Abstract
Study Objectives:
Social isolation has a multitude of negative consequences on human health including the ability to endure challenges to the immune system, sleep amount and efficiency, and general morbidity and mortality. These adverse health outcomes are conserved in other social species. In the fruit fly Drosophila melanogaster, social isolation leads to increased aggression, impaired memory, and reduced amounts of daytime sleep. There is a correlation between molecules affected by social isolation and those implicated in sleep in Drosophila. We previously demonstrated that acute sleep loss in flies and mice induced the unfolded protein response (UPR), an adaptive signaling pathway. One mechanism indicating UPR upregulation is elevated levels of the endoplasmic reticular chaperone BiP/GRP78. We previously showed that BiP overexpression in Drosophila led to increased sleep rebound. Increased rebound sleep has also been demonstrated in socially isolated (SI) flies.
Methods:
D. melanogaster were used to study the effect of social isolation on cellular stress.
Results:
SI flies displayed an increase in UPR markers; there were higher BiP levels, increased phosphorylation of the translation initiation factor eIF2α, and increased splicing of xbp1. These are all indicators of UPR activation. In addition, the effects of isolation on the UPR were reversible; pharmacologically and genetically altering sleep in the flies modulated the UPR.
Conclusions:
The reduction in sleep observed in SI flies is a cellular stressor that results in UPR induction.
Keywords: Drosophila, unfolded protein response (UPR), social isolation, sleep, cellular stress.
Statement of Significance
Research has clearly shown that social isolation is a public health concern because of its drastic effects on general morbidity and mortality; however, the mechanisms underlying these conditions are unknown. This study is the first to link the reduction in sleep seen during social isolation to protein homeostasis and upregulation of the unfolded protein response (UPR). Both social isolation and the UPR have been implicated in the pathophysiology of psychiatric disorders such as bipolar disorder, schizophrenia, and depression. These results suggest that chronic UPR induction as a result of social isolation could be an underlying mechanism in major psychiatric diseases and that understanding the interactions between social isolation and sleep could lead to new insights into the etiology of mental health disorders as well as new strategies for disease management.
INTRODUCTION
Perceived positive social interactions are associated with improved health. Conversely, social isolation has been shown to adversely impact behavior and health. Studies have demonstrated that social isolation leads to a variety of negative effects on human health including, but not limited to, the ability to withstand an immune system challenge,1,2 myocardial infarctions, stroke recurrence or survival after an initial stroke,3 the amount and efficiency of sleep,4–6 and general morbidity and mortality.7,8 The negative outcomes of social isolation are conserved in other social species. Behavioral disturbances such as increased aggression,9,10 anxiety,11,12 obesity,13 and deficits in learning and memory14,15 are all consequences of a socially isolated (SI) environment. Post-stroke survival was significantly decreased in mice that were SI in comparison to their socialized controls.16 Hyperactivity, another outcome of social isolation, is common to a number of species.17 In humans, the perception of being excluded from social interaction is sufficient for increased neural activity18 and leads to poor sleep quality.4
Sleep or sleep-like states have been observed in the majority of studied animals, although its fundamental role has not yet been elucidated. Several hypotheses have emerged to explain the central role/function of sleep, including macromolecule synthesis,19 synaptic homeostasis,20,21 and memory and learning.22 What is known, however, is that sleep is vital to promoting health. Deficiencies in sleep have been linked to a number of adverse health outcomes including decreases in immune function,23 cardiovascular disorders,24 and metabolic dysfunction.25 While sleep itself is affected by social isolation, the mechanisms underlying the negative outcomes of social isolation on sleep are poorly understood.
In this study, we used Drosophila melanogaster to study the effects of social isolation on sleep and cellular stress. Flies that are isolated from the point of eclosion demonstrate increased aggression,26 impaired memory, and reduced sleep.27 Lifespan is also significantly reduced in both male and female flies that have been SI.28 In the current study, we discovered that social isolation upregulates the unfolded protein response (UPR).29,30 Activation of the UPR is an adaptive response to endoplasmic reticular (ER) stress which increases transcription of genes involved in ER protein folding and degradation capacity and attenuates global protein translation.29–31 Briefly, protein homeostasis is maintained by three signaling cascades upon dissociation from immunoglobulin binding protein/glucose-regulated protein 78(BiP/GRP78), the major chaperone in the ER.32 This includes upregulation of serine–threonine kinase protein kinase RNA-like ER kinase (PERK) and activation of the inositol-requiring enzyme-1 (IRE1) and transcription factor 6 (ATF) pathways. Activated PERK phosphorylates the eukaryotic initiation factor 2α (eIF2α), resulting in translational suppression while activation of ATF6 and IRE1 upregulate chaperone expression. The IRE1 effect is mediated through spliced XBP1 (x-box-binding protein).32 Prolonged or sustained ER stress leads to a secondary maladaptive response which initiates inflammatory and proapoptotic signaling.29–32 Chronic induction of the UPR may be the mechanism underlying the negative health outcomes observed in social isolation. We wanted to determine if sleep loss that occurs as a result of social isolation would activate the UPR. Data presented here demonstrate that social isolation results in both reduced sleep and UPR induction. Our data also indicate that the effects of isolation on cellular stress are reversible. Pharmacologically and genetically, increasing sleep in SI flies reduced activation of the UPR. Conversely, decreasing sleep using similar methods in socially enriched (SE) flies increased cellular stress and markers of the UPR.
METHODS
Fly Stocks and Maintenance
Drosophila melanogaster strains white Canton-Special (wCS10), a gift from Ronald Davis, (Scripps Research Institute Florida, USA) and w1118ex from Bloomington Stock Center (Indiana, USA) 7–10 days were used in the majority of these studies. For the zolpidem (ZOL) studies, animals were aged to 4 weeks to avoid a ceiling effect. Flies were maintained at room temperature on standard food that contained dextrose, cornmeal, and yeast on a 12:12 hour light:dark (L:D) cycle. The flies were transferred onto new food every 2 to 3 days.
Social Isolation and Social Enrichment
For these studies, flies were separated into either grouped/SE or SI. SE flies were placed in vials and housed in groups of 20–30 flies from eclosion until their behavior was recorded. SI flies were isolated from pupae, sexed, and then individually housed in 2-mL Eppendorf tubes containing food as previously described.27 Flies were maintained at these conditions until 7 days after eclosion, when they were placed in individual 65 mm glass tubes and sleep behavior was recorded for 3 days using the video method.33
Drug Administration
ZOL and caffeine were used to increase and decrease sleep, respectively. For both ZOL and caffeine treatment, flies were placed into locomotor tubes containing 5% sucrose agar media and drug (0.01 mg/mL ZOL or caffeine 0.5 mg/mL) or vehicle (0.8% ethanol or distilled deionized water). We also tested for dose-dependent effects of ZOL on sleep at concentrations ranging from 0.01 to 5 mg/mL. Caffeine dosage was based on a previously published study.34 Caffeine treatment was carried out in both L:D and dark:dark (D:D) conditions. The L:D experiments were carried out by video monitoring whereas the D:D experiments were carried out using the Trikinetics DAMS system as previously described.34 Sleep analyses of the flies in D:D were generated by insomniac 3.3.
Genetic Manipulation of Sleep
Upstream activation sequence-galactose-responsive transcription factor 4 Binary System
We expressed the upstream activation sequence (UAS) construct under the control of an RU486-inducible mushroom body (MB) galactose-responsive transcription factor 4 (GAL4) driver, MB-Switch as described by Joiner et al.35 Briefly, virgin male and female flies of UAS Kir2.1 or NaChBac were crossed with virgin male or female MB switch flies. The resulting progeny (P{MB-Switch}/UAS-Kir2.1) or (P{MB-Switch}/UAS-NaChBac) were placed on either drug (RU486 100 µM) or vehicle (0.8% ethanol). UAS/GeneSwitch GAL4 genetic crosses were placed in locomotor tubes on drug (100 μM RU486) or vehicle (0.8% ethanol) in dextrose food at 7 days of age.
Behavioral Sleep Assays
Flies were collected 1 day after eclosion and either housed in groups (SE) or housed separately (SI) until behavior was recorded using video. For all behavioral experiments, D. melanogaster virgin females were placed in individual locomotor activity tubes on plates with a 28 animal capacity. Flies were allowed to acclimate to the tubes at least 24 hours before the recordings began. Sleep was recorded for a 3-day period. Sleep is defined as a 5-minute bin without activity.36 We performed four experiments of 28 flies each with wCS10 flies and four experiments with w1118ex.
Video Recording and Analysis
Flies were recorded using the video system previously described.33 Images were acquired at 5-second intervals using a Retiga 2000R camera (Qimaging, Surrey, British Columbia) and custom software written using MatLab (Mathworks, Natick, Massachusetts). Infrared LED lamps (Lilin Corp.) at a peak wavelength of 850 nm were used for camera illumination during the dark period. Video analysis: custom software written with Matlab and C computer languages was used to analyze the video images using subtraction analysis.33 Corresponding pixels from two temporally adjacent images are subtracted and each pixel in the DIFFERENCE image has the value: GS (XiYj) = [(GS2(XiYj) − GS1(XiYj))/2] + 127, where GS(XiYj) is the DIFFERENCE image grayscale value centered around a value of 127 at pixels X position i and Y position j and GS2 and GS1 are the grayscale values at that same pixel for the second and first video frames, respectively, in a pair of temporally adjacent frames.
Fly Head Preparation and Western Blotting
Molecular Analysis
Flies were sacrificed at the end of the 3-day recording period. Protein was extracted using a standard cell lysis protocol as described previously37 (groups of 10 pooled fly heads). Protein concentrations were determined using the Pierce micro-BCA assay kit. We also used single fly heads in some studies. Single fly heads were homogenized in lysis buffer and Laemli buffer as previously described.37,38 Protein samples from pooled head homogenate (15 µg/well) or single heads were run on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels (10% Tris-HCl), transferred to nitrocellulose membranes (Bio-Rad), and incubated with primary antibodies. Primary antibodies and dilutions used were: Hsc 3 (heat shock cognate 3)-BiP/GRP78 (Brabaham, United Kingdom) 1:2500, anti-phospho-eIF2α (Ser51) polyclonal antibody, and β-actin (Cell Signaling Technology, Tokyo, Japan), 1:1000. This was followed by a 1-hour incubation with secondary antibodies (anti-rat 1:20 000, Sigma, for BiP/GRP78 and anti-rabbit 1:10 000 for all other antibodies, except β-actin [mouse, 1:10 000]). Protein bands were detected and analyzed by the Odyssey Infrared Scanner (Li-Cor). Phosphoproteins were detected first and then NewBlot™ Nitro stripping buffer (Li-Cor, 30 minutes at room temperature) was used to reprobe the membranes with an antibody recognizing the nonphosphorylated form.
Reverse Transcriptase Polymerase Chain Reaction
Total RNA from flies was isolated using TRIzol reagent (Invitrogen) in conjunction with the RNeasy Mini Kit (Qiagen). We synthesized complementary DNA from 1 microgram of total RNA samples using Superscript III (Invitrogen). XBP1 complementary DNA was amplified by reverse transcription polymerase chain reaction (RT-PCR) using primers that flanked the unconventional splice site (Sidrauski and Walter, 1997) in xbp1 messenger RNA (Xbp1_F, 50-CGCCAGCGCAGGCGCTGAGG-30 and Xbp1_R, 50-CT GCTCCGCC-AGCAGACGCGC-30). The protocol was 25 PCR cycles long. PCR product was run on a 3% agarose gel and stained with Ethidium bromide to visualize the bands. The unspliced band was observed at 127 base pairs, and the spliced variant was observed at 104 base pairs.
Statistical Analysis
Analyses of sleep characteristics were performed separately for daytime and nighttime sleep. t tests were used when comparing results between two groups of interest. When comparing more than two groups, we tested the global null hypothesis of no differences among groups using analysis of variance. If this global hypothesis was rejected (p < .05), then we examined specific between-group comparisons of interest.
RESULTS
Social Isolation Reduces and Fragments Sleep
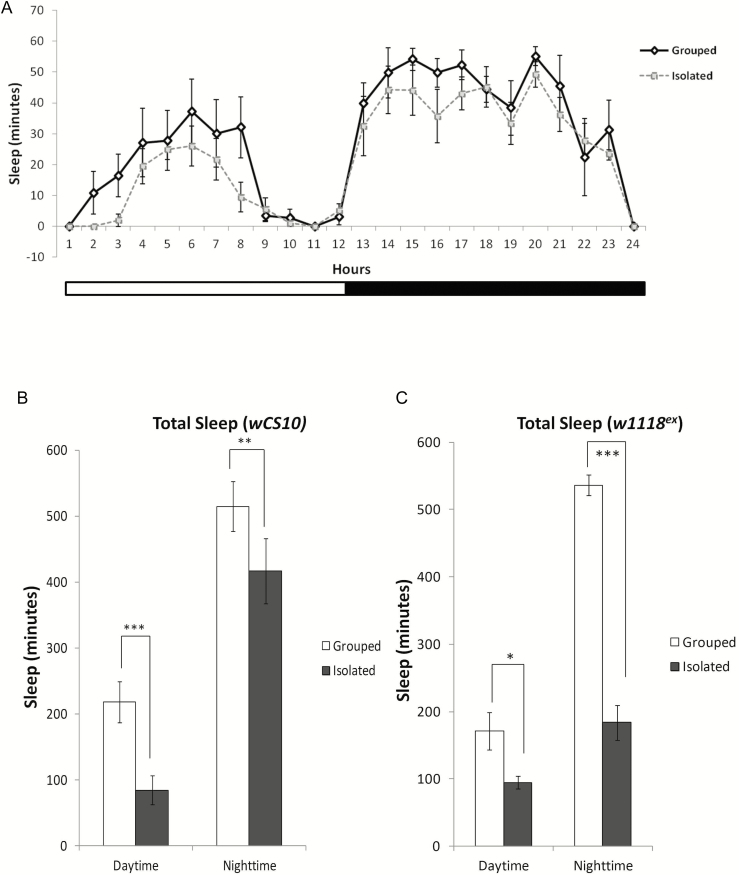
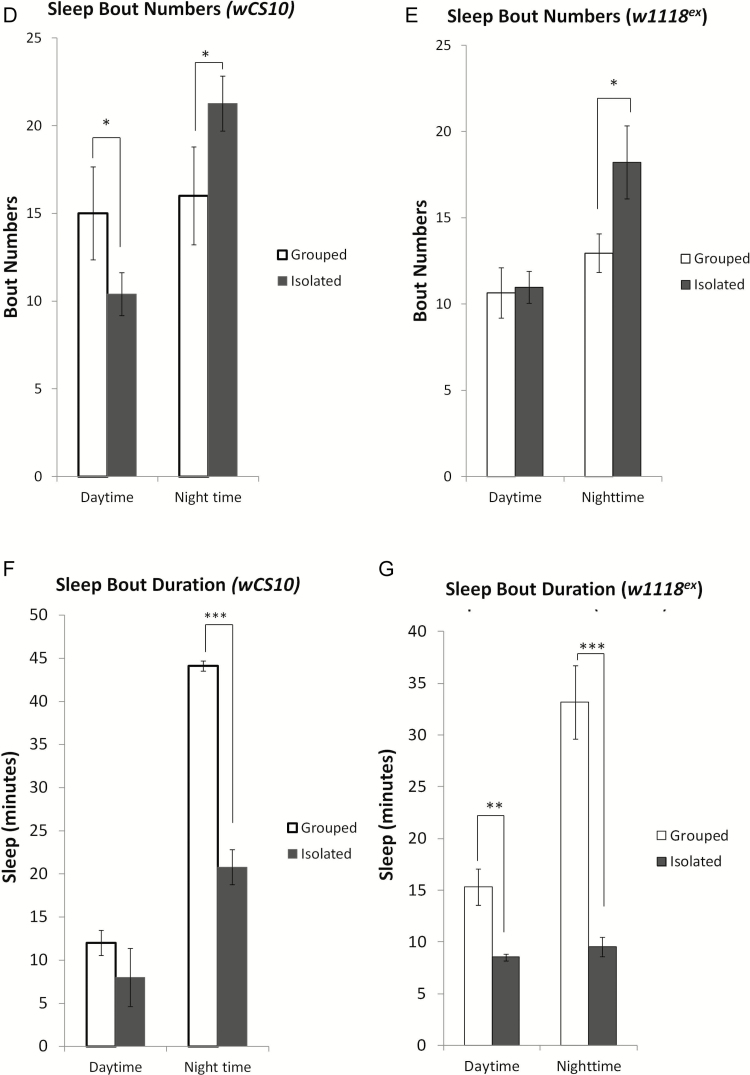
Similar to previous studies,27,39 we found that SI flies exhibited significant reductions in total sleep (Figure 1A) compared to grouped/SE flies. To confirm that the effect of social isolation was not strain specific, we used two wild-type strains and found that both wCS10 (Figure 1B) and w1118ex (Figure 1C) lose sleep when isolated. In addition to reductions in total sleep, we also observed that nighttime sleep in the SI flies was extremely fragmented, demonstrated by significant increases in sleep bout number (Figure 1, D and E; p < .05) and significant decreases in sleep bout duration (Figure 1, F and G; p < .001).
Figure 1.
Social isolation reduces and fragments sleep. (a) Representative graph for the wCS10 strain showing reduced sleep over 24 hours in flies that were socially isolated (SI) (n = 112) versus the grouped/socially enriched (SE) flies (n=112). Total sleep is significantly reduced during the day and night in both (b) wCS10 and (c) w118ex (n = 56) in the SI compared to the SE conditions. (d) Sleep bout numbers are significantly reduced during the day and increased at night in SI wCS10. (e) SI significantly increases sleep bout numbers during the night in w1118ex flies. (f) Sleep bout duration is significantly decreased during the night in the SI wCS10 flies compared to the SE flies. (g) Sleep bout duration is decreased during both the day and the night in w1118ex flies SI flies. Socially isolated (SI) = kept as single female; socially enriched (SE) = kept with 30 females; Daytime = total sleep during lights on; Nighttime = total sleep during lights off; mean ± SEM shown, *p < .05, **p < .01, ***p < 0.001.
Social Isolation Induces the UPR
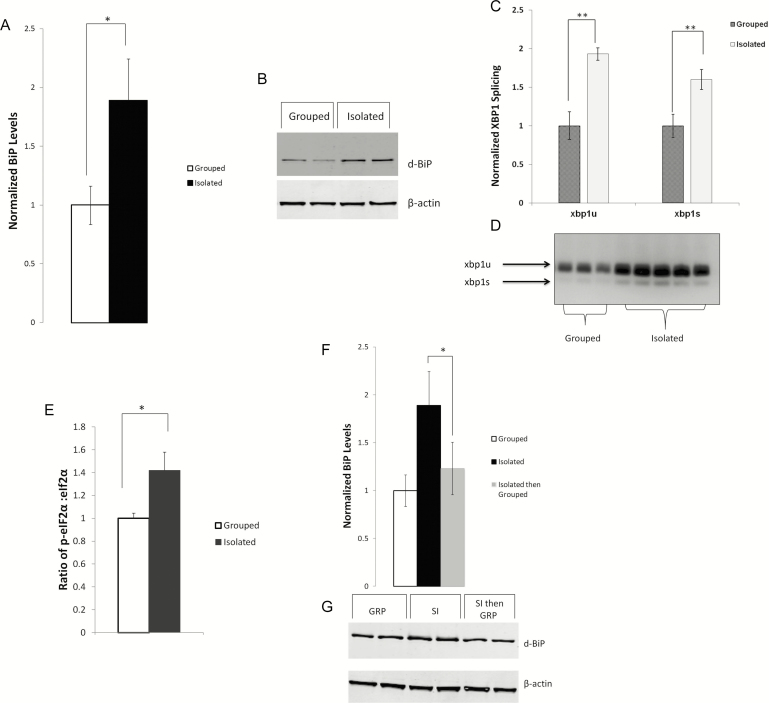
Previous studies in our laboratory demonstrated that sleep loss/ sleep deprivation induced the UPR.37,40 We hypothesized that the reduction in sleep due to social isolation would also activate the UPR. We found significant increases in BiP protein levels in SI flies compared to grouped/SE flies (Figure 2, A and B; p < .05). Increased BiP expression is the primary indicator of endoplasmic reticulum (ER) stress and UPR induction. Although we also observed elevated BiP levels in flies that were initially SE and later isolated, the observed difference was not significantly different from SE animals (data not shown).
Figure 2.
Social isolation induces the UPR. BiP expression is increased in flies that have been socially isolated (SI) in comparison to the grouped/socially enriched (SE) flies. SE flies were placed in vials and housed in groups of 20–30 flies from eclosion until their behavior was recorded. SI flies were isolated from pupae, sexed, individually housed in 2 ml eppendorf tubes and behavior was recorded 5–7 days later. (a) Densitometric quantification and (b) representative western blot of BiP expression in wCS10 fly heads (n = 4 trials, 10 heads/pool). Blot shows 10 µg of protein loaded per well from pooled socially enriched (SE) and socially isolated (SI) lysate; fold change (mean + standard error) in BiP expression in the SI flies over grouped p = .04. Dual lanes represent technical replicates. (c) xbp1 mRNA splicing is increased in SI flies compared to the grouped/SE flies. Splicing was measured by reverse transcription polymerase chain reaction (RT-PCR). Whole flies where used for these experiments (n = 24/condition; 2 flies/sample). Histogram represents data averaged from 3 PCR reactions and gels representing 12 independent samples per condition. Spliced and unspliced xbp1 were quantified from gels using Biorad image software and normalized across gels to the average of grouped xbp1. (d) Representative gel showing xbp1s and xbp1u; lanes represent independent biological replicates. Although xbp1 was found to be spliced in both grouped (SE) and socially isolated (SI) flies, more of both the unspliced and spliced variants were found under SI conditions. (e) p-eIF2α levels are increased in the SI flies compared to the grouped/SE flies (n = 10 per group, p = .02). (f and g) The effect of SI on BiP levels is reversible. Flies were grouped/SE, SI, or raised in SI then grouped for three (3) days. Placing SI flies in a grouped/SE environment both increased their sleep (data not shown) and reduced BiP levels (p < .05). Mean ± SEM shown, *p < .05, **p < .01, ***p < .001.
We next wanted to determine if other markers of the UPR were upregulated with SI. We found that xbp1 RNA was spliced (Figure 2, C and D) indicating activation of the IRE1 arm of the UPR. The PERK pathway was also activated as evidenced by increased phosphorylation of eIF2-α (Figure 2E) under conditions of SI.
It was previously demonstrated that placing SI flies into an enriched environment increased their sleep.27,41 Interestingly, we found that the effect of isolation on behavior tracked with changes in the UPR. Similar to what was observed in earlier studies,27 we saw that flies raised in isolation from pupae and later housed together for a minimum of 3 days, not only reverted to normal sleep behavior (data not shown) but also demonstrated reduced BiP levels (Figure 2, F and G).
Pharmacologically Increasing Sleep in SI Animals Suppresses UPR Activation
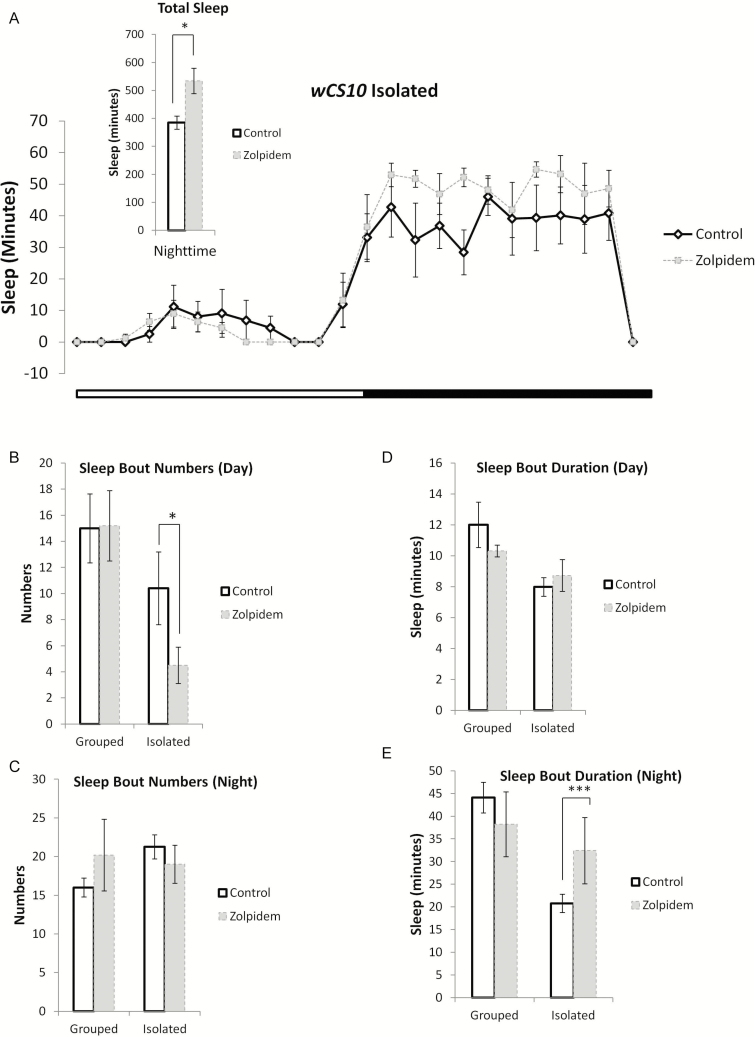
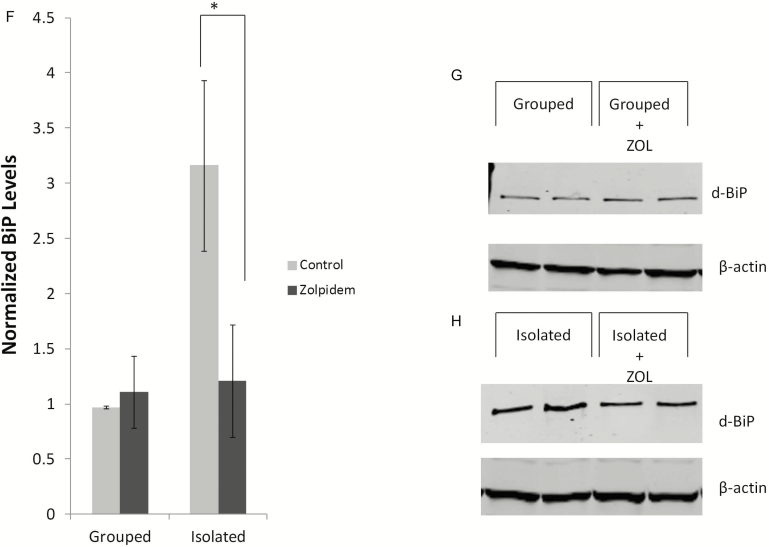
Because the sleep reduction caused by social isolation led to activation of the UPR, we wanted to ascertain whether pharmacologically inducing sleep in SI animals would repress the observed UPR induction. We chose the hypnotic ZOL, a nonbenzodiazepine allosteric modulator of GABA-A receptor subtypes, to induce sleep.42 To avoid a ceiling effect, we used 4-week-old wCS10 flies instead of the young flies for this set of experiments. As flies age, their sleep decreases and becomes fragmented.28 We found that ZOL increased total sleep in SI flies in a dose-dependent manner (0.05 mg/mL–0.5 mg/mL) (data not shown). The lowest effective dose (0.05 mg/mL), which was selected for the remainder of the ZOL experiments (Figure 3A) significantly increased sleep during the subjective night (p < .05). ZOL treatment significantly decreased sleep bout numbers in SI flies during the day (Figure 3B; p < .05) but had no significant effect on either SI or grouped flies that were treated with ZOL (GRP) during the night (Figure 3C). Sleep bout duration was not significantly altered during the day in grouped and SI flies treated with ZOL (Figure 3D); however, we observed a significant increase in sleep bout duration (Figure 3E; p < .001) during the nighttime in SI flies treated with ZOL [(n = 28) ZOL (isolated)] compared to the vehicle-treated SI flies indicating that ZOL consolidated sleep. We next determined the effect of ZOL treatment on the sentinel UPR marker, BiP. We found that ZOL significantly decreased BiP levels in SI flies (Figure 3F and H; p = .03).
Figure 3.
Pharmacologically increasing sleep in socially isolated animals suppresses UPR activation. (a) wCS10 flies were aged to 4 weeks for these experiments. Representative graph for the wCS10 strain showing increased sleep during the nighttime, over 24 hours in flies that were socially isolated (SI) with (n = 38) or without 0.05 mg/ml Zolpidem (ZOL) (n = 22) treatment. Inserted figure in the top left corner shows that ZOL treatment significantly increased sleep in SI flies during the 12 hour nighttime block (p < 0.05) when compared with their untreated age-matched controls. (b) Sleep bout numbers significant decreased during the day in SI flies treated with ZOL; however, during the night (c) there was no significant effect of drug on bout numbers. Sleep bout duration was not affected by ZOL treatment during the day (d); however, there was a significant increase in duration during the night time in the SI ZOL treated flies (p < .001) (e). (f) BiP expression was significantly decreased in the ZOL treated flies (p = .03), suggesting suppression of UPR activation in these flies. (g and h) ZOL treatment had no discernible effect on the grouped/SE flies. For the Westerns fly heads were pooled in groups of 10; two independent biological replicates shown for each condition. Grouped/socially enriched (SE) or (f) socially isolated (SI). Mean + SEM are shown. *p < .05, **p < .01, and ***p < .001.
Genetically Increasing Sleep in SI Flies Reduces Cellular Stress and UPR Induction
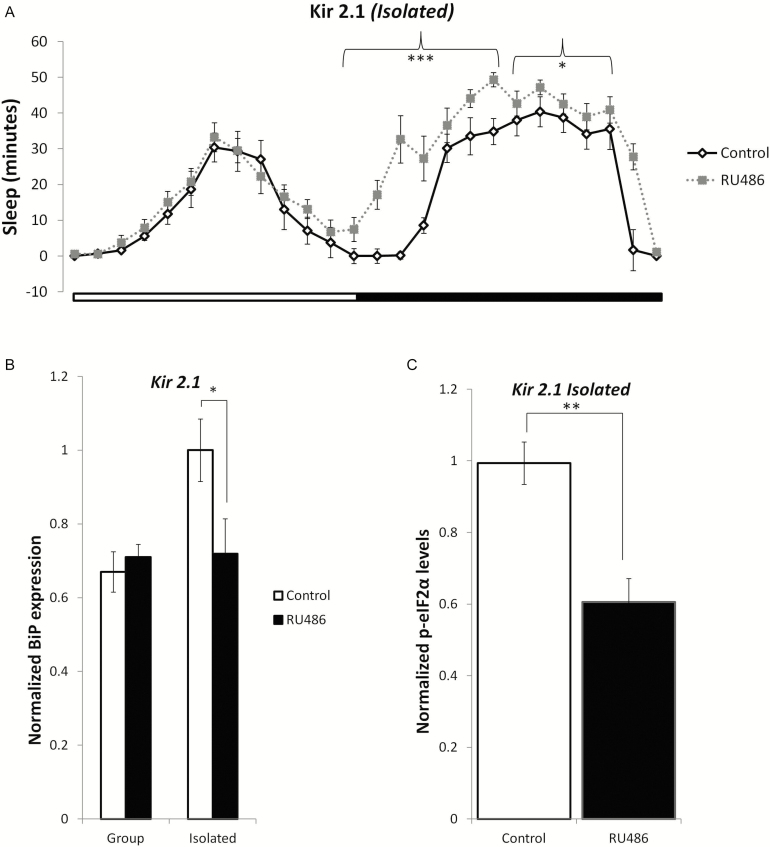
Because we observed a significant increase in sleep and a decrease in UPR activation in SI flies that were treated with ZOL, we hypothesized that a genetic manipulation that increased sleep in flies would also suppress activation of the UPR. We therefore inducibly expressed an inward rectifier potassium channel Kir2.143 under the control of the MB-Switch driver as previously described.44 Transgenic expression of Kir2.1 suppresses action-potential firing by hyperpolarizing neurons and decreasing membrane resistance, thus leading to reduced synaptic transmission. The induction of Kir2.1 in the MB has been shown to significantly increase sleep in drosophila.44 In P(MB−Switch)/UAS − Kir2.1 animals in which K+ channel expression in the MB was induced by RU486, sleep exceeded that of control animals of identical genotype. Activation of Kir2.1 significantly increased sleep during the night (Figure 4A; p < .05). Increasing sleep in SI flies resulted in a significant reduction in BiP protein levels (Figure 4B; p < .05) as well as diminished phosphorylation of eIF2α (Figure 4C; p < .01), suggesting that PERK activity and the UPR were suppressed.
Figure 4.
Genetically increasing sleep in socially isolated flies reduces UPR induction. (a) Activation of Kir2.1 using the P{MB-Switch}/UAS-Kir2.1, in which K+ channel expression in the mushroom body (MBs) was induced by RU486, increased sleep in SI flies (n = 28 ) during the night. Both (b) BiP (p < .05) and (c) p-eIF2-α (p < .01) expression levels were significantly decreased in the Kir2.1 flies that were raised in social isolation, suggesting suppression of the UPR. Mean ± SEM are shown. *p < .05 and **p < .01.
Pharmacologically Decreasing Sleep in Grouped Animals Leads to UPR Activation
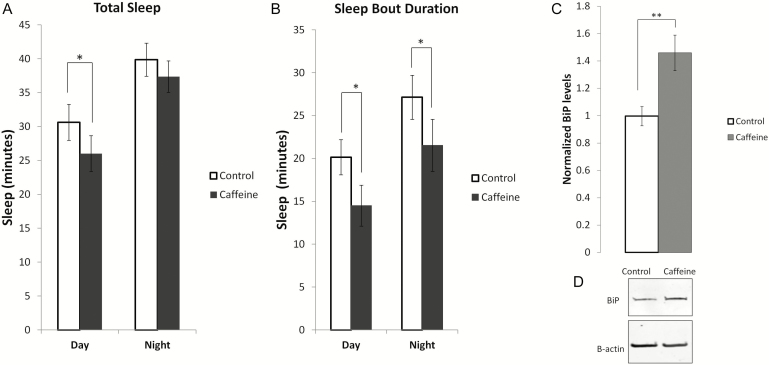
We used caffeine to pharmacologically reduce sleep in wildtype wCS10 flies. Total sleep was significantly reduced during the day in flies fed caffeine under L:D (Figure 5A; p < .05) conditions as well as the self-reported day under D:D (Supplementary Figure S1A) conditions. Sleep bout duration was reduced under both L:D and D:D conditions (Figure 5B and Supplementary Figure S1B; p < .05). To assess whether sleep loss due to caffeine administration upregulated the UPR, we probed BiP levels. Flies fed caffeine had an approximately 40% increase in BiP protein expression (Figure 5, C and D; p < .01) similar to BiP levels observed during sleep deprivation.
Figure 5.
Pharmacologically decreasing sleep in socially enriched animals leads to UPR activation. (a) Daytime sleep was significantly decreased in grouped/socially enriched (SE) flies that were fed 0.5 mg/ml caffeine (n = 18) versus the control flies (n = 10). (b) Total sleep was significantly reduced during the 12 hour daytime block (p = 0.034). (c) Sleep bout duration was significantly decreased during both the day and night in the caffeine-treated flies (p < .05). (d) BiP levels were significantly increased (p < .01) in caffeine-treated flies when compared to the controls. Mean ± SEM are shown. *p < .05 and **p < .01.
Genetically Decreasing Sleep in Grouped Flies Increases Cellular Stress and Induces the UPR
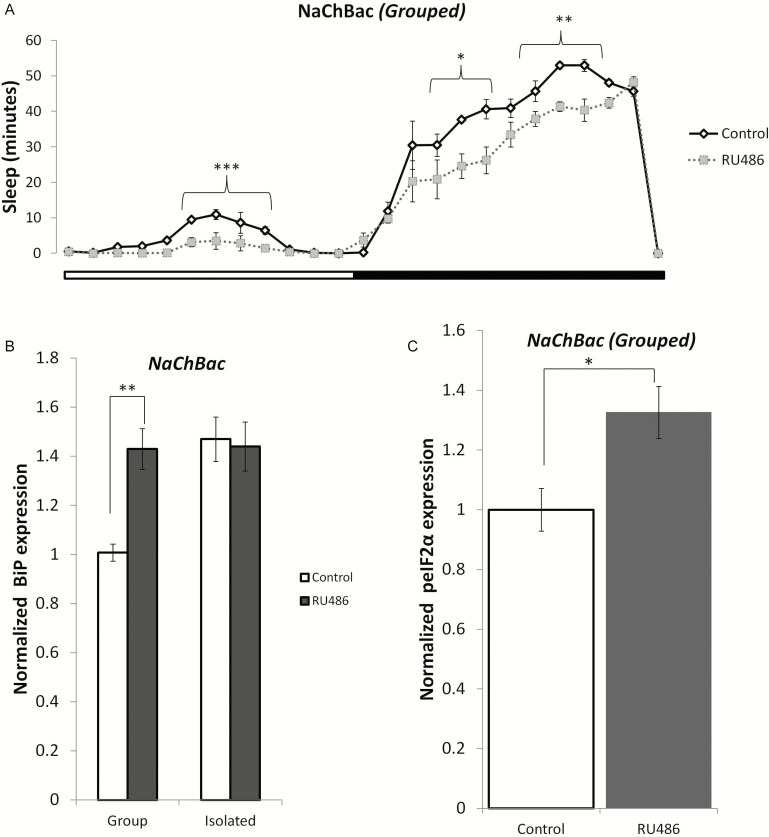
To test whether the loss of sleep in the isolated flies contributed to cellular stress and induction of the UPR, we genetically reduced sleep in grouped flies. The NaChBac transgene was expressed under the control of the MB-Switch driver as previously described.44 The UAS–NaChBac transgene is derived from a gene encoding a bacterial Na+ channel, which has the characteristics of high open probability and low inactivation. This drives membrane voltage to a more depolarized and easily excited state. When expressed, the sodium channel caused a decrease in sleep in the grouped RU486-treated flies compared to vehicle-treated flies. We found that both daytime (p < .001) and nighttime (p < .05) sleep were significantly reduced in the grouped NaChBac flies (Figure 6A). Further, the induction of NaChBac led to an increase in cellular stress and activation of the UPR. We observed a significant increase in BiP expression (p < .01) (Figure 6B) in the treated group relative to the untreated group, and this increase was not significantly different from BiP expression in isolated flies (p = .78). Subsequently, we also found increased phosphorylation of eIF2α in the RU486-treated grouped flies compared to vehicle-treated flies (Figure 6C, p < .05).
Figure 6.
Genetically decreasing sleep in grouped/socially enriched flies activates the UPR. The GAL4 construct P (MB-Switch) was used to drive the UAS-NaChBac construct and decrease sleep in flies. (a) Total sleep (both day and night) was significantly reduced in the NaChBac flies in comparison to their controls; p-values shown on figure. The UPR was induced in the NaChBac flies as indicated by increased (b) BiP expression levels (p < 0.01) and (c) phospho-eIF2α levels (p = 0.016) (n = 6). Mean ± SEM are shown. *p < .05, **p < .01, and ***p < 0.001.
DISCUSSION
Social isolation has been linked to a variety of adverse consequences that impact behaviors and overall health; impacted areas include inflammation, cardiovascular disease, depression, anxiety, aggression, and general morbidity and mortality.1,3,8,45,46 Many of the negative health outcomes of social isolation are conserved across social species including the following examples: aggression in macaques, anxiety and increased aggression in rodents,47 obesity,13 and post-stroke survival in mice.16 Hyperactivity is an outcome of social isolation common to a number of species.17 While the adverse consequences of social isolation have been well documented, the underlying mechanisms are still poorly understood.
Chronic induction of the UPR is a possible contributing factor to these undesirable effects. The UPR is a homeostatic ER stress response and an indicator of cellular stress.32 Induction of the UPR is an adaptive response which, if insufficient, leads to a secondary maladaptive response that activates inflammatory and proapoptotic signaling.48,49 Evidence in humans suggests that even perceived social isolation leads to increased neuronal activation18 and poor sleep.4,50 Reduced sleep is one of the adverse outcomes of social isolation common to humans,4,5 other mammals,51,52 and the fruit fly, D. melanogaster.27
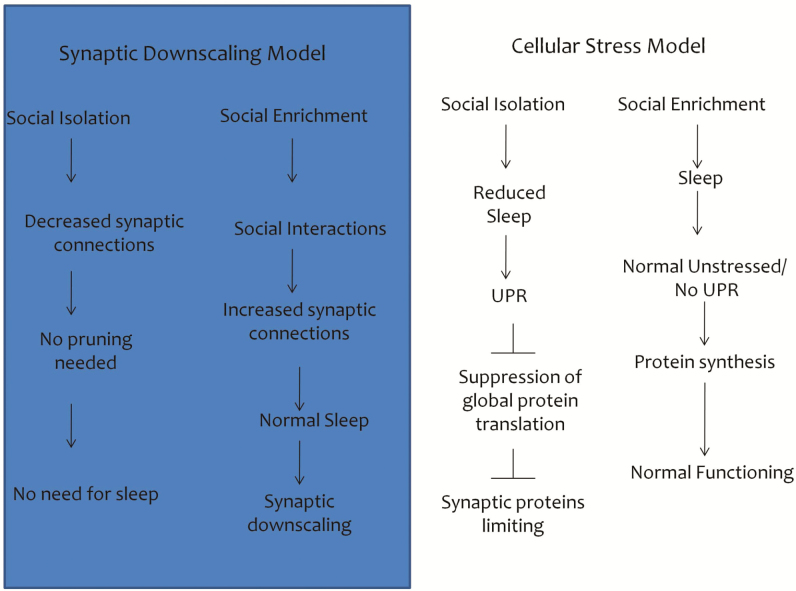
The synaptic homeostasis hypothesis, the current prevailing hypothesis for the function of sleep, speculates that the sleep state is necessary to downscale synaptic connections that are made in the wake state.21 It has been previously reported that flies in an SE environment (groups) have a greater number of synapses. Flies housed in a “fly mall” (about100 flies/vial) demonstrated increased branch length, branch number, and spine number.20 This hypothesis would predict that SI animals sleep less because they have a sensory-deprived environment and need less sleep than socially grouped animals. However, we found that social isolation also activates the UPR, which is demonstrated by upregulation of the molecular chaperone BiP, splicing of xbp1, and phosphorylation of eIF2α, all key indicators of the UPR. Induction of the UPR is an adaptive response to ER stress that attenuates global protein translation (reducing protein load) and increases transcription of genes involved in ER folding and degradation capacity.29,48,49 The results of our study suggest that the global reduction in protein synthesis may be a contributing factor to the reduced synapses observed in earlier studies20 (Figure 7). In fact, it has recently been shown that prolonged UPR activation results in reduced levels of synaptic proteins and induces synaptic loss and neurodegeneration.53,54 Additionally, pharmacologically and genetically increasing sleep in SI flies suppresses UPR activation. Conversely, decreasing sleep in SE flies using similar methods induces the UPR. We reproduced earlier results27 demonstrating increased sleep in flies that were initially isolated and later grouped. Furthermore, we found that grouping SI flies for 3 days not only increased their sleep but also decreased elevated BiP levels. These results suggest that sleep loss that occurs as a result of social isolation is a stressor that induces the UPR and that chronic induction of the UPR could potentially be a mechanism underlying the negative health outcomes of social isolation. Should the initial adaptive response be insufficient to alleviate ER stress, a secondary maladaptive response occurs that leads to inflammatory and proapoptotic signaling.29,48,49 Chronic induction of the UPR could provide a mechanism behind the negative health outcomes observed in cases of social isolation.
Figure 7.
Schematic demonstrating an alternative hypothesis to loss of sleep during social isolation. The synaptic downscaling hypothesis proposes that flies raised in socially isolated conditions sleep less because they were exposed to a sensory deprived environment and require less sleep than flies raised in a grouped or socially enriched environment. This study shows that the unfolded protein response (UPR) is activated in socially isolated flies—demonstrated by upregulation of the molecular chaperone BiP, splicing of xbp1 and phosphorylation of eIF2α—all key indicators of the UPR. UPR induction is an adaptive response to ER stress that attenuates global protein translation, concurrently decreasing protein load and increasing transcription of specific genes involved in ER folding and degradation. The global reduction in protein synthesis resulting from activation of the UPR could potentially be a contributing factor in the reduction of synapses observed in earlier reports investigating social isolation.
In addition to reproducing earlier studies that demonstrated reduced total sleep in flies that were SI,27 we also found that their sleep was extremely fragmented. The finding that reduced sleep and hyperactivity are both outcomes of social isolation is not surprising and could be evolutionarily conserved. Solitary animals would need to be more vigilant to ward off predators. Previous research has linked molecules affected by social isolation to Drosophila sleep. mRNA levels of the serotonin receptor 5-HT1A, are reduced in the heads of flies which have been SI versus those in groups.55 In addition, 5-HT1A mutant flies have reduced and fragmented sleep.56 Moreover, overexpression of octopamine, a wake promoting neurotransmitter in the fly,57 in Tyrosine decarboxylase 2 (Tdc2) neurons, alters aggression in group raised versus isolated flies without significantly modifying octopamine levels.58
The current study begins to elucidate some of the less understood mechanisms underlying the negative behaviors associated with social isolation. In this study, we found that the UPR was upregulated in flies that were SI. Earlier studies have shown that sleep loss upregulates the UPR.37,40 Both baseline and recovery sleep following sleep deprivation have also been shown to be affected by the UPR. Recovery sleep was increased in flies that overexpressed BiP. Conversely, in flies that had half the amount of functional BiP (dominant negative), their recovery sleep was decreased.37 In addition, application of a chemical chaperone, 4-phenylbuturayte, that acts similar to BiP by binding to exposed hydrophobic regions on misfolded/unfolded proteins, altered both baseline and recovery sleep in young and aged flies.38 Furthermore, pharmacologically inducing ER stress with tunicamycin resulted in fragmented sleep in young flies. Our finding that the effects of social isolation on sleep and the UPR are reversible could have implications for understanding the underlying mechanisms of the negative outcomes associated with isolation. These results are in line with an earlier study in rats that showed that the effects of social isolation on open-field emergence were reversed by resocialization prior to testing.59
Epidemiological studies suggest that social isolation increases the risk of death associated with several chronic diseases.60 Because chronic induction of the UPR activates apoptotic pathways, this could potentially be a mechanism that contributes to the negative health outcomes observed in cases of social isolation. Sleep is a modifiable risk factor for multiple maladies. Increasing the amount and efficiency of sleep could lead to positive health outcomes. Further research is needed to determine whether social enrichment might protect against the adverse effects of sleep deprivation on health outcomes.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
FUNDING
This project was supported by funding from NHLBI T32 HL07713 and NIA P01 AG17628.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
This article was prepared while Marishka K. Brown, Ph.D. was employed at the University of Pennsylvania. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
REFERENCES
- 1. Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM Jr. Social ties and susceptibility to the common cold. JAMA. 1997; 277: 1940–1944. [PubMed] [Google Scholar]
- 2. Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. 2005; 24(3): 297–306. [DOI] [PubMed] [Google Scholar]
- 3. Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005; 64(11): 1888–1892. [DOI] [PubMed] [Google Scholar]
- 4. Cacioppo JT, Ernst JM, Burleson MH et al. Lonely traits and concomitant physiological processes: the MacArthur social neuroscience studies. Int J Psychophysiol. 2000; 35(2-3): 143–154. [DOI] [PubMed] [Google Scholar]
- 5. Friedman EM. Sleep quality, social well-being, gender, and inflammation: an integrative analysis in a national sample. Ann N Y Acad Sci. 2011; 1231: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedman EM, Hayney MS, Love GD et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci U S A. 2005; 102(51): 18757–18762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. House JS. Social isolation kills, but how and why? Psychosom Med. 2001; 63(2): 273–274. [DOI] [PubMed] [Google Scholar]
- 8. Tomaka J, Thompson S, Palacios R. The relation of social isolation, loneliness, and social support to disease outcomes among the elderly. J Aging Health. 2006; 18(3): 359–384. [DOI] [PubMed] [Google Scholar]
- 9. Malick JB. The pharmacology of isolation-induced aggressive behavior in mice. Curr Dev Psychopharmacol. 1979; 5: 1–27. [PubMed] [Google Scholar]
- 10. Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996; 75(1–2): 27–32. [DOI] [PubMed] [Google Scholar]
- 11. Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav. 2003; 79(3): 471–478. [DOI] [PubMed] [Google Scholar]
- 12. Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998; 12(1–2): 129–162. [DOI] [PubMed] [Google Scholar]
- 13. Nonogaki K, Nozue K, Oka Y. Social isolation affects the development of obesity and type 2 diabetes in mice. Endocrinology. 2007; 148(10): 4658–4666. [DOI] [PubMed] [Google Scholar]
- 14. Jones GH, Marsden CA, Robbins TW. Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: neurochemical correlates. Behav Brain Res. 1991; 43(1): 35–50. [DOI] [PubMed] [Google Scholar]
- 15. Juraska JM, Henderson C, Müller J. Differential rearing experience, gender, and radial maze performance. Dev Psychobiol. 1984; 17(3): 209–215. [DOI] [PubMed] [Google Scholar]
- 16. Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A. 2009; 106(14): 5895–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008; 32(6): 1087–1102. [DOI] [PubMed] [Google Scholar]
- 18. Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003; 302(5643): 290–292. [DOI] [PubMed] [Google Scholar]
- 19. Mackiewicz M, Shockley KR, Romer MA et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007; 31(3): 441–457. [DOI] [PubMed] [Google Scholar]
- 20. Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011; 332(6037): 1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003; 62(2): 143–150. [DOI] [PubMed] [Google Scholar]
- 22. Maquet P. The role of sleep in learning and memory. Science 2001; 294: 1048–1052. [DOI] [PubMed] [Google Scholar]
- 23. Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012; 463(1): 121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol. 2005; 30(12): 625–662. [DOI] [PubMed] [Google Scholar]
- 25. Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004; 160: 521–530. [DOI] [PubMed] [Google Scholar]
- 26. Ueda A, Wu CF. Effects of social isolation on neuromuscular excitability and aggressive behaviors in Drosophila: altered responses by Hk and gsts1, two mutations implicated in redox regulation. J Neurogenet. 2009; 23(4): 378–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006; 313(5794): 1775–1781. [DOI] [PubMed] [Google Scholar]
- 28. Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci U S A. 2006; 103(37): 13843–13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002; 110(10): 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen X, Zhang K, Kaufman RJ. The unfolded protein response–a stress signaling pathway of the endoplasmic reticulum. J Chem Neuroanat. 2004; 28(1-2): 79–92. [DOI] [PubMed] [Google Scholar]
- 31. Naidoo N. The endoplasmic reticulum stress response and aging. Rev Neurosci. 2009; 20(1): 23–37. [DOI] [PubMed] [Google Scholar]
- 32. Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006; 66(2 Suppl 1): S102–S109. [DOI] [PubMed] [Google Scholar]
- 33. Zimmerman JE, Raizen DM, Maycock MH, Maislin G, Pack AI. A video method to study Drosophila sleep. Sleep. 2008; 31(11): 1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J Neurosci. 2009; 29(35): 11029–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mao Z, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc Natl Acad Sci U S A. 2004; 101(1): 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005; 393: 759–772. [DOI] [PubMed] [Google Scholar]
- 37. Naidoo N, Casiano V, Cater J, Zimmerman J, Pack AI. A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep homeostasis. Sleep. 2007; 30(5): 557–565. [DOI] [PubMed] [Google Scholar]
- 38. Brown MK, Chan MT, Zimmerman JE, Pack AI, Jackson NE, Naidoo N. Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiol Aging. 2014; 35(6): 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Alphen B, Yap MH, Kirszenblat L, Kottler B, van Swinderen B. A dynamic deep sleep stage in Drosophila. J Neurosci. 2013; 33(16): 6917–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005; 92(5): 1150–1157. [DOI] [PubMed] [Google Scholar]
- 41. Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009; 324(5923): 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009; 29(9): 1779–1794. [DOI] [PubMed] [Google Scholar]
- 43. Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001; 21(5): 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006; 441(7094): 757–760. [DOI] [PubMed] [Google Scholar]
- 45. Knox SS, Uvnäs-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998; 23(8): 877–890. [DOI] [PubMed] [Google Scholar]
- 46. Yang YC, McClintock MK, Kozloski M, Li T. Social isolation and adult mortality: the role of chronic inflammation and sex differences. J Health Soc Behav. 2013; 54(2): 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olsson A, Westlund K. More than numbers matter: The effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Applied Animal Behaviour Science 2007; 103: 229–254. [Google Scholar]
- 48. Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007; 18(6): 716–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011; 334(6059): 1081–1086. [DOI] [PubMed] [Google Scholar]
- 50. Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009; 13(10): 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matsumoto K, Ojima K, Watanabe H. Neurosteroidal modulation of social isolation-induced decrease in pentobarbital sleep in mice. Brain Res. 1996; 708(1–2): 1–6. [DOI] [PubMed] [Google Scholar]
- 52. Kaushal N, Nair D, Gozal D, Ramesh V. Socially isolated mice exhibit a blunted homeostatic sleep response to acute sleep deprivation compared to socially paired mice. Brain Res. 2012; 1454: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Freeman OJ, Mallucci GR. The UPR and synaptic dysfunction in neurodegeneration. Brain Res. 2016; 1648(Pt B): 530–537. [DOI] [PubMed] [Google Scholar]
- 54. Scheper W, Hoozemans JJ. The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol. 2015; 130(3): 315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson O, Becnel J, Nichols CD. Serotonin 5-HT(2) and 5-HT(1A)-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience. 2009; 158(4): 1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006; 16(11): 1051–1062. [DOI] [PubMed] [Google Scholar]
- 57. Crocker A, Sehgal A. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms. J Neurosci. 2008; 28(38): 9377–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008; 11(9): 1059–1067. [DOI] [PubMed] [Google Scholar]
- 59. Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977; 10(2): 123–132. [DOI] [PubMed] [Google Scholar]
- 60. Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013; 110(15): 5797–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.