Abstract
Objectives. To determine whether a novel measure of appendicular lean mass relative to fat mass is associated with physical functioning in RA.
Methods. In a cross-sectional design, three independent RA cohorts were retrospectively analysed. Whole-body DXA measures of appendicular lean mass index (ALMI, kg/m2) and fat mass index (FMI, kg/m2) were converted to age, sex and race-specific Z-scores using published National Health and Nutrition Examination Survey reference ranges. Adiposity-adjusted ALMI Z-scores (ALMIFMI) were determined using a published method to adjust for normal associations between ALMI and FMI Z-scores. Associations between ALMI Z-scores, ALMIFMI Z-scores and physical functioning were assessed after adjusting for age, sex and study. Functional outcomes assessed included the HAQ, Valued Life Activities assessment and Short Physical Performance Battery. Low lean for age was defined as a Z-score of −1 or less.
Results. Our sample consisted of 442 patients with RA. The combined cohort had a mean ALMI Z-score of − 0.51 (1.08) and a mean ALMIFMI Z-score of − 0.58 (1.53), suggesting muscle mass deficits compared with a nationally representative sample. Greater ALMIFMI Z-scores demonstrated stronger associations with better functional outcomes compared with ALMI Z-scores. Associations were not attenuated with adjustment for systemic inflammation or pain. The FMI Z-score was independently associated with physical functioning, with a stronger association seen among patients with greater FMI Z-score. Adiposity-adjusted definitions of low lean mass more clearly identified those with functional impairment.
Conclusion. Estimates of appendicular lean mass that are adjusted for adiposity demonstrate stronger positive associations with functional outcomes compared with unadjusted estimates.
Keywords: lean mass, fat mass, physical function, disability, rheumatoid arthritis
Rheumatology key messages
-
Lean mass relative to fat is a more informative construct for disability in RA than lean mass.
Associations between muscle and disability in RA have previously been underestimated due to confounding effects of adiposity.
Greater fat mass is associated with disability in RA, increasingly so among those with greater fat mass.
Introduction
Low muscle mass and low muscle strength have each been associated with functional impairment and poor long-term outcomes among the elderly [1–5]. Muscle deficits have been observed in patients with RA and have been associated with functional limitations in some studies [6, 7]. Studies evaluating muscle mass deficits in RA and other disease states have been limited by the methodologies available for quantifying and categorizing disease-related changes in skeletal muscle mass.
Patients with RA have been shown to have greater fat mass and are at increased risk of overfat and obesity [7–9]. Obesity is well-known to be associated with functional disability in other populations [10]. Therefore, patients with RA are affected by multiple body composition alterations that may contribute to disability. Under normal circumstances, there is a strong positive association between skeletal muscle mass and fat mass such that those with greater fat mass can be expected to have greater muscle mass [11]. This association confounds associations between muscle loss and disability (since obese subjects will be expected to have both greater muscle mass and greater disability due to excess adiposity). Therefore, methodologies for assessing muscle deficits relative to what would be expected given the extent of adiposity could help to uncover important relationships between muscle mass and physical function.
Previous studies have indeed demonstrated that a linear adjustment for adiposity improves the correlation of muscle mass estimates with physical functioning in the elderly [12–14]. We developed a comprehensive method that builds on this previous work to allow for the quantification of muscle mass relative to adiposity using reference data from the National Health and Nutrition Examination Survey (NHANES) [11]. Whole-body DXA measures of appendicular lean mass index (ALMI, kg/m2) for fat mass index (FMI, kg/m2) are compared with NHANES reference ranges and adjusted for previously described correlations within individual subgroups. The method allows for the quantification of deficits in individual subjects compared with the reference group for individuals of similar age, sex, race and FMI, taking into account altered relationships between lean and fat within particular subgroups.
We hypothesized that estimates of appendicular lean mass that are relative to fat mass would be more strongly associated with physical functioning and disability than estimates that do not consider fat. The objectives of the study were to determine the independent associations between adiposity-adjusted and unadjusted estimates of appendicular lean mass and physical functioning and to evaluate independent associations between fat mass and physical functioning in a large sample consisting of three distinct cohorts of patients with RA.
Methods
Study sample
We combined and assessed three independent cohorts of patients with RA. The internal review boards of the University of Pennsylvania, Philadelphia VA Medical Center, University of California San Francisco and John’s Hopkins University approved each respective study, and all subjects gave written informed consent.
University of California San Francisco Cohort (n = 141)
Details regarding this study cohort have been previously published [8, 15]. The majority of the research participants were drawn from the University of California San Francisco (UCSF) RA Panel Study. After telephone interviews in the study years 2007–09, RA Panel participants who lived in the greater San Francisco area were recruited for in-person assessments, including measurement of body composition. Exclusion criteria were non-English speaking, age <18 years, current daily oral prednisone dose >50 mg, current pregnancy, uncorrected vision problems that interfered with reading, and patients who had undergone joint replacement within 1 year.
University of Pennsylvania (Penn) Cohort (n = 111)
The University of Pennsylvania (Penn) cohort was initiated in 2012 to evaluate alterations in body composition and bone structure in patients with RA. Subjects composing the Penn cohort were recruited from the University of Pennsylvania Rheumatology practices and Philadelphia Veterans Affairs Medical Center and consisted of individuals with RA, ages 18–70 years, who met 2010 ACR criteria. Subjects with JIA (or another inflammatory arthritis), active cancer, a history of chronic diseases known to affect bone health (e.g. chronic kidney disease, liver disease, malabsorption syndromes) or pregnancy were excluded. One RA subject was excluded because her weight exceeded the limit for the DXA machine (300 pounds).
Evaluation of subclinical cardiovascular disease and predictors of events in RA Study Cohort (n = 190)
Subjects were men and women participating in the Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in RA (ESCAPE RA) cohort study between October 2004 and May 2006. This cohort has been previously described [16]. Briefly, 197 patients with RA followed at the Johns Hopkins Arthritis Centre or referred from local rheumatologists were enrolled, all of whom met ACR 1987 classification criteria for RA, were 45–84 years of age, and did not report any prior pre-specified cardiovascular events or procedures. Subjects weighing >300 pounds were excluded due to weight limitations of the imaging equipment.
Measures of ALMI and FMI
For the UCSF subjects, a Lunar Prodigy DXA system (software version 9.3) was used. The ESCAPE-RA study also utilized a Lunar Prodigy DXA system (Prodigy software, version 05.60.003) (GE/Lunar Radiation, Madison, WI, USA). In vivo coefficients of variation for measurement of lean mass by the Lunar Prodigy have been estimated at ⩽1% [17]. Body composition measures for the UCSF subjects and ESCAPE RA subjects were adjusted based on the method by Shepherd et al. [18] to facilitate comparison with NHANES data that were generated on Hologic equipment. Subjects from the Penn cohort underwent whole-body DXA assessment using a Hologic densitometer (Delphi Systems, Hologic, Inc., Bedford, MA, USA) and therefore did not require the adjustment. The in vitro coefficient of variation for Hologic measurement of lean mass was <0.6% and the in vivo coefficient of variation in adults was <1% [19]. Whole-body DXA was performed on all participants to estimate appendicular lean mass and total fat mass. ALMI and FMI were determined by dividing the respective estimate by height-squared, similar to the calculation of BMI.
Sex- and race/ethnicity-specific Z-scores were generated for FMI relative to age using Lambda, Mu, Sigma (LMS) curves previously published by Hologic Inc [6, 20], and for ALMI (not including bone mass) using LMS values provided by personal communication with the company. The LMS method helps to account for the non-linearity, heteroskedasticity and skew noted in body composition outcomes. We used a previously described method to generate adiposity-adjusted ALMI Z-scores (ALMIFMI) by utilizing the residuals from the regression of ALMI Z-score on FMI Z-score within age, sex and race categories [11].
Functional outcomes
This study utilized three validated measures of physical functioning, including the 20-question Multi-dimensional HAQ, the Valued Life Assessment (VLA) and the Short Physical Performance Battery (SPPB).
HAQ
The modified HAQ is widely used to identify and quantify disability in RA and is a mandated outcome measure in clinical trials [21]. Briefly, eight categories are assessed, including dressing and grooming, arising, eating, walking, hygiene, reach, grip and common daily activities. For each of these categories, patients report the amount of difficulty they have in performing two or three specific activities and whether they require assistance or the use of assistive devices to perform that activity.
VLA
The VLA is a validated questionnaire that assesses 29 domains of physical functioning and has been previously described in detail [22, 23]. A wide spectrum of activities is included, ranging from obligatory activities, such as self-care, to discretionary activities, such as recreation and social participation. This is in contrast to the HAQ, which queries fairly basic levels of functioning. Participants rate the difficulty of performing life activities, using a 4-point scale corresponding to the response scale of the HAQ, where 0 is no difficulty and 3 is unable to perform. For regression models, the VLA was log-transformed to fit a normal distribution.
SPPB
The SPPB is a widely used and simple test to measure lower extremity function through observed completion of tasks that mimic daily actions. Specifically, it examines an individual’s performance with regard to static balance, gait speed and timed chair-raises. It has been demonstrated to be reliable (intraclass correlations 0.88–0.92) and responsive to change [24]. Because SPPB scores are highly skewed, this variable was analysed as categories as previously defined (0–4, 5–8 and 9–12) [25, 26]. In contrast to the HAQ and VLA, higher values for the SPPB suggest better physical functioning.
Other disease measures
Detailed methods regarding data collection in the USCF [8], Penn [6] and ESCAPE RA cohort [16] have been previously published. Pain and patient global scores were assessed in all cohorts by a visual analogue scale (range: 0–100). CRP (mg/dl) and ACPA status (positive or negative) were measured using standard clinical assays at each institution.
Statistical analysis
Generation of adiposity-adjusted ALMIFMI Z-scores was performed as described in previous publications [11]. Briefly, these methods to generate ALMIFMI Z-scores were built on previously published methods and utilize the residuals of the regression of ALMI Z-score on FMI Z-score. The resulting score represents the difference between the actual ALMI Z-score and the predicted value given the observed FMI Z-score [12–14]. Estimating equations previously defined within NHANES based on this residual method were applied to adjust the ALMI Z-scores to create ALMIFMI Z-scores. The ALMIFMI Z-scores conceptually represent the number of s.d.s above or below the predicted value for a reference group of individuals of the same age, sex, race and FMI Z-score.
Correlations between unadjusted (ALMI) and adiposity-adjusted (ALMIFMI) Z-scores and physical functioning measures were determined using Spearman’s (for SPPB) and Pearson’s correlations (HAQ, VLA). Direct comparison of correlation coefficients was performed using the corcor command in Stata. Independent associations between lean mass estimates (ALMI, ALMIFMI Z-scores) and functional measures were assessed using linear and ordinal regression models adjusting for age and gender as well as pain, CRP and study cohort. Results were confirmed in mixed effects models with clustering on study cohort. We adjusted for age and sex, since these variables are associated with disability, and we previously identified differential disease impact on Z-scores by age and gender [15]. Brant tests were performed to test the parallel regression assumption in ordinal regression models. Non-linear associations between body composition measures and physical functioning were evaluated by visualization of LOWESS curves and by testing for the significance of quadratic terms in regression models.
Kappa statistics assessed agreement between ALMI and ALMIFMI definitions of low lean for age, previously defined as a Z-score of −1 or less (15.9th percentile for the reference population) [27]. Physical functioning was assessed among patients with low lean mass for age by both the unadjusted and adiposity-adjusted definitions by using t-tests and rank-sum tests for non-normally distributed data. Statistical analysis was performed using Stata 14.0 (StataCorp, LP, College Station, TX, USA).
Results
Basic characteristics of the three study cohorts are presented in Table 1. In each cohort, ALMI Z-scores and ALMIFMI Z-scores were low compared with those of the reference population. In the combined cohort (n = 442), the average ALMI, FMI and ALMIFMI Z-scores were low, with respective means of −0.51 (1.08) (31st percentile for NHANES population), −0.16 (1.16) (44th percentile) and −0.58 (1.53) (28th percentile), respectively.
Table 1.
Basic characteristics of the three study cohorts
| Participant Characteristics | Combined | UCSF | Penn | ESCAPE RA |
|---|---|---|---|---|
| n | 442 | 141 | 111 | 190 |
| Age, years | 58.3 (10.5) | 58.6 (10.8) | 54.2 (13.2) | 59.4 (8.7) |
| Female, n (%) | 258 (58) | 85 (60) | 46 (54) | 118 (60) |
| Race | ||||
| Black, n (%) | 61 (14) | 6 (4) | 29 (34) | 18 (10) |
| BMI, kg/m2 | 28.1 (5.9) | 27.1 (6.0) | 28.1 (6.8) | 28.4 (5.3) |
| ALMI Z-score | −0.51 (1.07) | −0.60 (1.14) | −0.22 (1.02) | −0.61 (1.03) |
| FMI Z-score | −0.16 (1.16) | −0.11 (1.23) | −0.11 (1.21) | −0.23 (1.06) |
| ALMIFMI Z-score | −0.58 (1.53) | −0.70 (1.90) | −0.27 (1.07) | −0.68 (1.44) |
| Disease duration, median (IQR), years | 12 (5–22) | 18 (11–27) | 8 (3–17) | 9 (4–17) |
| Current smoking, n (%) | 57 (13) | 8 (6) | 20 (24) | 23 (12) |
| CCPA positive, n/N (%) | 181/249 (72) | 92 (66) | 89 (82) | – |
| RADAI | – | 2.6 (1.8) | – | – |
| DAS28 (CRP) | – | – | 3.16 (1.19) | 3.66 (1.08) |
| CRP, median (IQR), mg/dl | 0.46 (0.13–0.90) | 0.19 (0.07–0.52) | 0.8 (0.5–1.4) | 0.27 (0.11–0.78) |
| Pain, 0–100, median (IQR) | 23 (10–48) | 20 (10–40) | 40 (15–65) | 21 (8–39) |
| Patient global, 0–100, median (IQR) | 62 (36–84) | – | 35 (15–50) | 78 (56–93) |
| Glucocorticoid use, n (%) | 155 (37) | 43 (32) | 49 (44) | 74 (39) |
| HAQ score | 0.85 (0.69) | 0.94 (0.67) | 0.79 (0.63) | 0.82 (0.74) |
| SPPB, median (IQR) | 10 (7–11) | 10 (8–11) | 11 (9–12) | 8 (4–10) |
| VLA score | 0.61 (0.54) | 0.62 (0.51) | – | 0.60 (0.57) |
Data presented as mean (s.d.) unless otherwise stated. UCSF: University of California San Francisco; ALMI: appendicular lean mass index; FMI: fat mass index; ALMIFMI: adiposity-adjusted appendicular lean mass index; IQR: interquartile range; RADAI: RA Disease Activity Index; VLA: Valued Life Activities Questionnaire; SPPB: short physical performance battery.
HAQ was strongly inversely correlated with the ALMIFMI Z-score (r = −0.24, P < 0.001), but was not associated with the ALMI Z-score (r = −0.074, P = 0.12). These correlations were statistically different from one another (P for comparison <0.001). These observations were similar in regression models adjusting for age and sex (Table 2) and were similar across each of the three study cohorts (data not shown). The association was not attenuated in a model further adjusting for CRP and pain levels (Table 2).
Table 2.
Age- and sex-adjusted associations between lean mass Z-scores and physical functioning
| HAQ | Ln(VLA) | Lower SPPB category | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | OR (95% CI) | P-value | |
| Model 1 | ||||||
| ALMI Z | −0.088 (−0.15, −0.027) | 0.005 | −0.048 (−0.11, 0.012) | 0.12 | 0.93 (0.76, 1.14) | 0.49 |
| ALMIFMI Z | −0.15 (−0.20, −0.11) | <0.001 | −0.12 (−0.17, −0.077) | <0.001 | 0.72 (0.62, 0.84) | <0.001 |
| Model 2 | ||||||
| ALMI Z | −0.087 (−0.14, −0.037) | 0.001 | −0.049 (−0.11, 0.016) | 0.14 | 0.91 (0.74, 1.12) | 0.36 |
| ALMIFMI Z | −0.11 (−0.15, −0.076) | <0.001 | −0.12 (−0.16, −0.076) | <0.001 | 0.77 (0.66, 0.90) | 0.001 |
Model 1: adjusted for age and sex. Model 2: Adjusted for age sex, ln(CRP) and pain levels. VLA: Valued Life Activities Questionnaire; SPPB: short physical performance battery; OR; odds ratio; UCSF: University of California San Francisco; ALMI: appendicular lean mass index; ALMIFMI: adiposity-adjusted appendicular lean mass index; FMI: fat mass index.
A significant inverse association was observed between VLA and ALMIFMI Z-score (r = −0.24, P < 0.001). In contrast, no association was observed between VLA scores and ALMI Z-scores (r = −0.049, P = 0.41). Pearson correlations were statistically greater between the ALMIFMI Z-score and VLA compared with the correlations observed between the ALMI Z-score and VLA (P for comparison <0.001). In regression models adjusting for age, sex, CRP and pain levels, the ALMIFMI was more strongly correlated with VLA (Table 2).
There was a positive correlation between SPPB and ALMIFMI Z-score (Spearman ρ = 0.17, P = 0.001), while no correlation was observed between SPPB and the ALMI Z-score (Spearman ρ = 0.0030, P = 0.95). Pearson correlations with SPPB were statistically stronger for the ALMIFMI Z-score compared with the ALMI Z-score (P for comparison <0.001). Individuals with greater ALMIFMI Z-score were less likely to have lower SPPB scores (suggesting greater physical limitations) after adjusting for age and sex (Table 2). In contrast, there was no significant association between ALMI Z-scores and SPPB category.
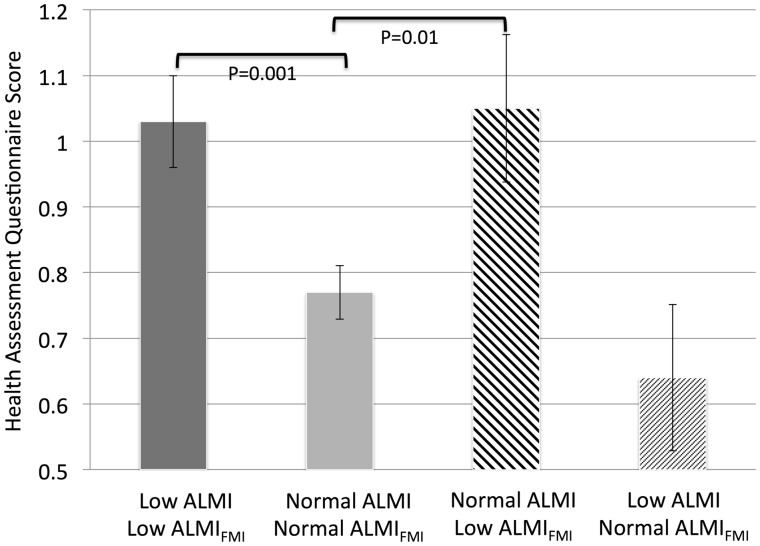
The correlation between ALMI and ALMIFMI definitions of low lean for age (i.e. below the 16th percentile) was good (κ 0.60, P < 0.001). Those subjects with an ALMIFMI Z-score of −1 or less had significantly greater HAQ and VLA scores and significantly lower SPPB scores. In contrast, subjects with an ALMI Z-score of − 1 or less did not have lower estimates of physical functioning by HAQ, VLA or SPPB (Table 3). Notably, those who had a normal ALMI but a low ALMIFMI Z-score had physical functioning that was comparable with that of those who were low by both definitions (Fig. 1).
Table 3.
Mean (s.d.) or median (IQR) physical functioning scores among those with low lean mass for age
| ALMIFMI Status | Low ALMI Z | Normal ALMI Z | Totals | P-value | |
|---|---|---|---|---|---|
| Health Assessment Questionnaire | |||||
| Low ALMIFMI Z | 1.03 (0.72) | 1.05 (0.76) | 1.04 (0.73) | <0.001 | |
| Normal ALMIFMI Z | 0.64 (0.62) | 0.77 (0.66) | 0.76 (0.66) | ||
| Totals | 0.94 (0.72) | 0.81 (0.68) | |||
| P = 0.07 | |||||
| Valued Life Activities Questionnaire | |||||
| Low ALMIFMI Z | 0.65 (0.30, 0.94) | 0.87 (0.54, 1.27) | 0.71 (0.32, 1.06) | <0.001 | |
| Normal ALMIFMI Z | 0.20 (0.10, 0.73) | 0.35 (0.083, 0.88) | 0.35 (0.083, 0.86) | ||
| Totals | 0.53 (0.2, 0.91) | 0.45 (0.12, 1) | |||
| P = 0.52 | |||||
| Short physical performance battery | |||||
| Low ALMIFMI Z | 9 (6, 11) | 8 (6, 10) | 9 (6, 11) | 0.004 | |
| Normal ALMIFMI Z | 10 (9, 12) | 10 (7, 11) | 10 (8, 11) | ||
| Totals | 9 (7, 11) | 10 (7, 11) | |||
| P = 0.82 | |||||
VLA: Valued Life Activities Questionnaire; SPPB: short physical performance battery; ALMIFMI: adiposity-adjusted appendicular lean mass index; FMI: fat mass index.
Fig. 1.
HAQ disability scores among individuals classified as low lean mass for age and normal
Those who were low by both definitions had high HAQ scores, while those who were normal by both definitions had low HAQ scores. Those who had a low appendicular lean mass index Z-score but normal adiposity-adjusted lean mass index Z-score had similar HAQ scores compared with individuals who were normal by both definitions. Those who had a normal appendicular lean mass index Z-score but a low adiposity-adjusted lean mass Z-score had HAQ scores that were similar to individuals who were low by both definitions.
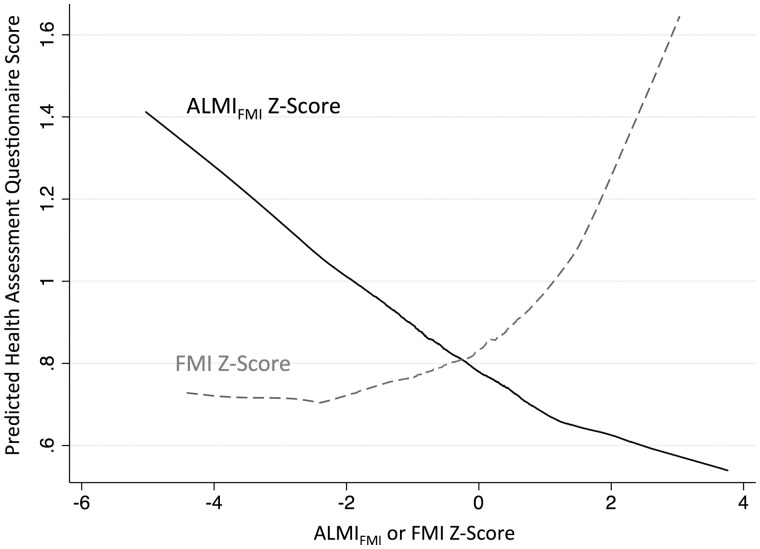
In the combined cohort, the associations between ALMIFMI Z-score and physical functioning were similar in models independent of the effect of the FMI Z-score (Table 4). In these models, a FMI Z2-score term was significant, suggesting stronger associations between FMI Z-score and physical functioning noted among those with greater FMI Z-scores. This non-linear association between FMI Z-score and HAQ is illustrated in the LOWESS curve in Fig. 2.
Table 4.
Independent associations between adiposity-adjusted lean mass estimates and physical functioning
| HAQ (n = 441) | Ln(VLA) (n = 292) | Lower SPPB category (n = 382) | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | OR (95% CI) | P-value | |
| ALMIFMI Z | −0.13 (−0.17, −0.089) | <0.001 | −0.083 (−0.13, −0.040) | <0.001 | 0.79 (0.68, 0.93) | 0.003 |
| FMI Z | 0.094 (0.037, 0.15) | 0.001 | 0.14 (0.074, 0.20) | <0.001 | 1.45 (1.17, 1,80) | <0.001 |
| FMI Z2 | 0.036 (0.009, 0.064) | 0.009 | 0.036 (0.0074, 0.064) | 0.01 | 1.12 (1.02, 1.23) | 0.02 |
All models are adjusted for age, sex and study. VLA: Valued Life Activities Questionnaire; SPPB: short physical performance battery; OR: odds ratio; ALMIFMI: adiposity-adjusted appendicular lean mass index; FMI: fat mass index.
Fig. 2.
Illustration of associations between body composition estimates and disability scores
The LOWESS curve illustrates associations between fat mass index Z-scores and adiposity-adjusted lean mass index Z-scores, with physical functioning as measured by HAQ (predicted from regression model in Table 4). The association between fat mass index Z-score and HAQ was greater among individuals with greater fat mass index Z-scores.
The 51 patients who reported active smoking had higher ALMIFMI Z-scores after adjusting for age, sex, race and study [β = 0.59 (0.20, 0.97) P = 0.003]. Smoking did not confound associations between ALMIFMI and physical function, and there were no statistical interactions (all P > 0.2).
Discussion
In this large study of three independent RA patient cohorts, adiposity-adjusted definitions of muscle mass deficits were more strongly associated with three separate measures of physical functioning. In regards to physical functioning, it appears to be more informative to determine the extent of the muscle deficit relative to that patient’s level of adiposity, rather than to determine the extent of the muscle deficit alone. Thus, our understanding of muscle loss in the context of RA should consider that adiposity is an important confounder. Overall, these results suggest that studies to date may have underestimated the impact of muscle loss on physical functioning in patients with RA.
Definitions of low lean mass for age based on the adiposity-adjusted estimates were more likely to identify patients with poor physical functioning. Therefore, an adiposity-adjusted definition of low lean mass for age is also demonstrated to have good construct validity as a dichotomous variable for clinical studies or potentially as a screening tool for at-risk individuals. Additional research is needed to better define clinically important deficits in adiposity-adjusted measures of appendicular lean mass. Patients with low lean mass for age have meaningful differences in function based on the minimally important differences previously described for HAQ [28] and SPPB [29], and an approximately half s.d. difference in the VLA [30]. While this study supports the hypothesis that muscle loss is an underappreciated risk factor for adverse physical functioning in RA, these cross-sectional associations might suffer from reverse causality. In other words, reduced physical functioning from arthritis could instead lead to relative muscle loss. Further study is needed to determine the impact of muscle loss relative to adiposity independent of comorbidities, disease severity and structural joint damage.
Importantly, the observations here also provide evidence of the construct validity of methods for considering adiposity in the estimation of muscle mass. A number of studies have demonstrated that adiposity may confound relationships between muscle loss and physical function [12–14]. The novel methods validated here build on previous evidence and represent an important construct either as a clinical or research outcome [11]. The current method builds on previous methods in several ways. This method can be replicated and compared across cohorts, considers known variation in associations in demographic groups, makes no assumptions about altered relationships between muscle and fat in disease states, and provides the ability to intuitively quantify deficits using s.d. scores compared with a large reference sample. For example, this cohort was observed, on average, to be at the 28th percentile for this measure compared with the reference population.
The method has implications for a research study or clinical setting where loss of lean and fat mass may be expected to occur through both healthy behaviors (i.e. exercise) and unintentionally (i.e. cachexia). While healthy and intentional weight loss might appear to reduce skeletal muscle mass, this healthy behaviour would be expected to have positive effects on these adiposity-adjusted estimates. The opposite is true for cachexia, where disproportional loss of lean mass is expected [6, 31].
To our knowledge, this study is the first to demonstrate a non-linear association between adiposity and physical functioning among patients with arthritis. In other words, the association between greater fat mass and worse physical functioning was most pronounced among those with the greatest fat mass. This study confirms previous studies that have observed higher HAQ scores among morbidly obese individuals with early arthritis and further demonstrates that elevated HAQ scores in obese RA patients are related to the excess adiposity in this group [32]. Here it is observed that the impact of fat mass on physical functioning is greatest among those who are more severely obese. Relatedly, it is intuitive that strategies to promote loss of fat mass are likely to have the greatest impact on those with more severe obesity. In contrast to associations between fat mass and disability, the relationship between adiposity-adjusted muscle mass estimates with disability was linear.
There are several limitations to consider in interpreting the findings of these analyses. First, the three previously derived cohorts studied were not designed to answer the question posed. However, the robustness of the results across all three cohorts is reassuring and therefore also a powerful strength. Second, because of the cross-sectional nature of the current study, it is difficult to draw conclusions regarding causality. In other words, these observations are not proof that the associations identified between muscle deficits and physical functioning represent a causal association. Longitudinal and interventional studies may be helpful in this setting. Similarly, it was out of the scope of this manuscript to assess the related impact of glucocorticoid use, comorbidity, and physical activity as contributors to these relationships. Third, the current study does not address how adiposity-adjusted estimates might be used in algorithms to define clinically important muscle loss. This may depend substantially on the clinical and research questions of interest. Whether and how to combine these estimates of skeletal muscle with other features of frailty, such as muscle strength, remains to be determined. Finally, because these methods of quantifying lean mass relative to fat mass utilize US national reference data, the generalizability to other populations is not clear and deserves further study.
There are several important strengths that are important to recognize. To our knowledge, this combined cohort is the largest group of patients with RA with both DXA and comprehensive functional assessments. The use of NHANES reference data for developing adiposity-adjusted Z-scores is also a novel and a critical strength. This method builds on previous methods of adjusting lean mass for adiposity [12, 14, 33].
In conclusion, adjustment of estimates of skeletal muscle mass for adiposity improves the strength of correlations with physical functioning and disability. Greater fat mass is also strongly and independently associated with physical functioning. Adiposity-adjusted definitions of muscle deficits are more discriminative of those with greater functional impairment.
Acknowledgements
Dr Baker would like to acknowledge the support of a Veterans Affairs Clinical Science Research and Development Career Development Award (IK2 CX000955). The contents of this work do not represent the views of the Department of the Veterans Affairs or the US Government. The authors would like to acknowledge Joan Bathon as the Principal Investigator on the ESCAPE RA study. Dr M.B.L. is supported by National Institutes of Health (NIH) grant K24 DK076808 (M.B.L.), and Dr D.W. is supported by a K12 HD068373. The University of California, San Francisco study cohort was funded by a National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant P60-AR053308) and the National Center for Research Resources (through a University of California San Francisco Clinical and Translational Sciences Institute grant UL1-RR024131). The ESCAPE RA Study was funded by NIH NIAMS AR050026-01 (P.I. Joan Bathon). Additional support was provided by the Johns Hopkins Bayview Medical Center General Clinical Research Center (Grant Number M01RR02719).
Funding: This work was supported by a Veterans Affairs Clinical Science Research and Development Career Development Award (IK2 CX000955) and the University of Pennsylvania Clinical and Translational Research Center (UL1 RR024134).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Bouchard DR, Héroux M, Janssen I.. Association between muscle mass, leg strength, and fat mass with physical function in older adults: influence of age and sex. J Aging Health 2011;23:313–28. [DOI] [PubMed] [Google Scholar]
- 2. Barbat-Artigas S, Rolland Y, Cesari M. et al. Clinical relevance of different muscle strength indexes and functional impairment in women aged 75 years and older. J Gerontol A Biol Sci Med Sci 2013;68:811–9. [DOI] [PubMed] [Google Scholar]
- 3. Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA.. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging 2008;12:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cawthon PM, Fox KM, Gandra SR. et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc 2009;57:1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lang T, Cauley JA, Tylavsky F. et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 2010;25:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker JF, Von Feldt J, Mostoufi-Moab S. et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res 2014;66:1612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giles JT, Ling SM, Ferrucci L. et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum 2008;59:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katz PP, Yazdany J, Trupin L. et al. Sex differences in assessment of obesity in rheumatoid arthritis. Arthritis Care Res 2013;65:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giles JT, Allison M, Blumenthal RS. et al. Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum 2010;62:3173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Visser M, Langlois J, Guralnik JM. et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr 1998;68:584–90. [DOI] [PubMed] [Google Scholar]
- 11. Weber DLJ, Long J, Leonard MB, Zemel B, Baker JF.. Development of novel methods to define deficits in appendicular lean mass relative to fat mass. PLoS One 2016;11:e0164385 10.1371/journal.pone.0164385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dufour AB, Hannan MT, Murabito JM, Kiel DP, McLean RR.. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. J Gerontol A Biol Sci Med Sci 2013;68:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newman AB, Kupelian V, Visser M. et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–9. [DOI] [PubMed] [Google Scholar]
- 14. Delmonico MJ, Harris TB, Lee JS. et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–74. [DOI] [PubMed] [Google Scholar]
- 15. Baker JF, Long J, Ibrahim S, Leonard MB, Katz P.. Are men at greater risk of lean mass deficits in rheumatoid arthritis? Arthritis Care Res 2015;67:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giles JT, Malayeri AA, Fernandes V. et al. Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Rheum 2010;62:940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toombs RJ, Ducher G, Shepherd JA, De Souza MJ.. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity 2012;20:30–9. [DOI] [PubMed] [Google Scholar]
- 18. Shepherd JA, Fan B, Lu Y. et al. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res 2012;27:2208–16. [DOI] [PubMed] [Google Scholar]
- 19. Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS.. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone 2004;34:1044–52. [DOI] [PubMed] [Google Scholar]
- 20. Kelly TL, Wilson KE, Heymsfield SB.. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolfe F, Michaud K, Pincus T.. Development and Validation of the Health Assessment Questionnaire II. Arthritis Rheum 2004;50:3296–305. [DOI] [PubMed] [Google Scholar]
- 22. Katz PP, Morris A, Yelin EH.. Prevalence and predictors of disability in valued life activities among individuals with rheumatoid arthritis. Ann Rheum Dis 2006;65:763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katz PP, Morris A.. Use of accommodations for valued life activities: prevalence and effects on disability scores. Arthritis Rheum 2007;57:730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM.. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women's Health and Aging Study. J Clin Epidemiol 2002;55:916–21. [DOI] [PubMed] [Google Scholar]
- 25. Mihalko SL, Wickley KL, Sharpe BL.. Promoting physical activity in independent living communities. Med Sci Sports Exerc 2006;38:112–5. [DOI] [PubMed] [Google Scholar]
- 26. Corsonello A, Lattanzio F, Pedone C. et al. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res 2012;15:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weber D, Long J, Leonard MB, Zemel B, Baker JF.. Characterization of lean-fat mass associations across age, sex, and racial groups and development of fat-adjusted measures of Sarcopenia Suppl. J Frailty Aging 2016. [Google Scholar]
- 28. Pope JE, Khanna D, Norrie D, Ouimet JM.. The minimally important difference for the health assessment questionnaire in rheumatoid arthritis clinical practice is smaller than in randomized controlled trials. J Rheumatol 2009;36:254–9. [DOI] [PubMed] [Google Scholar]
- 29. Kwon S, Perera S, Pahor M. et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging 2009;13:538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Norman GR, Sloan JA, Wyrwich KW.. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582–92. [DOI] [PubMed] [Google Scholar]
- 31. Challal S, Minichiello E, Boissier MC, Semerano L.. Cachexia and adiposity in rheumatoid arthritis. Relevance for disease management and clinical outcomes. Joint Bone Spine 2016;83:127–33. [DOI] [PubMed] [Google Scholar]
- 32. Humphreys JH, Verstappen SMM, Mirjafari H. et al. Association of morbid obesity with disability in early inflammatory polyarthritis: results from the Norfolk Arthritis Register. Arthritis Care Res 2013;65:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLean RR, Shardell MD, Alley DE. et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 2014;69:576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]