Dravet syndrome is a catastrophic intractable childhood epilepsy. Using a zebrafish Dravet syndrome model, Griffin et al. reveal that serotonergic signalling is likely to mediate the antiepileptic activity of clemizole. The serotonergic drugs trazodone and lorcaserin are also antiepileptic in this model, with the latter showing promising results in patients.
Keywords: epilepsy, zebrafish, drug-screening, serotonin, personalized medicine
Abstract
Dravet syndrome is a catastrophic childhood epilepsy with early-onset seizures, delayed language and motor development, sleep disturbances, anxiety-like behaviour, severe cognitive deficit and an increased risk of fatality. It is primarily caused by de novo mutations of the SCN1A gene encoding a neuronal voltage-activated sodium channel. Zebrafish with a mutation in the SCN1A homologue recapitulate spontaneous seizure activity and mimic the convulsive behavioural movements observed in Dravet syndrome. Here, we show that phenotypic screening of drug libraries in zebrafish scn1 mutants rapidly and successfully identifies new therapeutics. We demonstrate that clemizole binds to serotonin receptors and its antiepileptic activity can be mimicked by drugs acting on serotonin signalling pathways e.g. trazodone and lorcaserin. Coincident with these zebrafish findings, we treated five medically intractable Dravet syndrome patients with a clinically-approved serotonin receptor agonist (lorcaserin, Belviq®) and observed some promising results in terms of reductions in seizure frequency and/or severity. Our findings demonstrate a rapid path from preclinical discovery in zebrafish, through target identification, to potential clinical treatments for Dravet syndrome.
Dravet syndrome is a catastrophic intractable childhood epilepsy. Using a zebrafish Dravet syndrome model, Griffin et al. reveal that serotonergic signalling is likely to mediate the antiepileptic activity of clemizole. The serotonergic drugs trazodone and lorcaserin are also antiepileptic in this model, with the latter showing promising results in patients.
Introduction
Childhood epilepsies classified as catastrophic are often associated with a genetic mutation. Among these, Dravet syndrome has been linked to more than 600 de novo mutations in a single gene, SCN1A (Catterall et al., 2010; Escayg et al., 2010). Children suffering with Dravet syndrome exhibit seizures starting as early as 6 months of age, delayed language and motor development, sleep disturbances, anxiety-like behaviour and severe cognitive deficit (Dravet, 2011). Symptoms of autism spectrum disorder have also been reported (Li et al., 2011) and the risk for sudden unexplained death with epilepsy (SUDEP) in this population is estimated at 15-fold higher than other childhood epilepsies (Kearney, 2013). Available antiepileptic drugs do not offer adequate seizure control and resective neurosurgical procedures are not commonly an option. New treatments for Dravet syndrome remain an important unmet need despite some level of efficacy in limited clinical trials for cannabidiol (Epidiolex®) and stiripentol (Diacomit®), which can be associated with cognitive or appetite safety concerns, respectively (Perez et al., 1999; Chiron et al., 2000; Detyniecki et al., 2016; Devinsky et al., 2016).
Mutations in SCN1A, a gene encoding the pore-forming α-subunit of a voltage-gated sodium channel (Nav1.1), have been identified in nearly 85% of patients with Dravet syndrome (Dravet, 2011). Nav1.1 channels contribute to the fast depolarization of neuronal membranes seen during action potential generation (Hodgkin et al., 1952). Mice heterozygous for a loss-of-function mutation in Nav1.1 develop spontaneous and temperature-sensitive seizures early in life, and die prematurely around postnatal Day 25 (Yu et al., 2006; Oakley et al., 2009; Cheah et al., 2012). Acute electrophysiology studies in these and related Scn1a-deficient mice suggest a reduction in sodium current density and an associated decrease in the firing activity for a subpopulation of GABA-expressing inhibitory neurons (but not excitatory principal cells) culminating in reduced synaptic inhibition and network hyperexcitability (Yu et al., 2006; Kalume et al., 2007; Han et al., 2012). This ‘interneuronopathy’ hypothesis is consistent with other forms of catastrophic childhood epilepsies and was confirmed in mice where Scn1a was selectively deleted from parvalbumin- or somatostatin-expressing interneuron subpopulations (Dutton et al., 2013; Tai et al., 2014). Autistic-like behaviours were also reported in these mice (Han et al., 2012). Interestingly, initial studies on human excitatory and inhibitory neurons derived using induced pluripotent stem cell technology from two patients with Dravet syndrome reported deficits in voltage-activated sodium current for both cell types, suggesting either homeostatic compensation for the early loss-of-function of a critical brain-specific sodium channel, or additional mechanisms contributing to the epileptic phenotype observed in these patients (Jiao et al., 2013; Liu et al., 2013).
Although mice and human induced pluripotent stem cell-derived neurons contribute to our understanding of the underlying pathophysiology of Dravet syndrome, these systems are not well suited for the rapid identification of new therapies due to the variability of these models and reproducibility of quantitative measurements. As zebrafish are an ideal vertebrate model system for performing small molecule phenotype-based screens (MacRae et al., 2015), and are amenable to genetic manipulations, we focused our efforts on a zebrafish sodium channel mutant. Zebrafish mutants harbouring a loss-of-function missense mutation in the SCN1A orthologue, scn1Lab, were identified in a mutagenesis screen (Schoonheim et al., 2010). Due to an ancestral whole genome duplication, zebrafish scn1Lab mutants are haploinsufficient for Nav1.1 and analogous to Scn1a+/− mice or patients with Dravet syndrome. Convulsive behaviours and episodes of brief interictal and long-duration polyspike ictal-like electrographic discharge are observed in mutant larvae as early as 3 days post-fertilization (dpf) with progression to more robust seizure phenotypes between 4 and 7 dpf (Baraban et al., 2013; Hong et al., 2016). Mutant larvae die prematurely, exhibit metabolic deficits (Kumar et al., 2016), and are resistant to many antiepileptic drugs (AEDs) (Dinday et al., 2015). Similar to the clinical management of Dravet syndrome, some attenuation of seizure activity can be obtained with valproate, benzodiazepines, bromides, stiripentol, as well as a ketogenic diet (Baraban et al., 2013). Using scn1Lab mutant zebrafish larvae and a two-stage phenotype-based screening strategy, we have now screened more than 2300 compounds. Clemizole, a first-generation histamine receptor (H1) antagonist, was identified as a potent inhibitor of behavioural and electrographic seizure activity (Baraban et al., 2013). Antihistamines, however, are contraindicated in paediatric epilepsy populations (Miyata et al., 2011) and the H1 receptor analogue in zebrafish shows less than 50% similarity to human (Peitsaro et al., 2007). Here, we use preclinical zebrafish models to demonstrate that clemizole, but not antihistamines, exert antiepileptic activity. On the basis of ligand binding and additional targeted drug screening in scn1 mutant zebrafish, we identified several serotonin (5-HT) modulators as effective in suppressing seizures, including two (trazodone and lorcaserin) FDA-approved compounds. Lorcaserin (Belviq®) was prescribed under a compassionate use programme to children with Dravet syndrome and resulted in reduced seizure activity in some patients. We propose that modulation of 5-HT signalling represents a novel therapeutic intervention for this catastrophic childhood epilepsy.
Materials and methods
Zebrafish maintenance
Zebrafish were maintained in a light- and temperature-controlled aquaculture facility under a standard 14:10 h light/dark photoperiod. Adult zebrafish were housed in 1.5 l tanks at a density of 5–12 fish per tank and fed twice per day (dry flake and/or flake supplemented with live brine shrimp). Water quality was continuously monitored: temperature, 28–30°C; pH 7.4–8.0; conductivity, 690–710 mS/cm. Zebrafish embryos were maintained in round Petri dishes in ‘embryo medium’ consisting of 0.03% Instant Ocean (Aquarium Systems, Inc.) and 0.0002% methylene blue in reverse osmosis-distilled water. Zebrafish larvae were obtained from crosses of wild-type (TL strain) or scn1Lab (didys552) or scn1Laa (sa1674) heterozygous animals that had been outcrossed to the TL strain. Homozygous scn1Lab mutants (n = 2800) have dispersed melanosomes and appear visibly darker by 3 dpf compared to wild-type larvae. Homozygous scn1Laa mutants were initially identified using behavioural and electrophysiology assays on all offspring (n = 288; Fig. 1) and confirmed by post hoc polymerase chain reaction (PCR) as they appear visibly similar to wild-type larvae. The care and maintenance protocols comply with requirements outlined in the Guide for the Care and Use of Animals (ebrary Inc., 2011) and were approved by the Institutional Animal Care and Use Committee (protocol # AN108659-02).
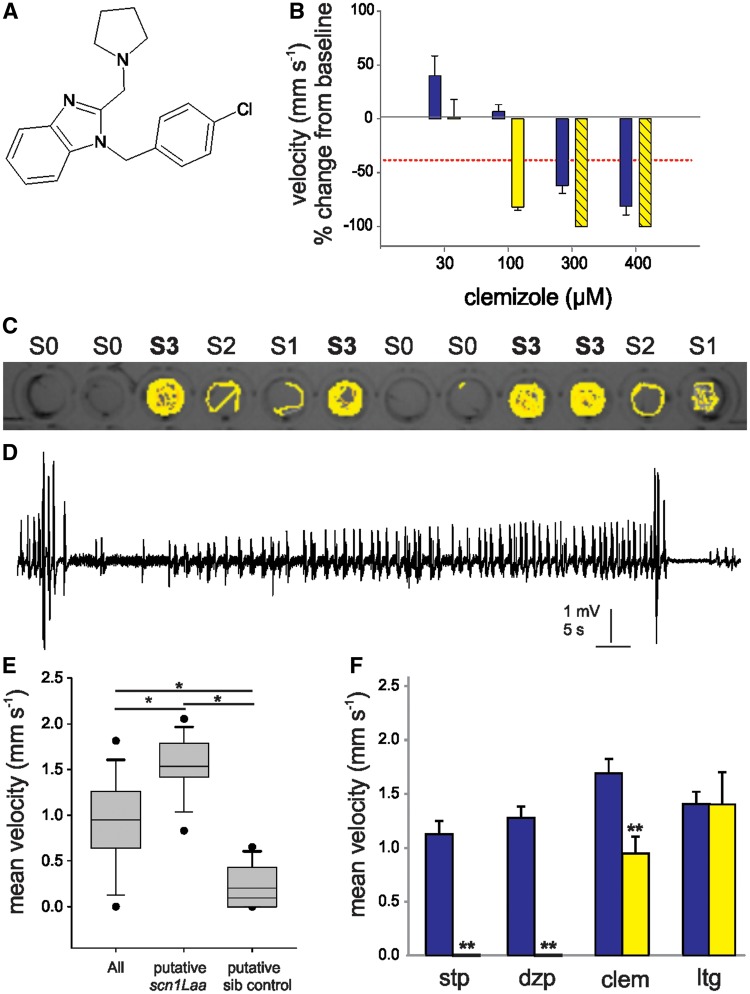
Figure 1.
Confirmation of the antiepileptic activity of clemizole. (A) The chemical structure of clemizole. (B) Graph showing the change in mean velocity of 5 dpf scn1Lab mutant larvae treated with four concentrations of clemizole. Locomotion was recorded for 10 min after an exposure of 30 min (blue bars) and 90 min (yellow bars). Each bar represents the mean change in velocity ± SEM from three independent experiments of six treated larvae. The threshold for significant decrease in velocity is ≥40% (red line). Hatched bars indicate toxicity was observed. (C) Locomotion tracking plot for 5 dpf larvae from a scn1Laa heterozygous cross. Larvae were scored on their swim behaviour (Stage 0 to Stage III). (D) A representative local field potential recording from the forebrain of agar-embedded Stage III classified larva. Small and large amplitude spontaneous burst discharge were observed. (E) Graph showing the mean swim velocity of 12 larvae, Stage III ‘putative scn1Laa mutants’ and ‘putative sibling controls’. Putative scn1Laa mutants were confirmed by PCR. Significance was determined by one-way ANOVA followed by Holm-Sidak test. (F) Graph showing the velocity of untreated scn1Laa mutants (blue bars) and subsequent treatment with 250 µM of stiripentol (stp), diazepam (dzp), clemizole (clem) and lamotrigine (ltg) (yellow bars). Each bar represents the mean velocity ± SEM. Student’s paired t-test was used to determine significance. *P < 0.05; **P < 0.01.
Seizure monitoring
At 5 dpf individual zebrafish larvae were placed into a single well of a clear flat-bottomed 96-well microplate containing embryo media. Larvae were selected randomly as sex determination is not possible at this stage. Microplates were placed inside the DanioVision motion-tracking device and acclimated for 20 min at room temperature. Locomotion plots were obtained for each well during a recording epoch of 10 min using a DanioVision system running EthoVision XT software (DanioVision, Noldus Information Technology); threshold detection settings to identify objects darker than the background were optimized for each experiment. Seizure scoring was performed using the following three-stage scale established for pentylenetetrazole-induced seizures (Baraban et al., 2005): Stage 0, no or very little swim activity; Stage I, increased, brief bouts of swim activity; Stage II, rapid ‘whirlpool-like’ circling swim behaviour; and Stage III, paroxysmal whole-body clonus-like convulsions, and a brief loss of posture. Wild-type fish are normally scored at Stage 0 or I. Plots were analysed for distance travelled (in millimetres) and mean velocity (in millimetres per second). As reported previously (Winter et al., 2008; Baraban et al., 2013), velocity changes were the most sensitive assay of seizure behaviour.
For electrophysiology studies, zebrafish larvae were briefly paralysed with α-bungarotoxin (1 mg/ml) and immobilized in 1.2% agarose; local field potential recordings were obtained from forebrain structures using a single-electrode technique, as previously described (Baraban et al., 2005; Hong et al., 2016). Agarose-embedded local field potential recording sessions of 10 to 30 min were obtained for each fish at 1 kHz. The iZAP system (Hong et al., 2016) was used for long-term non-invasive monitoring of zebrafish in the absence of a paralysing agent. The system autonomously traps several zebrafish larvae underneath multiple integrated surface electrodes within the microfluidic chambers. scn1Lab larvae were continuously monitored for 5 h. Electrical field potential was recorded at 1 kHz continuously except 2- to 3-min breaks for media change for compound treatment and washing. The recorded data was analysed by using MATLAB for field potential graphs and frequency analysis.
Compound library screening
Compounds for drug screening were purchased from Selleck Chemicals and were provided as 10 mM DMSO solutions. Selleck’s Ion Channel Ligand Library (Catalogue #L2700), GPCR Compound Library (Catalogue #L2200) and a customized 5-HT modulating library were used for screening. Library compounds are listed in Supplementary Table 1. In all drug library screens, compounds were coded and experiments were performed by investigators who were blind to the nature of the compound. Baseline recordings of locomotion behaviour were obtained from mutants in embryo media, as described above; a second locomotion plot was then obtained following a solution change to a test compound and an equilibration period of 20 min. Compounds for locomotion studies were dissolved in embryo media and were tested at a concentration of 250 µM, with a final DMSO concentration of 2.5%.
Criteria for a positive hit designation were as follows: (i) a decrease in mean velocity of ≥40%; and (ii) a reduction to Stage 0 or Stage I seizure behaviour in the locomotion plot for at least 50% of the test fish. Each test compound classified as a ‘positive hit’ in the locomotion assay was assessed for toxicity by direct visualization on a stereomicroscope following a 90 min drug exposure. Toxicity (or mortality) was defined as no visible heartbeat or movement in response to external stimulation in at least 50% of the test fish. Hyperexcitability was defined as a compound causing a ≥40% increase in swim velocity and/or Stage III seizure activity in at least 50% of the test fish. Positive hits identified in the primary locomotion screen were confirmed using the locomotion screening method in a second assay with an independent clutch of zebrafish. Compounds were then purchased separately from Sigma-Aldrich and tested using the locomotion screening method for a third time on an independent clutch of zebrafish. Drugs that reduced the mean swim velocity above threshold and were non-toxic in the three independent locomotion assays were further analysed using the electrophysiological assay. In electrophysiology studies, drugs were first confirmed at a concentration of 250 µM using the locomotion assay and then the same zebrafish was evaluated using a local field potential recording. All screening was done with coded compounds and analysed by investigators blinded to the compound identity.
Phylogenetic analysis
Phylogenetic analysis of human HTR2 and zebrafish Htr2 protein sequences were performed with the PhyML software under the SH-like likelihood-ratio test parameters (http://www.phylogeny.fr/) (Dereeper et al., 2008). Protein sequences were derived from Ensembl human HTR2A (ENST00000542664), HTR2B (ENST00000258400), HTR2C (ENST00000276198), and zebrafish htr2aa (ENSDART00000141502), htr2ab (ENSDART00000150982), htr2b (ENSDART00000104569), htr2cl1 (ENSDART00000024191) sequences.
Quantitative real-time mRNA expression analysis
The expression levels of zebrafish htr2 genes were examined using RNA pooled from 25 heads or tails from 5 dpf wild-type or scn1Lab homozygous mutant larvae, and dissected brains from individual wild-type adult male zebrafish. Total RNA was extracted using TRIzol® Reagent (Invitrogen), according to the manufacturer’s protocol and treated with DNase I (Invitrogen). Purified mRNA was retrotranscribed to cDNA using SuperScript®III First-Strand Synthesis System (Invitrogen) with a mix of oligo(dT)20. The expression levels of zebrafish htr2 genes and the housekeeping gene eukaryotic translation elongation factor 1 alpha 1, like 1 (eef1a1l1) was determined using a StepOne™ Real-Time PCR machine (Applied Biosystems). Reactions were performed in 20 µl volumes on 96-well plates using SYBR®Green Master Mix (Applied Biosystems), with 250 nM primer and 3 µl of cDNA. Oligonucleotide sequences are listed in Supplementary Table 2. Data were analysed from three independent experiments. Data were expressed as Ct values and used to determine ΔCt values.
Human studies
After successful identification of compounds in our zebrafish model and consideration of pharmacokinetics, children were prescribed Belviq® (lorcaserin) under a compassionate use protocol at Children’s Hospital Colorado (IND 125307). Children qualified for use of Belviq® if they had an SCN1A mutation or a clinical diagnosis of Dravet syndrome, and failed at least two medications including stiripentol in some cases and excluding sodium channel blockers. Children were required to have an electrocardiogram and echocardiogram at baseline and every 6 months during use of the product. In addition, they were required to have follow-up visits every 3 months to ensure adequate growth, as well as assess for additional side effects. Laboratory testing was required every 6 months to include haematologic testing, liver function testing and renal function testing. Belviq® dose was initiated at 2.5 mg at bedtime and gradually increased weekly as needed to a maximum dose of 10 mg twice a day or 0.3 mg/kg/day—whichever occurred first.
Institutional review board approval was obtained for retrospective data collection including a waiver of consent. Data were extracted from a retrospective review of electronic medical records at Children’s Hospital Colorado including age, seizure types and frequency prior and after use of Belviq®, adverse events, dose of Belviq® and concomitant medication use.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM), unless otherwise stated. For comparison between two groups Student’s t-test were used. When variance did not have a normal distribution the non-parametric Mann-Whitney U-test was used. One-way ANOVA after either Dunnett’s multiple comparison test for analysis against a control sample or Holm-Sidak pairwise multiple comparisons between means. Differences considered statistically significant are indicated with asterisks (*P < 0.05; **P < 0.01).
Results
Effect of clemizole on seizure behaviour in zebrafish
We treated scn1Lab mutant larvae (5 dpf) with clemizole at concentrations between 30 and 400 µM and then monitored the effect on spontaneous seizure behaviour using automated locomotion tracking software. Based on 250 repeated locomotion control trials in untreated scn1Lab mutants, a reduction in mean swim velocity ≥40% (>1.5 × SD) from baseline was set as the threshold for positive suppression of seizure behaviour. Clemizole (Fig. 1A) exhibited antiepileptic activity at 300 and 400 µM (30-min exposure) and at 100 µM (90-min exposure) (Fig. 1B); prolonged exposures were toxic at the higher concentrations. To determine if clemizole can suppress spontaneous seizure behaviour in a second zebrafish scn1 mutant, we screened mutant scn1Laa larvae (5 dpf) in the locomotion tracking assay. Larvae identified as Stage III seizure behaviour (e.g. full body convulsions, high-speed swim activity and a brief loss of posture; Fig. 1C) were confirmed as exhibiting electrographic discharges with interictal- and ictal-like components in subsequent field recordings from the forebrain (Fig. 1D). Mean swim velocity for larvae identified as S3 or ‘putative scn1Laa mutants’ zebrafish was significantly higher than for sibling controls, or all larvae tested (Fig. 1E); mutants were confirmed as scn1Laa homozygotes by post hoc PCR. We then tested drugs previously shown to suppress spontaneous seizures in Dravet syndrome and scn1Lab mutants (250 µM stiripentol and 250 µM diazepam) as well as 250 µM lamotrigine (an AED that can aggravate seizures in Dravet syndrome). As expected stiripentol and diazepam, but not lamotrigine, significantly suppressed seizure behaviour in scn1Laa mutant larvae; 250 µM clemizole was also effective in this assay (Fig. 1F). Together, these studies demonstrate that clemizole can suppress seizure behaviour in two different scn1 mutant zebrafish lines.
Investigation of the mechanism of action for clemizole
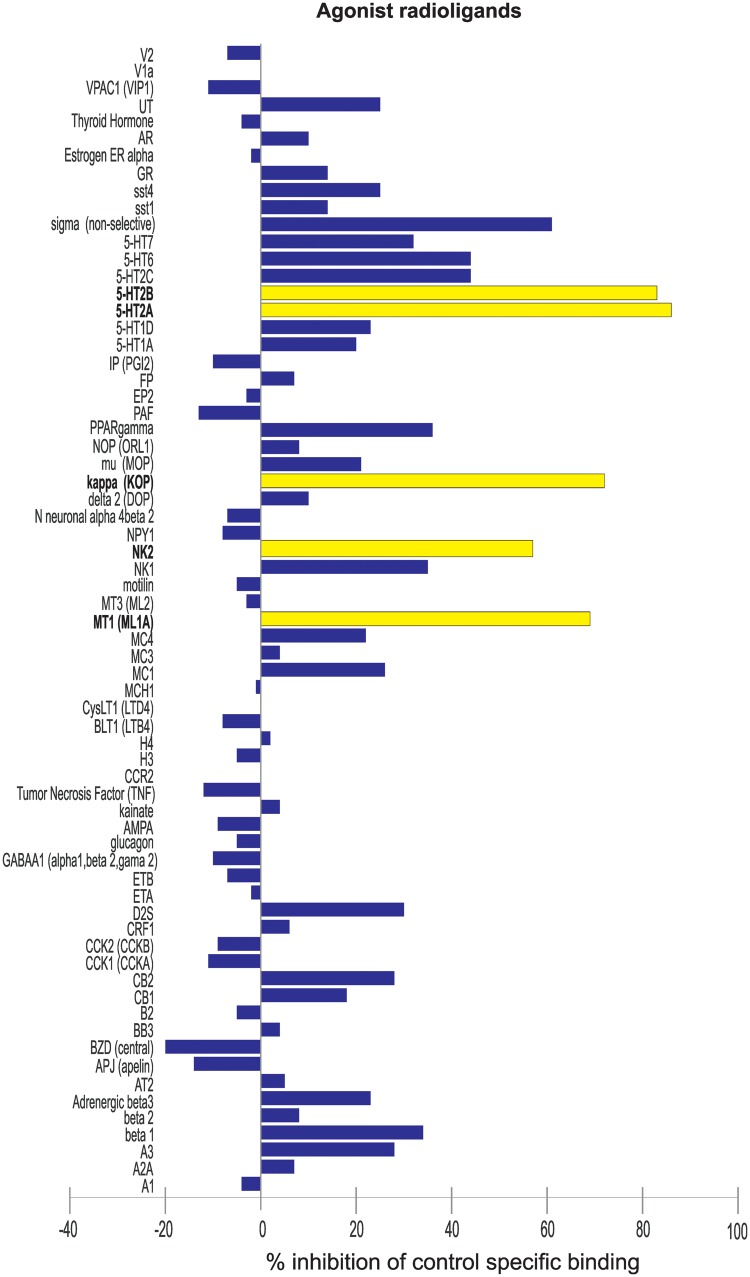
Because antihistamines are known to aggravate paediatric epilepsies and zebrafish H1 shows poor homology with human (Peitsaro et al., 2007), we hypothesized that clemizole does not exert antiepileptic activity via an anti-histaminergic mechanism of action. To test this hypothesis, we searched our database for all compounds with anti-histimanergic properties. Forty-nine drugs from our screening database of over 2300 compounds were identified. None of these were effective in suppressing scn1Lab mutant seizure behaviour in the locomotion assay. Several increased locomotor activity (thioperamide, A4730, mepyramine maleate, R-methylhistamine, mebhydrolin napthalenesulphonate, clemastine fumarate, azatadine dimaleate, chlorpheniramine maleate, and clemastine fumarate) or were found to be toxic (chlorpheniramine maleate, cinnarazine, promethazine hydrochloride, desloratadine, hydroxyzine, and cyclizine) (Supplementary Fig. 1). Next, a radioligand binding assay was performed on 132 targets, including various receptors, ion channels, transporters, enzymes and second messengers (Fig. 2 and Supplementary Fig. 2). As expected, clemizole has a very high antagonist binding affinity for the H1 receptor (99%). The next highest affinity, as an agonist, was for two postsynaptic 5-HT receptor subunits, HTR2A (86%) and HTR2B (83%), respectively. Other positively identified targets with percent inhibition values between 50 and 78% included ion channel modulators and other G-protein-coupled receptors (GPCRs).
Figure 2.
Radioligand binding assay to identify binding targets of clemizole. Clemizole was subjected to radioligand binding assay against 132 targets. The functional agonist activity of clemizole against 67 targets is shown. Compound binding was calculated as % inhibition of the binding of a radioactively-labelled ligand specific for each target. Inhibition or stimulation higher than 50% and are represented in yellow and are considered to represent significant effects of clemizole.
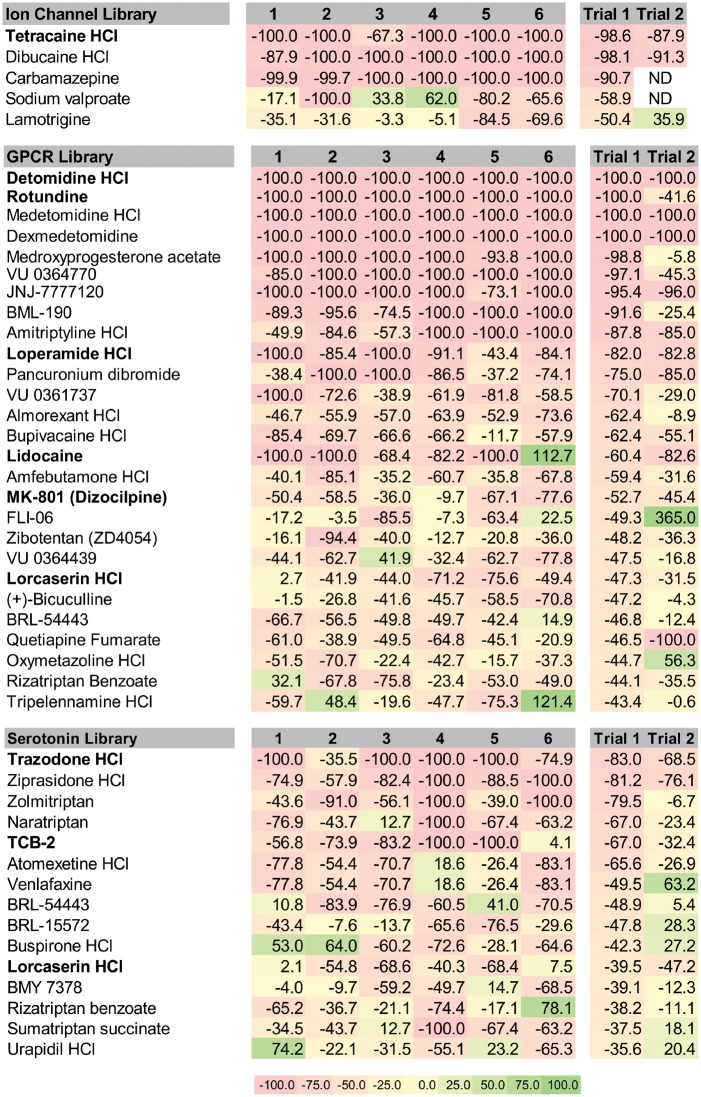
To determine if drugs modulating ion channels, GPCRs or 5-HT signalling could recapitulate the seizure suppressive activity of clemizole in scn1Lab mutant larvae, we obtained commercially available compound libraries spanning these three categories. In blinded phenotype-based screening, we tested compounds for their ability to reduce mean swim velocity of 5 dpf scn1Lab mutants at a concentration of 250 µM (n = 6 fish per drug). Plots for the first-pass assay on all 368 compounds are shown in Fig. 3A–C. Compounds that decreased movement (measured as a change in mean velocity) by ≥40% were considered significant. Five compounds from the ion channel ligand library (9.6%), 27 from the GPCR compound library (10.6%), and 10 from the 5-HT compound library (16.1%), were identified as positive hits. Subsequent retesting of these compounds was performed on a separate clutch of scn1Lab mutants at 250 µM; an additional five borderline compounds from the 5-HT library were also retested. AEDs (sodium valproate and carbamazepine) previously evaluated in scn1Lab larvae (Baraban et al., 2013) were not considered for further testing (Fig. 4). Of the retested compounds, one from the ion channel library, six from the GPCR compound library and two from the 5-HT compound library were confirmed to decrease velocity. Next, all identified compounds were unblinded and sourced from commercial suppliers for a third behavioural assay screen at 250 µM. TCB-2 was also further characterized given its reported identification in decreasing epileptiform activity (Sourbron et al., 2016). Across the three libraries, 100 compounds (27.1%) were identified as ‘toxic’ and 53 compounds (14.4%) were classified as ‘hyperexcitatory’ i.e. resulted in increased swim velocity (Supplementary Table 1). Positive hits that were non-toxic in three independent locomotion assays moved on to a secondary electrophysiology assay.
Figure 3.
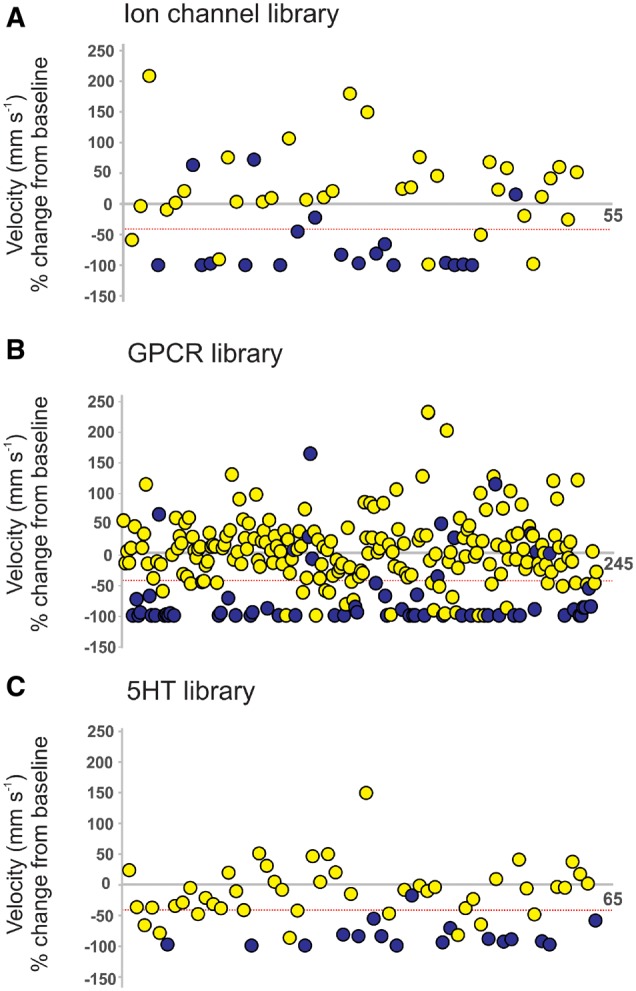
Summary of behavioural locomotion library screening using scn1Lab mutant zebrafish larvae. Plots of locomotor seizure behaviour for 5 dpf scn1Lab mutants screened against (A) 52 ion channel ligands, (B) 254 compound GPCR ligands, and (C) 65 5-HT modulating compounds. Threshold for inhibition of seizure activity (positive hits) was determined as a reduction in mean swim velocity of ≥40% (red line). Blue data points represent compounds that were classified as toxic as treated larvae have no visible heartbeat or movement in response to touch after 90-min exposure.
Figure 4.
Heat map of positive compounds identified from the three targeted libraries. The % change in velocity is shown for six individual larva from the first pass trial (1–6). Mean velocity data from six fish is shown for trial one and trial two. Drugs that reduced the mean swim velocity above threshold and were non-toxic in third trial using separately sourced compound are highlighted in bold. These positive compounds were considered for additional testing. Note: Lorcaserin was identified positive in both the GPCR and 5-HT libraries so it was also considered for further testing.
Secondary drug screen using scn1Lab mutants
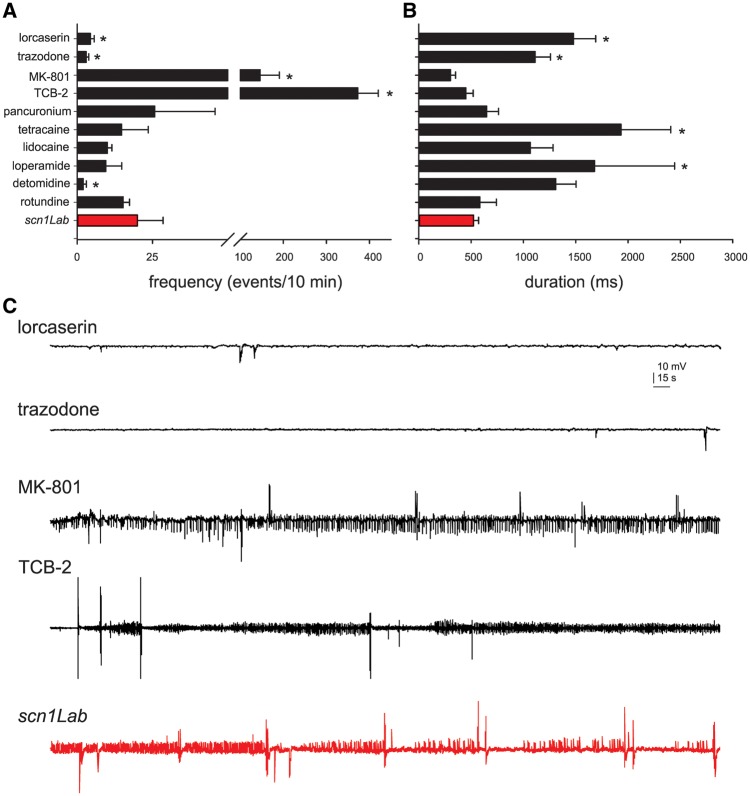
Monitoring electrographic brain activity is a ‘gold standard’ in the epilepsy field, and can be achieved by placing a microelectrode into a visually identified brain region of an agar immobilized zebrafish. At 5 dpf, local field potential recordings of scn1Lab zebrafish larvae show an average of 20 abnormal electrographic seizure events during a 10-min epoch with an average duration around 500 ms. scn1Lab zebrafish larvae were treated with compounds first confirmed to suppress locomotion behaviour and then subjected to local field potential monitoring. Trazodone, lorcaserin and detomidine suppressed the frequency of these electrographic seizure events (Fig. 5A). Lorcaserin and trazodone also increased the duration of these electrographic seizure events, though in these experiments epileptic events were rare (Fig. 5B and C). Representative EEG recording epochs are shown in Fig. 5C; MK-801 and TCB-2 increased the frequency of electrographic seizure events. Pancuronium (nicotinic acetylcholine receptor antagonist paralysing agent), tetracaine (local anaesthetic), lidocaine (sodium channel blocker anaesthetic), loperamide (peripheral opioid receptor agonist) and rotundine (D1 receptor antagonist), were classified as ‘false-positives’ as they failed to suppress electrographic seizure activity.
Figure 5.
Electrophysiological assay to identify drugs that rescue the scn1Lab mutant epilepsy phenotype. Bar graphs showing the (A) number, and (B) duration of epileptiform events in a 10-min recording epoch for scn1Lab larvae exposed to lorcaserin (n = 8), trazodone (n = 10), MK-801 (n = 4), TCB-2 (n = 9), pancuronium (n = 8), tetracaine (n = 4), lidocaine (n = 6), loperamide (n = 8), detomidine (n = 5), rotundine (n = 4), or scn1Lab mutants (n = 20). Graph represent mean ± SEM. Student’s unpaired t-test or Mann–Whitney rank sum test were used *P < 0.05. (C) Representative field electrode recording epochs (10 min) are shown for four compounds with significant changes in the frequency of events compared to untreated scn1Lab mutant zebrafish (red). Recordings were obtained with an electrode placed in the forebrain of agar-immobilized scn1Lab larvae that had previously showed suppressed seizure-like behaviour in the locomotion assay.
Tertiary screening to identify promising lead compounds for the clinic
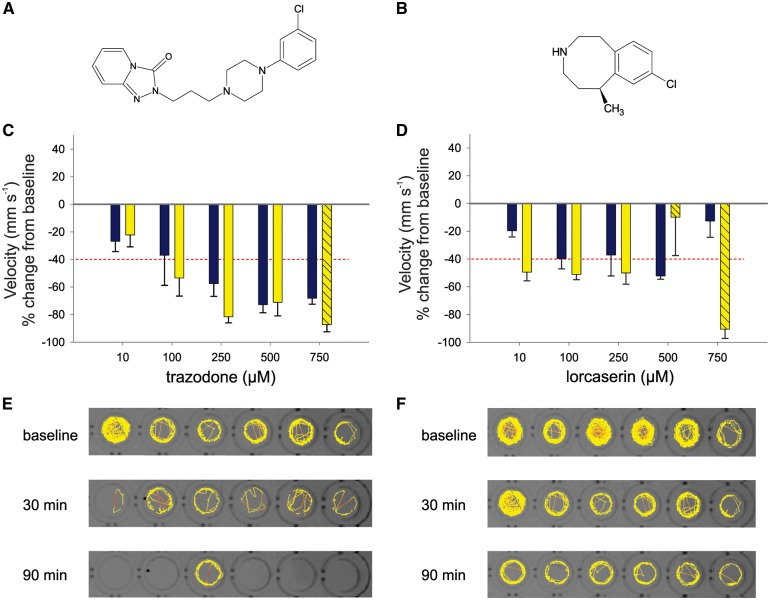
Trazodone and lorcaserin, two FDA-approved compounds with the potential for off-label application in Dravet syndrome, were screened across a range of concentrations (10–750 µM) at two time points (30 and 90 min) in the locomotion assay. Detomidine, a horse tranquilizer with little translational potential, was not considered further. Trazodone was effective in a concentration-dependent manner and reached maximal efficacy of ∼80% reduction at 250 µM (90 min) and 500 µM (30 mins). Toxicity was observed at 750 µM (Fig. 6A). Lorcaserin had a maximal efficacy of ∼50% reduction of velocity at 10 µM (90 min). This suppression of seizure behaviour was maintained at 100 and 250 µM (90 mins) and toxicity was observed above 500 µM (Fig. 6B); 30 min exposure was only effective at a concentration of 500 µM. Trazodone and lorcaserin moved on to an additional safety-efficacy test, incorporating a washout phase, in our microfluidic-based integrated Zebrafish Activity Platform (iZAP) monitoring system (Hong et al., 2016). Here scn1Lab mutant larvae are trapped in recording channels and monitored non-invasively using integrated surface electrodes. Baseline seizure activity was established for mutants simultaneously, then 250 µM trazodone or lorcaserin was perfused into the microfluidic chambers. Trazodone reduced seizure activity by 89.0 ± 9.1% (n = 5), and treatment with lorcaserin showed 27.2 ± 15.7% suppression (n = 5) during the 2-h treatment period. Activity returned to baseline levels during a subsequent washout period (Fig. 7). Mutant larvae were then released from the iZAP system and observed to be healthy and freely swimming. As a control experiment, similar studies were performed with exposure to 250 µM ethosuximide—an AED not effective in suppressing seizures associated with Dravet syndrome. No suppression of electrographic seizure activity was noted (4.5 ± 3.2 % increase; n = 6 scn1Lab mutants; Supplementary Fig. 3).
Figure 6.
Dose response evaluation of putative antiepileptic drugs in scn1Lab mutant zebrafish. Putative antiepileptic compounds trazodone and lorcaserin were tested for efficacy in 5 dpf scn1Lab mutant zebrafish. Chemical structure for each compound is shown (A and B). Graphs show the change in mean velocity over five concentrations of (C) trazodone and (D) lorcaserin. Locomotion was recorded for 10 min after an exposure of 30 min (blue bars) and 90 min (yellow bars). Toxicity is indicated by dashed bars. Each bar represents the mean change in velocity ± SEM from three independent experiments. The threshold for a decrease in velocity is ≥ 40% (red line). Representative tracking plots are shown from a single experiment of six individual 5 dpf scn1Lab zebrafish at baseline and following a 30 min and 90 min exposure of 250 µM (E) trazodone or (F) lorcaserin. Total movement is shown for a 10 min recording epoch.
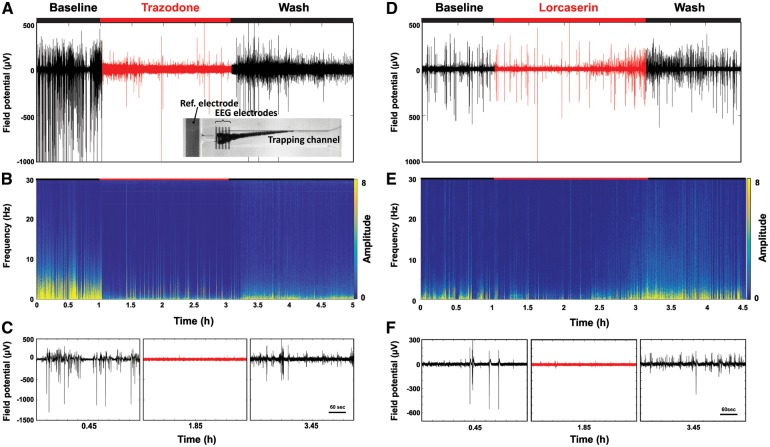
Figure 7.
iZAP EEG measurements of scn1Lab during treatment and washout with trazodone and lorcaserin. (A) Time-domain and (B) frequency-domain graphs of a representative field potential measured from one 5 dpf scn1Lab mutant treated with 250 µM trazodone. The inset photograph shows the larva positioned underneath the integrated surface electrodes of the iZAP, the reference electrode, and the trapping channel. (C) Representative zoomed field potential plots of baseline, trazodone and washing phase. The same data are shown for a representative individual scn1Lab mutant larva treated with 250 µM lorcaserin. During the 2-h treatment window there was a trend toward decreased efficacy with prolonged lorcaserin exposure (D–F).
5-HT receptor expression in zebrafish larvae
As clemizole has significant binding affinity to HTR2A and HTR2B, and both trazodone and lorcaserin are 5-HT signalling modulators, we sought to confirm the expression of these receptors in zebrafish. Protein sequence alignment of human and zebrafish HTR2 receptors revealed evolutional conservation with zebrafish orthologues, with Htr2aa and Htr2ab both exhibiting 59.3% protein identity with the human HTR2A; and a single HTR2B orthologue, Htr2b showing 62.0% protein identity. Quantification of htr2 expression level using isolated heads or tails of 5 dpf wild-type or scn1Lab mutant larvae revealed enriched htr2a and htr2cl1 expression in the head. Similar results were obtained from adult wild-type zebrafish brain, as mutant larvae do not survive to adulthood (Supplementary Fig. 4).
Reduction of seizure frequency in patients with Dravet syndrome
Dravet syndrome is a catastrophic childhood epilepsy and rare disorder (http://www.rarediseases.org/) with debilitating outcomes including intractable epilepsy, severely limited cognitive development and risk of SUDEP. Our preclinical data provide confirmation that modulation of 5-HT signalling can suppress seizures associated with SCN1A loss-of-function mutations. Because identified 5-HT modulators are FDA-approved compounds with known safety profiles, treatment with these repurposed drugs could alter seizure frequency in children with Dravet syndrome. This translational approach targets rare and devastating diseases for which large-scale clinical trials are not feasible (Dunoyer, 2011; Parker et al., 2013).
As clemizole is not currently manufactured or available in clinical grade formulation and trazodone can act as a 5-HT receptor agonist, or antagonist, depending on concentration (Maj et al., 1979; Marcoli et al., 1998), we chose to evaluate Belviq® (lorcaserin) under a compassionate use off-label programme in a small population of children with Dravet syndrome. These children were shown to be resistant to at least five approved AEDs. Five children (mean age: 11.8 years; range: 7–18 years) heterozygous for a deletion in SCN1A were treated prospectively with Belviq® and followed longitudinally at the Children’s Hospital Colorado (Aurora, CO). The treatment protocol was approved by the Colorado Medical Institutional Review Board (COMIRB), and parents of Dravet syndrome patients consented in writing to their child’s participation. We retrospectively reviewed the diary-reported number of atonic, myoclonic and generalized tonic-clonic (GTC) seizures, side effects and concurrent AEDs.
The clinical characteristics of Dravet syndrome children treated with Belviq® are summarized in Table 1. There were no deaths among the five Belviq®-treated patients, and Belviq® was well tolerated without serious adverse events causing cessation of therapy. During off-label Belviq® treatment, one patient was initially seizure-free for 3 weeks, one patient was seizure-free for 2 weeks, and a third patient had 1–2 seizure-free days per week. All five patients exhibited a reduction in the total number of seizures. Generalized tonic-clonic seizures were significantly reduced in Patients 1, 2 and 3. Indeed, Patient 2 experienced a 90% reduction in generalized tonic-clonic seizures with no need for rescue medications. Two patients remain on Belviq® with no increase in seizure frequency and, as expected, the most common side effect noted was a decreased appetite. One patient restarted medication a second time with interim improvement for a short period of time and then tapered off.
Table 1.
Dravet Syndrome patients treated with Belviq® (lorcaserin) show reduced seizure frequency
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (years) | 10 | 18 | 10 | 7 | 14 |
| Weight (kg) | 28 | 46 | 23 | 24 | 35 |
| Dose (mg/kg/day) | 0.25 | 0.27 | 0.19 | 0.32 | 0.31 |
| Prior treatments | CLZ CZP KD LMT LVT PRM OXC RUF TPX VPA | CBZ CBD CLZ CLB CZP FBM LMT LVT PRM PHB TPM VPA CC KD VNS | ESM FBM LMT LVT MSM VPA VMP ZNM KD | CZP ESM LVT LZP STP TPM ZNM KD | CBZ FBM GBP LCM LMT LVT OXC PHB PRED RUF STP VNS VPM ZNM KD |
| Concurrent AEDs | CLB STP VPA | CZP STP ZNM | KD TPM VPA | BRO CBD CLB VPA | CLB TPX VPA |
| Prior seizure frequency | FS: 50/day | MS: numerous daily | MS: daily | AS: 12/h | MS: constant throughout the day |
| GTC clusters: 1/ month | FS + GTC: 10/ month (requires rescue medications) | GTC seizures: 100/month (clusters 7–10) | FS: 3–5 / week | GTC seizures: 1–2/ week | |
| NCS: 1/ month | |||||
| Seizure frequency after treatment: first 3 months | Seizure free initial 3 weeks, cluster of seizures then again seizure free for 2 weeks | Seizure free for 2weeks | GTC seizures: 46/ month (GTC clusters of 1–3 seizures) | 1–2 seizure free days/ week | MS: initially reduced in the morning then increases to constant throughout the late afternoon |
| Cluster of seizures once a month with (FS, GTC) | MS: occasional | MS: daily | AS or FS: 3/ month | GTC: 1–2/ week | |
| FS + GTC: 1/month (no rescue medications) | GTC: 1–2 /day | ||||
| NCS: 1/ month | |||||
| Seizure frequency after treatment: following first 3 months | Gradual increase in seizures with return to baseline frequency | MS: clusters 1–2/ week | Seizures gradually decreased to 16/ month with some seizure free nights then seizures increased to baseline | Gradual increase in seizures, seizure free days stopped 9 months after treatment | Unchanged, Belviq® tapered off with no change in seizure frequency |
| FS + GTC: 1–2/ month and (no rescue medica tions required) | No increase in seizure when medication stopped | ||||
| Duration treat ment (months) | 12 months, still taking | 12 months, still taking | 14 months | 13 months | 9 months |
| Restarted due to increased seizures treated for 2 months, stopped to participate in other drug study | |||||
| Side effects | none | none | Vomiting and decreased appetite | Decreased appetite | Decreased appetite |
AS = atonic seizures; BRO = bromides; CBD = cannabidiol; CBZ = carbamazepine; CLB = clobazam; CZP = clonazepam; CLZ = clorazepate; CC = corpus callosotomy; ESM = ethosuximide; FBM = felbamate; FS = focal seizures; GBP = gabapentin; GTC = generalized tonic clonic seizures; KD = ketongenic diet; LCM = lacosamide; LMT = lamotrigine; LVT = levitiracetam; LZP = lorazepam; MS = myoclonic seizures; MSM = methosuximide; NCS = non-convulsive status; OXC = oxcarbazipine; PHB = phenobarbital; PRM = primodone; PRED = predinisone; RFM = rufinamide; STP = stiripentol; TPM = topiramate; VPA = valproic acid; VNS = vagus nerve stimulator; VPM = verapamil; ZNM = zonisamide.
Discussion
Clemizole, a first-generation antihistamine discovered in the 1950 s (Zierz et al., 1952), was identified as a potential therapeutic for the treatment of Dravet syndrome using scn1Lab mutant zebrafish to screen repurposed drug libraries (Baraban et al., 2013; Dinday et al., 2015). Here we confirmed an antiepileptic activity for clemizole using a second scn1 zebrafish mutant model. Unfortunately, clemizole is rapidly metabolized in mice with a plasma half-life of <10 min (compared to 3.4 h in humans) (Nishimura et al., 2013), limiting its evaluation in murine models. Successfully used as an antihistamine (Zierz et al., 1952; Jacques et al., 1960) with acute and chronic studies reporting a low order of toxicity (Finkelstein et al., 1960), clemizole is no longer manufactured and not currently available for off-label clinical administration. Lacking a means to efficiently evaluate clemizole in preclinical rodent models, we used zebrafish for target engagement (5-HT receptors) and identification of related drugs (trazodone and lorcaserin) with appropriate safety profiles facilitating rapid translation to a clinical application. Compassionate use, off-label prescription of Belviq® (lorcaserin) to medically intractable Dravet syndrome patients is also described.
Antihistamine H1-receptor antagonists are normally contraindicated in paediatric patient populations (Miyata et al., 2011) and screening these drugs confirmed their inability to suppress (and in some cases exacerbate) seizures in scn1 zebrafish. Binding data uncovered a previously unknown clemizole affinity for HTR2A and/or HTR2B receptors. A subsequent phenotypic screen of targeted libraries identified two 5-HT-modulating compounds, trazodone and lorcaserin, capable of suppressing behavioural and electrophysiological seizures in a manner comparable to clemizole. Belviq® (lorcaserin) is an FDA-approved HTR2C agonist prescribed for chronic weight management (Thomsen et al., 2008). Desyrel® (trazodone) is also an FDA-approved antidepressant commonly prescribed for sleep disorders (Mendelson, 2005). It is frequently classified as a HTR2A and HTR2C inverse antagonist and 5-HT uptake inhibitor (Stahl, 2009). However, studies in rats suggest trazodone, or its metabolite meta-chlorophenylpiperazine (mCPP), can act as a HTR2C agonist at higher concentrations (Maj et al., 1979; Marcoli et al., 1998). Notably, chronic trazodone treatment was previously shown to be protective against electroconvulsive induced seizures in mice (Chavan et al., 2010; Borowicz et al., 2012), supporting our data from scn1Lab mutant zebrafish. A 5-HT receptor-mediated action for clemizole is also consistent with a recent phenotypic screen where modulators of serotonergic signalling (including clemizole) were shown to be an effective treatment in a preclinical model of Machado-Joseph disease (Teixeira-Castro et al., 2015); and studies here suggest that clemizole and trazodone warrants further development for off-label testing in patients with Dravet syndrome.
Our results also add to a growing body of evidence suggesting modulation of serotonergic signalling as a potent suppressor of seizure activity, especially in catastrophic childhood epilepsies like Dravet syndrome. Recently, 7 of 10 patients on low-dose treatment with the 5-HT reuptake blocker fenfluramine were self-reported to be seizure-free for 1 year (Ceulemans et al., 2016). A slight thickening of one or two heart valves was reported in two of these patients consistent with a possible relationship between the use of fenfluramine and pulmonary hypertension (Douglas et al., 1981; Ceulemans et al., 2016). In humans, HTR2A and HTR2C are expressed in the CNS, while HTR2B expression is enriched in the heart (Lambe et al., 2011; Meltzer et al., 2013). More specifically, HTR2C is expressed on a subpopulation of inhibitory interneurons (Liu et al., 2007) and activation of these receptors with 5-HT increases GABA-mediated synaptic inhibition (Boothman et al., 2006) i.e. the antiepileptic mechanism of action underlying many commonly prescribed antiepileptic drugs. Indeed, most preclinical studies suggest activation of HTR2A and/or HTR2C receptors has antiepileptic effects (Gharedaghi et al., 2014; Guiard et al., 2015), which is a reasonable mechanism of action linking clemizole, lorcaserin, trazodone, and the 5-HT reuptake blocker fenfluramine (Dinday et al., 2015; Ceulemans et al., 2016). Interestingly, htr2b expression in the zebrafish brain was relatively low, further suggesting that these drugs potentially exert antiepileptic activity via HTR2A or HTR2C receptor activation. Interestingly, studies in Drosophila knock-in flies carrying the K1270T SCN1A human mutation in the para sodium channel gene have shown that supplementation with a 5-HT precursor (5-hydroxytryptophan) rescues the heat induced seizure phenotype (Schutte et al., 2014). Additionally, a recent study using scn1Lab mutants evaluated 13 5-HT signalling compounds and also suggested a potential antiepileptic role for modulators of 5-HT signalling (Sourbron et al., 2016). However, these latter zebrafish studies used a fundamentally different protocol (24 h versus 30–90 min drug exposure) not previously validated as successfully identifying AEDs used in Dravet syndrome (benzodiazepines, valproate, stiripentol, bromides, and ketogenic diet). Additionally, drug concentrations some 10-fold lower than we demonstrate to be effective in zebrafish (Baraban et al., 2005, 2013; Dinday et al., 2015), and reporting an antiepileptic action for the hallucinogen TCB-2 (Fig. 5), suggests that direct comparison of data from laboratories using different procedures should be interpreted with caution.
Overall, we conclude that mutant zebrafish are a suitable model for the rapid screening and discovery of novel AEDs that, with appropriate safety profiles, can directly inform clinical care for at risk patient populations such as Dravet syndrome.
Supplementary Material
Acknowledgements
We would like to thank members of the Baraban laboratory, Brian Grone and Matthew Dinday in particular, for useful discussions during the course of these studies.
Funding
S.C.B acknowledges funding from NINDS R01 grant no. NS079214, UCSF Catalyst Award and the Raymond & Beverley Sackler Centre Sabbatical Fund.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- 5-HT
serotonin
- AED
antiepileptic drug
- dpf
days post fertilization
- GPCR
G-protein-coupled receptor
- iZAP
integrated zebrafish analysis platform
References
- Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun 2013; 4: 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005; 131: 759–68. [DOI] [PubMed] [Google Scholar]
- Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br J Pharmacol 2006; 149: 861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowicz KK, Gurdziel E, Czuczwar SJ. Trazodone reduces the anticonvulsant action of certain classical antiepileptics in the mouse maximal electroshock model. Pharmacol Rep 2012; 64: 1136–45. [PubMed] [Google Scholar]
- Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol 2010; 588: 1849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans B, Schoonjans AS, Marchau F, Paelinck BP, Lagae L. Five-year extended follow-up status of 10 patients with Dravet syndrome treated with fenfluramine. Epilepsia 2016; 57: e129–34. [DOI] [PubMed] [Google Scholar]
- Chavan VR, Wali R, Totad R. Studies on the role of 5-HT2A and 5-HT2C receptor antagonist and effects of co-administration of Fluoxetines in regulating generalized seizures in albino rats. Al Ameen J Med Sci 2010; 3: 201–7. [Google Scholar]
- Cheah CS, Yu FH, Westenbroek RE, Kalume FK, Oakley JC, Potter GB. et al. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA 2012; 109: 14646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Marchand MC, Tran A, Rey E, d’Athis P, Vincent J. et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO Study Group. Lancet 2000; 356: 1638–42. [DOI] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F. et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 2008; 36: W465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detyniecki K, Hirsch LJ. Cannabidiol for epilepsy: trying to see through the haze. Lancet Neurol 2016; 15: 235–7. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J. et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 2016; 15: 270–8. [DOI] [PubMed] [Google Scholar]
- Dinday MT, Baraban SC. Large-scale phenotype-based antiepileptic drug screening in a zebrafish model of dravet syndrome(1,2,3). eNeuro 2015; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JG, Munro JF, Kitchin AH, Muir AL, Proudfoot AT. Pulmonary hypertension and fenfluramine. Br Med J (Clin Res Ed) 1981; 283: 881–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravet C. The core Dravet syndrome phenotype. Epilepsia 2011; 52 (Suppl 2): 3–9. [DOI] [PubMed] [Google Scholar]
- Dunoyer M. Accelerating access to treatments for rare diseases. Nat Rev Drug Discov 2011; 10: 475–6. [DOI] [PubMed] [Google Scholar]
- Dutton SB, Makinson CD, Papale LA, Shankar A, Balakrishnan B, Nakazawa K. et al. Preferential inactivation of Scn1a in parvalbumin interneurons increases seizure susceptibility. Neurobiol Dis 2013; 49: 211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 2010; 51: 1650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M, Kromer CM, Sweeney SA, Delahunt CS. Some aspects of the pharmacology of clemizole hydrochloride. J Am Pharm Assoc Am Pharm Assoc 1960; 49: 18–22. [PubMed] [Google Scholar]
- Gharedaghi MH, Seyedabadi M, Ghia JE, Dehpour AR, Rahimian R. The role of different serotonin receptor subtypes in seizure susceptibility. Exp Brain Res 2014; 232: 347–67. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Di Giovanni G. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: the missing link?. Front Pharmacol 2015; 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB. et al. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature 2012; 489: 385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 1952; 117: 500–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Lee P, Baraban SC, Lee LP. A novel long-term, multi-channel and non-invasive electrophysiology platform for Zebrafish. Sci Rep 2016; 6: 28248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques AA, Fuchs VH. Clinical evaluation of clemizole in allergic rhinitis. Int Rec Med 1960; 173: 88–91. [PubMed] [Google Scholar]
- Jiao J, Yang Y, Shi Y, Chen J, Gao R, Fan Y. et al. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum Mol Genet 2013; 22: 4241–52. [DOI] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci 2007; 27: 11065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. Sudden unexpected death in dravet syndrome. Epilepsy Curr 2013; 13: 264–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MG, Rowley S, Fulton R, Dinday MT, Baraban SC, Patel M. Altered glycolysis and mitochondrial respiration in a zebrafish model of dravet syndrome. eNeuro 2016; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Fillman SG, Webster MJ, Shannon Weickert C. Serotonin receptor expression in human prefrontal cortex: balancing excitation and inhibition across postnatal development. PLoS One 2011; 6: e22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BM, Liu XR, Yi YH, Deng YH, Su T, Zou X. et al. Autism in Dravet syndrome: prevalence, features, and relationship to the clinical characteristics of epilepsy and mental retardation. Epilepsy Behav 2011; 21: 291–5. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience 2007; 146: 1677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lopez-Santiago LF, Yuan Y, Jones JM, Zhang H, O’Malley HA. et al. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann Neurol 2013; 74: 128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov 2015; 14: 721–31. [DOI] [PubMed] [Google Scholar]
- Maj J, Palider W, Rawlow Trazodone, a central serotonin antagonist and agonist. J Neural Transm 1979; 44: 237–48. [DOI] [PubMed] [Google Scholar]
- Marcoli M, Maura G, Tortarolo M, Raiteri M. Trazodone is a potent agonist at 5-HT2C receptors mediating inhibition of the N-methyl-D-aspartate/nitric oxide/cyclic GMP pathway in rat cerebellum. J Pharmacol Exp Ther 1998; 285: 983–6. [PubMed] [Google Scholar]
- Meltzer HY, Roth BL. Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J Clin Invest 2013; 123: 4986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry 2005; 66: 469–76. [DOI] [PubMed] [Google Scholar]
- Miyata I, Saegusa H, Sakurai M. Seizure-modifying potential of histamine H1 antagonists: a clinical observation. Pediatr Int 2011; 53: 706–8. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Hu Y, Wu M, Pham E, Suemizu H, Elazar M. et al. Using chimeric mice with humanized livers to predict human drug metabolism and a drug-drug interaction. J Pharmacol Exp Ther 2013; 344: 388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci USA 2009; 106: 3994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WE, Orlova KA, Parker WH, Birnbaum JF, Krymskaya VP, Goncharov DA. et al. Rapamycin prevents seizures after depletion of STRADA in a rare neurodevelopmental disorder. Sci Transl Med 2013; 5: 182ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsaro N, Sundvik M, Anichtchik OV, Kaslin J, Panula P. Identification of zebrafish histamine H1, H2 and H3 receptors and effects of histaminergic ligands on behavior. Biochem Pharmacol 2007; 73: 1205–14. [DOI] [PubMed] [Google Scholar]
- Perez J, Chiron C, Musial C, Rey E, Blehaut H, d’Athis P. et al. Stiripentol: efficacy and tolerability in children with epilepsy. Epilepsia 1999; 40: 1618–26. [DOI] [PubMed] [Google Scholar]
- Schoonheim PJ, Arrenberg AB, Del Bene F, Baier H. Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J Neurosci 2010; 30: 7111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte RJ, Schutte SS, Algara J, Barragan EV, Gilligan J, Staber C. et al. Knock-in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current. J Neurophysiol 2014; 112: 903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourbron J, Schneider H, Kecskes A, Liu Y, Buening EM, Lagae L. et al. Serotonergic modulation as effective treatment for dravet syndrome in a zebrafish mutant model. ACS Chem Neurosci 2016; 7: 588–98. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr 2009; 14: 536–46. [DOI] [PubMed] [Google Scholar]
- Tai C, Abe Y, Westenbroek RE, Scheuer T, Catterall WA. Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA 2014; 111: E3139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Castro A, Jalles A, Esteves S, Kang S, da Silva Santos L, Silva-Fernandes A. et al. Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease. Brain 2015; 138: 3221–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D. et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 2008; 325: 577–87. [DOI] [PubMed] [Google Scholar]
- Winter MJ, Redfern WS, Hayfield AJ, Owen SF, Valentin JP, Hutchinson TH. Validation of a larval zebrafish locomotor assay for assessing the seizure liability of early-stage development drugs. J Pharmacol Toxicol Methods 2008; 57: 176–87. [DOI] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA. et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 2006; 9: 1142–9. [DOI] [PubMed] [Google Scholar]
- Zierz P, Greither H. Clinical evaluation of allercur, a new antihistaminic [Article in Undetermined Language]. Arztl Wochensch 1952; 7: 704–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.