Abstract
Introduction:
Abstinence reinforcement is efficacious for improving smoking treatment outcomes, but practical constraints related to the need for multiple in-person carbon monoxide (CO) breath tests daily to verify smoking abstinence have limited its use. This study tested an mHealth procedure to remotely monitor and reinforce smoking abstinence in individuals’ natural environment.
Methods:
Eligible treatment-seeking smokers (N = 90) were randomized to (1) usual care and ecological monitoring with abstinence reinforcement (mHealth reinforcement) or (2) without reinforcement (mHealth monitoring). Usual care was 8 weeks of transdermal nicotine and twice-weekly telephone counseling. Following training, an interactive voice response system prompted participants to conduct CO tests 1–3 daily at pseudorandom times (7 am to 10 pm) for 4 weeks. When prompted, participants used a study cell phone and CO monitor to complete a CO self-test, video record the process, and submit videos using multimedia messaging. mHealth reinforcement participants could earn prizes for smoking-negative on-time CO tests. The interactive voice response generated preliminary earnings immediately. Earnings were finalized by comparing video records against participants’ self-reports.
Results:
mHealth reinforcement was associated with a greater proportion of smoking-negative CO tests, longest duration of prolonged abstinence, and point-prevalence abstinence during the monitoring/reinforcement phase compared to mHealth monitoring (p < .01, d = 0.8–1.3). Follow-up (weeks 4–24) analyses indicated main effects of reinforcement on point-prevalence abstinence and proportion of days smoked (p ≤ .05); values were comparable by week 24.
Conclusions:
mHealth reinforcement has short-term efficacy. Research on methods to enhance and sustain benefits is needed.
Implications:
This study suggests that mHealth abstinence reinforcement is efficacious and may present temporal and spatial opportunities to research, engage, and support smokers trying to quit that do not exist with conventional (not technology-based) reinforcement interventions.
Introduction
Reinforcement interventions motivate behavior change through systematic reinforcement of objectively verified target behaviors like abstinence.1,2 Abstinence reinforcement is highly efficacious for improving substance use treatment outcomes.3–6 Applying reinforcement interventions to the treatment of cigarette smoking has received less attention than that for illicit drug use disorders. One obstacle has been the logistical problems arising from the relatively short half-life of breath carbon monoxide (CO) (approximately 4 hours in adults7) which necessitates multiple breath CO tests daily to objectively verify smoking status in the context of reinforcement programs. An additional complication is the predictability that occurs with scheduled visits for breath tests, as smokers may adjust their smoking behavior to avoid detection. One alternative to multiple tests daily is to monitor smoking with CO tests during the initial days of the quitting process while abstinence is established and then transition to cotinine testing. Cotinine is a major metabolite of nicotine with a half-life of about 19 hours,7 making it possible to limit testing to about twice weekly. Establishing initial abstinence with frequent breath tests and then transitioning to visits 2 to 3 times weekly for cotinine-based reinforcement can be efficacious,8,9 but cotinine-based reinforcement cannot be used concurrently with nicotine replacement pharmacotherapy.
Another option is to use Internet-based services to monitor and reinforce smoking abstinence multiple times daily in individuals’ natural environment.10–16 Using basic cell phone functions (not requiring a smartphone and without Internet or data plan) to monitor and reinforce smoking abstinence has even greater potential than requiring smart phone Internet services in terms of increased reach at lower costs,17,18 if efficacious. Importantly, basic cell phone multimedia strategies generally can improve treatment response, follow-up, and reach,19 and monitoring substance use with automated data capture (i.e., interactive voice response technology [IVR]) on such phones is feasible and cost-effective.20–22 Patients also find it an acceptable form of communication with treatment providers in the context of reporting smoking status and healthcare-related needs.23 Notably, combining automated services with human interaction may be important.24
In this study, we built on our initial work developing a mobile health (mHealth) system of using mobile devices to monitor and reinforce pro-health behaviors ecologically.25,26 According to the World Health Organization, mHealth, for example, involves the provision of health services and information via mobile technologies such as mobile phones, tablet computers, and personal digital assistants.27 The purpose of the current study was to use mHealth reinforcement to target smoking abstinence and examine outcomes compared to mHealth monitoring without reinforcement. Starting on the target quit date, 8 weeks of transdermal nicotine began, as well as 4 weeks of twice-weekly, telephone-based practical counseling along with daily monitoring of smoking status via IVR, cell phone, and CO monitor. Those in the mHealth reinforcement condition received vouchers for 4 weeks based on CO readings. We hypothesized that mHealth reinforcement would improve smoking outcomes during the 4-week daily monitoring/reinforcement period, and examined outcomes through week 24 (a 20-week follow-up period).
Methods
Participants and Setting
Participants (N = 90) were recruited for a study including transdermal nicotine and counseling through E-mail, flyers, and print advertisements. Participants attended an in-person baseline interview in which eligibility was established. Inclusion criteria were (1) at least 10 cigarettes daily verified by CO ≥ 8 parts per million (ppm), (2) no past-year abstinence exceeding 3 months, (3) intent to quit within 3 weeks (score ≥ 7 out of 10, “How much do you want to quit smoking within the next 3 weeks?”15, (4) aged 18 years or older, and (5) mailing address and valid photo ID. Exclusion criteria were (1) past month behavioral or pharmacotherapy for smoking, (2) serious and unstable psychiatric illness (e.g., schizophrenia, non-nicotine substance use disorder) or medical disease, or contraindication for transdermal nicotine, (3) pregnant, nursing a child, or not using effective contraceptive if female, (4) ongoing use of monoamine oxidase inhibitors, antipsychotics, mood stabilizers, bupropion, or naltrexone, and (5) not English speaking.
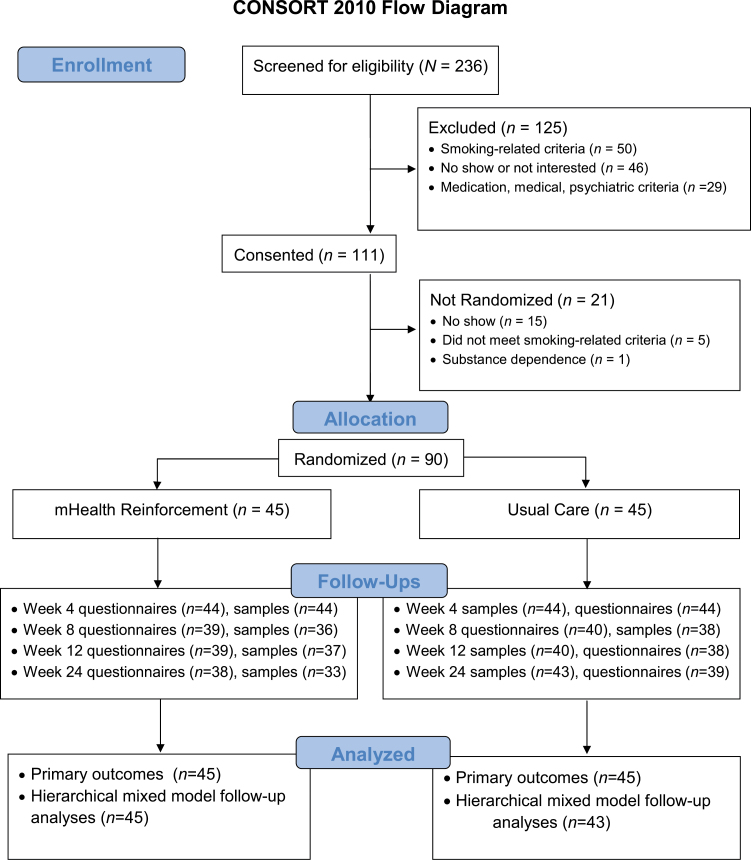
Participants provided written informed consent for study procedures and a second optional consent for audiotaping for staff oversight purposes only (none declined). A Masters-level licensed research therapist completed the UMASS Center for Tobacco Treatment and Control online course on working with smokers prior to providing telephone counseling. Physician and nursing staff completed medical procedures, and a Baccalaureate-level research assistant completed remaining assessments. Procedures were approved by an Institutional Review Board and occurred at a university health center between January 2012 and December 2014. See Figure 1 for participant flow. The trial ended when the target sample size was reached. Follow-up rates did not differ between conditions (p > .05).
Figure 1.
The flow of participants through the study.
Assessments
A patient form captured gender, ethnicity, race, smoking history, and eligibility criteria. Additional measures completed at intake were the Fagerström Test of Nicotine Dependence28 and the Readiness to Change Questionnaire29. The presence of past-year and past 6-month substance use disorder was determined using checklists based on Diagnostic and Statistical Manual of Mental Disorders-IV-TR criteria.30
At intake, baseline, and each counseling session, the timeline followback procedure31,32 captured frequency and intensity of smoking, other substance use, and medication use in the past 30 days or since the last visit. CO breath tests were conducted using a calibrated Micro Plus Smokerlyzer (Bedfont Scientific Ltd, Kent, England), which stores ID, test time, and up to about 100 tests. The abstinence criterion was CO ≤ 6 ppm. These procedures were repeated at the follow-up visits at weeks 4, 8, 12, and 24. In addition, urine samples were tested for cotinine at intake, week 12, and week 24 using the semi-quantitative lateral flow enzyme immunoassay onsite test strip Accutest NicAlert (JANT Pharmacal Corporation, Encino, CA), with values ≤ level 1 (0–30mg/mL) coded smoking-abstinent. Patient satisfaction was assessed at the end of the monitoring/reinforcement phase with an in-house form.
Procedures
Following intake, participants completed two quit preparation behavioral counseling in-person sessions, submitted a CO and cotinine sample, and set a target quit date for within 2 weeks. Telephone counseling, transdermal nicotine, and daily monitoring of smoking status with or without abstinence-contingent reinforcement started on the quit date.
Telephone Counseling and Transdermal Nicotine
For all participants, brief counseling33 (~10 minutes) was scheduled to occur twice weekly for 4 weeks by phone. Discussion included personal reasons for quitting, skills-based items, and craving control strategies. Self-reported smoking status was documented. The study also provided 8 weeks of transdermal nicotine (typically 21mg patches for 4 weeks, 14mg for 2 weeks, and 7mg for 2 weeks). Use was encouraged but not required for continued participation. Adverse events and pregnancy status were monitored at each visit. Clinical supervision was aided by review of audio recorded counseling sessions.
On the target quit date, participants (N = 90) were randomly assigned (allocated 1:1) to one of two treatment conditions using an urn procedure34 and stratified on (1) at least one smoking-negative CO during baseline (Reinforcement/Monitoring n = 3/2, p = .14) and (2) at least 20 cigarettes daily at intake (Reinforcement/Monitoring n = 15/21, p = .23).
Usual Care With Abstinence Monitoring (mHealth Monitoring)
Following training and independent demonstration of CO and video procedures, participants used the study cell phone to complete CO tests when prompted by IVR for 4 weeks. Participants were instructed that CO test prompts would occur up to 3 times daily between 7 am and 10 pm, with the exact number and timing not disclosed. In actuality, prompts occurred 3 times daily in week 1 and typically 1 but up to 3 times daily in weeks 2–4 (52 tests total), with a minimum of 4 hours between prompts.
CO testing was a five-step process requiring about 3 minutes to complete. First, at participants’ preferred time (e.g., 7 am), they received an IVR reminder to keep study equipment on hand throughout the day. Second, when a test came due, the IVR called participants to request the submission for a CO test within 2 hours. For calls not received, a reminder call occurred 1 hour later. Third, the CO self-test was video recorded. To that end, a phone with a front-facing lens is held at about arms-length (or placed in a stand at about head height) and the video record function is activated. The CO monitor is reset to zero (audible beep) and displayed to the lens. A deep breath is drawn, and the test button on the monitor is pushed. After a 15-second countdown, the breath is exhaled for at least 4 seconds, the result is displayed to the lens, and the recording is stopped. The video is reviewed for quality and the process is repeated if needed (e.g., too blurry) (anecdotally, rarely occurred). Fourth, the date- and time-stamped CO video is submitted within the 2-hour window to staff using multimedia messaging. Fifth, the participant calls the IVR (or the system calls the participant) to report results. If smoking-negative, a congratulatory statement follows, with a reminder to expect the same procedures throughout the day. Staff manually compared video recorded test results against IVR reports to confirm accuracy (only one instance of inaccuracy occurred in each condition). CO test results were discussed with participants during counseling sessions in weeks 1–4.
Usual Care With Abstinence Monitoring Plus Reinforcement (mHealth Reinforcement)
For mHealth reinforcement participants, in addition to the above, smoking-negative CO tests resulted in chances for prizes. CO monitoring steps 4 and 5 (above) were modified as follows. Abstinence-contingent reinforcement was guaranteed (100% likelihood) in weeks 1 and 2 and occurred 50% of the time in weeks 3 and 4. For each draw earned in weeks 1 and 2, the computer program selected at random 1 of 70 solutions (equivalent to drawing 1 card from a 70-card bowl in a typical in-person prize reinforcement program). Sixty-five solutions were a “small” ~$1 prize (e.g., sugar-free gum, mints), four were a “large” ~$20 prize (e.g., target gift card), and one was a “jumbo” ~$100 prize (e.g., bluetooth headset). Weeks 3 through 4, 80 draws were possible for on-time smoking-negative tests. For this phase, the computer randomly selected 1 of 280 solutions, 50% of which were worth a prize: 130 small prizes, 9 large, and 1 jumbo.
Draws escalated for consecutive negative tests and reset with a missed or drug-positive test, to promote high rates of abstinence.35 In this study, the IVR was programmed to “draw” at least one solution from many possible solutions for each on-time and negative test. One “draw” was earned for the first day of all tests submitted on-time and reading smoking-negative. To promote sustained abstinence, each day with all tests smoking-negative and on-time resulted in draws per test increasing by 1 for the following day (one draw per test meeting criteria on day 1 and two draws per test on day 2, etc.). Draws increased in this manner for the first 4 consecutive days of all on-time smoking-negative tests and thereafter remained at four draws per consecutive test. A positive or missed test reset draws to 1 for the next negative test. Transition from the 100% winning prize program to the 50% winning prize program did not affect the number of draws available. Participants were aware of the probabilities of winning and of changes in probabilities over time.
Participants received a catalog to take home that contained photos and listed examples of types of prizes so that prizes could be selected when earned. Staff encouraged participants to redeem earnings at least weekly but most elected for one-time redemption at the end. mHealth reinforcement participants could earn up to 190 draws in total (110 in weeks 1–2) for on-time smoking-negative CO tests, with an expected value of about $502.
Compensation
Participants earned $25 for the in-person intake, $35 for each in-person follow-up (weeks 4, 12, and 24), $10 per cotinine sample (intake, week 12, and week 24), and $50 for returning study equipment in good condition (100% done). To promote adherence, participants in both treatment conditions also received $1 for each CO sample submitted and a $10 bonus for submitting all CO samples each week.
Data Analysis
Demographic and baseline data were examined36 prior to proceeding with outcome analyses. Transdermal nicotine and counseling services received, and adherence with submitting CO tests, were examined for differences by condition with analysis of covariance. Variables that may affect outcomes were included as covariates, including age, gender, days of transdermal nicotine use, and percent of CO tests submitted and percent of counseling sessions received. The latter two variables were not normally distributed and were therefore coded according to whether they met ≥ 80% (no/yes).
To assess effects of mHealth reinforcement compared to mHealth monitoring during the 4-week monitoring/reinforcement intervention period, primary outcomes were as follows: (1) percent of smoking-negative CO tests, (2) longest duration of prolonged abstinence, and (3) 7-day point-prevalence abstinence (PPA; days 22–28). Percent of negative CO tests was computed with the number submitted in the denominator. Longest duration of abstinence was defined as the greatest number of days of no smoking on self-report and all CO tests reading negative, based on all available data. Percent negative CO and longest duration of abstinence were examined for group differences twice, with the covariates noted above (analysis of covariance) and without (analysis of variance). PPA (based on all available CO and self-report data) was examined for group differences using logistic regression, also with and without simultaneous entry of the covariates noted above. The study was powered with 1 − B = .80 and alpha less than .05 to detect about a 10% difference between conditions on PPA based on previous smoking abstinence reinforcement studies.37–39
Secondary outcomes included 7-day PPA at weeks 8, 12, and 24 and continuous abstinence between the quit date and each follow-up (no/yes). Hierarchical mixed models specified for dichotomous outcomes examined changes over time and between conditions, using Laplace approximation and reporting the unit-specific estimates. Changes in timeline followback proportion of days smoked and mean cigarettes per day on days smoked week 4 through week 24 were also examined with hierarchical linear mixed models.
Cohen’s d and odds ratios are presented as effect size estimates. Differences between conditions were evaluated at alpha = .05. Analyses were conducted with IBM SPSS Statistics version 21 and SuperMix version 1.2 for hierarchical models.
Results
Demographic and Baseline Characteristics
Demographic and baseline data did not differ significantly between conditions (Table 1).
Table 1.
Demographic and Baseline Characteristics
| Variables | mHealth reinforcement | mHealth monitoring | p |
|---|---|---|---|
| (n = 45) | (n = 45) | ||
| Age (mean, SD) | 44.4 (10.3) | 45.5 (11.6) | .64 |
| Female (n) | 55.6% (25) | 60.0% (27) | .67 |
| Ethnicity (n) | |||
| Not Hispanic | 88.9% (40) | 91.1% (41) | .34 |
| Hispanic | 6.7% (3) | 8.9% (4) | |
| Not reported | 4.4% (2) | 0.0% (0) | |
| Race (n) | .54 | ||
| European American | 71.1% (32) | 77.8% (35) | |
| African American | 15.6% (7) | 11.1% (5) | |
| Asian/More than one/not reported | 13.3% (6) | 11.1% (5) | |
| Cigarette smoking characteristics | |||
| Age first smoked (mean, SD) | 16.7 (4.2) | 16.5 (3.6) | .81 |
| Past 30 days cigarettes per day (mean, SD) | 17.6 (9.5) | 20.0 (8.4) | .23 |
| Packyearsa (mean, SD) | 358.6 (302.2) | 466.1 (385.6) | .15 |
| Fagerström score (mean, SD) | 3.5 (1.4) | 2.9 (1.5) | .71 |
| First cigarette within 5 minutes of waking (n) | 37.8% (17) | 35.6% (16) | .83 |
| Intake CO value (mean, SD) | 16.0 (8.3) | 16.4 (7.3) | .84 |
| Intake cotinine levelb (mean, SD) | 5.8 (0.4) | 5.9 (0.5) | .81 |
| Lives with smoker(s) (n) | 53.3% (24) | 35.6% (16) | .09 |
| At least 1 baseline CO ≤ 6 ppm (n)c | 6.7% (3) | 4.4% (2) | .65 |
| Prior quit history | |||
| ≥24-hour voluntary quit attempts (mean, SD) | 6.9 (20.4) | 6.9 (11.6) | .99 |
| Longest quit attempt (months; median, IQR) | 3.3 (8.3) | 1.6 (8.2) | .18 |
| Previous use of nicotine replacement (%, n) | 48.9% (22) | 55.6% (25) | .53 |
| Previous use non-nicotine medication (%, n) | 24.4% (11) | 33.3% (15) | .35 |
| Readiness to change (mean, SD) | 6.2 (1.8) | 6.0 (1.9) | .61 |
Readiness to Change scores range from 2 to 14.
CO = carbon monoxide; IQR = interquartile range; SD = standard deviation.
aPack years is defined as (Current cigarettes smoked per day) / (Years smoked that amount).
bThe highest salivary cotinine test result possible was 6, equivalent to >2000ng/mL cotinine equivalents per the manufacturer.
cStratification variable obtained during the baseline period.
Treatment Exposure and Adherence
Mean (SD) percent of days of transdermal nicotine use was 59.9% (2.7%) in the mHealth reinforcement condition and 53.2% (28.0%) in mHealth monitoring, F(1, 88) = 1.49, p = .23 (mean (SD) days of use was 33.6 (13.3) days and 29.8 (15.7) days, respectively). Three mHealth monitoring participants elected not to use the study-provided transdermal nicotine at all compared to no participants in the mHealth reinforcement condition. Conditions did differ on the mean (SD) percent of counseling sessions received, at 82.2% (27.5%) and 68.6% (34.6%), respectively, F(1, 88) = 4.27, p = .04, and mean (SD) percent of CO tests submitted, at 84.5% (19.6%) and 63.6% (33.2%), respectively, F(1, 88) = 13.29, p = .00. All participants returned study equipment in good working order. mHealth reinforcement condition participants earned a mean (SD) $344.18 ($187.87) for on-time smoking-negative CO tests.
Smoking Outcomes
4-Week Monitoring/Reinforcement Period
Abstinence during the monitoring/reinforcement phase was greater with mHealth reinforcement compared to mHealth monitoring on percent of negative CO tests, longest duration of abstinence, and week 4 PPA, with effect sizes ranging d = 0.8 to 1.3. Conclusions were similar when analyses included baseline and demographic covariates. See Table 2.
Table 2.
28-Day Behavioral Phase Outcomes
| Outcomes | mHealth reinforcement | mHealth monitoring | Significance test | Effect size estimate |
|---|---|---|---|---|
| (n = 45) | (n = 45) | |||
| Univariate analyses | ||||
| Percent negative CO tests (mean, SD) | 89.1% (19.5%) | 65.9% (38.0%) | F(1,88) = 13.23, p < .01 | d = 0.8 |
| Longest duration of abstinence (days)a | 27.0 (12.0) | 15.2 (11.9) | F(1,88) = 9.17, p < .01 | d = 1.3 |
| Week 4 PPA (%, n) | 82.2% (37) | 40.9% (18%) | χ2(df = 1) = 16.08, p < .01 | d = 0.9 |
| Covariate analysesb | ||||
| Percent negative CO tests | — | — | F(1,82) = 7.43, p = .01 | d = 0.8 |
| Longest duration of abstinence | — | — | F(1,82) = 4.27, p = .04 | d = 0.6 |
| PPAc | — | — | β(SE) = 1.8 (0.53), Wald = 10.96, p < .01 | OR (95% CI) = 5.83 (2.052–16.553) |
CI = confidence interval; CO = carbon monoxide; OR = odds ratio; PPA = point-prevalence abstinence.
Bold values indicate p < .05.
aValues are median days and interquartile range.
bCovariates included in analyses were age, gender, nicotine dependence severity, percent of CO tests submitted (at least 80%, no/yes), percent of counseling sessions completed (at least 80%, no/yes), and days of transdermal nicotine use.
cLogistic regression analysis.
Follow-up
Raw descriptive statistics for PPA, continuous abstinence since the quit date, timeline followback proportion of days on which smoking occurred, and mean number of cigarettes on days smoked at weeks 4, 8, 12, and 24 by study condition are in Table 3. Results of hierarchical mixed model analyses are in Table 4. There was an overall increased likelihood of PPA with mHealth reinforcement (main effect). The likelihood of PPA decreased over time (main effect), and the rate of change was steeper in the mHealth reinforcement condition (interaction effect). Proportion of days on which smoking occurred was lower overall with mHealth reinforcement (main effect) and increased over time (main effect), with no interaction effect. In both cases, abstinence was comparable between study conditions by the final follow-up. For remaining outcomes, the likelihood of continuous abstinence decreased over time and mean cigarettes on days smoked increased (main effects of time and condition), with no interaction effects. Other effects were nonsignificant (p > .05).
Table 3.
Weeks 4 Through 24 Follow-up Phase Outcomes: Raw Descriptive Statistics
| Outcomes | Week 4 | Week 8 | Week 12 | Week 24 |
|---|---|---|---|---|
| PPA (%, n) | ||||
| mHealth reinforcement | 82.2% (n = 37 of 45) | 50.0% (22 of 44) | 29.3% (n = 12 of 41) | 21.1% (n = 8 of 38) |
| mHealth monitoring | 40.9% (n = 18 of 45) | 44.2% (19 of 43) | 25.6% (n = 11 of 43) | 16.3% (n = 7 of 43) |
| Continuous abstinence (%, n) | ||||
| mHealth reinforcement | 48.9% (n = 22 of 45) | 40.9% (n = 18 of 44) | 26.8% (n = 11 of 41) | 18.4% (n = 7 of 38) |
| mHealth monitoring | 35.6% (n = 16 of 45) | 32.6% (n = 14 of 43) | 23.3% (n = 10 of 43) | 16.3% (n = 7 of 43) |
| Proportion of days smokeda | ||||
| mHealth reinforcement | 0.0 (8.5; n = 45) | 0.0 (39.0; n = 44) | 14.8 (100.0; n = 41) | 38.9 (100.0; n = 38) |
| mHealth monitoring | 6.2 (46.6; n = 44) | 13.3 (100.0; n = 43) | 48.6 (100.0; n = 43 | 96.8 (91.8; n = 43) |
| Cigarettes on days smokeda | ||||
| mHealth reinforcement | 3.6 (5.0; n = 20) | 4.7 (8.6; n = 21) | 5.3 (7.7; n = 24) | 9.3 (13.1; n = 26) |
| mHealth monitoring | 4.0 (8.6; n = 28) | 5.0 (7.8; n = 27) | 6.2 (10.3; n = 29) | 9.0 (8.7; n = 33) |
PPA = point-prevalence abstinence.
aMedian (interquartile range).
Table 4.
Weeks 4 Through 24 Follow-up Phase Outcomes: Results of Hierarchical Mixed Model Analyses
| Effecta | Condition | Time | Condition × Time |
|---|---|---|---|
| PPA | β01 = 2.27 (0.83), t(86) = 2.75, p < .01 | β10 = −0.04 (0.01), t(86) = −6.93, p < .01 | β11 = −0.02 (0.01), t(86) = −2.07, p = .04 |
| OR = 9.71 (1.87–50.30) | OR = 0.96 (0.96–0.98) | OR = 0.98 (0.97–0.999) | |
| Continuous abstinence | β01 = 0.93 (0.58), t(87) = 1.61, p = .11 | β10 = −0.03 (0.01), t(87) = −2.96, p < .01 | β11 = −0.01 (0.01), t(87) = −0.54, p = .59 |
| OR = 2.53 (0.81–7.95) | OR = 0.96 (0.94–0.99) | OR = 0.99 (0.98–1.01) | |
| Proportion of days smoked | β01 = −14.56 (7.4), t(87) = −1.98, p = .05 | β10 = 0.23 (0.04), t(87) = 5.61, p < .01 | β11 = 0.001 (0.07), t(87) = 0.02, p = .98 |
| Mean cigarettes on days smoked | β01 = −2.22 (0.01), t(68) = −1.51, p = .14 | β10 = 0.03 (0.001), t(68) = 1.04, p < .01 | β110.01 (0.01), t(68) = 1.04, p = .30 |
OR = odds ratio; PPA = point-prevalence abstinence.
To facilitate interpretation of estimated coefficients, mHealth monitoring was coded as the reference group (zero) and mHealth reinforcement coded as 1; and, Week 4 day was coded time 0 with subsequent days elapsed relative to that day.
Bold values indicate p < .05.
aReported for each effect: the estimated coefficient (SE), t ratio (approximate df), and p value. For binary outcomes, the associated OR (95% confidence interval) is also reported.
Safety and Tolerability
No study withdrawals occurred. One serious adverse event was for an overnight hospitalization for food poisoning. Adverse events were primarily for minor physical complaints related to transdermal nicotine (primarily dermatologic irritation; 34 events) and sleep disturbance possibly or probably related to transdermal nicotine (25 events). Other adverse events were for emergency room visits and physical complaints unrelated to study participation and not unexpected (27 events).
Discussion
This study examined effects of a novel strategy to ecologically monitor and reinforce objective evidence of cigarette smoking abstinence using mobile devices and common telephone functions. Results indicate increased abstinence with mHealth reinforcement during the period of contingencies, with notable treatment effect sizes. These results are particularly noteworthy given the active and intensive control condition which itself yielded relatively high rates of smoking abstinence, consistent with expectations.40,41 Increased PPA and lower proportion of days smoked in the mHealth reinforcement compared to monitoring condition persisted as a main effect through follow-up; relapse was not uncommon and no strong benefits of mHealth reinforcement remained at the most distal follow-up. Overall, the results of this first study support the feasibility and efficacy of our mHealth smoking abstinence reinforcement procedures for increasing abstinence during active treatment and indicate the need for research on methods to sustain benefits following active treatment.
This study is one of two randomized trials on smoking abstinence reinforcement delivered using mobile devices in the literature to date. In a study by Hertzberg et al.,42 veterans (N = 22) with post-traumatic stress disorder received two counseling sessions, switched to a low-nicotine cigarette prior to quitting, and received bupropion and nicotine replacement (transdermal form and choice of an acute “rescue” form). Participants were randomized to 4 weeks of abstinence-contingent payments for CO ≤ 8 ppm or noncontingent yoked payments for submitting samples twice per day (up to $690 available). Cell phone-based video clips of the CO testing process were submitted through a web portal, with the timing of tests at least partially at participants’ discretion (unprompted, and, what minimum time between samples was required is unclear in the report). Week 4 PPA was 82% in the reinforcement condition compared to 45% in the control condition. The current study builds on that study by supporting the feasibility and initial efficacy of mHealth reinforcement procedures (1) in general population smokers, and that (2) use basic phone features (i.e., not requiring internet or computers), (3) involve up to three tests daily to verify abstinence, and (4) request tests on an unpredictable pseudorandom schedule. This study also adds to the growing literatures on web-based abstinence reinforcement interventions10–16 and mobile phone-based smoking cessation interventions more generally (not reinforcement-based).43–47 Importantly, the large during-treatment effect sizes observed in the current study are generally on par with recent studies similarly involving frequent bio-verification and conventional (not technology-based) monitoring and reinforcement of smoking abstinence9,37,39,48 but carry with them the advantages discussed herein.
In the current study, CO test submission rates differed between the reinforcement and control conditions. Such differences were not observed in the study by Herzberg et al.42 Response effort requirements can influence response rates. Although these two studies did not differ substantially on the total number of CO tests requested (48 and 52 samples), they did differ on how test requests were distributed throughout the day and over the 4-week monitoring period. Other possible factors include participants’ agency versus study-determined structure around submitting tests and the amount of compensation for submitting CO tests in the control condition ($92 possible herein versus $314 earned on average in the study by Hertzberg et al.). We previously used a similar compensation structure for submitting alcohol breath tests in a study with frequent drinkers not seeking treatment and observed good adherence, at a mean (SD) 88.6% (10.4%), without differences between conditions.25 In the absence of substantial methodological differences between that and this study, it may be that differences in outcome expectations and/or efficacy expectations related to treatment- versus not-treatment-seeking relate to the differences in adherence. Nevertheless, relatively few tests were missed in the reinforcement condition, allowing for rigorous measurement of abstinence status.
During-treatment effects on abstinence are expected with reinforcement interventions based on theory and empirical evidence. Ultimately, however, long-term improvements are the goal. In this study, self-reported proportion of days on which smoking occurred remained lower in the reinforcement than control condition throughout the most distal follow-up, but other outcomes during follow-up were modest or nonsignificant. That there was a signal of benefits of reinforcement beyond the period of contingencies in the current study is promising, but clearly more work needs to focus on extending the benefits of reinforcement interventions. Counseling (typically four or more sessions) and transdermal nicotine have independent40,49,50 and additive effects40,41 on the likelihood and odds of quitting compared to placebo control conditions. Six-month quit rates are about twofold higher with transdermal nicotine compared to placebo.33 Adding behavioral support can further increase abstinence rates, up to 10% to 25% with increasing intensity of support, although effects are weaker when all conditions get behavioral support as opposed to no contact.41,51 Increased exposure and adherence to these treatment components increases long-term outcomes, and likely did so in the current study (although that cannot be concluded without the appropriate control condition), although there seems to be a point of diminishing returns.33 In the current study, effects of reinforcement, perhaps in both the short- and long-term, were likely attenuated by the efficacious background treatment. Other studies to compare abstinence reinforcement against usual care smoking treatment have sometimes observed persistent benefits of reinforcement at follow-up39,52 and sometimes not observed these effects.9,48,53 Considerations including reinforcement exposure, transdermal nicotine adherence, method of group assignment (not random39 versus random9,48), and participant characteristics (general population smokers,52 pregnant smokers9,39,48, and methadone patients53) may account for differences between studies. The current study was an initial investigation with a focus on exploring effects of mHealth reinforcement on during-treatment abstinence, and the study was appropriately powered for that aim. mHealth reinforcement solutions may also have implications for improving long-term smoking cessation outcomes. For example, mHealth technologies may allow for reinforcement frequency, timing, and duration options that have potential to extend the benefits of reinforcement interventions but that are not as easily accomplished with conventional in-person procedures.
One limitation of this study is that fewer CO tests were submitted in the monitoring compared to reinforcement condition. In this scenario, coding missed tests as smoking-positive would differentially deflate abstinence rates in the monitoring condition and inflate differences between conditions. Instead, the proportion of negative CO tests outcome was computed with the number of tests submitted in the denominator and missed tests omitted (and conclusions are unchanged). Engagement in counseling also differed between conditions in the current study, and increased counseling adherence is associated with improved smoking treatment outcomes.51 Importantly, differences between conditions on abstinence outcomes remained significant when effects of counseling, sample submission rates, and other variables were statistically controlled. Neither submission of cotinine samples nor submission of self-reports differed between conditions. Medication adherence rates also did not differ between conditions and were not substantially different than adherence expectations in relatively naturalistic settings54 or in the context of intensive intervention.55 This is important because premature discontinuation and incomplete adherence are strong predictors of relapse to smoking.33,56 Still, the results here suggest that greater compensation (or other motivation) is needed to maximize the submission of CO tests with the procedures investigated in this study.
There are also several strengths of this study. The background and comparison treatment platform was usual smoking cessation care consistent with recommended treatment guidelines plus monitoring and was not a placebo control condition. Abstinence status was biochemically verified throughout the study, consistent with expert recommendations.57 Adherence with transdermal nicotine and counseling was encouraged but not required for continued participation, thus producing conditions typical of pharmacotherapy and counseling treatment (i.e., variable adherence rates) and increasing the generalizability of findings. Furthermore, remote monitoring of breath tests and smoking status occurred in both conditions, and differences that nevertheless occurred were statistically controlled in analyses, allowing the effects of reinforcement to be isolated. Overall, the results of this study strongly indicate the feasibility and initial efficacy of mHealth smoking abstinence reinforcement, with implications for research on methods to utilize mobile technology to engage and support smokers trying to quit.
Funding
The National Institutes of Health grants R21-DA029215 and R01-DA01344 funded this study and preparation of this report.
ClinicalTrials.gov identifier: NCT01484717
Declaration of Interests
None declared.
Acknowledgments
We thank the research participants, without whom this study would not have been possible. We thank Sarah Coughlin, Karen Gilliam, Amy Novotny, and Sean Sierra for their efforts on study procedures and oversight, Ellen Ciesielski for assistance with the Institutional Review Board and other regulatory communications, and Laura LaBlanc and Wendy Soneson for administrative support.
References
- 1. Higgins ST, Petry NM. Contingency management. Alcohol Res Heal. 1999;23(2):122–127. [PMC free article] [PubMed] [Google Scholar]
- 2. Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend. 2000;58(1–2):9–25. doi:10.1016/S0376-8716(99)00071-X. [DOI] [PubMed] [Google Scholar]
- 3. Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi:10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 4. Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi:10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 5. Dutra L, Dutra L, Stathopoulou G, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–187. doi:10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 6. Cahill K, Hartmann-Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database Syst Rev. 2015;5:CD004307. doi:10.1002/14651858.CD004307.pub5. [DOI] [PubMed] [Google Scholar]
- 7. Grabowski J, Bell CS. Measurement in the analysis and treatment of smoking behaviour. NIDA Res Monogr. 1983;48:6–26.6443145 [Google Scholar]
- 8. Higgins ST, Washio Y, Heil SH, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012;55(suppl):S33–S40. doi:10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med. 2014;68:51–57. doi:10.1016/j.ypmed.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stoops WW, Dallery J, Fields NM, et al. An internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug Alcohol Depend. 2009;105(1–2):56–62. doi:10.1016/j.drugalcdep.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dallery J, Raiff BR, Grabinski MJ. Internet-based contingency management to promote smoking cessation: a randomized controlled study. J Appl Behav Anal. 2013;46(4):750–764. doi:10.1002/jaba.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raiff BR, Jarvis BP, Turturici M, Dallery J. Acceptability of an internet-based contingency management intervention for smoking cessation: views of smokers, nonsmokers, and healthcare professionals. Exp Clin Psychopharmacol. 2013;21(3):204–213. doi:10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reynolds B, Harris M, Slone SA, et al. A feasibility study of home-based contingency management with adolescent smokers of rural Appalachia. Exp Clin Psychopharmacol. 2015;23(6):486–493. doi:10.1037/pha0000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dallery J, Glenn IM. Effects of an Internet-based voucher reinforcement program for smoking abstinence: a feasibility study. J Appl Behav Anal. 2005;38(3):349–357. doi:10.1901/jaba.2005.150-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dallery J, Glenn IM, Raiff BR. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend. 2007;86(2–3):230–238. doi:10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 16. Meredith SE, Grabinski MJ, Dallery J. Internet-based group contingency management to promote abstinence from cigarette smoking: a feasibility study. Drug Alcohol Depend. 2011;118(1):23–30. doi:10.1016/j.drugalcdep.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The World Bank. Mobile phone access reaches three quarters of Planet’s population. World Bank Group Press Release. July 17, 2012. [Google Scholar]
- 18. International Telecommunications Union. The World in 2014: ICT Facts and Figures. 5 May, 2014.
- 19. Brendryen H, Kraft P. Happy ending: a randomized controlled trial of a digital multi-media smoking cessation intervention. Addiction. 2008;103(3):478–484. doi:10.1111/j.1360-0443.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- 20. Searles JS, Helzer JE, Walter DE. Comparison of drinking patterns measured by daily reports and timeline follow back. Psychol Addict Behav. 2000;14(3):277–286. doi:10.1037/0893-164X.14.3.277. [DOI] [PubMed] [Google Scholar]
- 21. Searles JS, Helzer JE, Rose GL, Badger GJ. Concurrent and retrospective reports of alcohol consumption across 30, 90 and 366 days: interactive voice response compared with the timeline follow back. J Stud Alcohol. 2002;63(3):352–362. [DOI] [PubMed] [Google Scholar]
- 22. Olmstead TA, Alessi SM, Kline B, Pacula RL, Petry NM. The price elasticity of demand for heroin: matched longitudinal and experimental evidence. J Health Econ. 2015;41:59–71. doi:10.1016/j.jhealeco.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDaniel AM, Benson PL, Roesener GH, Martindale J. An integrated computer-based system to support nicotine dependence treatment in primary care. Nicotine Tob Res. 2005;7(suppl 1):S57–S66. doi:10.1080/14622200500078139. [DOI] [PubMed] [Google Scholar]
- 24. Corkrey R, Parkinson L. Interactive voice response: review of studies 1989–2000. Behav Res Methods Instrum. 2002;34(3):342–353. doi:10.3758/BF03195462. [DOI] [PubMed] [Google Scholar]
- 25. Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction. 2013;108(5):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petry NM, Alessi SM, Byrne S, White WB. Reinforcing adherence to antihypertensive medications. J Clin Hypertens. 2015;17(1):33–38. doi:10.1111/jch.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Digital Health for the End TB Strategy: An Agenda for Action. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 28. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 29. Rollnick S, Heather N, Gold R, Hall W. Development of a short “readiness to change” questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict. 1992;87(5):743–754. doi:10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 30. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. IV-TR ed Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 31. Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68(1):134–144. doi:10.1037/0022-006X.68.1.134. [DOI] [PubMed] [Google Scholar]
- 32. Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litter RZ, Allen J, eds. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992:41–72. doi:10.1007/978-1-4612-0357-5_3. [Google Scholar]
- 33. Fiore M, Jaén C, Baker T, Bailey W, Bennet G, Benowitz N. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35(2):158–176. doi:10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stout RL, Wirtz PW, Carbonari, Joseph P, Del Boca FK, Carbonari JP. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol. 1994;12:70–75. [DOI] [PubMed] [Google Scholar]
- 35. Roll JM, Huber A, Sodano R, Chudzynski JE, Moynier E, Shoptaw S. A comparison of five reinforcement schedules for use in contingency management-based treatment of methamphetamine abuse. Psychol Rec. 2006;56(1):67–81. [Google Scholar]
- 36. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi:10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 37. Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: significant other supporter (SOS) program. Tob Control. 2000;9(suppl 3):67–69. doi:10.1136/tc.9.suppl_3.iii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: a pilot study. J Appl Behav Anal. 2008;41(4):527–538. doi:10.1901/jaba.2008.41–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins ST, Heil SH, Solomon LJ, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6(6):1015–1020. doi:10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- 40. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi:10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 41. Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2012;12:1–76. doi:10.1002/14651858.CD009670.pub2. [DOI] [PubMed] [Google Scholar]
- 42. Hertzberg JS, Carpenter VL, Kirby AC, et al. Mobile contingency management as an adjunctive smoking cessation treatment for smokers with posttraumatic stress disorder. Nicotine Tob Res. 2013;15(11):1934–1938. doi:10.1093/ntr/ntt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Free C, Knight R, Robertson S, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011;378(9785):49–55. doi:10.1016/S0140-6736(11)60701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi:10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rodgers A, Corbett T, Bramley D, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tob Control. 2005;14(4):255–261. doi:10.1136/tc.2005.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Balmford J, Borland R, Benda P, Howard S. Factors associated with use of automated smoking cessation interventions: findings from the eQuit study. Health Educ Res. 2013;28(2):288–299. doi:10.1093/her/cys104. [DOI] [PubMed] [Google Scholar]
- 47. Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A. Mobile phone-based interventions for smoking cessation. In: Whittaker R, ed. Chichester, UK: John Wiley; 2012. doi:10.1002/14651858.CD006611.pub2. [DOI] [PubMed] [Google Scholar]
- 48. Heil SH, Higgins ST, Bernstein IM, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1019. doi:10.1055/s-0029-1237430.Imprinting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fiore MC, Jaen CR, Baker TB, et al. US Department of Health and Human Services. Treating tobacco use and dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; May 2008. [Google Scholar]
- 50. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi:10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviews. Addiction. 2013;108(10):1711–1721. doi:10.1111/add.12291. [DOI] [PubMed] [Google Scholar]
- 52. Secades-Villa R, García-Rodríguez O, López-Núñez C, Alonso-Pérez F, Fernández-Hermida JR. Contingency management for smoking cessation among treatment-seeking patients in a community setting. Drug Alcohol Depend. 2014;140:63–68. doi:10.1016/j.drugalcdep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 53. Shoptaw S, Rotheram-Fuller E, Yang X, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97(10): 1317–1328. doi:10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 54. Jolicoeur DG, Ahluwalia JS, Richter KP, et al. The use of nicotine patches with minimal intervention. Prev Med. 2000;30(6):504–512. doi:10.1006/pmed.2000.0670. [DOI] [PubMed] [Google Scholar]
- 55. Yingst JM, Veldheer S, Hrabovsky S, Sciamanna C, Foulds J. Reasons for non-adherence to nicotine patch therapy during the first month of a quit attempt. Int J Clin Pract. 2015;69(8):883–888. doi:10.1111/ijcp.12644. [DOI] [PubMed] [Google Scholar]
- 56. Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med. 2008;34(3):212–215. doi:10.1016/j.amepre.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 57. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. doi:10.1080/1462220031000070552. [PubMed] [Google Scholar]