Abstract
Melanization of Histoplasma capsulatum remains poorly described, particularly in regards to the forms of melanin produced. In the present study, 30 clinical and environmental H. capsulatum strains were grown in culture media with or without L-tyrosine under conditions that produced either mycelial or yeast forms. Mycelial cultures were not melanized under the studied conditions. However, all strains cultivated under yeast conditions produced a brownish to black soluble pigment compatible with pyomelanin when grew in presence of L-tyrosine. Sulcotrione inhibited pigment production in yeast cultures, strengthening the hyphothesis that H. capsulatum yeast forms produce pyomelanin. Since pyomelanin is produced by the fungal parasitic form, this pigment may be involved in H. capsulatum virulence.
Keywords: Histoplasma capsulatum, L-tyrosine, pyomelanin
The dimorphic fungus Histoplasma capsulatum is the etiologic agent of histoplasmosis, a systemic mycosis with a worldwide distribution. Infection with H. capsulatum produces manifestations that range from asymptomatic acquisition to flu-like illnesses to severe disseminated disease. Immunocompromised patients are at greatest risk for life-threatening disease and fatality rates can approach 50%.1 Some well-characterized H. capsulatum virulence factors include the cell wall associated α-(1,3)-glucan, proteins such as Hsp60, Hsp82, CatB (M antigen), YPS3, calcium binding protein (CBP1), histone 2B, superoxide dismutase (SOD3), and hydroxamate siderophores.2
Melanins are virulence factors described in several fungal species such as Cryptococcus neoformans, Paracoccidioides brasiliensis and Sporothrix schenckii, among others.3–5 They are brown to black pigmented polymers, which can be produced by different metabolic pathways. Some of their functions are related to defense against environmental and parasitic stresses such as ultraviolet radiation, oxidizing agents and antifungal drugs.3 The most common types of fungal melanins are DHN-melanin, eumelanin, and pyomelanin.4 The latter is a water-soluble pigment produced during the catabolism of L-tyrosine, where the excess of homogentisate gathered from tyrosine catabolism is oxidized to benzoquinoneacetate, and then polymerized, leading to pyomelanin synthesis. Its production has been described in some fungi such as Aspergillus fumigatus, Madurella mycetomatis, Yarrowia lipolytica, and Sporothrix spp., with implications in fungal resistance to harsh conditions.4,6–8
Only a few studies have addressed aspects related to melanin production by H. capsulatum.9–12 In brief, these studies describe DHN-melanin production in the conidia cell-wall and eumelanin production by H. capsulatum yeast cells using L-DOPA as a substrate. To our knowledge, there are no reports of pyomelanin production by this fungus. In order to check whether this dimorphic fungus can produce this soluble type of melanin, 30 clinical and environmental H. capsulatum strains were selected (Table 1). Strains were grown in the mycelial phase at an initial concentration of 1 × 103 cells/ml in 100 ml minimal medium [MM] (15 mM glucose, 10 mM MgSO4, 29.4 mM K2HPO4, 13 mM glycine, and 3.0 mM thiamine, pH 5.5) supplemented with 10 mM L-tyrosine (Sigma-Aldrich Co., St. Louis, Missouri, USA). Yeast cells at same concentration were grown in HAM’s F12 nutrient mixture (Invitrogen Corporation, Grand Island, New York, USA), prepared according to the manufacturer instructions and also supplemented with 10 mM L-tyrosine (Sigma-Aldrich Co., St. Louis, Missouri, USA). Controls of mycelial and yeast forms were performed in standard MM or HAM’s F12 medium, respectively. The mycelial phase of the fungus was incubated at 30 °C and the yeast-phase at 37 °C on a rotary incubator at 150 rpm for 14 days.
Table 1.
Profile of dark pigment production of 30 Histoplasma capsulatum strains used in this study.
| Strain | Origin | Source of strains | Pigment production (A 340 nm) | |
|---|---|---|---|---|
| 30 °C | 37 °C | |||
| IPEC22/11 | Human | Bone marrow aspirate | 0.153 | 3.164 |
| IPEC24/11 | Human | Blood | 0.161 | 1.209 |
| IPEC25/11 | Human | Blood | 0.157 | 1.015 |
| IPEC27/11 | Human | Bone marrow aspirate | 0.149 | 0.668 |
| IPEC01/12 | Human | Bone marrow aspirate | 0.165 | 2.126 |
| IPEC04/12 | Human | Bone marrow aspirate | 0.168 | 0.820 |
| IPEC05/12 | Cat | Wound swabs | 0.160 | 0.945 |
| IPEC06/12 | Human | Bone marrow aspirate | 0.150 | 0.534 |
| IPEC07/12 | Dog | Lymph node biopsy | 0.149 | 0.488 |
| IPEC09/12 | Human | Bronchoalveolar lavage | 0.152 | 0.480 |
| IPEC11/12 | Human | Skin biopsy | 0.166 | 0.500 |
| 20231 | Human | Mucosae scrapings | 0.160 | 0.201 |
| 38874 | Human | Bone marrow aspirate | 0.151 | 0.398 |
| 39130 | Human | Bone marrow aspirate | 0.157 | 0.578 |
| 39439 | Human | Bone marrow aspirate | 0.155 | 0.293 |
| 42247 | Human | Blood | 0.168 | 1.389 |
| 44938 | Human | Bone marrow aspirate | 0.153 | 1.700 |
| 46028 | Human | Bone marrow aspirate | 0.160 | 1.089 |
| 46176 | Human | Skin biopsy | 0.154 | 0.900 |
| 46693 | Human | Skin biopsy | 0.156 | 0.847 |
| 36Gal | Human | Blood | 0.150 | 1.070 |
| RS36 | Rat | Liver and spleen biopsy | 0.164 | 0.497 |
| RPS35 | Environmental | Soil | 0.150 | 0.620 |
| RPS86 | Environmental | Soil | 0.152 | 0.300 |
| CO4 | Environmental | Soil | 0.163 | 2.218 |
| IT04 | Environmental | Soil | 0.167 | 0.698 |
| TI01 | Environmental | Soil | 0.159 | 0.955 |
| TI05 | Environmental | Soil | 0.154 | 0.520 |
| EP02 | Environmental | Soil | 0.167 | 0.480 |
| IGS4/5 | Environmental | Soil | 0.161 | 1.654 |
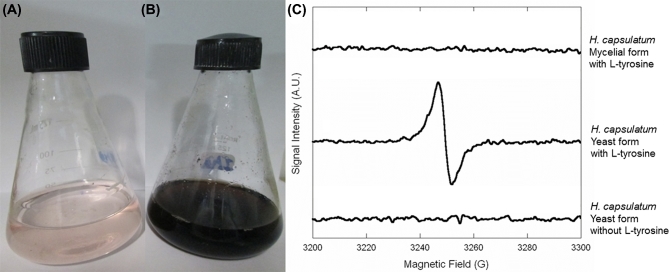
After 14 days of cultivation, there were no visual changes in the culture supernatants from any mycelial cultures (Fig. 1A). However, the supernatants from H. capsulatum yeast cultures were from brown to black color (Fig. 1B). To confirm this visual observation, aliquots of 0.5 ml of the supernatants were collected by centrifugation at 2,300 g, and absorbances at 340 nm were measured in triplicate with an ELISA plate reader (Bio-Tek model μQuant). As depicted in Table 1, absorbances of supernatants of mycelial cultures (range: 0.149–0.168) were close to the uninoculated medium (0.161), whereas spectrophotometry results of supernatants from the yeast cultures (range: 0.201–3.164) confirmed the production of pigments with absorbance at 340 nm, the wavelength of pyomelanin absorption.13 The yeast cells of the H. capsulatum strains were also dark in the presence of L-tyrosine.
Figure 1.
Pyomelanin production by H. capsulatum: (A) strain IPEC22/11 (1 × 103 cells/ml) inoculated in MM added with L-tyrosine at 30 °C, (B) production of a soluble melanoid pigment by this same strain when the yeast form of the fungus was incubated at 37 °C in HAM’s F12 medium supplemented with L-tyrosine, (C) EPR spectrum from supernatants of H. capsulatum cultures under different conditions. This Figure is reproduced in color in the online version of Medical Mycology.
To further confirm the nature of the pigment as melanin, its resistance to acid treatment was checked. Supernatants were filtered through 0.22 μm membranes, acidified to pH 2.0 using HCl 0.5 mol/l, and incubated overnight in the dark at room temperature. All yeast cultures yielded precipitated pigments that were harvested through centrifugation (12,800 g) and were soluble in distilled water, as characteristic of pyomelanins. Moreover, electron paramagnetic resonance (EPR) was performed on the supernatants of the IPEC22/11 strain as previously described14 using a Varian E112X-Band model spectrometer with a Gunn diode as the microwave source. The supernatant generated a distinctive signal on the EPR analysis, demonstrating the presence of unpaired electrons in the polymer, which is characteristic of melanin. On the other hand, the supernatants of the mycelial form cultured in the presence of L-tyrosine or the supernatants from the yeast form in the absence of this precursor did not generate EPR signals at a magnetic field of 3250 G (Fig. 1C).
Lastly, we have checked whether sulcotrione, an inhibitor of the 4-hydroxyphenyl-pyruvate-dioxygenase15 that regulates pyomelanin formation,13 interferes with pigment production by H. capsulatum yeast cells in the presence of L-tyrosine. Spectrophotometric analysis of all cultures in HAM’s F12 nutrient mixture supplemented with 10 mM L-tyrosine and 16 mg/L sulcotrione (Sigma-Aldrich Co., St. Louis, Missouri, USA) revealed that the yeast cells were unable to produce the melanoid pigment, with absorbances ranging from 0.149 to 0.163.
The results herein presented have shown pyomelanin production by the yeast form of H. capsulatum, which is supported by the following evidences: (i) appearance of a black pigment in the supernatants of stationary cultures only when L-tyrosine is present, (ii) pigment resistance to acid degradation, (iii) absorbance of the pigments at a 340 nm wavelength, (iv) EPR analysis demonstrating the presence of stable free radicals, and (v) inhibition of pigment formation by a specific pyomelanin inhibitor.
The differences in pigment production by different strains is remarkable. Recent studies have demonstrated a high degree of genetic variation among H. capsulatum strains, suggesting at least 17 cryptic phylogenetic species hidden within H. capsulatum sensu stricto.16 As reported, expression of some H. capsulatum virulence factors, such as α-glucan, Hsp60, Histone-2B, and CBP1, is variable among different phylogenetic groups of Histoplasma, whereas YPS3 and serine proteases have strain-specific differences.2 For this reason, we suggest that the variation in pyomelanin is also strain-dependent.
Interestingly, H. capsulatum conidia can produce melanin in the absence of phenolic exogenous substrates, whereas yeast cells require phenolic compounds to melanize in vitro.9 Here, we demonstrate that L-tyrosine can also be used by the fungal parasitic form to produce another type of melanin. Significantly, compared to mycelia cells, there is overexpression of the 4-hydroxyphenyl-pyruvate-dioxygenase encoding gene by H. capsulatum yeast cells,17 which supports the findings of the present study. This gene is also induced under infectious conditions in A. fumigatus, P. brasiliensis, and Talaromyces marneffei.6,18,19 The addition of an inhibitor of 4-hydroxyphenyl-pyruvate-dioxygenase to macrophages infected with T. marneffei prevents the formation of yeasts within macrophages.19 To our knowledge, there is no information about sulcotrione inhibition of H. capsulatum mycelium-to-yeast transition in macrophages.
Other dimorphic fungal pathogens have a similar pattern of pyomelanin production: Sporothrix brasiliensis produces more pyomelanin in the parasitic form13 and yeast-specific production of pyomelanin is observed in T. marneffei,19 suggesting that this is a conserved mechanism in dimorphic fungi. Since pyomelanin is only produced by the H. capsulatum yeast form in our studied conditions, this pigment is probably related to fungal virulence and pathogenicity. Melanin has been shown to protect fungal cells from free-radicals generated by host macrophages and also influence phagocytosis, phagolysosomal maturation and the release of proinflammatory cytokines during infection.3 Moreover, as observed with other dimorphic fungi13,20 and demonstrated previously with H. capsulatum eumelanin,10 pyomelanin may have a role in protection of H. capsulatum against some antifungal agents and immune effector responses in the infected host.
Acknowledgements
This work was supported by Conselho Nacional de desenvolvimento Científico e Tecnológico [grant number 449184/2014-5 to R. A.-P.]. R. A.-P. and R. M. Z.-O. are supported in part by Conselho Nacional de desenvolvimento Científico e Tecnológico [grant numbers 305487/2015-9, and 304976/2013-0, respectively]. J. D. N. is partially supported by NIH R21 AI124797 and AI052733.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Adenis A, Nacher M, Hanf M et al. HIV-associated histoplasmosis early mortality and incidence trends: from neglect to priority. PLoS Negl Trop Dis. 2014; 8: e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holbrook ED, Rappleye CA. Histoplasma capsulatum pathogenesis: making a lifestyle switch. Curr Opin Microbiol. 2008; 11: 318–324. [DOI] [PubMed] [Google Scholar]
- 3. Taborda CP, Silva MB, Nosanchuk JD, Travassos LR. Melanin as a virulence factor of Paracoccidioides brasiliensis and other dimorphic pathogenic fungi: a minireview. Mycopathologia 2008; 165: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almeida-Paes R, Borba-Santos LP, Rozental S, Marco S, Zancopé-Oliveira RM, Cunha MML. Melanin biosynthesis in pathogenic species of Sporothrix. Fungal Biol Rev. 2017; 31: 50–59. [Google Scholar]
- 5. Alspaugh JA. Virulence mechanisms and Cryptococcus neoformans pathogenesis. Fungal Genet Biol. 2015; 78: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keller S, Macheleidt J, Scherlach K et al. Pyomelanin formation in Aspergillus fumigatus requires HgmX and the transcriptional activator HmgR but is dispensable for virulence. PLoS One 2011; 6: e26604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van de Sande WWJ, Kat J, Coppens J et al. Melanin biosynthesis in Madurella mycetomatis and its effect on susceptibility to itraconazole and ketoconazole. Microbes Infect. 2007; 9: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 8. Carreira A, Ferreira LM, Loureiro V. Production of brown tyrosine pigments by the yeast Yarrowia lipolytica. J Appl Microbiol. 2001; 90: 372–379. [DOI] [PubMed] [Google Scholar]
- 9. Nosanchuk JD, Gómez BL, Youngchim S et al. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect Immun. 2002; 70: 5124–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Duin D Casadevall A, Nosanchuk JD. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob Agents Chemother. 2002; 46: 3394–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guimarães AJ, Cerqueira MD, Nosanchuk JD. Surface architecture of Histoplasma capsulatum. Front Microbiol. 2011; 2: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dadachova E, Bryan RA, Howell RC et al. The radioprotective properties of fungal melanin are a function of its chemical composition, stable radical presence and spatial arrangement. Pigment Cell Melanoma Res. 2008; 21: 192–199. [DOI] [PubMed] [Google Scholar]
- 13. Almeida-Paes R, Frases S, Araujo Gde S et al. Biosynthesis and functions of a melanoid pigment produced by species of the Sporothrix complex in the presence of L-tyrosine. Appl Environ Microbiol. 2012; 78: 8623–8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enochs WS, Nilges MJ, Swartz HMA. Standardized test for the identification and characterization of melanins using electron paramagnetic resonance (EPR) spectroscopy. Pigment Cell Res. 1993; 6: 91–99. [DOI] [PubMed] [Google Scholar]
- 15. Secor J. Inhibition of Barnyardgrass 4-Hydroxyphenylpyruvate dioxygenase by sulcotrione. Plant Physiol. 1994; 106: 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teixeira MM, Patané JS, Taylor ML et al. Worldwide phylogenetic distributions and population dynamics of the genus Histoplasma. PLoS Negl Trop Dis. 2016; 10: e0004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hwang L, Hocking-Murray D, Bahrami AK, Andersson M, Rine J, Sil A. Identifying phase-specific genes in the fungal pathogen Histoplasma capsulatum using a genomic shotgun microarray. Mol Biol Cell. 2003; 14: 2314–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nunes LR, Oliveira RC, Leite DB et al. Transcriptome analysis of Paracoccidioides brasiliensis cells undergoing mycelium-to-yeast transition. Eukaryot Cell 2005; 4: 2115–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyce KJ, McLauchlan A, Schreider L, Andrianopoulos A. Intracellular growth is dependent on tyrosine catabolism in the dimorphic fungal pathogen Penicillium marneffei. PLoS Pathog. 2015; 11: e1004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almeida-Paes R, Figueiredo-Carvalho MHG, Brito-Santos F, Almeida-Silva F, Oliveira MME, Zancopé-Oliveira RM. Melanins protect Sporothrix brasiliensis and Sporothrix schenckii from the antifungal effects of terbinafine. PloS One 2016; 11: e0152796. [DOI] [PMC free article] [PubMed] [Google Scholar]