Abstract
Individual differences in neuroanatomy are associated with intellectual ability and psychiatric risk. Factors responsible for this variability remain poorly understood. We tested whether 17 major demographic and obstetric variables were associated with individual differences in brain volumes in 756 neonates assessed with MRI. Gestational age at MRI, sex, gestational age at birth, and birthweight were the most significant predictors, explaining 31% to 59% of variance. Unexpectedly, earlier born babies had larger brains than later born babies after adjusting for other predictors. Our results suggest earlier born children experience accelerated brain growth, either as a consequence of the richer sensory environment they experience outside the womb or in response to other factors associated with delivery. In the full sample, maternal and paternal education, maternal ethnicity, maternal smoking, and maternal psychiatric history showed marginal associations with brain volumes, whereas maternal age, paternal age, paternal ethnicity, paternal psychiatric history, and income did not. Effects of parental education and maternal ethnicity are partially mediated by differences in birthweight. Remaining effects may reflect differences in genetic variation or cultural capital. In particular late initiation of prenatal care could negatively impact brain development. Findings could inform public health policy aimed at optimizing child development.
Keywords: birthweight, neonate, neuroimaging, premature birth, socioeconomic status
Individual differences in brain structure, as assessed by quantitative magnetic resonance imaging (MRI), are associated with intellectual ability (Deary et al. 2010) and risk for neurological and psychiatric disorders (Fusar-Poli et al. 2011; Braskie and Thompson 2014). Identifying the factors responsible for individual variability in brain structure is a key scientific question that the emerging discipline of population neuroscience endeavors to address (Paus 2010). While genetic differences play an important role, twin studies demonstrate that environmental factors account for a substantial portion of inter-individual variance in brain structure (Peper et al. 2007). The so-called “envirome” encompasses an almost infinite variety of exposures (Anthony 2001), but within this vast search space, prenatal influences are likely of particular importance.
The prenatal period represents a foundational phase of human brain development, involving a complex cascade of neurodevelopmental processes including cell differentiation and growth, neuronal migration, dendritic arborization, synaptogenesis, gyrification, myelinization, and programmed cell death. These processes produce dramatic changes in gray and white matter volumes as indexed by MRI (Gilmore et al. 2007; Knickmeyer et al. 2008). Furthermore, early aberrations relevant to adult-onset disorders can be captured via MRI as evidenced by a recent study showing that common polymorphisms in putative psychiatric risk genes are associated with brain changes at birth (Knickmeyer et al. 2013).
Overt prenatal insults such as maternal exposure to radiation (Schull et al. 1990) or starvation (Hulshoff Pol et al. 2000; de Rooij et al. 2010) have robust effects on brain development, but given their relative rarity are unlikely to explain much variance at the population level. In this report, we examine the impact of 17 major demographic and obstetric history variables on inter-individual variation in global brain and tissue volumes in a unique sample of 756 neonates who received high resolution MRI scans at 2 weeks of age. Studying infants allows us to assess pre- and perinatal variables of interest with a high degree of accuracy and provides a better understanding of age-specific effects. In addition, the neonatal brain is highly plastic and thus a particularly promising target for therapeutic interventions.
Demographic variables included maternal age, paternal age, maternal education, paternal education, maternal ethnicity, paternal ethnicity, maternal psychiatric history, paternal psychiatric history, and total household income. Medical history variables included birthweight, gestational age at birth, 5-minute APGAR scores, stay in neonatal intensive care unit over 24 h, gestation number (twin or singleton), and delivery method (vaginal or caesarian-section). Maternal smoking during pregnancy was also assessed. Outcomes included total gray matter volume (GM), total white matter volume (WM), total cerebrospinal fluid (CSF), and intracranial volume (ICV, sum of GM, WM, and CSF) as well as cerebellum volume.
Materials and Methods
Subjects
756 neonates (406 males, 350 females; 356 singletons, 400 twins) between 37 and 57 weeks gestational age are included in this analysis. All children were participating in prospective longitudinal studies of early brain development being carried out at the University of North Carolina at Chapel Hill (UNC). Mothers were recruited during the second trimester of pregnancy from the outpatient obstetrics and gynecology clinics at UNC hospitals. Exclusion criteria at enrollment were the presence of abnormalities on fetal ultrasound or major medical illness in the mother. Demographic variables (maternal age, paternal age, maternal education, paternal education, maternal ethnicity, paternal ethnicity, maternal psychiatric history, paternal psychiatric history, and total household income) were collected via maternal report. For the purpose of the current study maternal psychiatric history and paternal psychiatric history were treated as binary variables. Individuals were counted as positive for psychiatric history if they reported a diagnosis in any of the following DSM-V categories, or if medical record review indicated such a diagnosis: schizophrenia spectrum and other psychotic disorders, bipolar and related disorders, depressive disorders, anxiety disorders, obsessive-compulsive and related disorders, attention-deficit hyperactivity disorders, Tourette's syndrome, or autism spectrum disorders. Medical history variables (birthweight, gestational age at birth, 5-minute APGAR scores, stay in neonatal intensive care unit over 24 h, gestation number, and delivery method) were collected from maternity and pediatric medical records shortly after birth. Maternal smoking during pregnancy was collected via maternal report at two time points during pregnancy and shortly after birth. Demographic and obstetric history data are summarized in Table 1. Experiments were undertaken with the understanding and written consent of each subject's mother or father, with the approval of the Institutional Review Board of the UNC School of Medicine.
Table 1.
Descriptive statistics for demographic and medical history variables
| Continuous variables | Mean | SD | Min | Max |
|---|---|---|---|---|
| Gestational age at MRI (days) | 294 | 16 | 262 | 401 |
| Age since DOBa (days) | 33 | 20 | 6 | 161 |
| Gestational age at birth (days) | 261 | 19 | 192 | 295 |
| Birthweight (gr) | 2847 | 703 | 790 | 4732 |
| 5-minute APGAR score | 9 | 1 | 3 | 10 |
| Maternal age (years) | 30 | 6 | 16 | 47 |
| Paternal age (years) | 32 | 7 | 17 | 64 |
| Maternal education (years) | 15 | 4 | 0 | 26 |
| Paternal education (years) | 15 | 4 | 0 | 26 |
| Categorical variables | No. | Percent |
| Gender | ||
| Male | 406 | 54 |
| Female | 350 | 46 |
| Gestation number | ||
| Twin | 356 | 47 |
| Singleton | 400 | 53 |
| NICU stay >24 h | ||
| No | 591 | 78 |
| Yes | 165 | 22 |
| Caesarian section | ||
| No | 354 | 47 |
| Yes | 402 | 53 |
| Maternal ethnicity | ||
| White | 578 | 77 |
| Black | 159 | 21 |
| Asian | 16 | 2 |
| Native American | 3 | <1 |
| Paternal ethnicity | ||
| White | 551 | 73 |
| Black | 176 | 23 |
| Asian | 23 | 3 |
| Native American | 6 | <1 |
| Maternal psychiatric history | ||
| No | 472 | 62 |
| Yes | 284 | 38 |
| Paternal psychiatric history | ||
| No | 672 | 89 |
| Yes | 84 | 11 |
| Incomeb | ||
| High | 235 | 31 |
| Middle | 192 | 25 |
| Low | 284 | 38 |
| Missing | 45 | 6 |
| Maternal smoking | ||
| No | 685 | 91 |
| Yes | 71 | 9 |
aDOB = date of birth.
bLow income: at or below 200% of federal poverty level (FPL), middle income: between 200% and 400% of FPL, high income: above 400% of FPL.
Image Acquisition
Scans were acquired with either a Siemens Allegra head-only 3 T scanner using a circular polarization head coil (N = 668) or a Siemens TIM Trio 3 T scanner using a 32 channel head coil (N = 89) (Siemens Medical Supplies, Erlangen, Germany). For Allegra, structural T1-weighted images were initially acquired with a fast low angular shot sequence (TR [time repetition] = 15 ms, TE [time echo]= 7 ms, flip angle = 25°, spatial resolution = 0.9 × 0.9 × 1 mm3) (N = 36). An improved MRI protocol was acquired in the remaining subjects (N = 632) with a 3D magnetization prepared rapid gradient echo sequence (MP-RAGE, TR = 1820 ms, TE = 4.38 ms, flip angle = 7°, spatial resolution = 1 × 1 × 1 mm3). For Trio, T1-weighted images were acquired with a 3D MP-RAGE sequence (TR = 1820 ms, TE = 3.75 ms, flip angle = 7°, spatial resolution = 1 × 1 × 1 mm3) (N = 89). Proton density and T2-weighted images were acquired on Allegra using turbo-spin echo (TSE) sequences. For the first 11 subjects the parameters were TR = 7000, TE1 = 18, TE2 = 108, flip angle = 150o, spatial resolution = 1 × 1 × 3.9 mm3. An improved protocol was acquired in the remaining subjects (N = 657) (TR range = 5270–6200 ms, TE1 range = 20–21 ms, TE2 range = 119–124 ms, flip angle = 150°, spatial resolution = 1.25 × 1.25 × 1.95 mm3). The first 12 subjects scanned on the Trio also used a TSE sequence (TR = 6200 ms, TE1 = 17, TE2 = 116 ms, flip angle = 150°, spatial resolution = 1.25 × 1.25 × 1.95 mm3). We then upgraded to a 3DT2 SPACE protocol (TR = 3200 ms, TE = 406, flip angle = 120°, spatial resolution = 1 × 1 × 1 mm3) (N = 76). We performed an ANOVA in 561 unrelated subjects to determine if acquisition parameters or platform (Allegra or TIM Trio) impacted brain volumes. Results indicated that platform did have a significant impact on GM volume and a marginal effect on ICV. Acquisition parameters within each platform did not significantly affect brain volumes. These results are in keeping with prior studies (Jovicich et al. 2009). Platform was included as a covariate in all subsequent analyses.
Image Analysis (Global Brain Volumes)
Segmentation of brain tissues was performed using an atlas-based expectation-maximization segmentation algorithm based on both T1 and T2 weighted images specifically adapted to the neonate brain setting (Prastawa et al. 2005). Cortical tissues were automatically segmented into GM, WM, and CSF as previously described (Gilmore et al. 2007). Parcellation of the cerebellum was achieved by nonlinear warping of a parcellation atlas template as previously described (Gilmore et al. 2007).
Statistical Analysis (Global Brain Volumes)
In order to determine the potential impact of major demographic and medical history variables on inter-individual variation in global brain and tissue volumes, we used a moment-based method to select fixed effects in linear mixed effects models (Ahn et al. 2012). Twins are treated as repeated measures. For fixed effects selection, we applied an adaptive Lasso penalty using the feasible generalized least squares estimator as an initial. We used the BIC statistic to select the tuning parameter of the adaptive Lasso. Before applying our variable selection method, we standardized all covariates and centered the response variable. We also applied bootstrap methods 1000 times to assess the stability of our results. Variables were considered significant if they were selected more than 800 times. After model selection, we ran mixed effects models using the selected independent variables for significance testing and to estimate r2 values. Mixed effects models were also run including all variables for comparison. To account for subject correlation among MZ and DZ twins, we applied the linear mixed effects model described previously (Xia et al. 2014) which considers additive genetic effects (A), common environmental effects (C), and random environmental effects (E).
Image Analysis (Deformation Based Morphometry)
Next, we used deformation-based morphometry (DBM) to determine how variables selected by adaptive lasso (gestational age at MRI, sex, birthweight, and gestational age at birth) impacted local GM volumes. Local GM changes provide greater insight into potential functional consequences both in terms of cognitive development and risk for psychopathology. For this analysis brain tissue was extracted from T2-weighted images. Images were corrected for intensity inhomogeneity, skull stripped, and aligned using both rigid and affine registration methods. Intensity histogram matching was then applied on the affinely aligned images and an unbiased large deformation nonrigid group-wise registration method (Joshi et al. 2004) was applied to produce the atlas and corresponding deformation fields.
Statistical Analysis (DBM)
To test the impact of each predictor, we used a multiscale adaptive generalized estimation equation with the Jacobian determinant of the deformation matrix as response at each GM voxel (Li et al. 2013). ICV and scanner type were included as covariates in addition to gestational age at MRI, sex, gestational age at birth, and birthweight. Cluster-based inference was used to identify significant associations (Hayasaka et al. 2004). Following the recommendation of Silver et al. (2011), we used a relatively high cluster-forming threshold (P < 0.001) and a cluster extent criterion of P < 0.05.
Marginal Effects
The failure of a particular predictor to be included in the final model as selected by adaptive lasso does not mean it has no relationship with global brain tissue volumes. Unselected predictors may exert effects, which are mediated by other predictors with greater explanatory power. To address this issue, we tested the marginal effect of each variable not selected in our primary analysis on global tissue volumes, adjusting for gestational age at MRI and scanner. Linear mixed effects models were used as before.
Mediation Analyses
To evaluate whether marginal associations were mediated by gestational age at birth or birthweight, we performed mediation analysis (Baron and Kenny 1986) by modeling gestational age at birth or birthweight as mediators with environmental factors as predictors and scanner and gestational age at MRI as covariates. In selecting which associations to probe in the mediation analysis, we considered the correlation matrix for all variables (see Supplementary Table S1), with a particular focus on those selected by adaptive lasso. Here, we denote environmental factors as X, mediators as M, covariates as C, and brain volumes as response variable Y. Four steps are involved in the mediation analysis: Step 1: Show that the predictor is correlated with the response. Use Y as the response variable in a regression equation and X as a predictor (Y − X + C). This step establishes that there is an effect that may be mediated. Step 2: Show that the predictor is correlated with the mediator and use regression model M − X + C. This step essentially involves treating the mediator as if it were a response variable. Step 3: Show that the mediator affects the response variable Y and use regression model Y − X + M + C. This step evaluates the effect size of predictor when mediator is present. Step 4: Sobel test is used to evaluate the significance of the mediation. All the regression models were analyzed using linear regression model on a subset of independent subjects (e.g., all singletons plus one randomly selected individual from each twin pair).
Sensitivity Analyses
We also ran the following sensitivity analyses: 1) For all global brain volume outcome variables except the cerebellum (where sex was not selected in the bootstrapping analysis), we ran mixed effects models in order to test for interaction effects between sex and other selected predictors. 2) We performed our primary analyses (variable selection, full mixed models, and DBM) replacing gestational age at birth with days since birth. 3) We performed our primary analyses (variable selection, full mixed models, and DBM) as well as the marginal association analysis stratifying by gestational age at birth. Specifically, we split the cohort into two groups: a full term group with gestational age at birth ≥37 weeks (N = 473) and a preterm group with gestational age at birth <37 weeks (N = 283). 4) We ran full mixed models and selected models excluding birthweight as a predictor.
Results
Primary Analyses (Variable Selection and DBM)
Gestational age at MRI and gestational age at birth emerged as the most important predictors for all brain volumes examined, being selected over 80% of the time over 1000 bootstrap samples (See Supplementary Table S2). Birthweight was selected over 80% of the time for all outcomes except CSF. Sex was selected over 80% of the time for all outcomes except cerebellar volume. Paternal education was selected for total WM only. Mixed effects models containing all possible predictors confirmed the importance of gestational age at MRI, sex, gestational age at birth, and birthweight (See Supplementary Tables S3–S7). Paternal education was not a significant predictor of total WM in the full mixed effect model, but was in the selected mixed effect model (see Supplementary Table S4). The selected models explain 59% of the variance in total GM, 46% of the variance in total WM, 31% of the variance in total CSF, 55% of the variance in ICV, and 41% of the variance in cerebellar volume.
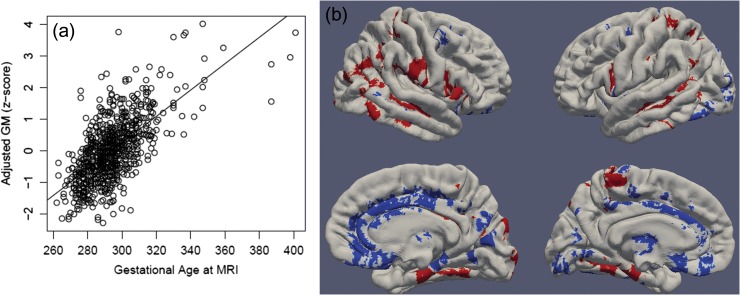
Gestational age at MRI was the strongest predictor of brain volumes with ICV increasing by 0.55% every day. Relative effect sizes were similar for GM, WM, CSF, and cerebellar volumes (See Fig. 1 and Supplementary Table S8). Deformation based morphometry (DBM) showed that lateral temporal cortex, right lateral occipital cortex, postcentral gyrus and paracentral lobules, insular and inferior frontal cortex, medial prefrontal cortex, the putamen, anterior portions of the fusiform gyrus, and lateral portions of the thalamus grew significantly faster than would be predicted based on ICV alone. The anterior cingulate gyrus, medial occipital cortex, medial portions of the thalamus, and posterior portions of the left fusiform gyrus grew significantly slower than would be predicted based on ICV alone (See Fig. 1 and Supplementary Table S9).
Figure 1.
Gestational age at MRI is strongly associated with GM volumes. Panel a: Multiple regression showed that gestational age at MRI has a strong, positive association with total GM volume in infancy (adjusted for birthweight, sex, gestational age at birth, and scanner). Panel b: DBM identifies associations between gestational age at MRI and local GM volumes (adjusting for birthweight, sex, gestational age at birth, scanner, and ICV). Positive associations, indicating fast-growing regions are shown in red and negative associations, indicating slow-growing regions are shown in blue. Significant clusters projected onto surface-rendered views of the left and right hemispheres; lateral view (top), medial view (bottom).
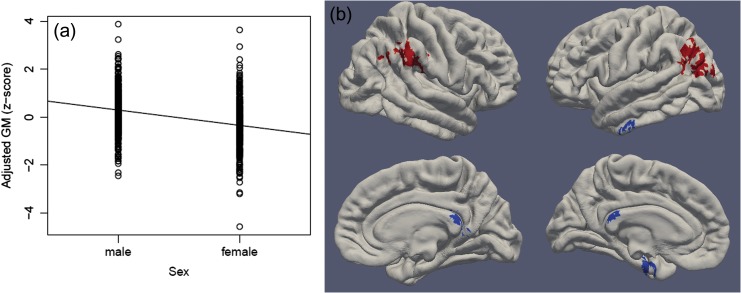
Males had 5% larger ICV than females. Relative effect sizes were similar for GM, WM, and CSF (See Fig. 2 and Supplementary Table S8). DBM revealed that males had relatively larger GM volumes than females in left medial temporal cortex, left anterior inferior temporal gyrus, and posterior cingulate cortex (See Fig. 2 and Supplementary Table S9). Females had relatively larger GM volumes than males in the posterior middle temporal gyri and parietal cortex. These clusters appear to correspond to the temporal-parietal junction (TPJ).
Figure 2.
Child sex is associated with GM volumes. Panel a: Multiple regression showed that males have larger total GM volume in infancy compared with females (adjusted for birthweight, gestational age at MRI, gestational age at birth, and scanner). Panel b: DBM identifies associations between sex and local GM volumes (adjusting for birthweight, gestational age at MRI, gestational age at birth, scanner, and ICV). Regions where females have relatively larger GM volumes are shown in red and regions where males have relatively larger GM volumes are shown in blue. Significant clusters projected onto surface-rendered views of the left and right hemispheres; lateral view (top), medial view (bottom).
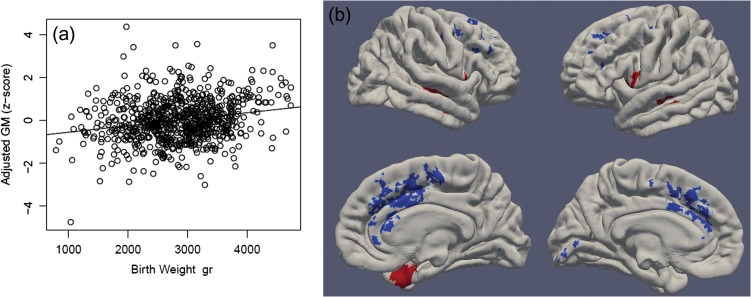
Birthweight also showed strong positive associations with brain volumes with every 500 g increase in birthweight producing a 4% increase in ICV. Relative effect sizes were similar for GM, WM, and cerebellum volumes (See Fig. 3 and Supplementary Table S8). DBM revealed that heavier babies had relatively larger GM volumes than lighter babies in lateral temporal, inferior frontal, and insular cortices. Heavier babies had relatively smaller volumes than lighter babies in medial frontal cortex, particularly in the anterior cingulate gyri, medial superior and middle frontal gyri, and supplementary motor areas. They also had smaller volumes in calcarine cortex and right angular gyrus (See Fig. 3 and Supplementary Table S9).
Figure 3.
Birthweight is associated with GM volumes. Panel a: Multiple regression showed that birthweight has a positive association with total GM volume (adjusted for sex, gestational age at MRI, gestational age at birth, and scanner). Panel b: DBM identifies associations between birthweight and local GM volumes (adjusting for sex, gestational age at MRI, gestational age at birth, scanner, and ICV). Positive associations are shown in red and negative associations are shown in blue. Significant clusters projected onto surface-rendered views of the left and right hemispheres; lateral view (top), medial view (bottom).
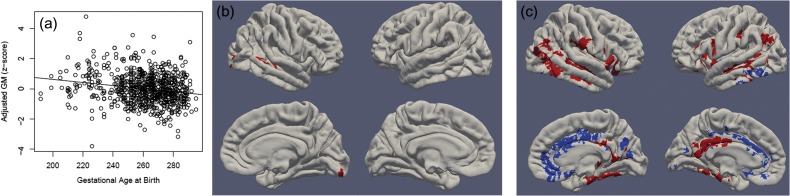
Gestational age at birth showed a strong negative correlation with brain volumes within the context of the final model. In other words, later born babies had smaller brain volumes than earlier born babies when controlling for gestational age at scan, birthweight, and sex. Within the age-range examined in this study (gestational age at birth between 27 and 42 weeks), for every additional day in the womb, ICV decreased by 0.35%. Relative effect sizes were similar for GM, WM, CSF, and cerebellar volumes (See Fig. 4 and Supplementary Table S8). DBM revealed 3 small clusters in right superior temporal and middle temporal cortex, right posterior fusiform gyrus/occipital cortex, and the right caudate where earlier born babies had relatively smaller volumes than later born babies (See Fig. 4 and Supplementary Table S9).
Figure 4.
Gestational age at birth is associated with GM volumes. Panel a: Multiple regression showed that earlier born babies had larger total GM volume (adjusted for sex, gestational age at MRI, birthweight, and scanner). Panel b: DBM identifies associations between gestational age at birth and local GM volumes (adjusting for sex, gestational age at MRI, birthweight, scanner, and ICV). Panel c: DBM identifies associations between gestational age at birth and local GM volumes (adjusting for sex, days post-birth, birthweight, scanner, and ICV). Positive associations are shown in red and negative associations are shown in blue. Significant clusters projected onto surface-rendered views of the left and right hemispheres; lateral view (top), medial view (bottom).
Secondary Analyses (Marginal Associations and Mediation Analysis)
Regarding demographic factors, the following variables were never significant: maternal age, paternal age, paternal ethnicity, paternal psychiatric history, and income. Maternal education was positively associated with total WM and GM. Paternal education was positively associated with total GM and with ICV (See Supplementary Table S10). Note that WM was not included in the marginal association analyses for paternal education, as it was selected in the adaptive lasso. Maternal education effects on brain volumes are mediated, at least in part, by birthweight (See Supplementary Fig. S1). Birthweight explains 20% of the total effect of maternal education on WM and 29% of the total effect on GM (P = 0.047 and 0.004, respectively). Birthweight also explains 16% of the total effect of paternal education on GM (P = 0.02). The association between paternal education and ICV was not mediated by birthweight. Maternal ethnicity was associated with ICV and GM. Offspring of Asian mothers have larger brain volumes than offspring of white mothers, while offspring of African American and Native American mothers have smaller brain volumes than offspring of white mothers. The differences in GM between white and African American mothers are mediated, at least in part, by birthweight (see Supplementary Fig. S1); other ethnic groups could not be included in the mediation analysis due to small subject numbers, but see Table S11 for descriptive data and ANOVA of ethnicity effects on birthweight. Birthweight explained 28% of the total effect of maternal ethnicity on GM (P = 0.003). The association between maternal ethnicity and ICV was not mediated by differences in birthweight. Maternal psychiatric history was associated with reductions in ICV and GM. As maternal psychiatric history was not associated with either birthweight or gestational age, it was not included in the mediation analyses.
Regarding medical history factors, high APGAR scores were associated with reductions in ICV, WM, and CSF. Gestation number was positively associated with ICV, WM, and CSF; twins had larger brain volumes than singletons adjusting for gestational age at MRI. Delivery method was positively associated with ICV, GM, and CSF; children delivered via caesarian section had larger brain volumes than those delivered vaginally adjusting for gestational age at MRI. NICU stay was positively associated with ICV and CSF. The majority of these relationships were mediated by gestational age at birth (see Supplementary Fig. S1). Gestational age at birth explains 47% of the total effect of APGAR scores on ICV and 78% of the total effect on CSF (P = 0.002 and 3.16 × 10–9, respectively). WM was not examined in the mediation analysis as APGAR scores did not predict WM values in the subset of unrelated cases included in the mediation analysis. Gestational age at birth explains 70% of the total effect of gestation number on ICV and 93% of the total effect on CSF (P = 0.008 and 2.71 × 10–12, respectively). The association between gestation number and WM was not mediated by birthweight or gestational age at birth. Gestational age at birth explains 42% of the total effect of delivery method on ICV, and 62% of the total effect on CSF (P = 0.003, and 5.61 × 10–9, respectively). GM was not examined in the mediation analysis as gestational age at birth did not predict GM values in the subset of unrelated cases included in the mediation analysis. Gestational age at birth explains 100% of the effect of NICU stay on CSF (P = 2.71 × 10–12).
Maternal smoking was associated with reduced ICV, WM, and GM. Effects on GM were mediated by birthweight (see Supplementary Fig. S1). Birthweight explained 28% of the effect on GM (P = 0.009). The association between maternal smoking and ICV and WM was not mediated by birthweight.
Sensitivity Analysis 1 (Sex Interaction)
No significant interaction effects between sex and the other selected variables were observed for ICV, WM, or GM. For CSF, there was a significant interaction effect between sex and gestational age at MRI (see Supplementary Table S12). The direction of the relationship was the same in males and females, with males showing a slightly steeper slope (see Supplementary Fig. S2).
Sensitivity Analysis 2 (Replacing Gestational Age at Birth with Days Since Birth)
The overall pattern of results was quite similar with the exception of gestational age at birth (see Supplementary Tables S13–S19 and Supplementary Figs S3–S5). In these models, gestational age at birth shows a positive correlation with global brain volumes. In addition, many more significant clusters emerged in the DBM analysis for gestational age at birth (see Fig. 4). Earlier born babies had smaller volumes in the lateral temporal cortex, fusiform gyrus, inferior frontal cortex, postcentral gyrus, insular cortex, posterior cingulate, putamen, thalamus, and left inferior parietal cortex and larger volumes in the medial frontal, parietal, and occipital cortices as well as the left inferior temporal gyrus.
Sensitivity Analysis 3 (Stratifying by Gestational Age at Birth)
Results of variable selection were highly similar in the full sample and the subset of children born ≥37 weeks (see Supplementary Table S20). In the subset of preterm children, fewer variables crossed the selection threshold of 80%. Gestational age at MRI was selected for all brain volume outcomes; birthweight was selected for WM and GM, and gestational age at birth and sex were selected for CSF (see Table S21). This is likely a consequence of the smaller sample size for the preterm subset which makes the variable selection less stable. Comparison of the full mixed models (see Supplementary Tables S22–S31) indicates that gestational age at MRI, gestational age at birth, sex, and birthweight are important variables for all outcome variables regardless of whether the children are full term or preterm. Of particular note, the strong negative correlation between gestational age at birth and brain volumes is present in both the full and preterm subsets. Regarding the DBM analyses, fewer clusters are observed in the sensitivity analyses, which is to be expected given the smaller sample size and reduced power when subdividing the sample. For gestational age at MRI, the relatively slow growth of the anterior cingulate and relatively rapid growth of middle temporal cortex is consistent across the full and preterm subset (see Supplementary Figs S6 and S7). For sex, larger volumes in the TPJ of females were observed in both the full and preterm subset although the laterality varies (e.g., it is observed in the left hemisphere in the full term subset and the right hemisphere of the preterm subset). Male larger volumes in the medial temporal cortex were only observed in the full term subset. Male larger volumes in posterior cingulate were only observed in the preterm subset (see Supplementary Figs S8 and S9). Birthweight does not show any regional effects in the full term subset. Positive relationships between birthweight and GM volumes in lateral temporal cortex and negative relationships between birthweight and GM volumes in medial frontal cortex, similar to those observed in the full sample, were present, though less pronounced, in the preterm subset (see Supplementary Fig. S10). Regional effects of gestational age at birth are not observed in either subset. Marginal association analyses were highly similar between the full sample and the full term subset with the following exceptions: 1) no associations were observed with NICU stay in the full term subset. 2) Cerebellar volume was associated with income (individuals with low income had smaller cerebellums, findings would not survive multiple comparison corrections), and 3) paternal ethnicity showed nominal associations with ICV and GM. In contrast to the full term sample, in the preterm subsample, no relationships were observed with maternal or paternal ethnicity, APGAR scores, gestation number, delivery method, or maternal smoking. Paternal psychiatric history showed a nominal association with CSF volumes (paternal psychiatric history was associated with less CSF).
Sensitivity Analysis 4 (Removing Birthweight as a Predictor)
In order to test the possibility that the negative correlation of brain volume with gestational age at birth is an artifact created by the assumption that correcting for weight will be the same across gestational age, we ran full mixed models and selected models excluding birthweight as a predictor. Significant negative correlations between brain volumes and gestational age at birth were still evident (see Tables S36–S40).
Discussion
The present results detail the impact of 17 major demographic and obstetric history variables on inter-individual variation in neonatal brain tissue volumes. While some of these variables have been examined in other studies, this is the first, in our knowledge, to apply a population neuroscience approach which considers a large number of environmental variables simultaneously. The current study is also unique in its focus on the infant brain. Converging evidence indicates that psychiatric illnesses including autism and schizophrenia have roots in the prenatal and early postnatal period (Fatemi and Folsom 2009; Chen et al. 2015). Even neurodegenerative disorders such as Alzheimer's appear to have a developmental component (Knickmeyer et al. 2013). In addition, neuroimaging phenotypes in early life predict cognitive ability (Short et al. 2013; Alcauter et al. 2014; Deoni et al. 2014). These converging lines of evidence strongly suggest that studying infants will provide greater insight into the ultimate origins of individual differences in cognitive performance and psychiatric risk.
In keeping with the extremely rapid pace of early postnatal brain development (Knickmeyer et al. 2008), gestational age at MRI was the strongest predictor of brain volumes. Studies of volumetric growth from birth to 2 years of age (Gilmore et al. 2012), as well as studies of human brain development during later childhood and adolescence (Amlien et al. 2014), suggest that the development of the cerebral cortex mirrors its hierarchical organization, with primary sensory areas maturing earlier than higher-order association areas. The current results suggest a more complicated picture, at least in early infancy. While fast-growing regions do include a number of primary sensory cortices (primary somatosensory cortex in the postcentral gyri and paracentral lobules, primary gustatory cortex in the insula, and perhaps primary olfactory cortex in the medial temporal lobe), multi-modal integration areas such as the middle temporal gyri are also growing rapidly. Likewise, slow-growing regions include both primary sensory cortex (e.g., primary visual cortex around the calcarine sulcus) as well as multi-modal areas in the cingulate gyri. Fast-growing regions may be particularly vulnerable to injury in early life. The potential developmental vulnerability of insular cortex is of particular interest as a recent meta-analytic study identified insular cortex as part of a shared biological substrate for multiple psychiatric illnesses, including schizophrenia, bipolar disorder, depression, addiction, obsessive-compulsive disorder, and anxiety (Goodkind et al. 2015).
Males had larger global brain volumes than females, but the magnitude of this difference was smaller than that reported in children (De Bellis et al. 2001) and adults (Gur et al. 1999; Nopoulos et al. 2000), suggesting that males experience accelerated brain growth in the first several years of life when compared with females. Males also had relatively larger GM volumes than females in left medial temporal cortex, left anterior inferior temporal gyrus, and posterior cingulate cortex. Sexual dimorphism in posterior cingulate may be relevant to sex differences in visceral pain, though we note that studies of neonatal responses to pain are somewhat inconsistent with regards to sex (Sternberg et al. 2004). Females had relatively larger GM volumes than males in the TPJ, an area engaged in lower-level computational processes associated with the sense of agency and reorienting attention to salient stimuli, as well as higher level social processes, such as perspective taking, empathy, and theory of mind (Decety and Lamm 2007). Greater volumes in females may explain why newborn girls show better social interactive capacities than newborn boys on the Brazelton Scale (Lundqvist and Sabel 2000; Boatella-Costa et al. 2007) and prefer looking at a human face over a mechanical mobile (Connellan et al. 2001). It may also help explain why girls display communicative gestures, productive vocabulary, word-gesture combinations, and word-word combinations earlier than boys (Ozcaliskan and Goldin-Meadow 2010; Eriksson et al. 2012) and are less likely to be diagnosed with autism spectrum disorders (Baron-Cohen et al. 2011).
Birthweight also showed strong positive associations with global brain volumes. This effect was particularly pronounced in lateral temporal, inferior frontal, and insular cortices, regions which are growing at a particularly fast rate in early infancy and, by extrapolation, in late pregnancy. As fetal weight increases exponentially in the third trimester (Johnsen et al. 2006), environmental factors influencing weight gain may also have a pronounced effect on brain regions undergoing rapid development in this period. Similar relationships between birthweight and surface area in the lateral temporal cortex were reported by Walhovd et al. (2012) and Raznahan et al. (2012) in individuals ranging from 3 to 30 years of age, suggesting the impact of birthweight on temporal cortex persists from infancy into adulthood. Walhovd et al. (2012) also reported strong positive relationships between birthweight and cortical surface area on the medial surface of the brain in the anterior cingulate, supplementary motor areas, and superior frontal and medial orbitofrontal cortices. Raznahan et al. (2012) reported more circumscribed effects on medial frontal cortex (limited to the superior frontal gyrus). The authors hypothesized that this was a developmentally stable effect with origins in the second trimester of pregnancy when environmental factors, such as poor maternal nutrition, might reduce amplified progenitor cell division within the subventricular zone. We also observed strong associations between birthweight and brain structure in the medial frontal lobes, but the direction of effect was opposite of that reported by Walhovd et al. (2012) and Raznahan et al. (2012). This suggests that the impact of birthweight on volumes of medial prefrontal cortex in adolescence and adulthood is not a consequence of reduced progenitor cell division prenatally, though this may explain the overall larger brain size in larger babies.
Perhaps the most striking and unexpected finding of the current study is that later born babies had smaller brain volumes than earlier born babies when controlling for gestational age at scan and birthweight. Many studies have reported reduced brain volumes in preterm children at term-equivalent age (Padilla et al. 2014) and throughout childhood and adolescence (de Kieviet et al. 2012). However, the majority of existing studies focused on extreme rather than normative variation in gestational age, and rarely disambiguated low birthweight from prematurity. Our results suggest that earlier born children experience accelerated brain growth, either as a consequence of the richer sensory environment they encounter outside the womb or in response to other factors associated with delivery. This interpretation is supported by a secondary analysis in which we replaced gestational age at MRI with days post-birth. In these models, the coefficients for gestational age at birth and days post-birth are both positive and the coefficient for days since birth is always greater than the coefficient for gestational age at MRI, consistent with the hypothesis that the brain grows faster after birth than during pregnancy. Interestingly, a recent longitudinal neuroimaging study of 87 healthy term-born or term-equivalent preterm-born infants, aged 2 to 90 days, suggests that earlier born babies continue to show accelerated brain growth compared with later born babies for at least the first 3 months, as the expected brain size of an infant born 1 week earlier than average was 5% smaller than average at term, but only 2% smaller than average at 90 days (Holland et al. 2014).
Despite the overall larger brain volume in earlier born babies, DBM indicated that earlier born babies had relatively smaller GM volumes in visual association cortex and primary auditory and auditory association cortex, suggesting these brain regions are disrupted by early delivery. Regarding the latter finding, impaired performance on basic semantic tasks has been reported in school-age preterm children with functional recovery during adolescence, despite persistent structural and functional abnormalities in language networks (Schafer et al. 2009). We note that more extensive regions of GM reduction have been reported in extremely preterm babies (Padilla et al. 2014). In sensitivity analyses using days post-birth rather than gestational age at MRI, earlier born babies had larger volumes in the medial frontal, parietal, and occipital cortices as well as the left inferior temporal gyrus. These regions are involved in control of autonomic functions such as blood pressure and heart rate (anterior cingulate cortex), control of movement and postural stability (supplementary motor areas), somatosensory input from the distal limbs (paracentral lobule), self-referential processing and motor imagery (precuneus), basic visual processing (cuneus and calcarine cortex), and visual object recognition (inferior temporal gyrus)—all functions that might be enhanced by early exposure to the extra-uterine environment. Larger GM volumes in the occipital, parietal, and frontal cortices of extremely preterm infants have also been reported by Padilla et al. (2014) using voxel-based morphometry.
Maternal age, paternal age, paternal ethnicity, paternal psychiatric history, and income were never significant predictors of global brain volumes even when testing for marginal associations. The negative finding for income is especially intriguing as socioeconomic disparities are clearly associated with differences in cognitive development and family income is positively associated with cortical surface area in children and adolescents (Noble et al. 2015). In addition, low income to needs ratios are associated with reduced cortical GM and WM volumes in school-age children (Luby et al. 2013). The mechanisms responsible for these relationships are unclear, but prenatal conditions, levels of cognitive and psycho-social stimulation, availability of nutritious food, and exposure to stress, infections, and environmental toxins in infancy and early childhood have all been suggested as mediators. The current results suggest that prenatal factors do not play a major role in the relationship of poverty to global brain tissue volumes. This hypothesis is supported by a recent study showing that children from low income households have similar GM volumes to children from medium and high income households at 5 months of age, but display slower trajectories of GM growth during later infancy and early childhood (Hanson et al. 2013). Thus, postnatal interventions are likely to produce important positive gains in brain development for children from low income households.
Maternal and paternal education did show marginal associations with global brain volumes which were partially mediated by differences in birthweight. Given the negative findings for income this is unlikely to reflect differences in financial resources, but may reflect differences in cultural capital. In particular a lack of information regarding the importance of prenatal care, proper nutrition, and avoidance of environmental pollutants, including maternal cigarette smoke, could negatively impact both birthweight and brain development. In addition, shared heritable factors influencing brain volume and educational achievement may explain the association between paternal education and ICV as well as some of the remaining effect of paternal education on GM volumes after accounting for birthweight.
Maternal ethnicity also showed marginal associations with global brain volumes which were partially mediated by differences in birthweight. Our results are in keeping with previous studies demonstrating that African American mothers are at increased risk for delivering low birthweight infants (Herd et al. 2015), a health disparity which is also apparent in our sample. The remaining effect after controlling for birthweight could be due to genetic variation between ethnicities. It could also arise as a consequence of other factors that segregate with ethnicity such as access to and utilization of prenatal care. As of 2008, early initiation into prenatal care (first trimester) was less common among African American women (60.2%) compared with white women (76.7%) (Osterman et al. 2013). Given that the basic structures of the brain and central nervous system are already established by 8 weeks post conception and the majority of neurons are produced by mid-gestation (Stiles and Jernigan 2010), late initiation of prenatal care could have significant consequences for fetal brain development which go above and beyond influences on birthweight.
Regarding maternal smoking, multiple studies have reported that cigarette exposure results in general, symmetrical growth restriction including decreased head circumference (Roza et al. 2007). While brain maturation is generally preserved in mild fetal growth restriction, our results suggest there is a downstream impact on GM volumes. In addition, smoking appears to exert effects on ICV and WM that are independent of birthweight. This is in keeping with the largest ultrasound study of maternal smoking and fetal growth carried out to date, which showed reduced growth of fetal head circumference and biparietal diameter in offspring exposed to maternal smoking, even after controlling for the effects of maternal smoking on abdominal circumference (Roza et al. 2007).
Maternal psychiatric history showed marginal associations with ICV and GM that were not mediated by birthweight or gestational age at birth. This may reflect an unmeasured heritable component linking psychiatric risk and brain volumes. Alternatively, this relationship may arise from adverse environmental effects of being carried by a mother with a psychiatric illness.
Finally, we observed marginal associations between global brain volumes and APGAR scores, gestation number, delivery method, and NICU stay. The direction of these effects may appear somewhat paradoxical (e.g., higher APGAR scores, indicative of better physical condition (Apgar 1953), were associated with smaller brain volumes), but further analyses indicated that the majority of these relationships were mediated by gestational age at birth and reflect rapid brain growth in earlier born babies. The one exception is the association between gestation number and WM volumes where birthweight did not achieve significance in the mediation analyses. Thus, there may be some independent effect of twin gestation on WM development.
In conclusion, our results highlight the importance of prenatal and perinatal factors in explaining individual differences in neonatal brain volumes. The relationships we observed may help explain individual variation in cognitive ability and risk for psychiatric and neurological disorders. Consequently, they have important public health policy implications. Additional research with longitudinal follow-up is needed to address the stability of these findings across the lifespan and their functional consequences, as well as interactions with postnatal environmental influences and genetic influences.
Supplementary Material
Notes
We thank the participating families that made this project possible as well as the staff of the UNC MRI Research Center, the UNC Neuro Image Research and Analysis Laboratories, and the UNC Early Brain Development Program. We would especially like to thank Joseph Blocher, Rachel Steiner, and Dianne Evans. Conflict of Interest: None declared.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institutes of Health (MH064065, MH070890, and HD053000 to Dr. Gilmore, MH083045 to Dr. Knickmeyer, SES-1357666, DMS-1407655, and MH086633 to Dr. Zhu, HD003110, EB005149 to Dr. Styner, and NS007431 to Ms. Jha).
References
- Ahn M, Zhang HH, Lu W. 2012. Moment-based method for random effects selection in linear mixed models. Stat Sin. 22:1539–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W. 2014. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci. 34:9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlien IK, Fjell AM, Tamnes CK, Grydeland H, Krogsrud SK, Chaplin TA, Rosa MG, Walhovd KB. 2014. Organizing principles of human cortical development-thickness and area from 4 to 30 years: insights from comparative primate neuroanatomy. Cereb Cortex. 26:257–267. [DOI] [PubMed] [Google Scholar]
- Anthony JC. 2001. The promise of psychiatric enviromics. Br J Psychiatry Suppl. 40:s8–s11. [DOI] [PubMed] [Google Scholar]
- Apgar V. 1953. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. 32:260–267. [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. 2011. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 9:e1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- Boatella-Costa E, Costas-Moragas C, Botet-Mussons F, Fornieles-Deu A, De Caceres-Zurita ML. 2007. Behavioral gender differences in the neonatal period according to the Brazelton scale. Early Hum Dev. 83:91–97. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Thompson PM. 2014. A focus on structural brain imaging in the Alzheimer's disease neuroimaging initiative. Biol Psychiatry. 75:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JA, Penagarikano O, Belgard TG, Swarup V, Geschwind DH. 2015. The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol. 10:111–144. [DOI] [PubMed] [Google Scholar]
- Connellan J, Baron-Cohen S, Wheelwright S, Ba'tki A, Ahluwalia J. 2001. Sex differences in human neonatal social perception. Infant Behav Dev. 23:113–118. [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. 2001. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 11:552–557. [DOI] [PubMed] [Google Scholar]
- de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. 2012. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 54:313–323. [DOI] [PubMed] [Google Scholar]
- de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. 2010. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci USA. 107:16881–16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. 2010. The neuroscience of human intelligence differences. Nat Rev Neurosci. 11:201–211. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. 2007. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 13:580–593. [DOI] [PubMed] [Google Scholar]
- Deoni SC, O'Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, Dirks H, Piryatinsky I, Dean DC 3rd, Jumbe NL. 2014. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct. 221:1189–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Marschik PB, Tulviste T, Almgren M, Perez Pereira M, Wehberg S, Marjanovic-Umek L, Gayraud F, Kovacevic M, Gallego C. 2012. Differences between girls and boys in emerging language skills: evidence from 10 language communities. Br J Dev Psychol. 30:326–343. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. 2009. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 35:528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, Mc Guire P, Sacchetti E. 2011. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 35:1175–1185. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, et al. 2007. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 27:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Zhu H, Hamer RM, Styner M, Shen D. 2012. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 22:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, et al. 2015. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. 1999. Sex differences in brain gray and white matter in healthy young adults: Correlations with cognitive performance. J Neurosci. 19:4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, Pollak SD. 2013. Family poverty affects the rate of human infant brain growth. PLoS One. 8:e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. 2004. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 22:676–687. [DOI] [PubMed] [Google Scholar]
- Herd D, Gruenewald P, Remer L, Guendelman S. 2015. Community level correlates of low birthweight among African American, hispanic and white women in California. Matern Child Health J. 19:2251–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Chang L, Ernst TM, Curran M, Buchthal SD, Alicata D, Skranes J, Johansen H, Hernandez A, Yamakawa R, et al. 2014. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol. 71:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Hoek HW, Susser E, Brown AS, Dingemans A, Schnack HG, van Haren NE, Pereira Ramos LM, Gispen-de Wied CC, Kahn RS. 2000. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 157:1170–1172. [DOI] [PubMed] [Google Scholar]
- Johnsen SL, Rasmussen S, Wilsgaard T, Sollien R, Kiserud T. 2006. Longitudinal reference ranges for estimated fetal weight. Acta Obstet Gynecol Scand. 85:286–297. [DOI] [PubMed] [Google Scholar]
- Joshi S, Davis B, Jomier M, Gerig G. 2004. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 23(Suppl 1):S151–S160. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, et al. 2009. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 46:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. 2008. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 28:12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Wang J, Zhu H, Geng X, Woolson S, Hamer RM, Konneker T, Lin W, Styner M, Gilmore JH. 2013. Common variants in psychiatric risk genes predict brain structure at birth. Cereb Cortex. 24:1230–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gilmore JH, Shen D, Styner M, Lin W, Zhu H. 2013. Multiscale adaptive generalized estimating equations for longitudinal neuroimaging data. Neuroimage. 72:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D. 2013. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 167:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist C, Sabel KG. 2000. Brief report: The Brazelton Neonatal Behavioral Assessment Scale detects differences among newborn infants of optimal health. J Pediatr Psychol. 25:577–582. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, et al. 2015. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 18:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O'Leary D, Andreasen NC. 2000. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 98:1–13. [DOI] [PubMed] [Google Scholar]
- Osterman MJ, Martin JA, Curtin SC, Matthews TJ, Wilson EC, Kirmeyer S. 2013. Newly released data from the revised U.S. birth certificate, 2011. Natl Vital Stat Rep. 62:1–22. [PubMed] [Google Scholar]
- Ozcaliskan S, Goldin-Meadow S. 2010. Sex differences in language first appear in gesture. Dev Sci. 13:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla N, Alexandrou G, Blennow M, Lagercrantz H, Aden U. 2014. Brain growth gains and losses in extremely preterm infants at term. Cereb Cortex. 25:1897–1905. [DOI] [PubMed] [Google Scholar]
- Paus T. 2010. Population neuroscience: why and how. Hum Brain Mapp. 31:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. 2007. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 28:464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin W, Gerig G. 2005. Automatic segmentation of MR images of the developing newborn brain. Med Image Anal. 9:457–466. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Greenstein D, Lee NR, Clasen LS, Giedd JN. 2012. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci USA. 109:11366–11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza SJ, Verburg BO, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EA, Witteman JC, Verhulst FC, Tiemeier H. 2007. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur J Neurosci. 25:611–617. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT, et al. 2009. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 132:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schull WJ, Norton S, Jensh RP. 1990. Ionizing radiation and the developing brain. Neurotoxicol Teratol. 12:249–260. [DOI] [PubMed] [Google Scholar]
- Short SJ, Elison JT, Goldman BD, Styner M, Gu H, Connelly M, Maltbie E, Woolson S, Lin W, Gerig G, et al. 2013. Associations between white matter microstructure and infants’ working memory. Neuroimage. 64:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M, Montana G, Nichols TE, Alzheimer's disease, neuroimaging I. 2011. False positives in neuroimaging genetics using voxel-based morphometry data. Neuroimage. 54:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg WF, Smith L, Scorr L. 2004. Nociception and antinociception during the first week of life in mice: sex differences and test dependence. J Pain. 5:420–426. [DOI] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. 2010. The basics of brain development. Neuropsychol Rev. 20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ Jr, Roddey JC, Erhart M, McCabe C, Akshoomoff N, et al. 2012. Long-term influence of normal variation in neonatal characteristics on human brain development. Proc Natl Acad Sci USA. 109:20089–20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K, Yu Y, Ahn M, Zhu H, Zou F, Gilmore JH, Knickmeyer RC. 2014. Environmental and genetic contributors to salivary testosterone levels in infants. Front Endocrinol (Lausanne). 5:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.