Abstract
Background: Risk factors may differentially influence development of estrogen receptor (ER)–positive vs –negative breast cancer. We examined associations with strong, prevalent risk factors by ER subtype.
Methods: Of 1 279 443 women age 35 to 74 years participating in the Breast Cancer Surveillance Consortium, 14 969 developed ER-positive and 3617 developed ER-negative invasive breast cancer. We calculated hazard ratios (HRs) using Cox regression and compared ER subtype hazard ratios at representative ages or by menopausal status using Wald tests. All statistical tests were two-sided.
Results: For women age 40 years, compared with no prior biopsy, ER-positive vs ER-negative HRs were 1.53 (95% CI = 1.30 to 1.81) vs 1.26 (95% CI = 0.90 to 1.76) for nonproliferative disease, 1.63 (95% CI = 1.23 to 2.17) vs 1.41 (95% CI = 0.78 to 2.57) for proliferative disease without atypia, and 4.47 (95% CI = 2.88 to 6.96) vs 0.20 (95% CI = 0.02 to 2.51) for proliferative disease with atypia. Benign disease proliferation risk was stronger for ER-positive than ER-negative cancer for women age 35 years (Wald P = .04), age 40 years (Wald P = .04), and age 50 years (Wald P = .06). Among pre/perimenopausal women, body mass index (BMI) had a stronger association with ER-negative than ER-positive cancer (obese II/III vs. normal weight: HR = 1.52, 95% CI = 1.19 to 1.94; vs 1.21, 95% CI = 1.08 to 1.36). Increasing BMI similarly increased ER-positive and ER-negative cancer risk among postmenopausal hormone users (Wald P = .15) and nonusers (Wald P = .08). Associations with ER subtype varied by race/ethnicity across all ages (P < .001) and by family history of breast cancer and breast density for specific ages.
Conclusions: Strength of risk factor associations differed by ER subtype. Separate risk models for ER subtypes may improve identification of women for targeted prevention strategies.
Primary prevention with selective estrogen receptor modulators (SERM) decreases the risk of estrogen-receptor (ER)–positive, but not ER-negative, breast cancer, and detection with digital mammography varies by ER status (1–3). Available risk prediction models, whose implementation is a key aspect of guiding prevention strategies, predict invasive cancer risk overall rather than by ER subtype (4–6).
Few studies of risk factors used in risk models have examined associations by ER subtype and, of those that have, results are inconsistent. Studies have reported history of benign breast disease (BBD) increases risk of ER-positive and -negative cancer among pre- (7) and postmenopausal women (8). While one study of postmenopausal women reported a stronger association of BBD with ER-negative than ER-positive cancer (9) and another of premenopausal and postmenopausal women reported similar associations (7), neither study stratified by BBD diagnosis. A BBD cohort study reported women with atypical hyperplasia had a higher frequency of ER-positive cancer compared with women with proliferative disease without atypia or nonproliferative disease (10).
Several studies, including a recent meta-analysis (11), report no difference in associations between breast density and breast cancer risk by ER subtype (11–17), while others report stronger associations for ER-positive cancer (18–20). In contrast, a large study suggests breast density is more strongly associated with ER-negative (vs ER-positive) breast cancer, but only among women younger than age 55 years compared with older women (21).
Elevated body mass index (BMI) is associated with increased ER-positive cancer risk in postmenopausal women (22–24), while results for ER-negative cancer are inconsistent. One study of postmenopausal women not using hormone therapy (HT) reported an association with ER-negative cancer and elevated BMI (25), while other studies have found no association (22–24). Studies have reported no association or a decreased risk with elevated BMI by ER subtypes in premenopausal women (23,25,26), but ER-negative studies have limited statistical power (23–25).
We used data from the large, prospective Breast Cancer Surveillance Consortium (BCSC) cohort to examine the associations of ER-positive and ER-negative invasive cancer with strong, prevalent risk factors used in risk prediction models (4–6) to assess if risk factors are differentially associated with ER subtypes.
Methods
Study Population
The National Cancer Institute (NCI)-funded BCSC (http://breastscreening.cancer.gov) (27) is a community-based, geographically diverse cohort study that broadly represents the population of women undergoing mammography in the United States (28). Our sample consisted of 1 279 443 women age 35 to 74 years who had at least one mammogram with American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) breast density reported between 1994 and 2014 and complete information on race/ethnicity, family history of breast cancer, and history of breast biopsy, as well as, for those who developed invasive breast cancer, ER status. We excluded women with a diagnosis of breast cancer prior to their first eligible mammogram or within the first three months of follow-up and women who had breast implants or who had undergone mastectomy. Each registry obtains annual institutional review board approval for consenting processes or a waiver of consent, enrollment of participants, and data linkages for research purposes. All registries received a Federal Certificate of Confidentiality that protects the identities of research participants.
Measurement of Risk Factors
Self-reported age, first-degree family history of breast cancer, race/ethnicity, prior breast biopsy history, height, weight, menopause status, and current postmenopausal HT use were obtained at the time of each mammography examination. Postmenopausal women were those with both ovaries removed, whose periods had stopped naturally, with current HT use, or age 55 years or older. Premenopausal women reported a period within the last 180 days, were younger than age 40 years, or were birth control hormone users, while perimenopausal were not sure if their periods had stopped or their last menstrual period was 180 to 364 days ago. Women were considered to have missing menopausal status if they had a hysterectomy without bilateral oophorectomy or surgical menopause and were not using HT or if their menopause status could not be determined based on available information (29–31). Height and weight were used to calculate BMI by dividing weight in kilograms by height in meters squared (kg/m2). BMI was examined as a continuous variable and stratified into five standard categories (underweight < 18.5 kg/m2, normal weight = 18.5–24.95 kg/m2, overweight = 25.0–29.95 kg/m2, grade I: obese = 30.0–34.95 kg/m2; grade II/III: obese > 35.05 kg/m2) using nationally defined cut-points (32). Race and ethnicity were coded using the expanded definition currently used in Surveillance, Epidemiology, and End Results (SEER) and US Vital statistics (non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Native American/Alaskan Native, Hispanic, other/mixed race).
Benign Breast Disease
Community pathologists at each registry classified breast biopsy results using their local clinical practice. We grouped each diagnosis from pathology reports into one of four categories: nonproliferative, proliferative without atypia, proliferative with atypia, and lobular carcinoma in situ (LCIS) using the taxonomy proposed by Dupont and Page (33–35). Details of benign diagnoses included in each category have been described previously (https://tools.bcsc-scc.org/bc5yearrisk/calculator.htm) (4). If there was more than one diagnosis per biopsy or multiple biopsies were performed within three months, we chose the diagnosis with the highest grade (LCIS > atypical hyperplasia > proliferative without atypia > nonproliferative). We classified the biopsy as diagnosis unknown if a woman reported a prior biopsy but pathology results were not available.
Mammographic Breast Density
Community radiologists classified breast density as part of routine clinical practice using the four BI-RADS density categories (36): a = almost entirely fat; b = scattered fibroglandular densities c = heterogeneously dense; d = extremely dense.
Ascertainment of Breast Cancer Cases
Breast cancers diagnosed at least three months after index mammograms were obtained through linkage with the regional population-based SEER programs, state tumor registries, and pathology databases. Invasive cancers were categorized by ER status.
Vital Status
Vital status was obtained through linkage to SEER and state tumor registries and state death records.
Statistical Analysis
We estimated hazard ratios (HRs) for each risk factor using partly conditional Cox proportional hazards regression to incorporate biopsies occurring after study entry and to facilitate risk prediction (37). We fit separate models for ER-positive and ER-negative invasive breast cancer. We used a robust sandwich estimator for repeated measures survival data to account for multiple observations per woman (38). The model time scale started three months after the index screening or diagnostic mammogram and possibly again three months after each new, more severe biopsy result. Age and risk factors were updated each time women entered the model. Each observation was censored at the time of death, diagnosis of invasive cancer of the other ER subtype or DCIS, mastectomy, at the end of complete cancer capture for her BCSC registry, or at 10 years of follow-up. All models were adjusted for risk factors and interactions in the BCSC model including age at study entry, age-squared and race/ethnicity, and interactions between age and breast density, family history, BBD, and race/ethnicity, all which had a P value of less than .10 in the ER-positive model. Hazard ratios are reported at representative ages because of statistically significant interactions of age with other risk factors. As BMI was missing for 43% of women, we fit separate models to examine the association of BMI by ER subtype including interactions between BMI and menopause status. We report Pinteraction values for linear and quadratic age and BMI separately, which makes the type I error rate higher than 5% but provides more information for descriptive purposes. We assessed the proportional hazards assumption by calculating interval-specific hazard ratios (ie, zero to three months, three to six months, six months to one year, one to two years, etc.) for each predictor variable and comparing for clinically meaningful changes over time. The proportional hazards assumption appeared reasonable for all predictors except BBD, where hazard ratios within six months of study entry were higher than in subsequent years. Excluding these data did not change the model results in a clinically significant manner.
We used Wald chi-square tests in a competing risks model to compare ER-positive and ER-negative model parameters (39). Comparisons were made within levels of age and menopausal status for risk factors involving interactions.
Analyses were performed using R version 3.0.3 and SAS version 9.3. Statistical tests were two-sided, and the cut-point for statistical significance was .05.
Results
Study Characteristics
During a median follow-up of 8.3 years, 18 586 women developed invasive breast cancer, 14 969 ER-positive (80.5%) and 3617 ER-negative (19.5%). Black women had the highest proportion of ER-negative tumors (37.7%) (Table 1).
Table 1.
Baseline characteristics of study cohort
| No breast cancer | ER-positive cancer | ER-negative cancer | |
|---|---|---|---|
| Characteristics | No. (%) | No. (%) | No. (%) |
| Total | 1 260 857 (98.6) | 14 969 (1.2) | 3617 (0.3) |
| Age groups, y | |||
| 35–39 | 111 400 (8.8) | 658 (4.4) | 238 (6.6) |
| 40–44 | 287 067 (22.8) | 2145 (14.3) | 606 (16.8) |
| 45–49 | 222 884 (17.7) | 2213 (14.8) | 689 (19.1) |
| 50–54 | 204 094 (16.2) | 2462 (16.5) | 581 (16.1) |
| 55–59 | 152 357 (12.1) | 2327 (15.6) | 524 (14.5) |
| 60–64 | 116 788 (9.3) | 2012 (13.4) | 394 (10.9) |
| 65–69 | 95 224 (7.6) | 1792 (12.0) | 332 (9.2) |
| 70–74 | 71 043 (5.6) | 1360 (9.1) | 253 (7.0) |
| Race/ethnicity | |||
| White, non-Hispanic | 936 413 (74.3) | 12 220 (81.6) | 2622 (72.5) |
| Black, non-Hispanic | 94 725 (7.5) | 884 (5.9) | 536 (14.8) |
| Asian, Native Hawaiian, or Pacific Islander | 84 122 (6.7) | 644 (4.3) | 167 (4.6) |
| American Indian or Alaska Native | 11 801 (0.9) | 75 (0.5) | 27 (0.8) |
| Hispanic | 115 082 (9.1) | 962 (6.4) | 220 (6.1) |
| Other, mixed (2+ races) | 18 714 (1.5) | 184 (1.2) | 45 (1.2) |
| 1st-degree relatives with breast cancer | |||
| No | 1 109 616 (88.0) | 12 187 (81.4) | 3036 (83.9) |
| Yes | 151 241 (12.0) | 2782 (18.6) | 581 (16.1) |
| Benign breast disease | |||
| No prior biopsy | 1 060 408 (84.1) | 11 173 (74.6) | 2783 (76.9) |
| Prior biopsy, diagnosis unknown | 158 967 (12.6) | 3073 (20.5) | 704 (19.5) |
| Nonproliferative | 29 038 (2.3) | 446 (3.0) | 89 (2.5) |
| Proliferative without atypia | 10 349 (0.8) | 193 (1.3) | 30 (0.8) |
| Proliferative with atypia | 1662 (0.1) | 52 (0.4) | 7 (0.2) |
| Lobular carcinoma in situ | 433 (0.03) | 32 (0.2) | 4 (0.1) |
| BI-RADS breast density* | |||
| Almost entirely fat | 106 244 (8.4) | 713 (4.8) | 157 (4.3) |
| Scattered densities | 528 925 (42.0) | 5675 (37.9) | 1350 (37.3) |
| Heterogeneously dense | 499 894 (39.7) | 6821 (45.6) | 1679 (46.4) |
| Extremely dense | 125 794 (10.0) | 1760 (11.8) | 431 (11.9) |
| Body mass index, kg/m2† | |||
| Underweight (<18.5) | 13 137 (1.8) | 140 (1.7) | 22 (1.3) |
| Normal (18.5–24.9) | 328 162 (46.0) | 3581 (44.6) | 722 (43.2) |
| Overweight (25.0–29.9) | 201 841 (28.3) | 2402 (29.9) | 521 (31.2) |
| Obese, grade I (30.0–34.9) | 100 197 (14.0) | 1152 (14.3) | 226 (13.5) |
| Obese, grade II/III (≥35.0) | 70 406 (9.9) | 761 (9.5) | 181 (10.8) |
| Menopausal status‡ | |||
| Pre- or perimenopausal | 501 293 (46.2) | 4335 (32.5) | 1243 (39.4) |
| Postmenopausal with HT | 229 557 (21.2) | 3895 (29.2) | 824 (26.1) |
| Postmenopausal no HT | 353 610 (32.6) | 5115 (38.3) | 1089 (34.5) |
BI-RADS density categories: a = almost entirely fat; b = scattered fibroglandular densities; c = heterogeneously dense; d = extremely dense. BI-RADS = Breast Imaging Reporting and Data System; ER = estrogen receptor; HT = hormone therapy.
Missing body mass index: 547 114 no breast cancer, 6933 ER-positive, 1945 ER-negative.
Missing menopausal status: 176 397 no breast cancer, 1624 ER-positive, 461 ER-negative; 95% premenopausal and 5% perimenopausal.
BBD Associations by ER Subtypes
The strength of BBD associations with ER-positive (Pinteraction = .08 for linear age and P = .07 for quadratic age) and ER-negative cancer (Pinteraction = .17 for linear age and P = .18 for quadratic age) did not vary with age. ER-positive cancer risk increased with extent of BBD proliferation for all ages of women (Table 2). For women age 40 years, compared with no prior biopsy, ER-positive vs ER-negative hazard ratios were 1.53 (95% CI = 1.30 to 1.81) vs 1.26 (95% CI = 0.90 to 1.76) for nonproliferative disease, 1.63 (95% CI = 1.23 to 2.17) vs 1.41 (95% CI = 0.78 to 2.57) for proliferative disease without atypia, 4.47 (95% CI = 2.88 to 6.96) vs 0.20 (95% CI = 0.02 to 2.51) for proliferative disease with atypia, and 9.93 (95% CI = 4.79 to 20.6) for LCIS. BBD proliferation increased ER-negative cancer risk the most in women age 60 years (Table 2). BBD proliferation associations were stronger for ER-positive than ER-negative cancer for women younger than age 50 years (Wald P = .04, age 35 years; P = .04, age 40 years; P = .06, age 50 years).
Table 2.
Cox proportional hazards model results for benign breast disease by estrogen receptor–positive or estrogen receptor–negative events
| HR (95% CI)* |
|||||
|---|---|---|---|---|---|
| Characteristic | Age 35 y | Age 40 y | Age 50 y | Age 60 y | Age 70 y |
| ER-positive | |||||
| Benign breast disease | |||||
| No prior biopsy | (ref) | (ref) | (ref) | (ref) | (ref) |
| Prior biopsy, diagnosis unknown | 1.39 (1.17 to 1.65) | 1.41 (1.27 to 1.57) | 1.46 (1.40 to 1.53) | 1.52 (1.45 to 1.59) | 1.58 (1.48 to 1.68) |
| Nonproliferative | 1.61 (1.22 to 2.13) | 1.53 (1.30 to 1.81) | 1.49 (1.36 to 1.63) | 1.58 (1.44 to 1.74) | 1.84 (1.60 to 2.11) |
| Proliferative without atypia | 1.53 (0.95 to 2.46) | 1.63 (1.23 to 2.17) | 1.83 (1.62 to 2.08) | 2.03 (1.78 to 2.33) | 2.23 (1.85 to 2.69) |
| Proliferative with atypia | 5.05 (2.44 to 10.43) | 4.47 (2.88 to 6.96) | 3.68 (2.98 to 4.54) | 3.21 (2.59 to 3.97) | 2.97 (2.10 to 4.19) |
| LCIS | 19.79 (6.09 to 64.35) | 9.93 (4.79 to 20.57) | 4.32 (3.04 to 6.12) | 3.90 (2.75 to 5.52) | 7.30 (4.75 to 11.21) |
| ER-negative | |||||
| No prior biopsy | (ref) | (ref) | (ref) | (ref) | (ref) |
| Prior biopsy, diagnosis unknown | 1.56 (1.13 to 2.15) | 1.52 (1.26 to 1.84) | 1.47 (1.34 to 1.61) | 1.44 (1.30 to 1.59) | 1.43 (1.23 to 1.67) |
| Nonproliferative | 1.08 (0.60 to 1.94) | 1.26 (0.90 to 1.76) | 1.53 (1.27 to 1.84) | 1.60 (1.31 to 1.96) | 1.43 (1.00 to 2.06) |
| Proliferative without atypia | 1.33 (0.47 to 3.75) | 1.41 (0.78 to 2.57) | 1.53 (1.15 to 2.02) | 1.57 (1.14 to 2.17) | 1.54 (0.92 to 2.58) |
| Proliferative with atypia | NE† | 0.20 (0.02 to 2.51) | 1.23 (0.55 to 2.75) | 2.33 (1.22 to 4.48) | 1.34 (0.43 to 4.22) |
| LCIS | NE† | NE† | 1.05 (0.17 to 6.30) | 3.73 (1.39 to 10.05) | 1.52 (0.23 to 9.94) |
| P, Wald test§ | .04 | .04 | .06 | .61 | .16 |
Adjusted for age at entry (linear and quadratic terms) and race/ethnicity, with interactions between Breast Imaging Reporting and Data System density and age at entry (linear), first-degree relatives and age at entry (linear and quadratic), benign breast disease (linear and quadratic terms), and race/ethnicity and age at entry (linear). Analyses include 1 361 990 mammograms associated with 16 749 estrogen receptor (ER)–positive and 3990 ER-negative cancers. CI = confidence interval; ER = estrogen receptor; HR = hazard ratio; LCIS = lobular carcinoma in situ.
Nonestimable because of small sample sizes.
Compares estrogen receptor–positive vs –negative cancer parameters within age group. This test was two-sided.
BMI Associations by ER Subtypes
Menopausal status was statistically significantly associated with ER-positive cancer (P < .001) and ER-negative cancer (P = .03), with statistically significant interactions with BMI (Table 3). Pre/perimenopausal and postmenopausal overweight/obese women were at increased risk for ER-positive cancer, while underweight women were at decreased risk compared with normal weight women. Pre/perimenopausal women and postmenopausal overweight/obese HT nonusers were at increased ER-negative cancer risk, while underweight women were at decreased risk compared with normal weight women. For example, pre/perimenopausal obese, grade II/III vs normal weight women had a stronger association with ER-negative vs ER-positive cancer (HR = 1.52, 95% CI = 1.19 to 1.94; vs HR = 1.21, 95% CI = 1.08 to 1.36) (Table 3). Also, postmenopausal obese, grade II/III HT users were at increased ER-negative cancer risk compared with normal weight women.
Table 3.
Cox proportional hazards model results for 687 601 examinations and 8386 estrogen receptor–positive or 1715 estrogen receptor–negative events by body mass index
| HR (95% CI)* |
|||
|---|---|---|---|
| ER subtype by BMI | Pre- or perimenopausal | Postmenopausal current HT use | Postmenopausal no current HT use |
| ER-positive† | |||
| BMI, kg/m2‡ | |||
| 18 (underweight) | 0.93 (0.87 to 0.98) | 0.90 (0.83 to 0.97) | 0.79 (0.74 to 0.84) |
| 22 (normal) | (ref) | (ref) | (ref) |
| 27 (overweight) | 1.08 (1.03 to 1.14) | 1.11 (1.05 to 1.18) | 1.28 (1.21 to 1.35) |
| 32 (obese, grade I) | 1.15 (1.06 to 1.25) | 1.20 (1.09 to 1.32) | 1.53 (1.41 to 1.66) |
| 39 (obese, grade II/III) | 1.21 (1.08 to 1.36) | 1.28 (1.11 to 1.47) | 1.78 (1.61 to 1.98) |
| ER-negative§ | |||
| BMI, kg/m2‡ | |||
| 18 (underweight) | 0.76 (0.66 to 0.87) | 0.96 (0.85 to 1.09) | 0.88 (0.79 to 0.98) |
| 22 (normal) | (ref) | (ref) | (ref) |
| 27 (overweight) | 1.28 (1.14 to 1.44) | 1.08 (0.95 to 1.22) | 1.17 (1.06 to 1.30) |
| 32 (obese, grade I) | 1.48 (1.25 to 1.77) | 1.19 (0.96 to 1.47) | 1.38 (1.16 to 1.63) |
| 39 (obese, grade II/III) | 1.52 (1.19 to 1.94) | 1.44 (1.06 to 1.94) | 1.72 (1.36 to 2.17) |
| P‖ | .03 | .15 | .08 |
Adjusted for age at entry (linear and quadratic terms) and race/ethnicity, with interactions between Breast Imaging Reporting and Data System density and age at entry (linear), first-degree relatives and age at entry (linear and quadratic), benign breast disease (linear and quadratic terms), and race/ethnicity and age at entry (linear). BMI = body mass index; CI = confidence interval; ER = estrogen receptor; HR = hazard ratio.
P values for continuous BMI linear and quadratic terms = .06 and .19, respectively; P value for menopausal status ≤ 0.001; Pinteraction values between continuous BMI linear and quadratic terms and menopausal status = .02 and .12, respectively. All statistical tests were two-sided.
Median of values within range of values for category.
P values for continuous BMI linear and quadratic terms = .002 and .009, respectively; P value for menopausal status = .03; Pinteraction values between continuous BMI linear and quadratic terms and menopausal status = .02 and .02, respectively. All statistical tests were two-sided.
Compares estrogen receptor–positive vs –negative cancer parameters within menopausal group. P values were calculated using a two-sided Wald test.
Increasing BMI similarly increased ER-positive and ER-negative cancer risk among postmenopausal hormone users (Wald P = .15) and nonusers (Wald P = .08), but associations were stronger with ER-negative than ER-positive cancer for pre/perimenopausal women (Wald P = .03) (Table 3).
Race/Ethnicity, Family History, and Breast Density Associations by ER Subtypes
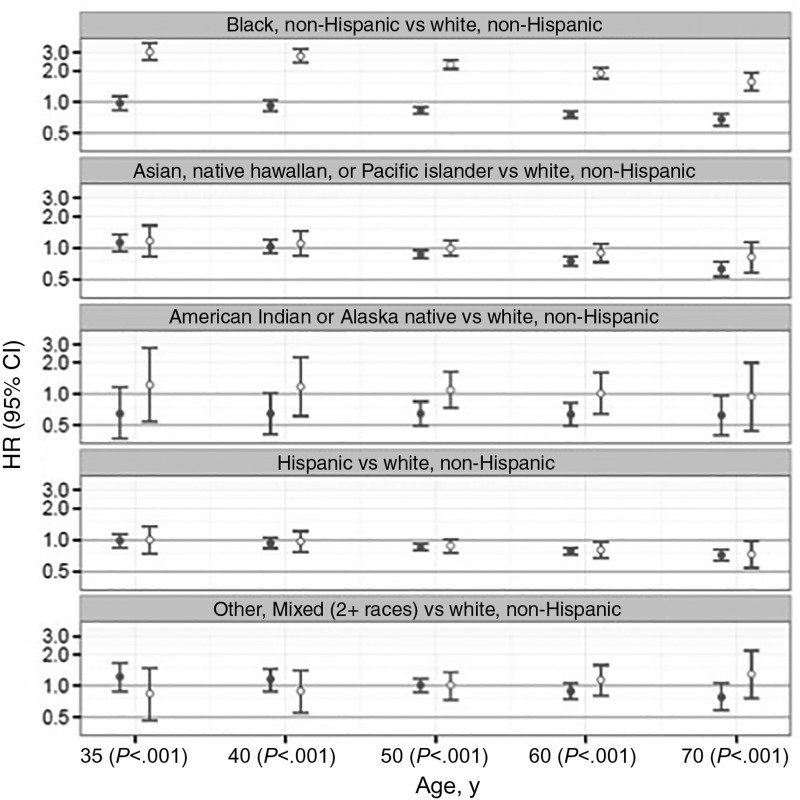
The strength of race/ethnicity associations with ER subtype (Figure 1) statistically significantly declined with age for ER-positive (Pinteraction < .001) and ER-negative cancer (Pinteraction = .004). Black, non-Hispanic women had an increased ER-negative cancer risk for all ages compared with white, non-Hispanic women with the highest hazard ratio for women age 35 years (3.05, 95% CI = 2.52 to 3.70) and lowest for women age 70 years (1.56, 95% CI = 1.28 to 1.90). The effect of race/ethnicity on risk differed by ER subtype for all ages (Wald P < .001) because of an increased ER-negative cancer risk for black, non-Hispanic women.
Figure 1.
Association of race/ethnicity by estrogen receptor subtype. Cox proportional hazards model results for estrogen receptor–positive (solid circle) and –negative (open circle) invasive breast cancers comparing non-Hispanic black, Asian/Pacific Islander, Native American/Alaskan Native, Hispanic, and other/mixed race to non-Hispanic white women by age. The numbers in parentheses along the x-axis are P values calculated by two-sided Wald test comparing estrogen receptor–positive vs –negative cancer parameters within age group. The error bars represent the 95% confidence intervals. CI = confidence interval; HR = hazard ratio.
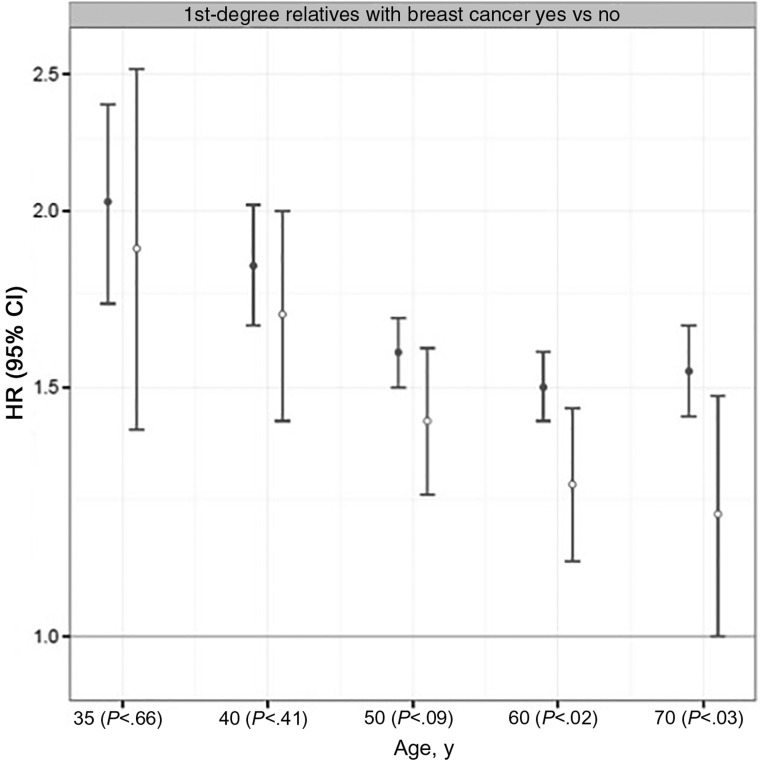
The strength of family history associations with ER-positive cancer (Figure 2) statistically significantly declined with age (Pinteraction = .02 for linear age and P = .03 for quadratic age), but not for ER-negative cancer (Pinteraction = .33 for linear age and P = .46 for quadratic age). Family history of breast cancer associations was stronger for ER-positive compared with ER-negative cancer for women older than age 50 years (Wald P = .09, age 50 years; P = .02, age 60 years; P = .03, age 70 years).
Figure 2.
Association of first-degree family history of breast cancer by estrogen receptor subtype. Cox proportional hazards model results for estrogen receptor–positive (solid circle) and –negative (open circle) invasive breast cancers comparing women with and without a first-degree relative with breast cancer. The numbers in parentheses along the x-axis are P values calculated using a two-sided Wald test result to compare estrogen receptor–positive vs –negative cancer parameters within each age group. The error bars represent the 95% confidence intervals. CI = confidence interval; HR = hazard ratio.
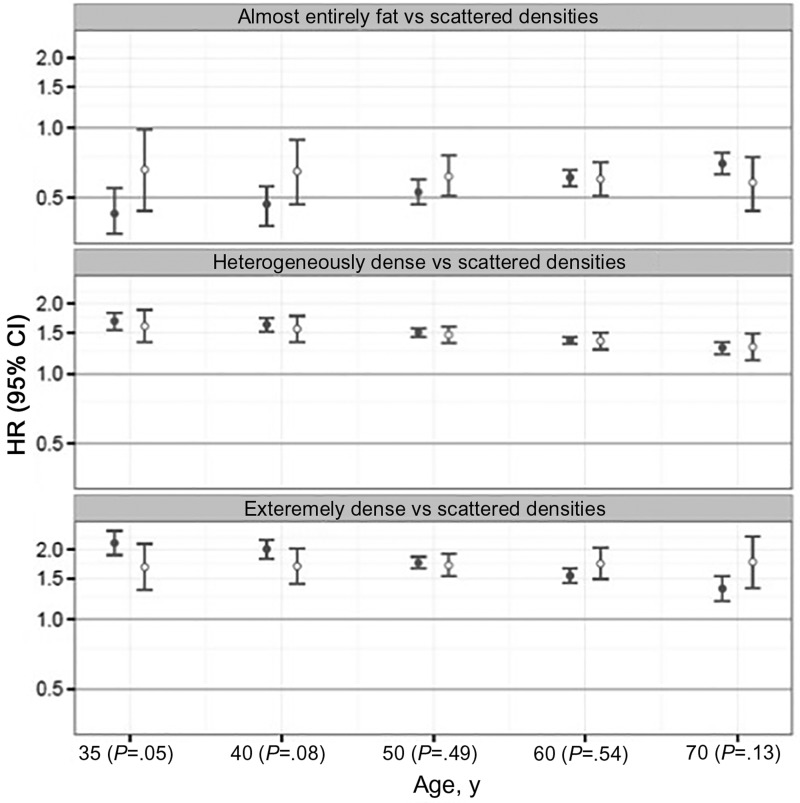
The strength of BI-RADS density associations with ER-positive cancer (Figure 3) statistically significantly declined with age (Pinteraction < .001), but not for ER-negative cancer (Pinteraction = .34). For example, hazard ratios for extremely dense breasts compared with scattered fibroglandular densities remained elevated at 1.68 (95% CI = 1.34 to 2.11) at age 35 years and 1.76 (95% CI = 1.36 to 2.27) at age 70 years for ER-negative cancer, but declined from 2.13 (95% CI = 1.89 to 2.40) at age 35 years to 1.35 (95% CI = 1.20 to 1.53) at age 70 years for ER-positive cancer. Women with dense breasts had a stronger association with ER-positive compared with ER-negative cancer for women younger than age 40 years (Wald P = .05, age 35 years; P = .08, age 40 years).
Figure 3.
Association of breast density by ER subtype. Cox proportional hazards model results for estrogen receptor–positive (solid circle) and –negative (open circle) invasive breast cancers comparing women with almost entirely fat, heterogeneously dens,e and extremely dense breasts compared with scattered fibroglandular density. The numbers in parentheses along the x-axis are P values calculated using a two-sided Wald test result to compare estrogen receptor–positive vs –negative cancer parameters within each age group. The error bars represent the 95% confidence intervals. CI = confidence interval; HR = hazard ratio.
Discussion
We examined strong, prevalent risk factors included in risk prediction models and their associations with ER subtypes because women’s risk of ER subtypes can inform targeted prevention strategies. We found that family history of breast cancer, BBD, breast density, and race/ethnicity increased the risk for both ER-positive and ER-negative invasive breast cancer but the strength of associations varied by age. For example, associations with high breast density decreased with age for ER-positive cancer and remained elevated for ER-negative cancer, whereas associations with family history of breast cancer decreased with age for ER-negative cancer to a greater extent than for ER-positive cancer. In comparison, elevated postmenopausal BMI similarly increased ER-positive and ER-negative cancer risk while elevated peri/premenopausal BMI increased ER-negative cancer risk to a greater extent than ER-positive risk. Our results suggest that there are important differences in associations by ER subtype with age and menopausal status for common risk factors and that these associations should be taken into account in developing risk prediction models (8).
Observational studies consistently report that obesity is associated with increased breast cancer risk in postmenopausal women (40) and weight gain is associated with increased risk in women of all ages (40–42). Consistent with our results, Ritte et al. reported that elevated BMI increases ER-negative cancer risk among overweight/obese postmenopausal women who never used HT, but Ritte et al. did not find an association of BMI and ER-negative cancer diagnosed among premenopausal women (25). In the Shanghai Breast Cancer Study, premenopausal breast cancer was not associated with increasing BMI, but both ER-negative and ER-positive cancers were associated with increasing waist-to-hip ratios (23). Similarly, measures of fat distribution were associated with greater incidence of ER-negative cancer in the Nurses’ Health Study, but not elevated BMI (26). A meta-analysis reported a statistically significant association between BMI greater than 30 kg/m2 and triple-negative breast cancer among premenopausal women (43). Our study extends the literature by showing that premenopausal and postmenopausal obesity is associated with an increase in ER-positive and ER-negative cancer risk in the next 10 years. Our large sample size, ability to account for race/ethnicity, breast density, and BBD diagnoses, as well as our measurement of premenopausal BMI up to 10 years prior to pre- or postmenopausal breast cancer diagnosis, could account for our observed associations between elevated BMI among premenopausal women and ER-negative cancer. Because weight loss among pre- and postmenopausal women not at ideal body weight has been shown to decrease postmenopausal breast cancer risk (44), all women who are overweight or obese should be encouraged to lose weight to decrease their breast cancer risk.
Tamoxifen and raloxifene block the ER receptor to decrease risk of ER-positive tumors (45,46). Thus, risk factors associated with estrogen metabolism are likely to be associated with ER-positive cancer. For example, postmenopausal women with high BMIs have twofold higher circulating estrogens and lower sex hormone–binding globulin levels and thus more bioavailable estrogens to promote tumor growth compared with normal weight women (47). Our results are consistent with this hypothesis because ER-positive cancer risk increases with increasing BMI, in particular among postmenopausal women. Elevated BMI’s association with ER-negative disease is likely by a different mechanism given the strong association among premenopausal women. High blood levels of insulin and insulin-like growth factor and chronic low-grade inflammation have been hypothesized as possible mechanisms by which obesity modulates increased breast cancer risk (40).
Women at highest risk in our study had proliferative breast disease with atypia or LCIS and were at highest risk of ER-positive cancer. A recent meta-analysis reported that proliferative BBD with or without atypia is associated with a statistically significantly increased risk of developing breast cancer (48). The magnitude of the associations we report for ER subtypes and proliferative BBD with or without atypia are consistent with this report, but the associations we report for nonproliferative disease are twofold higher. Pooled studies of nonproliferative disease had statistically significant heterogeneity and publication bias, potentially underestimating breast cancer risk (48), while our study grouped specific nonproliferative diagnoses. Tamoxifen reduces incidence of clinically detected BBD (49), and individuals with BBD have ER-positive epithelium (50). Thus, biologically it is consistent that breast cancer that develops after a BBD diagnosis may likely be ER-positive. Our results highlight that women with proliferative lesions with atypia or LCIS are likely to benefit from tamoxifen because they are primarily at increased ER-positive cancer risk.
High breast density is a heritable risk factor (51–53), and family history of breast cancer is a marker of genetic susceptibility to disease. Both of these risk factors are associated with ER subtypes. The strength of association of family history of breast cancer and breast density decreases with older age for ER-positive breast cancer. Yet, postmenopausal incidence of ER-positive cancer continues to increase with age. One explanation is that ER-positive breast cancer among older women is driven more by processes associated with aging such as the accumulation of somatic mutations that are independent of genetics (54,55). In comparison, the incidence of ER-negative cancer is stable with increasing age and the association with breast density remains elevated across all ages, suggesting that there could be continued genetic influence of breast density for the development of ER-negative cancer as women age. In support of this, at least some single-nucleotide polymorphisms associated with breast density are also preferentially associated with ER-negative cancer (56,57).
This study included large numbers of ER-positive and ER-negative cancers in a nationally representative sample, which should enhance its generalizability. But our study was not without limitations. For proliferative disease with atypia and LCIS, the numbers of ER-negative cancers in these groups were small, limiting our ability to identify important associations. Correlation of self-reported height and weight with measured height and weight has been reported to be high (58,59). Misclassification of obese women to lower BMI categories because of inaccurate self-report and exclusion of women who did not report their weight may have led to an over- or underestimation of the true association between obesity and breast cancer. However, the dose response we observe with increasing BMI and the magnitude of increased risk is consistent with studies that measured BMI and suggest minimal underestimation of risk. We did not account for HT regimen type, but this is unlikely to impact our results given there is no interaction between HT regimen type and BMI (60). Several studies have documented poor agreement between pathologists for some histologic diagnoses (61–66). The decreased precision from lack of pathology standardization would tend to bias results toward the null and lead to an underestimate of the true strength of the associations between BBD and ER subtype.
We found that strong, prevalent risk factors have different strength associations by age, menopausal status, and ER subtype. Risk models that incorporate race/ethnicity, BBD diagnoses, family history of breast cancer, breast density, and BMI with interactions with age and menopausal status could improve prediction of ER subtypes. The BCSC risk model (4) includes these factors except menopausal status and BMI, and the Tyrer-Cuzick model (5) includes these factors except race/ethnicity, and nonproliferative BBD. ER subtype risk models should be compared with standard risk models to determine if they improve risk prediction and better identify women for targeted prevention strategies.
Funding
This work was supported by a National Institutes of Health, National Cancer Institute–funded Program Project (P01 CA154292). Data collection was additionally supported by the Breast Cancer Surveillance Consortium (HHSN261201100031C), Vermont Breast Cancer Surveillance System data collection was also supported by U54CA163303. KK was supported in part by grant R01 CA140286.
Notes
The National Cancer Institute had no role in the study’s design; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html. We thank the BCSC investigators, participating mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes is provided at: http://breastscreening.cancer.gov/.
References
- 1. Henderson L, Miglioretti D, Kerlikowske K, Wernli K, Sprague B, Lehman C.. Breast cancer characteristics associated with digital versus screen-film mammography for screen-detected and interval cancers. AJR. 2015;205(3):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirsh V, Chiarelli A, Edwards S, et al. Tumor characteristics associated with mammographic detection of breast cancer in the Ontario breast screening program. J Natl Cancer Inst. 2011;103(12):942–950. [DOI] [PubMed] [Google Scholar]
- 3. Kerlikowske K, Hubbard R, Miglioretti D, et al. Comparative-effectiveness of digital vs film-screen mammography in community practice in the U.S. Ann Intern Med. 2011(8);155:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tice J, Miglioretti D, Li C, Vachon C, Gard C, Kerlikowske K.. Benign breast disease, mammographic breast density and the risk of breast cancer. J Clin Oncol. 2015;33(28):3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tyrer J, Duff SW, Cuzick J.. A breast cancer prediction model incorporating familial and personal risk factors. Statist. Med. 2004;23(7):1111–1130. [DOI] [PubMed] [Google Scholar]
- 6. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. [DOI] [PubMed] [Google Scholar]
- 7. Rosner B, Glynn R, Tamimi R, et al. Breast cancer risk prediction with heterogeneous risk profiles according to breast cancer tumor markers. Am J Epidemiol. 2013;178(2):296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chlebowski RT, Anderson GL, Lane DS, et al. Predicting risk of breast cancer in postmenopausal women by hormone receptor status. J Natl Cancer Inst. 2007;99(22):1695–1705. [DOI] [PubMed] [Google Scholar]
- 9. Barnes B, Steindorf K, Hein R, Flesch-Janys D, Chang-Claude J.. Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemiol. 2011;35(4):345–352. [DOI] [PubMed] [Google Scholar]
- 10. Visscher D, Frost M, Hartmann L, et al. Clinicopathologic features of breast cancers that develop in women with previous benign breast disease. Cancer. 2016;122(3):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antoni S, Sasco A, dos Santos Silva I, McCormack V.. Is mammographic density differentially associated with breast cancer according to receptor status? A meta-analysis. Breast Cancer Res Treat. 2013;137(2):337–347. [DOI] [PubMed] [Google Scholar]
- 12. Ghosh K, Brandt K, Sellers T, et al. Association of mammographic density with the pathology of subsequent breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17(4):872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aiello E, Buist D, White E, Porter P.. Association between mammographic breast density and breast cancer tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2005;14(3):662–668. [DOI] [PubMed] [Google Scholar]
- 14. Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K.. Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090–2095. [PubMed] [Google Scholar]
- 15. Yang WT, Dryden M, Broglio K, et al. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat. 2008;111(3):405–410. [DOI] [PubMed] [Google Scholar]
- 16. Chen JH, Hsu F, Shih H, et al. Does breast density show difference in patients with estrogen receptor-positive and estrogen receptor-negative breast cancer measured on MRI? Ann Oncol. 2009;20(8):1447–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pollán M, Ascunce N, Ederra M, et al. Mammographic density and risk of breast cancer according to tumor characteristics and mode of detection: A Spanish population-based case-control study. Breast Cancer Res. 2013;15(1):R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding J, Warren R, Girling A, Thompson D, Easton D.. Mammographic density, estrogen receptor status and other breast cancer tumor characteristics. Breast J 2010;16(3):279–289. [DOI] [PubMed] [Google Scholar]
- 19. Conroy S, Pagano I, Kolonel L, Maskarinec G.. Mammographic density and hormone receptor expression in breast cancer: The Multiethnic Cohort Study. Cancer Epidemiol. 2011;35(5):448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eriksson L, Hall P, Czene K, et al. Mammographic density and molecular subtypes of breast cancer. Br J Cancer. 2012;107(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertrand K, Tamimi R, Scott C, et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013;15(6):R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerlikowske K, Walker R, Miglioretti D, Desai A, Ballard-Barbash R, Buist D.. Obesity, mammography use and accuracy, and advanced breast cancer risk. J Natl Cancer Inst. 2008;100(23):1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bao P, Shu X, Gao Y, et al. Association of hormone-related characteristics and breast cancer risk by estrogen receptor/progesterone receptor status in the Shanghai Breast Cancer Study. Am J Epidemiol. 2011;174(6):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neuhouser M, Aragaki A, Prentice R, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the Women's Health Initiative Randomized Clinical Trials. Jama Oncol. 2015;1(5):611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ritte R, Lukanova A, Berrino F, et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: A large prospective cohort study. Breast Cancer Res. 2012;14(3):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris H, Willett W, Terry K, Michels K.. Body fat distribution and risk of premenopausal breast cancer in the nurses’ health study II. J Natl Cancer Inst. 2011;103(3):273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: A national mammography screening and outcomes database. Am J Roetengol. 1997;169(4):1001–1008. [DOI] [PubMed] [Google Scholar]
- 28. Sickles E, Miglioretti D, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology. 2005;235(3):775–790. [DOI] [PubMed] [Google Scholar]
- 29. Ahn J, Schatzkin A, Lacey JV Jr, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167(19):2091–2102. [DOI] [PubMed] [Google Scholar]
- 30. Kerlikowske K, Miglioretti D, Ballard-Barbash R, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. J Clin Oncol. 2003;21(23):4314–4321. [DOI] [PubMed] [Google Scholar]
- 31. Kerlikowske K, Miglioretti D, Buist D, Walker R, Carney P.. Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. J Natl Cancer Inst. 2007;99(17):1335–1339. [DOI] [PubMed] [Google Scholar]
- 32. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158(17):1855–1867. [DOI] [PubMed] [Google Scholar]
- 33. Dupont W, Page D.. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–151. [DOI] [PubMed] [Google Scholar]
- 34. Page D, Dupont W, Rogers L, et al. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55(11):2698–2708. [DOI] [PubMed] [Google Scholar]
- 35. Page D, Schuyler P, Dupont W, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: A retrospective cohort study. Lancet. 2003;361(9352):125–129. [DOI] [PubMed] [Google Scholar]
- 36. Sickles E, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. Reston, VA: 2013. [Google Scholar]
- 37. Zheng Y, Heagerty P.. Partly conditional survival models for longitudinal data. Biometrics. 2005;61(2):379–391. [DOI] [PubMed] [Google Scholar]
- 38. Lee E, Wei L, Amato D.. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 39. Lagakos SW. A covariate model for partially censored data subject to competing causes of failure. J Royal Stat Soc Series C (Appl Stat). 1978;27(3):235–241. [Google Scholar]
- 40. Amadou A, Hainaut P, Romieu I.. Role of obesity in the risk of breast cancer: Lessons from anthropometry. J Oncol. 2013;906495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colditz G, Bohlke K.. Priorities for the primary prevention of breast cancer. CA Cancer J Clin. 2014;64(3):186–194. [DOI] [PubMed] [Google Scholar]
- 42. Renehan A, Roberts D, Dive C.. Obesity and cancer: Pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114(1):71–83. [DOI] [PubMed] [Google Scholar]
- 43. Pierobon M, Frankenfeld CL.. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137(1):307–314. [DOI] [PubMed] [Google Scholar]
- 44. Harvie M, Howell A, Vierkant RA, et al. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev. 2005;14(3):656–661. [DOI] [PubMed] [Google Scholar]
- 45. Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: Extended longterm follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281(23):2189–2197. [DOI] [PubMed] [Google Scholar]
- 47. Key TJ, Appleby PN, Reeves GK. et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. [DOI] [PubMed] [Google Scholar]
- 48. Dyrstad SW, Yan Y, Fowler AM, Colditz GA.. Breast cancer risk associated with benign breast disease: Systematic review and meta-analysis. Breast Cancer Res Treat. 2015;149(3):569–575. [DOI] [PubMed] [Google Scholar]
- 49. Tan-Chiu E, Wang J, Costantino J, et al. Effects of tamoxifen on benign breast disease in women at high risk for breast cancer. J Natl Cancer Inst. 2003;95(4):302–307. [DOI] [PubMed] [Google Scholar]
- 50. Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ.. Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst. 1998;90(1):37–42. [DOI] [PubMed] [Google Scholar]
- 51. Boyd NF, Dite G, Stone J, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347(12):886–894. [DOI] [PubMed] [Google Scholar]
- 52. Ziv E, Shepherd J, Smith-Bindman R, Kerlikowske K.. Mammographic breast density and family history of breast cancer. J Natl Cancer Inst. 2003;95(7):556–558. [DOI] [PubMed] [Google Scholar]
- 53. Crest AB, Aiello EJ, Anderson ML, Buist DS.. Varying levels of family history of breast cancer in relation to mammographic breast density (United States). Cancer Causes Control. 2006;17(6):843–850. [DOI] [PubMed] [Google Scholar]
- 54. Kennedy SR, Loeb LA, Herr AJ.. Somatic mutations in aging, cancer and neurodegeneration. Mech Ageing Dev. 2012;133(4):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tomasetti C, Vogelstein B, Parmigiani G.. Half or more of the somatic mutations in cancers of self-renewing tissues originate prior to tumor initiation. Proc Natl Acad Sci U S A. 2013;110(6):1999–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dunning A, Michailidou K, Kuchenbaecker KB, et al. Breast cancer risk variants at 6q25 display different phenotype associations and regulate ESR1, RMND1 and CCDC170. Nat Genet. 2016;48(4):374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fejerman L, Ahmadiyeh N, Hu D, et al. Genome-wide association study of breast cancer in Latinas identifies novel protective variants on 6q25. Nat Commun. 2014;5:5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Niedhammer I, Bugel I, Goldberg M, Leclere A.. Validity of self-reported weight and height in the French Gazel cohort. Int J Obesity. 2000;24(9):1111–1118. [DOI] [PubMed] [Google Scholar]
- 59. Stevens J, Keil JE, Waid R, Gazes PD.. Accuracy of current 4-year and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132(6):1156–1163. [DOI] [PubMed] [Google Scholar]
- 60. Chlebowski RT, Anderson GL, Aragaki AK, Prentice R.. Breast cancer and menopausal hormone therapy by race/ethnicity and body mass index. J Natl Cancer Inst. 2015;108(2):doi:10.1093/jnci/djv327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bodian CA, Perzin KH, Lattes R, Hoffmann P.. Reproducibility and validity of pathologic classifications of benign breast disease and implications for clinical applications. Cancer. 1993;71(12):3908–3913. [DOI] [PubMed] [Google Scholar]
- 62. Jain RK, Mehta R, Dimitrov R, et al. Atypical ductal hyperplasia: Interobserver and intraobserver variability. Mod Pathol. 2011;24(7):917–923. [DOI] [PubMed] [Google Scholar]
- 63. Masood S, Rosa M.. Borderline breast lesions: Diagnostic challenges and clinical implications. Adv Anat Pathol. 2011;18(3):190–198. [DOI] [PubMed] [Google Scholar]
- 64. Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15(3):209–221. [DOI] [PubMed] [Google Scholar]
- 65. Sidawy MK, Stoler MH, Frable WJ, et al. Interobserver variability in the classification of proliferative breast lesions by fine-needle aspiration: Results of the Papanicolaou Society of Cytopathology Study. Diagn Cytopathol. 1998;18(2):150–165. [DOI] [PubMed] [Google Scholar]
- 66. Sloane JP, Ellman R, Anderson TJ, et al. Consistency of histopathological reporting of breast lesions detected by screening: Findings of the U.K. National External Quality Assessment (EQA) Scheme. U. K. National Coordinating Group for Breast Screening Pathology. Eur J Cancer. 1994;30A(10):1414–1419. [DOI] [PubMed] [Google Scholar]