Abstract
Background: With rising cancer care costs, including high-priced cancer drugs, financial hardship is increasingly documented among cancer survivors in the United States; research findings have not been synthesized.
Methods: We conducted a systematic review of articles published between 1990 and 2015 describing the financial hardship experienced by cancer survivors using PubMed, Embase, Scopus, and CINAHL databases. We categorized measures of financial hardship into: material conditions (eg, out-of-pocket costs, productivity loss, medical debt, or bankruptcy), psychological responses (eg, distress or worry), and coping behaviors (eg, skipped medications). We abstracted findings and conducted a qualitative synthesis.
Results: Among 676 studies identified, 45 met the inclusion criteria and were incorporated in the review. The majority of the studies (82%, n = 37) reported financial hardship as a material condition measure; others reported psychological (7%, n = 3) and behavioral measures (16%, n = 7). Financial hardship measures were heterogeneous within each broad category, and the prevalence of financial hardship varied by the measure used and population studied. Mean annual productivity loss ranged from $380 to $8236, 12% to 62% of survivors reported being in debt because of their treatment, 47% to 49% of survivors reported experiencing some form of financial distress, and 4% to 45% of survivors did not adhere to recommended prescription medication because of cost.
Conclusions: Financial hardship is common among cancer survivors, although we found substantial heterogeneity in its prevalence. Our findings highlight the need for consistent use of definitions, terms, and measures to determine the best intervention targets and inform intervention development in order to prevent and minimize the impact of financial hardship experienced by cancer survivors.
The number of cancer survivors in the United States in 2014 was approximately 14.5 million (1), and with an aging population and improvements in early detection and treatment, cancer survivorship is expected to increase to 18 million by 2022 (2). The costs of new cancer therapies are increasing as well (3–5). When compared with individuals without a cancer history, cancer survivors have greater out-of-pocket (OOP) costs, even many years after initial diagnosis (6), reflecting ongoing cancer care as well as care for any late or lasting treatment effects. Cancer survivors are also more likely to have limitations in ability to work (7) and reduced resources to pay for medical care, thereby increasing the financial impact of cancer. Facing high OOP costs and reduction in income, cancer survivors are at risk for medical debt, bankruptcy, and increased stress, anxiety, and worry about their financial situation (6–13). Cancer survivors reporting financial hardship have been shown to be more likely to delay, forgo, and have poorer adherence to care (14), and a recent study found that bankruptcy increased the risk of death for cancer survivors, even after controlling for socioeconomic and clinical characteristics (15). The mechanism for this increased mortality risk is not entirely clear. Poorer quality of life and overall well-being, increased stress, restricted choices associated with limited resources (eg, food and housing insecurity), and decreased treatment adherence are among the hypothesized mechanisms (15,16).
Increasingly, researchers have documented the prevalence of different aspects of financial hardships among cancer survivors (17,18). A prior review highlighted the heterogeneity in measures of financial hardship in cancer survivorship research (19); however, that review focused exclusively on terminal cancer patients and only included studies published through 2006. Little research has been conducted to synthesize findings from these studies across the cancer care continuum. In an effort to build on existing research and close this gap, we conducted a systematic review of the published literature guided by a financial hardship typology to inform future research in cancer survivorship and intervention development in order to minimize the effects of financial hardship.
Methods
Financial Hardship Typology
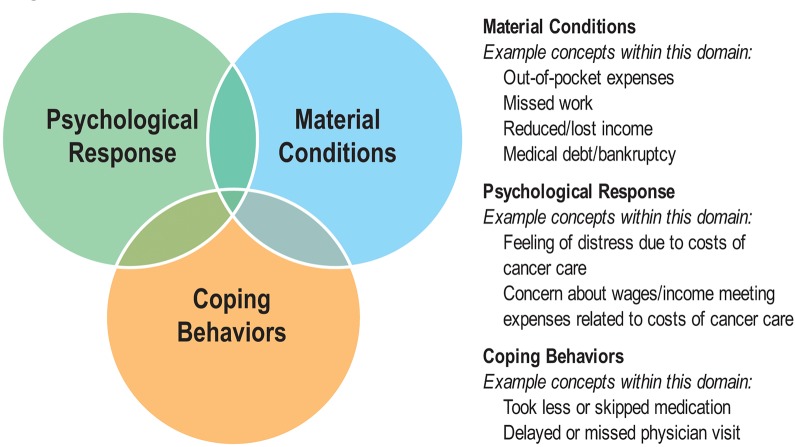
Building on theoretical work in health disparities research (16,20,21), we developed a typology for conceptualizing financial hardship in cancer survivors (22). Previous research has shown material (23), psychological (24), and behavioral (25) aspects of the financial hardship experience among cancer patients, and recent studies have begun to differentiate the multiple domains of financial hardship (18), thus heeding the calls in the literature for greater conceptual clarity to inform both financial hardship measurement and intervention development for cancer survivors (22,26). As described in Figure 1, financial hardship can be characterized as: 1) the material conditions that arise from the increased OOP expenses and lower income that can result from the inability to work during/following cancer treatment, 2) the psychological response to the increase in household expenses that must now be managed as patients navigate cancer care, or 3) the coping behaviors that patients adopt to manage their medical care while experiencing increased household expenses during/following cancer care. We used this typology to guide our literature search, abstract data from identified studies, and synthesize results from the underlying studies.
Figure 1.
Financial hardship typology. The typology illustrates and provides examples of the three broad domains of financial hardship: the material conditions that arise from the increased out-of-pocket expenses and potentially lower income that results from the inability to work during/following cancer treatment; the psychological response to the increase in household expenses that must now be managed as patients navigate cancer care; and the coping behaviors that patients adopt to manage their medical care while experiencing increased household expenses during/following cancer care.
As indicated in Figure 1, there may be some overlap across these domains of the underlying multidimensional construct of financial hardship; while the psychological response measures may be clearly distinct from the other two domains, the material conditions and coping behavior measures may seem more similar. However, we contend that there is not complete overlap among the material conditions and coping behavior domains in our typology. Full overlap between these two domains would suggest perfect correlation between material conditions measures and coping behavior measures. The distinction in our typology between material conditions measures and coping behavior measures is that the former attempts to capture the financial costs of cancer care to the patient and/or the depletion of financial resources as a result of cancer care; however, the latter attempts to capture the purposeful effort (27) used by the patient to manage the financial situation produced by the reduction of financial resources. The purposeful efforts or actions to economize that are used to manage the depletion of financial resources distinguish the coping behavior domain from the other domains. Though our typology does not include a temporal aspect, the coping behavior (eg, economizing strategies such as taking less medication and/or forgoing care because of cost) measures are assumed to capture the patient’s behavioral response to the reduction in material conditions and the stressful psychological response caused by the substantial financial burden of cancer care.
Literature Search
We used the PubMed, Embase, Scopus, and CINAHL databases to identify articles describing the financial burden of cancer that were written in English and published between January 1, 1990, and December 31, 2015. In the PubMed database, the search strategy used a combination of the Medical Subject Heading (MeSH) terms “cost of illness,” “healthcare costs,” “health expenditures,” and “neoplasm,” and related keywords (ie, bankruptcy, debt, OOP, mortality cost, cancer, economic burden, economic hardship, financial burden, financial hardship, financial stress, financial distress, material hardship, and financial toxicity). These terms also reflect concepts in the cost-effectiveness literature. We replicated the search strategy in the Embase, Scopus, and CINAHL databases according to their search parameters. The combined searches from all four databases yielded 676 unique articles (Supplementary Methods and Supplementary Table 1, available online).
Articles were excluded if the study was conducted outside of the United States (n = 239) because of important differences in health care systems, including the absence of universal health insurance coverage and the potential for very high patient OOP costs in the United States compared with other developed countries. Editorials, commentaries, and literature reviews were excluded (n = 381). Articles were also required to include some aspect of the financial hardship of cancer, present results from quantitative data analysis, and include individual-level data for at least 10 survivors. Articles describing a specific treatment or procedure (eg, economic burden of repeat renal surgery) were also excluded. The abstracts from the remaining 35 articles were selected for full text review. Reference lists were also reviewed, and 10 additional articles were identified for inclusion. A total of 45 articles were included in the literature review (6–10,12,14,23,28–64) (Supplementary Figure 1, available online).
Data Abstraction
Data were abstracted on study characteristics, cancer patient characteristics, and measures of financial hardship from each article. Study characteristics included data source, geographic setting, service type, payer type, indirect cost, study design, and comparison group. Data on cancer patient characteristics included method of identification (eg, registry, self-report), sample size, age, stage, and cancer site. We grouped measures of financial hardship into the three categories defined in our typology in Figure 1: material conditions, psychological responses, and coping behaviors. Material conditions included measures of OOP costs, indirect costs, and productivity loss (ie, the loss of economic resources and opportunities associated with morbidity because of cancer and its treatment), medical debt, and bankruptcy experienced by cancer survivors. Psychological responses were measured as any psychological, emotional, and social impact experienced by cancer survivors because of financial hardship (eg, feeling of distress because of costs of cancer care; concern about wages/income meeting expenses related to costs of cancer care). Coping behavior measures included assessments of treatment nonadherence and forgoing medical care because of cost. Some studies used composite or summary measures across multiple domains of financial hardship, which we grouped separately.
A single author reviewed abstracts for objective information (eg, conducted in United States, included cancer survivors), and three of the authors collectively made decisions about whether studies should be included or excluded. A single author abstracted data from the underlying studies, and three authors reviewed these data. Any disagreements were resolved by consensus.
Results
Study Characteristics
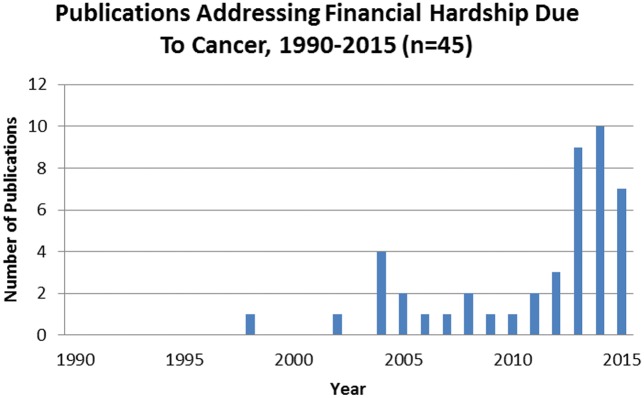
Over half of the studies (58%, n = 26) were published in 2013, 2014, and 2015 (Figure 2). The majority of studies were conducted using national (47%, n = 21) or multistate-level data (29%, n = 13) (Table 1), while others used data from a single institute/city (20%, n = 9) or single state (4%, n = 2). Studies used data from a variety of sources, including the Medical Expenditures Panel Survey (MEPS; 22%, n = 10) and the linked Surveillance, Epidemiology, and End Results Program (SEER)–Medicare data (16%, n = 7). Most studies featured cross-sectional (64%, n = 29) or cohort (29%, n = 13) designs. Many studies included comparison groups comprised of noncancer controls (38%, n = 17) or other defined groups (ie, patients with chronic heart disease; 16%, n = 7); however, 47% (n = 21) of the studies did not include a comparison group. Study populations included newly diagnosed patients in the initial/treatment phase (58%, n = 26) with few studies specifically addressing end of life/last year of life (4%, n = 2).
Figure 2.
Studies by year of publication. The figure depicts the distribution of peer-reviewed manuscripts from studies that measure financial hardship from cancer between 1990–2015.
Table 1.
Study characteristics*
| Characteristics | No. (%) |
|---|---|
| Study characteristics | |
| Data source | |
| Other | 17 (38) |
| MEPS | 10 (22) |
| SEER or SEER-Medicare | 7 (16) |
| Academic Cancer Institute | 5 (11) |
| MarketScan | 3 (7) |
| NHIS | 3 (7) |
| Geographic setting | |
| National | 21 (47) |
| Multiple cities and/or states | 13 (29) |
| Single institute/single city | 9 (20) |
| Single state | 2 (4) |
| Service type | |
| Outpatient | 26 (58) |
| Inpatient | 20 (44) |
| Pharmacy | 19 (42) |
| Not listed/NA | 16 (36) |
| Other | 12 (27) |
| Payer type | |
| Private insurance | 29 (64) |
| Medicare | 27 (60) |
| Medicaid | 23 (51) |
| Out of pocket | 22 (49) |
| Uninsured | 19 (42) |
| Other | 16 (36) |
| Not listed | 7 (16) |
| Military/VA | 4 (9) |
| Study design | |
| Cross-sectional | 29 (64) |
| Cohort | 13 (29) |
| Based on randomized controlled trial | 3 (7) |
| Phase of cancer care | |
| Initial treatment of incident disease | 26 (58) |
| Surveillance, continuing, or monitoring | 10 (22) |
| Last year of life | 2 (4) |
| Other | 0 (0) |
| Comparison group | |
| No comparison group | 21 (47) |
| Non cancer controls | 17 (38) |
| Other comparison group | 7 (16) |
| Cancer patient characteristics | |
| Cancer patient identification | |
| Self-report | 15 (33) |
| Medical record review | 12 (27) |
| Registry | 8 (18) |
| Other | 5 (11) |
| Claims | 5 (11) |
| Cancer stage | |
| Stage I–III/localized regional | 9 (20) |
| Stage IV/distant | 0 (0) |
| Other | 0 (0) |
| All | 22 (49) |
| Not listed | 14 (31) |
| Number of cancer survivors | |
| <100 | 2 (4) |
| 100–999 | 20 (44) |
| 1000–9999 | 19 (42) |
| 10 000+ | 4 (9) |
| Patient age group | |
| <18 | 3 (7) |
| 18–39 | 36 (80) |
| 40–64 | 38 (84) |
| 65+ | 33 (73) |
| Not listed | 2 (4) |
| Cancer site | |
| Other cancer site | 30 (68) |
| Breast | 19 (42) |
| All cancer sites | 16 (36) |
| Colon | 17 (38) |
| Lung | 11 (24) |
| Prostate | 9 (20) |
| Uterine | 5 (11) |
| Ovarian | 4 (9) |
| Financial burden measures | |
| Material measures | |
| Direct costs/out-of-pocket costs | 20 (44) |
| Indirect costs/productivity loss | 18 (40) |
| Medical debt/depletion of assets | 12 (27) |
| Health related spending >20% of income | 4 (9) |
| Decline in financial status | 3 (7) |
| Patient time cost | 3 (7) |
| Bankruptcy | 3 (7) |
| Psychological response measures | |
| Financial distress/worry | 3 (7) |
| Coping behavior measures | |
| Prescription drug management/treatment nonadherence | 7 (16) |
| Composite or summary measures | 13 (29) |
*MEPS = Medical Expenditures Panel Survey; NHIS = National Health Interview Survey; SEER = Surveillance, Epidemiology and End Results program.
Cancer survivors were identified by self-report (33%, n = 15), medical record review (27%, n = 12), registry (18%, n = 8), and insurance claims (11%, n = 5) (Table 1). Approximately half of the studies included patients at all stages of disease at diagnosis (49%, n = 22), whereas 20% (n = 9) included stages I–III and 31% (n = 14) of studies did not list study patients’ stage of cancer. The most common cancer sites reported were breast (42%, n = 19), colon (38%, n = 17), lung (24%, n = 11), prostate (20%, n = 9), uterine (11%, n = 5), and ovarian (9%, n = 4). Over a third of the studies included all cancer sites (36%, n = 16).
Measures of Financial Hardship of Cancer
Across studies, there was a large degree of inconsistency in the terms used, including financial distress, financial stress, financial hardship, financial problem, financial toxicity, financial burden, economic burden, economic hardship, and financial impact. The majority of studies reported material condition measures (82%, n = 37), and there was considerable variation in the measures, including direct medical costs or OOP costs (44%, n = 20), indirect cost or cost because of productivity loss (40%, n=18), medical debt/depletion of assets (27%, n = 12), health-related spending as a percentage of total household income (ie, >20% of income) (9%, n = 4), decline in financial status (7%, n = 3), patient time costs (7%, n = 3), and bankruptcy (7%, n = 3) (Table 1). Approximately 7% (n = 3) of the studies reported financial hardship as a psychological response measure (ie, assess subjective financial distress, stress and worry in survivors), while a total of 16% (n = 7) of studies reported financial hardship as a coping behavior measure, evaluating the association between cancer-related expenses and utilization of medical care services (ie, skipped treatment appointments or filled only part of a medication prescription). Thirteen studies (29%) reported financial hardship using composite or other summary measures.
Few studies included in our review used validated measures or commented about the validation process for financial hardship measures; a single study (43) included the comprehensive score for financial toxicity (COST) measure developed specifically as a patient-reported outcome in oncology care (20), and three studies (10,44,50) used the InCharge Financial Distress/Financial Well-Being Scale (IFDFW), which was tested for validity and reliability but is not health care specific (65). Both the COST measure and the IFDFW scales include aspects of material and psychological hardship.
Even objective measures that addressed the same basic financial hardship concept were inconsistently reported across studies and had items that were worded differently or used different response options. For example, OOP measurements varied across studies and included costs associated with both direct medical services (eg, inpatient and outpatient services, mental health services, counseling, and medication) and direct nonmedical costs (eg, transportation, restaurant meals, cleaning, insurance premium increases, home maintenance, and child care). Some studies compared OOP costs to family income, whereas others only reported absolute levels of OOP costs. Finally, the reference period for evaluating OOP costs ranged from one month to a calendar year to a monthly average from the past two years.
Material Condition Measures of Financial Hardship
OOP medical costs
Patient OOP costs were included in 20 of the studies (6–8,23,28–30,35,40–42,45–52,57) (Table 2). Of the studies that included OOP costs, there was notable variation in the estimates reported, with studies stratifying estimates by treatment type, sex, age group (ie, age <65 years and 65+ years), time since diagnosis or treatment, insurance type, service type, and cancer site. Estimates of total OOP cost of cancer survivors ranged on average per month from $316 (2008 US dollars) in breast cancer survivors of all ages (52) to $741 (years of dollars not stated) in a general cancer survivor population (51).
Table 2.
Material measures of financial hardship: Out-of-pocket medical costs
| First author, y | Sample size (n) | Setting, data source, and time frame (duration of spending) | Measures | Key findings |
|---|---|---|---|---|
| Arozullah, 2004 (28) | 154 breast cancer survivors | Chicago; Northwestern University; 1999 to 2002 (3 mo) | OOP expenses related to direct medical, direct nonmedical, and indirect costs | Average monthly OOP and lost income costs of $1455 during a 3-mo period (y of dollars not stated); OOP expenditures for medications (80%), transportation (78%), physician visits (66%), and restaurant meals (51%) |
| Banthin, 2006 (29) | 7519 cancer survivors (all sites) | National; MEPS; 1996 and 2003 (annual) | Family OOP burden and family health care services OOP burden; spending >20% of income | Total annual family OOP burden (2003 US dollars): 28.8% paid >10% and, 11.4% paid >20% of disposable income; family health care services OOP burden: 16% paid >10% and 6.7% paid >20% of disposable income |
| Bernard, 2011 (30) | 4243 cancer survivors (all sites) | National; MEPS; 2001 to 2008 (annual) | OOP burden, high health care total burden of > 20% of income | Cancer survivors had annual OOP of $3881 (2008 dollars); high health care total burden in 13.4% of cancer survivors, 9.7% with and 4.4% without chronic conditions |
| Davidoff, 2013 (35) | 1868 cancer survivors (all sites) | National; MCBS; 1997 to 2007 (2 y) | Total OOP spending and OOP spending >20% of income | Beneficiaries with cancer paid $4727 (cumulative 2-y spending, 2007 US dollars) in OOP, while comparison group paid $3209; high OOP burden in 28% of cancer survivors and 16% of beneficiaries without a cancer history |
| Ekwueme, 2014 (7) | 6722 cancer survivors (all sites) | National; MEPS; 2008 to 2011 (annual) | OOP spending | Annual OOP expenditures: $751 for male cancer survivors, $600 for male controls, $973 for female cancer survivors, and $833 for female controls (2011 US dollars) |
| Finkelstein, 2007 (40) | 1940 cancer survivors (all sites) | National; MEPS; 2000 to 2005 (annual) | OOP expenditures | Annual OOP expenditures for cancer survivors in active care $870 higher than survivors not currently receiving treatment, and $1190 higher than those without a cancer history (2005 US dollars) |
| Guy, 2013 (6) | 4960 cancer survivors (all sites) | National; MEPS; 2008 to 2010 (annual) | OOP expenditures | For adults age 18–64 y, annual adjusted OOP expenditure was $1107 (2010 US dollars) for recently diagnosed, $747 for previously diagnosed, and $617 for no history of cancer; for age 65 y and older, annual adjusted OOP expenditure was $1711 for recently diagnosed, $1529 for previously diagnosed, and $1220 for no history of cancer |
| Guy, 2014 (41) | 1464 cancer survivors (all sites) | National; MEPS; 2008 to 2011 (annual) | OOP expenditures | Annual adjusted OOP expenditures for adult survivors of cancer diagnosed age 15–39 y was $765 (2011 US dollars) compared with $686 for adults without a cancer history |
| Guy, 2015 (42) | 4271 cancer survivors (all sites) | National; MEPS; 2008 to 2012 (annual) | Total annual OOP spending on healthcare >20% of annual income | Survivors were more likely to report a high OOP burden (4.3%) compared with those without a cancer history (3.4%) |
| Jacobsen, 2012 (45) | 3918 oral, oral pharyngeal, and salivary gland cancer survivors | National; Marketscan CCAE databases; 2004 to 2008 (annual) | OOP payments | Annual OOP payments for survivors with commercial insurance and Medicare were $2133 and $785 (2009 US dollars) more than for controls |
| Jagsi, 2014 (8) | 1502 breast cancer survivors | Los Angeles, CA, and Detroit, MI; SEER; 2005 to 2007 (4 y) | OOP expenditures | 65% of breast cancer survivors paid <$2000 in OOP expenditures, 18% paid $2001–<$5000 and 17% paid >$5000 (y of dollars not stated) |
| Jayadevappa, 2010 (46) | 512 prostate cancer patients | Urology clinics of an academic medical center and the Veterans Administration Medical Center; 2002 to 2005 (2 y) | OOP expenditures (medical costs and nonmedical costs) | OOP costs for patients receiving radical prostatectomy and radiation at 24-month follow-up were $330 and $661, respectively (y of dollars not stated) |
| Kilgore, 2007 (47) | 781 cancer survivors (all sites) | National; Cost of Cancer Treatment Study; y not stated (6 mo) | OOP expenditures for prescription drugs and other health care | No statistically significant difference in OOP expenditures for clinical trial participants compared with nonparticipants |
| Langa, 2004 (48) | 988 cancer survivors (all sites) | National; Health and Retirement Study; 1995 (2 y) | OOP expenditures for nursing home stays, outpatient services, home care and prescription medication | Adjusted annual OOP expenditures for the no cancer, cancer/no treatment, and cancer current treatment groups were $1210, $1450, and $1880, respectively (1995 US dollars); prescription medication ($1120) and home care services ($250) accounted for most of the additional OOP expenditures in treatment group |
| Li, 2014 (49) | 5944 cancer survivors (all sites) | National; MEPS; 2008 to 2011 (annual) | OOP expenditures for mental health | For cancer survivors age 18–64 and 65+ y, annual OOP MH expenditure was $13 and $28 (y of dollars not stated) |
| Meisenberg, 2015 (50) | 132 cancer survivors (all sites) | Single cancer institute in Annapolis, MD; y not stated (not listed) | OOP expenditures | Mean and median OOP costs for survivors were $938 and $250 monthly, respectively (y of dollars not listed) |
| Moore, 1998 (51) | 20 cancer survivors (all sites) | Single Midwest medical clinic; y not stated (not listed) | OOP expenditures for clinic visits, symptom and side effects, support/assistance, administrative, and quality of life | Mean OOP expenditures was $741 per month (range = $12–$3130; y of dollars not listed) |
| Pisu, 2011 (52) | 262 cancer survivors | Southern states; BCEI; y not stated (monthly) | Medical and nonmedical OOP costs | OOP costs were $316 per month (2008 US dollars); direct medical monthly OOP cost was $281, and direct nonmedical monthly OOP cost was $66 |
| Teitelbaum, 2013 (57) | 2642 multiple myeloma patients | National; Optuminsight (1 y after treatment episode*) | OOP costs, including copayment, coinsurance, and deductibles for three target agents: BOR, THAL, and LEN | Mean unadjusted OOP costs per episode for patients treated with agents: BOR ($3846) THAL ($4666), LEN ($4483), and other ($3900) per treatment episode (y of dollars not listed) |
| Zafar, 2013 (23) | 258 cancer survivors with breast, colorectal, lung, or other solid tumors | Multiple cities and states; HealthWell Foundation and Duke University Medical Center; 2010 to 2011 (up to 4 mo) | Cancer-related OOP expenses | Median monthly OOP of $456, including $28 in travel, $15 in nonprescription medication, $120 in insurance premium, $56 in prescription medication (2011 US dollars) |
*Study evaluated three novel target agents: the proteasome inhibitor bortezomib and the immunomodulatory agents thalidomide and its analog lenalidomide. BCEI = Breast Cancer Education Intervention clinical trials; BOR = bortezomib; CCAE = Commercial Claims and Encounters; LEN = lenalidomide; MCBS = Medicare Current Beneficiary Survey; MEPS = Medical Expenditures Panel Survey; NHIS = National Health Interview Survey; OOP = out of pocket; SEER = Surveillance, Epidemiology and End Results program; THAL = thalidomide.
Four studies (29,30,35,42) reported the proportion of cancer survivors whose OOP costs were greater than 20% of their annual income, with estimates between about 11% in a nationally representative population of cancer survivors of all ages (29) and 28% of Medicare beneficiaries with cancer (35). The majority of studies’ findings underscored the higher OOP costs among individuals diagnosed with cancer compared with those without a cancer history.
Indirect costs/productivity loss and patient time costs
Indirect costs/productivity loss were reported in 18 studies (40%) (6–9,28,32,34,37,40,41,43,46,51,55,59,60,61,62). As shown in Table 3, studies measured indirect costs/productivity loss as loss of income, missed or lost days from work because of illness (ie, absenteeism, short- and long-term disability, days spent in bed), patient time costs (7%, n = 3) (46,60,62), and limitations in ability to do activities related to work and outside of work. Mean annual indirect cost ranged from $380 in a sample of prostate cancer survivors (year of dollars not stated) (46) to $8236 in breast cancer survivors (2000 US dollars) (55). Arozullah et al. (28) reported that 45% of breast cancer survivors from a single cancer center had job-related income loss, with a maximum loss of $13 462 per month during a three-month period (1999 to 2002 data; dollars not adjusted for inflation). A nationally representative population-based study of cancer survivors found that nearly one in three survivors had limitations in usual daily activities outside of work and one in four felt less productive at work (2008 to 2011 data) (7). In a cohort of breast cancer survivors, Sasser et al. (55) estimated an average of 42.1 total work loss days, comprised of 18.1 absenteeism days and 23.9 disability days, which translated to annual totals of $8236, $3634, and $4602 (in 2000 US dollars), respectively (1998 to 2000 data). Guy et al. (41), the only study to assess lost productivity explicitly in adult survivors of childhood cancers, reported an annual per capita lost productivity of $4564 (2011 US dollars) in a nationally representative sample (2008 to 2011 data), approximately double the lost productivity of adults without a history of cancer during the same time period ($2314).
Table 3.
Material measures of financial hardship: Indirect cost/productivity loss and patient time costs*
| First author, y | Sample size (n) | Setting, data source, and collection time frame (duration of spending) | Measure | Key findings |
|---|---|---|---|---|
| Arozullah, 2004 (28) | 154 breast cancer survivors | Chicago; Northwestern University; 1999 to 2002 (3 mo) | Lost income and missed work d | Mean monthly loss in income of $727 and 7 h missed per wk during a 3-mo period (y of dollars not listed) |
| Chang, 2004 (32) | 603 brain, colorectal, lung, ovarian, pancreatic, prostate, or non-Hodgkin’s lymphoma cancer survivors | National; MarketScan–CCAE, Medicare, Health and Productivity Management; 1998 to 2000 (≤2 y) | Days absent from work and short-term disability d | Compared with controls, cancer survivors had a higher monthly absenteeism ($373 vs $101; y of wages not listed) and more mean monthly short-term disability days (5.2 vs 0.2), translating to higher monthly costs ($698 vs $25); compared with controls, cancer caregivers had more absentee days per mo (2.2 vs 1.4), translating to higher costs ($161 vs $255) |
| Chirikos, 2002 (34) | 105 breast cancer survivors 5 y after treatment | Single institute; 1995 and 2000 (5 y) | Indirect morbidity/disability costs | Breast cancer survivors had a reduction in earnings of about $3600, while the noncancer comparison group had earning rise by $1800 (2000 US dollars) over the study period; among those who worked, survivors reduced their work h more than comparison group (440 vs 259 annual h) |
| Dowling, 2013 (37) | 4960 cancer survivors (all sites) | National; MEPS; 2008–2010 (annual) | Employment; limitations in work, housework, or school because of health | In past years cancer survivors age 18–64 y with another chronic disease (heart disease or diabetes) experienced higher levels of burden (lower employment and higher limitations in work, housework, or school) compared with individuals with a history of cancer only, chronic disease only, and neither cancer, heart disease, nor diabetes; cancer site–specific reports of any limitation in work/housework/school ranged from 8.8%–17.5%, completely unable to do activities ranged from 4.7%–11.7%, cognitive limitation ranged from 3.4%–8.5% |
| Ekwueme, 2014 (7) | 6722 cancer survivors (all sites) | MEPS; 2008-2011 (annual) | Lost productivity measured by inability to work, missed workdays, additional d spent in bed; and valued with 2011 median wage, change in work, inability to perform physical tasks required by job, mental tasks required by job, feeling less productive at work | Cancer survivors had greater annual productivity losses than individuals without a cancer history (men: $3719 vs $2260; women: $4033 vs $2703) Among employed cancer survivors, cancer interfered with physical tasks (25%) and mental tasks (14%) required by the job, with nearly 25% feeling less productive at work; although men were more likely than women to have been employed (62% and 55%, respectively), females were more likely to make changes in work because of cancer than males (48% and 34%, respectively) |
| Finkelstein, 2007 (40) | 1940 cancer survivors (all sites) | National; MEPS; 2000–2005 (annual) | D lost from work | Among the employed, those undergoing cancer care missed 22.3 more workdays per y than those without a cancer history |
| Guy, 2013 (6) | 4960 cancer survivors (all sites) | National; MEPS; 2008 to 2010 (annual) | Lost productivity measured by inability to work, d of work missed, and d spent in bed and valued with 2010 median wage | For age 18–64 y, annual per capita lost productivity was $4694 (2010 US dollars) among recently diagnosed cancer survivors, $3593 among previously diagnosed, and $2040 among individuals without a cancer history; for age 65 y and older, the annual per capita lost productivity was $6133 among recently diagnosed, $5295 among previously diagnosed, and $4409 among individuals without a cancer history |
| Guy, 2014 (41) | 1464 adult survivors of adolescent and young adult cancers | National; MEPS; 2008 to 2011 (annual) | Lost productivity measured by inability to work, missed workdays, additional d spent in bed; and valued with 2011 median wage | Annual per capita lost productivity was $4564 (2011 US dollars) for adult survivors of adolescent and young adult cancers, compared with $2314 among adults without a history of cancer |
| Huntington, 2015 (43) | 100 multiple myeloma cancer patients | Philadelphia, PA; Academic Medical Center; August 2014 to January 2015 (not listed) | Change in employment since diagnosis, including reduction in work h and increase in work h | Of the 100 respondents, 38% stated they stopped working, 12% reduced h worked, 20% had no change in work h, and 2% increased work h |
| Jagsi, 2014 (8) | 1502 breast cancer survivors | Los Angeles, CA, and Detroit, MI; SEER; 2005 to 2007 (4 y) | Change in work h | Of the respondents who worked at some time after diagnosis (n = 741), 27% decreased work h, whereas 7% increased h; another 7% were denied job opportunities because of cancer |
| Jayadevappa, 2010 (46) | 512 prostate cancer patients | Urology clinics of an academic medical center and the Veterans Administration Medical Center; 2002 to 2005 (≤2 y) | Time and indirect costs were measured as travel time, number of missed workdays, and total imputed indirect costs | Mean indirect costs and time costs at 12 mo for prostate cancer patients receiving radical prostatectomy were $256 and $341, respectively; indirect and time costs were $380 and $187, respectively, for those receiving radiation therapy (y of dollars not listed) |
| Meneses, 2012 (9) | 137 breast cancer survivors | Southeast states; BCEI; y not listed (6 mo) | Changes in economic motivation to work, productivity and quality of work, and missing d of work; changes in economic lifestyle | At baseline and 6 mo, survivors reported change in motivation to work (23% and 12%), productivity (27% and 12%), quality of work (17% and 7%), and missed d from work (28% and 19%); survivors reported changes in economic lifestyle at baseline (22%) and 6 mo (18.3%) |
| Moore, 1998 (51) | 20 cancer survivors (all sites) | Single Midwest medical clinic; y not listed (not listed) | Lost income | Mean lost income for one month of chemotherapy was $801 (y of dollars not listed) |
| Sasser, 2005 (55) | 555 breast cancer survivors | National; employer admin medical claims; 1998 to 2000 (annual) | Indirect costs calculated from employer's perspective as sum of disability and absenteeism costs | Per capita annual number of work loss d was 42.1, an annual average cost of $8236 to the employer (2000 US dollars); annual absenteeism was 18.1 days, an average annual cost of $3634, and disability was 23.9 d and $4602 annually for the employer |
| Wan, 2013 (59) | 1984 breast cancer survivors and 1375 family members | National; MarketScan CCAE and Health and Productivity Management databases; 2005 to 2009 (annual) | Survivors' indirect costs (sick leave from absentiseem and short-term disability) and family members' indirect costs (personal leave and leave under FMLA) | Annual per capita sick leave indirect costs were $2383 for early-stage breast cancer survivors, $1775 for metastatic breast cancer survivors, and $1282 for controls; short-term disability indirect costs for EBC, MBC, and controls were $6165, $3690, and $558, respectively; costs of family member leave d for MBC and EBC were $1075 and $808, respectively |
| Yabroff, 2004 (61) | 1823 cancer survivors (all sites) | National; NHIS; 2000 (annual) | Ability to work, limitations in work, d lost from work because of health | When compared with the noncancer controls, cancer survivors were more likely to be unable to work (18.0% vs 10.3%), be limited in work (27.3% vs 17.6%), and have more days lost from work (13.2 vs 5.7) in the past years because of health |
| Yabroff, 2005 (62) | 75 470 colorectal cancer survivors | Multiple states and cities; SEER-Medicare; 1995-1998 (1 y) | Net patient time costs by phase of care | Net patient time costs $4592 for 12 mo of initial phase, $25 per month in continuing phase, and $3788 for 12 mo of the terminal phase of care (2002 US dollars) |
| Yabroff, 2007 (60) | 763 527 cancer survivors (multiple sites) | Multiple states and cities; SEER; 1995 to 2001 (1 y) | Net patient time costs for initial, continuing, and last-year-of-life phases of care | Net patient time costs in the 12 mo of initial phase of care were lowest for melanoma of the skin ($271) and prostate cancers ($842) and highest for gastric ($5348) and ovarian ($5605) cancers (2002 US dollars); net patient time cost during continuing care phase was less than $60 per month for all cancers; net patient time costs for 12 mo of last-year-of-life phase were lowest for melanoma of the skin ($1509) and highest for gastric ($7799), lung ($7435), and ovarian ($7388) cancers |
*BCEI = Breast Cancer Education Intervention clinical trials; CCAE = Commercial Claims and Encounters; EBC = early-stage breast cancer; FMLA = Family and Medical Leave Act; MBC = metastatic breast cancer; MEPS = Medical Expenditures Panel Survey; NHIS = National Health Interview Survey; OOP = out of pocket; SEER = Surveillance, Epidemiology and End Results program.
Medical debt, depletion of assets, and bankruptcy
Twelve studies reported medical debt, depletion of assets, or bankruptcy because of cancer (8,9,12,23,31,43,44,50,54,56,58,63) (Table 4). Bankruptcy claims ranged from 2% to 3% in the two years after diagnosis (9). Furthermore, Ramsey et al. (12) found that cancer survivors in Western Washington State were 2.65 times more likely to file for bankruptcy than those without cancer and that younger survivors (ie, <40 years of age) had higher rates of bankruptcy than their older counterparts based on cancer surveillance data collected from 1995 to 2009. Meneses et al. (9) found that 12% of survivors borrowed money to pay for their medical expenses and 24% used up savings over a six-month period (data years not listed). Jagsi et al. (8) provided details regarding how the survivors financed their medical expenses—80% used income or savings, 10% increased credit card debt, and 7% borrowed from family or friends (2005 to 2007 data). In addition, several studies noted that survivors had trouble paying for medical bills and basic necessities (ie, utilities, mortgage, food, clothes) (8,23,43,44,50,54,56,58,63). The study by Regenbogen et al. (54) reported that from 2011 to 2013 colorectal cancer survivors with complications were more likely to spend savings and borrow or take loans than survivors without complications (40% vs 31% and 18% vs 11%, respectively). Shankaran et al. (56) reported that 62% of colorectal cancer survivors were in debt, with a mean amount of $26 860 (2009 US dollars, 2008 to 2010 data), and Veenstra et al. (58) reported that a total of 34% of colorectal cancer survey respondents used savings and 13% had to borrow money or take out a loan as a result of their cancer treatment (2011 to 2013 data).
Table 4.
Material measures of financial hardship: medical debt, depletion of assets, and bankruptcy*
| First author, y | Sample size (n) | Setting, data source, and collection time frame (duration of spending) | Measure | Key findings |
|---|---|---|---|---|
| Cagle, 2015 (31) | 158 cancer survivors or family member (all sites) | National; Kaiser Foundation, Harvard School of Public Health, and USA Today; 2006 (not listed) | Treatment-related financial stress, assessed by 9 (1 = yes/0 = no) questions | A third of the respondents used all or most of their savings |
| Huntington, 2015 (43) | 100 multiple myeloma cancer patients | Philadelphia, PA; Academic Medical Center; August 2014 to January 2015 (31 mo) | Treatment-related financial burden | A total of 46% used savings to pay for cancer care, 36% of survivors reported applying for financial assistance to pay for treatment-related expenses, 21% borrowed money to cope with the costs, 55% decreased spending on basic goods (eg, food and clothing), and 64% decreased spending on leisure activities |
| Irwin, 2014 (44) | 134 breast cancer survivors | North Carolina; Duke University; 2012 (not listed) | Treatment-related financial hardship | A total of 16% of survivors reporting difficulty paying for basic necessities, and 19% used all/most of their savings |
| Jagsi, 2014 (8) | 1502 breast cancer survivor | Los Angeles, CA, and Detroit, MI; SEER; 2005 to 2007 (4 y) | Debt from cancer treatment and financing of medical expenses | 4 y after diagnosis, 12% of survivors were in debt because of breast cancer treatment; to finance medical expenses, 80% used income and/or savings, 7% borrowed from family or friends, 2% borrowed against house, 5% left some medical bills unpaid, and 10% increased credit card debt; to cope with cost during a 12-month period, 6% went without health insurance, 5% had their utilities turned off because of unpaid bills, and 4% had to move out of their homes because they could not afford to stay |
| Meisenberg, 2015 (50) | 132 cancer survivors (all sites) | Single cancer institute in Annapolis, MD; y not stated (not listed) | Debt, delinquency on personal bills, and bankruptcy because of cancer treatment | A quarter of participants (25.8%) increased their debt, 22.0% became delinquent on personal bills, and 1.5% declared bankruptcy as a result of their treatment costs |
| Meneses, 2012 (9) | 137 breast cancer survivors | Southeastern US states; BCEI; y not listed (6 mo) | Financing of medical expenses and declaration of bankruptcy | At baseline and 6 mo, survivors borrowed money (14% and 12%), declared bankruptcy (2% and 3%), and used up savings (27% and 24%) |
| Ramsey, 2013 (12) | 197 840 cancer survivors (multiple sites) | Washington; SEER, Cancer Surveillance system; 1995 to 2009 (up to 5 y) | Filing for bankruptcy | 5 y after diagnosis, 1.7% of cancer survivors filed for bankruptcy; cancer survivors were 2.65 times more likely to go bankrupt than those without cancer |
| Regenbogen, 2014 (54) | 937 colorectal cancer survivors | SEER registries of Detroit, MI, and GA; 2011 to 2013 (up to 1 y post surgery) | Debt because of cancer treatment | Survivors with complications were more likely to spend savings (40% vs 31%), borrow or take loans (18% vs 11%), fail to make credit card payments (18% vs 11%), reduce spending for food or clothes (38% vs 27%), and decrease recreational activities (41% vs 33%) than survivors without complications |
| Shankaran, 2012 (56) | 284 colon cancer survivors | Washington; SEER, Cancer Surveillance system; 2008 to 2010 (not listed) | Debt and treatment-related financial hardship; decline in income | A total of 38% of survivors reported at least one treatment-related financial hardship (selling, refinancing, or obtaining a second mortgage, accruing debt, borrowing money, experiencing a > 20% decline in income); as a result of treatment-related expenses, 23% of survivors were in debt at the time of the study, with a mean debt of $26 860 (2009 US dollars), and 42% reported a decline in income; approximately 27% of respondents who did not experience financial hardship reported other financial impacts, such as needing to sell stocks or investments, using savings or retirement accounts, or having a less than 20% income decline as a result of cancer treatment |
| Veenstra, 2014 (58) | 956 colorectal cancer survivors | SEER registries Detroit, MI, and GA); NHIS; 2011 to 2013 (not listed) | Financing of medical expenses and strategies to cope with other financial burdens | A total of 34% of respondents used savings, 13% had to borrow money/take out loans, 13% could not make payments on credit cards, 30% cut down spending on food/clothes, 35% cut down on recreational activities, and 48% cut down on expenses in general |
| Zafar, 2015 (63) | 987 colorectal and lung cancer survivors | Multiple cities and states; CanCORS II 2003 to 2006 (not listed) | Financial burden defined as greater likelihood of difficulty living on household income | A total of 48% (n = 482) of cancer survivors stated they experienced some level of difficulty in living on total household income (32% found it somewhat difficult, 10% found it difficult or can barely get by, 5% found it very difficult, and 3% found it extremely difficult or impossible) |
| Zafar, 2013 (23) | 258 cancer survivors (multiple sites) | Multiple cities and states; HealthWell Foundation and Duke University Medical Center; 2010 to 2011 (up to 4 mo) | Financing of medical expenses and strategies to cope with the financial burdens | To cope with expenses, 68% cut back on leisure activities, 46% reduced spending on basics, 46% used savings, and 17% sold possessions or property |
*BCEI = Breast Cancer Education Intervention clinical trials; NHIS = National Health Interview Survey; SEER = Surveillance, Epidemiology and End Results program
Psychological Response Measures of Financial Hardship
Three studies (33,38,54) included psychological response measures of financial hardship (eg, measures of distress, stress, and worry) (Table 5). A longitudinal study conducted by Ell et al. (38) among low-income women undergoing treatment or follow-up for breast/gynecological cancer found that 68% of cancer survivors had medical cost concerns, 47% had wage concerns, and 49% had financial stress; furthermore, those survivors who reported medical cost concerns, wage worry, or financial stress had poorer functional, emotional, and physical well-being, as well as a greater risk of depression (data years not listed). A multiregional study by Chino et al. (33) reported a total of 47% of cancer survivors experienced high levels of financial distress associated with dissatisfaction with general aspects of health care, technical quality of cancer care delivery, and the financial aspects of health care from 2010 to 2011.
Table 5.
Psychological Response Measures (distress, stress, and worry) and Coping Behavioral Measures*
| First author, y | Sample size (n) | Setting, data source, and collection time frame (duration of spending) | Measure | Key findings |
|---|---|---|---|---|
| Bestvina, 2014 (10) | 300 cancer survivors (multiple sites) | Duke Cancer Institute, and 3 affiliated rural oncology clinics; 2012 to 2013 (not listed) | Behavioral; medication nonadherence because of cost | Survivors reporting high or overwhelming financial distress were more likely be nonadherent (OR = 1.64, 95% CI = 1.38 to 1.96, P = .01) compared with those with low or average financial distress |
| Chino, 2014 (33) | 168 cancer survivors (multiple sites) | Multiple cities and states; Duke University and Health Well Foundation; 2010 to 2011 (not listed) | Psychological; subjective financial distress, 5-point likert scale ranging from "not a financial burden at all" to "catastrophic financial burden" | 47% reported catastrophic financial burden; high financial burden was associated with dissatisfaction with general aspects of health care, technical quality of cancer care delivery, and the financial aspects of health care |
| Ell, 2008 (38) | 329 cancer survivors (multiple sites) | Outpatient oncology clinic (location not listed); y not listed (1 y) | Psychological; financial stress, wage concern | At 12 mo, 49% of cancer survivors reported financial stress and 47% reported wage concern |
| Huntington, 2015 (43) | 100 multiple myeloma cancer survivors | Philadelphia, PA; Academic Medical Center; August 2014 to January 2015 (not listed) | Behavioral; forgoing treatment, nonadherence | A total of 17% of survivors delayed the start of treatment, 16% filled only part of noncancer prescription because of cost, 12% filled only part of cancer prescription because of cost, 11% stopped filling prescription because of cost, 10% refused recommended test because of cost, 6% skipped clinical visit because of cost |
| Irwin, 2014 (44) | 134 breast cancer survivors | North Carolina; Duke University; 2012 (not listed) | Behavioral: behavior changes because of financial distress/cost of care | A total of 14% changed medical decisions and 12% avoided treatment of non-cancer-related health issues because of costs |
| Jagsi, 2014 (8) | 1502 breast cancer survivors | Los Angeles, CA, and Detroit, MI; SEER 2005 to 2007 (1 y) | Behavioral; strategies to cope with the cost of prescription medications and cancer care expenses | To cope with costs during a 12-mo period, 8% missed a doctor's appointment, 5% went without medication, and 4% took less than prescribed amount |
| Kent, 2013 (14) | 1556 cancer survivors (all sites) | National; NHIS; 2010 (1 y) | Behavioral; financial problems and forgoing or delaying care | During a 12-mo period, cancer survivors with financial problems were more likely to delay (18.3% vs 7.4%) or forgo medical care (13.8% vs 5.0%), prescription medications (14.2% vs 7.6%), dental care (19.8% vs 8.3%), eyeglasses (13.9% vs 5.8%), or mental health care (3.9% vs 1.6%) than survivors who did not have financial problems |
| Regenbogen, 2014 (54) | 937 colorectal cancer survivors | SEER registries of Detroit, MI, and GA; 2011 to 2013 (up to 1 y post surgery) | Psychological; worry about financial problems (5-point Likert scale where 4–5 = high) | Survivors with complications from surgery were more likely to report high levels of worry about their financial circumstances than survivors without complications (61% vs 52%, P = .01) |
| Zafar, 2013 (23) | 258 cancer survivors (multiple sites) | Multiple cities and states; HealthWell Foundation and Duke University Medical Center; 2010 to 2011 (up to 4 mo) | Behavioral; strategies to cope with the cost of prescription medications and cancer care expenses | To cope with expenses, 20% of took less than prescribed medication, 19% filled part of a prescription, 24% did not fill prescriptions, 7% avoided procedures, 9% avoided tests, 7% spread out appointments, and 4% skipped appointments |
| Zullig, 2013 (64) | 164 cancer survivors (multiple cancer sites) | Multiple cities and states; Health Well Foundation and Duke University; 2010 to 2011 (not listed) | Behavioral; prescription medication nonadherence | Overall, 45% of survivors were nonadherent with medication because of cost; a total of 36% used at least 2 of the 4 coping strategies; prescription drug plan and older age were protective against nonadherence; unemployment was associated with increased odds of nonadherence |
*CI = confidence interval; NHIS = National Health Interview Survey; OR = odds ratio; SEER = Surveillance, Epidemiology, and End Results program.
Coping Behavior Measures of Financial Hardship
Seven studies included coping behavior measures of financial hardship (8,10,14,23,43,44,64) (Table 5). Examples of coping behavioral measures include treatment nonadherence and forgoing/delaying cancer-related and non-cancer-related medical care because of cost. Many studies highlight a negative effect on behavior, such that survivors with higher levels of financial hardship are at greater risk of treatment nonadherence and delaying or forgoing medical care (14,23,64). Among specific financial sacrifices and cost-coping strategies of cancer survivors faced with financial hardship, several national and multistate studies evaluated general populations of cancer survivors and found that between 4% to 45% of survivors either skip, take less, or avoid filling prescription medication (8,14,23,64), and 5% to 20% reduce spending on both non-cancer-related health care or health care of other family members (14,44).
Composite and Other Summary Measures of Financial Hardship
In 13 studies (9,10,14,23,31,36,39,43,44,50,53,54,58) (Table 6), financial hardship was measured as a composite or summary of responses to several questions or survey items related to household finances that cut across our material conditions, psychological responses, and coping behavior categories. For example, Davidoff et al. (36) measured financial hardship as either 1) financial burden (OOP expenditures divided by adjusted gross income) or 2) delays or unmet need for medical, prescription, or dental care and found that 18% of cancer survivors reported experiencing some type of financial hardship from 2008 to 2010. Huntington et al. (43) used the COST measure to evaluate financial toxicity (score range = 0–44, with a lower value denoting greater financial burden) and reported that 90% of survivors with a COST score below the mean score of 23 experienced at least a minor level of financial burden (data years not listed). Meneses et al. (9) used the sum of affirmative responses to 19 items as a proxy measure of overall financial burden (eg, changes in work productivity, lifestyle, income, or debt) and reported that breast cancer survivors experienced a mean of 2.94 items at baseline and 2.25 items at six months. Regenbogen et al. (54) measured financial burden (score range = 0–7, with higher scores denoting increased financial burden) by summing responses to seven statements: “I had to use savings,” “I had to borrow money or take out a loan,” “I could not make payments on credit cards or other bills,” “I cut down on spending for food and/or clothes,” “I cut down on spending for health care for other family members,” “I cut down on recreational activities,” and “I cut down on expenses in general.” Mean scores for colorectal cancer survivors with complications following surgery were statistically significantly greater than for survivors without complications in multivariable Poisson regression analyses with two-sided tests of significance (2.21 vs 1.69, P < .001).
Table 6.
Composite and other summary measures of financial hardship*
| First author, y | Sample size (n) | Setting, data source, and collection time frame (duration of spending) | Measure | Key findings |
|---|---|---|---|---|
| Bestvina, 2014 (10) | 300 cancer survivors (multiple sites) | Duke Cancer Institute, and 3 affiliated rural oncology clinics; 2012 to 2013 (not listed) | Subjective financial distress (IFDFW) | A total of 16% of survivors reported high or overwhelming financial distress |
| Cagle, 2015 (31) | 176 cancer survivors (all sites) | National; Kaiser Foundation, the Harvard School of Public Health, and USA Today; 2006 (not listed) | Subjective measure of financial strain (score range: 1 = not a burden at all; 2 = minor burden; 3 = major burden) | A total of 22.7% of survivors reported that the cost of care was a major financial burden, 28.4% a minor burden, and 48.9% not a burden at all |
| Davidoff, 2015 (36) | 2527 cancer survivors (all sites) | National; MEPS; 2008 to 2010 (annual) | Financial hardship measure (financial burden [OOP expenditures divided by unadjusted gross income] and/or reported delays or unmet need for medical, prescription, or dental care) | Overall, 18% of cancer survivors reported experiencing financial hardship, and 37% of uninsured reported financial hardship |
| Fenn, 2014 (39) | 2108 cancer survivors (all sites) | National; NHIS; 2010 (not listed) | Cancer-related financial problems and their effect on quality of life | Survivors who reported “a lot” of financial problems (8.6%) were more likely to rate their physical health, mental health, and satisfaction with social activities and relationships as poor compared with those with no financial problems (69.6%) |
| Huntington, 2015 (43) | 100 multiple myeloma cancer patients | Philadelphia, PA; Academic Medical Center; August 2014 to January 2015 (not listed) | Financial toxicity by COST measure (score range = 0–44, lower value equals greater burden); self-reported level of financial burden (not at all, minor, moderate, significant) | Survivors had a mean COST score of 23; COST scores were highly correlated with patient-reported use of strategies to cope with treatment expenses; a total of 90% of survivors with a COST score below 23 experienced at least a minor level of financial burden |
| Irwin, 2014 (44) | 134 breast cancer survivors | North Carolina; Duke University; 2012 (not listed) | Subjective financial distress (IFDFW) | About one-third of survivors reported hardship as a result of their cancer costs |
| Kent, 2013 (14) | 1556 cancer survivors (all sites) | National; NHIS; 2010 (not listed) | Cancer-related financial problems | Approximately 32% of survivors reported any cancer-related financial problems |
| Meisenberg, 2015 (50) | 132 cancer survivors (all sites) | Single cancer institute in Annapolis, MD; y not stated (not listed) | InCharge Financial Distress/Financial Well-Being Scale (8 items, score range = 1–10, with low numbers indicating higher distress) | Average financial distress score was 5.11; nearly half of respondents reported high levels of financial distress |
| Meneses, 2012 (9) | 137 breast cancer survivors | Southeast states; BCEI; y not listed (6-mo duration) | 19 economic burden items related to work and financial hardship events | Survivors reported a mean of 2.94 economic burden items at baseline and 2.25 economic burden items at month 6 |
| Pisu, 2015 (53) | 1364 lung cancer survivors and 2068 colorectal cancer survivors | CanCORS sites; 2003 to 2005 (1 y after diagnosis) | Self-reported economic hardship (3 items related to difficulty of living on current income and anticipated future hardship) | A total of 52.7% of lung cancer and 46.1% of colorectal cancer survivors experienced some form of economic hardship (eg, difficulty of living on current income, anticipated hardship, and anticipated reduction in living standards), with a higher proportion of African Americans and Hispanics reporting economic hardship than whites |
| Regenbogen, 2014 (54) | 937 colorectal cancer survivors | SEER registries of Detroit, MI, and GA; 2011 to 2013 (up to 1 y post surgery) | Composite measure of financial burden (score range = 0–7, with higher scores denoting increased financial burden) | Survivors who reported complications after surgery had statistically significantly higher composite financial burden than survivors without complications, with adjusted scores of 2.21 and 1.69, respectively (P < .001) |
| Veenstra, 2014 (58) | 956 colorectal cancer survivors | SEER registries of Detroit, MI, and GA; 2011 to 2013 (not listed) | Personal financial burden (7 questions about how cancer or its treatment affected finances) | Mean financial burden score was 1.72, range = 0–6, and 38% of respondents endorsed no measures of financial burden; a total of 29% reported 1–2 measures, 23% reported 2–4 and 9% reported 5 or more measures of financial burden |
| Zafar, 2013 (23) | 258 cancer survivors (multiple sites) | Multiple cities and states; HealthWell Foundation and Duke University Medical Center; 2010 to 2011 (up to 4 mo) | Subjective financial burden resulting from cancer-related OOP expenses | The vast majority of survivors reported at least some financial burden, ranging from minor (15%), to moderate (37%), significant (33%), or catastrophic (9%) |
*BCEI = Breast Cancer Education Intervention clinical trials; CanCORS = Cancer Care Outcomes Research and Surveillance Consortium; IFDFW = InCharge Financial Distress/Financial Well-Being Scale; MEPS = Medical Expenditures Panel Survey; NHIS = National Health Interview Survey; OOP = out of pocket; SEER = Surveillance, Epidemiology and End Results program.
Discussion
The continued rise in costs of cancer care in the United States over the past few decades (66–69), alongside practice and payment reform initiatives (69–73), have driven the need for metrics to evaluate the financial hardship associated with cancer. In this systemic review, we identified and summarized the measures of financial hardship among US cancer survivors in the published literature. We found that the number of published studies addressing at least one aspect of financial hardship increased dramatically over the past 25 years, especially in 2013–2015, which accounts for over half of the published literature included in this review. The increasing number of studies coincides with the increasing costs of cancer care and reflects a rapidly evolving field focusing on the impact of the costs of cancer care for patients. In our review of the published literature, we observed substantial heterogeneity in both the methods employed and the terms used to describe financial hardship, including financial distress, financial stress, financial hardship, financial problem, financial toxicity, financial burden, economic burden, economic hardship, and financial impact. The noticeable overlap in definitions and inconsistency in terms and measures used across studies underscores the need for consistent use of terms and defining measures in order to maximize the value of evidence produced from studies on the financial hardship among cancer survivors and to ensure that interventions, policies, and programs developed based on the results of these studies are targeting the most relevant aspect of financial hardship for patients and their families.
Previous studies have also highlighted the lack of conceptual clarity for the construct of financial hardship in cancer-related research (22). In our review, we observed that studies rarely stated explicitly which of the following financial hardship domains the construct was attempting to capture: 1) the material conditions that arise from the increased OOP expenses and potentially lower income that results from the inability to work during/following cancer treatment, 2) the psychological response to the increase in household expenses that must now be managed as patients navigate cancer care, or 3) the coping behaviors that patients adopt to manage their medical care while experiencing increased household expenses during/following cancer care. Future research should be conducted to further refine this typology and the measurement of financial hardship to inform intervention development. To move this effort forward, we recommend future cancer care research that includes financial hardship measures to explicitly state whether the measure is attempting to capture the material conditions, psychological response, and/or coping behaviors of the financial hardship experience of the cancer patient.
Importantly, few studies of financial hardship in cancer research used validated measures or commented about the validation process. The most commonly used validated measures were the COST measure (20) and the InCharge Financial Distress/Financial Well-Being Scale (65). The nationally representative MEPS includes standard quality-of-life measures, and the 2016–2017 MEPS Experiences with Cancer survey, which is currently being fielded, includes many items about financial hardship, in addition to the Patient Reported Outcomes Measurement Information System (PROMIS) global measures. Further validation of financial hardship measures for the typology we propose in large samples across diverse populations will be important in future research.
Regardless of how financial hardship has been measured, the 25 years of published research illustrate that the experience of financial hardship across multiple domains is a common problem for cancer survivors. A study recently published in 2016 (after the timeframe for this review) reported that in a nationally representative sample of working-age cancer survivors approximately one in four reported ever having any material financial hardship and one in three reported psychological financial hardship (18). Many more recently available therapies have price tags of more than $100 000 per year and are commonly used in combination with other therapies. As medical costs are increasingly shifted to patients through higher health insurance premiums, deductibles, and greater cost sharing, the ongoing surveillance of the multiple domains of financial hardship will be critical as cancer patients navigate treatment and survivorship. Appropriately targeted interventions will need to be developed to ensure such financial hardships do not negatively impact treatment-related outcomes (eg, survival).
Our results indicate that material condition measures were, most often, considered objective indicators that provided information related to: 1) OOP costs—costs associated with both direct medical (eg, medication) and nonmedical (eg, transportation) needs; 2) indirect costs and productivity loss—costs associated with the reduction in work (eg, loss of income), time, and activities (eg, work-related or usual daily activities) because of the occurrence of cancer; and 3) medical debt and bankruptcy—the economic consequences of financial hardship. However, it is important to note that there is a lack of consistency in measurement approaches and definitions; thus any meaningful comparisons across studies using material condition indicators of financial hardship are severely hampered.
The studies using psychological response measures of financial hardship aim to estimate both the subjective (eg, financial distress) and financial consequences of a cancer diagnosis. Further, these psychological response measures illustrate the different areas of a cancer patient’s well-being (eg, emotional, functional, mental, and physical) that can be influenced when experiencing financial hardship (38,54). A better understanding of quality of life and patient-reported outcomes associated with the survivor’s psychological response to financial hardship is warranted, especially given the need for evidence for efforts like the American Society of Clinical Oncology (ASCO) Value in Cancer Care Initiative, which aims to incorporate data on both patient cost and quality of life.
Use of coping behavior measures in reviewed studies highlights the cost-coping strategies resulting from the financial hardship of cancer, illustrating trade-offs between paying for basic life needs (eg, food and shelter) and spending on health care for cancer survivors as well as other family members by cutting back on health care use (eg, forgoing or skipping medication) (8,14,44,58). Increased knowledge about financial coping behaviors that families use to manage cancer care will be especially useful in developing interventions to improve adherence to recommended treatment.
Many researchers combined components of the three different aspects of financial hardship to create a composite or summary measure, with items summarized into a single score/unit (eg, combining used savings, borrowed money or took out a loan, missed bill payments [8,23,43,44,54]). Although composite or summary measures can be useful for ranking the overall severity of financial hardship, one limitation in these approaches is the extent to which individual items are ordered or weighted. Moreover, the interpretation of the score and what action to take based on the score are unclear. For instance, do cancer survivors who must mortgage their homes have the same level of financial hardship as those who cut down on vacation expenses? Additionally, should the survivor’s consumption preference and risk tolerance be considered when interpreting the financial hardship scores? The use of different questions and combinations of questions across studies to construct composite or summary measures also limits the validity across populations. Consequently, while composite or summary measures may be useful in studies that have several items associated with a specific dimension of financial hardship (eg, creating an overall score for a psychological response measure such as financial stress), the use of composite measures may not clearly highlight whether the hardship issue is related to the financial resources available, how the patient feels about their lack of financial resources, or an issue with managing the financial resources available.
How might financial hardship be incorporated in cost-effectiveness and value frameworks when evaluating cancer control strategies? These frameworks offer transparent approaches to quantifying and summarizing the costs and benefits (or consequences) associated with alternative strategies, for use by health care programs, health plans, and other policy makers operating in settings with limited resources. Some of the material financial hardship measures we identified, such as patient OOP costs, are traditional measures of cost from the perspective of the patient and society, more broadly. Other material measures, such as productivity losses, may also be measured from the patient or societal perspective, yet represent areas of some controversy within the field. Nevertheless, these material financial hardship measures could be used as inputs in some cost effectiveness estimates, such as the incremental cost-effectiveness ratio (ICER), where the numerator is cost (eg, out-of-pocket costs because of particular intervention or treatment). Psychological financial hardship measures might be conceptualized as a component of patient utility, as part of the denominator of the ICER. Financial hardship concepts are important when considering the costs and benefits of different strategies and will be important for future cancer care research.
Although we included studies published over the past 25 years in this review of the published literature, we did not include studies of financial hardship in informal caregivers, including spouses and partners, siblings, parents, children, and friends of cancer survivors. The few studies that have been conducted suggest that caregivers also face considerable OOP costs (74–77) and productivity losses (74–78) associated with cancer. They may also experience psychological distress and delay or forgo their own medical care as a behavioral response to financial hardship. Improving understanding of financial hardship for the entire household and support network will be an important area for additional research.
We limited our systematic review to studies conducted in the United States because of substantial differences between health systems in other countries and the United States. The majority of other developed countries have universal health insurance coverage, making the differences in health care delivery, particularly for the uninsured, quite distinct in the United States. Differences in health care systems between the United States and other developed countries also affect the insured with respect to health insurance premiums, deductibles, copayments, coinsurance, and limitations in coverage. Thus, the potential for very high OOP payments for cancer survivors is quite different in the United States than in other countries. Nonetheless, several studies have evaluated financial hardship outside of the United States (79–83), and comparative studies in similar groups of patients will be important for future research for improving understanding of how differences in health systems can impact financial hardship.
An important strength of our systematic review is that we used a multiple database and keyword search strategy to identify articles related to financial hardship in cancer survivors over the past 25 years. However, despite the use of multiple databases and replicating each search strategy for each database, we were unable to capture every relevant article because of different indexing used by the databases and the inconsistent terminology used to define financial hardship. To help minimize this limitation, we hand-searched the reference lists for each article for any additional studies that were not captured in the initial electronic search process. The fact that we identified 10 studies with our hand-search reflects a lack of specificity with the financial hardship search terms available in major publication databases.
Data abstraction by a single author is another limitation to this review; however, we did our best to standardize the data abstraction process by using objective measures of financial hardship and multiple reviewers to resolve any disagreements or inconsistencies in the data. Yet, despite our efforts, there still may be some unavoidable subjectivity in classifying some of the measures. Finally, we included studies of financial hardship published over several decades and it is possible that estimates published in earlier years are not directly comparable with those published more recently. However, our synthesis of findings was qualitative, rather than quantitative. In addition, our exhaustive systematic review offers a comprehensive picture of an increasingly recognized issue of great importance to patients and a rapidly emerging area of research.
In summary, the complexity of measuring the financial hardship of cancer has led to substantial heterogeneity in methods and measures. Our findings highlight the need for consistent use of definitions, terms, and measures to better inform development of interventions in order to prevent and minimize the financial hardship experienced by cancer survivors.
Funding
Dr. Tucker-Seeley was supported by a National Cancer Institute K01 Career Development Grant (K01 CA169041).
Notes
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
References
- 1. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. http://seer.cancer.gov/csr/1975_2013/. Accessed September 30, 2015.
- 2. de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conti RM, Fein AJ, Bhatta SS. National trends in spending on and use of oral oncologics, first quarter 2006 through third quarter 2011. Health Aff (Millwood). 2014;33(10):1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradley CJ, Yabroff KR, Warren JL, et al. Trends in the treatment of metastatic colon and rectal cancer in elderly patients. Med Care. 2016;54(5):490–497. [DOI] [PubMed] [Google Scholar]
- 5. Shih YC, Smieliauskas F, Geynisman DM, et al. Trends in the cost and use of targeted cancer therapies for the privately insured nonelderly: 2001 to 2011. J Clin Oncol. 2015;33(19):2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guy GP, Jr, Ekwueme DU, Yabroff KR, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31(30):3749–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ekwueme DU, Yabroff KR, Guy GP, Jr, et al. Medical costs and productivity losses of cancer survivors--United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2014;63(23):505–510. [PMC free article] [PubMed] [Google Scholar]
- 8. Jagsi R, Pottow JA, Griffith KA, et al. Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol. 2014;32(12):1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meneses K, Azuero A, Hassey L, et al. Does economic burden influence quality of life in breast cancer survivors? Gynecol Oncol. 2012;124(3):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10(3):162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy people 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives. Accessed June 10, 2015.
- 12. Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood). 2013;32(6):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwartz K, Claxton G, Martin K, et al. Spending to Survive: Cancer Patients Confront Holes in the Health Insurance System. Menlo Park, CA: Kaiser Family Foundation; 2009. [Google Scholar]
- 14. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zafar SY. Financial toxicity of cancer care: It's time to intervene. J Natl Cancer Inst. 2016;108(5). [DOI] [PubMed] [Google Scholar]
- 17. Banegas MP, Guy GP, Jr, de Moor JS, et al. For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Aff (Millwood). 2016;35(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yabroff KR, Dowling EC, Guy GP, Jr, et al. Financial hardship associated with cancer in the united states: Findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34(3):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanratty B, Holland P, Jacoby A, et al. Financial stress and strain associated with terminal cancer--a review of the evidence. Palliat Med. 2007;21(7):595–607. [DOI] [PubMed] [Google Scholar]
- 20. de Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer: The COST measure. Cancer. 2014;120(20):3245–3253. [DOI] [PubMed] [Google Scholar]
- 21. Choi SK, Adams SA, Eberth JM, et al. Medicaid coverage expansion and implications for cancer disparities. Am J Public Health. 2015;105(suppl 5):S706–S712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tucker-Seeley RD, Yabroff KR. Minimizing the "financial toxicity" associated with cancer care: Advancing the research agenda. J Natl Cancer Inst. 2016;108(5). [DOI] [PubMed] [Google Scholar]
- 23. Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist. 2013;18(4):381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin MY, Fouad MN, Oster RA, et al. What do cancer patients worry about when making decisions about treatment? Variation across racial/ethnic groups. Support Care Cancer. 2014;22(1):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meropol NJ, Schulman KA. Cost of cancer care: Issues and implications. J Clin Oncol. 2007;25(2):180–186. [DOI] [PubMed] [Google Scholar]
- 26. de Souza JA, Yap B, Ratain MJ, et al. User beware: We need more science and less art when measuring financial toxicity in oncology. J Clin Oncol. 2015;33(12):1414–1415. [DOI] [PubMed] [Google Scholar]
- 27. Lazarus R, Folkman S. The concept of coping In Stress, Appraisal and Coping. New York: Springer Publishing Company; 1984:117–140. [Google Scholar]
- 28. Arozullah AM, Calhoun EA, Wolf M, et al. The financial burden of cancer: Estimates from a study of insured women with breast cancer. J Support Oncol. 2004;2(3):271–278. [PubMed] [Google Scholar]
- 29. Banthin JS, Bernard DM. Changes in financial burdens for health care: national estimates for the population younger than 65 years, 1996 to 2003. JAMA. 2006;296(22):2712–2719. [DOI] [PubMed] [Google Scholar]
- 30. Bernard DS, Farr SL, Fang Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J Clin Oncol. 2011;29(20):2821–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cagle JG, Carr DC, Hong S, et al. Financial burden among US households affected by cancer at the end of life. Psychooncology. 2016;25(8):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang S, Long SR, Kutikova L, et al. Estimating the cost of cancer: Results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol. 2004;22(17):3524–3530. [DOI] [PubMed] [Google Scholar]
- 33. Chino F, Peppercorn J, Taylor DH, Jr, et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19(4):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chirikos TN, Russell-Jacobs A, Cantor AB. Indirect economic effects of long-term breast cancer survival. Cancer Pract. 2002;10(5):248–255. [DOI] [PubMed] [Google Scholar]
- 35. Davidoff AJ, Erten M, Shaffer T, et al. Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer. 2013;119(6):1257–1265. [DOI] [PubMed] [Google Scholar]
- 36. Davidoff AJ, Hill SC, Bernard D, et al. The Affordable Care Act and expanded insurance eligibility among nonelderly adult cancer survivors. J Natl Cancer Inst. 2015;107(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dowling EC, Chawla N, Forsythe LP, et al. Lost productivity and burden of illness in cancer survivors with and without other chronic conditions. Cancer. 2013;119(18):3393–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ell K, Xie B, Wells A, et al. Economic stress among low-income women with cancer: Effects on quality of life. Cancer. 2008;112(3):616–625. [DOI] [PubMed] [Google Scholar]
- 39. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors' quality of life. J Oncol Pract. 2014;10(5):332–338. [DOI] [PubMed] [Google Scholar]
- 40. Finkelstein EA, Tangka FK, Trogdon JG, et al. The personal financial burden of cancer for the working-aged population. Am J Manag Care. 2009;15(11):801–806. [PubMed] [Google Scholar]
- 41. Guy GP, Robin Yabroff K, Ekwueme DU, et al. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Affairs. 2014;33(6):1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guy GP, Jr, Yabroff KR, Ekwueme DU, et al. Healthcare expenditure burden among non-elderly cancer survivors, 2008-2012. Am J Prev Med. 2015;49(6 suppl 5):S489–S497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huntington SF, Weiss BM, Vogl DT, et al. Financial toxicity in insured patients with multiple myeloma: A cross-sectional pilot study. Lancet Haematol. 2015;2(10):e408–e416. [DOI] [PubMed] [Google Scholar]
- 44. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19(11):1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jacobson JJ, Epstein JB, Eichmiller FC, et al. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: Commercial insurance, Medicare, and Medicaid. Head Neck Oncol. 2012;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jayadevappa R, Schwartz JS, Chhatre S, et al. The burden of out-of-pocket and indirect costs of prostate cancer. Prostate. 2010;70(11):1255–1264. [DOI] [PubMed] [Google Scholar]
- 47. Kilgore ML, Goldman DP. Drug costs and out-of-pocket spending in cancer clinical trials. Contemp Clin Trials. 2008;29(1):1–8. [DOI] [PubMed] [Google Scholar]
- 48. Langa KM, Fendrick AM, Chernew ME, et al. Out-of-pocket health-care expenditures among older Americans with cancer. Value Health. 2004;7(2):186–194. [DOI] [PubMed] [Google Scholar]
- 49. Li C, Li C, Forsythe L, et al. Mental health services utilization and expenditures associated with cancer survivorship in the United States. J Cancer Surviv. 2015;9(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20(10):1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moore K. Out-of-pocket expenditures of outpatients receiving chemotherapy. Oncol Nurs Forum. 1998;25(9):1615–1622. [PubMed] [Google Scholar]
- 52. Pisu M, Azuero A, Meneses K, et al. Out of pocket cost comparison between Caucasian and minority breast cancer survivors in the Breast Cancer Education Intervention (BCEI). Breast Cancer Res Treat. 2011;127(2):521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pisu M, Kenzik KM, Oster RA, et al. Economic hardship of minority and non-minority cancer survivors 1 year after diagnosis: Another long-term effect of cancer? Cancer. 2015;121(8):1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Regenbogen SE, Veenstra CM, Hawley ST, et al. The personal financial burden of complications after colorectal cancer surgery. Cancer. 2014;120(19):3074–3081. [DOI] [PubMed] [Google Scholar]
- 55. Sasser AC, Rousculp MD, Birnbaum HG, et al. Economic burden of osteoporosis, breast cancer, and cardiovascular disease among postmenopausal women in an employed population. Womens Health Issues. 2005;15(3):97–108. [DOI] [PubMed] [Google Scholar]
- 56. Shankaran V, Jolly S, Blough D, et al. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: A population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608–1614. [DOI] [PubMed] [Google Scholar]
- 57. Teitelbaum A, Ba-Mancini A, Huang H, et al. Health care costs and resource utilization, including patient burden, associated with novel-agent-based treatment versus other therapies for multiple myeloma: Findings using real-world claims data. Oncologist. 2013;18(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Veenstra CM, Regenbogen SE, Hawley ST, et al. A composite measure of personal financial burden among patients with stage III colorectal cancer. Med Care. 2014;52(11):957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wan Y, Gao X, Mehta S, et al. Indirect costs associated with metastatic breast cancer. J Med Econ. 2013;16(10):1169–1178. [DOI] [PubMed] [Google Scholar]
- 60. Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99(1):14–23. [DOI] [PubMed] [Google Scholar]
- 61. Yabroff KR, Lawrence WF, Clauser S, et al. Burden of illness in cancer survivors: Findings from a population-based national sample. J Natl Cancer Inst. 2004;96(17):1322–1330. [DOI] [PubMed] [Google Scholar]
- 62. Yabroff KR, Warren JL, Knopf K, et al. Estimating patient time costs associated with colorectal cancer care. Med Care. 2005;43(7):640–648. [DOI] [PubMed] [Google Scholar]
- 63. Zafar SY, McNeil RB, Thomas CM, et al. Population-based assessment of cancer survivors' financial burden and quality of life: A prospective cohort study. J Oncol Pract. 2015;11(2):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zullig LL, Peppercorn JM, Schrag D, et al. Financial distress, use of cost-coping strategies, and adherence to prescription medication among patients with cancer. J Oncol Pract. 2013;9(6 SUPPL.):60s–63s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Prawitz AD, Garman ET, Sorhaindo B, et al. The incharge financial distress/financial well-being scale: Establishing validity and reliability. Fin Counsel Plan. 2006;17:34–50. [Google Scholar]
- 66. Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000-2014. JAMA Oncol. 2016;2(7):960–961. [DOI] [PubMed] [Google Scholar]
- 67. Lee JA, Roehrig CS, Butto ED. Cancer care cost trends in the United States: 1998 to 2012. Cancer. 2016;122(7):1078–1084. [DOI] [PubMed] [Google Scholar]
- 68. Tangka FK, Trogdon JG, Richardson LC, et al. Cancer treatment cost in the United States: Has the burden shifted over time? Cancer. 2010;116(14):3477–3484. [DOI] [PubMed] [Google Scholar]
- 69. American Society of Clinical Oncology. The state of cancer care in America, 2016: A report by the American Society of Clinical Oncology. J Oncol Pract. 2016;12(18): 339–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kline RM, Bazell C, Smith E, et al. Centers for medicare and medicaid services: Using an episode-based payment model to improve oncology care. J Oncol Pract. 2015;11(2):114–116. [DOI] [PubMed] [Google Scholar]
- 71. Newcomer LN. Innovative payment models and measurement for cancer therapy. J Oncol Pract. 2014;10(3):187–189. [DOI] [PubMed] [Google Scholar]
- 72. Page RD, Newcomer LN, Sprandio JD, et al. The patient-centered medical home in oncology: From concept to reality. Am Soc Clin Oncol Educ Book. 2015;e82–e89. [DOI] [PubMed] [Google Scholar]
- 73. Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23):2563–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fluchel MN, Kirchhoff AC, Bodson J, et al. Geography and the burden of care in pediatric cancers. Pediatr Blood Cancer. 2014;61(11):1918–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Given BA, Given CW, Stommel M. Family and out-of-pocket costs for women with breast cancer. Cancer Pract. 1994;2(3):187–193. [PubMed] [Google Scholar]
- 76. Van Houtven CH, Ramsey SD, Hornbrook MC, et al. Economic burden for informal caregivers of lung and colorectal cancer patients. Oncologist. 2010;15(8):883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Warner EL, Kirchhoff AC, Nam GE, et al. Financial burden of pediatric cancer for patients and their families. J Oncol Pract. 2015;11(1):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li C, Zeliadt SB, Hall IJ, et al. Burden among partner caregivers of patients diagnosed with localized prostate cancer within 1 year after diagnosis: An economic perspective. Support Care Cancer. 2013;21(12):3461–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Girgis A, Lambert S, Johnson C, et al. Physical, psychosocial, relationship, and economic burden of caring for people with cancer: A review. J Oncol Pract. 2013;9(4):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lauzier S, Levesque P, Mondor M, et al. Out-of-pocket costs in the year after early breast cancer among Canadian women and spouses. J Natl Cancer Inst. 2013;105(4):280–292. [DOI] [PubMed] [Google Scholar]
- 81. Longo CJ, Fitch M, Deber RB, et al. Financial and family burden associated with cancer treatment in Ontario, Canada. Support Care Cancer. 2006;14(11):1077–1085. [DOI] [PubMed] [Google Scholar]
- 82. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22(4):745–755. [DOI] [PubMed] [Google Scholar]
- 83. Sharp L, Timmons A. Pre-diagnosis employment status and financial circumstances predict cancer-related financial stress and strain among breast and prostate cancer survivors. Support Care Cancer. 2016;24(2):699–709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.