Abstract
Background:
Fatigue adversely impacts quality of life in old age. The relationship between subjective and objective measurements of fatigue, however, is poorly understood. We examined whether subjective fatigue moderated the expression of objective fatigue during locomotion. Associations between objective and subjective measures of fatigue were predicted to manifest only under dual-task conditions that maximized cognitive demands.
Methods:
Participants were 314 nondemented older adults (age = 76.8±6.7 years; % female = 56). Functional near-infrared spectroscopy was used to assess oxygenated hemoglobin (HbO2) levels during walking. A 4×14-foot Zeno electronic walkway was utilized to assess stride velocity (cm/s). Objective fatigue was operationalized as attenuation in HbO2 levels and decline in stride velocity (cm/s) during six continuous straight walks under single- (normal-walk) and dual-task (walk-while-talk) conditions. The Brief Fatigue Inventory assessed subjective fatigue.
Results:
Worse subjective fatigue was associated with attenuated increase in HbO2 levels (estimate = 0.175; p < .05) but not with decline in stride velocity (estimate = 0.394; p > .05) from normal-walk to walk-while-talk conditions. Objective fatigue did not manifest and was not associated with subjective fatigue during the course of normal-walk. Worse subjective fatigue was associated with attenuated HbO2 levels in the fourth (estimate = −0.178; p < .05), fifth (estimate = −0.230; p < .01), and sixth (estimate = −0.231; p < .01) straight walks compared to the first during walk-while-talk.
Conclusion:
Dual-task walking paradigms provide a unique environment to simultaneously assess different facets of fatigue. The prefrontal cortex subserves both subjective and objective measurements of fatigue as defined in the context of attention-demanding locomotion.
Keywords: Locomotion, Aging, Brain, Fatigue
Fatigue is common among older adults. While 27%–50% of community-dwelling older adults report moderate to severe levels of fatigue, the prevalence is even higher for those residing in long-term care settings (1–4). In aging, fatigue is associated with increased risk of functional decline, hospitalization, and mortality (4,5). Despite robust evidence for the epidemiological importance of fatigue in older adults (6), its multidimensional presentation has posed significant challenges for proper risk assessment and intervention.
There is no universally accepted definition for fatigue but recent conceptual frameworks emphasize the distinction between subjective and objective assessments of fatigue (7). To date, most clinical and research measurements of fatigue have relied upon self-reported perception of sensations such as exhaustion or weariness. In contrast, objective fatigue is performance based and determined by decline or attenuation on a quantifiable measure. Although subjective reports of fatigue can occur independently of changes in performance (8), evidence for strong associations between self-reported fatigue and performance on a cognitive dual-task paradigm has been reported (9). Clearly, further research incorporating both subjective and objective measurements of fatigue is necessary to develop a more comprehensive understanding of this complex phenomenon.
A theoretical model proposed that dysfunction of frontal-basal ganglia circuitry is implicated in the pathogenesis of fatigue (10). This model further distinguished between peripheral and central fatigue with the latter conceptualized as a cognitive phenomenon. Specifically, cognitive fatigue was defined as a failure to sustain attention to optimize task performance during acute mental effort (though different experimental conditions may elicit cognitive fatigue). Objective cognitive fatigue is typically operationalized as a decline or attenuation on a quantifiable measure over the time course of a functional task. Consistent with the above definition, evidence suggests that measures of executive control are particularly sensitive to cognitive fatigue effects; among three attention networks, cognitive fatigue was observed in executive attention, but not alerting or orienting (11).
Dual-tasking is a distinct component of executive functions requiring the individual to divide attention between competing task demands (12). The walk-while-talk (WWT) paradigm has been studied extensively in older adults as an attention-demanding mobility stress test shown to predict disability and mortality (13). WWT presents several advantages in the assessment of fatigue. First, functional neuroimaging studies of WWT suggest differential involvement of the prefrontal cortex, one of the purported brain substrates implicated in fatigue, in single-task condition compared to dual-task walking conditions (14). Using functional near-infrared spectroscopy (fNIRS), we recently demonstrated robust, sustained increases in oxygenated hemoglobin (HbO2) levels in the prefrontal cortex during WWT compared to normal-walk (NW) (15). Second, by experimentally manipulating cognitive demands, WWT offers an optimal environment to assess interactions of subjective and objective aspects of fatigue thus addressing the inherently multifaceted nature of this construct, which has been largely absent in current literature. Third, the contrast between single- and dual-task performance is critical for the study of cognitive fatigue; whereas single-task performance can become automated over time, dual-tasking imposes high cognitive demands that require continuous effort. Hence, cognitive fatigue would be expected to manifest under dual-task conditions but not single-task conditions. This notion is especially relevant when using brain activation patterns during a functional task as a proxy for cognitive fatigue. The decline in oxygenation levels over the course of a single task may represent greater brain efficiency secondary to task habituation (14) and such a decline would not be expected to correlate with reports of fatigue. In contrast, attenuation in oxygenation levels during the course of an attention-demanding task can represent cognitive fatigue and may thus correlate with subjective fatigue.
The current study was designed to determine whether subjective fatigue was associated with objective fatigue measures, assessed during single- and attention-demanding dual-task walking conditions, in nondemented older adults. The Brief Fatigue Inventory (BFI), a self-report measure, was used to assess subjective fatigue (16). The trajectories of stride velocity and HbO2, assessed during six continuous straight walks on an instrumented walkway, were used to operationalize objective fatigue. Whereas stride velocity can be automated under single-task conditions, the cognitive components of gait, notably under dual-task conditions, have been firmly established (17). Task-related oxygenation levels are commonly used as proxies for mental operations and as such HbO2 trajectories may represent an operational definition of cognitive fatigue that is more proximal to its underlying brain circuitry. Therefore, we hypothesized that evidence for objective fatigue, by means of the above trajectories, would be observed only under conditions that maximize cognitive demands. Specifically, we evaluated the following two predictions: (i) objective fatigue would manifest under WWT but not under NW conditions and (ii) subjective fatigue would moderate HbO2 and stride velocity trajectories under WWT but not under NW conditions.
Methods
Participants
Participants were community-residing older adults (age ≥65 years) enrolled in “Central Control of Mobility in Aging” (CCMA), a cohort study designed to determine cognitive and brain predictors of mobility. CCMA procedures were described in previous publications (17). In brief, potential participants were identified from population lists of lower Westchester County, NY. Structured telephone interviews were administered to obtain verbal assent and determine initial eligibility including screens for dementia and assessments of medical history and current physical function. Individuals who passed the telephone interview were invited to two annual in-person study visits during which trained research assistants administered comprehensive neuropsychological, cognitive, psychological, and mobility assessments. Dementia diagnoses were assigned at consensus diagnostic case conferences (18). Exclusion criteria were: current or history of severe neurological or psychiatric disorders, inability to ambulate independently, significant loss of vision and/or hearing, and recent or anticipated medical procedures that may affect ambulation. Written informed consents were obtained in-person and approved by the IRB.
Measures
Brief Fatigue Inventory
The BFI is a 9-item, 11-point rating scale developed to assess subjective reports of fatigue over a 24-hour period preceding the testing (16). Higher scores are indicative of worse fatigue. The BFI and walking paradigm were administered on the same day. The reliability and validity of the BFI in older adults have been established (19).
Walking paradigm
Our validated dual-task walking paradigm (13) was used to operationalize objective performance-based fatigue. The paradigm consists of two single-task conditions (NW; Cognitive – Alpha) and one dual-task condition. In the NW condition, participants were asked to walk around the electronic walkway at their “normal pace” for three consecutive loops. In the Alpha condition, participants were required to stand still while reciting alternate letters of the alphabet out aloud, starting with the letter B, for 30 seconds. In the WWT condition, participants were instructed to walk around the walkway for three consecutive loops at their normal pace while reciting alternate letters of the alphabet starting with the letter “B.” Participants were instructed to pay equal attention to both tasks to minimize task prioritization effects (17,20). The three test conditions were presented in a counterbalanced order using a Latin-square design to minimize task order effects on the outcome measures. None of the participants required assistive devices during the experimental walking conditions.
Quantitative gait assessment
Zenometrics.
A 4×14-foot Zeno electronic walkway was utilized to assess quantitative measures of gait during NW and WWT (Zenometrics, LLC, Peekskill, NY). The quantitative gait measure used in the current study, stride velocity (cm/s), was measured based on the location and mathematical parameters between footfalls on the instrumented walkway. Split-half intraclass correlations for stride velocity (cm/s) in NW and WWT were greater than 0.95 revealing excellent internal consistency.
fNIRS system.
fNIRS measures changes in cortical HbO2 levels using light–tissue interaction properties of light within the near-infrared range. Changes in hemodynamic activity in the prefrontal cortex were assessed using fNIRS Imager 1000 (fNIRS Devices, LLC, Potomac, MD). The system collects data at a sampling rate of 2 Hz. The fNIRS sensor consists of 4 LED light sources and 10 photodetectors, which cover the forehead using 16 voxels, with a source-detector separation of 2.5cm. The light sources on the sensor (Epitex Inc. type L4X730/4X805/4X850-40Q96-I) contain three built-in LEDs having peak wavelengths at 730, 805, and 850nm, with an overall outer diameter of 9.2±0.2mm. The photodetectors (Bur Brown, type OPT101) are monolithic photodiodes with a single-supply transimpedance amplifier. We implemented a standard sensor placement procedure. Details and visual depiction of the fNIRS device and procedures have been previously described (15).
Preprocessing and hemodynamic signal extraction.
Raw data were inspected to identify and remove raw intensity measurements (ie, voltage values obtained from the photodetectors) at 730 and 850nm that reached saturation or dark current conditions. To eliminate possible respiration, heart rate signals, and unwanted high-frequency noise, raw intensity measurements at 730 and 850nm were low-pass filtered with a finite impulse response filter with a cutoff frequency of 0.14 Hz (21). Saturation or dark current conditions were excluded. Using modified Beer–Lambert law (21), HbO2 levels, which are more reliable and sensitive to locomotion-related changes in cerebral oxygenation (22), were calculated for the current study. Separate 10-second baselines were used to determine relative changes in HbO2 concentrations during each experimental condition (15).
Epoch and feature extraction.
Based on the 16 fNIRS channels, mean HbO2 data, per participant, were extracted separately for NW and WWT overall and stratified by the six consecutive straight walks. A second-level postprocessing time synchronization method was implemented using the first recorded foot contact with the walkway as a time stamp. The recording of fNIRS was algorithmically terminated at the end of the sixth and final straight walk. HbO2 data in NW and WWT within this range were extracted and used for comparisons between task conditions and between the six straight walks within NW and WWT. Oxygenated hemoglobin levels per task condition and straight walking segment (1–6) were expressed in micromolar units (µM). Internal consistency of HbO2 measurements, determined by split-half intraclass correlations, was excellent for NW (0.830), Alpha (0.864), and WWT (0.849) (15).
Objective fatigue
Using ProtoKinetics Movement Analysis Software (PKMAS) technology, a validated computer algorithm determined entry and exit points from turns during the three consecutive loops that each participant was required to walk under single- and then dual-task conditions (23). This procedure yielded six consecutive straight walks that served as the time course based upon which objective fatigue trajectories in gait velocity were operationalized for NW and WWT for each participant. Mean straight walks length in feet for NW (8.76±0.88) and WWT (8.82±1.4) were very similar. As expected, average walk time, in seconds, was shorter in NW (38.73±14.23) compared to WWT (48.08±18.56). Attenuation in HbO2 levels during the time course of WWT operationalized objective cognitive fatigue. HbO2 levels show an initial increase with a subsequent decline due to habituation during NW (15).
Covariates.
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to assess overall level of cognitive function (24). A comorbidity summary score (range 0–10) including the presence of diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’ s disease, chronic obstructive lung disease, angina, and myocardial infarction was used to characterize disease burden (18).
Statistical Analysis
Linear mixed-effects and generalized estimating equations Poisson models were used to assess the moderating effect of perceived fatigue on the change in stride velocity and rate of letter generation in dual compared to single-task conditions, respectively. A separate linear mixed-effect model was used to assess the moderating effect of perceived fatigue on HbO2 levels in WWT compared to NW and Alpha. Separate linear mixed-effects models were run to examine trajectories of stride velocity and HbO2 levels and whether perceived fatigue moderated their expression during NW and WWT. Specifically, the six straight walks within each task served as the within-person repeated measure. HbO2 levels and stride velocity in each straight walk served as the dependent measures. Moderation effects on the course of gait velocity and HbO2 levels were determined by interactions of perceived fatigue and the change in the dependent measures in the first straight walk compared to the subsequent five walks. Analyses controlled for gender, age, education, disease comorbidity, and total RBANS index score. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
A total of 314 nondemented participants (mean age in years = 76.8±6.7; mean education in years = 14.42±3.00; % female = 56) were included. The mean RBANS total score (91.38±11.74) was indicative of average cognitive function. Mean BFI score was 1.5±1.5, indicating relatively low subjective fatigue levels (Table 1).
Table 1.
Sample Characteristics
| Total Sample | Low Perceived Fatigue | High Perceived Fatigue | |
|---|---|---|---|
| Participants, n | 314 | 160 | 154 |
| Women, n (%) | 176 (56) | 86 (54) | 89 (58) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age, y | 76.8 (±6.67) | 76.20 (±6.64) | 77.41 (±6.66) |
| Education, y | 14.42 (±3.00) | 14.57 (±2.85) | 14.27 (±3.14) |
| BFI | 1.5 (±1.5) | 0.39 (±0.34) | 2.5 (±1.43) |
| Disease Comorbidity Index | 1.62 (±1.08) | 1.46 (±1.04) | 1.78 (±1.10) |
| RBANS (total score) | 91.38 (±11.74) | 92.29 (±11.57) | 90.44 (±11.86) |
| Gait velocity NW, cm/s | 79.82 (±17.28) | 83.35 (±17.12) | 76.46 (±16.80) |
| Gait velocity WWT, cm/s | 64.96 (±18.63) | 68.12 (±18.31) | 61.93 (±18.48) |
| Alpha: rate of letter generation per minute | 33.6 (±12) | 33.6 (±12.6) | 32.4 (±10.8) |
| WWT: rate of letter generation per minute | 33.6 (±15) | 34.2 (±15.6) | 31.2 (±12) |
| Alpha: rate of errors generation per minute | 3.83 (±5.5) | 4.09 (±6.80) | 3.57 (±3.54) |
| WWT: rate of error generation per minute | 6.94 (±7.39) | 7.47 (±8.93) | 6.40 (±5.39) |
| NW: HbO2 levels | 0.11 (±1.25) | 0.11 (±1.35) | 0.12 (±1.14) |
| Alpha: HbO2 levels | 0.69 (±0.98) | 0.73 (±1.05) | 0.63 (±.90) |
| WWT: HbO2 levels | 0.73 (±1.41) | 0.79 (±1.27) | 0.63 (±1.50) |
Note: BFI = Brief Fatigue Inventory; NW = normal-walk; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; WWT = walk-while-talk.
For visual depiction and interpretation of the data, subjective fatigue was dichotomized based on median split. Analyses using subjective fatigue as a continuous variable were not materially different.
Dual-Task Effects on Behavioral Outcomes and HbO2 Levels
Mean stride velocity (cm/s) in WWT compared to NW was significantly reduced (estimate = −14.912; p < .001; 95% CI = −16.655 to −13.170), demonstrating the expected dual-task effect. The main effect (estimate = −2.724; p = .122; 95% CI = −6.183 to 0.735) and interaction (estimate = −0.394; p = .754; 95% CI = −2.084 to 2.873) of subjective fatigue × task were not significant. High levels of subjective fatigue were, however, associated with slower stride velocity (cm/s) irrespective of walking task condition (estimate = −3.709; p = .032; 95% CI = −7.108 to −0.311). The rate of correct letter generation per minute was not significantly reduced in WWT compared to Alpha (estimate of log of rate ratio = −0.025; p = .476; 95% CI = −0.094 to 0.044). The error rate per minute, however, was significantly increased in WWT compared to Alpha (estimate of log of rate ratio = 0.550; p < .001; 95% CI = 0.372 to 0.728). The main effects and interactions of perceived fatigue status with task were not significant for the rate of correct letters or errors (data not shown).
HbO2 levels were significantly increased in WWT compared to NW (estimate = −0.637; p < .001; 95% CI = −0.754 to −0.519) but not compared to Alpha (estimate = −0.071; p = .285; 95% CI = −0.203 to 0.059). Worse subjective fatigue attenuated the increase in HbO2 levels from NW to WWT (interaction of task × subjective fatigue estimate = 0.175; p < .034; 95% CI = 0.012 to 0.337) but not from alpha to WWT (interaction of task × subjective fatigue estimate = 0.052; p = .582; 95% CI = −0.134 to 0.238).
Subjective Fatigue and HbO2 Trajectories
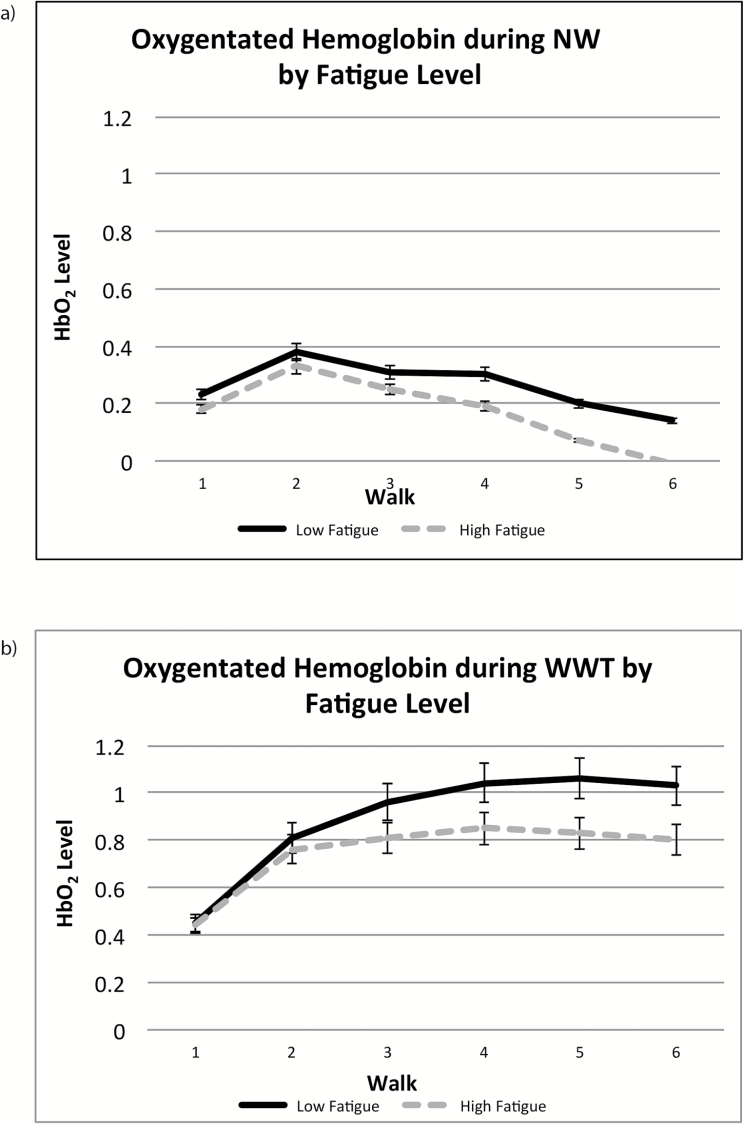
Subjective fatigue did not moderate changes in HbO2 levels during the course of NW (Table 2, (A); Figure 1a). Worse subjective fatigue, however, attenuated the change in HbO2 levels during WWT (Table 2, (B); Figure 1b).
Table 2.
Effect of Time and Subjective Fatigue on HbO2 Levels During NW and WWT
| Estimate | t | 95% CI | p | |
|---|---|---|---|---|
| (A) HbO2 levels during NW | ||||
| Walk 1 vs Walk 2 | 0.151 | 2.95 | 0.050 to 0.252 | <.01 |
| Walk 1 vs Walk 3 | 0.081 | 1.58 | −0.019 to 0.182 | .114 |
| Walk 1 vs Walk 4 | 0.064 | 1.25 | −0.036 to 0.164 | .210 |
| Walk 1 vs Walk 5 | −0.038 | −0.75 | −0.139 to 0.062 | .452 |
| Walk 1 vs Walk 6 | −0.091 | −1.78 | −0.193 to 0.009 | .074 |
| Subjective fatigue | −0.064 | −0.55 | −0.296 to 0.166 | .582 |
| Subjective fatigue × Walk 1 vs Walk 2 | −0.004 | −0.01 | −0.144 to 0.143 | .995 |
| Subjective fatigue × Walk 1 vs Walk 3 | −0.003 | −0.05 | −0.147 to 0.140 | .964 |
| Subjective fatigue × Walk 1 vs Walk 4 | −0.049 | −0.68 | −0.194 to 0.094 | .496 |
| Subjective fatigue × Walk 1 vs Walk 5 | −0.070 | −0.96 | −0.215 to 0.073 | .335 |
| Subjective fatigue × Walk 1 vs Walk 6 | −0.095 | −1.29 | −0.240 to 0.049 | .195 |
| (B) HbO2 levels during WWT | ||||
| Walk 1 vs Walk 2 | 0.356 | 6.04 | 0.240 to 0.472 | <.0001 |
| Walk 1 vs Walk 3 | 0.506 | 8.59 | 0.390 to 0.622 | <.0001 |
| Walk 1 vs Walk 4 | 0.588 | 9.98 | 0.472 to 0.704 | <.0001 |
| Walk 1 vs Walk 5 | 0.615 | 10.39 | 0.499 to 0.731 | <.0001 |
| Walk 1 vs Walk 6 | 0.584 | 9.87 | 0.468 to 0.700 | <.0001 |
| Subjective fatigue | −0.004 | −0.03 | −0.274 to 0.265 | .973 |
| Subjective fatigue × Walk 1 vs Walk 2 | −0.037 | −0.45 | −0.203 to 0.127 | .652 |
| Subjective fatigue × Walk 1 vs Walk 3 | −0.137 | −1.63 | −0.302 to 0.027 | .103 |
| Subjective fatigue × Walk 1 vs Walk 4 | −0.178 | −2.12 | −0.344 to −0.013 | .033 |
| Subjective fatigue × Walk 1 vs Walk 5 | −0.230 | −2.73 | −0.396 to −0.064 | .0065 |
| Subjective fatigue × Walk 1 vs Walk 6 | −0.231 | −2.74 | −0.397 to −0.065 | .0062 |
Note: NW = normal-walk; WWT = walk-while-talk.
Figure 1.
HbO2 trajectories during NW (a) and WWT (b). NW = normal-walk; WWT = walk-while-talk.
Subjective Fatigue and Stride Velocity (cm/s) Trajectories
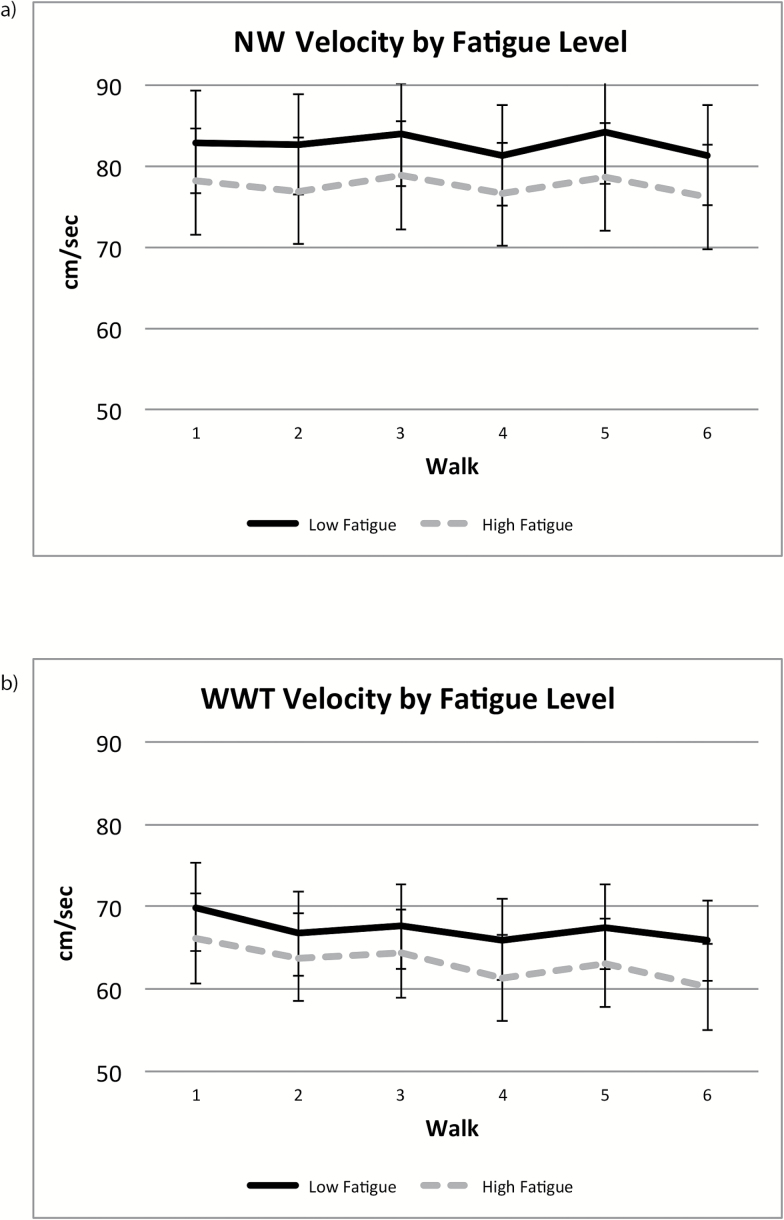
Stride velocity (cm/s) declined during WWT but not during NW, and in both conditions, the moderation effects of subjective fatigue were not significant (Table 3; Figure 2).
Table 3.
Effect of Time and Subjective Fatigue on Stride Velocity During NW and WWT
| Estimate | t | 95% CI | p | |
|---|---|---|---|---|
| (A) Stride velocity during NW | ||||
| Walk 1 vs Walk 2 | −0.250 | −0.44 | −1.363 to 0.862 | .658 |
| Walk 1 vs Walk 3 | 0.930 | 1.56 | −2.458 to 2.107 | .120 |
| Walk 1 vs Walk 4 | −1.655 | −2.45 | −2.986 to −0.325 | .014 |
| Walk 1 vs Walk 5 | 0.673 | 1.68 | −0.195 to 2.455 | .094 |
| Walk 1 vs Walk 6 | −1.694 | −2.30 | −3.144 to −0.245 | .021 |
| Subjective fatigue | −1.913 | −1.06 | −5.480 to 1.653 | .292 |
| Subjective fatigue × Walk 1 vs Walk 2 | −0.896 | −1.11 | −2.486 to 0.692 | .267 |
| Subjective fatigue × Walk 1 vs Walk 3 | −0.217 | −0.25 | −1.896 to 1.462 | .799 |
| Subjective fatigue × Walk 1 vs Walk 4 | 0.083 | 0.09 | −1.816 to 1.983 | .931 |
| Subjective fatigue × Walk 1 vs Walk 5 | −0.465 | −0.48 | −2.359 to 1.427 | .628 |
| Subjective fatigue × Walk 1 vs Walk 6 | −0.544 | −0.52 | −2.615 to 1.527 | .605 |
| (B) Stride velocity during WWT | ||||
| Walk 1 vs Walk 2 | −3.185 | −4.85 | −4.477 to −1.891 | <.0001 |
| Walk 1 vs Walk 3 | −2.318 | −3.31 | −3.696 to −0.939 | .001 |
| Walk 1 vs Walk 4 | −3.984 | −5.27 | −5.473 to −2.495 | <.0001 |
| Walk 1 vs Walk 5 | −2.756 | −3.53 | −4.293 to −1.220 | .0005 |
| Walk 1 vs Walk 6 | −4.392 | −5.51 | −5.962 to −2.832 | <.0001 |
| Subjective fatigue | −1.362 | −0.70 | −5.183 to 2.458 | .483 |
| Subjective fatigue × Walk 1 vs Walk 2 | 0.887 | 0.95 | −0.958 to 2.733 | .344 |
| Subjective fatigue × Walk 1 vs Walk 3 | 0.472 | 0.48 | −1.489 to 2.446 | .632 |
| Subjective fatigue × Walk 1 vs Walk 4 | −0.808 | −0.75 | −2.934 to 1.317 | .454 |
| Subjective fatigue × Walk 1 vs Walk 5 | 0.018 | 0.02 | −2.175 to 2.212 | .986 |
| Subjective fatigue × Walk 1 vs Walk 6 | −1.231 | −1.08 | −3.473 to 1.009 | .280 |
Note: NW = normal-walk; WWT = walk-while-talk.
Figure 2.
Stride velocity (cm/s) trajectories during NW (a) and WWT (b). NW = normal-walk; WWT = walk-while-talk.
Discussion
Fatigue is a multifaceted construct and relations between its multiple components are poorly understood. Using a dual-task paradigm, we have found that worse subjective fatigue was associated with attenuation in both the increase of HbO2 levels from NW to WWT and the trajectory of HbO2 during the course of WWT. The trajectory of HbO2 during NW, however, was not associated with subjective fatigue. Confirming our prediction, a meaningful relationship between subjective reports of fatigue and objective cognitive fatigue was observed only under walking conditions that maximized executive demands. This finding is consistent with existing models implicating frontal-basal ganglia circuits in fatigue (10) and further illustrates the advantages of utilizing the dual-task environment to evaluate performance-based cognitive fatigue and it’s relation to subjective fatigue. Specifically, dual-tasks impose additional demands on the attention system (17,25) and require differential involvement of the prefrontal cortex relative to the single tasks (15,26). The individual’s performance on the dual-task can be directly compared to the single task where objective fatigue is not expected to manifest. Moreover, our previous work revealed that oxygenation levels declined after initial elevation only during NW due to habituation (15). Hence, task context and demands play a critical role in the interpretation of the findings, in this case distinguishing performance-based cognitive fatigue, as defined by HbO2 levels, from habituation.
Stride velocity (cm/s) declined over time but only under the dual-task condition that maximized executive demands. We suggest that this decline is consistent with an operational definition of objective cognitive fatigue. While decline in gait speed can be used to define physical or peripheral fatigue (10), it is noteworthy that under the current experimental conditions such a decline was not observed during single-task walking. NW and WWT were counterbalanced precluding the possibility that practice or test order effects accounted for this finding. Stride velocity (cm/s) is a robust and reliable measure and thus optimal for repeated assessments necessary to establish the presence of objective fatigue. Separating the objective physical and cognitive fatigue components that are inherent in stride velocity is a challenge despite the compelling comparison between the single- and dual-task walking conditions. Assessing concurrently physical aspects of fatigue that are less likely to be influenced by cognition maybe advantageous. The decline in stride velocity during WWT was not related to subjective fatigue. While speculative, it could be argued that compared to gait speed, the change in task-related HbO2 levels constitutes a more proximal measure of brain function and may thus be more sensitive to fatigue. Future studies, however, should examine whether other quantitative measures of gait have differential sensitivity to fatigue effects.
Limitations and Future Directions
Subjective fatigue did not moderate the change in the rate of correct letters or errors from single- to dual-task conditions. We did not, however, assess performance on Alpha as a function of time, which precluded a determination of whether or not objective fatigue on this measure could be established and related to subjective fatigue. The sample in the current study consisted of relatively healthy and independent older adults whose subjective fatigue was relatively low (stratification into fatigue groups was done based on sample distribution). The generalizability of these findings to patient populations, especially those with neurological diseases with a high prevalence and severity of fatigue symptoms, remains to be evaluated. Whether or not activity-related subjective fatigue is differentially related to objective fatigue should be explored in future research. While our participants were community-dwelling, ambulatory, and dementia free, our study design did not include imaging in all participants that would enable further insights into underlying brain substrates and will be examined in future studies. Fatigue is a multifaceted construct and consensus regarding the terminology of its various components is clearly absent. Under these circumstances, we deliberately elected to parsimoniously operationalize perception (subjective report) and objective (performance-based) measurements of fatigue in the context of the reported work. We note, however, that performance-based fatigue, as defined herein, is also consistent with the term performance fatigability.
Conclusion
Subjective fatigue influenced locomotion-related brain activation patterns implicated in cognitive fatigue. The utility of these findings for risk assessment and treatment of individuals at risk of mobility impairments should be further evaluated. Whether improving subjective or objective fatigue may improve locomotion in healthy and impaired older adults requires additional investigation.
Funding
This research was supported by the National Institutes on Aging R01AG036921 (PI: R. Holtzer) and R01 AG044007 (PI: J Verghese).
Conflict of Interest
M.I. has a very minor share in the company that manufactures the fNIRS device used in this study. All other authors have no conflicts of interest to report in relation to the current article.
References
- 1. Hellstrom Y, Hallberg IR. Determinants and characteristics of help provision for elderly people living at home and in relation to quality of life. Scand J Caring Sci. 2004;18:387–395. doi:10.1111/j.1471-6712.2004.00291.x [DOI] [PubMed] [Google Scholar]
- 2. Reyes-Gibby CC, Mendoza TR, Wang S, Anderson KO, Cleeland CS. Pain and fatigue in community-dwelling adults. Pain Med. 2003;4:231–237. [DOI] [PubMed] [Google Scholar]
- 3. Wijeratne C, Hickie I, Brodaty H. The characteristics of fatigue in an older primary care sample. J Psychosom Res. 2007;62:153–158. doi:10.1016/j.jpsychores.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 4. Liao S, Ferrell BA. Fatigue in an older population. J Am Geriatr Soc. 2000;48:426–430. [DOI] [PubMed] [Google Scholar]
- 5. Schultz-Larsen K, Avlund K. Tiredness in daily activities: a subjective measure for the identification of frailty among non-disabled community-living older adults. Arch Gerontol Geriatr. 2007;44:83–93. doi:10.1016/j.archger.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 6. Egerton T, Chastin SF, Stensvold D, Helbostad JL. Fatigue may contribute to reduced physical activity among older people: an observational study. J Gerontol A Biol Sci Med Sci. 2016;71:670–676. doi:10.1093/gerona/glv150 [DOI] [PubMed] [Google Scholar]
- 7. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–416. doi:10.1212/WNL.0b013e31827f07be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bailey A, Channon S, Beaumont JG. The relationship between subjective fatigue and cognitive fatigue in advanced multiple sclerosis. Mult Scler. 2007;13:73–80. [DOI] [PubMed] [Google Scholar]
- 9. Holtzer R, Foley F. The relationship between subjective reports of fatigue and executive control in multiple sclerosis. J Neurol Sci. 2009;281:46–50. doi:10.1016/j.jns.2009.02.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179:34–42. [DOI] [PubMed] [Google Scholar]
- 11. Holtzer R, Shuman M, Mahoney JR, Lipton R, Verghese J. Cognitive fatigue defined in the context of attention networks. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011;18:108–128. doi:10.1080/13825585.2010.517826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit Psychol. 2000;41:49–100. doi:10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- 13. Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60(10):1901–1905. doi:10.1111/j.1532-5415.2012.04145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi:10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage. 2015;112:152–159. doi:10.1016/j.neuroimage.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. [DOI] [PubMed] [Google Scholar]
- 17. Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordrecht, Netherlands). 2014;36:373–381. doi:10.1007/s11357-013-9570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300:823–830. doi:10.1001/jama.300.7.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shuman-Paretsky MJ, Belser-Ehrlich J, Holtzer R. Psychometric properties of the Brief Fatigue Inventory in community-dwelling older adults. Arch Phys Med Rehabil. 2014;95:1533–1539. doi:10.1016/j.apmr.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor Control. 2012;16:64–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Izzetoglu M, Chitrapu P, Bunce S, Onaral B. Motion artifact cancellation in NIR spectroscopy using discrete Kalman filtering. Biomed Eng Online. 2010;9:16. doi:10.1186/1475-925X-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193:445–454. doi:10.1007/s00221-008-1643-y [DOI] [PubMed] [Google Scholar]
- 23. England SE, Verghese J, Mahoney JR, Trantzas C, Holtzer R. Three-level rating of turns while walking. Gait Posture. 2015;41:300–303. doi:10.1016/j.gaitpost.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clinical Neuropsychol. 2008;23:603–612. doi:10.1016/j.acn.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holtzer R, Mahoney J, Verghese J. Intraindividual variability in executive functions but not speed of processing or conflict resolution predicts performance differences in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:980–986. doi:10.1093/gerona/glt180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR. Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topogr. 2016;29:334–343. doi:10.1007/s10548-015-0465-z [DOI] [PMC free article] [PubMed] [Google Scholar]