Abstract
Taste cells in taste buds are epithelial sensory cells. Old taste bud cells die and are replaced by new ones generated from taste stem cells. Identifying and characterizing adult taste stem cells is therefore important to understand how peripheral taste tissues are maintained. SOX2 is expressed in oral epithelium including gustatory papillae and has been proposed to be a marker of adult taste stem/progenitor cells. Nevertheless, this hypothesis has never been directly tested. Here, by single-color genetic lineage tracing using Sox2-CreERT2 strain, we reveal that all types of taste bud cells distributed throughout the oral epithelium are derived from stem cells that express SOX2. Short-term tracing shows that SOX2-positive taste stem cells actively supply taste bud cells. At the base of epithelium outside taste buds are distributed proliferation marker- and SOX2-positive cells. Consistently, taste stem cells identified by Lgr5 expression in the circumvallate papillae also express SOX2. Together, taste stem cells distributed in oral epithelia express SOX2.
Keywords: lineage tracing, oral epithelium, stem cell, taste bud cell
Introduction
Epithelial cells of the alimentary tract, including the oral cavity, are maintained by continuous turnover in adult vertebrates. Multiple types of locally distributed stem cells continuously supply epithelial cells under normal conditions and replenish them after injury (Barker et al. 2012; Kretzschmar and Watt 2012). In oral epithelium, Lgr5+ stem cells give rise to taste bud cells and nongustatory papillary epithelial cells juxtaposed to taste buds in circumvallate and foliate papillae of the posterior tongue (Takeda et al. 2013; Yee et al. 2013). Although short-term (≤1 month) lineage tracing using a transgenic mouse with human Krt14 promoter suggests that both taste bud cells and surrounding epithelial cells are supplied from cells in the basal epithelium outside taste buds (Okubo et al. 2009), the identity and stemness of the transgene-expressing cells remain elusive due to lack of in-depth analyses including long-term lineage tracing (≥3 months; some taste bud cells live more than ~6 weeks) (Perea-Martinez et al. 2013). Short-term lineage tracing reveals that Lgr6+ cells supply a subset of taste bud cells in both anterior and posterior tongue (Ren et al. 2014), but not all taste bud cells in any gustatory regions are derived from Lgr6+ cells or from Lgr5+ or Krt14+ cells in vivo (Okubo et al. 2009; Takeda et al. 2013; Yee et al. 2013; Ren et al. 2014), probably because knock-in and transgenic mice used in the previous studies yield mosaic expression of CreERT2 in Lgr5+, Lgr6+, and Krt14+ taste stem cells. Therefore, identification of other taste stem cell markers that are expressed in all taste stem cells and/or CreERT2 strains that efficiently express CreERT2 in taste stem cells not only enables us to understand the lineage(s) and turnover mechanisms of taste bud cells but also enable us to generate mice with conditional knockout (cKO) of genes of interest in taste bud cells.
Sox2 is expressed in the tongue epithelium and Sox2+ cell population contains stem cells of epithelial cells in nongustatory epithelium in adult mice (Arnold et al. 2011). Although Sox2 is also expressed in a subset of taste bud cells and surrounding epithelial cells (Suzuki 2008), it is still unclear whether Sox2+ cell population in the tongue epithelium also contains taste stem cells. Here, we report that taste stem cells express Sox2, and that the knock-in strain that encodes CreERT2 in its Sox2 locus is useful for generating cKO of taste bud cell genes.
Materials and methods
Animals
C57BL/6J (stock no. 000664), Sox2CreERT2/+ (stock no. 017593) (Arnold et al. 2011), Rosa26lsl-Tom/lsl-Tom (stock no. 007908) (Madisen et al. 2010), and Lgr5EGFP-ires-CreERT2/+ (stock no. 008875) (Barker et al. 2007) mice were purchased from the Jackson Laboratory. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center and performed in accordance with National Institutes of Health guidelines.
Tamoxifen administration
Tamoxifen (10 mg/mL in corn oil; Sigma–Aldrich) was intraperitoneally injected to young adult mice (100 mg/kg body weight): once for short-term chase (3 days) and 5 times over 5 consecutive days for moderate- and long-term chase (24 days and 6 and 21 months) in gustatory tissues in lineage tracing studies.
EdU labeling
A nucleoside analog of thymidine, EdU (5-ethynyl-2′-deoxyuridine; 0.5 mg/mL in PBS; Thermo Fisher Scientific), was intraperitoneally injected to adult mice (5 mg/kg body weight). Tissues were dissected from mice perfused with 4% paraformaldehyde (PFA) in PBS 4 h after EdU administration. EdU incorporated into nuclei in tissue sections was detected and visualized using the Click-iT EdU Alexa Fluor 488 imaging kit (Thermo Fisher Scientific), according to the manufacturer’s protocol.
Tissue preparation
Mice were deeply anesthetized with urethane and transcardially perfused with PBS followed by 4% PFA in PBS. Oral epithelia dissected from them were postfixed, cryoprotected, and frozen as described previously (Ohmoto et al. 2008). Cryosections (8 µm thickness) were prepared using a Leica CM1900 cryostat (Leica Microsystems), mounted on tissue-adhesive-coated glass slides (Fisher Scientific), and preserved in at −80 °C until use.
Immunohistochemistry
Immunohistochemistry using 4% PFA-fixed sections was carried out as described previously (Ohmoto et al. 2008). Sections were treated in a preheated target retrieval solution pH 9 (Agilent Technologies) at 80 °C for 20 min before blocking. As primary antibodies, rabbit anti-KCNQ1 (1:1000, Millipore), goat anti-KCNQ1 (1:300, Santa Cruz Biotechnology), goat anti-SOX2 (1:300, Santa Cruz Biotechnology), rabbit anti-TRPM5 (1:3000, Alomone Labs), rabbit anti-DDC (1:2000, GeneTex), mouse anti-PCNA (1:100 Millipore), and mouse anti-GFP (1:1000, Clontech Laboratories) antibodies were used. Alexa 488-, 555-, and 647-conjugated antibodies (1:500, Thermo Fisher Scientific) were used as secondary antibodies. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent images were acquired by a Leica TCS SP2 confocal microscope (Leica Microsystems) with a pinhole size of 1.5 airy units. All images were captured under the similar conditions except the fluorescence of tdTomato with a low laser power. Optical confocal images were processed with Photoshop (Adobe Systems) and analyzed on a computer screen.
Results
Stem cells to generate taste bud cells for long-term express Sox2
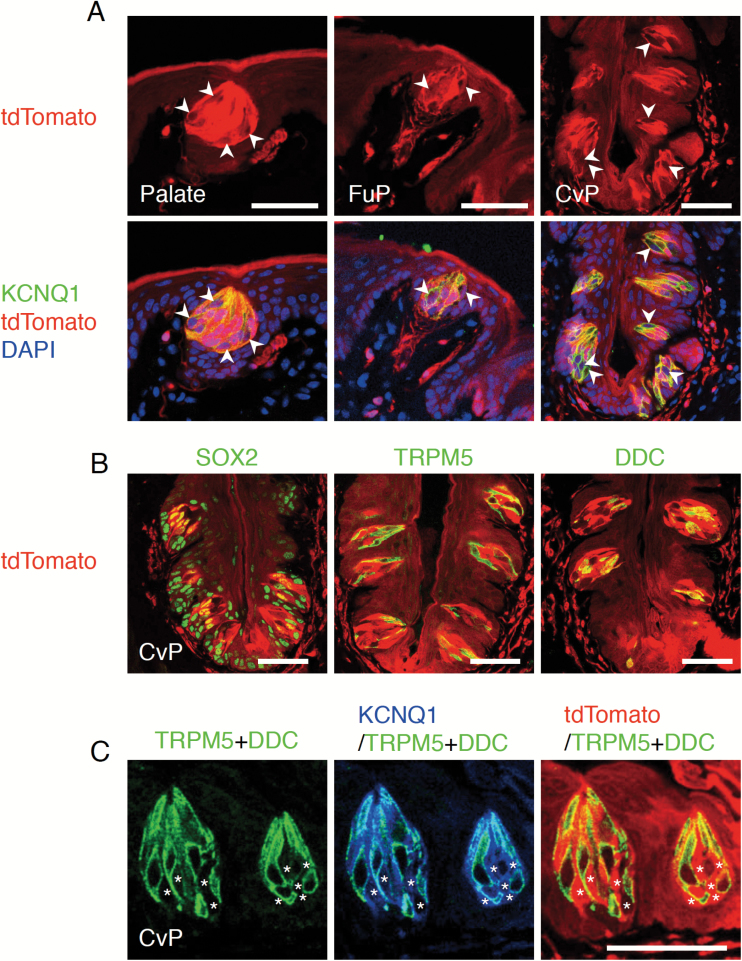
Genetic lineage tracing using a drug-inducible Cre recombinase and a conditional reporter allele is a powerful method to examine whether the cells of interest are stem cells (Barker et al. 2012). In tongue epithelia, Bmi1+ and Sox2+ stem cells are involved in the turnover of nongustatory oral epithelial cells (Arnold et al. 2011; Tanaka et al. 2013). Bmi1+ stem cells do not generate any taste bud cells (Tanaka et al. 2013; Yee et al. 2013). However, the involvement of Sox2+ stem cells in taste bud cell turnover remains to be unclear (Arnold et al. 2011). To examine whether Sox2+ cells give rise to any taste bud cells over the long term, we carried out single-color genetic lineage tracing using young adult Sox2CreERT2/+; Rosa26loxP-stop-loxP-tdTomato/+ (Rosa26lsl-Tom/+) mice. In gustatory tissues where taste buds composed of tens of cells are distributed, such as soft palate, fungiform papillae of anterior dorsal tongue, and circumvallate papillae of posterior dorsal tongue, all epithelial cells we examined exhibit tdTomato fluorescence at 21 months after intraperitoneal tamoxifen injection, although intensity of tdTomato fluorescence varies among cells (e.g., fluorescence intensities in nongustatory epithelial cells are weaker than in taste bud cells and some taste bud cells express lower level of fluorescence than other taste bud cells) (Figure 1A). Immunohistochemical detection of taste bud cells with the pan-taste-bud-cell marker KCNQ1 (Ohmoto et al. 2006; Matsumoto et al. 2011) indicates that all taste bud cells exhibit tdTomato fluorescence (Figure 1A). Indeed, TRPM5-expressing cells responsible for sweet, umami, or bitter tastes and DDC-expressing cells responsible for sour taste, most of which do not express SOX2 (Suzuki 2008), are tdTomato+ in circumvallate papillae at 6 months after tamoxifen injection (Figure 1B). Taste bud cells lacking TRPM5 and DDC expression are also tdTomato+ (Figure 1C), demonstrating that all types of taste bud cells express tdTomato. Considering that average life spans of taste bud cells are estimated to be a few weeks (Beidler and Smallman 1965; Perea-Martinez et al. 2013), 6 months is long enough for several rounds of complete taste bud cell turnover. Thus, these analyses demonstrate that the Sox2+ cell population includes the taste stem cells that supply taste bud cells in any gustatory tissues over the long term.
Figure 1.
Long-term lineage tracing of Sox2+ cells in the gustatory areas of oral epithelium. (A) Fluorescence of tdTomato in the oral epithelium of Sox2CreERT2/+; Rosa26lsl-Tom/+ mice at 21 months after tamoxifen injections for 5 consecutive days: fluorescent labeling of pan-taste-bud-cell marker KCNQ1 (green, bottom) and nuclei (stained with DAPI, blue, bottom) in the soft palate (left), fungiform papillae (FuP, middle), and circumvallate papillae (CvP, right) with tdTomato fluorescence (red). All taste bud cells are labeled with tdTomato at 21 months after tamoxifen injection. Taste bud cells marked by arrowheads are the representative cells exhibiting lower tdTomato fluorescence than other taste bud cells. (B) Fluorescent labeling of SOX2 (green, left), TRPM5 (green, middle), and DDC (green, right) and tdTomato (red) in the CvP of Sox2CreERT2/+; Rosa26lsl-Tom/+ mice at 6 months after tamoxifen injections for 5 consecutive days. (C) Fluorescent labeling of the combination of TRPM5 and DDC (green) and KCNQ1 (blue) with tdTomato (red) in the taste buds of CvP of Sox2CreERT2/+; Rosa26lsl-Tom/+ mice at 6 months after tamoxifen injections for 5 consecutive days. Taste bud cells marked by asterisks (*) are the cells positive for KCNQ1 but negative for TRPM5 and DDC and labeled with tdTomato. Scale bar: 50 µm.
Perigemmal basal proliferative Sox2+ cells give rise to taste bud cells
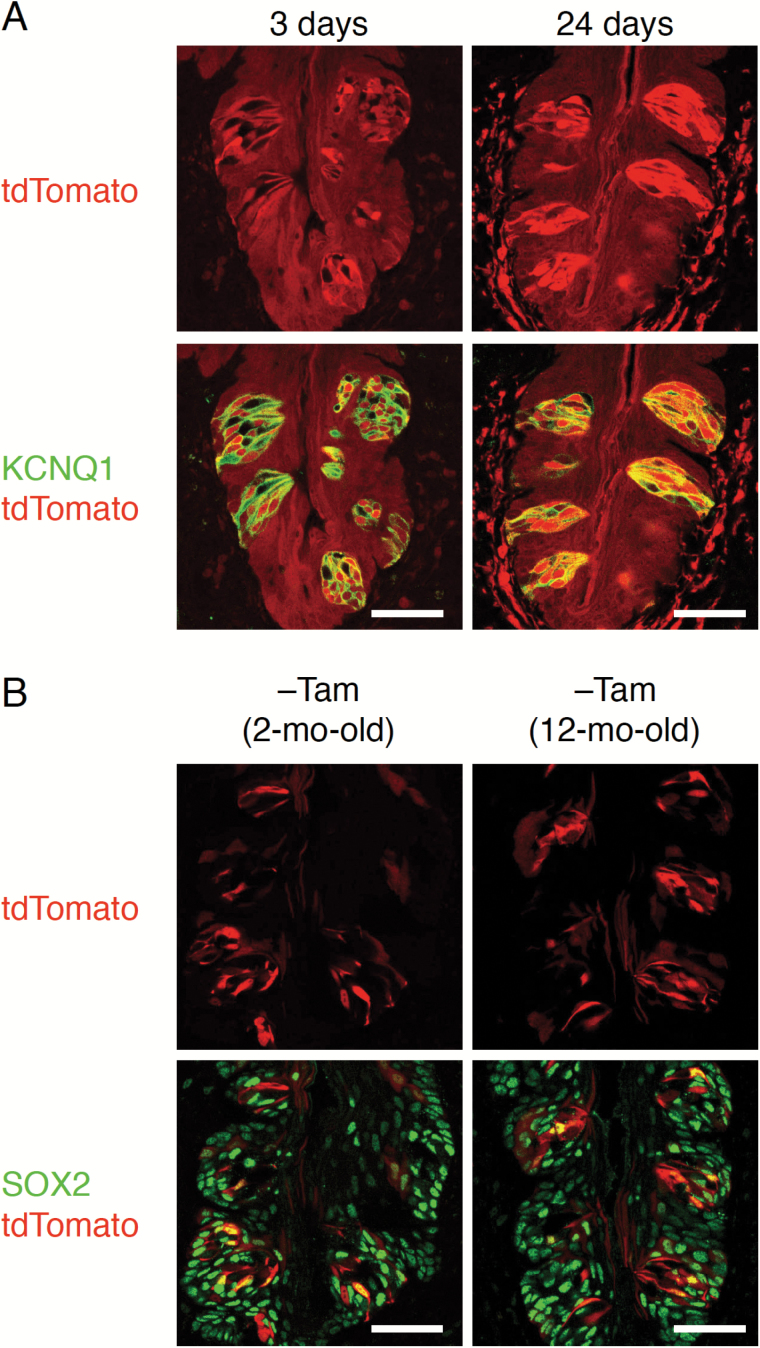
At about 3 weeks after tamoxifen injection, a time period sufficient to replace most taste bud cells under normal turnover conditions in adult mice (Beidler and Smallman 1965; Perea-Martinez et al. 2013), most taste bud cells are tdTomato+ (Figure 2A). However, rare but significant spontaneous expression of tdTomato is detected in circumvallate papillary epithelium of tamoxifen-untreated Sox2CreERT2/+; Rosa26lsl-Tom/+ mice (Figure 2B) and also detected in the palate and fungiform papillae (data not shown). Similar phenomena were reported in other tissues of tamoxifen-untreated Sox2CreERT2/+; Rosa26lsl-EYFP/+ mice, with the same Sox2-Cre driver allele and different Rosa26-reporter allele (Arnold et al. 2011). If these spontaneously labeled taste bud cells were slow-cycling stem cells, tdTomato+ cell population in taste buds should increase over time. However, in taste buds of mice that are older by 10 months, the frequency of tdTomato+ cells appears unchanged, strongly suggesting that tdTomato-labeled taste bud cells are not stem cells or don’t contribute to the increase of tdTomato+ cells. Although it is difficult to distinguish spontaneously expressed tdTomato from tdTomato of newly generated cells from Sox2+ stem cells, frequency of tdTomato+ taste bud cells seems to be increased compared to untreated Sox2CreERT2/+; Rosa26lsl-Tom/+ mice at 3 days after tamoxifen injection (Figure 2). Thus, these results suggest that taste bud cells are actively supplied from stem cells expressing Sox2, as are nongustatory epithelial cells in gustatory papillae.
Figure 2.
Short-term lineage tracing of Sox2+ cells in the circumvallate papillae. (A) Fluorescent labeling of KCNQ1 (green, bottom) with tdTomato fluorescence (red) in CvP of Sox2CreERT2/+; Rosa26lsl-Tom/+ mice. Most KCNQ1+ cells are tdTomato-positive at 24 days after tamoxifen injections for 5 consecutive days (right), although some KCNQ1+ cells are tdTomato-negative at 3 days after one tamoxifen injection for 1 day (left). (B) Fluorescence of tdTomato (red) and merged images labeled with SOX2 (green, bottom) in the CvP of Sox2CreERT2/+; Rosa26lsl-Tom/+ mice without tamoxifen treatment at the age of 2 months (left) and 12 months (right). Some SOX2+ cells and a subset of taste bud cells that do not express SOX2 are tdTomato+. Spontaneous labeling with tdTomato is observed in taste bud cells and nongustatory epithelial cells, showing no strict correlation between spontaneous expression of tdTomato and SOX2 expression. Scale bar: 50 µm.
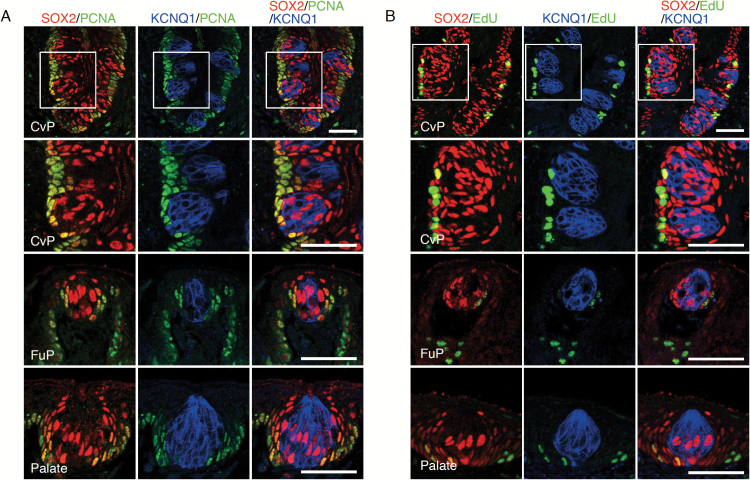
The actively proliferating cells immunohistochemically detected by Ki67 expression and short-term 5-bromo-2′-deoxyuridine (BrdU) incorporation are distributed at the basal region of epithelium in the circumvallate papillae (Asano-Miyoshi et al. 2008). Although a subset of SOX2+ cells is positive to BrdU after short-term BrdU incorporation (Suzuki 2008), it is unclear whether they are inside or outside taste buds. To address this, we carried out immunohistochemical analysis of proliferating cell nuclear antigen (PCNA) and EdU labeling in combination with immunohistochemistry for SOX2 and KCNQ1. PCNA+ cells and EdU-incorporated cells, which are observed only at the bottom of the epithelial layer outside taste buds in all gustatory areas examined, also express SOX2 (Figure 3), as observed in previous reports using the same or other methods to identify proliferating cells (Beidler and Smallman 1965; Hamamichi et al. 2006; Asano-Miyoshi et al. 2008; Perea-Martinez et al. 2013). These results uncover that SOX2+ cells outside taste buds, at least a subpopulation of them, are active and long-term taste stem cells, consistent with the previous finding of local distribution of taste stem cells (Stone et al. 1995).
Figure 3.
Identification of actively proliferating cells in the gustatory areas of oral epithelium. (A) Triple fluorescent labeling of SOX2 (red), PCNA (green), and KCNQ1 (blue) in the CvP (top and second-top), FuP (second-bottom), and the soft palate (bottom). Actively proliferating PCNA+ cells are observed in the basal epithelial cells outside taste buds, which are colabeled with SOX2. (B) Nucleoside analog (EdU) incorporation in actively proliferating cells in CvP (top and second-top), FuP (second-bottom), and soft palate (bottom): triple fluorescent labeling of EdU (green), SOX2 (red), and KCNQ1 (blue) at 4 h after EdU administration. Cells positive for both SOX2 and EdU are observed in the basal epithelium outside KCNQ1+ cells. Areas of magnified fluorescent images in row 2 (second-top) are indicated by white squares in row 1 (top). Scale bar: 50 µm.
Lgr5 + stem cells in posterior gustatory papillae express SOX2
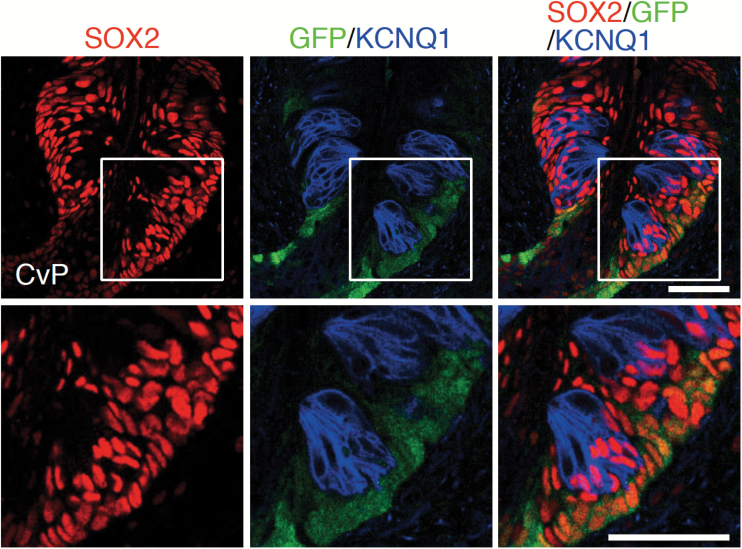
Taste bud cells in the foliate and circumvallate papillae in the posterior tongue are derived from Lgr5+ taste stem cells whose distribution is confined to those gustatory papillae (Takeda et al. 2013; Yee et al. 2013). Triple fluorescence immunohistochemistry of SOX2, KCNQ1, and GFP in circumvallate papillae of Lgr5EGFP-ires-CreERT2/+ mice shows that GFP signals are observed in a subpopulation of SOX2+ cells outside taste buds (Figure 4), indicating the coexpression of SOX2 in all Lgr5+ taste stem cells. Taken together, this study demonstrates that taste stem cells express SOX2.
Figure 4.
Relationship between Lgr5+ taste stem cells and SOX2+ cells in the circumvallate papillae. Triple fluorescence labeling of SOX2 (red), green fluorescent protein (GFP; green), and KCNQ1 (blue) in the CvP of Lgr5EGFP-ires-CreERT2/+ mice. All GFP+ cells are observed outside taste buds and colabeled with SOX2. Areas of magnified fluorescent images (bottom) are indicated by white squares. Scale bar: 50 µm.
Discussion
In this article, we performed single-color lineage tracing using Sox2-CreERT2 strain and revealed that SOX2 is a stem cell marker of taste bud cells in adult mice. SOX2 expression is observed throughout the basal layer of nongustatory oral epithelium and also in the subset of taste bud cells. SOX2+ cell population in the basal epithelium outside the taste buds is proliferative and contains taste stem cells, as observed in the lineage tracing studies using Lgr5-CreERT2 and Krt14-CreERT2 strains (Okubo et al. 2009; Takeda et al. 2013; Yee et al. 2013).
Since Lgr5 expression is confined to the posterior tongue (Takeda et al. 2013; Yee et al. 2013), molecular characteristics of taste stem cells are different among the locations of taste buds: Sox2+Lgr5+ in the circumvallate and foliate papillae and Sox2+Lgr5– in the palate and fungiform papillae. Methods to develop organoids in vitro have been established for many tissues from their stem cells. Interestingly, tissue organoids generated from Lgr5+ stem cells of small intestine, stomach, and circumvallate papillae under the same culture conditions display features of in vivo intestinal, gastric, and circumvallate papillae, respectively (Sato et al. 2009; Barker et al. 2010; Ren et al. 2014), suggesting that Lgr5+ stem cells intrinsically have their own different blueprints for cell lineages. It would not be surprising if Sox2+ stem cells distributed near fungiform taste buds generate taste organoids, and if they can, would be interesting to examine the molecular features and characteristics of those organoids. Taste buds show regionally different features of gene expression and therefore regional differences in the composition of taste bud cells (Hoon et al. 1999; Huang et al. 2006; Ishimaru et al. 2006; Shindo et al. 2008; Ohmoto et al. 2011), and such regional differences are also found in their stem cells. By comparing taste organoids generated from Sox2+ stem cells harvested from spatially different gustatory regions, we could obtain important insights into whether the region-specific molecular features and composition of taste bud cells are intrinsically destined in the local Sox2+ stem cells or depend on communication with their local environment, such as niche.
Both taste bud cells and surrounding epithelial cells are supplied from Sox2+ stem cells, showing the multipotency of Sox2+ stem cells, which is consistent with the data suggested by the previous lineage tracing with Krt14-CreER transgenic mice (Okubo et al. 2009). However, it is unclear whether each Sox2+ stem cell is multipotent, that is, can give rise to taste bud cells as well as nongustatory epithelial cells or is unipotent, producing only taste bud cells or nongustatory epithelial cells. It is also unclear whether one stem cell generates only one type of taste bud cells or multiple types of cells. Multi-color lineage tracing using reporter strains such as Thy1-Brainbow (Livet et al. 2007) or Rosa26-Confetti (Snippert et al. 2010) strains would address these questions.
This study demonstrates strong evidence showing that taste stem cells express SOX2. Although SOX2 is not a specific marker of taste stem cells, this finding is sufficient to suggest the availability of Sox2-CreERT2 strain in taste research. Conventional knockout mice of genes for taste receptors and intracellular signaling molecules are viable and fertile so that their functions in taste cells were well studied in vivo. However, the roles of molecules that have been thought to be important for taste bud cell turnover such as PROX1, NKX2-2, ASCL1, and SHH remains largely elusive, probably because their conventional knockout mice are embryonic or perinatal lethal or die at early postnatal days (Guillemot et al. 1993; Chiang et al. 1996; Sussel et al. 1998; Wigle et al. 1999), which hampers studying their roles in taste bud cells in vivo. Sox2 locates on the chromosome 3 in mouse different from Prox1 (chr. 1), Nkx2-2 (chr. 2), Ascl1 (chr. 10), and Shh (chr. 5), and thus the Sox2-CreERT2 strain, with their floxed strains (Dassule et al. 2000; Harvey et al. 2005; Pacary et al. 2011; Mastracci et al. 2013), will be useful to induce cKO of those genes in taste stem cells, which culminates in the generation of taste bud cells devoid of their expression. However, it may be pragmatically difficult to generate such cKO of the gene which locates in the same chromosome as Sox2, especially when they are very close, because recombination between 2 genes integrated closely in the same chromosome rarely occurs. Also, Sox2 is expressed in other epithelial stem cells and some neural cells. If genes of interest are expressed not only in taste bud cells but also other cells where Sox2 is expressed such as stomach and pituitary gland, it may be difficult to distinguish phenotypes of their deficiency induced using Sox2-CreERT2 allele in taste bud cells from those in other tissues. It would be helpful if there is another CreERT2 knock-in strain that expresses CreERT2 in taste stem cells from the chromosome other than chromosome 3, because we will be able to obtain cKO mice of any genes of interest in taste bud cells using this strain or Sox2-CreERT2 strain. If CreERT2 expression is regulated by the gene that is expressed in the taste stem cell but not in the other stem cells such as epithelial stem cells, the strain would be more useful for taste research. Identifying and characterizing taste stem cells will bring us important information about taste bud cell turnover and enable us to obtain useful genetic tools which contribute to the molecular genetic studies in taste.
Funding
This work was supported in part by the National Institute for Deafness and Other Communication Disorders at the National Institute of Health [R01DC015491 to I.M. and R01DC013807 to P.J.]. We thank the Monell Genotyping and Histology and Cellular Localization Cores, supported in part by the funding from the National Institute for Deafness and Other Communication Disorders at the National Institute of Health [P30DC011735 to Robert F. Margolskee, Monell Chemical Senses Center], for facilities.
References
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. 2011. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 9:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano-Miyoshi M, Hamamichi R, Emori Y. 2008. Cytokeratin 14 is expressed in immature cells in rat taste buds. J Mol Histol. 39:193–199. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M et al. 2010. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449:1003–1007. [DOI] [PubMed] [Google Scholar]
- Barker N, van Oudenaarden A, Clevers H. 2012. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 11:452–460. [DOI] [PubMed] [Google Scholar]
- Beidler LM, Smallman RL. 1965. Renewal of cells within taste buds. J Cell Biol. 27:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. 1996. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 383:407–413. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. 2000. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 127:4775–4785. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. 1993. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 75:463–476. [DOI] [PubMed] [Google Scholar]
- Hamamichi R, Asano-Miyoshi M, Emori Y. 2006. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 141:2129–2138. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. 2005. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 37:1072–1081. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. 1999. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 96:541–551. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. 2006. The cells and logic for mammalian sour taste detection. Nature. 442:934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. 2006. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 103:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K, Watt FM. 2012. Lineage tracing. Cell. 148:33–45. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. 2007. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 450:56–62. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR et al. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 13:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastracci TL, Lin CS, Sussel L. 2013. Generation of mice encoding a conditional allele of Nkx2.2. Transgenic Res. 22:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. 2011. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 14:685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmoto M, Matsumoto I, Misaka T, Abe K. 2006. Taste receptor cells express voltage-dependent potassium channels in a cell age-specific manner. Chem Senses. 31:739–746. [DOI] [PubMed] [Google Scholar]
- Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. 2008. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol Cell Neurosci. 38:505–517. [DOI] [PubMed] [Google Scholar]
- Ohmoto M, Okada S, Nakamura S, Abe K, Matsumoto I. 2011. Mutually exclusive expression of Gαia and Gα14 reveals diversification of taste receptor cells in zebrafish. J Comp Neurol. 519:1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Clark C, Hogan BL. 2009. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 27:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary E, Heng J, Azzarelli R, Riou P, Castro D, Lebel-Potter M, Parras C, Bell DM, Ridley AJ, Parsons M et al. 2011. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron. 69:1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Martinez I, Nagai T, Chaudhari N. 2013. Functional cell types in taste buds have distinct longevities. PLoS One. 8:e53399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P. 2014. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci U S A. 111:16401–16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ et al. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265. [DOI] [PubMed] [Google Scholar]
- Shindo Y, Miura H, Carninci P, Kawai J, Hayashizaki Y, Ninomiya Y, Hino A, Kanda T, Kusakabe Y. 2008. G alpha14 is a candidate mediator of sweet/umami signal transduction in the posterior region of the mouse tongue. Biochem Biophys Res Commun. 376:504–508. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD et al. 2010. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 143(1):134–144. [DOI] [PubMed] [Google Scholar]
- Stone LM, Finger TE, Tam PP, Tan SS. 1995. Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc Natl Acad Sci U S A. 92:1916–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. 1998. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 125:2213–2221. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. 2008. Expression of Sox2 in mouse taste buds and its relation to innervation. Cell Tissue Res. 332:393–401. [DOI] [PubMed] [Google Scholar]
- Takeda N, Jain R, Li D, Li L, Lu MM, Epstein JA. 2013. Lgr5 identifies progenitor cells capable of taste bud regeneration after injury. PLoS One. 8:e66314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Komai Y, Tokuyama Y, Yanai H, Ohe S, Okazaki K, Ueno H. 2013. Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells. Nat Cell Biol. 15:511–518. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. 1999. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 21:318–322. [DOI] [PubMed] [Google Scholar]
- Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, Jiang P. 2013. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells. 31:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]