Abstract
Global developmental delay (GDD), often accompanied by intellectual disability, seizures and other features is a severe, clinically and genetically highly heterogeneous childhood-onset disorder. In cases where genetic causes have been identified, de novo mutations in neuronally expressed genes are a common scenario. These mutations can be best identified by exome sequencing of parent-offspring trios. De novo mutations in the guanine nucleotide-binding protein, beta 1 (GNB1) gene, encoding the Gβ1 subunit of heterotrimeric G proteins, have recently been identified as a novel genetic cause of GDD. Using exome sequencing, we identified 14 different novel variants (2 splice site, 2 frameshift and 10 missense changes) in GNB1 in 16 pediatric patients. One mutation (R96L) was recurrently found in three ethnically diverse families with an autosomal dominant mode of inheritance. Ten variants occurred de novo in the patients. Missense changes were functionally tested for their pathogenicity by assaying the impact on complex formation with Gγ and resultant mutant Gβγ with Gα. Signaling properties of G protein complexes carrying mutant Gβ1 subunits were further analyzed by their ability to couple to dopamine D1R receptors by real-time bioluminescence resonance energy transfer (BRET) assays. These studies revealed altered functionality of the missense mutations R52G, G64V, A92T, P94S, P96L, A106T and D118G but not for L30F, H91R and K337Q. In conclusion, we demonstrate a pathogenic role of de novo and autosomal dominant mutations in GNB1 as a cause of GDD and provide insights how perturbation in heterotrimeric G protein function contributes to the disease.
Introduction
Severe, early onset disorders such as intellectual disability (ID) and global developmental delay (GDD) impact on reproductive fitness and are often caused by denovo mutations that can be best identified by next generation sequencing of parent-offspring trios (1). Recently, denovo mutations in the guanine nucleotide-binding protein, beta 1 (GNB1) gene have been identified as a novel genetic cause of developmental delay (2). Among the first 13 patients, a wide range of additional symptoms and signs were reported including hypotonia in 11 and seizures in 10 of the patients (2). GNB1 encodes the guanine nucleotide-binding protein subunit beta-1, Gβ1, a G protein that is involved in signal transduction and forms a heterotrimer complex with Gα and Gγ subunits (3).
With the advent of next generation sequencing, challenges for the detection of disease-causing mutations have changed (4). In the era of Sanger sequencing, the experimental efforts were enormous to detect one likely pathogenic variant in sometimes hundreds of successively sequenced candidate genes. Nowadays, nearly all human genes can be sequenced in parallel by exome sequencing with data interpretation, i.e. to find the disease causing-mutation among thousands of variants, being the greatest challenge. Even when focusing on rare variants (minor allele frequency <0.01) that are predicted to change the protein sequence (such as missense, nonsense, frameshift or splice site alterations), there is typically more than one possible disease-causing variant per patient (5). In addition, not all rare and protein-changing variants in a given disease gene are necessarily pathogenic requiring functional characterization of such changes (6).
For signaling molecules, functional impact can be determined by analyzing their interaction with protein partners in the transmission cascade as well as by the ability to propagate receptor-initiated signal. In the case of Gβ, the functionality of wild-type and mutant protein can be investigated by studying its ability to interact with Gγ and Gα subunits and to undergo receptor-driven rearrangements. Changes in these interactions can be monitored in vitro by bioluminescence resonance energy transfer (BRET) between fluorescent-tag labeled components of the cascade. This strategy has proved useful in evaluating the pathogenicity of variants in the GNAL gene encoding the Gα subunit in the Gαβγ complex that mediates signaling of the D1 dopamine receptor (D1R), linking this deficiency to a form of adult-onset dystonia (7,8).
Here, we report 14 novel variants in GNB1 including 10 missense changes which were assessed for trimeric G protein complex formation and signaling by BRET-based cellular assays to characterize their functional impact. These revealed that only 7 of the 10 missense variants altered functionality, suggesting that the remaining three substitutions represent possibly benign changes.
Results
Genetic analysis
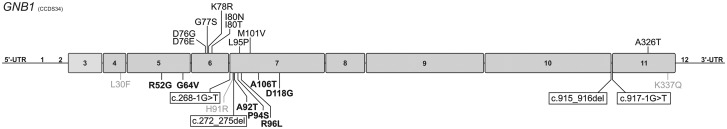
We identified 16 carriers of 14 different rare variants including 2 frameshifts, 2 splice sites and 10 missense changes (Table 1). Most of these variants (n = 8) were located in Exon 7 or its splice sites (Fig. 1). Only the recurrent substitution, p.R96L, was shown to be inherited in an autosomal dominant manner. For 10 of the variants a denovo origin could be confirmed since the change was not found in either of the parents. Mode of inheritance could not be determined for three variants owing to lack of parental DNA samples and unknown family history (Table 1). All 14 variants were detected in patients and were absent in 4361 in-house exome datasets at Centogene AG (Rostock, Germany). Furthermore, none of the changes was reported in public databases such as the 60 706 exomes of the Exome Aggregation Consortium (ExAC, http://exac.broadinstitute.org/gene/ENSG00000078369; date last accessed November 10, 2016) except for L30F that was found in 2 of ∼8000 South Asian individuals (http://exac.broadinstitute.org/variant/1-1749284-G-A; date last accessed November 10, 2016).
Table 1.
Clinical, genetic and functional information of mutation carriers
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | F | F | M | M | M | F | M | F | F | M | M | F | M | F | M |
| Age at diagnosis | 4 | 2.5 | 4 | 2.4 | 5 | 8 | 2.9 | 4 | 6 | 1 | Unk | 8 | 12 | 8 | 6 | 8 |
| Ethnicity | Saudi Arabian | Qatar | Egypt | Saudi Arabian | Israeli | Mexican | Saudi Arabian | Kuwaiti | Israeli | Indian | Mexican | Algerian | German | Saudi Arabian | Indian | Saudi Arabian |
| Var Build 19 | 1:1749284 | 1:1747244 | 1:1747207 | 1:1736021 | 1:1736013 | 1:1736016 | 1:1736014 | 1:1736008 | 1:1736001 | 1:1736001 | 1:1736001 | 1:1735972 | 1:1735935 | 1:1720492 | 1:1718877 | 1:1718784 |
| NM_002074 | c.88C>T | c.154C>G | c.191G>T | c.268-1G>T | c.272_275del | c.272A>G | c.274G>A | c.280C>T | c.287G>T | c.287G>T | c.287G>T | c.316G>A | c.353A>G | c.915_916del | c.917-1G>T | c.1009A>C |
| NP_002065 | p.L30F | p.R52G | p.G64V | p.? | p.? | p.H91R | p.A92T | p.P94S | p.R96L | p.R96L | p.R96L | p.A106T | p.D118G | p.? | p.? | p.K337Q |
| Location of mut. | Exon 4 | Exon 5 | Exon 5 | Intron 6 | Exon 7 | Exon 7 | Exon 7 | Exon 7 | Exon 7 | Exon 7 | Exon 7 | Exon 7 | Exon 7 | Exon 10 | Intron 10 | Exon 11 |
| Type of mut. | ms | ms | ms | ss | fs | ms | ms | ms | ms | ms | ms | ms | ms | fs | ss | ms |
| Change in function | No | Yes | Yes | Not tested | Not tested | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not tested | Not tested | No |
| CADD score (v1.3) | 22 | 21 | 33 | 27 | 35 | 18 | 25 | 23 | 26 | 26 | 26 | 24 | 29 | 35 | 26 | 24 |
| Inheritance | De novo | De novo | De novo | De novo | De novo | Unk | De novo | Unk | Dominant | Dominant | Dominant | De novo | De novo | De novo | Unk | De novo |
| GDD | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Growth delay | Yes | No | No | No | Yes | Yes | No | No | Yes | No | No | No | No | No | Yes | No |
| Muscular hypotonia | Yes | No | No | Yes | No | Yes | Yes | No | No | No | No | No | Yes | Yes | Yes | No |
| ID | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Seizures | Yes | No | No | No | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| Cranniosynostosis | No | No | No | Yes | Yes | No | No | No | No | No | No | Yes | No | No | No | No |
| Cerebellar hypoplasia | No | Yes | No | No | No | Yes | Yes | No | No | No | No | No | No | No | No | No |
| Abnormal myelination | No | No | No | Yes | Yes | No | No | No | Yes | No | Yes | No | No | Yes | No | Yes |
| Ophthalmoplegia | No | Yes | No | Yes | No | No | No | Yes | Yes | No | No | No | No | No | No | No |
| Nystagmus | Yes | Yes | No | No | Yes | Yes | No | No | Yes | No | Yes | No | No | Yes | Yes | Yes |
| Ataxia | Yes | Yes | No | No | Yes | No | Yes | No | No | No | No | No | No | No | Yes | No |
| Chorea | No | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No |
| Dystonia | No | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No |
Unk, unknown; ss, splice site; fs, Frameshift; ms, missense; GDD, global developmental delay; ID, intellectual disability; CADD, combined annotation dependent depletion.
Figure 1.
Schematic representation of GNB1 variants. The exons of the GNB1 gene are shown to scale with the nine coding exons highlighted by boxes and flanked by the respective untranslated regions (UTR). Previously reported mutations (2) are shown above the gene in black and newly identified variants in this study are indicated below the gene including truncating mutations (black, boxed), missense changes which alter the functionality of Gβ1 (black, bold), and likely benign variants (grey).
First symptoms were noted in the patients at a mean age of 5.3 ± 2.8 years (range 1–12 years) and nine patients were male. Patients were of diverse ethnic background including Arabic, South Asian, European and Hispanic (Table 1). All variants were found among about 4500 individuals referred for genetic testing by exome sequencing at Centogene AG (Rostock, Germany) but neither among the 51 German patients with epilepsy and ID nor among the 40 Serbian Rett-like patients.
Interaction of Gβ1 with Gα and Gγ proteins and propagation of D1R signaling
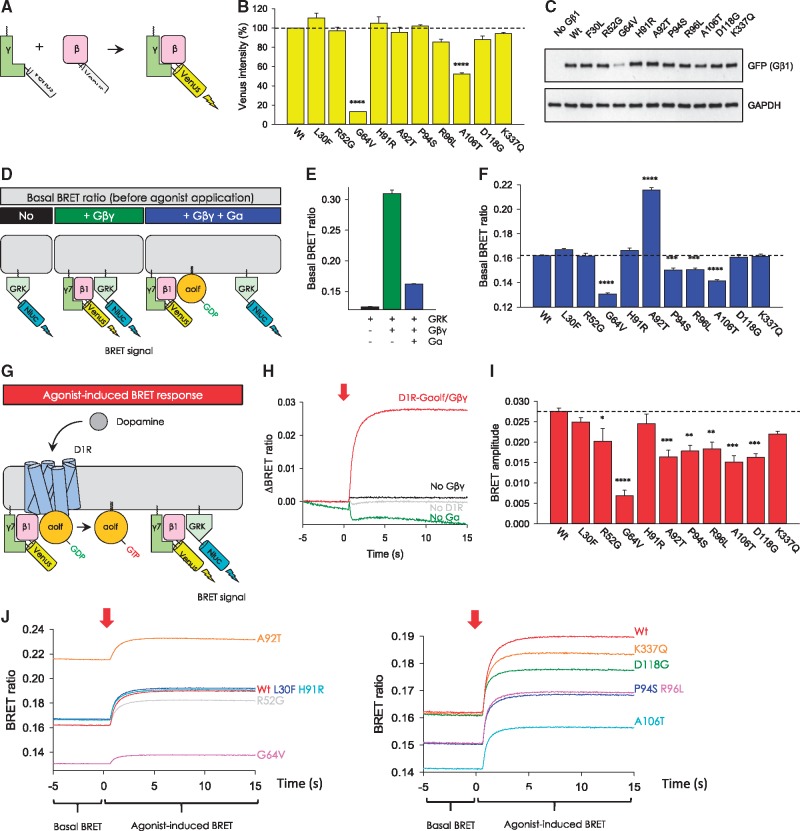
For the 10 GNB1 missense variants, we examined the functional effects on D1R signaling via Gαolfβ1γ7. First, we determined the impact on the ability of Gβ1 to form constitutive dimers with the Gγ7 subunit by bimolecular fluorescence complementation (BiFC) (9) (Fig. 2A). Gγ7 was chosen for its dominant role in mediating Gαolf signaling in the striatum (10), a key brain region involved in a wide range of pathologies prominently including movement disorders (11,12). In this assay, productive formation of a complex between Gγ7 and Gβ1 reconstitutes Venus protein, detected by its fluorescence. We found that G64V and A106T mutants significantly diminished Venus intensity (Fig. 2B). These two mutants also exhibited lower expression levels when analyzed by Western blotting (Fig. 2C), suggesting deficits in stability and Gβγ dimer formation. All other Gβ1 mutants exhibited normal expression and dimerization with Gγ7 comparable to wild-type construct (Fig. 2B and 2C).
Figure 2.
Effect of missense mutations in Gβ1 on Gβγ dimer formation, trimeric Golf formation and agonist-induced G protein activation. (A) Schematic presentation of the BiFC assay to test Gβγ dimer formation. Two non-fluorescent fragments of Venus fused to Gβ and Gγ, respectively, are brought together by interactions between Gβ and Gγ and produces a yellow fluorescent protein, Venus. (B) Quantitative assessment of Gβγ dimer formation of Gβ1 mutants. HEK293T/17 cells were transiently transfected with D1R, Gαolf, Venus-156-239-Gβ1, Venus-1-155-Gγ7 and effector reporter (masGRK3ct-Nluc), and Venus intensity was measured indicating impaired dimer formation for G64V and A106T. (C) Western blot analysis of protein expression in HEK293T/17 cells. Cell lysates of the transfected cells used to measure Venus intensity in panel B were subjected to Western blotting. Anti-GFP antibody was used to detect Venus 156-239-Gβ1. Western blotting with anti-GAPDH antibody was performed as a loading control. (D) Schematic presentation of the experimental set-up to assess basal BRET levels (before agonist application) to test for trimer formation. Expression of masGRK3ct-Nluc with Venus-Gβγ produces GRK3-bound Gβγ-induced increase of Bioluminescence Resonance Energy Transfer (BRET) between Nluc and Venus. Additional expression of the Gα subunit sequestrates Gβγ from masGRK3ct-Nluc. (E) This additional expression of Gα decreases BRET signal and disruption of αβγ trimer formation increases BRET signal. (F) Basal BRET ratio for the 10 tested missense changes demonstrated decreased BRET signal for G64V, P94S, R96L and A106T as well as an increased basal BRET ratio for A92T. (G) Schematic BRET assay design for monitoring G protein activation. Dopamine stimulation of cells transiently transfected with D1R, Gαolf with BRET sensors results in the dissociation of Gαolf from the heterotrimer. Released Venus-tagged Gβγ subunits become available for interaction with the masGRK3ct-Nluc reporter, producing the BRET signal. (H). Upon agonist-induced stimulation (red arrow), the BRET signal increases. (I) BRET amplitude for the 10 tested missense changes demonstrated decreased BRET signal also for R52G and D118G. (J) Characterization of mutant Gβ1 subunits by BRET assay. Trace lines represent D1R-Golf signaling before (basal BRET) and after agonist application (agonist-induced BRET) to cells transfected with wild-type or mutant Gβ1. Responses to application of dopamine (100 µM) were recorded. Dopamine application is indicated by a red arrow. Expression of all signaling components is required to see an agonist-induced response, indicating the reconstitution of D1R-Golf signaling by exogenous expression of signaling molecules. Data are means of six replicates. Values represent means ± SEM from three independent experiments each performed with six replicates. One-way ANOVA followed by Dunett's post hoc test was conducted with GraphPad Prism Ver. 6 (*P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001).
Second, we investigated the impact of missense changes on the association of β1γ7 with Gαolf using a cell-based BRET assay (Fig. 2D). In this assay, interaction of Venus-tagged Gβ1γ7 dimer with the luciferase-tagged GRK reporter results in a BRET signal. When introduced, Gαolf competes with GRK-based reporter for Venus-Gβ1γ7 binding which lowers the BRET signal indicating productive Gαolf-β1γ7 trimer formation (Fig. 2D and E). We detected lower basal BRET ratios for G64V and A106T, the mutants with disturbed Gβγ dimer formation, and a milder decrease for P94S and R96L (Fig. 2F). The A92T mutant showed higher basal BRET signal, also pointing to deficits in heterotrimer assembly (Fig. 2F).
Third, we examined the mutants for their ability to propagate the signal upon stimulation of D1R using an extended cell-based BRET assay (Fig. 2G) by monitoring increases in agonist-induced BRET. Stimulation of D1R with agonist dissociates the Gαolf-β1γ7 trimer, leading to a rapid increase in BRET signal, which serves as a measure for the functional activity of Gβ1γ7 (Fig. 2G and H). We found that in addition to deficient mutants (G64V, A92T, P94S, R96L, A106T), two other substitutions (R52G and D118G) showed significantly lower response amplitudes, suggesting deficits in receptor-driven G protein activation (Fig. 2I and J). In an additional control experiment, we examined basal BRET signal and Venus intensity of Gβ1 mutants in the absence of Gα subunit (Supplementary Material, Fig. S1). We found correlation of these two measurements, indicating that all Gβ1 mutants maintained association with the GRK3-based reporter proportionately scaling according to their expression level. Thus, we concluded that the deficiency observed in functional analysis cannot be explained by the loss of reporter binding. Of note, we detected normal behavior indistinguishable from wild-type Gβ1 for three of the variants including L30F, H91R and K337Q by all three measures (Fig. 2B, F and I).
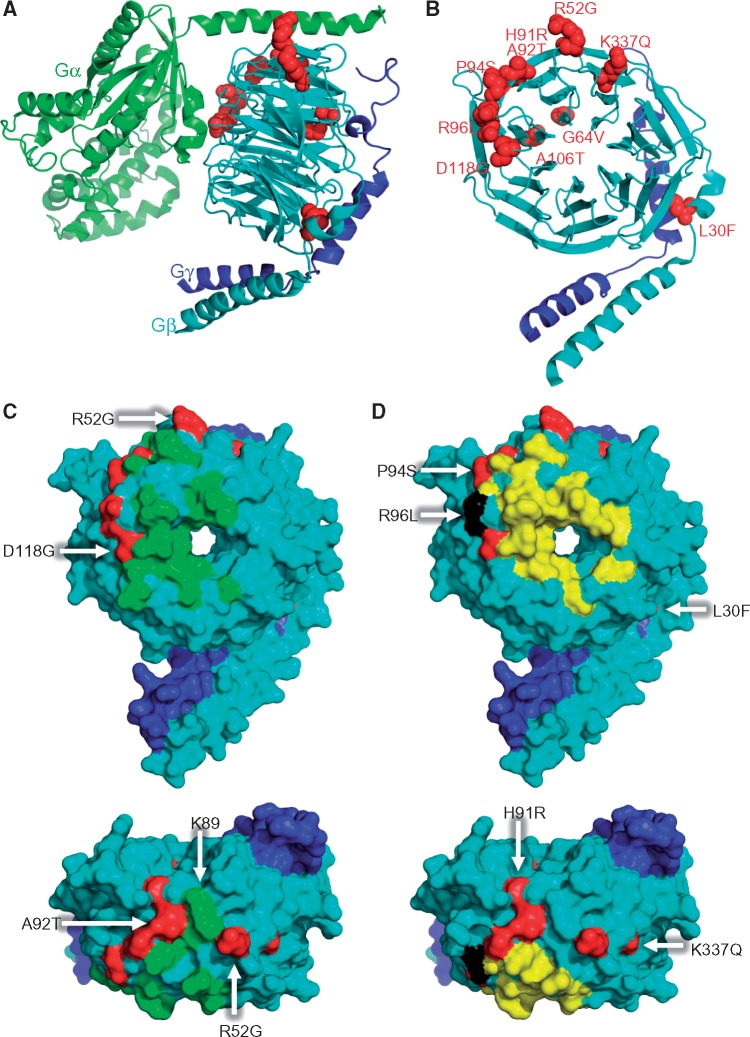
Finally, we investigated the 3D structure of the G protein heterotrimer containing Gβ1 and the location of identified variants (Fig. 3). This revealed that the amino acid residues G64 and A106 are located close to each other within the inner β-propeller structure (Fig. 3B). Furthermore, amino acid residues R52, A92, P94, R96 and D118 are located close to interaction sites with Gα or effector molecules (Fig. 3C and D). In contrast, the likely benign variants L30F, H91R and K337Q are more distantly located from these interaction sides (Fig. 3D).
Figure 3.
Mutant residues of Gβ1 on three-dimensional crystal structures. (A) Cartoon representation of crystal structure of the Gαβγ trimer complex with mutations shown in red spheres. Gα, Gβ and Gγ are shown in green, cyan, and blue, respectively. The published crystal structure of Gαi1/Gβ1γ2 (PDB ID: 1GP2) was chosen as a model of the trimer (16). (B) Mutant residues in Gβ1γ2 dimer found in dystonia patients are highlighted and labeled by red spheres. (C, D) Binding sites on the molecular surface of Gα (C, green) and GRK (D, yellow) and on Gβ1 (cyan), respectively. Mutations are indicated in red. GRK2-Gβ1γ2 complex (PDB ID: 1OMW) (15) and Gαi1β1γ2 (PDB ID: 1GP2) were used to obtain the footprints. The residue colored in black indicates the overlap residue of the GRK binding site and the R96L mutation. The bird´s eye perspectives are shown at the bottom panels.
Clinical findings in patients with pathogenic mutations
Based on the results of the functional studies in the BRET assays, the missense variants R52G, G64V, A92T, P94S, R96L, A106T and D118G lead to a loss of function and thus can be considered as disease-causing (Fig. 1, Table 1). In addition, we interpreted the splice-site and frameshift variants identified in four patients to be pathogenic, as well, because of the predicted complete loss of functional protein. These 11 changes were present in 13 patients who had a mean age at diagnosis of 5.0 ± 2.8 years (range 1–12 years) and all had GDD. Among these, 10 patients (87%) were diagnosed with ID. Seizures were reported in 7 (54%), nystagmus in 6 (46%), muscular hypotonia in 5 (38%) and ophthalmoplegia in 4 (31%) patients. In addition, abnormal myelination, craniosynostosis and cerebellar hypoplasia were observed in 5, 3 and 2 patients, respectively. Ataxia was noted in 4, chorea and dystonia (13) in one patient each (Table 1). Of note, the age at diagnosis and the phenotypic presentation did not vary between patients with functionally proven mutations or with variants of unknown significance, except for the absence of GDD in the patient with the L30F variant.
Discussion
Here, we present clinical findings on 16 carriers of 14 different rare, protein-changing variants in GNB1. Most of these changes occurred de novo and have not been reported previously. GNB1 encodes the G protein subunit Gβ1 which requires forming heterotrimers with Gα and Gγ subunits for function as a transducer of signals from G protein-coupled receptors. It has previously been noted that many of the reported GNB1 mutations are located at the interface of Gβγ and Gα (2) based on the crystal structure of the heterotrimer.
To differentiate between likely pathogenic and benign missense variants (14), we investigated the effects of the mutations on G protein heterotrimer formation and its ability to mediate D1R signaling using live-cell BRET assays. In addition, we modeled the 3D structure of respective protein complexes and evaluated the location of the variants in these assemblies. First, we tested Gβ and Gγ dimer formation by BiFC and demonstrated deficits in Gβγ dimer formation through G64V and A106T mutations. Since the amino acid residues G64 and A106 are buried in the β-propeller structure (Fig. 3A and B), it is likely that introducing larger side chains by the mutations disrupt the structural integrity impeding Gβ1 folding. Indeed, Western blot analysis showed low expression of these mutants (Fig. 2C). Next, we investigated the effects of mutations on the ability of trimeric Golf to propagate D1R signal by cell-based BRET assays. As expected from the observed deficits in protein expression, G64V and A106T mutants showed a low basal BRET ratio and small agonist-induced BRET response (Fig. 2F, I and J). A similar, yet milder decrease in basal BRET ratio was also observed for the P94S and R96L mutants suggesting increased binding to Gαolf (Fig. 2F and J). Although the region encompassing P94 and R96 is known to be involved in interactions with the effector molecules (15) (Fig. 3D, upper panel), we found no deficiency in GRK3 binding (Supplementary Material, Fig. S1). Accordingly, we speculate that these mutations enhance Gβ1γ7’s ability to interact with Gα subunit, lowering basal BRET ratio and agonist-induced BRET response. In contrast, the A92T mutant showed high basal BRET signal (Fig. 2F), suggesting deficits in association with Gαolf. Indeed, the neighboring residue K89 is known to be essential for stabilizing Gαβγ trimer through a tripartite interaction with the N-terminal helix of Gα subunit (16) (Fig. 3C, lower panel). Preservation of GRK binding to A92T Gβ1 suggests a selective role of A92 in discriminating between Gα and effector interactions. Therefore, we speculate that the A92T mutation likely produces constitutively active Gβγ dimer. This is expected to possibly result in a gain-of-function in engaging Gβγ effectors, similarly to what has been reported for several GNB1 mutations linked to diverse human tumors (17). However, elevation of the baseline at rest also diminishes the extent of the receptor mediated signaling and thus the pathological effect of this mutation could still be originating from the loss-of-function as far as transduction of neurotransmitter signals is concerned. Finally, when using agonist-induced BRET, we demonstrated lower response amplitudes for R52G and D118G in addition to the above-mentioned five missense variants with lower basal BRET signal. This may be caused by deficits in agonist-induced G protein activation. Notably, these residues surround the Gα binding interface and thus likely play a role in receptor-induced re-arrangement (Fig. 3C, upper panel). In contrast, the likely benign variants L30F, H91R, and K337Q that did not show any differences in behavior compared with wild-type Gβ1 in the BRET assays, are more distantly located from interaction sides with Gα or effector molecules (Fig. 3D).
Thus, we conclude that only 7 of the 10 missense mutations found in patients in this study are likely pathogenic owing to altered Gβ1 functionality. In addition, the two splice site mutations affecting the conserved last intronic base pair at the acceptor splice site of Introns 6 and 10, respectively, likely lead to a splicing defect and subsequent degradation of the erroneous RNA by nonsense-mediated decay. The two frameshift mutations are also considered to result in a premature stop codon, similarly leading to haploinsufficiency. Of note, we did not observe any obvious correlation between altered behavior of the mutations in the functional assays and the clinical phenotypes. In this context, it is also worth noting that the degree of functional alterations in vitro may not fully reflect the situation in vivo since we tested in our model system functionality of one multimeric complex in response to a single signal molecule and a single effector protein. Along the same lines, although, we did not observe any effect for L30F, H91R and K337Q in the assays that we used, it is still conceivable that these variants may impact Gβ1 function, i.e. reactions that were not tested in our experiments such as interactions with other effector proteins and/or other α and γ subunits, and thus may still be causative for the disease in the respective carriers. However, our structural modeling indicates that these amino acid substitutions are not located at the common protein interaction interface of Gβ1, making this possibility less likely. Further, L30F has been reported in two individuals who are free of severe pediatric disease in ExAC (http://exac.broadinstitute.org/about) also suggesting that it represents a rare but benign variant. In agreement with these considerations, the patient carrying the L30F variant did—unlike all other patients—not present with GDD.
Combining the previously published (2) and the present study, almost 30 carriers of germline mutations in GNB1 have been identified to date. The mutation frequency (<0.5%) is low and consistent in both studies which included >10 000 exomes of individuals with various undiagnosed, likely genetic disorders [5866 in (2) and 4377 in our in-house database]. Thus, it is not unexpected that we did not observe another GNB1 mutation carrier among the about 100 additional patients that we screened for mutations by conventional Sanger sequencing in a candidate-based approach.
Phenotypically, GDD was present in all 26 mutation carriers (including only the functionally confirmed ones from our study). Frequent symptoms that were present in more than half of the reported patients include seizures (17/26), muscular hypotonia (17/26), ophthalmological disorder (17/26) and ID (14/26). Although abnormalities on brain MRI were only reported in 12 of the patients (46%), this frequency is probably an underestimate since not all patients underwent MRI and several were still very young [<1 year (2)] at the time of examination. Growth delay was reported in 9 (35%) and movement disorders including tics, ataxia, dystonia or chorea were noticed in at least 9 patients. This illustrates that the phenotypic spectrum in GNB1 mutation carriers seems to be broad and additional signs and symptoms may be part of the clinical spectrum. For our patients, the clinical information was limited and re-examination of mutation carriers was not possible, except for one patient who was reported in detail previously (13).
Taken together, our study further underlines the importance and power of exome sequencing, especially in genetically highly heterogeneous disorders such as GDD and ID. None of the at least 13 mutation carriers was identified in a candidate-based analysis of about 100 patients with a similar phenotype. Although the detection of a GNB1 mutation in an affected individual currently does not influence treatment choices and no causative therapy is available as yet, it reduces uncertainty in the families by establishing a diagnosis (18) and has a major influence on genetic counseling and family planning since almost all of the mutations occurred de novo.
Materials and Methods
Exome sequencing
Exome sequencing was carried out at Centogene AG (Rostock, Germany) according to standard diagnostic procedures. Prior to exome sequencing, all participants gave written informed consent. In brief, genomic DNA was extracted from whole blood. Exome capture was carried out with Illumina’s Nextera Rapid Capture Exome Kit followed by massively parallel sequencing on a NextSeq500 Sequencer (Illumina, San Diego, CA, USA). Raw sequencing reads were converted to fastq format using bcl2fastq software (Illumina). Using an in-house developed pipeline for exome data analysis, the reads were aligned to the human reference genome (GRCh37, hg19 build) using bwa software with the mem algorithm. Alignments were converted to binary bam file and variant calling was performed using three different variant callers (GATK HaplotypeCaller, freebayes and samtools). Variants were annotated using Annovar and in-house ad hoc bioinformatics tools. All data were fed into an in-house database that was searched for rare (frequency in publically available and the in-house database <0.01) and protein-changing variants in GNB1.
Mutational analysis by Sanger sequencing
The study was approved by the ethics committee at the University of Lübeck (Germany). For mutational analysis of GNB1 as a candidate gene, we Sanger sequenced all 9 coding exons of GNB1 (NM_001282539) in a total of 91 patients. This included 51 unrelated, mainly German patients (21 male, mean age: 14.4 ± 11.1 years, age range 2–50 years) with a combination of epileptic seizures, psychomotor retardation and ID with delayed speech development. These patients had previously been tested negative for mutations in SCN2A (19). Furthermore, we included 40 patients with a clinical presentation of Rett syndrome who were diagnosed from 2002 to 2015 (37 females, mean age: 10.2 ± 4.0 years, range 4–20 years). Mutations in MECP2 were previously excluded in these patients.
cDNA constructs and antibodies
Dopamine D1 receptor and Gαolf in pcDNA3.1+ were purchased from the cDNA Resource Center. Plasmid encoding Venus 156-239-Gβ1 was a gift from N. Lambert (Georgia Regents University) (20). Venus 1–155-Gγ7 encodes amino acids 1–155 of Venus fused to a GGSGGG linker at the N-terminus of human Gγ7 (AF493874). Plasmids encoding Flag-Ric-8B were gifts from B. Malnic (University of São Paulo) (21). PTX-S1 was kindly provided by Eitan Reuveny (Weizmann Institute of Science, Rehovot, Israel) (22). masGRK3ct-Nluc were previously described (23). Venus 156-239-Gβ1 mutants were constructed with site-directed mutagenesis using the QuikChange II® Site-directed Mutagenesis Kit (Agilent Technologies) and Venus 156–239-Gβ1 as a template. All mutations were confirmed in the plasmids by Sanger sequencing. GFP (clones 7.1 and 13.1) and GAPDH (clone 6C5) antibodies were purchased from Roche and Millipore, respectively.
Real-time monitoring of G protein activity by fast kinetic BRET assay
BRET experiments were performed as previously reported with slight modifications (23,24). Briefly, 293T/17 cells were grown in DMEM supplemented with 10% FBS, minimum Eagle's medium non-essential amino acids, 1 mM sodium pyruvate and antibiotics (100 units/ml penicillin and 100 µg/ml streptomycin) at 37 °C in a humidified incubator containing 5% CO2. Cells were transfected with Lipofectamine LTX transfection reagents (12 µl/6-cm dish) and PLUS (7.5 µl/6-cm dish). Dopamine D1 receptor, Gαolf, Venus-156-239-Gβ1, Venus-1-155-Gγ7, Flag-Ric-8B, masGRK3ct-Nluc and PTX-S1 constructs (total 7.5 μg) were used at a 1:6:1:1:1:1:1 ratio (ratio 1 = 0.42 μg of plasmid DNA). Since promiscuous nature of G protein-coupling of GPCRs is reported (23), PTX-S1 were transfected to inhibit the possible coupling of endogenous Gi/o to D1R. BRET and Venus intensity measurements were performed using a microplate reader (POLARstar Omega, BMG Labtech) equipped with two emission photomultiplier tubes. All measurements were performed at room temperature. The BRET signal is determined by calculating the ratio of the light emitted by Venus-Gβ1γ7 (535 nm) over the light emitted by masGRK3ct-Nluc (475 nm). The average baseline value recorded before agonist stimulation was subtracted from BRET signal values, and the resulting difference (ΔBRET ratio) was plotted as traces.
Western blotting
For each sample, about 5 × 106 cells were lysed in 500 µl of sample buffer [125 mM Tris–HCl (pH 6.8), 4 M urea, 4% SDS, 10% 2-mercaptoethanol, 20% glycerol, bromophenol blue (0.16 mg/ml)]. Western blotting analysis of proteins was performed after samples were resolved by SDS–polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Blots were blocked with 5% skim milk in PBS containing 0.1% Tween 20 (PBST) for 30 min at room temperature, which was followed by a 90-min incubation with specific antibodies diluted in PBST containing 1% skim milk. Blots were washed in PBST and incubated for 45 min with a 1:10 000 dilution of secondary antibodies conjugated with horseradish peroxidase in PBST containing 1% skim milk. Proteins were visualized on X-ray film by SuperSignal West Femto substrate (Pierce).
Visualization of 3D models of Gαβγ heterotrimers and effector molecule binding
To investigate the 3D structure of the G protein heterotrimer containing Gβ1 and the location of identified variants, we modeled this complex based on the crystal structure of Gαi1β1γ2 (PDB ID: 1GP2) (16) which is one of only two reported crystal structures of G protein heterotrimers. In addition, footprints of Gα and effector molecule GRK binding sites on the molecular surface of Gβ1 were generated. GRK2-Gβ1γ2 complex (PDB ID: 1OMW) (15) and Gαi1β1γ2 (PDB ID: 1GP2) were used to obtain the footprints. Figures (Fig. 3) were drawn using PyMOL (http://www.pymol.org/).
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
Funding
This study was supported by NIH grants (NS081282, HL105550, to K.M.), the German Ministry of Education and Research (BMBF, DYSTRACT consortium, 01GM1514B, to A.M., C.K. and K.L.), the Ministry of Education, Science and Technological Development, Republic of Serbia (grant no. 173016, 175090 to V.D.). C.K. is a recipient of a career development award from the Hermann and Lilly Schilling Foundation.
Supplementary Material
References
- 1. Vissers L.E., Gilissen C., Veltman J.A. (2016) Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet, 17, 9–18. [DOI] [PubMed] [Google Scholar]
- 2. Petrovski S., Kury S., Myers C.T., Anyane-Yeboa K., Cogne B., Bialer M., Xia F., Hemati P., Riviello J., Mehaffey M., et al. (2016) Germline de novo mutations in GNB1 cause severe neurodevelopmental disability, hypotonia, and seizures. Am. J. Hum. Genet., 98, 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freissmuth M., Casey P.J., Gilman A.G. (1989) G proteins control diverse pathways of transmembrane signaling. FASEB J., 3, 2125–2131. [PubMed] [Google Scholar]
- 4. Lohmann K., Klein C. (2014) Next generation sequencing and the future of genetic diagnosis. Neurotherapeutics, 11, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilissen C., Hoischen A., Brunner H.G., Veltman J.A. (2012) Disease gene identification strategies for exome sequencing. Eur. J. Hum. Genet., 20, 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lohmann K., Uflacker N., Erogullari A., Lohnau T., Winkler S., Dendorfer A., Schneider S.A., Osmanovic A., Svetel M., Ferbert A., et al. (2012) Identification and functional analysis of novel THAP1 mutations. Eur. J. Hum. Genet., 20, 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar K.R., Lohmann K., Masuho I., Miyamoto R., Ferbert A., Lohnau T., Kasten M., Hagenah J., Bruggemann N., Graf J., et al. (2014) Mutations in GNAL: a novel cause of craniocervical dystonia. JAMA Neurol., 71, 490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchs T., Saunders-Pullman R., Masuho I., Luciano M.S., Raymond D., Factor S., Lang A.E., Liang T.W., Trosch R.M., White S., et al. (2013) Mutations in GNAL cause primary torsion dystonia. Nat. Genet., 45, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu C.D., Kerppola T.K. (2003) Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat. Biotechnol., 21, 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwindinger W.F., Mihalcik L.J., Giger K.E., Betz K.S., Stauffer A.M., Linden J., Herve D., Robishaw J.D. (2010) Adenosine A2A receptor signaling and golf assembly show a specific requirement for the gamma7 subtype in the striatum. J. Biol. Chem., 285, 29787–29796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corvol J.C., Muriel M.P., Valjent E., Feger J., Hanoun N., Girault J.A., Hirsch E.C., Herve D. (2004) Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J. Neurosci., 24, 7007–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Girault J.A. (2012) Signaling in striatal neurons: the phosphoproteins of reward, addiction, and dyskinesia. Prog. Mol. Biol. Transl. Sci., 106, 33–62. [DOI] [PubMed] [Google Scholar]
- 13. Steinrucke S., Lohmann K., Domingo A., Rolfs A., Baumer T., Spiegler J., Hartmann C., Munchau A. (2016) Novel GNB1 missense mutation in a patient with generalized dystonia, hypotonia, and intellectual disability. Neurol. Genet., 2, e106.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med., 17, 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lodowski D.T., Pitcher J.A., Capel W.D., Lefkowitz R.J., Tesmer J.J. (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science, 300, 1256–1262. [DOI] [PubMed] [Google Scholar]
- 16. Wall M.A., Coleman D.E., Lee E., Iniguez-Lluhi J.A., Posner B.A., Gilman A.G., Sprang S.R. (1995) The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell, 83, 1047–1058. [DOI] [PubMed] [Google Scholar]
- 17. Yoda A., Adelmant G., Tamburini J., Chapuy B., Shindoh N., Yoda Y., Weigert O., Kopp N., Wu S.C., Kim S.S., et al. (2015) Mutations in G protein beta subunits promote transformation and kinase inhibitor resistance. Nat. Med., 21, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lingen M., Albers L., Borchers M., Haass S., Gartner J., Schroder S., Goldbeck L., von Kries R., Brockmann K., Zirn B. (2015) Obtaining a genetic diagnosis in a child with disability: impact on parental quality of life. Clin. Genet., 89, 258–266. [DOI] [PubMed] [Google Scholar]
- 19. Baasch A.L., Huning I., Gilissen C., Klepper J., Veltman J.A., Gillessen-Kaesbach G., Hoischen A., Lohmann K. (2014) Exome sequencing identifies a de novo SCN2A mutation in a patient with intractable seizures, severe intellectual disability, optic atrophy, muscular hypotonia, and brain abnormalities. Epilepsia, 55, e25–e29. [DOI] [PubMed] [Google Scholar]
- 20. Hollins B., Kuravi S., Digby G.J., Lambert N.A. (2009) The c-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell. Signal, 21, 1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Von Dannecker L.E., Mercadante A.F., Malnic B. (2006) Ric-8B promotes functional expression of odorant receptors. Proc. Natl. Acad. Sci. U. S. A., 103, 9310–9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raveh A., Cooper A., Guy-David L., Reuveny E. (2010) Nonenzymatic rapid control of GIRK channel function by a G protein-coupled receptor kinase. Cell, 143, 750–760. [DOI] [PubMed] [Google Scholar]
- 23. Masuho I., Ostrovskaya O., Kramer G.M., Jones C.D., Xie K., Martemyanov K.A. (2015) Distinct profiles of functional discrimination among G proteins determine the actions of G protein-coupled receptors. Sci. Signal, 8, ra123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Masuho I., Martemyanov K.A., Lambert N.A. (2015) Monitoring G protein activation in cells with BRET. Methods Mol. Biol, 1335, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.