Abstract
Background: Recipients of faecal microbiota transplantation (FMT) in treatment of recurrent Clostridium difficile infection (RCDI) remain at markedly increased risk of re-infection with C. difficile with new antibiotic provocations. Urinary tract infections (UTIs) are common indications for antibiotics in these patients, often resulting in C. difficile re-infection.
Methods: We present a case series of 19 patients treated with parenteral aminoglycosides for UTI following FMT for RCDI. A 3 day outpatient regimen of once-daily intramuscular administration of gentamicin was used to treat 18 consecutive FMT recipients with uncomplicated UTI. One other patient was treated for a complicated UTI with intravenous amikacin. Profiling of 16S rRNA genes was used to track changes in faecal microbial community structure during this regimen in three patients.
Results: The protocol was highly effective in treating UTI symptoms. None of the patients suffered a re-infection with C. difficile. The faecal microbial communities remained undisturbed by treatment with intramuscular administration of gentamicin.
Conclusions: Despite falling out of favour in recent years, aminoglycoside antibiotics given parenterally have the advantage of minimal penetration into the gut lumen. A brief (3 day) course of parenteral gentamicin was safe and effective in curing UTI in patients at high risk of C. difficile infection without perturbing their gut microbiota.
Introduction
Antibiotic treatment of urinary tract infections (UTIs) is a common trigger of Clostridium difficile infection (CDI) and both diseases commonly become recurrent, requiring multiple cycles of antibiotics. The risk of CDI recurrence progressively rises with cumulative antibiotic exposure.1 Recurrent UTIs constitute a formidable challenge for such patients, as all oral antibiotics currently available to treat UTIs are also associated with increased risk of triggering CDI.2,3
A significant fraction of CDI patients develop a syndrome of recurrent cycles of C. difficile infections (RCDIs) that cannot be broken with antibiotics alone.4 Faecal microbiota transplantation (FMT), a treatment that restores the normal microbial community structure in the gut, has proven to be highly successful in preventing CDI recurrence.5 However, patients with the RCDI syndrome remain at a very high risk (∼25%) of re-infection with C. difficile following new antibiotic provocations during the initial years following FMT.6 Our experience is consistent with that risk. Between 2008 and 2015 we performed FMTs in ∼300 patients in treatment of RCDI and regularly encountered post-FMT re-infections with C. difficile following uncomplicated UTI treatment with all commonly used oral antibiotics, including quinolones, penicillins, cephalosporins, macrolides, sulfamethoxazole/trimethoprim, nitrofurantoin and fosfomycin. Therefore, we sought an antibiotic treatment for UTIs in our FMT recipients that would not disrupt the microbial communities in the gut and would reduce the risk of re-infection with CDI.
Aminoglycoside antibiotics are particularly well suited for treatment of most Gram-negative uropathogens because of their renal elimination and high concentrations in the urine. In recent decades, however, their use has been largely abandoned and only occasionally considered as initial parenteral treatment of severe complicated UTIs and pyelonephritis.7 Even in these cases, aminoglycosides are typically discontinued after 48–72 h, once there is evidence of clinical improvement and results of the urine cultures with organism susceptibilities allow a switch to oral antibiotic therapy. Such short-term use of aminoglycosides minimizes concerns about ototoxicity and nephrotoxicity associated with these agents.
Aminoglycosides are not suitable as oral agents for treatment of UTIs because of their poor intestinal absorption. In addition, when given parenterally, aminoglycosides are not actively excreted into bile and therefore have minimal penetration into the gastrointestinal lumen.8 However, these characteristics make them potentially attractive alternatives when preservation of the gut microbiota is especially critical. Therefore, we established an aminoglycoside-based outpatient UTI treatment protocol for our RCDI patients that underwent FMT. Here we report the clinical outcomes in the first 19 consecutive FMT recipient patients treated for their UTIs with aminoglycosides. In addition, we tested whether the treatment affected the integrity of gut microbiota in three patients by analysing bacterial composition in their pre- and post-treatment faecal samples.
Methods
Patients
All patients in this study were recipients of FMT in treatment of RCDI at the University of Minnesota. FMT in our programme is offered as rescue therapy for patients who suffer at least two spontaneous recurrences of CDI following the initial infection and fail at least one extended antibiotic regimen to clear the infection. Extended antibiotic regimens include vancomycin pulse/taper protocols or chaser protocols with rifaximin or fidaxomicin that total at least 6 weeks. Detailed inclusion and exclusion criteria in our programme were described previously.9 One of these exclusion criteria is anticipated antibiotic treatment for a non-CDI indication within 3 months, which includes patients with recurrent UTIs (two or more infections in 6 months or three or more infections in 1 year). FMT was performed using either an encapsulated oral preparation or colonoscopic delivery of microbiota. All our FMT recipients were routinely informed about the high risk of C. difficile re-infection associated with antibiotic treatments for any indications in the initial years post-FMT. The problem of UTIs and availability of the aminoglycoside treatment option were specifically highlighted. The aminoglycoside regimen as well as oral antibiotic alternatives were discussed as options with all patients who developed post-FMT UTI.
Ethics
Known and potential risks and benefits associated with antibiotic treatments for UTI, oral and parenteral, were discussed in detail with each patient and informed consent for treatment and outcome data collection was obtained. The study was approved by the University of Minnesota Institutional Review Board (Protocol 0901M56962).
Aminoglycoside UTI treatment protocol
The outpatient treatment protocol for uncomplicated UTI included: (i) clinical evaluation for the possibility of UTI and documentation of infection by urinalysis and culture, preferably from a catheterized urine specimen; and (ii) gentamicin administered intramuscularly at 160 mg on day 1 (two injections in separate sites) and single 80 mg injections on days 2 and 3. Intravenous infusion of gentamicin with the same dosing schedule could be offered as an alternative. A different aminoglycoside antibiotic could be offered in cases of gentamicin resistance based on culture susceptibility results. A pharmacist was consulted for dosage adjustment if a patient had an estimated glomerular filtration rate <40 mL/min/1.73 m2. In cases of complicated UTI, we recommended hospitalization for extended intravenous antibiotic treatment with careful drug level monitoring.
Collection of faecal samples
Faecal samples were collected by patients into sterile containers just prior to initiation of UTI treatment (day 0), at its completion (∼days 3–4) and ∼1 week after initiation (∼days 6–8). The samples were kept frozen until retrieval by the research assistant, transported on ice to the laboratory and stored at −80 °C until used.
Analysis of the faecal microbiome
To characterize the bacterial community, DNA was extracted from 250–300 mg faecal samples, using the MoBio PowerSoil DNA isolation kit (MoBio Laboratories, Inc., Carlsbad, CA, USA). The V5–V6 hypervariable regions of the 16S rRNA gene were amplified using the BSF784/1065R primer set and paired-end sequenced at the University of Minnesota Genomics Center to a read length of 300 nt.10 Raw sequence data were deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession number SRP077993. Sequence processing and analysis were performed using mothur software (version 1.34.0).11 Sequences were paired-end joined and trimmed for quality as described previously.12 High-quality sequences were aligned against the SILVA database (version 119) and subjected to a 2% pre-clustering step, and chimeras were removed using UCHIME.13–15 Operational taxonomic units (OTUs) were assigned at the 97% similarity level using the furthest-neighbour algorithm and taxonomic classification was performed against the Ribosomal Database Project classifier (version 14).16 For unbiased group comparisons, samples were rarefied by random subsample to 65 450 sequences per sample.17
Bacterial communities from UTI patient samples were also evaluated against those in faecal samples of 26 FMT patients (28 pre-FMT and 21 post-FMT samples), collected >1 month following FMT, as well as a series of three samples from one patient treated with piperacillin/tazobactam for a complicated UTI within days of her FMT. In addition, to compare normal fluctuations within an individual, the UTI patient communities were compared with faecal samples from five healthy FMT donors collected during the same range (7–8 days). FMT patient sample data were processed as described above with rarefaction to 11 500 reads for comparison and raw data were deposited in the SRA under BioProject accession numbers SRP070464 and SRP064361 for patients and healthy individuals, respectively.
The α diversity was measured using the Shannon index and the abundance-based coverage estimate (ACE) for richness was calculated using mothur, and ANOVA analysis was performed using XLSTAT (version 2015.1.01) software (Addinsoft, Belmont, MA, USA). Ordination by principal coordinate analysis of Bray–Curtis dissimilarity matrices and comparison of β diversity using analysis of similarity (ANOSIM) were also performed in mothur.18,19 Variance partitioning was performed using constrained redundancy analyses, as described previously.20
Results
Efficacy of the 3 day outpatient UTI treatment protocol
We offered parenteral aminoglycoside treatment to 20 consecutive recipients of FMTs for RCDI syndrome who developed UTI after the FMT procedure within our programme. One patient with renal insufficiency declined the option because of her concern about nephrotoxicity; she was uneventfully treated with sulfamethoxazole/trimethoprim for her UTI. All other patients preferred the aminoglycoside regimen. The majority (18/19) of patients had uncomplicated UTIs. Not unexpectedly, all patients were female (Table 1). The median time of the first UTI episode from their FMT was 2 months. Three patients had more than one UTI episode during the post-FMT follow-up. All uncomplicated UTIs were successfully treated with gentamicin and had prompt resolution of their urinary symptoms. None of the patients had recurrence of CDI.
Table 1.
Clinical patient characteristics
| Patient | Age (years) | Sex | Interval between FMT and treatment of first UTI (months) | Interval between FMT and subsequent treatments for recurrent UTI (months) | Uropathogen | Antibiotic resistance |
|---|---|---|---|---|---|---|
| 1a | 35 | female | 1 | 2, 12, 17 | Escherichia coli | ampicillin, trimethoprim/sulfamethoxazole |
| 2 | 58 | female | 23 | Escherichia coli and Klebsiella pneumoniae | ||
| 3a | 73 | female | 2 | Klebsiella pneumoniae | ampicillin and nitrofurantoin | |
| 4a | 66 | female | 18 | Escherichia coli | ||
| 5 | 78 | female | 1.5 | 12, 13 | Escherichia coli | ampicillin |
| 6 | 75 | female | 2 | Escherichia coli | ampicillin, trimethoprim/sulfamethoxazole, piperacillin | |
| 7 | 80 | female | 0.25 | Klebsiella pneumoniae | nitrofurantoin | |
| 8b | 77 | female | 4 | 6, 9 | Proteus mirabilis and Pseudomonas aeruginosa | ampicillin, cefazolin, ciprofloxacin, gentamicin, levofloxacin, nitrofurantoin, tobramycin, trimethoprim/sulfamethoxazole |
| 9 | 53 | female | 2 | Escherichia coli | NA | |
| 10 | 82 | female | 6 | Klebsiella pneumoniae | ampicillin, nitrofurantoin | |
| 11 | 34 | female | 2 | 5 | Escherichia coli and Klebsiella pneumoniae | ampicillin |
| 12 | 34 | female | 4 | Escherichia coli | ||
| 13 | 60 | female | 2 | Klebsiella oxytoca | ampicillin, cefazolin, nitrofurantoin | |
| 14 | 62 | female | 11 | NA (lost specimen) | ||
| 15 | 50 | female | 32 | β-haemolytic Streptococcus | ND | |
| 16 | 29 | female | 5 | Klebsiella oxytoca | ampicillin | |
| 17 | 33 | female | 0.5 | Escherichia coli | ampicillin | |
| 18 | 58 | female | 2 | Escherichia coli | ampicillin, ampicillin/sulbactam | |
| 19 | 49 | female | 2 | Klebsiella pneumoniae | ampicillin, nitrofurantoin |
NA, not available; ND, not done.
Faecal samples collected from these three patients underwent faecal microbiome analysis by 16S rRNA gene profiling in the course of gentamicin treatment.
This patient had a complicated UTI post-FMT and was initially treated with piperacillin/tazobactam (faecal samples collected with this treatment) and subsequently was treated with amikacin.
One patient in this series had a complicated UTI with gentamicin-resistant Proteus mirabilis and Pseudomonas aeruginosa. This patient had a history of nephrolithiasis and urinary stents placed to relieve ureteral stenosis caused by prior radiation treatment of endometrial cancer. She had a history of frequent UTIs prior to FMT, but at the time of initial evaluation she had not had a UTI episode for over 3 months, having been placed by her infectious disease physician on a regimen of methenamine hippurate and ascorbic acid. Two days after her FMT she had a urinary stent exchange and was given piperacillin/tazobactam during the procedure. She relapsed with CDI within a week. As she was participating in the faecal analysis study, she provided samples during this sequence of events. Ultimately she received another FMT after treatment with fidaxomicin, which was chosen due to persistent diarrhoeal symptoms with a positive test for C. difficile toxin B by PCR while on vancomycin. Four months after her second FMT she developed another UTI, for which she was hospitalized at the University of Minnesota hospital and treated with amikacin dosed every 24 h in consultation with the hospital pharmacist based on her renal function. She was discharged after 3 days of treatment with amikacin without further antibiotics. Subsequently, over a period of 8 months she developed two more episodes of UTIs, both of which were treated uneventfully with amikacin at her local hospital.
Adverse events
The intramuscular injection regimen was well tolerated by patients. One patient (Patient 5, Table 1) had two UTIs within a month and developed tenderness at the gentamicin injection sites over the gluteal muscles. This was treated with warm packs and a 1 week course of ibuprofen. Her intramuscular injections of gentamicin were changed to intravenous infusions, following the same dosing regimen.
Gut bacterial community structure during UTI treatment
In order to test our presumption that parenteral administration does not disrupt the gut microbial communities, we analysed faecal microbiota composition in three patients (indicated with ‘a’ in Table 1) in the course of their UTI treatment with gentamicin. Although there was some variability in α diversity between individuals (Table 2), it was not statistically affected by this UTI treatment (P = 0.810 and 0.744 for Shannon and ACE indices, respectively).
Table 2.
α diversity indices (mean ± SEM) for patient samples collected on three dates
| Patient no. | Coverage (%) | Number of OTUs observed | Shannon | ACE |
|---|---|---|---|---|
| 1 | 99.5 ± 0.1 | 733 ± 42 | 4.30 ± 0.04 | 1893 ± 344 |
| 2 | 99.8 ± 0.0 | 356 ± 27 | 3.08 ± 0.25 | 817 ± 89 |
| 3 | 99.6 ± 0.0 | 583 ± 42 | 4.02 ± 0.08 | 1613 ± 310 |
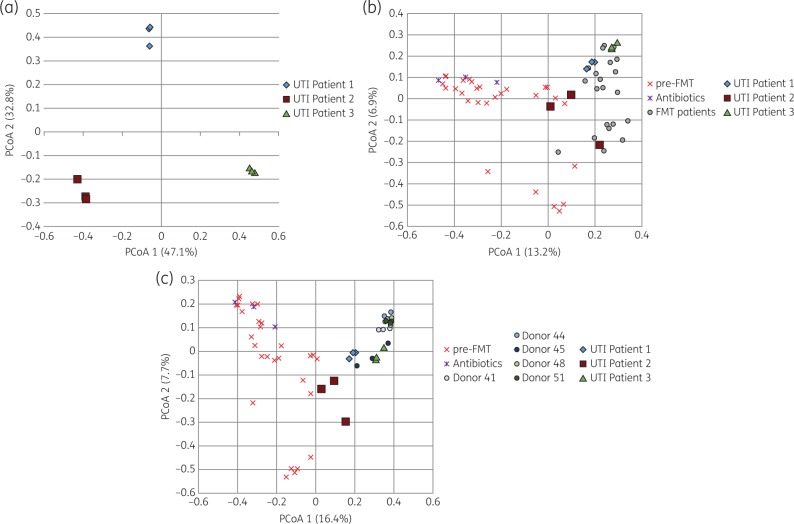
Ordination of samples by principal coordinate analysis (Figure 1a) revealed separation of samples by patient, and β diversity (community composition) was also significantly different between patients (P = 0.003). Administration of gentamicin did not result in substantial shifts in faecal bacterial composition since pre-gentamicin and post-gentamicin samples from individual patients clustered together. When compared against the bacterial communities of FMT patients, UTI patient samples (pre- and post-gentamicin) fell within the normal range of other post-FMT patient samples (Figure 1b), although Patient 2 samples were near the border between pre-FMT and post-FMT patient samples. A similar pattern was seen when comparing UTI patient samples (pre- and post-gentamicin) against the bacterial communities of healthy FMT donors (Figure 1c). The samples of the post-FMT patient treated for her complicated UTI with piperacillin/tazobactam clustered together with pre-FMT patient samples (Figure 1b and c).
Figure 1.
Principal coordinate analysis (PCoA) of faecal samples. (a) Three individual post-FMT patients treated with gentamicin. Each patient submitted three samples, one before gentamicin and two during or after treatment. (b) The same samples (as in a) from the three patients are shown within the principal coordinate analysis that contains pre-FMT (red crosses) and post-FMT (grey circles) faecal samples from patients who did not get any new antibiotics after FMT and one patient who received piperacillin/tazobactam shortly after her FMT (blue crosses). (c) The same samples (as in a) from the three patients are shown within the principal coordinate analysis that contains pre-FMT faecal samples (red crosses) and healthy donors (grey circles). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
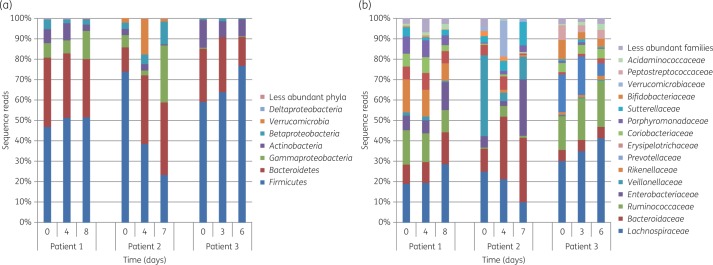
Variance partitioning of differences in relative abundances of phyla among samples revealed that the difference between patients accounted for 71.6% of the variation, while time alone accounted for 28.4%. The taxonomic composition of bacteria at the phylum and family levels in faecal samples remained relatively constant throughout the observation period in Patients 1 and 3, but showed greater fluctuation in Patient 2 (Figure 2). The dominant distal gut phyla, Bacteroidetes and Firmicutes, remained intact in all patients, although in Patient 2 there was prominent variability in the content of Veillonellaceae and Enterobacteriaceae (Figure 2b).
Figure 2.
Taxonomic analysis of the faecal microbiota from gentamicin-treated UTI patients at phylum (a) and family (b) levels. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
While FMT is remarkably effective at treating RCDI and restores the normal composition and functionality of gut microbiota, FMT recipients continue to experience high risk of re-infection with C. difficile in the initial months and years after the procedure following new antibiotic provocations.6 Although the reasons for this are not entirely clear, one potential factor is contamination of the patient home environment by C. difficile spores.21 Such contamination is likely greatest in patients with multiply recurrent CDI who require FMT as rescue therapy because of the long duration of their struggle with CDI and episodic faecal incontinence experienced by the majority of these patients during active infections.9
Treatments of UTIs are common triggers of CDI and both infections preferentially affect female patients.22,23 We were frustrated with the problem of post-FMT re-infections with CDI following UTI treatments in our programme and sought an effective solution that would minimize perturbation of protective gut microbiota. We introduced the parenteral aminoglycoside regimen into our clinical programme after failure to find a safe oral antibiotic for UTIs.
Aminoglycosides were once commonly used to treat UTIs, but were largely abandoned because of the associated risks of nephrotoxicity and ototoxicity as well as the inconvenience associated with parenteral administration.7 Although aminoglycosides are traditionally administered several times per day, animal experiments and clinical studies demonstrate that once-daily regimens are associated with lower toxicity without loss of antibacterial efficacy.24 Less frequent dosing results in less contact of the drug with the target host tissue binding sites and enables a period of cellular processing of internalized aminoglycoside. Notably, the fall of the drug level below the MIC during once-daily dosing does not impair its efficacy, possibly due to the persistent antibiotic effect (PAE). Less frequent dosage also results in higher peak drug concentrations and longer PAE.25 The once-daily dosing is also feasible in an outpatient setting. Although our regimen involves three consecutive nurse clinic visits, it was clearly preferable to our patients relative to the risk of having another episode of CDI.
The investigation of the faecal bacterial composition during UTI treatment in three patients from this series was consistent with minimal penetration of the aminoglycoside antibiotic into the colon microbial compartment. We have previously demonstrated that FMT results in prompt normalization of faecal bacterial community structure configuration and subsequent compositional fluctuation within this normal range.26 The same held true in this study, with little evidence that intramuscular administration of gentamicin impacted the gut microbiota in any way. In the case in which piperacillin/tazobactam was administered shortly after FMT, donor microbiota failed to engraft.
Illustrative of the challenge associated with antibiotic UTI treatment after FMT, Patient 2 was a recipient of FMT in 2009 and developed a UTI 5 years later. The UTI was treated with ciprofloxacin, which triggered a re-infection with C. difficile. The CDI became recurrent following vancomycin therapy and she ultimately underwent another FMT. She then developed a new UTI 2 months later. Her post-FMT faecal microbiome composition was intermediate between healthy and pre-FMT control samples. Interestingly, it showed a spike in Enterobacteriaceae, a microbial family that harbours the most common potential uropathogens, following completion of gentamicin treatment. Most members of this family are susceptible to aminoglycosides and the observed rise of Enterobacteriaceae following treatment is consistent with minimal penetration of this antibiotic into the gut. Interestingly, we have previously reported a patient, enrolled in a different study tracking the composition of faecal microbiota after FMT, who developed a spike in the relative abundance of Enterobacteriaceae prior to becoming symptomatic with a UTI.27 In the future it may be interesting to explore the dynamics of gut microbial communities, especially among members of Enterobacteriaceae, in contributing to recurrent UTI pathogenesis.
Our study has multiple limitations. It is an uncontrolled case series limited to one centre. We found resistance to gentamicin only in one patient. Other geographical locations may harbour greater prevalence of gentamicin resistance, although that typically does not translate into resistance to other members of the aminoglycoside antibiotic class.28 The faecal microbiome studies with gentamicin treatment were limited to three patients, although the results were clearly consistent with minimal (if any) effects of parenteral gentamicin on gut microbiota, as expected from the drug pharmacological properties. Microbiome studies, by themselves, may also not be entirely reassuring and may require supplementation with additional data. For example, while nitrofurantoin administration was shown to cause only relatively subtle perturbation of the gut microbiome,29,30 it is still associated with increased risk of CDI.3
In conclusion, we report a practical outpatient treatment regimen for uncomplicated UTIs that may be especially useful in patients at great risk of CDI. Our experience thus far was limited to patients treated previously with FMT for RCDI, which certainly fit into that group. It is possible that this regimen may also be useful for patients that are not anticipated to benefit from FMT for RCDI due to frequent antibiotic treatments for recurrent UTIs. In fact, in our programme anticipated antibiotic usage within 3 months, including an established syndrome of recurrent UTIs, is a contraindication for FMT. The regimen described here might be an option for such patients, although safety of this regimen needs to be determined with respect to renal function and ototoxicity if it is to be used three or more times per year. Currently, there are few antibiotic options that can selectively target pathogens and avoid disrupting the indigenous gut microbiota. Yet gut microbiota play critical roles in protecting the host from pathogens by providing colonization resistance. Therefore, singular focus on pathogens without consideration of the bystander effect that treatments may have on the gut microbiota is short-sighted. The regimen described here is one option that can be considered in specific clinical situations and worthy of further multicentre clinical trials.
Funding
This research was made possible by support of grants from the National Institutes of Health (NIH) 1R21-AI114722-01 (A. K., M. J. S.) and the Minnesota’s Discovery, Research, and InnoVation Economy grant from the University of Minnesota (A. K., M. J. S.).
Transparency declarations
None to declare.
References
- 1. Stevens V, Dumyati G, Fine LS. et al. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011; 53: 42–8. [DOI] [PubMed] [Google Scholar]
- 2. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40: 1–15. [DOI] [PubMed] [Google Scholar]
- 3. Hirschhorn LR, Trnka Y, Onderdonk A. et al. Epidemiology of community-acquired Clostridium difficile-associated diarrhea. J Infect Dis 1994; 169: 127–33. [DOI] [PubMed] [Google Scholar]
- 4. Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2012; 9: 88–96. [DOI] [PubMed] [Google Scholar]
- 5. van Nood E, Vrieze A, Nieuwdorp M. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407–15. [DOI] [PubMed] [Google Scholar]
- 6. Brandt LJ, Aroniadis OC, Mellow M. et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107: 1079–87. [DOI] [PubMed] [Google Scholar]
- 7. Nicolle LE. Urinary tract infection: traditional pharmacologic therapies. Am J Med 2002; 113Suppl 1A: 35S–44S. [DOI] [PubMed] [Google Scholar]
- 8. Pitt HA, Roberts RB, Johnson WD., Jr. Gentamicin levels in the human biliary tract. J Infect Dis 1973; 127: 299–302. [DOI] [PubMed] [Google Scholar]
- 9. Khoruts A, Rank KM, Newman KM. et al. Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol 2016; 14: 1433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Claesson MJ, Wang Q, O'Sullivan O. et al. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res 2010; 38: e200.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schloss PD, Westcott SL, Ryabin T. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009; 75: 7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Staley C, Gould TJ, Wang P. et al. Evaluation of water sampling methodologies for amplicon-based characterization of bacterial community structure. J Microbiol Methods 2015; 114: 43–50. [DOI] [PubMed] [Google Scholar]
- 13. Pruesse E, Quast C, Knittel K. et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 2007; 35: 7188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huse SM, Welch DM, Morrison HG. et al. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol 2010; 12: 1889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edgar RC, Haas BJ, Clemente JC. et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011; 27: 2194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cole JR, Wang Q, Cardenas E. et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 2009; 37: D141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gihring TM, Green SJ, Schadt CW. Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ Microbiol 2012; 14: 285–90. [DOI] [PubMed] [Google Scholar]
- 18. Bray J, Curtis J. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 1957. [Google Scholar]
- 19. Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust Ecol 1993; 18: 117–43. [Google Scholar]
- 20. Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology 1992; 73: 1045–55. [Google Scholar]
- 21. Shaughnessy MK, Bobr A, Kuskowski MA. et al. Environmental contamination in households of patients with recurrent Clostridium difficile infection. Appl Environ Microbiol 2016; 82: 2686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lessa FC, Mu Y, Bamberg WM. et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372: 825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother 2000; 46Suppl 1: 1–7. [PubMed] [Google Scholar]
- 24. Gilbert DN. Once-daily aminoglycoside therapy. Antimicrob Agents Chemother 1991; 35: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapusnik JE, Hackbarth CJ, Chambers HF. et al. Single, large, daily dosing versus intermittent dosing of tobramycin for treating experimental pseudomonas pneumonia. J Infect Dis 1988; 158: 7–12. [DOI] [PubMed] [Google Scholar]
- 26. Weingarden A, Gonzalez A, Vazquez-Baeza Y. et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 2015; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamilton MJ, Weingarden AR, Unno T. et al. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 2013; 4: 125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. George CE, Norman G, Ramana GV. et al. Treatment of uncomplicated symptomatic urinary tract infections: resistance patterns and misuse of antibiotics. J Family Med Prim Care 2015; 4: 416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stewardson AJ, Gaia N, Francois P. et al. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin Microbiol Infect 2015; 21: 344.e1–11. [DOI] [PubMed] [Google Scholar]
- 30. Vervoort J, Xavier BB, Stewardson A. et al. Metagenomic analysis of the impact of nitrofurantoin treatment on the human faecal microbiota. J Antimicrob Chemother 2015; 70: 1989–92. [DOI] [PubMed] [Google Scholar]