction; Pf, Plasmodium falciparum.
During the ensuing 6-month malaria season, clinical malaria episodes were detected by weekly active surveillance and self-referral. Consistent with prior studies [18–22], asymptomatic P. falciparum infection at the end of the dry season was associated with lower risk of febrile malaria during the ensuing malaria season (P < .0001; Figure 1C), an association that remained significant after adjusting for age, sex, and hemoglobin (Hb) type (P < .0001; Figure 1D). The risk of febrile malaria was not significantly different between PCR-positive, smear-positive subjects and PCR-positive, smear-negative subjects (P = .51; Figure 1C), indicating that the difference in baseline parasitemia between these groups did not affect subsequent malaria risk. Hereafter, all analyses focus on children ≤11 years of age—the age group that experiences the majority of clinical malaria episodes in this cohort [27].
Treatment of Chronic Asymptomatic P. falciparum Infection Does Not Change the Risk of Clinical Malaria During the Subsequent Malaria Season
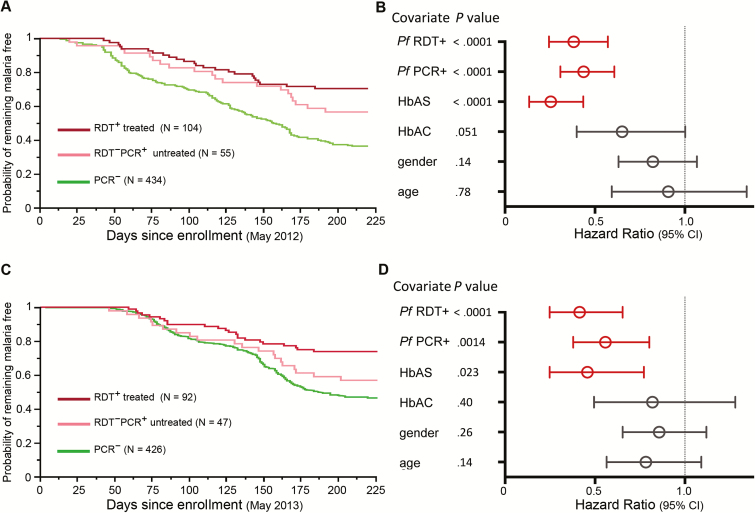
The association between asymptomatic P. falciparum infection during the dry season and subsequent protection from febrile malaria suggests that treatment of asymptomatic infection at the end of the dry season could increase the risk of clinical malaria during the ensuing malaria season. To test this hypothesis, we screened the same cohort for P. falciparum infection (all asymptomatic) at the end of the second dry season (May 2012) using an RDT with a sensitivity comparable to that of blood smear [29]. All subjects found to be P. falciparum infected by RDT (n = 104) were treated with a standard 3-day course of AL, the first daily dose of which was directly observed by study staff. Dried blood spots collected from RDT-negative subjects at the same timepoint (n = 489) were later analyzed by PCR to retrospectively identify 2 additional groups that did not receive antimalarials: RDT-negative, PCR-positive subjects (n = 55) and RDT-negative, PCR-negative subjects (n = 434).
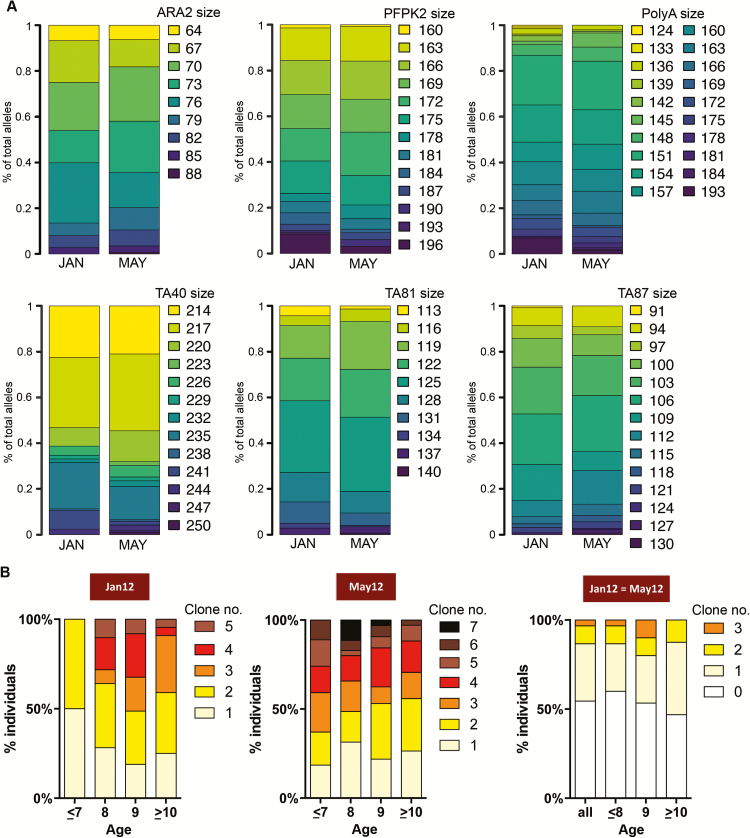
Because asymptomatic P. falciparum infections detected during cross-sectional surveys can become symptomatic within days or weeks of initial detection [3, 4, 30], we sought to confirm that P. falciparum infections detected at the end of the dry season in May 2012 were chronic and asymptomatic. We found that subjects infected with P. falciparum in May 2012 were highly likely to have been infected at the start of the dry season (January 2012) (odds ratio [OR], 842.6 [95% confidence interval {CI}, 200.2–3546.3]; P < .0001); and through the mid–dry season (March 2012) (OR, 172.9 [CI, 79.8–374.5]; P < .0001); conversely, uninfected subjects at the start of the dry season remained uninfected at the end of the dry season (Supplementary Figure 1). During the same time period, no cases of clinical malaria were detected. We also examined parasites collected in January and May 2012 from individuals who tested PCR positive in May 2012, for 6 microsatellite loci previously used to characterize the genetic diversity of P. falciparum [31], and we obtained low fixation index (Fst) values between the populations (Fst January vs May = 0.004), indicating that the 2 populations were genetically very similar (Figure 2A). Additionally, analysis of the polymorphic region of the P. falciparum msp2 locus in January and May 2012 indicated that asymptomatic infections were polyclonal at both timepoints and 47% of subjects harbored at least 1 common parasite clone at both timepoints (Figure 2B). Together with our observation that the entomological inoculation rate is near zero during the dry season, these data indicate that asymptomatic P. falciparum infections detected at the end of the dry season had persisted as chronic asymptomatic infections throughout the preceding dry season.
Figure 2.
Genetic evidence that Plasmodium falciparum infections persist throughout the 6-month dry season. A, Six P. falciparum microsatellite loci were examined in peripheral blood samples collected from 91 P. falciparum–infected subjects in January and May 2012. Each color represents different allele sizes after adjustment to 3 bp bins. B, Proportion of subjects with different number of P. falciparum clones determined by size differences in the polymorphic region of msp2 in January (n = 124) and May 2012 (n = 128) and overlapping at the 2 cross-sectional time points (n = 90) in the respective age groups.
The characteristics of the 3 groups defined in May 2012 (RDT-positive treated; RDT-negative, PCR-positive untreated; and RDT-negative, PCR-negative untreated) are shown in Table 1. Consistent with the first year of the study, the RDT-negative, PCR-negative group had the highest risk of febrile malaria during the second malaria season (Figure 3A), while febrile malaria risk in the RDT-positive treated and RDT-negative, PCR-positive untreated groups was similar in both univariate (Figure 3A) and multivariate (Figure 3B) analyses. We observed the same pattern during the third malaria season (Figure 3C and 3D), indicating that treatment of chronic asymptomatic P. falciparum infection at the end of the dry season does not change the risk of clinical malaria during 2 subsequent malaria seasons.
Table 1.
Characteristics of Study Participants Stratified by Infection Status at the End of the Second Dry Season
| Parameter | RDT–PCR– (n = 434) | RDT–PCR+ (n = 55) | RDT+ (n = 104) | RDT–PCR+ vs RDT+ |
|---|---|---|---|---|
| % of total | 73.20 | 9.30 | 17.50 | |
| Age, y, mean (95% CI) | 5.64 (5.3–5.9) | 8.53 (8.0–9.0) | 8.23 (7.9–8.5) | NS |
| Female sex, % | 48.85 | 52.73 | 41.75 | NS |
| Weight, kg, mean (95% CI) | 19.61 (19.0–20.3) | 25.93 (24.6–27.3) | 24.44 (23.5–25.4) | NS |
| HbAS, % | 10.83 | 10.91 | 4.81 | NS |
| Hb, g/dL, mean (95% CI) | 11.99 (11.9–12.1) | 12.17 (11.9–12.4) | 12.03 (11.8–12.2) | NS |
Abbreviations: CI, confidence interval; Hb, hemoglobin; HbAS, hemoglobin type AS; NS, non significant; PCR, polymerase chain reaction; RDT, rapid diagnostic test.
Figure 3.
Treatment of chronic asymptomatic Plasmodium falciparum infection does not change the subsequent risk of febrile malaria. A, Kaplan-Meier analysis of time to first febrile malaria episode during the 2012 malaria season stratified by P. falciparum infection and treatment status at the end of the dry season in May 2012. Pairwise comparisons by log-rank test: RDT+/treated vs PCR– (P < .0001); RDT–PCR+/untreated vs PCR– (P < .0001); RDT+/treated vs RDT–PCR+/untreated (P = .26). B, Cox model showing the effect of P. falciparum infection and treatment status in May 2012 on the risk of febrile malaria during the ensuing 2012 malaria season, adjusted for covariates. C, Kaplan-Meier analysis of time to first febrile malaria episode during the 2013 malaria season stratified by P. falciparum infection and treatment status at the end of the dry season in May 2012. Pairwise comparisons by log-rank test: RDT+/treated vs PCR– (P < .0001); RDT–PCR+/untreated vs PCR– (P < .0001); RDT+/treated vs RDT–PCR+/untreated (P = .066). D, Cox model showing the effect of P. falciparum infection and treatment status in May 2012 on the risk of febrile malaria during the 2013 malaria season, adjusted for covariates. Hazard ratios and 95% confidence intervals are represented by open circles and horizontal bars, respectively. Abbreviations: CI, confidence interval; HbAC, hemoglobin type AC; HbAS, hemoglobin type AS; PCR, polymerase chain reaction; Pf, Plasmodium falciparum; RDT, rapid diagnostic test.
Plasmodium falciparum–Specific Humoral Immunity Decreases Similarly With or Without Chronic Asymptomatic Infection
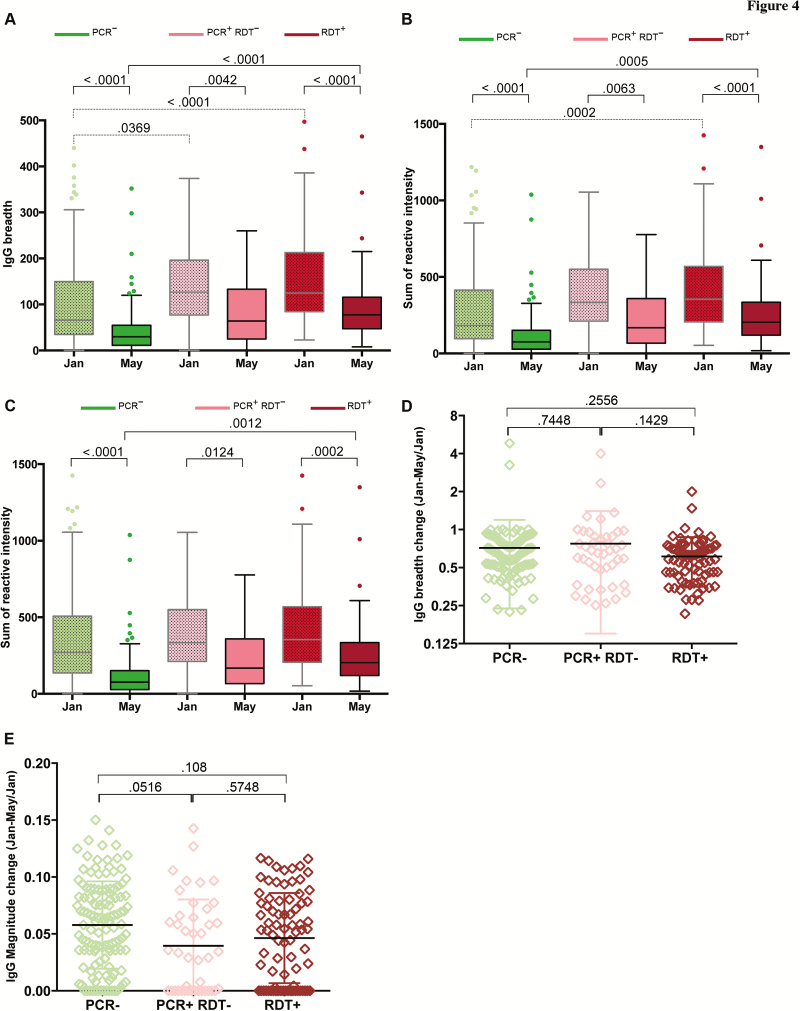
Because treatment of asymptomatic P. falciparum infection at the end of the dry season did not increase the subsequent risk of febrile malaria, we hypothesized that chronic asymptomatic P. falciparum infection per se does not maintain malaria immunity but is instead a marker of higher past P. falciparum exposure and thus higher cumulative immunity. We tested this hypothesis by comparing antibody responses to 862 P. falciparum proteins before and after the dry season in age-matched children who did or did not harbor asymptomatic P. falciparum over the same time period. At both timepoints, the breadth and magnitude of P. falciparum–specific antibodies were higher in subjects who carried parasites through the dry season (Figure 4A–C); however, both antibody breadth and magnitude decreased similarly during the dry season in infected and uninfected subjects (Figures 4D and 4E), suggesting that chronic asymptomatic P. falciparum infection per se does not contribute significantly to the maintenance of humoral immunity to malaria.
Figure 4.
Plasmodium falciparum–specific immunoglobulin G (IgG) reactivity decreases during the dry season irrespective of P. falciparum infection status. A, Breadth of IgG response in January and May 2012, stratified by P. falciparum infection status in May 2012. B, Magnitude of IgG reactivity in January and May 2012, stratified by P. falciparum infection status in May 2012. C, Magnitude of IgG response in January and May 2012 for antigens that were reactive at both timepoints (2 standard deviations (SDs) above the no DNA control), stratified by P. falciparum infection status in May 2012. D, Proportion of antigens to which the level of IgG reactivity fell below the level of detection between January and May 2012, stratified by P. falciparum infection status in May 2012. E, Change in magnitude of IgG reactivity for antigens that were reactive in January and May 2012, stratified by P. falciparum infection status in May 2012. Breadth is defined as the number of antigens to which the level of IgG reactivity exceeds 2 SDs above the no DNA control. Magnitude is defined as the sum of log2-IgG intensity values for all antigens per sample. Boxes indicate median and 25th and 75th percentiles. Values >1.5 times the interquartile range are plotted as individual points (Tukey method). P values less than .05 were considered statistically significant. Abbreviations: IgG, immunoglobulin G; PCR, polymerase chain reaction; RDT, rapid diagnostic test.
DISCUSSION
Here we investigated the impact of treating chronic asymptomatic P. falciparum at the end of the dry season on the subsequent risk of clinical malaria. In doing so we tested the long-standing hypothesis that asymptomatic P. falciparum infection maintains immunity to malaria [15–17, 32, 33]. We found that treatment of asymptomatic P. falciparum infection at the end of the dry season did not increase clinical malaria risk at the individual level during 2 subsequent malaria seasons. Moreover, P. falciparum–specific antibodies declined at a similar rate in children who did or did not harbor asymptomatic P. falciparum over the dry season. Together these findings challenge the notion that asymptomatic P. falciparum infection maintains clinical and humoral immunity to malaria and suggest that MDA during the dry season should not increase the subsequent risk of clinical malaria at the individual level. In contrast, seasonal malaria chemoprevention [34]—which prevents the progression of new blood-stage infections during the transmission season—has been associated in some studies with increased malaria risk (ie, rebound) after discontinuation of seasonal malaria chemoprevention [35, 36]. Therefore, we hypothesize that recently transmitted P. falciparum parasites more effectively induce immune responses relative to parasites that have persisted in blood for several months during the dry season. A differential capacity to trigger host immune responses could reflect epigenetic, transcriptional, and metabolic differences between newly transmitted parasites and parasites that persist during long periods of asexual replication in blood.
We found that the breadth and magnitude of immunoglobulin G (IgG) specific for 862 P. falciparum proteins/polypeptides declined at a similar rate in children who did and those who did not carry asymptomatic P. falciparum infection during the dry season. Similarly, consistent with our antibody data, a study comparing Gambian children who did or did not carry P. falciparum parasites during the dry season found no difference in the rate of decline of IgG specific for 3 P. falciparum merozoite antigens (AMA1, EBA175, MSP119), whereas IgG specific for the merozoite antigen MSP2 declined more rapidly in uninfected children [37], suggesting interactions between specific antigens and infection status that require further investigation. We cannot exclude a role for chronic P. falciparum infection in maintaining other facets of host immunity such as cell-mediated immunity or regulatory mechanisms that attenuate malaria-induced inflammation. Our prior work in Mali suggests that asymptomatic infection during the dry season maintains P. falciparum–inducible IL-10 production capacity in some individuals; however, the magnitude of this response is much lower than that observed in the same children during the preceding transmission season 1 week after acute febrile malaria [38]. This is consistent with other studies in this population that showed a marked increase in P. falciparum–specific memory B cells and antibodies during acute malaria that waned rapidly during the subsequent dry season [39, 40]. Together these observations suggest that the maintenance of malaria immunity depends on repeated exposures to newly transmitted parasites.
Of note, we observed no difference in baseline Hb levels among uninfected subjects and asymptomatically infected subjects, possibly explained in part by the exclusion of subjects with Hb <7 g/dL from this study. Moreover, treatment of asymptomatic P. falciparum infection did not change the prevalence of anemia 1 year later (Supplementary Figure 2), which is consistent with a study in Kenya [41] but at odds with other studies [24, 42].
This study has limitations. First, subjects were not blinded to treatment status, which could have led to differences in treatment-seeking behavior. However, this was likely mitigated by weekly active surveillance for symptomatic malaria. Second, subjects were not randomized to treatment or no treatment groups, but were classified as such based on the RDT result at the end of the dry season, which may have led to differences between groups in known and unknown factors that affect malaria risk. The most important factors known to influence malaria risk in this cohort are age and Hb type [43], which did not differ significantly between the RDT-positive treated and RDT-negative, PCR-positive untreated groups. Moreover, subjects who were blood smear positive or blood smear negative, PCR positive at the end of the first dry season had the same risk of clinical malaria during the first year of the study. Although we did not control for socioeconomic factors, the study population was an age-stratified random sample of individuals residing in a rural community where socioeconomic conditions are relatively uniform and where the research clinic was the only local source of antimalarials. Moreover, it seems unlikely that socioeconomic factors confounded the results such that children who were infected with P. falciparum before the malaria season were more likely to experience malaria during the transmission season, as we observed the opposite effect in this study. Third, a larger study may have detected smaller differences in the risk of clinical malaria between groups. Finally, the average age of the RDT-positive treated and RDT-negative, PCR-positive untreated groups was approximately 8 years, so further studies are needed to determine the impact of treating chronic asymptomatic P. falciparum infection in younger children.
Recent studies in endemic areas have shown that more sensitive detection methods reveal larger reservoirs of asymptomatic P. falciparum infection than previously appreciated [44, 45]. Therefore, it is possible that some subjects in this study had parasite densities below the detection limit of our PCR assay. However, the primary objective of this study was to compare febrile malaria risk in RDT-positive treated subjects vs RDT-negative, PCR-positive untreated subjects, so the possibility that some PCR-negative subjects were infected is unrelated to the major conclusions of this study. Moreover, if a significant proportion of PCR-negative subjects were actually infected, it would be difficult to reconcile their superior ability to suppress parasitemia during the dry season with their lower breadth and magnitude of P. falciparum–specific antibodies and higher risk of febrile malaria during the ensuing malaria season.
Because this study does not support a causal link between chronic asymptomatic P. falciparum infection and protection from febrile malaria, the question remains: What underlies the association between asymptomatic infection and decreased malaria risk? Longitudinal analysis of P. falciparum–specific IgG responses in this study suggests that asymptomatic infection during the dry season is simply a marker of higher past P. falciparum exposure and thus higher cumulative humoral immunity to malaria. It is also possible that the protective immunomodulatory effects of asymptomatic infection persist beyond antimalarial treatment at the end of the dry season into the subsequent transmission season—a possibility that could be tested by treating asymptomatic infections at the beginning of the dry season and ensuring that clearance is sustained by repeated screening and MDA.
In summary, treatment of chronic asymptomatic P. falciparum infection at the end of the dry season did not change the subsequent risk of clinical malaria, and P. falciparum–specific antibodies declined similarly in children who did or did not harbor chronic asymptomatic P. falciparum infection during the dry season. These findings challenge the notion that chronic asymptomatic P. falciparum infection maintains malaria immunity and suggest that MDA during the dry season may not increase the subsequent risk of clinical malaria at the individual level.
Supplementary Material
Notes
Acknowledgments. We thank the residents of Kalifabougou, Mali, for participating in this study, and Dr Richard Sakai and the Mali Service Center for logistic support.
Financial support This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Protein microarray experiments were funded by NIAID grants (award numbers U19AI089686 and R01AI095916 to A. J., D. H. D., C. H., L. L., and P. L. F.).
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2. Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greenwood BM. Asymptomatic malaria infections—do they matter? Parasitol Today 1987; 3:206–14. [DOI] [PubMed] [Google Scholar]
- 4. Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 2014; 12:833–40. [DOI] [PubMed] [Google Scholar]
- 5. Ashley EA, White NJ. The duration of Plasmodium falciparum infections. Malar J 2014; 13:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bousema T, Dinglasan RR, Morlais I, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One 2012; 7:e42821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Churcher TS, Bousema T, Walker M, et al. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife 2013; 2:e00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drakeley C, Sutherland C, Bousema JT, Sauerwein RW, Targett GA. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol 2006; 22:424–30. [DOI] [PubMed] [Google Scholar]
- 9. Ouédraogo AL, Bousema T, Schneider P, et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One 2009; 4:e8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newby G, Hwang J, Koita K, et al. Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg 2015; 93:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okell LC, Griffin JT, Kleinschmidt I, et al. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS One 2011; 6:e20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sturrock HJ, Hsiang MS, Cohen JM, et al. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med 2013; 10:e1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kern SE, Tiono AB, Makanga M, et al. Community screening and treatment of asymptomatic carriers of Plasmodium falciparum with artemether-lumefantrine to reduce malaria disease burden: a modelling and simulation analysis. Malar J 2011; 10:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bousema JT, Gouagna LC, Drakeley CJ, et al. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J 2004; 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith T, Felger I, Tanner M, Beck HP. Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg 1999; 93(suppl 1):59–64. [DOI] [PubMed] [Google Scholar]
- 16. Baird JK. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today 1995; 11:105–11. [DOI] [PubMed] [Google Scholar]
- 17. Sergent E. Definition of immunity and premunition. Ann Inst Pasteur (Paris) 1950; 79:786–97. [PubMed] [Google Scholar]
- 18. Bereczky S, Liljander A, Rooth I, et al. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect 2007; 9:103–10. [DOI] [PubMed] [Google Scholar]
- 19. Crompton PD, Traore B, Kayentao K, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis 2008; 198:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doumbo S, Tran TM, Sangala J, et al. Co-infection of long-term carriers of Plasmodium falciparum with Schistosoma haematobium enhances protection from febrile malaria: a prospective cohort study in Mali. PLoS Negl Trop Dis 2014; 8:e3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Males S, Gaye O, Garcia A. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin Infect Dis 2008; 46:516–22. [DOI] [PubMed] [Google Scholar]
- 22. Sondén K, Doumbo S, Hammar U, et al. Asymptomatic multiclonal Plasmodium falciparum infections carried through the dry season predict protection against subsequent clinical malaria. J Infect Dis 2015; 212:608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Seidlein L, Walraven G, Milligan PJ, et al. The effect of mass administration of sulfadoxine-pyrimethamine combined with artesunate on malaria incidence: a double-blind, community-randomized, placebo-controlled trial in The Gambia. Trans R Soc Trop Med Hyg 2003; 97:217–25. [DOI] [PubMed] [Google Scholar]
- 24. Tiono AB, Ouédraogo A, Ogutu B, et al. A controlled, parallel, cluster-randomized trial of community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malar J 2013; 12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larsen DA, Bennett A, Silumbe K, et al. Population-wide malaria testing and treatment with rapid diagnostic tests and artemether-lumefantrine in southern Zambia: a community randomized step-wedge control trial design. Am J Trop Med Hyg 2015; 92:913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laishram DD, Sutton PL, Nanda N, et al. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J 2012; 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tran TM, Li S, Doumbo S, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis 2013; 57:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell D, Wongsrichanalai C, Barnwell JW. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol 2006; 4:S7–20. [DOI] [PubMed] [Google Scholar]
- 29. Bharti PK, Silawat N, Singh PP, et al. The usefulness of a new rapid diagnostic test, the first response malaria combo (pLDH/HRP2) card test, for malaria diagnosis in the forested belt of central India. Malar J 2008; 7:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nsobya SL, Parikh S, Kironde F, et al. Molecular evaluation of the natural history of asymptomatic parasitemia in Ugandan children. J Infect Dis 2004; 189:2220–6. [DOI] [PubMed] [Google Scholar]
- 31. Anderson TJ, Su XZ, Bockarie M, Lagog M, Day KP. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology 1999; 119(pt 2):113–25. [DOI] [PubMed] [Google Scholar]
- 32. Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009; 22:13–36, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol 2006; 28:51–60. [DOI] [PubMed] [Google Scholar]
- 34. Greenwood B. Review: Intermittent preventive treatment—a new approach to the prevention of malaria in children in areas with seasonal malaria transmission. Trop Med Int Health 2006; 11:983–91. [DOI] [PubMed] [Google Scholar]
- 35. Wilson AL; IPTc Taskforce A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc). PLoS One 2011; 6:e16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenwood BM, David PH, Otoo-Forbes LN, et al. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans R Soc Trop Med Hyg 1995; 89:629–33. [DOI] [PubMed] [Google Scholar]
- 37. Akpogheneta OJ, Duah NO, Tetteh KK, et al. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun 2008; 76:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Portugal S, Moebius J, Skinner J, et al. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog 2014; 10:e1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crompton PD, Kayala MA, Traore B, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A 2010; 107:6958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiss GE, Traore B, Kayentao K, et al. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog 2010; 6:e1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Halliday KE, Okello G, Turner EL, et al. Impact of intermittent screening and treatment for malaria among school children in Kenya: a cluster randomised trial. PLoS Med 2014; 11:e1001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tiono AB, Ouédraogo A, Remy C, Hamed K. Treatment of asymptomatic carriers of Plasmodium falciparum with artemether-lumefantrine: impact on the prevalence of anemia. Infect Dis Ther 2013; 2:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tran TM, Ongoiba A, Coursen J, et al. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J Infect Dis 2014; 209:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 2015; 12:e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parr JB, Belson C, Patel JC, et al. Estimation of Plasmodium falciparum transmission intensity in Lilongwe, Malawi, by microscopy, rapid diagnostic testing, and nucleic acid detection. Am J Trop Med Hyg 2016; 95:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.