Abstract
Rett syndrome (RTT) is an X-linked neurodevelopmental disorder in which the MECP2 (methyl CpG-binding protein 2) gene is mutated. Recent studies showed that RTT-derived neurons have many cellular deficits when compared to control, such as: less synapses, lower dendritic arborization and reduced spine density. Interestingly, treatment of RTT-derived neurons with Insulin-like Growth Factor 1 (IGF1) could rescue some of these cellular phenotypes. Given the critical role of IGF1 during neurodevelopment, the present study used human induced pluripotent stem cells (iPSCs) from RTT and control individuals to investigate the gene expression profile of IGF1 and IGF1R on different developmental stages of differentiation. We found that the thyroid hormone receptor (TRalpha 3) has a differential expression profile. Thyroid hormone is critical for normal brain development. Our results showed that there is a possible link between IGF1/IGF1R and the TRalpha 3 and that over expression of IGF1R in RTT cells may be the cause of neurites improvement in neural RTT-derived neurons.
Introduction
Rett syndrome (RTT; OMIM 312750) is an X-linked neurodevelopmental disease that affects mainly girls (1–3). Boys with the disease are severely affected and, in most cases, die early in the development (3). Mutations on the methyl-CpG binding protein 2 (MECP2) gene are found in nearly all the cases of RTT. MECP2 expression occurs in all tissues, however its major complication affects the central nervous system (4). The MeCP2 protein works by binding into the genome and controlling the expression of several genes, such as Insulin-like Growth Factor 1 (IGF1), brain-derived neurotrophic factor (BDNF) and N-methyl-D-aspartate (NMDA) (5). Moreover, MECP2 gene duplication also yields to a severe neurodevelopmental disorder (MECP2 Duplication Syndrome), suggesting that its expression should be tightly controlled to a normal development of the nervous system (6,7).
IGF1 deficiency in mice causes reduced postnatal brain growth. The brain growth arrest is likely a result of reduced neuronal size, since the number of cells is similar to control animals (8,9). It was already shown (10) that IGF1 treatment could significantly increase dendritic growth of cortical slices. Glutamatergic neurons, derived from RTT patient iPSCs, have decreased synapses, reduced dendritic spines, and an imbalance in the neural network (11). Interestingly, IGF1 treatment was able to increase the number of synapses on those neurons. Furthermore, studies also revealed an improvement in cognition and interactions with the surrounding environment in RTT patients treated with IGF1 (2,12).
The action of IGF1 is through IGF1 receptor (IGF1R), but could also interact with the insulin receptor. IGF1R is found in all tissues. The interaction between IGF1 and IGF1R is modulated by IGF binding proteins (IGFBPs) (13–15). IGF1R is widely expressed in the brain and can co-localize, in many regions, with the insulin receptor (8,16). IGF1 is an endocrine hormone mainly produced by the liver (8,17,18). This hormone mRNA is abundant in the brain, with a higher expression during postnatal development (19). IGF1 is found mainly in growing projection neurons in sensory and the cerebellar relay system, where IGF1R is also highly expressed, suggesting an autocrine or paracrine mode of action (8). IGF1 protects neurons, reducing neurodegeneration and prolonging life span of cells (20,21). It is also important to neurodevelopment modulation, being fundamental to proliferation and neural maturation (16).
Production of IGF1 is stimulated by growth hormone (GH) produced by pituitary (8,17,18,20,22). Oestrogen is another hormone that mediates regulation of IGF1 (23). Thyroid hormone also affects IGF1 concentration in plasma (24), since this hormone influences GH/IGF1 axis (25). In an elegant study (26) it was shown that TH controls IGF1 action throughout an integrin ανβ3. In Muscle cells, IGF1 binds to its receptors and stimulate tyrosine kinase and PI3K activities. Interestingly, T4 may inhibit the action of IGF1 in glucose homeostasis, impairing cell proliferation, signalling and growth.
IGF1 is an important hormone to insulin homeostasis and is sensitive to minor alterations, such as bisphenol A and dexamethasone exposure during rats’ pregnancy resulted in offspring with TH, GH and IGF1 serum concentration altered, which may lead to a delay in the neurodevelopment (27,28).
Brain is an important target of thyroid hormones (THs) (pro hormone tiroxine, T4 and the active form 3,5,3’- triiodotironine, T3). During brain maturation THs influence the development process, differentiation, myelination, neural and glial signalling (29–31). Deficiency on TH function may impair neural differentiation, survive and neurogenesis and its replacement restore cognition and all defects caused by TH deficit (32,33). TH actions are mediated by nuclear receptors located in specific regions of DNA, when THs are bonded to their receptors there is transcriptional activation or inhibition of target genes and proteins that they encode (34,35).
Two genes, THRalpha and THRbeta, originated by alternative splicing, encode different forms of TH receptors: TRalpha 1, TRalpha 2, TRbeta 1, TRbeta 2. These isoforms are expressed in different tissues (36,37). TRalpha 3 is encoded by transcript variant 3. Studies using knockout mice of different TH receptors showed that, besides the similarity of these receptors, they have different functions in TH signalling. The variant 3 resembles to variant 2 (NM_001190918.1), however TRalpha 2 is an isoform that is not responsive to TH, it does not bind to T3, since it lacks functional ligand-binding domain (38,39).
The aim of this study was to investigate the gene expression profile of IGF1 and its receptor from the stage of iPSC until neural cells from a derived line cell from a male RTT patient carrying a stop codon mutation with no protein synthesis. Furthermore, it was exhibited in the rescue of the neurites length in RTT neurons after treatment with IGF1, and this result is possible since RTT neurons have an increased expression of IGF1R. Finally, our data revealed a possible correlation between the expression levels of TRalpha 3 and IGF1, revealing a likely mechanism of IGF1/IGF1R regulation.
Since there are an overlap of RTT features and other diseases and/or neurodevelopmental syndromes in which intellectual impairment, such as the autism spectrum disorder (ASD) is included (40), it is important to understand RTT and find a therapeutic exit to this syndrome that may contribute to the treatment of a variety of neurodevelopmental disease.
Results
Cells characterization
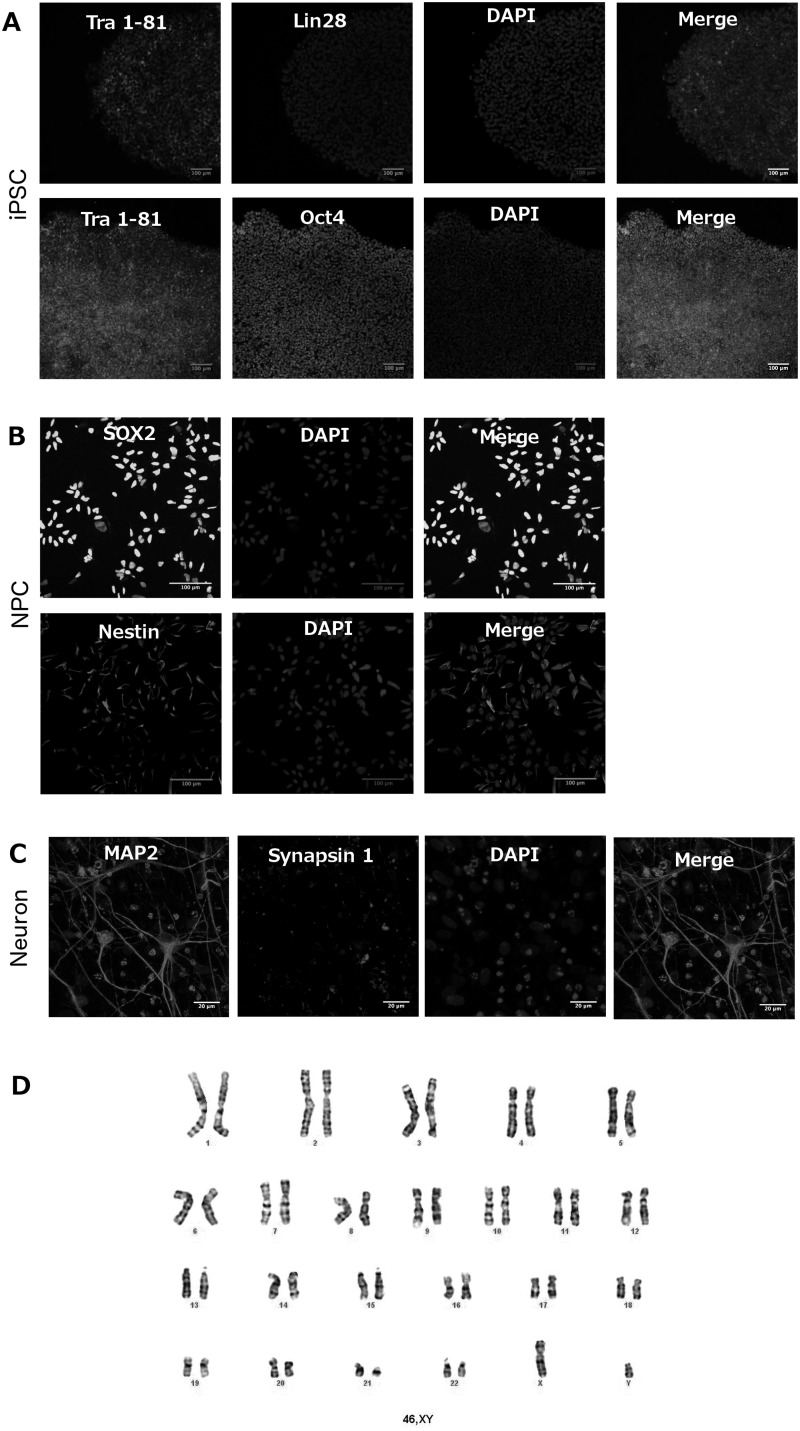
The canonical markers of pluripotent state Oct4, Tra 1-81 and Lin28 were used to characterize RTT and control iPSCs (Fig. 1A). NPCs were stained with antibodies targeting Sox2 and Nestin, two markers of neural precursors (Fig. 1B). Finally, 6 weeks old neural culture cells were stained with Map2 and Synapsin 1 antibodies, targeting two classical markers of neurons (Fig. 1C).
Figure 1.
Cell characterization shows specific markers for each cell stage - iPSC, NPC and neurons – according to the cell type, chromosome number matches with normal karyotyping. (A) Immunofluorescence images of induced Pluripotent Stem Cells (iPSC) showing the expression of specific markers for this type of cell after approximately 5–6 days of colony passage. Top: Tra 1-81, Lin28, DAPI and Merge. Bottom: Tra 1-81, Oct4, DAPI and, Merge. Scale bar = 100 μm. (B) Immunofluorescence images of Neural Progenitor Cells (NPC) expressing specific markers for this type of cell after confluence of approximately 50%. Top: SOX2, DAPI and Merge. Bottom: Nestin – NES, DAPI and, Merge. Scale bar = 100 μm. (C) Immunofluorescence images of Neurons showing the expression of specific markers for this type of cell after six weeks of induced differentiation. Synapsin 1, MAP2, DAPI and, Merge. Scale bar = 20 μm. (D) Images representing cell karyotyping at NPC stage showing that the studied cells present normal chromosome number (46,XY) and that its origin is from male control and affected subjects. Banding method (resolution): G-Banding; number of cells counted: 20; number of cells karyotyped: 5.
Maintenance of iPSCs and NPCs in culture requires several cell passages, a procedure that can lead to chromosomal abnormalities. To verify any chromosomal aberrancy acquired during the process of maintenance of the cells, karyotyping was performed at the NPC stage, before the cells were differentiated in neurons. The results show a normal karyotype for all cells used on this study (Fig. 1D).
As part of cell characterization it was also performed a qRT-PCR at the stages of iPSC, NPC and Neural cells to show specific gene expression at each stage. The gene expression profiles of the three cellular stages were compared to each other. Supplementary Material, Fig. S1A–C shows three genes that are markers for pluripotency. As it is presented, OCT4 gene expression is increased in iPSC (1.0650 ±0.2166) compared to NPC (0.0077 ±0.0008), NANOG gene expression is higher in iPSCs (1.0440 ±0.1765) than in NPCs (0.0142 ±0.0022) and neurons (0.1415 ± 0.0316) and LIN28 gene expression is also higher in iPSC (1.0040 ±0.0505) than in NPC (0.0608 ±0.0176) and neurons (0.0445 ±0.0037). The expression of genes related to neural lineage shows that PAX6 gene expression up-regulated in NPCs (4.0300 ±0.1360) and neurons (4.9250 ±0.2837) when compared to iPSCs (1.0210 ±0.1176) (Supplementary Material, Fig. S1D), with the expression in neurons increased when compared to NPCs. Neurons display an increased expression of MAP2 (337.3000 ± 19.7800) compared to iPSCs (1.0310 ±0.1407) and NPCs (4.8110 ±1.1950). Synapsin1 gene expression was also higher in neurons (22.0600 ±5.7250) than in iPSCs (1.0340 ±0.1637) and NPCs (1.2770 ± 0.0503), confirming the successful differentiation towards neural lineage in our culture (Supplementary Material, Fig. S1G and H).
RTT neuron gene expression of IGF1 and TRalpha 3 do not change at neural stage
An open question in the field is if IGF1 expression is altered during the development of the nervous system. Interestingly, we did not detect any difference between RTT and control neurons (Fig. 2A). We also checked the expression of thyroid hormone receptor alpha 3 (TRalpha 3). Similarly to IGF1, this gene did not present a significant difference between RTT and control neurons (Fig. 2B).
Figure 2.
IGF1 and TRalpha3 gene expression do not change in Rett Syndrome affected subject compared to control. (A) Graphic representation of IGF1 gene expression on stage of neural cells. There is no significant change in Rett Syndrome subject (Q83X) IGF1 gene expression compared to control (WT83). P = 0.1981 (n = 5–9). (B) Graphic representation of TRalpha3 gene expression on stage of neural cells. There is no significant change in Rett Syndrome subject (Q83X) TRalpha3 gene expression compared to control (WT83). P = 0.2863 (n = 5–9). All graphical data are ± SEM. Unpaired t test with equal SD.
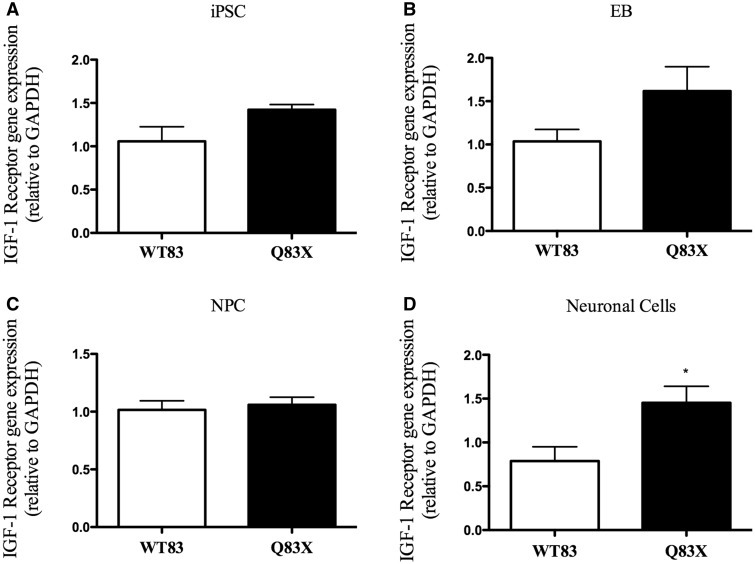
IGF1 receptor gene expression is increased in Rett syndrome at neural stage
Analyses of IGF1 Receptor (IGF1R) gene expression profile in different stages of development showed no difference of expression in iPSC, EB and NPC stages when comparing RTT and control cells (Fig. 3A, B and C). Interestingly, at neural stage IGF1R increased 1.8 times in RTT cells compared to control, as shown at Fig. 3D.
Figure 3.
IGF1 receptor gene expression is increased in Rett Syndrome at neural cell stage. (A) Graphic representation of IGF1 receptor gene expression in iPSC. There is no significant change in gene expression in Q83X compared to WT83. P = 0.0680 (n = 6). (B) Graphic representation of IGF1 receptor gene expression in Embryoid Body (EB). There is no significant change in gene expression in Q83X compared to WT83. P = 0.1350 (n = 6–9). (C) Graphic representation of IGF1 receptor gene expression in Neural Progenitor Cells (NPC). There is no significant change in gene expression in Q83X compared to WT83. P = 0.6957 (n = 6–12). (D) Graphic representation of IGF1 receptor gene expression in neural cells. At this stage of cell differentiation IGF1 receptor is increased in Rett Syndrome (Q83X) compared to control (WT83). *P < 0.05 (n = 5–9). All graphical data are ± SEM. Unpaired t test with equal SD of IGF1 Receptor.
TRalpha 3 may be related to IGF1 receptor for IGF1 and neural IGF1
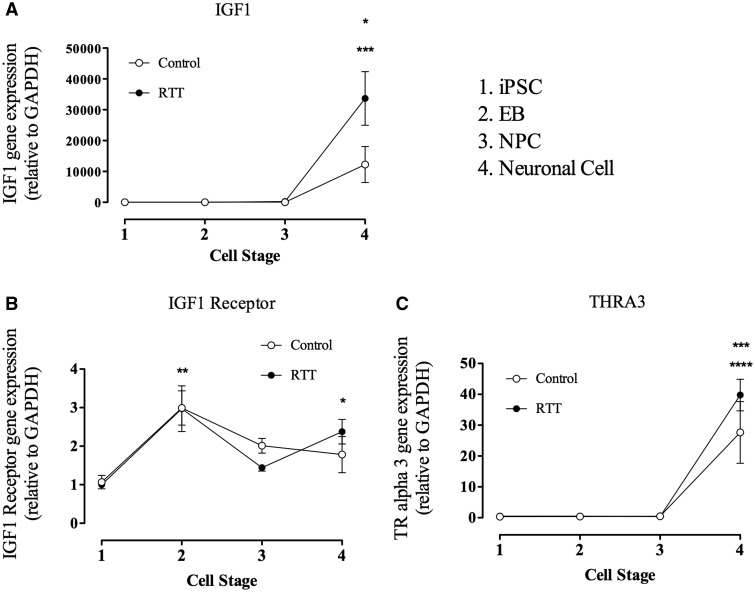
Gene expression analyses were performed in RTT and control cells at different developmental stages (iPSC, EB, NPC and neurons). In Fig. 4A is possible to observe that detectable IGF1 expression only occurs in neurons (cell stage 4). In control, the increasing of IGF1 expression was approximately 15000 times compared to the other developmental stages. In RTT the increasing was approximately 35000 times compared to the other stages. Meanwhile, IGF1R expression was present in all stages in both control and RTT, as shown in Fig. 4B. Interestingly, at the EB stage (cell stage 2), both control and RTT had an increase of approximately 3 times the expression found on iPSC stage. However, only RTT displays an increase in the neural stage when compared to iPSC.
Figure 4.
IGF 1 receptor gene expression is presented during all cell differentiation, since iPSC until Neural cells, while IGF 1 gene expression occurs only during neural mature cell which may be related with TRalpha3 expression. (A) Graphic representation of IGF 1 gene expression from iPSC until neural cells. The graphic is representing control (WT83) and Rett Synrome (RTT) gene expression. Data shown as mean ± SEM (n = 5–12). (open circle) *P = 0,0141 vs. all; (closed circle) ***P < 0.0001 vs. all. (B) Graphic representation of IGF 1 receptor gene expression from iPSC until neural cells. The graphic is representing control (WT83) and Rett Synrome (RTT) gene expression. Data shown as mean ± SEM (n = 5–12). (open circle) **P = 0.0053 vs. iPSC; (closed circle) **P = 0.0007 vs. iPSC and NPC, *P < 0.05 vs. iPSC. (C) Graphic representation of TRalpha3 gene expression from iPSC until neural cells. The graphic is representing control (WT83) and Rett Synrome (RTT) gene expression. Data shown as mean ± SEM (n = 5–12). (open circle) **P = 0.0018 vs. all; (closed circle) ***P < 0.0001 vs. all.
We also studied the expression of TRalpha 3, a thyroid hormone receptor. In our results it was shown that this gene expression is similar to the IGF1 expression. TRalpha 3 gene expression was only detected in neural stage in both control and RTT cells (Fig. 4C) and is increased approximately 35–40 times compared to the other stages.
To test the concentration of IGF1 during the neural differentiation, we performed ELISA analysis at 3 weeks of differentiation. The levels of this hormone in the media did not change when compared to control media (media without cells; Table 1). On the other hand, the concentration of IGF1 from medium of 6 week-mature neurons decreases significantly compared to control media and to 3 week-differentiated cells. Comparing the concentration of IGF1 from the medium of 3 week-old cells with 6 week-old cells, in both (control and RTT) is possible to observe that at six weeks of differentiation concentration decreases 2.6 times compared to 3 week-differentiated neural cell medium. When these cells were treated with thyroid hormone at 3 weeks of differentiation the concentration of IGF1 did not change after two days of treatment. However, at 6 weeks of differentiation, when these cells were treated with thyroid hormone, the consumption of IGF1 was higher in RTT T3 10−6 M (0.07072 ±4.7330 10–5) compared to controls (no treatment with T3), in both cell lines: control (0.07098 ±1.0570 10–5) and RTT (0.07098 ±5.9700 10–6). It is important to notice that there is no significant difference between these controls in IGF1 consumption. Q83X T3 10–6 M has also an increased consumption of IGF1 compared to cells treated with medium without thyroid hormone (hypo) in both control (0.07098 ±6.1950 10–5) and RTT (0.07090 ±5.8880 10–5), and RTT rT3 (0.07092 ±3.6550 10–5).
Table 1.
IGF1 dosage for verification of media concentration. Consumption of IGF1 by Neural cells of the media in two distinctive stage of differentiation, 3 and 6 weeks, control cells (WT83) and affected cells (Q83X) and, six different treatments.
| WT83 | Q83X | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | No Hormone | T3 10−6 | T3 10−12 | T4 | rT3 | Control | No Hormone | T3 10−6 | T3 10−12 | T4 | rT3 | |
| 3 weeks | 0.1872 | 0.1872 | 0.1872 | 0.1872 | 0.1872 | 0.1872 | 0.1872 | 0.1872 | 0.1872 | 0.1872 | 0.1872 | 0.1872 |
| ±2.125 10−5 | ±4.650 10−5 | ±3.510 10−5 | ±5.946 10−5 | ±2.135 10−5 | ±5.315 10−5 | ±6.018 10−5 | ±4.283 10−5 | ±1.890 10−5 | ±1.368 10−5 | ±3.896 10−5 | ±1.917 10−5 | |
| 6 weeks | 0.07098 | 0.07097 | 0.07085 | 0.07090 | 0.07086 | 0.07089 | 0.07098 | 0.07090 | 0.07072 | 0.07081 | 0.07088 | 0.07092 |
| ±1.057 10−5**** | ±6.195 10−5 | ±6.452 10−6 | ±4.583 10−5 | ±2.377 10−5 | ±2.490 10−5 | ±5.970 10−6d | ±5.888 10−5 | ±4.733 10−5b,c,e | ±1.063 10−5b | ±1.842 10−5 | ±3.655 10−5c |
avalues of NG = 0,1874 pg/ml e N2 = 0,1874 pg/ml; Q83X:bP < 0,05 Control vs. T3 10–6 M; bP < 0,05 Control vs. T3 10–12 M and rT3 vs. T3 10–12 M; cP < 0,005 rT3 vs T3 10–6 M; eP < 0,05 no Hormone vs. T3 10–6 M and T4 vs. T3 10–6 M; WT83 vs. Q83X:bP < 0,05 Control WT83 vs. T3 10–6 M Q83X; dP = 0,0001 Control WT 3 weeks vs 6 weeks and Control Q83X 3 weeks vs 6 weeks. One-way ANOVA, ± S.E.M.
Treatment of neural cells with IGF1 increases neurite length of Rett syndrome neurons
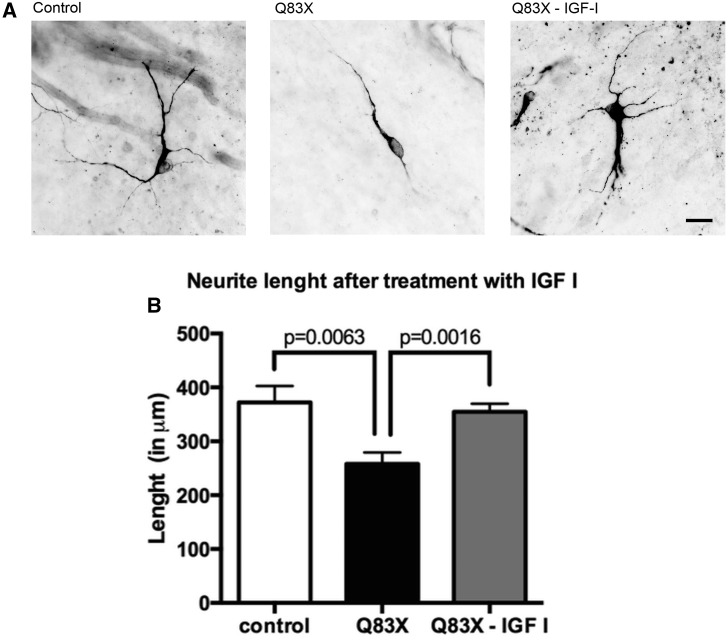
In Rett syndrome one of the characteristics of the subject is the abnormal neurodevelopment. Several studies had shown that IGF1 could improve neural morphology and function. Here we took maturate neural cells, and treated them with this hormone Fig. 5A. These cells were treated with IGF1 for 14 days at a concentration of 10 μM. Control neurons that were treated with IGF1 did not present any significant difference (data not shown) compared to untreated control. The length of neurites (Fig. 5B) from control was 380 μm, and the length from RTT was 250 μm, these results showed that the RTT neurites length is approximately 35% shorter than those from control. On the other hand, when RTT cells were treated with IGF1 the length of neurites increased in treated cells compared to untreated RTT neurons, practically rescuing the total neurite length to control levels.
Figure 5.
Rett Syndrome neurons treated with IGF1 recover their neurite length compared to control and untreated cells. (A) Representative images of neurites length of control cells, affected cells (Q83X) and affected cells treated with IGF1 (Q83X + IGF1). Neurons were stained with MAP2 ab and imaged under 40X Zeiss microscope. The scale bar represents 20 μm. Tracing in NeuronJ (ImageJ). (B) Graphic representation of neurite length in each condition tested. Total length sum (in μm) of primary and secondary neurites are shown. S.E.M. was used to plot the error bar. 4 weeks neurons (n = 10).
The absence of MECP2 function alters the expression of thyroid hormone receptors
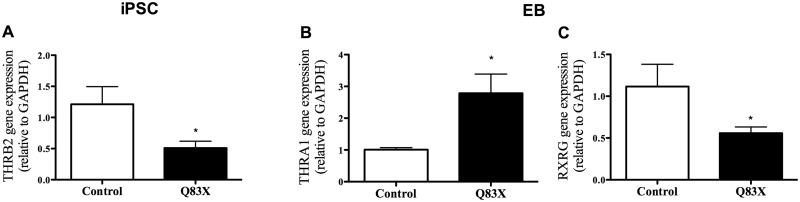
Thyroid hormone receptors are important to cell genomic response to the hormone and its biological action (41). In Rett syndrome, it was shown that the cause of the syndrome is the absence of MECP2 function (11) and in our study we demonstrated significant alteration in expression of TH receptors. At the iPSC stage (Fig. 6A), THRB2 gene expression was 2.4-fold lower in RTT (0.5098 ±0.1101) compared to control (1.2120 ±0.2837). At the EB stage (Fig. 6B), THRA1 expression was 2.8-fold higher in RTT (2.7860 ±0.6023) compared to control (1.008 ±0.0634). RXRG (Fig. 6C) expression is 2-fold lower in RTT (0.5586 ±0.0735) compared to control (1.1150 ±0.2667).
Figure 6.
Thyroid hormone receptors gene expression is altered in RTT. (A–C) Graphic representation of gene expression of Thyroid Hormone Receptors in iPSC (THRB2) and EB (THRA1 and RXRG) compared control and RTT cells. *P < 0.05 vs. control. Values are the mean ± SEM (n = 5–12).
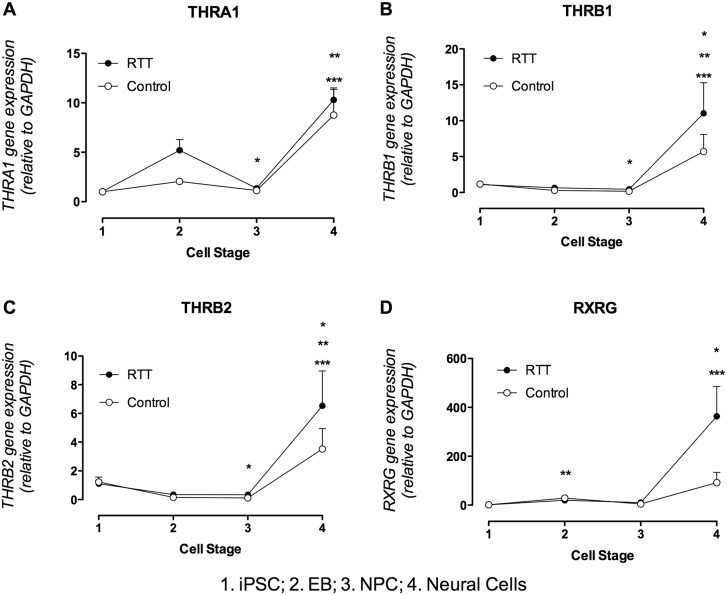
The gene expression profile of TH receptors was analysed. THRA1 expression during cell development in control cells (Fig. 7A) increased 8.7-fold and 7.7-fold at the neural cell stage (8.7580 ±2.7550) compared to iPSC (1.0070 ±0.0529) and NPC (1.1380 ±0.3377), respectively. In RTT cells (Fig. 7A) expression increased 9.9-fold and 7.6-fold at the neural cell stage (10.3000 ±1.0450) compared, respectively, to iPSC (1.0390 ±0.1202) and NPC (1.3540 ±0.1829), and decreased 3.9-fold in the NPC stage compared to the EB stage (5.1980 ±1.0870).
Figure 7.
Thyroid hormone receptors gene expression profile. (A) Graphic representation of THRA1 gene expression during cell differentiation. There are significant changes in gene expression during differentiation of control cells (open circle) and RTT derived cells (filled circle). Control - **P < 0.01 vs. iPSC and NPC. RTT - ***P < 0.001 vs. iPSC and NPC; *P < 0.05 vs. EB. (B) Graphic representation of THRB1 gene expression during cell differentiation. There are significant changes in gene expression during differentiation of control cells (open circle) and RTT derived cells (filled circle). Control - **P < 0.01 vs. NPC; *P < 0.05 Neural cells vs. EB and NPC vs. iPSC. RTT - ***P < 0.001 vs. NPC; *P < 0.05 vs. EB. (C) Graphic representation of THRB2 gene expression during cell differentiation. There are significant changes in gene expression during differentiation of control cells (open circle) and RTT derived cells (filled circle). Control - **P < 0.001 vs. NPC; *P < 0.05 Neural cell vs. EB and NPC vs. iPSC. RTT - ***P < 0.001 vs. NPC; **P < 0.01 vs. EB. (D) Graphic representation of RXRG gene expression during cell differentiation. There are significant changes in gene expression during differentiation of control cells (open circle) and RTT derived cells (filled circle). Control - **P < 0.001 vs. iPSC; *P < 0.05 vs. iPSC. RTT - ***P < 0.001 vs. iPSC and NPC.
The gene expression of THRB1 in control cells (Fig. 7B) during cell differentiation increased 33.6-fold and 19.1-fold, respectively, in neural cells (5.7210 ±2.3890) compared to NPC (0.1746 ±0.0158) and EB (0.2952 ±0.0849), and decreased 6.9-fold in NPC compared to iPSC (1.1770 ±0.2823). In RTT cells (Fig. 7B), the expression of THRB1 significantly increased in neural cells (11.0200 ±4.2610), 24-fold compared to NPC (0.4582 ±0.1707) and 10.4-fold compared to EB (0.6519 ±0.2192).
THRB2 gene expression was also analysed and in control cells (Fig. 7C), at the neural cell stage (3.5200 ±1.4270), the expression increased 32-fold compared to NPC (0.1133 ±0.0135) and 22-fold compared to EB (0.1569 ±0.0617). NPC gene expression decreased 11.2-fold compared to iPSC expression (1.2310 ±0.3381). In RTT cells (Fig. 7C) THRB2 expression increased 19.8-fold and 18.7-fold in neural cells (6.5340 ±2.4230) compared to NPC (0.3335 ±0.0983) and EB (0.3455 ±0.0951), respectively.
Thyroid hormone receptors may form dimers with other nuclear receptors, such as RXR family receptors (Retinoid X Receptor). The expression of RXRG in control cells (Fig. 7D) increased at the EB stage (28.8500 ±9.3730) 20.56-fold compared to iPSC (1.4130 ±0.4357), and also increased at the neural cell stage (91.9100 ±42.4400) 65.2-fold compared to iPSC. In RTT (Fig. 7D) the expression at the neural cell stage (363.4000 ±121.7000) increased 300.3-fold compared to iPSC (1.2070 ±0.2678) and 34.6-fold compared to NPC (10.5000 ±3.3040).
Correlation analysis of investigated genes
In addition to the presented results, the correlation of the expression of studied genes at neural cell stage was also evaluated. Pearson correlation analysis showed a highly significant association between IGF1 Receptor gene expression and TRalpha 3 gene expression (r = 0.644) (Table 2). The expression of IGF1 has a significant positive correlation with THRB1 (r = 0.725) and THRB2 (r = 0.638). THRA1 has a significant positive correlation with IGF1R (r = 0.833) and THRA3 (r = 0.699). THRB1 has a strong correlation with THRB2 (r = 0.976). RXRG has a negative correlation with THRB2 (r=−0.583) and THRB1 (r=−0.535), which means that the expression of these genes is inversely proportional.
Table 2.
Pearson correlation matrix of neural cells gene expression.
| r | IGF1 | IGF1 receptor | THRA3 | THRA1 | THRB1 | THRB2 | RXRG |
|---|---|---|---|---|---|---|---|
| IGF1 | 1 | ||||||
| IGF1 receptor | 0.057 | 1 | |||||
| THRA3 | −0.073 | 0.644a | 1 | ||||
| THRA1 | 0.355 | 0.833c | 0.699b | 1 | |||
| THRB1 | 0.725b | 0.369 | −0.134 | 0.348 | 1 | ||
| THRB2 | 0.638a | 0.342 | −0.206 | 0.270 | 0.976c | 1 | |
| RXRG | −0.025 | −0.466 | −0.260 | −0.310 | −0.535a | −0.583a | 1 |
r, Pearson coefficient, and correlations (two-tailed) that are significant at the 0.05a, 0.01b and 0.001c
Discussion
In this study, we use human iPSCs to investigate the expression profile of IGF1 during the development of the nervous system in RTT and control cells, given the recent findings that IGF1 could rescue some of the phenotypes in RTT neurons (2,11) and its importance in normal brain function (42).
MeCP2 regulates IGF1 and BDNF levels, and when this gene is mutated both proteins have their expression decreased (22). In our study, we show that IGF1 gene expression was not different between control and RTT neurons. Interestingly, IGF1R has its gene expression increased in RTT neural cells compared to control.
Previous studies showed that IGF1 is expressed in the brain during the early stages of development. Our data revealed that the expression of IGF1 by the neural cells occurs at the final stages of differentiation of these cells, and there is no significant difference between IGF1 expression in control (WT83) and RTT (Q83X) neurons (8,19).
Several studies have already shown that IGF1 can recover neural function and morphology in Rett Syndrome after treatment (2,11,12,22). Additionally, IGF1R has been shown as having an important role in brain development. Mutations leading to loss-of-function of IGF1R may result in microcephaly, mental retardation and behavioural deficiency (20).
Taking into account IGF1 role in RTT, in this study we showed gene expression of IGF1 and IGF1R from iPSC until neuronal cell stage in control and RTT. It was demonstrated that there is an IGF1 gene expression only during the neuronal cell stage, at 6 weeks of cell differentiation, which means that this expression occurs mainly at mature stage. From iPSC until NPC, there is very low IGF1 gene expression. With the presented results some conclusions can be inferred, such as that the presence of IGF1R since iPSC stage does not necessary means that this receptor is related individually to IGF1 signalling. It was shown (43) that extracellular vimentin interacts with an IGF1 receptor to promote axonal growth in cultured cortical neurons; therefore the expression of IGF1R, before the expression of an IGF1 by the neurons, can relate this protein to other functions. Likewise, the presence of IGF1R is necessary for axonal regeneration in the adult central nervous system (CNS), as well as for embryonic CNS axonal development (44).
When the extracellular concentration of IGF1 was measured in the medium that neurons were cultured, no different changes in hormone concentration were detected at 3 weeks of differentiation. However, when we measured the IGF1 concentration on 6-weeks-old neural culture, the consumption of IGF1 increased considerably in both types of cells, control and RTT. Interestingly, when we examined the levels of IGF1R, we detected an increase of expression only in the RTT neurons. This increased expression of IGF1R may reflect the prompt response of these cells to treatment with IGF1, which is involved with the neuroprotective mechanisms after some neural damages (45) and is increased after injures in this fundamental tissue (46,47).
We hypothesized that TRalpha3, a subtype of thyroid hormone receptor, may be involved in regulating the expression of IGF1, since the expression of TRalpha3 occurs concomitantly with IGF1. Moreover, our work is the first to prove a strong correlation between IGF1R response to IGF1 and TRalpha3. It is noteworthy that there is a great possibility of the interaction among these genes. Unexpectedly, the treatment of neural cells with thyroid hormone at 3 and 6 weeks of differentiation seems to corroborate our hypothesis. The other subtypes of thyroid hormone receptors, such as TRalpha1, TRbeta1 and TRbeta2 are expressed in all studied stages of cell differentiation (iPSC, EB, NPC and neuronal cells – Fig. 7), after treatment of neuronal cells with TH only at Q83X 6-week-differentiated neural cells the consumption of IGF1 increased in both dosage of T3 treatments (10–6 M and 10–12 M). In order to assess whether the existing functional differences between THRA and THRB genes are derived from individual properties of each receptor, a study compared the responsive gene expression to T3 in two cell lines, one of which expresses the TRalpha1 and the other, the TRbeta1. This study showed that a considerable amount of target genes tested showed preference for one of the two receptors, indicating these receptors contribute unique characteristics and can determine its function, mainly during neurodevelopment (48).
To answer the new hypothesis, we will need more studies, but these present results give us a good hint that we are in the right direction to better understand the role of these studied genes.
This study has shown that the gene expression of TH receptors is affected in all cellular phases in RTT compared to the Control. They act differently in distinct stages of cell differentiation.
The effects of TH in the CNS are primarily mediated by TH receptors (TRs), members of the family of nuclear hormone receptors. The TRs bind to the regulatory regions of the DNA of target genes to activate or repress transcription through interactions with proteins known as co-regulators (49).
The major isoforms of TRs are TRalpha1 and TRbeta1, both bind to TH and also have different biological functions (49).
TRalpha1 corresponds to 70–80% of thyroid receptors in the brain, but there is still TRbeta1 and TRbeta2 expression (50). TRbeta2 comprises 10% of receptors in various tissues, including the brain (51). The expression of TRalpha1, which is important for CNS development, is higher in RTT compared with Control at the EB stage.
The lack of TRalpha1 in mice has a different result when compared to animals that have a mutation in this gene. The absence of TRalpha1 in the brain does not resemble hypothyroidism. In hypothyroidism, the receptor that is not bound to a hormone presents activity since the gene expression of some proteins that are negatively responsive to TH is induced. On the other hand, in the absence of the receptor, this activity is suppressed and hypothyroidism is not as damaging as in its presence (52).
TRs can bind to DNA in its entirety as protein dimers, forming heterodimers with other members of the nuclear receptor family, such as the RXR (Retinoic X Receptor) or homodimers with themselves. TRs have bimodal regulation, typically connecting co-repressors to inactivate transcription of target genes, in the absence of TH. TH can also release co-repressors and recruit co-activators to activate transcription of target genes, which respond positively to the presence of this hormone (49).
TRalpha1 is expressed early in embryonic development and then widespread in adults, while TRbeta1 is expressed late in embryonic development and displays a pattern of more restricted tissue expression in adults. TRalpha1 has a greater response to T4 than TRbeta1 (49). The expression of the nuclear receptor RXRG, which forms dimers with TH receptors, significantly increased expression at the neural cell stage, showing its importance at this stage.
In humans, TH receptors are present around week 10 of embryonic development and gradually increase until week 18. It is in this period that the brain increases considerably in size (53). This study found a pattern of increasing TH receptors during cell differentiation and the higher expression at the neural cell stage, corroborating with the literature.
Thyroid hormone has an important role in brain development and its function has rarely been studied in RTT, in which was shown to present prevalence of decreased concentration of serum free T4 (3). IGF1, a fundamental regeneration factor, was already shown as responsive to this hormone, such as other factors regulated by TH, Neuron Growth Factor (NGF) (54–57), Fibroblast Growth Factor (FGF), BDNF, Epidermal Growth factor (EGF), Vascular endothelial Growth factor (VEGF) (58).
We conclude that increased IGF1R expression may be the key role for the improvement experienced by RTT-derived cells after treatment with IGF1 and that thyroid hormone receptor alpha 3 (TRalpha 3) may be involved with the responsiveness onset of the IGF1R to IGF1.
Materials and Methods
Ethics committee
This study was reviewed and approved by the UCSD Human Research Protections Program in accordance with the requirements of the Code of Federal Regulations on the Protection of Human Subjects and its reference number is 090801ZF. Recruited individuals were aware about study description, written informed consent was signed.
Cell culture
Induced pluripotent stem cells (iPSCs)
IPSCs reprogrammed from skin fibroblasts were used in this study. Skin fibroblasts derived from male patient with Rett syndrome, with a nonsense mutation at position 83 of the protein (RTT), and a male control (WT83) were transduced with retroviral human vectors containing coding sequences of human OCT4, SOX2, KLF4 and C-MYC (59). After four days post infection, fibroblasts were trypsinized to single cells and plated on the inactivated mouse embryonic fibroblast feeders, and cultured using human embryonic stem cell medium. After 3–4 weeks, iPSC clones were manually picked and further propagated clonally on feeders, using mTeSR medium (StemCell Technologies, Tukwila, WA, EUA).
Generation of embryoid bodies (EBs)
The iPSCs were treated for two days in N2 medium (DMEM/F-12 50/50, with L-glutamine and 15 mM HEPES; insulin; progesterone, sodium selenate, L-glutamine, HEPES, Apo-transferrin) with Dorsomorphin (DM) and SB431542 (Stemgent, Cambridge, MA, EUA). After these two days, the cells were resuspended and maintained in suspension for approximately 1 week with N2 medium (6).
Neural rosettes generation
After 5–7 days EBs were collected and gently resuspended and seeded in 6 cm coated matrigel plate, so rosettes formation would occur. For approximately 4 days rosettes were kept in NGF medium (DMEM/F-12 supplemented with 0.5X N2, 0.5X Gem21, 1X Penicillin/Streptomycin and 20ng/ml of bFGF) and then manually collected. After dissociation to single cells, the obtained Neural Progenitor Cells (NPCs) were plated on Poly-ornithine/Laminin plates and cultured with NGF medium (6).
NPCs expansion and neural differentiation
NPCs were propagated until the fifth passage in a density of 106 cells in Poly-ornithine/laminin plates using NGF medium. After this, they were seeded in six-well plates in a density of 7 x 105 cells per well. When these cells achieved 90% of confluence the differentiation was induced with NG medium (DMEM/F-12 supplemented with 0.5X N2, 0.5X Gem21 and 1X Penicillin/Streptomycin) supplemented with Rock Inhibitor (Ri) for 48h, then the cells were kept in NG medium without Ri up to six weeks for neural maturation
RNA extraction and qRT-PCR
Total RNA was extracted using TRIzol® Reagent (Life Technologies). cDNA was synthesized by taking 1 μg of total RNA and QuantiTec Reverse Transcription Kit (Qiagen) was used as the manufacturer’s instruction. Quantitative reverse-transcriptase PCR (qRT-PCR) was performed using gene-specific primers (Supplementary Material, Table S1) and QuantiTect SYBR Green PCR Kit (Qiagen). Quantification was made at ABI Prism 5700 detector (Applied Biosystems). The expression of target genes and the endogenous control were measured with technical duplicates in each qRT-PCR reaction. To obtain statistical significance, values from a minimum of three independent differentiation qRT-PCR runs were considered. GAPDH (Supplementary Material, Table S1) was used as the endogenous control. The cycle threshold number (Ct) was calculated by (Life Technologies, USA). The relative expressions of each target gene across differentiation days were normalized using 2(−ΔΔCt) method compared with control (60).
Cell karyotyping
Cytogenetic analysis was performed in all clones to evaluate correct cell karyotype (Children's Hospital Los Angeles).
Immunocytochemistry
Cells grown on coverslips were fixed using 4% paraformaldehyde (PFA) for 20 min at room temperature (RT). Cells were permeabilized and blocked using a SuperBlock™ (Thermo Scientific) blocking buffer for 30 min at RT. Primary antibodies were diluted in antibody diluent (blocking buffer) and were incubated overnight at 4 °C. Secondary antibodies were incubated for 1 h at RT. Cells were washed three times with phosphate-buffered saline (PBS) + 0.1% Tween-20. DAPI (Dako) was used to visualize nuclei. Coverslips were mounted on slides using Prolong Gold mounting medium (Life Technologies). Images were acquired using confocal microscope Radiance 2100 (Zeiss, Oberkochen, Germany) equipped with an upright microscope (Eclipse E800; Nikon, Tokyo, Japan).
IGF1 hormonal dosage
After 48 h of cell treatment, the medium was collected in 1.5 ml vials and frozen until hormonal dosage. IGF1 concentration was determined by using an ELISA kit (Abnova, Taiwan) following the manufacturer’s protocol.
Statistical analysis
The results were first submitted to the Kolmogorov–Smirnov normality test. The parameters were analysed by ANOVA one way when passed in normality test followed by Newman-Keuls Multiple Comparison Test, and KRUSKAL-WALLIS, followed by Dunn’s multiple comparison test. Pearson correlation analysis was done to evaluate the relationship strength between variables obtained from the same sample. The r values range from +1 to −1, where +1 is an exact correlation and −1 is an exact inverse correlation. All analyses were performed with GraphPad Prism (La Jolla, California, USA). Statistical differences were considered significant when the value of P was lower than 0.05. The values were expressed as means and the standard error of the mean (± SEM).
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest Statement. None declared.
Funding
This work was supported by a grant from the California Institute for Regenerative Medicine (CIRM) TR2-01814, TR4-06747, the National Institutes of Health (NIH) R01MH094753, R01MH103134, U19MH107367, a NARSAD Independent Investigator Grant to A.R.M., and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasilia, Brazil) 18952-12-7 to J.S.S., and from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, São Paulo, Brazil) 2014/08049-1 to B.H.S.A.
Supplementary Material
References
- 1. Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet., 23, 185–188. [DOI] [PubMed] [Google Scholar]
- 2. Pini G., Scusa M.F., Congiu L., Benincasa A., Morescalchi P., Bottiglioni I., D., Marco P., Borelli P., Bonuccelli U., Della-Chiesa A., et al. (2012) IGF1 as a potential treatment for Rett Syndrome: Safety assessment in six Rett patients. Autism. Res. Treat., 679801. (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stagi S., Cavalli L., Congiu L., Scusa M.F., Ferlini A., Bigoni S., Benincasa A., Rossi B. (2015) Thyroid Function in Rett Syndrome. Horm. Res. Paediatr., 83, 118–125. [DOI] [PubMed] [Google Scholar]
- 4. Ross P.D., Guy J., Selfridge J., Kamal B., Bahey N., Tanner K.E., Gillingwater T.H., Jones R.A., Loughrey C.M., McCarroll C.S., et al. (2016) Exclusive expression of MeCP2 in the nervous system distinguishes between brain and peripheral Rett syndrome-like phenotypes. Hum. Mol. Genet., pii, ddw269.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnston M., Blue M.E., Naidu S. (2015) Recent advances in understanding synaptic abnormalities in Rett syndrome. F1000Res, pii, F1000 Faculty Rev-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nageshappa S., Carromeu C., Trujillo C.A., Mesci P., Espuny-Camacho I., Pasciuto E., Vanderhaeghen P., Verfaillie C.M., Raitano S., Kumar A., et al. (2015) Altered Neural network and rescue in human MECP2 duplication model. Mol. Psychiatry, 21, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Q., Zhao Y., Yang Y., Bao X. (2015) MECP2 duplication syndrome in Chinese family. BMC. Med. Genet., 16, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bondy C.A., Cheng C.M. (2004) Signaling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol., 490, 25–31. [DOI] [PubMed] [Google Scholar]
- 9. Netchine I., Azzi S., Houang M., Seurin D., Perin L., Ricort J.M., Daubas C., Legay C., Mester J., Herich R., et al. (2009) Partial primary deficiency of insulin-like growth factor (IGF)-I activity associated with IGF1 mutation demonstrates its critical role in growth and brain development. J. Clin. Endocrinol. Metab., 94, 3913–3921. [DOI] [PubMed] [Google Scholar]
- 10. Niblock M.M., Brunso-Bechtold J.K., Riddle D.R. (2000) Insulin-like growth factor I stimulates dendritic in primary somatosensory cortex. J. Neurosci., 20, 4165–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marchetto M.C., Carromeu C., Acab A., Yu D., Yeo G.W., Mu Y., Chen G., Gage F.H., Muotri A.R. (2010) A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell, 143, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pini G., Scusa M.F., Benincasa A., Bottiglioni I., Congiu L., Vadhatpour C., Romanelli A.M., Gemo I., Puccetti C., McNamara R., et al. (2014) Repeated insulin-like growth factor 1 treatment in a patient with Rett syndrome: a single case study. Front. Pediatr., 2, 52.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ocrant I., Fay C.T., Parmelee J.T. (1990) Characterization of insulin-like growth factor binding proteins produced in the rat central nervous system. Endocrinology, 127, 1260–1267. [DOI] [PubMed] [Google Scholar]
- 14. Binoux M., Roghani M., Hossenlopp P., Whitechurch O. (1991) Cerebrospinal IGF binding proteins: isolation and characterization. Adv. Exp. Med. Biol., 293, 161–170. [DOI] [PubMed] [Google Scholar]
- 15. Butler A.A., Yakar S., Gewolb I.H., Karas M., Okubo Y., LeRoith D. (1998) Insulin-like growth factor 1 receptor signal transduction: at the interference between physiology and cell biology. Comp. Biochem. Physiol. B. Biochem. Mol. Biol., 121, 19–26. [DOI] [PubMed] [Google Scholar]
- 16. Anderson M.F., Åberg M.A.I., Nilson M., Eriksson P.S. (2002) Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Dev. Brain. Res., 134, 115–122. [DOI] [PubMed] [Google Scholar]
- 17. Bondy C.A., Lee W.H. (1993) Patterns of insulin-like growth factor and IGF receptor gene expression in the brain. Functional implications. Ann. N. Y. Acad. Sci., 692, 33–43. [DOI] [PubMed] [Google Scholar]
- 18. Yan H., Mitschelen M., Bixler G.V., Brucklacher R.M., Farley J.A., Han S., Freeman W.M., Sonntag W.E. (2011) Circulating IGF1 regulates hippocampal IGF1 and brain gene expression during adolescence. J. Endocrinol., 211, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandez A.M., Torres-Alemán I. (2012) The many faces of insulin-like peptide signaling in the brain. Nat. Rev. Neurosci., 13, 225–239. [DOI] [PubMed] [Google Scholar]
- 20. Holzenberger M. (2011) IGF-I signaling and effects on longevity. Nestle. Nutr. Workshop. Ser. Pediatr. Program, 68, 237–245. discussion 246-249. [DOI] [PubMed] [Google Scholar]
- 21. White M.F. (2014) IRS2 integrates insulin/IGF1 signaling with metabolism, neurodegeneration and longevity. Diabetes. Obes. Metab., 16 Suppl 1, 4–15. [DOI] [PubMed] [Google Scholar]
- 22. Castro J., Garcia R.I., Kwok S., Banerjee A., Petravicz J., Woodson J., Mellios N., Tropea D., Sur M. (2014) Functional recovery with recombinant human IGF1 treatment in a mouse model of Ret Syndrome. Proc. Natl. Acad. Sci. U. S. A, 111, 9941–9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hewitt S.C., Li Y., Li L., Korach K.S. (2010) Estrogen-mediated regulation of IGF1 transcription and uterine growth involves direct biding of estrogen receptor alpha to estrogen-responsive elements. J. Biol. Chem., 285, 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rozario K.S., Lloyd C., Ryan F., Gh and Igh-1 physiology in childhood In: De Groot LJ, Beck-Peccoz P, Chrousos G., et al. editors. Endotext [Internet]. South Dartmouth (MA: ): MDText.com, Inc; 2000-2015. [Google Scholar]
- 25. Martin S., Sirbu A., Betivoiu M., Florea S., Barbu C., Fica S. (2015) IGF1 deficiency in newly diagnosed Graves’ disease patients. Hormones. (Athens), 14, 651–659. [DOI] [PubMed] [Google Scholar]
- 26. Incerpi S., Hsieh M.T., Lin H.Y., Cheng G.Y., De Vito P., Fiore A.M., Ahmed R.G., Salvia R., Candelotti E., Leone S., et al. (2014) Thyroid hormone inhibition in L6 myoblasts of IGF-1-mediated glucose uptake and proliferation: new roles for integrin ανβ3. Am. J. Physiol. Cell. Physiol., 307, C150–C161. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed R.G. (2016) Maternal bisphenol A alters fetal endocrine system: Thyroid adipokine dysfunction. Food. Chem. Toxicol., 95, 168–174. [DOI] [PubMed] [Google Scholar]
- 28. Ahmed R.G. (2016) Gestational dexamethasone alters fetal neuroendocrine axis. Toxicol. Lett., 258, 46–54. [DOI] [PubMed] [Google Scholar]
- 29. Manzano J., Bernal J., Morte B. (2007) Influence of thyroid hormones on maturation of rat cerebellar astrocytes. Int. J. Dev. Neurosci., 25, 171–179. [DOI] [PubMed] [Google Scholar]
- 30. Ahmed O.M., El-Gareib A.W., El-Bakry A.M., Abd El-Tawab S.M., Ahmed R.G. (2008) Thyroid hormones states and brain development interactions. Int. J. Dev. Neurosci., 26, 147–209. [DOI] [PubMed] [Google Scholar]
- 31. Mendes-de-Aguiar C.B., Alchini R., Decker H., Alvarez-Silva M., Tasca C.I., Trentin A.G. (2008) Thyroid hormone increases astrocytic glutamate uptake and protects astrocytes and neurons against glutamate toxicity. J. Neurosci. Res., 86, 3117–3125. [DOI] [PubMed] [Google Scholar]
- 32. DeSouza L.A., Ladiwala U., Daniel S.M., Agashe S., Vaidya R.A., Vaidya V.A. (2005) Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol. Cell. Neurosci., 29, 414–426. [DOI] [PubMed] [Google Scholar]
- 33. Bauer M., Goetz T., Glenn T., Whybrow P.C. (2009) The thyroid-brain interaction in thyroid disorders and mood disorders. J. Neuroendocrinol., 20, 1101–1114. [DOI] [PubMed] [Google Scholar]
- 34. Brent G.A., Moore D.D., Larsen P.R. (1991) Thyroid hormone regulation of gene expression. Annu. Rev. Physiol., 53, 17–35. [DOI] [PubMed] [Google Scholar]
- 35. Harvey C.B., Williams G.R. (2002) Mechanism of thyroid hormone action. Thyroid, 12, 441–446. [DOI] [PubMed] [Google Scholar]
- 36. Flamant F., Samarut J. (2003) Thyroid hormone receptors: lessons from knockout and knock-in mutante mice. Trends. Endocrino. Metab., 14, 85–90. [DOI] [PubMed] [Google Scholar]
- 37. Bernal J. (2007) Thyroid hormone receptors in brain development and function. Nat. Clin. Prat. Endocrinol. Metab., 3, 249–259. [DOI] [PubMed] [Google Scholar]
- 38. Ribeiro R.C., Apriletti J.W., Wagner R.L., West B.L., Feng W., Huber R., Kushner P.J., Nilsson S., Scanlan T., Fletterick R.J., et al. (1998) Mechanisms of thyroid hormone action: insights from X-ray crystallographic and functional studies. Recent. Prog. Horm. Res., 53, 351–394. [PubMed] [Google Scholar]
- 39. Sabatino L., Gliozheni E., Molinaro S., Bonotti A., Azzolina S., Popoff G., Carpi A., Iervasi G. (2007) Thyroid hormone receptor and IGF1/IGF1R system: possible relations in the human heart. Biomed. Pharmacother., 61, 457–462. [DOI] [PubMed] [Google Scholar]
- 40. Veeragavan S., Wan Y.W., Connolly D.R., Hamilton S.M., Ward C.S., Soriano S., Pitcher M.R., McGraw C.M., Huang S.G., Green J.R., et al. (2016) Loss of MeCP2 in the rat models regression, impaired sociability and transcriptional deficits of Rett Syndrome. Hum. Mol. Gent., pii, ddw178.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lazar M.A. (1993) Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr. Rev., 14, 184–193. [DOI] [PubMed] [Google Scholar]
- 42. Aleman A., Torres-Alemán I. (2009) Circulating insulin-like growth factor 1 and cognitive function: neuromodulation throughout the lifespan. Prog. Neurobiol., 89, 256–265. [DOI] [PubMed] [Google Scholar]
- 43. Shigyo M., Kuboyama T., Sawai Y., Tada-Umezaki M., Tahda C. (2015) Extracellular vimentin interacts with insulin-like growth factor 1 receptor to promote axonal growth. Sci. Rep., 5, 12055.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dupraz S., Grassi D., Karnas D., Guil A.F.N., Hicks D., Quiroga S. (2013) The insulin-like growth factor 1 receptor is essential for axonal regeneration in adult central nervous system neurons. Plos. One, 8, e54462.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guan J., Bennet L., Gluckman P.D., Gunn A.J. (2003) Insulin-like growth factor-1 and post-ischemic brain injury. Progr. Neurobiol., 70, 443–462. [DOI] [PubMed] [Google Scholar]
- 46. Madathil S.K., Evans H.N., Saatman K.E. (2010) Temporal and regional changes in IGF-1/IGF-1R signaling in the mouse brain after traumatic brain injury. J. Neurotrauma., 27, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Royo N.C., Conte V., Saatman K.E., Shimizu S., Belfield C.M., Soltesz K.M., Davis J.E., Fujimoto S.T., McIntosh T.K. (2006) Hippocampal vulnerability following traumatic brain injury: A potential role for neurotrophin-4/5 in pyramidal cell neuroprotection. Eur. J. Neurosci., 23, 1089–1102. [DOI] [PubMed] [Google Scholar]
- 48. Chatonnet F., Guyot R., Benoît G., Flamant F. (2013) Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells. Proc. Natl. Acad. Sci. U. S. A, 110, 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schroeder A.C., Privalsky M.L. (2014) Thyroid hormones, T3 and T4, in the Brain. Front. Endocrinol., 5, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hodin R.A., Lazar M.A., Wintman B.I., Darling D.S., Koenig R.J., Larsen P.R., Moore D.D., Chin W.W. (1989) Identification of a thyroid hormone receptor that is pituitary-specific. Science, 244, 76–79. [DOI] [PubMed] [Google Scholar]
- 51. Schwartz H.L., Lazar M.A., Oppenheimer J.H. (1994) Widespread distribution of immunoreactive thyroid hormone beta 2 receptor (TR beta 2) in the nuclei of extrapituitary rat tissues. J. Biol. Chem., 269, 24777–24782. [PubMed] [Google Scholar]
- 52. Bernal J., Morte B. (2013) Thyroid hormone receptor activity in the absence of ligand: physiological and developmental implications. Biochim. Biophys. Acta, 1830, 3893–3899. [DOI] [PubMed] [Google Scholar]
- 53. Iskaros J., Pickard M., Evans I., Sinha A., Hardiman P., Ekins R. (2000) Thyroid hormone receptor gene expression in the first trimester human fetal brain. J. Clin. Endocrinol. Metab., 85, 2620–2623. [DOI] [PubMed] [Google Scholar]
- 54. Gilbert M.E., Sanchez-Huerta K., Wood C. (2016) Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology, 157, 774–787. [DOI] [PubMed] [Google Scholar]
- 55. Sinha R.A., Pathak A., Kumar A., Tiwari M., Shrivastava A., Godbole M.M. (2009) Enhanced neuronal loss under perinatal hypothyroidism involves impaired neurotrophic signaling and increased proteolysis of p75(NTR). Mol. Cell. Neurosci., 40, 354–364. [DOI] [PubMed] [Google Scholar]
- 56. Roskoden T., Heese K., Otten U., Schwegler H. (1999) Modulation of mRNA expression of the neurotrophins of the nerve-growth-factor family and their receptors in the septum and hippocampus of rats after transient postnatal thyroxine treatment. II. Effects on p75 and trk receptor expression. Exp. Brain. Res., 127, 307–313. [DOI] [PubMed] [Google Scholar]
- 57. Jiménez-Cervantes C., Pichon B., Dumont J.E., Maenhaut C. (1998) Activation by thyroid stimulating hormone of nerve growth factor-induced gene-B expression in thyrocytes in culture: relation with proliferation and specific gene expression. Biochim. Biophys. Acta, 1403, 232–244. [DOI] [PubMed] [Google Scholar]
- 58. Wang Y., Zhang S. (2011) Expression and regulation by thyroid hormone (TH) of zebrafish IGF-I gene and amphioxus IGFl gene with implication of the origin of TH/IGF signaling pathway. Comp. Biochem. Physiol. A. Mol. Integr. Physiol., 160, 474–479. [DOI] [PubMed] [Google Scholar]
- 59. Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872. [DOI] [PubMed] [Google Scholar]
- 60. Dussault A.A., Pouliot M. (2006) Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol. Proced., 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.