Summary
We investigated breast carcinogenesis in rodent models of heritable fitness in the absence of induced physical activity and observed that cancer incidence is significantly lower in rats with high inherent aerobic capacity.
Abstract
Although regular physical activity is associated with improvement in aerobic capacity and lower breast cancer risk, there are heritable sets of traits that affect improvement in aerobic capacity in response to physical activity. Although aerobic capacity segregates risk for a number of chronic diseases, the effect of the heritable component on cancer risk has not been evaluated. Therefore, we investigated breast carcinogenesis in rodent models of heritable fitness in the absence of induced physical activity. Female offspring of N:NIH rats selectively bred for low (LIAC) or high (HIAC) inherent aerobic capacity were injected intraperitoneally with 1-methyl-1-nitrosurea (70 mg/kg body wt). At study termination 33 weeks post-carcinogen, cancer incidence (14.0 versus 47.3%; P < 0.001) and multiplicity (0.18 versus 0.85 cancers per rat; P < 0.0001) were significantly decreased in HIAC versus LIAC rats, respectively. HIAC had smaller visceral and subcutaneous body fat depots than LIAC and activity of two proteins that regulated the mammalian target of rapamycin, protein kinase B (Akt), and adenosine monophosphate-activated protein kinase were suppressed and activated, respectively, in HIAC. Although many factors distinguish between HIAC and LIAC, it appears that the protective effect of HIAC against breast carcinogenesis is mediated, at least in part, via alterations in core metabolic signaling pathways deregulated in the majority of human breast cancers.
Introduction
Lifestyle behaviors play an important role in the etiology of breast cancer (1). A wealth of observational data indicates that self-reported regular physical activity is associated with, on average, a 25% risk reduction in premenopausal as well as postmenopausal breast cancer incidence compared with inactive women even after controlling for important confounding variables (2,3). There is also evidence of a ‘dose–response’ relationship. Specifically, 2–3 h/week of moderately intense physical activity is associated with a 9% reduction in risk, whereas >6 h/week (of moderately intense physical activity) is associated with a 30% reduction in risk (3,4). On the basis of available data, national agencies recommend that all adult women participate in at least 150 min of moderately intense or 75 min of vigorously intense physical activity per week or a combination of these (5).
Self-reported assessment of physical activity has recognized limitations (6). In response, other assessment tools of physical activity exposure, such as exercise tolerance testing, have been developed and aerobic capacity, also referred to as cardiorespiratory fitness, has been identified as an objective measure of physical activity exposure. Aerobic capacity is an established independent predictor of cardiovascular and all-cause mortality in adults with and without cardiovascular disease (7). In contrast to the setting of cardiovascular disease, the clinical importance of aerobic capacity for the prediction of breast cancer risk has received scant attention. In a prospective cohort study of 14,811 women participating in the Cooper Center Longitudinal Study, aerobic capacity was associated with a reduced risk of dying from breast cancer in women (8). Moreover, in a review of 27 clinical trials and observational studies measuring aerobic capacity in the pre- and post-adjuvant treatment setting for breast cancer, aerobic capacity was substantially lower in women with a history of breast cancer compared with healthy women and this was most pronounced among breast cancer patients in the post-adjuvant setting (9). Although neither study assessed the relationship between aerobic capacity and incident breast cancer, it has been assessed in other cancer sites where higher aerobic capacity, assessed at mid-life, was associated with a 68 and 38% reduction in the risk of lung and colorectal cancer, respectively, compared with the lowest aerobic capacity quintile in 17,049 men (10).
For the work reported herein, the tenet that aerobic capacity is directly related to physical activity exposure was juxtaposed with a body of evidence showing that among populations of individuals undergoing the same exercise training protocol that marked differences exist in response relative to the improvement of aerobic capacity (11,12). These differential responses have been shown to have a genetic component with heritability accounting for 40–49% of the differences in improvement in aerobic capacity to the same exercise training protocol. Whether higher intrinsic aerobic capacity confers protection against the development of breast cancer has not been evaluated. Here, we investigated this question by examining the direct effect of inherited, as opposed to physical activity-induced, differences in aerobic capacity, using an established breeding program of rodents intentionally selected on the basis of their inherent ability to run on a treadmill (13–15). We tested the effects of low (LIAC) versus high (HIAC) inherent aerobic capacity (IAC): (i) on breast cancer incidence, multiplicity and latency and (ii) on the level of circulating growth factors and hormones and activity of proteins in mammary gland that interface with core signaling pathways frequently deregulated during the development of breast cancer.
Materials and methods
Study design
Breeding pairs
Sixteen breeding pairs of HIACs and LIACs from generation 29 of selection (N = 8 per cohort) were obtained from the Department of Anesthesiology at the University of Michigan directed by SLB and LGK (16). All animals were subjected to a maximal treadmill exercise test to determine aerobic capacity, as published previously (17). The mean distance and duration for HIACs and LIACs were 2106 ± 273 m (74.6 min) and 260 ± 60 m (18.3 min), respectively.
Experimental procedures
Female pups weaned from dams at 21 days of age were assigned into two groups: one with offspring from LIAC breeding pairs and the other with offspring from HIAC breeding pairs. Rats remained sedentary for the entire study, i.e. they had normal cage activity within the group housing setting (three rats per cage). Animals were maintained in solid bottomed polycarbonate cages and fed a standard laboratory diet (Harlan 2918 Teklad Lab Animal Diet) ad libitum. Rooms were maintained at 22 ± 1°C with 50% relative humidity and a 12-h light/12-h dark cycle. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee and conducted according to the committee guidelines at Colorado State University.
Carcinogen administration and tumor detection
At 21 days of age, LIACs (N = 55) and HIACs (N = 57) were injected intraperitoneally with 1-methyl-1-nitrosurea (70 mg/kg, Ash Stevens, Detroit, MI), as described previously (18). Animals were weighed weekly and palpated for mammary tumors twice weekly starting from 3 weeks post-carcinogen. The study was terminated 33 weeks post-carcinogen injection, following a period of 6 weeks during which no new mammary tumors were detected by palpation.
Necropsy
Following an overnight fast, all animals were euthanized between 8 a.m. and 11 a.m. via inhalation of gaseous carbon dioxide. Blood samples were drawn via retro-orbital sinus into ethylenediaminetetraacetic acid-coated tubes (Becton Dickinson, Franklin Lakes, NJ) for plasma. Rats were then skinned and the skin to which mammary gland chains were attached was examined under translucent light for detectable mammary pathologies at ×5 magnification. All detectable mammary gland pathologies were excised and a cross section prepared for histological classification according to published criteria (19). After excision of detectable pathologies, the abdominal–inguinal mammary gland chains were excised, one side snap frozen in liquid nitrogen and the other side processed as a whole mount preparation per our published method (19).
Plasma biomarkers
Plasma was isolated by centrifugation of blood obtained at necropsy at 1000g for 10 min at room temperature and then stored at −80°C until it was analyzed. Plasma was subjected to the assessment of the following molecules: insulin-like growth factor-1 (IGF-1), corticosterone, insulin and leptin using commercially available enzyme-linked immunosorbent assays (Diagnostic Systems Laboratory, Webster, TX; Cayman Chemicals, Ann Arbor, MI; and Millipore, Billerica, MA). Glucose was determined enzymatically using a commercially available kit (Pointe Scientific, Canton, MI.).
Digital analysis of mammary gland and fat pad
Mammary epithelial area and the area of the subcutaneous fat pad in which the mammary gland is embedded were determined using our previously published method for rat mammary gland (20). Briefly, digital images of the mammary gland whole mounts (prepared as described above) were captured using a semi-automated image acquisition system. A series of Z-stack images were automatically captured using a motorized stage creating a seamlessly merged single uniformly focused composite image of each mammary gland. Resulting images were analyzed using Image-Pro® plus 4.5 (Mediacybernetics, Silver Spring, MD) image analysis software to quantify mammary gland epithelial area.
Western blot-based immune nanocapillary electrophoresis
Frozen tissues were removed from −80°C storage and quickly ground to a fine powder in liquid nitrogen using ceramic mortars and pestles. Lysis buffer was prepared using ice-cold T-PER tissue protein extraction reagent (Thermo Fisher Scientific, Waltham, MA) with Halt protease and phosphatase inhibitor cocktail at 1:50 (Thermo Fisher Scientific), and 0.5M ethylenediaminetetraacetic acid at 1:100 to inhibit metalloproteases. Ice-cold 7.5 ml glass Dounce homogenizers (VWR, Radnor, PA) were filled with 1.5 ml of lysis buffer and tared on the scale. Frozen tissue powder was quickly weighed in the Dounce and then homogenized on ice using 10 full strokes of the glass pestle followed by vortexing for 5 s and sonication (Branson Sonifier S-250A, Fisher Scientific), using 1 pulse (10% duty cycle and control set at 5). The homogenization process was repeated four times and samples were allowed to sit on ice for an additional 10 min prior to transfer to 2.0 ml microfuge tubes and centrifugation at 12,000g for 20 min. A glass Pasteur pipet was used to extract the supernatant sandwiched between the cell pellet and surface lipid layer. The lysate supernatant was divided into 25 µl single use aliquots using 0.2 ml PCR tubes and stored at −80°C. Protein concentration was determined using the Bradford assay. Nano capillary electrophoresis was performed using the WES instrument and proprietary kits (ProteinSimple, San Jose, CA). The kits consisted of microplates with a prefilled section containing proprietary reagents, cartridge with 25 nano capillaries, lyophilized standard pack (DTT, biotinylated ladder and fluorescent standards), 10× sample buffer, secondary antibody, horseradish peroxidase-conjugated streptavidin (ladder only), luminol-S, peroxide, antibody diluent and wash buffer. Briefly, samples were prepared by adding 2.5 µl of diluted tissue lysate to a 0.2 ml PCR tube containing 1.5 µl of 5× fluorescent master mix and 3.5 µl of 0.1× sample buffer. Samples were denatured in a dry bath at 95°C for 5 min. Samples, biotinylated ladder, multiplexed primary antibodies, horseradish peroxidase-conjugated secondary antibody, chemiluminescent substrate (luminol-S peroxide) and wash buffer were pipetted into the appropriate wells according to kit instructions.
The capillary cartridge and microplate were loaded into the fully automated WES instrument. The entire assay was completed within each capillary as follows: the vacuum manifold loaded each capillary with a separation matrix, stacking matrix and sample; a voltage of 375 V was applied for 30 min to separate the proteins based on size followed by exposure to UV light in order to immobilize the proteins in the capillary prior to immuno-labeling and subsequent detection resulting in the chemiluminescent signal intensity of each target protein displayed as an electropherogram.
The electropherogram for each antibody used in this study, determined on a pooled sample of 10 mammary glands as part of our quality control protocol for antibody selection, is shown along with the vendor and catalog number for each antibody (Supplementary Figure 1 is available at Carcinogenesis Online).
Statistical analyses
The carcinogenesis experiment had 80% power to detect an effect size of 0.5 in incident cancer between the LIAC and HIAC groups based on an incidence of 50% in the N:NIH founder population determined in a preliminary experiment (data not shown). Incidence of mammary carcinomas in LIAC versus HIAC was compared using the Fischer exact test, whereas the between-group analysis of the number of mammary carcinomas per rat (multiplicity) was by Poisson regression (21). Cancer latency was evaluated by survival analysis using the Mantel–Haenszel method. Plasma biomarkers and protein expression data were evaluated by analysis of variance and multivariate techniques per our previously published approach (22).
Results
Carcinogenic response
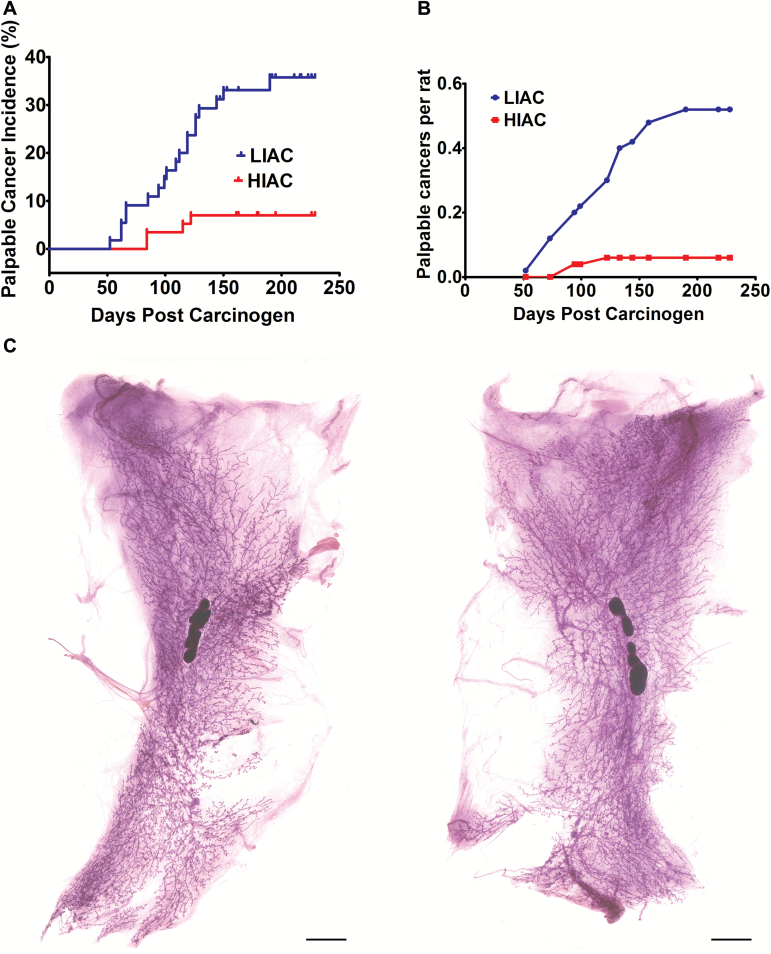
The occurrence of the first palpable carcinoma in a rat over time, i.e. cancer latency, was prolonged in HIAC versus LIAC [Figure 1A; Mantel hazard ratio = 4.01; 2.02–7.93, 95% confidence interval (95% CI); P = 4 × 10-4]. In order to assess whether tumor occurrence was simply delayed in HIAC versus LIAC, the experiment was allowed to continue for a long duration (33 weeks post-carcinogen injection). Inspection of the time course of tumor emergence (Figure 1A and B) showed that no additional palpable tumors were detected after 17 weeks post-carcinogen in HIAC, whereas tumors continued to emerge in LIAC until 27 weeks post-carcinogen. At the end of the study, the incidence of mammary cancer detectable by palpation was 7.0% in HIAC compared with 34.5% in LIAC (P = 3.5 × 10-4). Cancer multiplicity was 0.1 per animal (0.0–0.2, 95% CI) in HIAC compared with 0.5 per animal (0.3–0.8, 95% CI) in LIAC (P = 3.0 × 10-4) (Table 1). Moreover, of the eight tumor-bearing HIAC rats, only one animal had more than one carcinoma (three tumors), whereas 12 of the 26 LIAC rats had multiple tumors, ranging between two to five carcinomas per rat. Thus, even in animals that developed cancer, HIAC was associated with resistance to disease occurrence.
Figure 1.
Effect of inherent aerobic capacity on the carcinogenic response. The total number of observation days from time of carcinogen injection to the day an animal was euthanized was essentially the same for both groups (10,892 and 10,829 days, respectively, for HIAC and LIAC). (A) The incidence of palpable mammary cancer as determined by survival analysis over the 290-day interval post-carcinogen administration. The rate of new tumor occurrence was 4-fold higher in rats with low inherent running capacity versus those rats with high inherent running capacity. Mantel hazard ratio = 4.01; 2.02–7.93, 95% CI; P = 5 × 10-4. (B) The average number of palpable mammary cancers as a function of time post-carcinogen administration. (C) Mammary gland whole mounts prepared at necropsy. Mammary epithelial area determined via digital analysis of mammary gland whole mounts obtained at necropsy revealed that no statistically significant difference existed between LIAC and HIAC (0.829 ± 0.049 cm2 versus 0.791 cm2 ± 0.039, respectively; mean ± SEM; P = 0.582). Bars = 0.5 cm.
Table 1.
Effect of inherent aerobic capacity on the tumorigenic response in the mammary gland
| LIAC (n = 55) | HIAC (n = 57) | P-value | ||
|---|---|---|---|---|
| Mammary carcinoma, palpable | Incidence, % | 34.5 (19)a | 7.0 (4) | 3.5 × 10-4 |
| Number per rat | 0.5 (29)b (0.3–0.8)c |
0.1 (5) (0.0–0.2) |
3.0 × 10-4 | |
| Mammary carcinoma, microcarcinoma | Incidence, % | 25.5 (14)a | 8.8 (5) | 2.4 × 10-2 |
| Number per rat | 0.3 (18)b (0.16–0.49)c |
0.1 (5) (0.01–0.16) |
1.5 × 10-2 | |
| Mammary carcinoma, total | Incidence, % | 47.3 (26)a | 14.0 (8) | 1.8 × 10-4 |
| Number per rat | 0.85 (47)b (0.54–1.16)c |
0.18 (10) (0.04–0.31) |
7.0 × 10-5 | |
| Benign mammary pathologies | Incidence, % | 20.0 (11)a | 8.8 (5) | 1.1 × 10-1 |
| Number per rat | 0.24 (13)b (0.09–0.37)c |
0.11 (6) (0.01–0.16) |
9.3 × 10-2 | |
| Mammary pathologies, total, malignant and benign | Incidence, % | 72.7 (32)a | 27.3 (12) | 9.0 × 10-5 |
| Number per rat | 1.1 (60)b (0.8–1.4)c |
0.3 (16) (0.1–0.5) |
2.0 × 10-5 |
aNumber in parentheses is the number of animals bearing this pathology in the group.
bAverage number per rat; number in parentheses is the total number of pathologies of this type detected in the group.
cNumbers in parentheses are the 95% CIs about the mean.
Given that no new palpable tumors occurred in either group after 27 weeks until the end of the study, these findings support the possibility that HIAC provided absolute protection against cancer. To further examine this issue, data were also obtained on the occurrence of occult disease, i.e. microcarcinoma weighing <100 mg, which were detected via inspection of the mammary glands using 5× magnification at necropsy. The incidence and number of microcarcinomas was significantly lower in HIAC versus LIAC (8.8 versus 25.5%, P = 2.4 × 10-2 and 0.1 versus 0.3 cancers per rat, P = 1.5 × 10-2, respectively, HIAC versus LIAC). When the data on occurrence of microcarcinoma were combined with the incidence and multiplicity of carcinomas that were palpable, protection against the occurrence of cancer in HIAC rats was robust (14.0 versus 47.3%, P = 1.8 × 10-5 and 0.18 versus 0.85 cancers per rat, P = 7.0 × 10-5, respectively, HIAC versus LIAC).
Physiological parameters and the carcinogenic response
To determine if the amount of mammary epithelium at risk for the development of cancer was similar in both groups, mammary epithelial area was determined via digital analysis of mammary gland whole mounts in LIAC and HIAC rats at 21 days of age, i.e. the age at which carcinogen was administered (Figure 1C). No statistically significant difference existed between LIAC and HIAC (0.829 ± 0.049 cm2 versus 0.791 cm2 ± 0.039, mean ± SEM, respectively, P = 0.6).
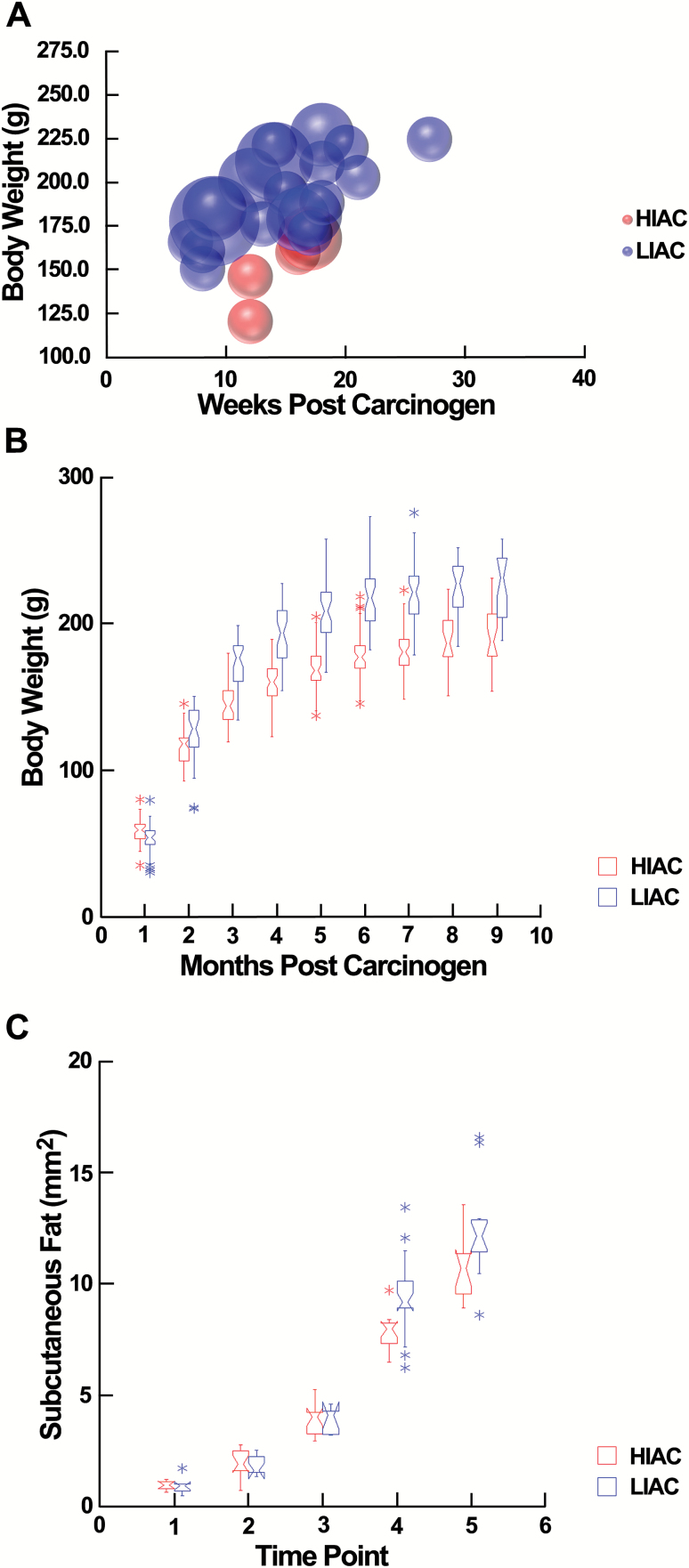
We examined potential contributions of body weight and differences in adiposity to the observed differences in the carcinogenic response. Consistent with other reports (23), final body weights of LIAC rats were 15% higher than HIAC rats (217 ± 4.9 versus 183 ± 4.1, mean ± SEM, P = 1 × 10-2); however, inspection of the time points at which tumors were detected by palpation (subsequently confirmed to be mammary carcinomas histologically) with body weight (Figure 2A) was consistent with statistical evidence that body weight was not a significant covariate in modeling effects of IAC on cancer incidence or multiplicity. Recognizing that there was considerable overlap in body weights of LIAC and HIAC throughout the study (Figure 2B), we also explored the potential influence of different growth rates by limiting the analysis of the carcinogenic response to animals with a final body weight between 185 and 225 g. This resulted in two groups of rats (N = 30 per group) that differed in final body weight by 5%. As shown in Table 2, no palpable tumors were detected in HIAC and the difference in the carcinogenic response between HIAC and LIAC was numerically greater than reported in Table 1 (cancer incidence: 3.3 versus 50.0%, HIAC versus LIAC, P < 6.0 × 10-4). We also measured the size of the subcutaneous fat pad in which the mammary epithelium is embedded and observed that the difference between LIAC and HIAC diverged over time primarily during the time interval beyond the emergence of the majority of tumors in either group (Figure 2C). At the end of the study, visceral and subcutaneous fat pads were larger in LIAC versus HIAC (69%, P = 0.001 and 16%, P = 0.02, respectively; Supplementary_Table_1, available at Carcinogenesis Online).
Figure 2.
Effect of inherent aerobic capacity on body weight and fat depots. (A) The body weight at which an animal was first detected bearing a palpable tumor and the week post-carcinogen that tumor was detected. The size of each bubble corresponds to the number of tumors the animal was confirmed to bear. (B) Box plots of body weight over the duration of the carcinogenesis experiment showing the extent of overlap in body weights between groups. (C) Box plots showing how the subcutaneous abdominal–inguinal fat pad increased in size over time. Age blocks: 1, 3 weeks of age (WOA); 2, 4 WOA; 3, 5 WOA; 4, 10 WOA and 5, 33 WOA. The size of the fat pad increased at a more rapid rate in LIAC versus HIAC, P = 0.001.
Table 2.
Effect of inherent aerobic capacity on the tumorigenic response in the mammary gland: subgroup analysis
| LIAC (n = 30) | HIAC (n = 30) | P-value | ||
|---|---|---|---|---|
| Mammary carcinoma, palpable | Incidence, % | 33.3 (10)a | 0.0 (0) | 5.3 × 10=4 |
| Number per rat | 0.4 (12)b (0.2–0.6)c |
0.0 (0) | 3 × 10-4 | |
| Mammary carcinoma, microcarcinoma | Incidence, % | 30.0 (9)a | 3.3 (1) | 5.6 × 10-3 |
| Number per rat | 0.3 (11)b (0.14–0.60) c |
0.03 (1) (−0.03–0.10) |
5.7 × 10-3 | |
| Mammary carcinoma, total | Incidence, % | 50.0 (15)a | 3.3 (1) | 4.0 × 10-5 |
| Number per rat | 0.77 (23)b (0.42–1.12)c |
0.03 (1) (−0.03–0.10) |
7.0 × 10-5 | |
| Final body weight | g | 205 ± 2.5d | 195 ± 2.4 | 5.0 × 10-3 |
aNumber in parentheses is the number of animals bearing this pathology of this type detected in the group.
bAverage number per rat; number in parentheses is the total number of pathologies of this type detected in the group.
cNumbers in parentheses are the 95% CIs about the mean.
dValues are means ± SEM.
The heritability of the difference in aerobic capacity in this model system and running behavior between generations is high and has been reported (24). Nonetheless, since the carcinogenesis study was performed using the offspring of rats phenotyped for maximal running capacity on a treadmill, we took nine HIAC and nine LIAC female rats at the mid-point of the carcinogenesis experiment that had not been injected with carcinogen and provided them access to our computer controlled non-motorized running wheels (25). This was a situation to which the rats had not previously been exposed. For four consecutive days, each rat was given access to its own running wheel during the dark phase of the light/dark cycle, which is the component of the photoperiod during which rats are most active. The average distance run was four times greater in HIAC than LIAC (4.1 ± 0.1 km, HIAC versus 0.9 ± 0.04 km, LIAC; mean ± SEM; P < 1 × 10-3). This demonstrates how differences in IAC translate into voluntary wheel running behavior.
Cell signaling pathways and circulating growth factors and hormones
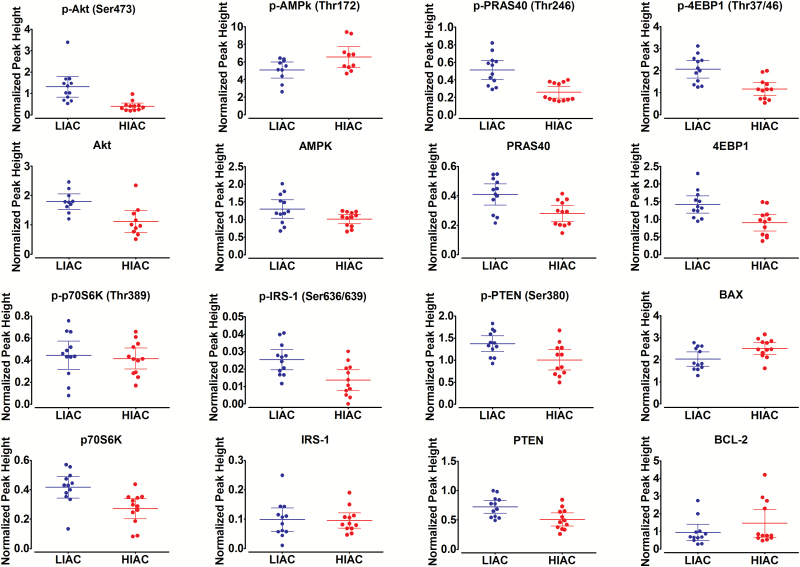
Mammalian target of rapamycin signaling network. Mammalian target of rapamycin (mTOR) is a master sensor of changes in nutrient and energy status. Initially, two key regulatory nodes for mTOR were investigated, protein kinase B (Akt), the activation of which induces mTOR activity and adenosine monophosphate-activated protein kinase (AMPK), which is a negative regulator of mTOR (Table 3 and Figure 3). Protection against cancer in HIAC rats was associated with a significant reduction in the level of phospho-Akt (Ser473) and an increased level of phospho-AMPK (Thr172). To evaluate whether the shifts in phosphorylation of Akt and AMPK had functional significance, we probed downstream targets involved in the regulation of mTOR kinase activity.
Table 3.
Protein expression in mammary gland
| Protein | LIACa (n = 12) | HIAC (n = 12) | P-value |
|---|---|---|---|
| p-Akt (Ser473) | 1.31 ± 0.22 | 0.41 ± 0.07 | 0.001 |
| Akt | 2.22 ± 0.26 | 1.23 ± 0.16 | 0.004 |
| p-Akt (Ser473)/Akt Ratio | 0.65 ± 0.11 | 0.36 ± 0.05 | 0.025 |
| p-AMPK (Thr172) | 5.08 ± 0.41 | 6.55 ± 0.53 | 0.040 |
| AMPK | 1.30 ± 0.12 | 1.01 ± 0.06 | 0.043 |
| p-AMPK (Thr172)/AMPK Ratio | 4.04 ± 0.54 | 6.44 ± 0.47 | 0.003 |
| p-PRAS40 (Thr246) | 0.51 ± 0.05 | 0.26 ± 0.03 | 0.001 |
| PRAS40 | 0.41 ± 0.03 | 0.28 ± 0.02 | 0.005 |
| p-PRAS40 (Thr246)/ PRAS40/ Ratio | 1.26 ± 0.07 | 0.92 ± 0.06 | 0.001 |
| p-4EBP1 (Thr37/46) | 2.06 ± 0.18 | 1.16 ± 0.14 | 0.001 |
| 4EBP1 | 1.42 ± 0.11 | 0.91 ± 0.11 | 0.003 |
| p-4EBP1 (Thr37/46)/4EBP1 Ratio | 1.47 ± 0.09 | 1.32 ± 0.07 | 0.204 |
| p-p70S6K (Thr389) | 0.44 ± 0.06 | 0.41 ± 0.04 | 0.676 |
| p70S6K | 0.42 ± 0.03 | 0.27 ± 0.03 | 0.004 |
| p-p70S6K (Thr389)/ p70S6K Ratio | 1.23 ± 0.23 | 1.70 ± 0.20 | 0.143 |
| p-IRS-1 (Ser636/639) | 0.03 ± 0.003 | 0.01 ± 0.003 | 0.005 |
| IRS-1 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.884 |
| p-IRS-1 (Ser636/639)/IRS-1 Ratio | 0.40 ± 0.12 | 0.14 ± 0.02 | 0.037 |
| p-PTEN (Ser380) | 1.37 ± 0.08 | 1.00 ± 0.10 | 0.011 |
| PTEN | 0.72 ± 0.05 | 0.51 ± 0.05 | 0.007 |
| p-PTEN (Ser380)/PTEN Ratio | 1.92 ± 0.04 | 1.97 ± 0.05 | 0.387 |
| BAX | 2.03 ± 0.15 | 2.51 ± 0.12 | 0.021 |
| BCL2 | 0.93 ± 0.21 | 1.46 ± 0.36 | 0.222 |
IRS-1, insulin receptor substrate-1.
aValues are peak heights normalized to a loading control, mean ± SEM.
Figure 3.
Analysis of protein expression in the mammary gland. Effects of HIAC or LIAC running on patterns of protein expression in the mammary gland. Normalized peak height determined via nano-capillary immuno-electrophoresis as implemented using WES system (Protein Simple). Dot density plots showing means and 95% CIs. Table 3 contains exact P-values for difference between LIAC and HIAC.
Proline-rich Akt substrate 40 (PRAS40) is a target of activated Akt as well as of activated mTOR (26). We focused on phospho-PRAS40 (Thr246), which is the site phosphorylated by Akt and observed it to be downregulated in HIAC breast tissue. Moreover, there was a strong linear relationship between levels of pAkt (Ser473) and pPRAS40 (Thr246) (r2 = 0.77; P < 1.0 × 10-3), thus, supporting the view that the downregulation of Akt activation in mammary gland associated with HIAC had regulatory significance. We also probed regulatory-associated protein of mTOR, a negative regulator of mTOR activity (27), as well as acetyl COA carboxylase, which is a rate limiting step in lipid synthesis. Activated AMPK negatively regulates both proteins; however, the observed numerical increases in phosphorylation of these targets predicted by AMPK activation in HIAC did not reach the level of statistical significance.
Given differences in PRAS40 phosphorylation distinguishing between HIAC and LIAC, we next investigated 4-EBP1 and p70S6 kinase, downstream targets of mTOR frequently upregulated in breast cancer. The 4-EBP1 encodes one member of a family of translation repressor proteins. The protein directly interacts with eukaryotic translation initiation factor 4E, which is a limiting component of the multi-subunit complex that recruits 40S ribosomal subunits to the 5′ end of mRNAs. Interaction of this protein with eIF4E inhibits complex assembly and represses translation. When this protein is phosphorylated, it dissociates from eIF4E and results in activation of cap-dependent mRNA translation (28). Both total protein and phospho-4EBP1 (Thr37/46) (but not there ratio) were lower in HIAC, consistent with suppression of mTOR activity. Ribosomal protein S6 kinase, also known as p70S6 kinase, is a serine/threonine kinase whose target substrate is the S6 ribosomal protein. Phosphorylation of S6 induces protein synthesis at the ribosome. The phosphorylation of p70S6K at threonine 389 has been used as a hallmark of activation by mTOR (29). Unlike the finding with 4EPB1, differences in phospho-p70S6K (Thr389) were not observed, although the total amount of p70S6K was reduced in HIAC versus LIAC. The contrasting pattern of expression of 4EBP1 and p70S6K indicate complexity in the regulation of mTOR network signaling that extends beyond the canonical pathways usually considered.
Since the mTOR-signaling cascade regulates cell proliferation and apoptosis, we next investigated effects on G1/S transition of the cell cycle (cyclin D1 and p27) as well as proteins involved in the induction of apoptosis via the intrinsic mechanism. The only difference that was detected was an increase in BAX protein in HIAC; BAX is considered a pro-apoptotic factor. This was observed in the absence of significant effects on antiapoptotic protein BCL2 or the inhibitor of apoptosis protein, XIAP. These data, though limited in scope, are consistent with a proapoptotic environment existing in breast tissue of animals with HIAC and point to the value of understanding how IAC regulates cell death pathways in future work.
PI3K–Akt regulation. Although the activation of AMPK in HIAC is consistent with the small but significant increase in concentration of circulating adiponectin in HIAC (Supplementary_Table_2, available at Carcinogenesis Online), the lack of difference in insulin or IGF-1 in plasma of HIAC versus LIAC rats indicated that cell autonomous processes were operative. Therefore, the regulation of the PI3K–Akt pathway was examined in greater detail. We reasoned that Akt activation could still be influenced by circulating levels of insulin and IGF-1 if cell surface receptors were increased. As an initial test of this idea, we measured the concentration of IGF receptor-1, a tyrosine kinase receptor that has been implicated in breast cancer. We also measured the level of insulin receptor substrate-1, which is a signaling adapter protein transmitting signals from the insulin and IGF-1 receptors to the PI3K–Akt pathway. However, neither the amount of IGF receptor-1 nor insulin receptor substrate-1 differed in the mammary gland of LIAC and HIAC (Supplementary Figure 2 and Table 3, available at Carcinogenesis Online). We also probed phospho-insulin receptor substrate (Ser636/639), which was found to be higher in LIAC (P = 0.005). This is consistent with activation of PI3K signaling (30). We also probed the levels of phosphatase and tensin homolog (PTEN), a protein that acts as a tumor suppressor and that is mutated with a high frequency in breast cancer. PTEN is a dual specificity protein tyrosine phosphatase that preferentially dephosphorylates phosphoinositide substrates. It negatively regulates intracellular levels of phosphatidylinositol-3,4,5-trisphosphate, thereby suppressing signaling mediated by Akt (31). PTEN and phosho-PTEN (Ser380) were significantly higher in LIAC versus HIAC. The phosphorylation of this site has been reported to reduce PTEN activity, which would be consistent with higher levels of phosho-Akt observed in LIAC mammary gland, although the ratio of phospho to total PTEN did not differ. Taken collectively, the data on mTOR network activity are consistent with cell autonomous regulation of PI3K–Akt signaling in accounting for the difference in cancer susceptibility between LIAC and HIAC.
Other pathway analyses
Since differences in body fat depots were noted (Supplementary_Table_1, available at Carcinogenesis Online), circulating leptin was measured in a subgroup of rats (Supplementary_Table_2, available at Carcinogenesis Online). Plasma leptin was 5-fold higher in LIAC, and thus, we reasoned that higher circulating leptin would be associated with activation of the JAK2/STAT3 pathway in the mammary gland, an observation that would be consistent with increased cancer susceptibility in LIAC, but no significant differences were found (Supplementary Figure 2 and Table 3, available at Carcinogenesis Online). Because the HIAC phenotype has been recently compared with the effects of caloric restriction (32), we also measured plasma corticosterone, which is elevated by caloric restriction. Plasma corticosterone was 3-fold higher in HIAC than LIAC (Table 3), and thus, we reasoned that stress-related signaling, presumably mediated via the binding of corticosterone to the glucocorticoid receptor, would be higher with higher plasma corticosterone and confer tumor suppressor activity within the mammary gland via activation of p38MAPK (33). However, using phospho-p38MAPK (Thr180/Tyr182) as an indicator of pathway activation, no differences were observed between concentrations of either p38MAPK total protein or phosho-p38MAPK, and further analysis of this pathway was not undertaken.
Discussion
This is, to our knowledge, the first report of a relationship between IAC and breast cancer risk. The LIAC/HIAC models have been widely studied with over 100 publications reporting their use, although not in the field of cancer research (34). Included in those reports are extensive characterizations of the phenotypes that distinguish between HIAC and LIAC (35–37). Female HIAC rats that are fed ad libitum generally have a body weight that is 15% lighter than LIAC and they have less body fat and display higher in-cage activity. These phenotypes are consistent with the cancer protective effects associated with reduced adiposity, caloric restriction, and induced physical activity but were sustained in the absence of restricted feeding or exercise in these models. Although our subgroup analysis (Table 2) is notable in that the protective effect of HIAC against breast cancer (relative to LIAC) was not diminished in rats with similar group mean body weights (differed by only 5%) in comparison with the carcinogenic response observed when all animals were considered (Table 1), the potential contributions of differences in caloric intake, adiposity or in cage activity to the observed effects are not known. However, the magnitude of the protective effect of HIAC against breast cancer (RR = 0.38; 95% CI, 0.2 to 0.8) was considerably greater than observed in response to
caloric restriction resulting in the same 15% difference in body weight (RR = 0.94; 95% CI 0.6–1.4; ref. 38), running on a non-motorized activity wheel (7650 m/day) (RR = 0.92; 95% CI 0.7–1.2; ref. 25) or resistance to excessive body fat accumulation with a 15% difference in body weights (RR = 0.83; 95% CI, 0.7–1.1; ref. 39) in other rat models of chemically induced mammary cancer.
Factors such as energy intake and physical activity that influence energy balance are linked to a signaling network known to be deregulated in the majority of human breast cancer, the mTOR network (40–42). The mTOR network signaling was downregulated in HIAC, identifying it as a potential metabolic hub that is integrating multiple signaling inputs associated with differences in IAC. Circulating factors have been frequently cited to explain differences in cancer risk attributed to physical activity as well as caloric restriction and diet-induced obesity (38,39,43,44). The fact that plasma concentrations of the growth factors and hormones (Supplementary_Table_2, available at Carcinogenesis Online) were not associated with signaling pathways implicated in carcinogenesis indicates that simply examining a snapshot of changes in these circulating factors that can vary over time is not sufficient to explain the potential effects of IAC on cancer outcomes. This is consistent with the possibility that changes in circulating concentrations of these factors do not reflect availability at the tissue or cellular level. The molecular data presented suggest that cell autonomous rather than host systemic effects are involved in mediating the observed differences in the carcinogenic response observed in HIAC versus LIAC. Specifically, it appears that the PI3k/Akt/mTOR signaling network, which is commonly deregulated in breast cancer (40–42,45), accounts at least in part for protection against cancer potentially through the maintenance of a pro-apoptotic microenvironment in mammary tissue of rats with HIAC.
Due to technical feasibility and cost in epidemiological studies, little attention has been given to how aerobic capacity, an objective indicator of physical activity exposure, is associated with cancer risk. This is a significant limitation given the recognized problems associated with the self-reported data used to conclude that physical activity protects against the development of breast cancer (2,3,6). Although care must be exercised not to over extend the implications of our data (Tables 1 and 2 and Figure 1) relative to evidence about protective effects of physical activity on cancer risk in human populations, it is notable that when given access to an activity wheel, HIAC rats voluntarily run much longer distances each day than LIAC (35), a finding that we have confirmed. The role of IAC and its effect on running behavior and cancer risk have not been investigated in epidemiological studies. Nonetheless, if the same type of relationship observed in rodents occurs in humans with HIAC, then at least a component of the protection against breast cancer that is associated with a physically active lifestyle may actually be a genetically determined cancer susceptibility trait that acts independent of physical activity behaviors. It remains to be determined how imposition of physical activity-induced improvements in fitness in the HIAC/LIAC model systems of IAC will affect the carcinogenic response.
Supplementary material
Supplementary data are available at Carcinogenesis online.
Funding
National Cancer Institute (R01 CA100693) to H.J.T.; National Institutes of Health (P40OD021331) to L.G.K. and S.L.B.
Supplementary Material
Acknowledgements
The authors thank Angie Neil, Weiqin Jiang and Zongjian Zhu for their technical assistance. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. Contact L.G.K. or S.L.B. for information on the LIAC and HIAC rats: these rat models are maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor, MI.
Conflict of Interest Statement: None declared.
Abbreviations
- 95% CI
95% confidence interval
- HIAC
high inherent aerobic capacity
- IAC
inherent aerobic capacity
- IGF-1
insulin-like growth factor-1
- LIAC
low inherent aerobic capacity
- PTEN
phosphatase and tensin homolog
References
- 1. Brenner D.R., et al. (2016) Breast cancer survival among young women: a review of the role of modifiable lifestyle factors. Cancer Causes Control, 27, 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lynch B.M., et al. (2011) Physical activity and breast cancer prevention. Recent Results Cancer Res., 186, 13–42. [DOI] [PubMed] [Google Scholar]
- 3. Wu Y., et al. (2013) Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res. Treat., 137, 869–882. [DOI] [PubMed] [Google Scholar]
- 4. Friedenreich C.M. (2011) Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res., 188, 125–139. [DOI] [PubMed] [Google Scholar]
- 5. Kushi L.H., et al. ; American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee. (2012) American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin., 62, 30–67. [DOI] [PubMed] [Google Scholar]
- 6. Shephard R.J. (2003) Limits to the measurement of habitual physical activity by questionnaires. Br. J. Sports Med., 37, 197–206; discussion 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aijaz B., et al. (2008) Long-term mortality with multiple treadmill exercise test abnormalities: comparison between patients with and without cardiovascular disease. Am. Heart J., 156, 783–789. [DOI] [PubMed] [Google Scholar]
- 8. Peel J.B., et al. (2009) A prospective study of cardiorespiratory fitness and breast cancer mortality. Med. Sci. Sports Exerc., 41, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peel A.B., et al. (2014) Cardiorespiratory fitness in breast cancer patients: a call for normative values. J. Am. Heart Assoc., 3, e000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lakoski S.G., et al. (2015) Midlife cardiorespiratory fitness, incident cancer, and survival after cancer in men: the Cooper Center Longitudinal Study. JAMA Oncol., 1, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarzynski M.A., et al. (2017) Genomic and transcriptomic predictors of response levels to endurance exercise training. J. Physiol., 595, 2931–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pitsiladis Y.P., et al. ; Athlome Project Consortium. (2016) Athlome Project Consortium: a concerted effort to discover genomic and other “omic” markers of athletic performance. Physiol. Genomics, 48, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koch L.G., et al. (2011) Intrinsic aerobic capacity sets a divide for aging and longevity. Circ. Res., 109, 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koch L.G., et al. (2008) Development of animal models to test the fundamental basis of gene-environment interactions. Obesity (Silver Spring)., 16 (suppl 3, S28–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wisløff U., et al. (2005) Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science, 307, 418–420. [DOI] [PubMed] [Google Scholar]
- 16. Koch L.G., et al. (2001) Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol. Genomics, 5, 45–52. [DOI] [PubMed] [Google Scholar]
- 17. Overmyer K.A., et al. (2015) Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab., 21, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson H.J., et al. (1995) Rapid induction of mammary intraductal proliferations, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis, 16, 2407–2411. [DOI] [PubMed] [Google Scholar]
- 19. Thompson H.J., et al. (2000) Classification of premalignant and malignant lesions developing in the rat mammary gland after injection of sexually immature rats with 1-methyl-1-nitrosourea. J. Mammary Gland Biol. Neoplasia, 5, 201–210. [DOI] [PubMed] [Google Scholar]
- 20. McGinley J.N., et al. (2011) Quantitative assessment of mammary gland density in rodents using digital image analysis. Biol. Proced. Online, 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sokal R.R., et al. (1995) Biometry: The Principles and Practice of Statistics in Biological Research. W.H. Freeman, New York, NY. [Google Scholar]
- 22. Thompson H.J., et al. (2016) Effect of low or high glycemic load diets on experimentally induced mammary carcinogenesis in rats. Mol. Nutr. Food Res., 60, 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vieira-Potter V.J., et al. (2015) Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol., 308, R530–R542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ren Y.Y., et al. (2013) Genetic analysis of a rat model of aerobic capacity and metabolic fitness. PLoS One, 8, e77588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu Z., et al. (2008) Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol. Biomarkers Prev., 17, 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiza C., et al. (2012) Role of PRAS40 in Akt and mTOR signaling in health and disease. Am. J. Physiol. Endocrinol. Metab., 302, E1453–E1460. [DOI] [PubMed] [Google Scholar]
- 27. Gwinn D.M., et al. (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell, 30, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peter D., et al. (2015) Molecular architecture of 4E-BP translational inhibitors bound to eIF4E. Mol. Cell, 57, 1074–1087. [DOI] [PubMed] [Google Scholar]
- 29. Galbaugh T., et al. (2006) EGF-induced activation of Akt results in mTOR-dependent p70S6 kinase phosphorylation and inhibition of HC11 cell lactogenic differentiation. BMC Cell Biol., 7, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tzatsos A., et al. (2006) Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol. Cell. Biol., 26, 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chu E.C., et al. (2004) PTEN regulatory functions in tumor suppression and cell biology. Med. Sci. Monit., 10, RA235–RA241. [PubMed] [Google Scholar]
- 32. Park S.J., et al. (2017) DNA-PK promotes the mitochondrial, metabolic, and physical decline that occurs during aging. Cell Metab., 25, 1135–1146.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avivar-Valderas A., et al. (2014) Stress signaling and the shaping of the mammary tissue in development and cancer. Oncogene, 33, 5483–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koch L.G., et al. (2017) Theoretical and biological evaluation of the link between low exercise capacity and disease risk. Cold Spring Harb. Perspect. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smyers M.E., et al. (2015) Physically active rats lose more weight during calorie restriction. Physiol. Behav., 139, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeMarco V.G., et al. (2012) Overweight female rats selectively breed for low aerobic capacity exhibit increased myocardial fibrosis and diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol., 302, H1667–H1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Novak C.M., et al. (2010) Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm. Behav., 58, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu Z., et al. (1997) Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis, 18, 1007–1012. [DOI] [PubMed] [Google Scholar]
- 39. Matthews S.B., et al. (2014) Excess weight gain accelerates 1-methyl-1-nitrosourea-induced mammary carcinogenesis in a rat model of premenopausal breast cancer. Cancer Prev. Res. (Phila)., 7, 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis N.M., et al. (2014) Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention. Oncotarget, 5, 4603–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson H.J., et al. (2009) Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life, 61, 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu Z., et al. (2009) Energetics and mammary carcinogenesis: effects of moderate-intensity running and energy intake on cellular processes and molecular mechanisms in rats. J. Appl. Physiol. (1985), 106, 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiang W., et al. (2009) Effects of physical activity and restricted energy intake on chemically induced mammary carcinogenesis. Cancer Prev. Res. (Phila), 2, 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McTiernan A. (2008) Mechanisms linking physical activity with cancer. Nat. Rev. Cancer, 8, 205–211. [DOI] [PubMed] [Google Scholar]
- 45. El-Chaâr D., et al. (2004) Inhibition of insulin signaling and adipogenesis by rapamycin: effect on phosphorylation of p70 S6 kinase vs eIF4E-BP1. Int. J. Obes. Relat. Metab. Disord., 28, 191–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.