Abstract
Progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta is the primary cause for motor symptoms observed in Parkinson’s disease (PD). Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most commonly linked contributor to familial PD. LRRK2 is suggested to be involved in a wide variety of cellular processes, but deciphering its role in the pathogenesis of PD has been difficult. Modelling PD in rodents has been a persistent challenge for the field. However, the fruit fly has been exploited to recapitulate PD gene related dopaminergic cell loss. Using the GAL4-UAS system and established models of hLRRK2 induced eye degeneration in Drosophila, we conducted an unbiased suppressor/enhancer screen to uncover genetic modifiers of LRRK2. We have identified 36 candidate interactors that modify LRRK2 induced toxicity in the Drosophila eye. Importantly, we determined that a subset of these interactors also modified hLRRK2(I2020T) induced dopaminergic neuronal loss in the fly brain and uncovered 16 candidates that modify dopaminergic cell loss. Our results suggest LRRK2 may be involved in a wide variety of cellular processes and the results from this screen provide an important genetic resource for further evaluation of LRRK2 function.
Introduction
Parkinson’s disease (PD) is predominantly characterized by the progressive degeneration of the dopaminergic (DA) neurons of the nigrostriatal pathway. Motor symptoms arise typically after at least fifty percent of the DA neurons in the substantia nigra pars compacta (SNc) have been lost. Currently, pharmacological treatment is predominantly limited to dopamine replacement strategies with no avenue to curb further cell loss. While the underlying etiology of PD has remained elusive, oxidative stress, mitochondrial function, protein quality control and inflammatory processes have all been implicated in PD pathogenesis. Importantly, an increasing number of familial PD genes have been identified. Depending on the population, LRRK2 mutations account for up to 42% of familial PD and potentially up to 10% of the sporadic disease making it the most commonly linked PD gene (1,2). It encodes a large, 2527 amino acid, multi-domain protein. Flanked by leucine-rich repeats and a WD40 domain at the N and C terminus, respectively, the catalytic core of LRRK2 consists of a ROCO GTPase and a kinase domain similar to both mitogen activated protein kinase kinase kinases (MAPKKKs) and receptor-interacting protein kinases (RIPKs) (3). LRRK2 has been implicated in a wide variety of cellular processes including: vesicular trafficking, mitochondrial dynamics, cytoskeleton dynamics, inflammatory response, translational control, protein degradation pathways and autophagy (4–9). Unfortunately, there is an incomplete understanding of the LRRK2 function as it relates to PD. A variety of LRRK2 mouse models, as with other PD genes, generally fail to recapitulate the primary characteristics of the disease. However, multiple groups have had success in modeling LRRK2 induced Parkinsonism in the fruit fly, Drosophila melanogaster (10,11). Our previous work has shown that human (h) LRRK2 mutant expression within the Drosophila tyrosine hydroxylase positive (TH+) neurons causes TH+ cell loss and locomotor deficits. We furthermore established a rapidly assessable model of degeneration by expressing the LRRK2(I2020T) PD mutant in the compound eye of flies using the GMR-GAL4 promoter (10). We have now used this model to conduct an unbiased, functional genetic screen for LRRK2 interacting genes that enhance or suppress LRRK2 induced degeneration. Identified interacting genes were also confirmed in flies expressing the wildtype (WT), R1441C and Y1699C pathogenic mutants, or the I1122V risk allele. Finally, we determined if the identified candidates from the eye screen also modified TH+ cell loss in our hLRRK2(I2020T) flies. These studies provide a critical source of candidate pathways regulated by LRRK2 for further exploration.
Results
The established GAL4-UAS (upstream activation sequence) system was utilized to ectopically express hLRRK2(I2020T) in the compound eye of D. melanogaster. We have previously shown that various hLRRK2 expressing flies maintained at 29°C display retinal degeneration marked by loss of red pigmentation and occasional black spots (10). To test if this phenotype could be suppressed or exacerbated by modifying the expression levels of other genes, we initially conducted an unbiased screen of chromosomes two, three, and four of the organism utilizing the deficiency kit from the Bloomington Drosophila Stock Center (BDSC). These regions encode approximately 96% of the autosomal genes in the Drosophila genome and the corresponding deficiency kit consists of a total of 297 fly lines that have large hemizygous chromosomal deletions that typically encompass 20–100 genes. Notably, taking advantage of the temperature dependency of the GAL4-UAS system, fly screening was conducted at both 29°C and 25°C. This was to ensure accurate sensitivity of the screen since hLRRK2 expressing flies at 25°C display a normal eye with no macroscopic degeneration (Fig. 1).
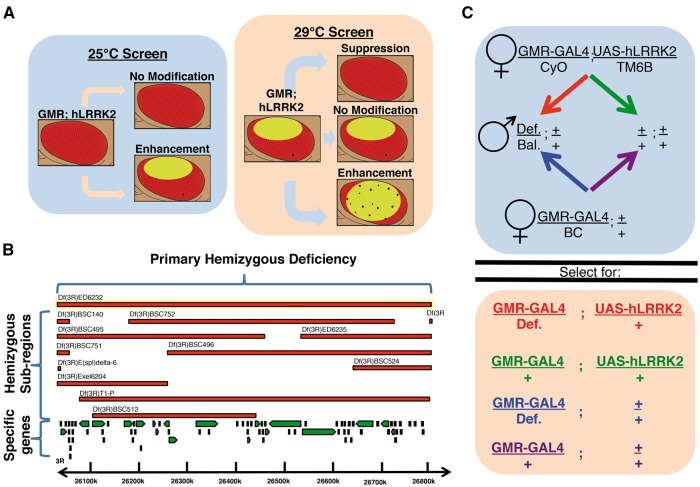
Figure 1.
Schematic representation of the screen phenotypic readout, process and Drosophila genetics. Schematic representation of parallel screens based on dose-dependent toxicity of LRRK2 expression in the eye due to the temperature dependency of the GAL4-UAS system (A) Diagram describing the screening process from large hemizygous deficiencies to narrowing with sub-region deficiencies and finally single gene disruptions (B) Example of Drosophila genetics underlying the screening process including control and experimental crosses for a second chromosome deficiency (C) Def. = Deficiency, Bal. = Balancer.
Eye Screen
Upon screening of the primary hemizygous deficiencies, we found 38 regions that phenotypically modified hLRRK2(I2020T) expression under the GMR-GAL4 eye promoter (Table 1, Supplementary Material, Table S1). Of these, there were 9 lines on the second chromosome, 29 lines on the third and none found on the fourth. We found seven deficiency lines that suppressed the loss of pigmentation, surface roughness and black lesions of the GMR-GAL4/+; UAS-hLRRK2(I2020T)/+ flies incubated at 29°C. There were no deficiencies found that led to an enhancement of the phenotype. In our parallel 25°C screen, we found 31 deficiencies that produced an obvious loss of pigmentation and damage to the retina when crossed with UAS-hLRRK2(I2020T). For candidates identified from both the 25°C and 29°C primary screens, and wherever possible, flies hemizygous for sub-region deletions were analyzed to narrow down the candidate interacting genetic zone(s). These sub-regions displayed consistent phenotypes to those observed in their corresponding primary deficiency (Table 1, Supplementary Material, Table S2). Representative optical and scanning electron microscope (SEM) images of deficiencies causing an enhancement at 25°C or a suppression at 29°C were obtained for Df(3L)BSC20 and Df(2L)C144, respectively (Fig. 2). The analyses of available sub-regions identified 474 potential LRRK2 interacting gene candidates that required further examination. For the assessment of these single gene candidates, we utilized more targeted approaches available: a) specific RNAi knock-down flies obtained from the Vienna Drosophila Resource Center (VDRC), b) disruption constructs (gene traps, mobile activating elements), and c) deletion generators from the BDSC, when available. As such, the majority of the flies employed were loss of function in nature, with the occasional over-expression lines tested, when possible. Upon completion of the single-gene screening at both 25°C and 29°C, we identified 36 genes that consistently modified the hLRRK2(I2020T) eye phenotype (Table 2, Supplementary Material, Table S3). It is important to note that a specific gene was not necessarily identified for each of the 38 primary deficiencies that modified the GMR-GAL4; UAS-hLRRK2(I2020T) eye phenotype. In fact, there were 15 primary deficiencies where no specific gene disruption could be uncovered and 11 deficiencies where multiple LRRK2 genetic modifiers were found. Of the 36 single-gene interactors, a total of 13 fly lines caused a suppression of hLRRK2(I2020T) toxicity at 29°C; 7 of these lines decreased the expression of their respective gene, while 6 were overexpressors. Again, there were no gene disruption lines that caused an enhancement of the hLRRK2(I2020T) induced eye damage at 29°C. Our parallel 25°C revealed 23 LRRK2 gene interactors that developed eye degeneration when crossed with the hLRRK2(I2020T) fly (20 were down-regulated; 3 were over-expressed). Optical and SEM images of specific gene interactors causing an enhancement at 25°C or a suppression at 29°C were obtained for the fly genes Cbl proto-oncogene ortholog (Cbl) and cathD, respectively (Fig. 3). To confirm our results and test if the phenotypic modification observed was mutation specific, we crossed our 36 candidate genes with four additional hLRRK2 expressing flies: WT, the pathogenic mutants - R1441C and Y1699C, and a risk variant - I1122V (Supplementary Material, Table S4). The screening conducted with ectopic expression of other hLRRK2 flies was fairly consistent in producing a similar modification (26/36 with at least one other hLRRK2 line showing a consistent phenotype). There were 8 gene disruptions that were hLRRK2(I2020T) specific, they did not modify hLRRK2-WT/I1122V/Y1699C or R1441C phenotypes, and 2 gene disruptions (Hrs and CG10809) that suggested potential opposing effects across the different hLRRK2 flies.
Table 1.
List of primary hemizygous deficiency lines with corresponding sub-regions that modify hLRRK2(I2020T) toxicity in the compound eye. Hemizygous Drosophila lines from the BDSC deficiency kit (in bold type face) or sub-regions (sans bold) are crossed to GMR-GAL4/CyO; UAS-hLRRK2(I2020T)/TM6B flies where the progeny may suppress, enhance or cause no change to GMR-GAL4/+; UAS-hLRRK2(I2020T)/+ toxicity at 29 °C or produce visible damage at 25 °C. Flies were analyzed by a minimum of two independent researchers and at least 20 male flies of each genotype were examined. NA = non applicable.
| Phenotypic modification | 25 °C | 29 °C |
|---|---|---|
| Enhancement | Df(2L)dp-79b, Df(2L)BSC147, Df(2R)en-A [Df(2R)BSC358], Df(2R)BSC40, Df(2R)Exel7131, Df(3L)BSC119, Df(3L)ED50002, Df(3L)ED4287, Df(3L)BSC23, Df(3L)M21, Df(3L)ED208, Df(3L)ED210, Df(3L)BSC20, Df(3L)6B-29+Df(3R)6B-29, Df(3R)10-65, Df(3R)BSC633, Df(3R)BSC466, Df(3R)ED5428, Df(3R)BSC515 [Df(3R)BSC569], Df(3R)Ubx109, Df(3R)BSC517, Df(3R)BSC489, Df(3R)ED6232 [Df(3R)BSC495, Df(3R)ED6235], Df(3R)L127, Df(3R)BSC620, Df(3R)R133, Df(3R)ED6361, Df(3R)ED50003 | Df(2L)dp-79b, Df(2L)ED250, Df(2R)Exel7131, Df(3R)L127 |
| Suppression | NA | Df(2L)C144 [Df(2L)BSC692, Df(2L)BSC180, Df(2L)Exel6008], Df(2L)Mdh [Df(2L)BSC251], Df(2L)BSC145, Df(3L)ED201 [Df(3L)BSC128], Df(3L)BSC35, Df(3L)BSC392 [Df(3L)BSC394], Df(3R)BSC819 |
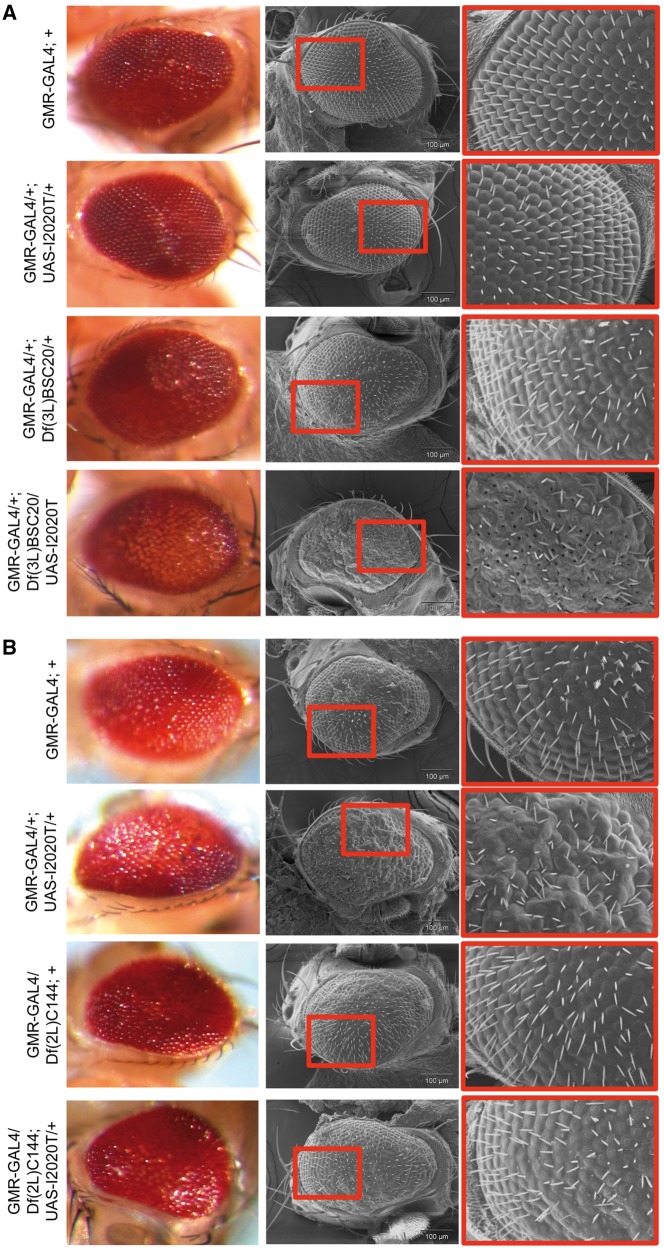
Figure 2.
Representative deficiency lines causing suppression or enhancement of GMR-GAL4/+; UAS-hLRRK2(I2020T)/+ eye phenotypes. Scanning electron microscope (SEM) and optical images of GMR-GAL4/+; UAS-hLRRK2(I2020T)/Df(3L)BSC20 causing an enhancement to the hLRRK2(I2020T) phenotype at 25°C (A) and GMR-GAL4/Df(2L)C144; UAS-hLRRK2(I2020T)/+ mediated suppression of bristle disorganization and structural abnormalities of the GMR-GAL4/+; UAS-hLRRK2(I2020T)/+ fly eye at 29°C (B).
Table 2.
List of specific genes that modify hLRRK2(I2020T) toxicity in the compound eye. Drosophila gene disruption lines (human homolog in brackets) for genes within the deficiency regions that modified hLRRK2(I2020T) eye phenotypes were screened. Multiple gene disruption lines for 474 genes were crossed to GMR-GAL4/CyO; UAS-hLRRK2(I2020T)/TM6B (Supplemental Material, Table S3) and 36 genes were found to either suppress or enhance GMR-GAL4/+; UAS-hLRRK2(I2020T)/+ toxicity at 29 °C or produce damage at 25 °C. Flies were analyzed by a minimum of two independent researchers with at least 20 male flies per group.
| Type of disruption | 25 °C (Enhancements) | 29 °C (Suppressions) |
|---|---|---|
| Down-regulated genes | CG31935 (RAB3GAP1), Scar (WAVE3), CG8888 (BDH1), convoluted (IGFALS), mdr50 (ABCB1), aly (LIN9), rasp (HHAT), CG32266 (SORBS2), rpd3 (HDAC2), CG32236 (PPIA), gef64c (ITSN1), Cbl (CBL), pipe (HS2ST1), vtd (RAD21), CG1091 (ZCCHC6), αTub85E (TUBA1C), CG5191 (FAAH), CG6154 (DPEP1), tx (ATOH1), Modulo (RBM39) | Hrs (HGS), Rbp9 (ELAVL2), Hsc70-5 (HSPA9), CG7028 (PRPF4B), rdl (GLRA3), cdk8 (CDK8), Atpα (ATP1A3) |
| Over-expressed genes | mlp84b (CSRP2), beat-VII (MUC18), CG2003 (SLC17A7) | CG2991 (MARCH3), CG5846 (RFXANK), CG13130 (UNC13B), cathD (CTSD), CG10809 (ANKRD54) |
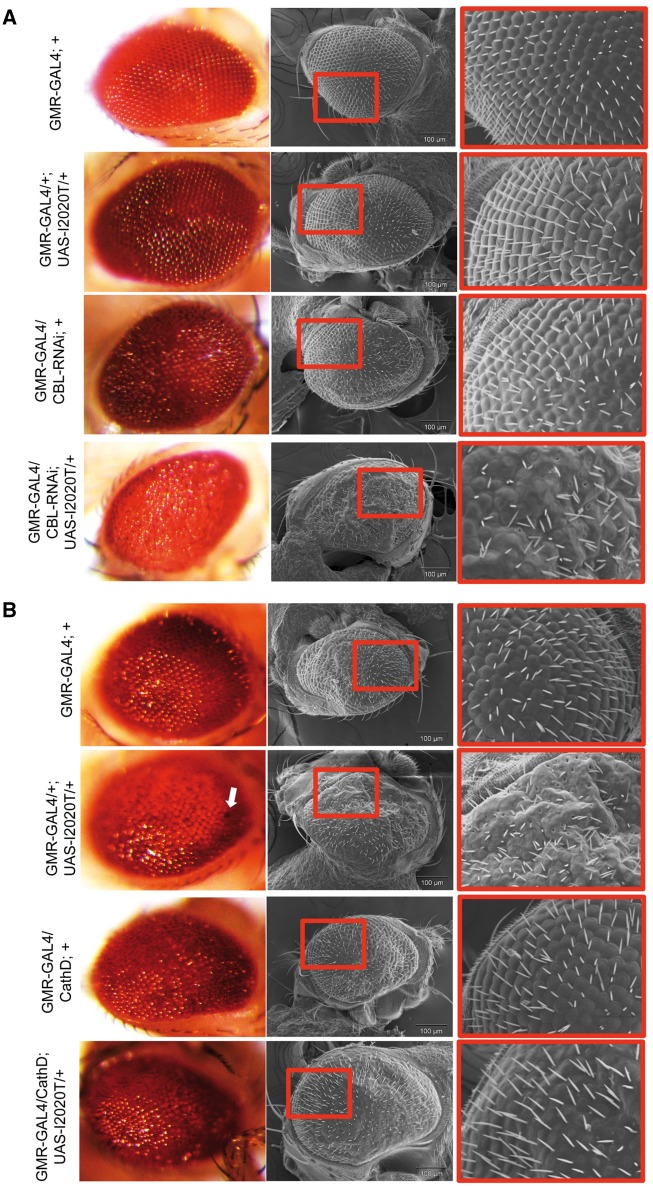
Figure 3.
Representative optical and SEM images of specific genes causing suppression or enhancement of hLRRK2(I2020T) eye phenotypes. Knockdown of Cbl via RNAi combined with overexpression of UAS-hLRRK2(I2020T) at 25 °C produces a loss of pigmentation phenotype with further structural abnormalities observable under SEM (A). CathD overexpression at 29 °C at least partially rescues loss of pigmentation and structural abnormalities of the GMR-GAL4/+; UAS-hLRRK2(I2020T)/+ fly (B).
Dopaminergic Loss
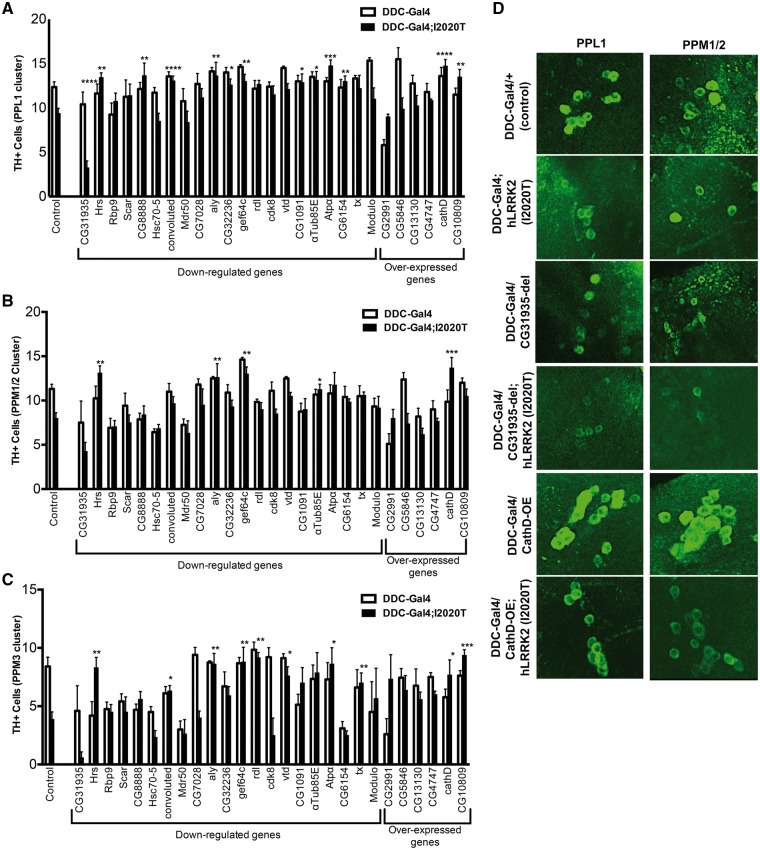
To extend our results from the eye to a more PD relevant context, a significant representative subset of candidates were examined within the DA system of the fly. Our goal was to determine if any of these genes could modify the loss of TH+ neurons and locomotor impairments that we previously demonstrated in TH-GAL4/UAS-hLRRK2(I2020T) flies (10). However, in this case, it was necessary to employ the dopamine decarboxylase (DDC-GAL4) promoter (located on the second chromosome) instead of the TH-GAL4 promoter (third chromosome). This was due to the fact that our hLRRK2 constructs are also found on the third chromosome, making the screening of third chromosome candidates difficult. Using the Drosophila Activity Monitor (DAM) system, we first confirmed that overexpression of hLRRK2(WT) or hLRRK2(I2020T) under the DDC-GAL4 promoter also causes a decrease in locomotor activity (Fig. 4D). Next, using confocal microscopy, we confirmed that 10-day old DDC-GAL4/+; UAS-hLRRK2(I2020T)/+ flies have a similar loss of TH+ staining as we previously reported with the TH-Gal4 driver (Fig. 4A–C). Specifically, we examined four DA clusters: protocerebral posterior lateral (PPL) 1, protocerebral posterior medial (PPM) 1/2, PPL2, and PPM3. Traditionally, these are the main DA clusters examined, since they are predicted to mediate motor function in Drosophila (12). We found that 16 of the 27 candidates screened in this manner modified the loss of TH+ cells observed in the DDC-GAL4/+; UAS-hLRRK2(I2020T)/+ fly (Fig. 5, Supplementary Material, Table S5). Unexpectedly, the vast majority of gene interactions (15/16) caused the suppression of the hLRRK2(I2020T) induced TH+ cell loss, while only one line (knockdown of the CG31935 gene) exacerbated loss of TH+ neurons. We did not observe any modification in the PPL2 region in any flies. Strikingly, of these 16 candidates, 6 were consistent with the modification observed in their corresponding eye phenotype but 10 were opposite in nature, indicating that genetic interactions with LRRK2 may have differing consequences or modes of action in different tissues. Alternatively, the lack of congruence between the eye and DA clusters may indicate potential false positive results. To filter this effect, we employed a more stringent statistical analysis, using an alpha value of 0.01. Using this level of significance, the 16 candidates narrow to 12 (Supplementary Material, Table S5) and the six directionally consistent interactors (CG31935, Hrs, rdl, Atpα, cathD, CG10809) survive tougher statistical stringency (Table 3). In order to offer insight on these six candidates, we performed Gene Ontology (GO) analysis using PANTHER software. We found these genes show a significant enrichment to the synapse (3/6), extracellular exosome (3/6) and vesicles (4/6) after GO analysis for cellular compartments (Table 4).
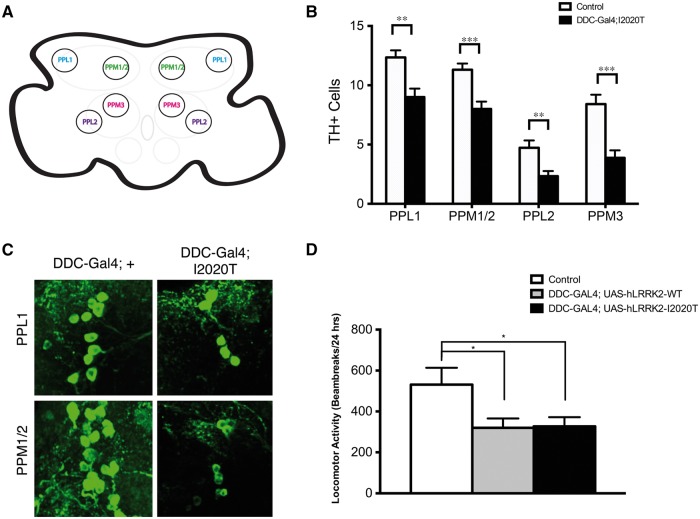
Figure 4.
DDC-GAL4/+; hLRRK2(I2020T)/+ flies display TH+ cell loss and locomotor deficits. Schematic diagram displaying the dopaminergic clusters of the Drosophila central nervous system (A). Quantification and representative confocal images of the PPL1, PPM1/2, PPL2, and PPM3 TH+ clusters of 10 day-old DDC-GAL4/+; UAS-hLRRK2(I2020T)/+ (n = 11) and DDC-GAL4/+ (n = 12) flies (B, C). Locomotor activity of DDC-GAL4/+ (n = 11), DDC-GAL4/+; UAS-hLRRK2-WT/+ (n = 15) and DDC-GAL4/+; UAS-hLRRK2(I2020T)/+ (n = 15) 10 day-old flies was analyzed with the Drosophila Activity Monitor (DAM) system over 24 h (D). All flies were incubated at 25°C. One-way ANOVA followed by Tukey’s least significant difference (LSD) post-test.
Figure 5.
Specific gene disruptions that modify loss of TH+ cells in the DDC-Gal4/+; UAS-hLRRK2(I2020T)/+ dopaminergic clusters of the Drosophila CNS. 27 specific gene disruptions found in the eye screen were crossed to the DDC-GAL4/+ and DDC-GAL4/+; UAS-hLRRK2(I2020T)/+ flies with a minimum of 3–16 brains quantified per group. After dissection and staining, quantification of TH+ cell clusters for PPL1, PPM1/2, PPL2, and PPM3 was performed on experimental and control flies including DDC-GAL4; + (n = 13) and DDC-GAL4/+; UAS-hLRRK2(I2020T)/+ (n = 17) flies. One-way ANOVA followed by Bonferroni’s multiple comparison post-test. NS = not significant. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Table 3.
Directionally consistent LRRK2 interactors. Drosophila (H. sapiens) genes that significantly (P < 0.01) modified hLRRK2(I2020T) toxicity in both the eye and dopaminergic system.
| Phenotypic modification | Enhancement | Suppression | |||
| Down-regulated genes | CG31935 (RAB3GAP1) | Hrs (HGS) rdl (GLRA3) Atpα (ATP1A3) | |||
| Over-expressed genes | cathD (CTSD) CG10809 (ANKRD54) | ||||
Table 4.
Gene ontology analysis of most consistent LRRK2 interactors. These six genes were examined for enrichment using Homo sapiens reference genome and performing cellular component GO analysis by PANTHER (http://www.pantherdb.org; date last accessed 24 Oct 2016).
| GO cellular component | # Genes in reference set | # Genes in query | Expected | Fold enrichment | P value |
| postsynapse (GO:0098794) | 397 | 3 | 0.11 | + 26.41 | 1.30 × 10−4 |
| → synapse part (GO:0044456) | 667 | 3 | 0.19 | + 15.72 | 5.99 × 10−4 |
| → synapse (GO:0045202) | 813 | 3 | 0.23 | + 12.9 | 1.07 × 10−3 |
| extracellular exosome (GO:0070062) | 2750 | 3 | 0.79 | + 3.81 | 3.31 × 10−2 |
| → extracellular vesicle (GO:1903561) | 2764 | 4 | 0.79 | + 5.06 | 3.62 × 10−3 |
Discussion
In an attempt to elucidate LRRK2 genetic interactors and associated pathways, we conducted an unbiased, functional genetic screen in Drosophila. This was accomplished using a two-tiered, double-pronged approach. First, we utilized previously established models of LRRK2 that induce toxicity in the eye and DA systems, which provided phenotypes that could be both suppressed or exacerbated. Secondly, we took the advantage of the temperature-dependent GAL4-UAS expression system, allowing us to increase and lower LRRK2 expression levels to assess whether potential genetic interactors clearly cause phenotypic alterations in the eye. The results from the eye screen revealed a number of signals associated with a range of cellular processes. Some of these pathways are expanded on below.
To begin, both LRRK2 and many of the interactors revealed in this study are associated with vesicular trafficking. In fact, the most consistent interactors showed enrichment for the synapse and extracellular vesicles. LRRK2’s role in trafficking has been connected with various RAB protein family members including RAB3 GTPase (13). Our screen revealed that down regulation of CG31935 (human RAB3GAP1) enhanced both eye toxicity and loss of TH+ neurons. Our screen also revealed Hrs, homologous to Hepatocyte Growth Factor-Regulated Tyrosine Kinase Substrate (HGS), as a LRRK2 interactor. HGS is crucial for recruitment of clathrin to early endosomes (14,15). Importantly, LRRK2 has been specifically implicated in clathrin-mediated endocytosis by direct binding to clathrin-light chains (15). Along these lines, we identified the interactor, Gef64c, which is similar to human intersectin1 (ITSN1); a protein that interacts with clathrin as well as other endocytic machinery (16–18).
Secondly, LRRK2 has been implicated in both actin and microtubule dynamics in cytoskeletal remodelling (19,20). One interesting interactor unveiled by our screen is αTub8E, which is homologous to α-tubulin1C (TUBA1C), since LRRK2 has been shown to directly interact with some tubulin family members involved in microtubule dynamics (19). Furthermore, we identified Scar and CG32266 that have human gene products WAVE3 and SORBS2, respectively, which are known to form a complex that controls the actin cytoskeleton (21).
Another common theme is mitochondrial health, which is implicated in both PD and LRRK2 function (5,22,23). Both Hsc70-5 (HSPA9) and CG8888 (BDH1) are involved in mitochondrial homeostasis and energy production and were revealed by the screen (24,25). Importantly, some of the interactors discovered by our screen have been implicated in PD by either proteomics or genome-wide association studies. For instance, the transcript for pre-mRNA processing factor 4B (PRPF4B), orthologous to CG7028 from our screen, has been found to be up-regulated in the SNc of PD patients (26). Additionally, CathD, CG13130, Atpα, Hsc70-5, and Mdr50 contain polymorphisms in their respective human homologs: CTSD, UNC13b, ATP1A3, HSPA9 and ABCB1 that have been associated with risk of developing PD (27–31). Overall, these results suggest LRRK2 may interact with other PD risk factors and lend confidence to our screen.
It is important to note that a few interactors could not be confirmed with LRRK2 WT or other pathogenic mutant expression in the eye. One important possibility for this may be that discrete mutations may differentially regulate target pathways or potential binding partners. Furthermore, the inhibition of LRRK2 kinase activity is thought to ameliorate its toxicity in the cell (32). However, it is not completely clear exactly how various LRRK2 mutations affect kinase activity.
Unexpectedly, many of the genetic interactors that led to an enhanced eye phenotype when combined with LRRK2(I2020T) revealed suppression phenotypes when examined in the DA system. However, the expression of LRRK2 and/or the specific fly gene disruption may result in a different cellular response depending on the cell type. The development and maintenance of both the compound eye and the dopaminergic system of the fly may have some distinct properties that make it challenging to draw comparison between them. This is particularly true when conducting ectopic expression studies. Furthermore, it may be difficult to compare these two niches, as our analysis was limited to qualitative gross morphology in the eye and quantification of a limited number of TH+ cells in the brain. This may explain why not all suppression phenotypes in the eye were recovered in the dopaminergic system.
During the course of our studies in the DA system, we discovered two genes in particular, CG2991 (MARCH3) and Mdr50 (ABCB1), that consistently played a role in DA cell death, independent of LRRK2. Admittedly, these types of results make functional genetic interactions for these candidates challenging to interpret in our model. There may be a floor effect based on the limited number of cells quantified in a given DA cluster, and may merit further investigation.
In summary, we have conducted a fully saturated, unbiased autosomal screen for LRRK2(I2020T) modifiers in the eye. Furthermore, we sub-screened the majority of the candidates in the DA system of the fly. As a result, our studies provide some important general insights on LRRK2 function. Firstly, our most consistent interactions were enriched for genes associated with synaptic vesicles, which is in agreement with the much of the current LRRK2 field. Within this subset, we identified novel LRRK2 genetic interactors that may be pursued in the mammalian system. On the other hand, we detected differential outcomes with identified genes both through pathway analyses and also in the direction of a phenotypic modification between the eye and the DA system. This suggests the importance of examining all candidate genes/pathways, including those identified presently, in diverse cellular contexts. This is particularly important in PD where multiple cell types including, but not limited to: neurons, astrocytes, and microglia likely interact to regulate disease manifestation and progression. Secondly, discrete LRRK2 mutations may not necessarily function in identical fashion. This would necessitate more careful study of potential downstream signals specific to each. Nevertheless, our study provides a comprehensive starting point for future studies characterizing candidate genes in a mammalian context to determine whether or how they may affect pathology related to PD.
Materials and Methods
Drosophila stocks
All fly stocks were maintained on a standard cornmeal/agar medium at ambient room temperature. Crosses were conducted at 25 °C or 29 °C when under experimental conditions. hLRRK2 flies were previously created and characterized in Venderova et al. (2009). The primary deficiency kit, sub-region lines, all elevated promoter (overexpression), deletion, transgenic transposon, enhancer trap, gene trap, DDC-GAL4 and some dsRNA lines were commercially obtained from the BDSC (Bloomington, Indiana). GMR-GAL4/BC and w1118 flies were a gift from Yong Rao (33) and Margaret Sonnenfeld (34), respectively. All other dsRNA lines were obtained from the VDRC.
Suppressor/enhancer screen genetics
hLRRK2 flies were kept balanced as GMR-GAL4/CyO;UAS-hLRRK2/TM6B, as GMR-GAL4 is homozygous lethal. The flies for the TH+ screen were kept as DDC-GAL4/CyO; UAS-hLRRK2/TM6B. These lines are crossed to deficiency or gene disruption lines and w1118 control flies. Importantly, deficiencies and gene disruption lines were also crossed to GMR-GAL4 or DDC-GAL4 lines to determine if they cause a phenotype sans hLRRK2. Finally, we always crossed GMR-GAL4 or DDC-GAL4 to w1118 flies to control for any driver-mediated effects.
Phenotypic scoring for the eye screen
All experimental and control flies were rapidly screened for loss of red pigmentation, obvious black lesions and any gross morphological changes to the ommatidia under optical microscopy at 25 °C. Due to the lack of degeneration in hLRRK2 flies at this temperature, scoring was clear among the users. The hLRRK2 fly eye at 29 °C has a variable phenotype (from simple loss of pigmentation on half of the eye to loss covering the entirety of the eye and the presence of black lesions). The LRRK2 phenotype was considered suppressed when disruption/overexpression of one of the tested genes in the mutant LRRK2 fly caused an eye phenotype that was not significantly different from the GMR-GAL4/+ eye at 29 °C (some visible surface roughness, minor loss of pigmentation). The threshold for enhancements at 29 °C was high due to the variable LRRK2 phenotype; no interactors that met this threshold were found. All interactors found in the screen were confirmed by at least two researchers, blinded to the genotype. Phenotypic modifications were complete in penetrance. All analyzed flies were male.
SEM imaging
Heads of 10 day-old male flies were fixed in 2% glutaraldehyde in 0.1M sodium cacodylate buffer (pH 7.3) for 2 hrs and dehydrated in ethanol. The SEM was performed by the Advanced Bioimaging Center, Mount Sinai Hospital, Toronto.
Quantification of TH± neurons
Male gene disruption lines on either a DDC-GAL4/+; UAS-hLRRK(I2020T)/+ or control, DDC-GAL4/+ background were aged to 10 days at 25 °C. Briefly, flies were dissected in PBS and fixed in 4% PFA and stained with polyclonal rabbit anti-TH antibody (EMD Millipore AB152) at 1:300 in PBT (0.3% Tween), 5% normal goat serum following standard fly brain protocol (35). Secondary antibody was donkey anti-rabbit conjugated Alexa 488 (Invitrogen). Whole brains were mounted to slides using the bridge method and confocal images (Zeiss LSM510) were taken (20X objective) in Z-stack to render a 3-D image used for counting the TH+ clusters of the fly brain.
Locomotor activity
Male DDC-GAL4/+; UAS-hLRRK-WT/+ (or I2020T) and DDC-GAL4; + flies were aged to 10 days at 25 °C and placed in the Drosophila Activity Monitor (DAM) system (TriKinetics). The apparatus has 32 chambers that house one fly in each chamber. Flies are able to walk back and forth within the chamber and have access to food. An infrared beambreak system sums activity every 5 min and flies were monitored for a 24-h period.
Gene Ontology
Gene Ontology analysis was classified based on the PANTHER (Protein Analysis Through Evolutionary Relationships) system (http://www.pantherdb.org; date last accessed October 24, 2016) (36). The PANTHER Protein Class ontology was adapted from the PANTHER/X cellular component ontology.
Statistical Analysis
The data were analyzed as specified, expressed as means ± standard error of means, and denoted as: * if P ≤ 0.05, ** if P ≤ 0.01, *** if P ≤ 0.001, and **** if P ≤ 0.0001.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
DSP is funded by the Network of Centers of Excellence in Neurodegeneration, the Heart and Stroke Foundation of Ontario, and the Neuroscience Brain Canada/Krembil Foundation. PCM was funded by fellowships from PSC and PRC. There is no declared conflict of interest with the contents of this article. We would like to thank the undergraduate students who helped to maintain fly stocks: Cindy Wei, Amanda Perozzo, Francis Lebrun, Yannick Lee, Shaheen Upal and Alaa Fanous. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We would like to thank Douglas Holmyard (Mount Sinai Hospital, Toronto) for SEM work.
Conflict of Interest statement. None declared.
Funding
Michael J. Fox Foundation for Parkinson Research, Canadian Institutes of Health Research, the Parkinson Society Canada (PSC), and the Parkinson Research Consortium (PRC) in Ottawa.
References
- 1. Bardien S., Lesage S., Brice A., Carr J. (2011) Genetic characteristics of leucine-rich repeat kinase 2 (LRRK2) associated Parkinson's disease. Parkinsonism Relat. Disord., 17, 501–508. [DOI] [PubMed] [Google Scholar]
- 2. Correia Guedes L., Ferreira J.J., Rosa M.M., Coelho M., Bonifati V., Sampaio C. (2010) Worldwide frequency of G2019S LRRK2 mutation in Parkinson's disease: a systematic review. Parkinsonism Relat. Disord., 16, 237–242. [DOI] [PubMed] [Google Scholar]
- 3. Mata I.F., Wedemeyer W.J., Farrer M.J., Taylor J.P., Gallo K.A. (2006) LRRK2 in Parkinson's disease: protein domains and functional insights. Trends Neurosci., 29, 286–293. [DOI] [PubMed] [Google Scholar]
- 4. MacLeod D.A., Rhinn H., Kuwahara T., Zolin A., Di Paolo G., McCabe B.D., MacCabe B.D., Marder K.S., Honig L.S., Clark L.N., et al. (2013) RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron, 77, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mortiboys H., Johansen K.K., Aasly J.O., Bandmann O. (2010) Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology, 75, 2017–2020. [DOI] [PubMed] [Google Scholar]
- 6. Häbig K., Gellhaar S., Heim B., Djuric V., Giesert F., Wurst W., Walter C., Hentrich T., Riess O., Bonin M. (2013) LRRK2 guides the actin cytoskeleton at growth cones together with ARHGEF7 and Tropomyosin 4. Biochim. Biophys. Acta, 1832, 2352–2367. [DOI] [PubMed] [Google Scholar]
- 7. Shin N., Jeong H., Kwon J., Heo H.Y., Kwon J.J., Yun H.J., Kim C.H., Han B.S., Tong Y., Shen J., et al. (2008) LRRK2 regulates synaptic vesicle endocytosis. Exp. Cell Res., 314, 2055–2065. [DOI] [PubMed] [Google Scholar]
- 8. Martin I., Kim J.W., Lee B.D., Kang H.C., Xu J.C., Jia H., Stankowski J., Kim M.S., Zhong J., Kumar M., et al. (2014) Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson’s disease. Cell, 157, 472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linhart R., Wong S.A., Cao J., Tran M., Huynh A., Ardrey C., Park J.M., Hsu C., Taha S., Peterson R., et al. (2014) Vacuolar protein sorting 35 (Vps35) rescues locomotor deficits and shortened lifespan in Drosophila expressing a Parkinson’s disease mutant of Leucine-rich repeat kinase 2 (LRRK2). Mol. Neurodegen., 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venderova K., Kabbach G., Abdel-Messih E., Zhang Y., Parks R.J., Imai Y., Gehrke S., Ngsee J., LaVoie M.J., Slack R.S., et al. (2009) Leucine-Rich Repeat Kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson's disease. Hum. Mol. Genet., 18, 4390–4404. [DOI] [PubMed] [Google Scholar]
- 11. Liu Z., Wang X., Yu Y., Li X., Wang T., Jiang H., Ren Q., Jiao Y., Sawa A., Moran T., et al. (2008) A Drosophila model for LRRK2-linked parkinsonism. Proc. Natl. Acad. Sci. U.S.A, 105, 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mao Z., Davis R.L. (2009) Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits, 3, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steger M., Tonelli F., Ito G., Davies P., Trost M., Vetter M., Wachter S., Lorentzen E., Duddy G., Wilson S., et al. (2016) Phosphoproteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. eLife Sciences, 5, e12813.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raiborg C., Bache K.G., Mehlum A., Stang E., Stenmark H. (2001) Hrs recruits clathrin to early endosomes. embo J., 20, 5008–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schreij A.M.A., Chaineau M., Ruan W., Lin S., Barker P.A., Fon E.A., McPherson P.S. (2015) LRRK2 localizes to endosomes and interacts with clathrin-light chains to limit Rac1 activation. EMBO Rep., 16, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu Y., Chu P.Y., Bowser D.N., Keating D.J., Dubach D., Harper I., Tkalcevic J., Finkelstein D.I., Pritchard M.A. (2008) Mice deficient for the chromosome 21 ortholog Itsn1 exhibit vesicle-trafficking abnormalities. Hum. Mol. Genet., 17, 3281–3290. [DOI] [PubMed] [Google Scholar]
- 17. Cirnaru M.D., Marte A., Belluzzi E., Russo I., Gabrielli M., Longo F., Arcuri L., Murru L., Bubacco L., Matteoli M., et al. (2014) LRRK2 kinase activity regulates synaptic vesicle trafficking and neurotransmitter release through modulation of LRRK2 macro-molecular complex. Front Mol Neurosci., 7, 49.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matta S., Van Kolen K., da Cunha R., van den Bogaart G., Mandemakers W., Miskiewicz K., De Bock P.J., Morais V.A., Vilain S., Haddad D., et al. (2012) LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron, 75, 1008–1021. [DOI] [PubMed] [Google Scholar]
- 19. Law B.M.H., Spain V.A., Leinster V.H.L., Chia R., Beilina A., Cho H.J., Taymans J.M., Urban M.K., Sancho R.M., Ramírez M.B., et al. (2014) A direct interaction between leucine-rich repeat kinase 2 and specific β-tubulin isoforms regulates tubulin acetylation. J. Biol. Chem., 289, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meixner A., Boldt K., Van Troys M., Askenazi M., Gloeckner C.J., Bauer M., Marto J.A., Ampe C., Kinkl N., Ueffing M. (2011) A QUICK screen for Lrrk2 interaction partners–leucine-rich repeat kinase 2 is involved in actin cytoskeleton dynamics. Mol. Cell Proteomics, 10, M110.001172.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cestra G., Toomre D., Chang S., De Camilli P. (2005) The Abl/Arg substrate ArgBP2/nArgBP2 coordinates the function of multiple regulatory mechanisms converging on the actin cytoskeleton. Proc. Natl. Acad. Sci. U.S.A, 102, 1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saha S., Guillily M.D., Ferree A., Lanceta J., Chan D., Ghosh J., Hsu C.H., Segal L., Raghavan K., Matsumoto K., et al. (2009) LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J. Neurosci., 29, 9210–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X., Yan M.H., Fujioka H., Liu J., Wilson-Delfosse A., Chen S.G., Perry G., Casadesus G., Zhu X. (2012) LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet., 21, 1931–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaul S.C., Deocaris C.C., Wadhwa R. (2007) Three faces of mortalin: A housekeeper, guardian and killer. Exp. Gerontol., 42, 263–274. [DOI] [PubMed] [Google Scholar]
- 25. Tieu K., Perier C., Caspersen C., Teismann P., Wu D.C., Yan S.D., Naini A., Vila M., Jackson-Lewis V., Ramasamy R., et al. (2003) D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Invest., 112, 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cowherd M., Lee I. (2015) Transcriptional regulators are upregulated in the substantia nigra of Parkinson's disease patients. J. Emerg. Invest., 1–7. [Google Scholar]
- 27. Schulte T., Böhringer S., Schöls L., Müller T., Fischer C., Riess O., Przuntek H., Berger K., Epplen J.T., Krüger R. (2003) Modulation of disease risk according to a cathepsin D/apolipoprotein E genotype in Parkinson's disease. J. Neural. Transm., 110, 749–755. [DOI] [PubMed] [Google Scholar]
- 28. Liu X., Cheng R., Verbitsky M., Kisselev S., Browne A., Mejia-Sanatana H., Louis E.D., Cote L.J., Andrews H., Waters C., et al. (2011) Genome-wide association study identifies candidate genes for Parkinson's disease in an Ashkenazi Jewish population. BMC Med. Genet., 12, 104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Carvalho Aguiar P., Sweadner K.J., Penniston J.T., Zaremba J., Liu L., Caton M., Linazasoro G., Borg M., Tijssen M.A.J., Bressman S.B., et al. (2004) Mutations in the Na+/K+-ATPase α3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron, 43, 169–175. [DOI] [PubMed] [Google Scholar]
- 30. De Mena L., Coto E., Sánchez-Ferrero E., Ribacoba R., Guisasola L.M., Salvador C., Blazquez M., Alvarez V. (2009) Mutational screening of the mortalin gene (HSPA9) in Parkinson’s disease. J. Neural. Transm., 116, 1289–1293. [DOI] [PubMed] [Google Scholar]
- 31. Westerlund M., Belin A.C., Anvret A., Håkansson A., Nissbrandt H., Lind C., Sydow O., Olson L., Galter D. (2009) Association of a polymorphism in the ABCB1 gene with Parkinson's disease. Parkinsonism Relat. Disord., 15, 422–424. [DOI] [PubMed] [Google Scholar]
- 32. Greggio E., Jain S., Kingsbury A., Bandopadhyay R., Lewis P., Kaganovich A., van der Brug M.P., Beilina A., Blackinton J., Thomas K.J., et al. (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis., 23, 329–341. [DOI] [PubMed] [Google Scholar]
- 33. Cafferty P., Yu L., Long H., Rao Y. (2006) Semaphorin-1a functions as a guidance receptor in the Drosophila visual system. J. Neurosci., 26, 3999–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun X., Morozova T., Sonnenfeld M. (2006) Glial and Neuronal Functions of the Drosophila Homolog of the Human SWI/SNF Gene ATR-X (DATR-X) and the jing Zinc-Finger Gene Specify the Lateral Positioning of Longitudinal Glia and Axons. Genetics, 173, 1397–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu J.S., Luo L. (2006) A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat. Protocols, 1, 2110–2115. [DOI] [PubMed] [Google Scholar]
- 36. Mi H., Poudel S., Muruganujan A., Casagrande J.T., Thomas P.D. (2016) PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res., 44, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.