Abstract
Background:
Several longitudinal studies found an inverse relationship between levels of physical activity and cognitive decline, dementia, and/or Alzheimer’s disease (AD), but results have been inconsistent. We followed an older, community-based cohort for over a decade to examine the association of physical activity with the risk of incident dementia and subclinical brain MRI markers of dementia.
Methods:
The physical activity index (PAI) was assessed in the Framingham Study Original and Offspring cohorts, aged 60 years or older. We examined the association between PAI and risk of incident all-cause dementia and AD in participants of both cohorts who were cognitively intact and had available PAI (n = 3,714; 54% women; mean age = 70±7 years). We additionally examined the association between PAI and brain MRI in the Offspring cohort (n = 1,987).
Results:
Over a decade of follow-up, 236 participants developed dementia (188 AD). Participants in the lowest quintile of PAI had an increased risk of incident dementia compared with those in higher quintiles (hazard ratio [HR] = 1.50, 95% confidence interval [CI] = 1.04–1.97, p = .028) in a multivariable-adjusted model. Secondary analysis revealed that this relation was limited to participants who were apolipoprotein (APO)E ε4 allele noncarriers (HR = 1.58, 95% CI = 1.08–2.32; p = .018) and strongest in participants aged 75 years or older. PAI was also linearly related to total brain and hippocampal volumes (β ± SE = 0.24±0.06; p < .01 and 0.004±0.001; p = .003, respectively).
Conclusion:
Low physical activity is associated with a higher risk for dementia in older individuals, suggesting that a reduced risk of dementia and higher brain volumes may be additional health benefits of maintaining physical activity into old age.
Keywords: Alzheimer’s, Epidemiology, Neurological disorders, Neuroimaging, Physical activity
In the Framingham Heart Study, low levels of physical activity have been associated with greater incidence of ischemic heart disease in men (1). Additionally, level of physical activity has been shown to have an inverse relationship with the incidence of stroke, peripheral vascular disease, and congestive heart failure. Several longitudinal epidemiological studies have also related physical activity to cognitive decline, dementia, and/or Alzheimer’s disease (AD), with the majority (2–4) demonstrating a significant and independent inverse relationship between physical activity and cognitive decline and/or risk of dementia. However, several other studies (5–7) failed to show an association, possibly due to insufficient length of follow-up, significant loss to follow-up, or inadequate physical activity assessment, such as neglecting to assess for a possible nonlinear relationship, between physical activity and dementia. Thus, studies with a longer-term follow-up in older persons are warranted to elucidate the true relationship.
In the Framingham Original and Offspring cohorts, physical activity was measured using the physical activity index (PAI). We examined the relationship between PAI and the risk of incident dementia over a decade of follow-up in both cohorts and identified associations among PAI and subclinical brain MRI markers of dementia in the Offspring cohort.
Methods
Study Population
The Framingham Study Original cohort is a longitudinal community-based sample evaluated biennially since 1948 for cardiovascular risk factors (8). The Framingham Offspring cohort comprises the Offspring of the Original participants or spouses of the Offspring who were enrolled in 1971–1975 and has been examined eight times over the past 40 years, approximately once every 4 to 8 years. Participants who attended one of the three baseline examinations—the Original cohort 20th cycle examination (1986–1987), the Offspring 4th (1988–1992), or Offspring 7th (1998–2001) cycle examination—were pooled. Participants eligible for the present investigation were dementia free and aged at least 60 years at the baseline examination and had available physical activity data (n = 3,714; 54% women; mean age = 70±7 years). All participants were followed prospectively for the development of incident dementia and AD for up to 10 years.
Physical Activity Index
Physical activity was assessed in the Original Framingham Heart Study cohort at the 20th examination cycle and at the 4th and 7th examination cycles in the Framingham Offspring cohort. The PAI was a composite score constructed for each participant by weighting each hour in their typical day based on the their activity level (based on oxygen consumption or metabolic equivalents) and summing up these weighted hours over a 24-hour period. Participants were asked to report the number of hours in a typical day spent sleeping (weighting factor [WF] = 1) and in sedentary (WF = 1.1), slight (WF = 1.5), moderate (WF = 2.4), and heavy activities (WF = 5) (9). Thus, based on a history of usual activity elicited by the examiner from each participant, a person who sleeps continually would receive a score of 24, an office worker with no outside exercise a 27, and a laborer involved in heavy physical activity a score of 42. In addition to the PAI, sedentary time/day and moderate + heavy time/day were used as independent variables. Validity of these variables, by correlation of comparable physical activity questionnaires to accelerometer-determined physical activity measures, has only been fair (0.3–0.4), but it has been suggested that this correlation may be as high a validity as can be expected from a short physical activity questionnaire (10,11). PAI scores, sedentary time and moderate + heavy time were divided into sex-specific quintiles, from low (Q1) to highest (Q5), with Q1 as the referent for PAI and moderate + heavy time and Q5 as the referent for sedentary time.
Outcomes
Dementia
Methods used for dementia screening and follow-up have been previously described (12). For this study, we followed the study population for the development of incident dementia over a period of 10 years from the baseline examination. We used data from the neurologist’s examination, neuropsychological test performance, Framingham study records, hospital records, information from primary care physicians, family interviews, CT and MRI records, and autopsy confirmation when available. All participants identified to have dementia satisfied the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria, had dementia severity equivalent to a Clinical Dementia Rating of one or greater, and had symptoms of dementia for a period of at least 6 months. All participants identified as having Alzheimer’s dementia met the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (13) criteria for definite, probable, or possible AD.
Brain imaging measures
2,063 Offspring participants who attended Examination Cyle 7 and have available PAI also agreed to have a brain MRI scan from 1999 to 2005. MRI acquisition, measurement techniques, and interrater reliability have previously been described (14). MRI measures assessed total cerebral brain volume (TCBV) and hippocampal volume (HPV). To further explore the potential link between physical activity and subclinical markers for dementia, we related PAI to TCBV and HPV in 1987 participants (after excluding 76 participants with prevalent stroke, dementia, or other neurological conditions). TCBV was determined by manual outlining in coronal images of the intracranial vault above the tentorium to determine the total cranial volume as a function of head size. Once the skull and other nonbrain tissues were removed from the image, mathematical modeling was performed to determine total parenchymal brain volume above the tentorium (cerebral). HPV was defined by the operator using manually traced boundaries. Both TCBV and HPV were calculated as ratios over total intracranial volume. Supplementary Figure 1 provides an overview of the timing of physical activity data collection and follow-up for dementia and brain imaging.
Statistical Analyses
We used Cox proportional hazards models to examine the relations of PAI, sedentary time, and moderate + heavy time (as a continuous variable and sex-specific quintiles) assessed at the baseline examination to the risk of incident AD and all-cause dementia over a follow-up of up to 10 years (time scale in years from baseline) in tables and figures. Participants who developed dementia were followed to the date of diagnosis of dementia; those who did not were censored at 10 years, or at the date last known not to be demented if less than 10 years after baseline. We used interactions with time to test for proportional hazards. We explored this potential relationship further by determining the risk of incident AD and all-cause dementia in participants who were in the lowest quintile (Q1) of reported PAI versus higher quintiles (Q2–Q5). The decision to treat physical activity variables as continuous and in quintiles was determined a priori due to previous suggestion of potential nonlinear relationships of physical activity to dementia risk (15). All analyses were adjusted for age and sex, and then additionally for apolipoprotein (APO)Eε4 allele status, plasma homocysteine, diabetes, and prevalent cardiovascular disease including stroke. The potential competing risk of death in these relations was also assessed, using methods described by Fine and Gray (16). Furthermore, to determine whether PAI affects longer-term dementia risk, we performed Cox proportional hazards models with the methods described earlier to test the relation of baseline PAI to dementia and AD at 22 years of follow-up in the Original cohort.
In the 10-year follow-up analyses, we tested for interactions by APOEε4 and age. However, given that in the Cardiovascular Health Cognition and the Age Gene/Environment Susceptibility—Reykjavik (AGES) studies the relationship between physical activity and risk of dementia was observed only in APOEε4 noncarriers (17,18), while the CAIDE (Cardiovascular Risk Factors, Aging and Incidence of Dementia) Study found an inverse relationship between physical activity and cognitive decline primarily in APOEε4 carriers (3), we decided a priori to construct separate models for ε4 carriers and noncarriers. We also decided a priori to perform analyses stratified by age (<75 years vs 75+ years).
We investigated the association of PAI, sedentary time, and moderate + heavy time with brain MRI measures using the same forms of PAI (continuous, quintiles, and Q1 vs Q2–Q5). The primary model was adjusted for age, sex, and the time between PAI measure and MRI. Secondary analyses additionally adjusted for systolic blood pressure, antihypertensive medication, smoking, history of atrial fibrillation, prevalent diabetes, and prevalent cardiovascular disease, including stroke. We chose the age- and sex-adjusted model as the primary analysis because we wished to assess whether any observed association was partly mediated through vascular risk factors. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
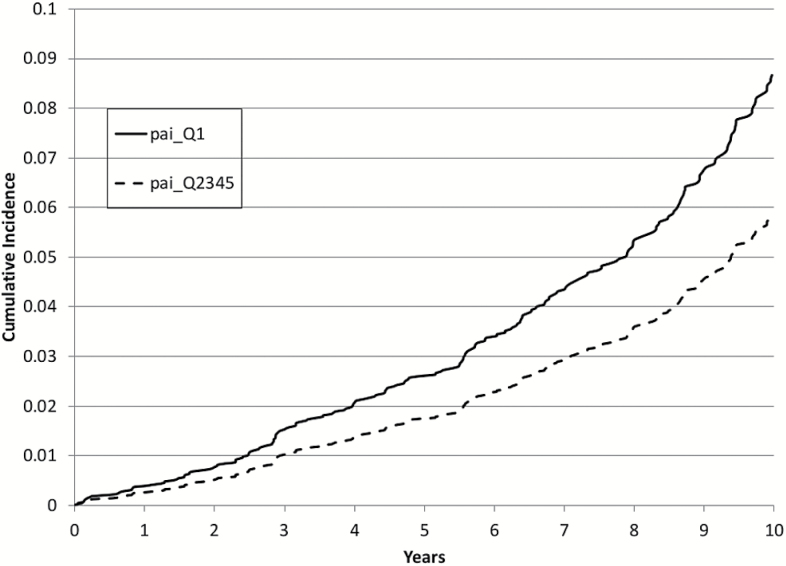
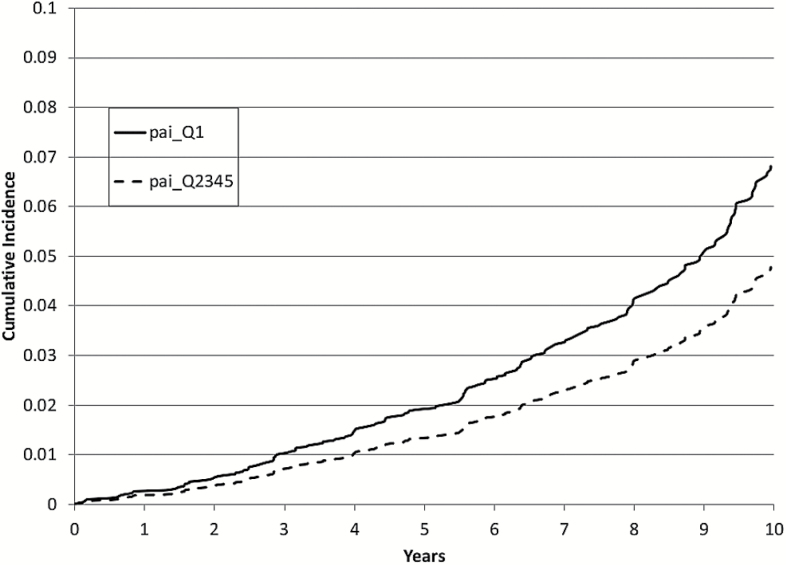
Baseline characteristics of the study population are presented in Table 1. After up to 10 years of follow-up (mean 7.5±2.7 years), 236 participants developed dementia (188 developed AD). We found that although PAI was not linearly related to either all-cause dementia or AD, there was a threshold effect such that participants in the lowest quintile (Q1) of PAI had an increased risk of incident dementia compared with those in higher quintiles (hazard ratio = 1.50, 95% confidence interval = 1.12–2.01, p = .006; Table 2), visually represented in Figure 1. Additional adjustment for APOEε4 status and multivariable adjustment did not change this relationship. Similar results were observed when analyses were limited to incident AD alone (Figure 2), except additional adjustment for APOEε4 allele status and multivariable adjustment made the relation nonsignificant (Table 3). Additional analysis assessing the competing risks of death (using methods described by Fine and Gray (16)) yielded similar results to those demonstrated in Tables 2 and 3 (data not shown). In contrast, in a longer follow-up period of up to 22 years in the Original cohort (n = 1,104, a subsample of our study population), the relations of baseline physical activity to dementia or AD were not significant (Supplementary Tables 1 and 2).
Table 1.
Baseline Characteristics of Study Population (Original cohort examination Cycle 20 [1986–1987] and Offspring cohort examination Cycles 4 and 7)
| Characteristic | Female (n = 2,022) | Male (n = 1,692) |
|---|---|---|
| Age (years ± SD) | 71±7 | 70±7 |
| High school degree(%) | 82 | 82 |
| APOEε4 (%) | 21 | 22 |
| Plasma homocysteine (µmol/L), median (range) | 9.3 (3.3–61.6) | 10.0 (4.1–84.3) |
| Prevalent cardiovascular disease (%) | 18 | 30 |
| Diabetes (%) | 11 | 18 |
| Stroke (%) | 3 | 4 |
| Body mass index (kg/m2 ± SD) | 27±5 | 28±4 |
| Physical activity index, median (range) | 34.4 (24.2–65.7) | 35.4 (24.6–74.5) |
| Sedentary time (hours) | 7.4±3.6 | 7.1±3.5 |
| Moderate + heavy time (hours) | 4.4±2.7 | 4.4±2.9 |
Table 2.
Physical Activity Index and the Risk of Dementia (Framingham Original and Offspring cohorts)
| Cases / N | 236 / 3,714 | p Value | 214 / 3,542 | p Value | 190 / 2,958 | p Value | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| Age and Sex Adjusted | Age, Sex, and APOEε4 Adjusted | Multivariable Adjusteda | |||||
| Dementia | Per SD | 1.01 (0.89–1.15) | .866 | 1.06(0.92–1.21) | .434 | 1.04 (0.90–1.20) | .570 |
| Q1 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | ||||
| Q2 | 0.52 (0.35–0.78) | .002 | 0.51 (0.33–0.80) | .003 | 0.44 (0.27–0.73) | .001 | |
| Q3 | 0.68 (0.46–1.00) | .047 | 0.73 (0.49–1.09) | .128 | 0.80 (0.52–1.22) | .292 | |
| Q4 | 0.64 (0.43–0.96) | .030 | 0.63 (0.41–0.97) | .035 | 0.63 (0.40–1.00) | .051 | |
| Q5 | 0.87 (0.60–1.26) | .458 | 0.94 (0.63–1.38) | .733 | 0.95 (0.63–1.41) | .518 | |
| Q1 vs Q2–5 | 1.50 (1.12–2.01) | .006 | 1.44 (1.05–1.96) | .022 | 1.47 (1.06–2.04) | .023 |
Notes: APO = apolipoprotein; CI = confidence interval; HR = hazard ratio; Q = quartile.
aAdjusted for age, sex, high school degree, APOEε4 allele status, log plasma homocysteine, systolic blood pressure, diastolic blood pressure, antihypertensive medication, total cholesterol, current smoking, prevalent cardiovascular disease, diabetes, stroke, and atrial fibrillation.
p Values less than .05 are bolded to highlight statistical significance.
Figure 1.
Ten-year cumulative incidence of dementia: Lowest quintile (Q1) of physical activity index (PAI) versus upper four quintiles (Q2–Q5), adjusted for age and sex.
Figure 2.
Ten-year cumulative incidence of Alzheimer’s disease: Lowest quintile (Q1) of physical activity index (PAI) versus upper four quintiles (Q2–Q5), adjusted for age and sex.
Table 3.
Physical Activity Index and the Risk of Alzheimer’s Disease (Framingham Original and Offspring cohorts)
| Cases / N | 188 / 3,714 | p Value | 171 / 3,542 | p Value | 153 / 2,958 | p Value | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| Age and Sex Adjusted | Age, Sex, and APOEε4 Adjusted | Multivariable Adjusteda | |||||
| Alzheimer’s disease | Per SD | 1.06 (0.92–1.23) | .709 | 1.11 (0.96–1.29) | .153 | 1.10 (0.95–1.29) | .214 |
| Q1 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | ||||
| Q2 | 0.49 (0.30–0.79) | .003 | 0.49 (0.29–0.83) | .008 | 0.44 (0.25–0.78) | .005 | |
| Q3 | 0.73 (0.48–1.13) | .157 | 0.81 (0.52–1.27) | .350 | 0.91 (0.57–1.46) | .705 | |
| Q4 | 0.71 (0.45–1.10) | .120 | 0.72 (0.45–1.15) | .163 | 0.74 (0.45–1.23) | .251 | |
| Q5 | 0.90 (0.59–1.37) | .612 | 0.98 (0.63–1.52) | .930 | 0.93 (0.58–1.49) | .758 | |
| Q1 vs. Q2–Q5 | 1.44 (1.04–2.00) | .029 | 1.35 (0.95–1.92) | .097 | 1.34 (0.92–1.95) | .129 |
Notes: APO = apolipoprotein; CI = confidence interval; HR = hazard ratio; Q = quartile.
aAdjusted for age, sex, high school degree, APOEε4 allele status, log plasma homocysteine, systolic blood pressure, diastolic blood pressure, antihypertensive medication, total cholesterol, current smoking, prevalent cardiovascular disease, diabetes, stroke, and atrial fibrillation.
p Values less than .05 are bolded to highlight statistical significance.
Stratified analyses (Supplementary Tables 3 and 4) showed that the relationship between physical activity and incident dementia risk was limited to participants who were non-APOEε4 allele carriers and to participants who were 75 years or older. Interactions by age and APOEε4 status were not significant (data not shown). Analysis testing components of the PAI (sedentary time and moderate + heavy time) revealed no significant relations to dementia in multivariable models (Supplementary Table 5).
On brain MRI measures, we found that PAI was linearly related to total cerebral brain and hippocampal volumes (β ± SE = 0.24±0.06; p < .001 and β ± SE = 0.004±0.001; p = .003, respectively). These relationships remained statistically significant in the multivariate model (Table 4).
Table 4.
Physical Activity Index and TCBV and HPV in Framingham Offspring Cohort, Examination Cycle 7
| β ± SE | p Value | β ± SE | p Value | |
|---|---|---|---|---|
| Age and Sex Adjusted | Multivariable Adjusted* | |||
| TCBV | ||||
| Per SD | 0.24 ± 0.06 | <.001 | 0.23 ± 0.06 | <.001 |
| Q1 | 1.00 (Referent) | 1.00 (Referent) | ||
| Q2 | 0.02±0.20 | .914 | -0.10±0.20 | .605 |
| Q3 | 0.26±0.20 | .191 | 0.22±0.20 | .288 |
| Q4 | 0.56 ± 0.20 | .005 | 0.42 ± 0.20 | .035 |
| Q5 | 0.54 ± 0.20 | .007 | 0.49 ± 0.20 | .014 |
| HPV | ||||
| Per SD | 0.004 ± 0.001 | .003 | 0.004 ± 0.001 | .003 |
| Q1 | 1.00 (Referent) | 1.00 (Referent) | ||
| Q2 | 0.001±0.004 | .713 | 0.001±0.004 | .772 |
| Q3 | 0.001±0.004 | .746 | 0.002±0.004 | .693 |
| Q4 | 0.006±0.004 | .107 | 0.006±0.004 | .109 |
| Q5 | 0.010 ± 0.004 | .007 | 0.011 ± 0.004 | .006 |
Notes: HPV = hippocampal volume; PAI = physical activity index; Q = quintile; TCBV = total cerebral volume.
aAdjusted for age, sex, high school degree, time from PAI to MRI measure, APOEε4 allele status, log plasma homocysteine, systolic blood pressure, diastolic blood pressure, antihypertensive medication, total cholesterol, current smoking, prevalent cardiovascular disease, diabetes, stroke, and atrial fibrillation.
p Values less than .05 are bolded to highlight statistical significance.
Discussion
Many longitudinal epidemiological studies have analyzed the possible relationship between physical activity and cognitive decline, dementia, and/or Alzheimer’s disease (AD) with varying results (19), hinging on a variety of factors: study design (eg, follow-up time), differences in cohort demographics, adjustment for confounders, and assessment of physical activity. The largest of the positive studies was performed in the Nurses Health Study cohort, wherein women in the highest quintile of physical activity were 20% less likely to show cognitive decline over a decade later, compared with the lowest quintile (2). Larson and colleagues found that older persons who participated in a minimal amount of aerobic physical activity (lasting only 45 minutes per week) had a decreased risk of dementia compared with those who were more sedentary (4). Recently, the Rotterdam Study reported an association between higher levels of physical activity and lower risk of dementia when follow-up was confined to up to 4 years, but no such association with longer follow-up (20). On the other hand a two-decade follow-up study, the CAIDE Study, found that participation in leisure-time physical activity for 20–30 minutes at least twice a week was associated with a reduced risk of dementia and AD (3). But not all studies that looked at this potential relationship have yielded positive results (5–7).
In the Bronx Aging Study, participation in leisure cognitive activities was found to be associated with a decreased risk of dementia over two decades while physical activity was not (when examined overall or dichotomized as low or high physical activity) (5). Similarly, Yamada and colleagues found that a self-assessment of physical activity (as a continuous variable) was not associated with the risk for AD and vascular dementia in older individuals (6). In the Religious Orders Study, an estimate of hours per week spent doing physical activities was not associated with incident AD or decline in cognitive function after 4 years of follow-up, despite a similar analytic design to the one employed in the current study where physical activity was divided into quartiles (7). Thus, although the preponderance of epidemiological evidence supports the link between physical activity and cognitive decline/dementia risk (15), further studies are needed to elucidate this relationship.
In the Framingham Heart Study, the incidence of ischemic heart disease in men had a statistically significant negative association with level of physical activity (1). Additionally, a trend toward an inverse relationship between physical activity and the incidence of stroke, peripheral vascular disease, and congestive heart failure has been observed, although the associations did not reach statistical significance. In the present investigation, participants in the pooled Framingham Original and Offspring cohorts (mean age of 70±7 years) who had lower levels of physical activity had a higher incidence of dementia and AD. We observed that this association was significant even after a follow-up of up to a decade, making reverse causality an unlikely explanation for the observed relationship. However, in follow-up longer than a decade (up to 22 years), only available in a subsample of our study population, the relations of baseline physical activity to dementia or AD were not significant and the effect sizes were attenuated. This observation was consistent with a recent systematic review suggesting a weaker protective effect of physical activity in studies with follow-up time longer than a decade (15).
In the 10-year follow-up analyses, our reported protective effect appeared strongest for the difference between the lowest physical activity group (Q1) and the more active participants (Q2–Q5). We interpret this to mean that even a modest amount of physical activity may be protective against dementia. Furthermore, our sensitivity analysis did not reveal a significant relation of self-reported sedentary or moderate + heavy time to dementia, suggesting that the association between PAI and dementia must be driven by a balance of all types of activities, not just single components of higher intensity activities. These data add to a growing body of literature identifying the importance of light activity for favorable health outcomes (21).
Another important aspect of the relation of low physical activity to higher dementia risk is that it has been suggested to be curvilinear with diminishing returns at higher levels of physical activity (15), which may explain why some studies (including the current study) did not report significant associations when physical activity is assessed as a continuous or dichotomous variable. The suggestion of diminishing returns with greater amounts of physical activity is also consistent with findings from two studies with follow-up of more than 20 years (3,18). Therefore, it is necessary for future studies to stratify by level of physical activity, or use nonlinear modeling, to accurately assess this relationship.
We found a linear association between physical activity levels and total cerebral and hippocampal brain volume, supporting reported relations of physical activity with brain volume in other cohorts (22). Our findings suggest it is the highest physical activity group driving the protective effect on total brain and hippocampal volumes. Our results are in agreement with a previous exercise intervention study (walking 2 hours per week) that was shown to prevent hippocampal atrophy in older adults (23). More research is necessary to understand the intensity and duration of physical activity necessary to slow the brain morphological changes that occur with age, but it is becoming clearer that a relation between physical activity and brain aging exists.
There are several theories to explain the pathophysiologic mechanisms underlying the relations observed between physical activity and brain aging or dementia. One possible mechanism is through exercise-induced increase in brain-derived neurotrophic factor, which may result in growth of new brain capillaries, increase in length and number of dendritic interconnections, and increase in hippocampal cell production (24,25). These structural changes from chronotropic aerobic activity observed in animal studies may explain our finding of a linear relationship between PAI and total cerebral and hippocampal brain volumes. It is possible that in addition, there are other mechanisms for the relationship between physical activity and dementia that may not be mediated by structural changes. These alternate mechanisms include exercise-induced alternations in oxidants/antioxidant systems (26) and the adrenergic system (27), which may not translate to structural brain changes.
Given the known beneficial effects of physical activity on overall cardiovascular health, several studies have likewise implicated vascular mechanisms, including increase in cerebral capillary density (28) and augmentation of cerebral blood flow by exercise. The brain is a highly metabolic organ that accounts for 20% of the oxygen and 25% of the glucose consumed even though it is only 2% of body weight (29), which makes it particularly vulnerable to alternations in blood flow. Resting cerebral blood flow has been shown to decline in normal healthy aging (30), whereas people older than 65 years who continued to work or elected to participate in regular physical activities maintained their cerebral blood flow for 3 years (31).
The limitation of the relationship between physical activity and dementia/AD in the present investigation only to noncarriers of the APOEε4 allele is consistent with observations in the Cardiovascular Health Cognition Study (17) and the AGES Study (18). Previous studies have shown that APOEε4 carriers do not derive the same blood pressure and lipid benefits from physical activity as noncarriers (32,33). APOEε4 being an established risk factor for dementia, this finding suggests that any beneficial effect of physical activity on cognition may not be sufficient to mitigate the effect of APOEε4 on dementia risk. However, the CAIDE Study reported a relation between physical activity and dementia only in APOEε4 allele carriers (3), and others have found no interaction between physical activity and the APOEε4 allele as they relate to dementia risk (34–36). Differences in cohort demographics or study design, previously discussed, may explain these inconsistent findings. One source of limitation in comparing our results to results of other observational studies is the ages of participants at physical activity assessment and follow-up for dementia. Our identification of a stronger relationship between physical activity and incident dementia in participants who were aged 75 years or older demonstrates the beneficial effects of physical activity especially in older persons.
The study’s strengths include the prospective design, the long follow-up period, the older mean age of the study population, and the availability of structural brain MRI measures. We also found no measurable effects of the competing risk of death in the observed relation of higher physical activity to lower dementia risk However, our study also has several limitations, including the primarily European American cohort that may limit generalizability to larger, more ethnically diverse samples. Also, there is potential measurement error inherent in self-reported physical activity used in this study; however, given that random measurement error tends to bias toward null results, we have reason to speculate that the relationships described in this study may actually be stronger than we reported. The results are consistent with the majority of studies published thus far and add to the weight of the evidence linking physical activity and dementia risk in older persons.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
This work was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute Contract (NIH/NHLBI, HHSN268201500001I, N01-HC-25195, N01HV28178, R01HL093029, R01 HL131029 U01 HL096917, and 2K24HL04334) and training grant (T32-HL07224), the National Institute on Aging (NIA, R01 AG016495, AG008122, AG031287, AG047645), the National Institute of Neurological Disorders and Stroke (NINDS, R01 NS017950), and the American Heart Association (AHA Award 11CRP4930020 and 16MCPRP30310001). The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS, NHLBI, NIA, NIH, or AHA.
Supplementary Material
References
- 1. Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–861. doi:10.1001/archinte.139.8.857 [PubMed] [Google Scholar]
- 2. Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi:10.1001/jama.292.12.1454 [DOI] [PubMed] [Google Scholar]
- 3. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi:10.1016/S1474-4422(05)70198-8 [DOI] [PubMed] [Google Scholar]
- 4. Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi:10.7326/0003-4819-144-2-200601170-00004 [DOI] [PubMed] [Google Scholar]
- 5. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi:10.1056/NEJMoa022252 [DOI] [PubMed] [Google Scholar]
- 6. Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc. 2003;51:410–414. doi:10.1046/j.1532-5415.2003.51117.x [DOI] [PubMed] [Google Scholar]
- 7. Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi:10.1001/jama.287.6.742 [DOI] [PubMed] [Google Scholar]
- 8. Dawber TR, Kannel WB. The Framingham study. An epidemiological approach to coronary heart disease. Circulation. 1966;34:553–555. doi:10.1161/01.CIR.34.4.553 [DOI] [PubMed] [Google Scholar]
- 9. Kannel WB, Belanger A, D’Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J. 1986;112:820–825. doi:10.1016/0002-8703(86)90480-1 [DOI] [PubMed] [Google Scholar]
- 10. Lee PH, Yu YY, McDowell I, Leung GM, Lam TH, Stewart SM. Performance of the international physical activity questionnaire (short form) in subgroups of the Hong Kong Chinese population. Int J Behav Nutr Phys Act. 2011;8:81. doi:10.1186/1479-5868-8-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46:99–106. doi:10.1249/MSS.0b013e3182a0595f [DOI] [PubMed] [Google Scholar]
- 12. Farmer ME, White LR, Kittner SJ, et al. Neuropsychological test performance in Framingham: a descriptive study. Psychol Rep. 1987;60:1023–1040. doi: 10.2466/pr0.1987.60.3c.1023 [DOI] [PubMed] [Google Scholar]
- 13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi:10.1212/WNL.34.7.939: 1526-632X [DOI] [PubMed] [Google Scholar]
- 14. DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi:10.1016/j.neurobiolaging.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 15. Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi:10.1186/1471-2458-14-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi:10.2307/2670170 [Google Scholar]
- 17. Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi:10.1093/aje/kwi092 [DOI] [PubMed] [Google Scholar]
- 18. Chang M, Jonsson PV, Snaedal J, et al. The effect of midlife physical activity on cognitive function among older adults: AGES--Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2010;65:1369–1374. doi:10.1093/gerona/glq152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer’s disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9:390–405. doi:10.1016/j.jamda.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 20. de Bruijn RF, Schrijvers EM, de Groot KA, et al. The association between physical activity and dementia in an elderly population: the Rotterdam Study. Eur J Epidemiol. 2013;28:277–283. doi:10.1007/s10654-013-9773-3 [DOI] [PubMed] [Google Scholar]
- 21. Hamer M, de Oliveira C, Demakakos P. Non-exercise physical activity and survival: English longitudinal study of ageing. Am J Prev Med. 2014;47:452–460. doi:10.1016/j.amepre.2014.05.044 [DOI] [PubMed] [Google Scholar]
- 22. Rovio S, Spulber G, Nieminen LJ, et al. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol Aging. 2010;31:1927–1936. doi:10.1016/j.neurobiolaging.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 23. Erickson KI, Voss MW, Prakashd RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi:10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30:75–79. doi:10.1097/00003677-200204000-00006 [DOI] [PubMed] [Google Scholar]
- 25. Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi:10.1093/gerona/61.11.1166 [DOI] [PubMed] [Google Scholar]
- 26. Corbi G, Conti V, Scapagnini G, Filippelli A, Ferrara N. Role of sirtuins, calorie restriction and physical activity in aging. Front Biosci. 2012;4:768–778. doi:10.2741/417 [DOI] [PubMed] [Google Scholar]
- 27. Corbi G, Conti V, Filippelli A, Di Costanzo A, Ferrara N. The role of physical activity on the prevention of cognitive impairment. Transl Med UniSa. 2015;13:42–46. Retrieved from http://www.translationalmedicine.unisa.it/ [PMC free article] [PubMed] [Google Scholar]
- 28. Bullitt E, Rahman FN, Smith JK, et al. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. AJNR Am J Neuroradiol. 2009;30:1857–1863. doi:10.3174/ajnr.a1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson BJ, Greenwood SJ, McCloskey D. Exercise as an intervention for the age-related decline in neural metabolic support. Front Aging Neurosci. 2010;2:30 doi:10.3389/fnagi.2010.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E. Resting cerebral blood flow, attention, and aging. Brain Res. 2009;1267:77–88. doi:10.1016/j.brainres.2009.02.053 [DOI] [PubMed] [Google Scholar]
- 31. Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38:123–128. doi:10.1111/j.1532-5415.1990.tb03472.x [DOI] [PubMed] [Google Scholar]
- 32. Hagberg JM, Ferrell RE, Dengel DR, Wilund KR. Exercise training-induced blood pressure and plasma lipid improvements in hypertensives may be genotype dependent. Hypertension. 1999;34:18–23. doi:10.1161/01.HYP.34.1.18 [DOI] [PubMed] [Google Scholar]
- 33. St-Amand J, Prud'homme D, Moorjani S, et al. Apolipoprotein E polymorphism and the relationships of physical fitness to plasma lipoprotein-lipid levels in men and women. Med Sci Sports Exerc. 1999;31:692–697. doi:10.1097/00005768-199905000-00011 [DOI] [PubMed] [Google Scholar]
- 34. Luck T, Riedel-Heller SG, Luppa M, et al. Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: analysis of gene-environment interaction for the risk of dementia and Alzheimer’s disease dementia. Psychol Med. 2014;44:1319–1329. doi:10.1017/s0033291713001918 [DOI] [PubMed] [Google Scholar]
- 35. Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12:2762–2771. doi:10.1111/j.1582-4934.2008.00296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paillard-Borg S, Fratiglioni L, Xu W, Winblad B, Wang HX.An active lifestyle postpones dementia onset by more than one year in very old adults. J Alzheimers Dis. 2012;31:835–842. doi:10.3233/JAD-2012-120724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.