Abstract
STUDY QUESTION
Does low molecular weight heparin (LMWH) require heparin-binding epidermal growth factor (EGF)-like growth factor (HBEGF) signaling to induce extravillous trophoblast differentiation and decrease apoptosis during oxidative stress?
SUMMARY ANSWER
LMWH increased HBEGF expression and secretion, and HBEGF signaling was required to stimulate trophoblast extravillous differentiation, increase invasion in vitro and reduce trophoblast apoptosis during oxidative stress.
WHAT IS KNOWN ALREADY
Abnormal trophoblast differentiation and survival contribute to placental insufficiency syndromes, including preeclampsia and intrauterine growth restriction. Preeclampsia often manifests as a pro-thrombotic state, with unsuccessful transformation of the spiral arteries that reduces oxygen supply and can produce placental infarction. LMWH improves placental function by increasing blood flow. Recent data suggest that the actions of LMWH transcend its anti-coagulative properties, but the molecular mechanism is unknown. There is evidence that LMWH alters the expression of human HBEGF in trophoblast cells, which regulates human trophoblast pathophysiology. HBEGF, itself, is capable of increasing trophoblast survival and invasiveness.
STUDY DESIGN, SIZE, DURATION
First-trimester placental explants and the HTR-8/SVneo cell line, established using extravillous trophoblast outgrowths from first-trimester villous explants, were treated in vitro with LMWH to examine the effects on HBEGF signaling and trophoblast function under normal physiological and pathological conditions. A highly specific antagonist of HBEGF and other inhibitors of HBEGF downstream signaling were used to determine the relationship between LMWH treatment and HBEGF.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Placental tissues (n = 5) were obtained with IRB approval and patient consent from first-trimester terminations. Placental explants and HTR-8/SVneo cells were cultured on plastic or Matrigel™ and treated with a therapeutic dose of LMWH (Enoxaparin; 10 IU/ml), with or without CRM197, pan Erb-B2 Receptor Tyrosine Kinase (ERBB) inhibitor, anti-ERBB1 or ERBB4 blocking antibodies, or pretreatment of cells with heparitinase I. Extravillous differentiation was assessed by immunocytochemistry to determine the relative levels of integrins α6β4 and α1β1. Trophoblast invasiveness was assessed in villous explants by measuring outgrowth from villous tips cultured on Matrigel, and by invasion assays with HTR-8/SVneo cells cultured on Matrigel-coated transwell insert. Placental explants and HTR-8/SVneo cells were exposed to oxidative stress in a hypoxia–reoxygenation (H–R) model, measuring cell death by TUNEL assay, caspase 3 cleavage, and BCL-2α expression.
MAIN RESULTS AND THE ROLE OF CHANCE
LMWH induced extravillous differentiation, according to trophoblast invasion assays and integrin (α6β4–α1β1) switching. Treatment with LMWH rescued cytotrophoblasts and HTR-8/SVneo cells from apoptosis during exposure to reoxygenation injury, based on TUNEL, caspase 3 cleavage and BCL-2α expression. Experiments using CRM197, ERBB1 and ERBB4 blocking antibodies, pan-ERBB inhibitor and removal of cell surface heparin demonstrated that the effects of LMWH on trophoblast invasion and survival were dependent upon HBEGF signaling.
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
The primary limitation of this study was the use of only in vitro experiments. Patient demographics from elective terminations were not available.
WIDER IMPLICATIONS OF THE FINDINGS
These data provide new insights into the non-coagulation-related aspects of perinatal LMWH treatment in the management of placental insufficiency disorders.
STUDY FUNDING/COMPETING INTEREST(S)
This research was supported by grants from the National Institutes of Health (HD071408 and HL128628), the March of Dimes, and the W. K. Kellogg Foundation. There were no conflicts or competing interests.
Keywords: LMWH, HBEGFimplantation, trophoblast, placenta, apoptosis, differentiation, integrins, caspase, BCL-2α
Introduction
Preeclampsia is a disorder characterized by hypertension and proteinuria that affects up to 5–8% of all pregnancies after 20 weeks of gestation (Roberts et al., 2003). It is a leading cause of maternal and perinatal morbidity and mortality (Roberts, 1998; Redman and Sargent, 2000; Steegers et al., 2010). Placental insufficiency syndromes often manifest as a pro-thrombotic state (Di Prima et al., 2011), and are a contributory factor for miscarriage and preeclampsia (Burton and Jauniaux, 2004). Preeclampsia is generally associated with intrauterine growth restriction (IUGR), and, along with miscarriage, originates from deficient extravillous trophoblast invasion (Burton and Jauniaux, 2004). Evidence suggests that non-physiological hypoxia/reoxygenation (H/R) occurs in the first trimester and is responsible for abnormal remodeling of the placenta and its membranes (Burton and Jauniaux, 2004). Unsuccessful transformation of the spiral arteries by the placental extravillous trophoblast is later associated with reduced oxygen supply and placental dysfunction as pregnancy progresses (Drewlo et al., 2011). Antiphospholipid syndrome (APS) can cause recurrent miscarriage due to poor transformation of spiral arteries (Tong et al., 2015), while unexplained pregnancy loss not related to anticoagulation pathways also occurs (Simcox et al., 2015).
Low molecular weight heparin (LMWH) is used clinically for the prevention of pregnancy complications associated with pro-thrombotic disorders (Oberkersch et al., 2010). LMWH has been suggested as an intervention to improve placental function by increasing blood flow toward the implantation site and reducing the presence of thrombotic lesions (Yinon et al., 2015). Additionally, LMWH appears to be a useful therapy for prevention of placenta-mediated pregnancy complications, including preeclampsia, IUGR (Rodger et al., 2014) and unexplained recurrent pregnancy loss (Girardi et al., 2004; Yuksel et al., 2014). A recent systematic review and meta-analysis found that LMWH significantly reduces the risk of recurrent placenta-mediated pregnancy complications in women with prior occurrences (Rodger et al., 2014). LMWH is often used as preventative therapy for these common, serious pregnancy disorders, for which other prevention strategies are unavailable.
Previous studies proposed that heparin directly influences trophoblast cells independently of its anticoagulant activity by interacting with key proteins and signaling pathways (Bose et al., 2004; Bose et al., 2005; Adiguzel et al., 2009; Rey et al., 2009; Kingdom and Drewlo, 2011). Unfractionated heparin and LMWH promote the differentiation and invasion of extravillous trophoblast cells (Chen et al., 2012; D'Ippolito et al., 2012), and decrease vascular resistance (Hills et al., 2006; Ganapathy et al., 2007; Oberkersch et al., 2010). The exact molecular mechanisms by which heparin could exert its potential therapeutic effects in human reproduction are not fully understood (Adiguzel et al., 2009). However, it has been reported that LMWH is associated with increased heparin-binding epidermal growth factor (EGF)-like growth factor (HBEGF) protein expression and secretion (Di Simone et al., 2007; Chen et al., 2012). HBEGF is a member of the EGF family, which includes proteins that bind to tyrosine kinase receptors of the human EGF receptor (ERBB) family (Riese and Stern, 1998). HBEGF specifically binds to ERBB1 and ERBB4, but is capable of activating ERBB2 through receptor transactivation (Holbro and Hynes, 2004). Heparin is a cofactor for proteins with heparin-binding domains, including HBEGF (Riese and Stern, 1998). In addition to HBEGF, heparin is required as a cofactor for fibroblast growth factor (FGF) 4 in the maintenance of rodent trophoblast stem cells (Tanaka et al., 1998). In humans, villous cytotrophoblasts (CTBs) proliferate in response to heparin and FGF treatment (Baczyk et al., 2006). These studies show that heparins activate numerous signaling pathways that could influence trophoblast survival and function.
HBEGF contributes to trophoblast survival and extravillous differentiation during early pregnancy, and is dysregulated in placental insufficiencies (Leach et al., 2002; Chobotova et al., 2005; Chen et al., 2012; Dodd et al., 2013). HBEGF is secreted from trophoblast cells of the human blastocyst (Aghajanova et al., 2012), induces trophoblast extravillous differentiation (Leach et al., 2004), regulates endometrial stromal cell motility at the implantation site (Schwenke et al., 2013), and provides cytoprotection in an adverse environment (Jessmon et al., 2009). Inhibition of HBEGF signaling by a specific antagonist, or by inhibiting ERBB receptors, abrogates its cytoprotective function, as does removal of its cofactor, heparan sulfate from the cell surface (Armant et al., 2006). HBEGF expression is significantly reduced in all trophoblast populations of placentas from women with preeclampsia (Leach et al., 2002), suggesting a contribution of this deficiency to the associated cell death and poor invasion of those cells. LMWH induces a significant increase in HBEGF protein expression and secretion, and reduces TNF-α–induced apoptosis (Di Simone et al., 2007). Therefore, we investigated whether LMWH acts through HBEGF signaling to induce trophoblast extravillous differentiation and decrease apoptosis caused by oxidative stress. We have examined this concept using both a human trophoblast cell line and first-trimester placental explants. Our findings suggest a mechanism for heparin-mediated reduction of placental disorders.
Materials and Methods
Cell culture
HTR-8/SVneo an extravillous trophoblast cell line was grown in DMEM/F12 medium containing 10% (v/v) fetal bovine serum. Culture medium was replaced with serum-free medium 24 h prior to all experiments. Cells were cultured in a humidified 5% CO2/95% air incubator for ambient culture (20% O2), or at 2% O2 as previously reported (Armant et al., 2006). HTR cell exposure to H/R was performed as previously described (Leach et al., 2008). Briefly, cells were cultured at 2% O2 for 2 h, and then medium was replaced with fresh medium pre-equilibrated to 20% O2 for an additional 6 h of culture at ambient conditions. Cells cultured at 20% or 2% O2 for 8 h served as controls.
Villous explant culture
Placental tissues (n = 5; mean gestational age 8.2 ± 0.7 weeks) were obtained with approval of Wayne State University Institutional Review Board and patient informed consent from first-trimester terminations at a Michigan Family Planning Facility. Fresh tissue was placed in ice cold PBS and immediately transported to the laboratory. The chorionic villi containing extravillous clusters were dissected under a microscope into pieces of ~5 mg wet weight (Drewlo et al., 2011), and cultured free floating for 24 h in DMEM/F12 medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, 100 IU penicillin and 100 μg/ml streptomycin. Villous explants exposed to H/R were cultured at 2% O2 for 2 h, and then medium was replaced with fresh medium pre-equilibrated to 8% O2 for an additional 6 h of culture at ambient conditions consistent with elevated oxygen concentrations (~60 mm Hg or 8%) at the end of the first trimester. Explants were then used for experimentation as described below.
Treatments
Cells and villous explant cultures were treated by supplementing culture medium with 10 IU/ml LMWH (Enoxaparin; Sigma, St. Louis, MO) for 24 h during ambient culture or during reoxygenation. LMWH was titrated to a therapeutic range based on the anti-factor Xa assay (Ignjatovic et al., 2007). Cells and villous explants were also treated with vehicle, 10 μg/ml CRM197 (a highly specific HBEGF antagonist; EMD Biosciences, San Diego, CA), 10 μg/ml EGFR/ERBB2/ERBB4 (pan-ERBB inhibitor) tyrosine kinase antagonist (Millipore, catalog no. 324 840), 0.1 U/ml heparitinase I (from Flavobacterium heparinum, EC 4.2.2.8; 100 704-1, Seikagaku America, East Falmouth, MA), and 10 μg/ml mouse anti-ERBB1 (Ab-2) or ERBB4 (Ab-3) blocking antibodies (Lab Vision, Fremont, CA). At the conclusion of each experiment, cells or explants were either lysed to make cell extracts or fixed. Extracts from an ~80% confluent T-25 tissue culture flask, or 20–30 mg of tissue, or were prepared using a lysis buffer containing 1% sodium dodecyl sulfate (SDS), or 1% Triton X-100. Cells or villous explants in each well were gently rinsed three times with PBS and fixed for 30 min in PBS containing 4% paraformaldehyde or 10% neutral buffered formalin for tissue. Fixative was removed by rinsing three times with 150 μl of PBS. Fixed villous explants were embedded in paraffin wax and 5 μm sections were cut and mounted on glass slides. Paraffin sections were deparaffinized with xylene and rehydrated into Tris-buffered saline before immunocytochemical or cell death assays.
Anchoring villous explant outgrowth experiments
Villous explants were cultured on Matrigel™-coated Millicell-CM inserts (12-mm diameter, 0.4-μm pores; Millipore, Burlington, MA) in a 24-well culture plate for a total of 72 h (Caniggia et al., 1997a, b). The bottom chamber contained 500 μl DMEM/F12 medium supplemented with 10% fetal bovine serum, penicillin and streptomycin, and the upper chamber contained ~25 μl of medium initially. Chorionic villi were added with 150 μl of medium to the upper chamber, with or without supplementation, and the culture was continued for 72 h. The villi were fixed in 4% paraformaldehyde, rinsed three times with PBS, and assessed for outgrowth. Streams of migrating extravillous trophoblast cells were measured at the tip of each villous explant using digital images captured with a Hamamatsu (Hamamatsu City, Japan) Orca digital camera mounted on a Leica (Wetzlar, Germany) DM IRB epifluorescence microscope. Outgrowth length was measured using Simple PCI (Hamamatsu) imaging software.
Invasion assay
HTR-8/SVneo cells (100 000 per well) were cultured for 72 h on Matrigel™ (Collaborative Research, Bedford, MA) in 6.5-mm transwell inserts (Corning Inc., Acton, MA), as previously reported (Kilburn et al., 2000). Cells that penetrated the Matrigel™ and populated the lower chamber were detached using trypsin-EDTA solution, fixed and counted (Jessmon et al., 2010).
ELISA
ELISA was performed using the HBEGF DuoSet ELISA kit (R&D Systems), as previously described (Armant et al., 2006, Leach et al., 2008). The optical density of the final reaction product was determined at 450 nm using a programmable multiplate spectrophotometer (Power Wave Workstation; Bio-Tek Instruments) with automatic wavelength correction. HBEGF concentrations were calculated from the corresponding standard curve.
Immunocytochemistry
Fixed HTR-8/SVneo cells were grown in 96-well plates or deparaffinized sections of placental explants were permeabilized for 10 min by incubation in PBS containing 0.1% Triton X-100. Overnight incubations with primary antibody diluted in antibody diluent (DAKO, Carpinteria, CA) were carried out with 5 μg/ml of goat polyclonal HBEGF antibody (R&D Systems), 10 μg/ml of rabbit anti-cleaved caspase 3 and mouse anti-BCL-2-α (Cell Signaling Technology; Danvers, MA), 1 μg/ml of mouse monoclonal antibodies against the integrin subunits α1 or α6 (Upstate Biotechnology, Lake Placid, NY), or 0.55 μg/ml anti-Ki-67 monoclonal antibody (Ki-S5; DAKO, Carpinteria, CA). Controls were incubated with 10 μg/ml non-immune IgG (Jackson ImmunoResearch, West Grove, PA). Antibody labeling was visualized with either fluorescent conjugated antibody or an Envision System™ peroxidase anti-mouse kit (DAKO). For immunofluorescence, primary antibodies were visualized with 0.3 μg/ml FITC- or Texas red-conjugated secondary antibody (Jackson), and counterstained with 5 µg/ml 4,6-diamidino-2-phenylindole, HCl (DAPI; EMD Biosciences). Staining was imaged using a Leica DM IRB epifluorescence microscope, and images were captured using a Hamamatsu Orca digital camera. Peroxidase labeled cells were quantified using Simple PCI imaging software, as previously described (Leach et al., 2012). Values obtained with non-immune IgG controls were subtracted from each sample.
Western blotting
Western blots were performed as previously described (Patel et al., 2015). Cellular lysates (35 µg protein) were diluted in SDS sample buffer containing 5% β-mercaptoethanol, run on precast 4–20% Tris-HCl gradient gels (BioRad), and blotted overnight at 4°C with primary antibody diluted 1:1000 against BCL-2α. Membranes were incubated with horse radish peroxidase-conjugated secondary antibodies diluted 1:1000 for 1 h at room temperature, and developed with Western Lightning® ECL Pro (PerkinElmer, USA). Signals were visualized using a ChemiDoc Imaging System (BioRad, USA) and Image Lab V.5.1 software (BioRad, USA). Densities of immuno-reactive bands were measured as arbitrary units by ImageJ software (NIH, USA). Protein levels were normalized to a housekeeping protein (β-actin, 1:20 000; Abcam).
Cell death and proliferation assays
Cell death was detected in HTR-8/SVneo cells and tissue sections by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL), using a fluorescein-based kit from Roche Applied Science (Indianapolis, IND) as previously described (Armant et al., 2006). Cells were counterstained with DAPI. Fluorescent nuclei were imaged at ×400 magnification and processed using Simple PCI imaging software, counting total nuclei (DAPI) and TUNEL-positive nuclei (fluorescein) for each field. The percentage of TUNEL/DAPI-labeled nuclei (TUNEL index) was calculated by averaging counts for triplicate fields in each well or tissue section. Cell proliferation was measured in nuclei of HTR-8/SVneo cells or tissue sections fluorescently (FITC) labeled with antibody against Ki-67 and counterstained with DAPI. We determined the percentage of Ki-67/DAPI-labeled nuclei (Ki-67 index), as previously described (Armant et al., 2006).
Statistical analysis
All experiments were conducted using triplicate samples and were repeated at least three times. Data were statistically compared using SPSS Version 22.0. (IBM Corp., 2012). Independent t-tests were used to compare the effects of treatments on HBEGF expression levels. One-way ANOVA followed by Tukey's post hoc test was used to compare the effects of treatments on cell proliferation and integrin switching. One-way ANOVA followed by Dunnett's post hoc test was used to compare the effects of treatments on cell invasion, outgrowth length and cell death. P < 0.05 was considered statistically significant.
Results
LMWH prevents apoptosis in first-trimester villous explants in vitro
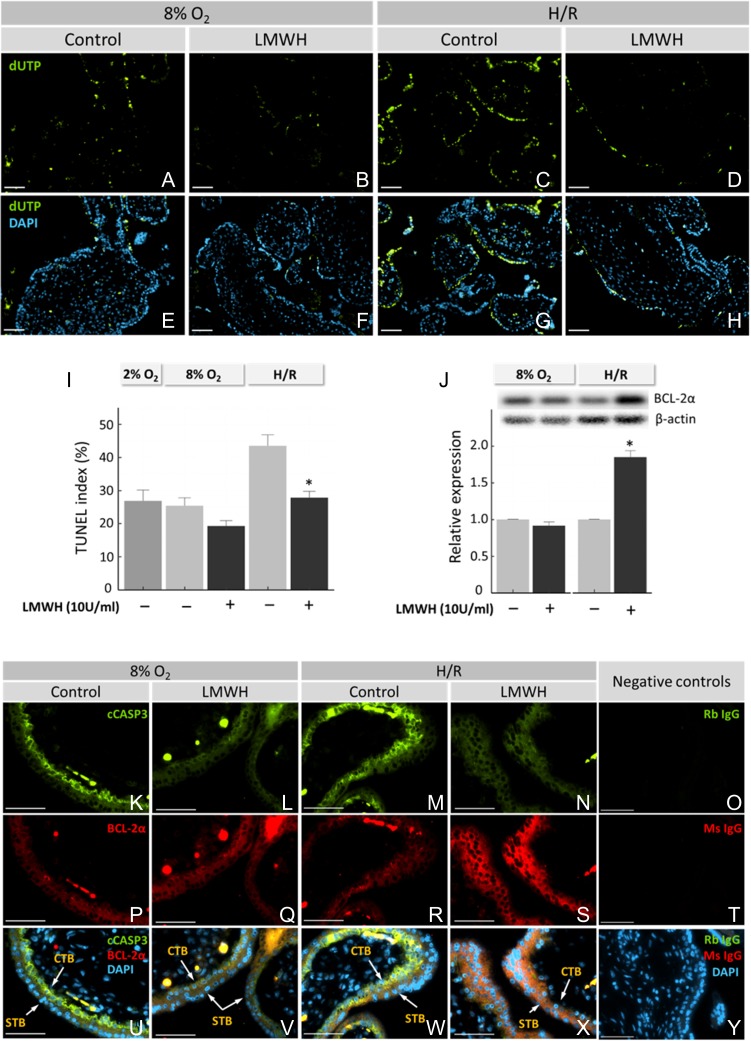
First-trimester villous explants exposed to H/R exhibited elevated cell death, as detected by TUNEL, compared to continuous culture at a physiological (8%) O2 concentration (Fig. 1A, C). However, treatment with LMWH during reoxygenation prevented cell death (Fig. 1C, D). DAPI nuclear staining indicated equivalent amounts of tissue present for each treatment (Fig. 1E–H). Quantification of TUNEL demonstrated increased (P < 0.05) cell death in villous explants exposed to H/R, rising from 21 ± 3% to 38 ± 5% (Fig. 1I). Supplementing the medium with 10 IU/ml LMWH during H/R exposure reduced (P < 0.05) TUNEL to 23 ± 2%. Addition of LMWH to explants cultured continuously at 8% O2 trended toward a reduction in apoptosis, but was not statistically significant (Fig. 1I). Culturing first-trimester explants at lower oxygen concentrations (2%) did not significantly change the TUNEL index from explants cultured at 8% O2.
Figure 1.
Effect of LMWH on cell death in first-trimester placental explants. Dissected villous explants were cultured at 8% O2, 2% O2 or during H/R, with or without addition of 10 IU LMWH, as indicated. Cell death was assessed using a TUNEL assay (A–I). Villi were double-labeled using fluorescent indicators to visualize DNA fragmentation (dUTP) by TUNEL (A–D) or nuclei with DAPI (E–H). TUNEL Index was quantified as a percentage by dividing the number of TUNEL-positive cells by the total cell number of DAPI-labeled nuclei (I). Western blot analysis of villous explant extracts was performed for BCL-2α expression, and densities of immuno-reactive bands were measured as arbitrary units by ImageJ software (J). Protein levels were normalized to β-actin. (K–Y) first-trimester villous explants were fluorescently labeled with antibodies against BCL-2α and cleaved caspase 3 (cCASP3). Immunofluorescence images are shown for BCL-2α (without treatment at ambient green, K–N), cCASP3 (red, P–S) and merged fields (U–Y), as indicated. Non-immune IgG negative controls are shown in O and T. Arrows indicate STB and CTB cells. Bars = 50 µM. Error bars denote SEM. *P < 0.05 compared to treatments without LMWH (I, J). LMWH, low molecular weight heparin; STB, syncytiotrophoblast; CBT, cytotrophoblast; H/R, hypoxia/reoxygenation.
The level of anti-apoptotic BCL-2α in response to LMWH stimulation was determined by western blot of whole cell extracts of villous explants cultured at 8% O2 or with H/R. BCL-2α was detected at 8% O2, and protein expression remained stable after LMWH stimulation, as determined by densitometry (Fig. 1J). BCL-2α was unaffected by LMWH at 8% O2. However, there was a significant increase in BCL-2α after LMWH treatment during H/R, compared to untreated tissue. First-trimester villous explants exposed to H/R exhibited a higher expression of the pro-apoptotic cleaved caspase 3 in cytotrophoblast cells compared to explants cultured continuously at 8% O2, as assessed by immunofluorescence (Fig. 1K, M). The expression of BCL-2α was unchanged in H/R compared to villi cultured continuously at 8% O2 (Fig. 1P, R). Supplementation with 10 IU/ml LMWH during H/R resulted in low expression of pro-apoptotic cleaved caspase 3 protein (Fig. 1N) and high expression of the anti-apoptotic BCL-2α protein (Fig. 1S). Without H/R, LMWH reduced cleaved caspase 3 (Fig. 1L), but had little effect on BCL-2α (Fig. 1Q) levels.
LMWH increases HBEGF protein expression and secretion
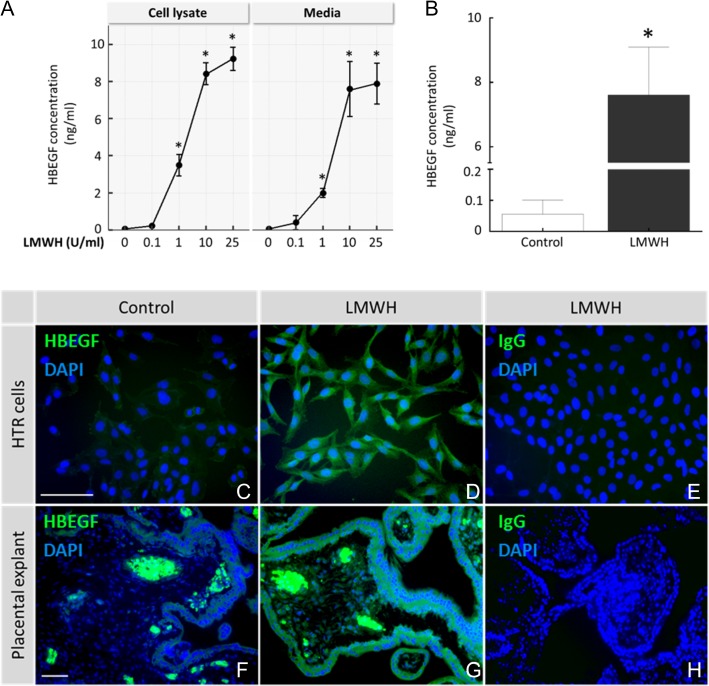
Secreted HBEGF was quantified by ELISA in conditioned medium after treating with LMWH for 24 h. We examined the LMWH dose dependency of HBEGF secretion with the human first-trimester trophoblast cell line, HTR-8/SVneo. LMWH was dose dependent over the concentration range of 0.1–25 IU/ml (Fig. 2A). HBEGF production and secretion significantly increased at or above 1 IU/ml LMWH. Cells cultured with 10 or 25 IU/ml LMWH showed an 8- to 10-fold increase in HBEGF secretion as assessed by ELISA, suggesting an optimal dosage of 10 IU/ml. HBEGF protein was nearly undetectable in untreated HTR-8/SVneo and villous explant media (0.06 ± 0.05 and 0.07 ± 0.03 ng/ml, respectively). However, it increased significantly in medium collected from HTR-8/SVneo cells (7.6 ± 1.5 ng/ml; Fig. 2A) and villous explants (17.9 ± 2.63 ng/ml; Fig. 2B) after 24 h of exposure to 10 IU/ml LMWH (P < 0.05). The effect of LMWH on the expression of HBEGF in the HTR-8/SVneo cell line and first-trimester villous explants was also examined by immunofluorescence. HTR-8/SVneo cells (Fig. 2C, D) and villi (Fig. 2F, G) expressed higher levels of HBEGF after treatment with LMWH compared to untreated controls. HBEGF antibody stained trophoblast and mesoderm at levels above the background staining of non-immune IgG (Fig. 2E, H). Fetal capillaries and blood cells exhibited high amounts of HBEGF staining that appeared as hot spots in villous explants (Fig. 2F, G).
Figure 2.
HBEGF protein expression and LMWH. HTR-8/SVneo (HTR) cells were cultured untreated (0, Control), or their medium was supplemented with 0.1–25 IU/ml LMWH for 24 h (A). Afterward, HBEGF was measured by ELISA in the cell lysates and media. Secreted HBEGF was measured by ELISA in first-trimester villous explants after 24 h of culture with 0 (Control) or 10 IU/ml of LMWH (B). HBEGF expression was localized by immunofluorescence in HTR cells (C–D) and first-trimester villous explants (F–G) for control and LMWH treatments, as indicated. DAPI nuclear counterstain (blue) is included in the images. Villi and cells treated with LMWH were labeled with non-immune goat IgG, as indicated (E and H). Bars = 50 μM. *P < 0.05, compared with control. HBEGF, heparin-binding epidermal growth factor-like growth factor.
LMWH increases cell invasion in vitro through HBEGF signaling
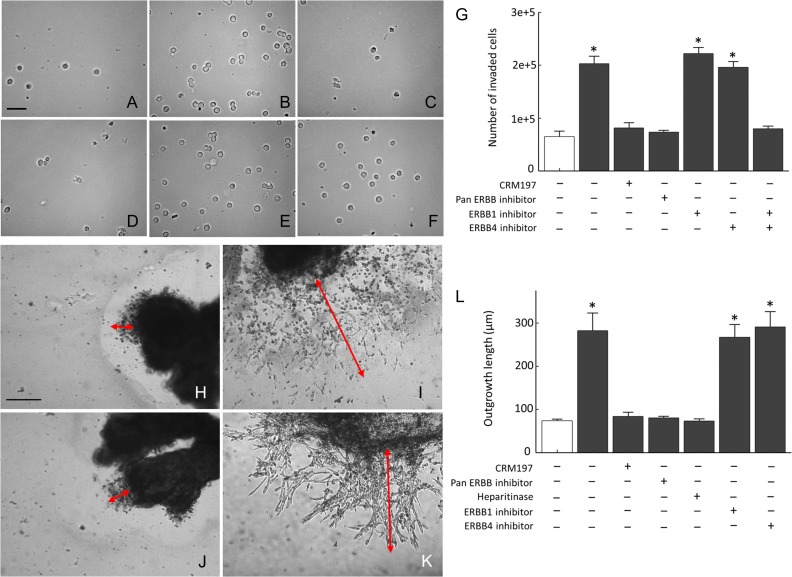
We examined the effect of LMWH on trophoblast invasion using a Matrigel invasion assay with the HTR-8/SVneo cell line and first-trimester placental explants. Supplementation with 10 IU/ml of LMWH stimulated (P < 0.05) invasion of HTR-8/SVneo cells cultured with LMWH (4.5-fold increase compared to control) assessed by penetration through Matrigel™ and cell counting (Fig. 3A–G). To examine the involvement of HBEGF in LMWH induction of invasion, we evaluated the effects of a specific antagonist, CRM197, a mutant diphtheria toxin that specifically antagonizes HBEGF by masking its EGF domain (Mitamura et al., 1995), inhibitors of its receptors (ERBB1 and 4), and heparitinase-mediated removal of cell surface heparan sulfate to deprive HBEGF of an essential cofactor for binding to its receptor. Increased invasion due to LMWH treatment was blocked with CRM197, pan-ERBB inhibitor and heparitinase. Inhibition of either ERBB1 or ERBB4 alone had no effect on LMWH's ability to increase HTR-8/SVneo invasion; however, a combination of antibodies blocking both receptors did abolish the effect of LMWH (Fig. 3G).
Figure 3.
Induction of trophoblast invasion and outgrowth by LMWH. HTR-8/SVneo cells were cultured on Matrigel™-coated transwell inserts for 72 h to determine their invasive capacity (A–G). Cells that invaded through the Matrigel™ were trypsinized from the bottom of the insert, and fixed. Examples of cells that penetrated through the Matrigel™ are shown after culture with no treatment (Control, A), 10 IU/ml LMWH (B), or combinations of LMWH and either 10 μg/ml CRM197 (C), pan-ERBB inhibitor (D), ERBB1 blocking antibody (E) or ERBB4 blocking antibody (F). The number of cells entering the lower chambers after 72 h was quantified for the indicated treatments that did (solid bars) or did not (open bars) include LMWH (G). First-trimester villous explants were cultured on Matrigel™-coated transwell inserts for 72 h, and assessed for trophoblast outgrowth from their distal ends (H–L). Examples of outgrowths from villous explants cultured with no treatment (Control, H), 10 IU/ml LMWH (I), and a combination of LMWH with either CRM197 (J), or ERBB4 blocking antibody (K) are shown. Outgrowth length was measured, as indicated by red arrows, using Simple PCI image analysis software, and is depicted graphically in L for triplicate experiments in which 10 villous explants were measured for each indicated treatment that did (solid bars) or did not (open bars) include LMWH. Size bars = 100 μM. *P < 0.05 compared to no treatment (open bars). Error bars represent SEM. ERBB, Erb-B2 Receptor Tyrosine Kinase.
To evaluate the direct effect of LMWH on human first-trimester extravillous explants, culture medium was supplemented with 10 IU/ml LMWH and extravillous trophoblast outgrowth on Matrigel™ was quantified, as described in the Materials and Methods section. Villi exposed to LMWH produced large outgrowths from their tips, while control villi had very small areas of extravillous trophoblast outgrowth (Fig. 3 H, I). The length of villous tips increased significantly after 72 h of culture in explants treated with LMWH, compared to untreated controls (73.9 ± 3.8 vs 281.9 ± 41.0; P < 0.05). The increased outgrowth due to LMWH was blocked with CRM197 (Fig. 3J). Increased invasion due to LMWH in villous explants was also blocked with pan-ERBB inhibitor and heparitinase (Fig. 3L), but not ERBB1 or ERBB4 alone (Fig. 3K), similar to HTR-8/SVneo cells.
LMWH promotes trophoblast extravillous differentiation through HBEGF
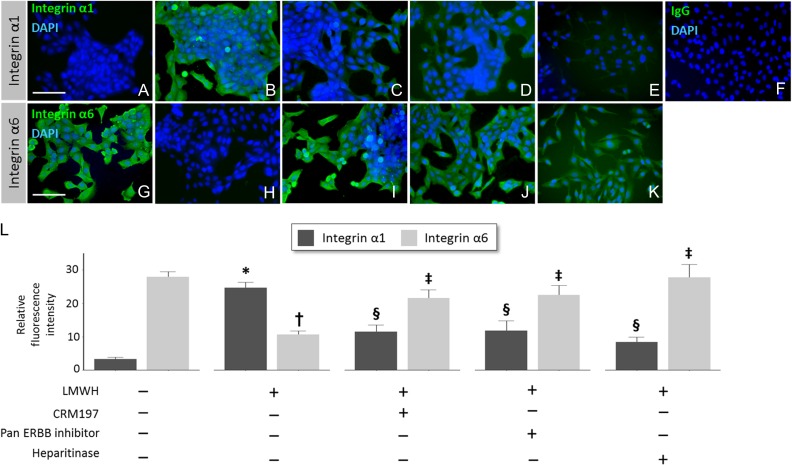
We and others previously found that integrin protein expression in HTR-8/SVneo and primary cytotrophoblast cells switches from predominantly α6β4 to α1β1 during culture on Matrigel as part of extravillous differentiation (Damsky et al., 1994; Kilburn et al., 2000). The integrin switch also occurs when trophoblast cells are induced to become invasive by other treatments (Leach et al., 2004; Bolnick et al., 2015). Since LMWH increased trophoblast invasion, we determined whether it also induced integrin switching (extravillous differentiation) in the trophoblast cell line. HTR-8/SVneo cells cultured without LMWH expressed low levels of integrin α1 (3.33 ± 0.52) and high levels of integrin α6 (27.9 ± 3.26), as shown in Fig. 4A,G, and L. Cells treated with 10 IU/ml of LMWH exhibited a clear switch (P < 0.05) to elevated integrin α1 (24.7 ± 1.61; Fig. 4B) and reduced integrin α6 (10.67 ± 1.07; Fig. 4B, H and L). The HBEGF antagonist CRM197 (Fig. 4C, I and L), pan-ERBB inhibitor (Fig. 4D, J and L) and heparitinase treatment of cells (Fig. 4E, K and L) each attenuated the ability of LMWH to induce integrin switching in HTR-8/SVneo cells, with different (P < 0.05) integrin expression levels than those of cells treated with LMWH only.
Figure 4.
Induction of integrin switching by LMWH, and HBEGF dependence. HTR-8/SVneo cells were cultured for 24 h with no treatment (Control; A, G), 10 IU/ml LMWH (B, H), and combinations of LMWH with 10 μg/ml CRM197 (C, I), pan-ERBB inhibitor (D, J) or heparitinase (E, K). Cells were assessed for α1 (A–E) or α6 (G–K) integrin subunits by immunofluorescence. Nuclei were counterstained with DAPI. Cells treated with LMWH were labeled with non-immune goat IgG (F) as a negative control. Bars = 100 μM. Integrin α1 and α6 protein expression was quantified by image analysis (L). * or †, P < 0.05 compared to no treatment. § or ‡, P < 0.05 compared to LMWH treatment.
LMWH does not affect trophoblast proliferation in vitro
Trophoblast proliferation, assessed by nuclear Ki-67 expression, was unaffected by LMWH in HTR-8/SVneo cells and first-trimester villous explants when comparing the relative percentage of Ki-67 expressing cells in LMWH-treated and untreated controls (Supplementary data, Fig S1). Treating HTR-8/SVneo cells with HBEGF also fails to increase proliferation significantly (Leach et al., 2004).
HBEGF signaling is needed for LMWH to prevent apoptosis induced by H/R
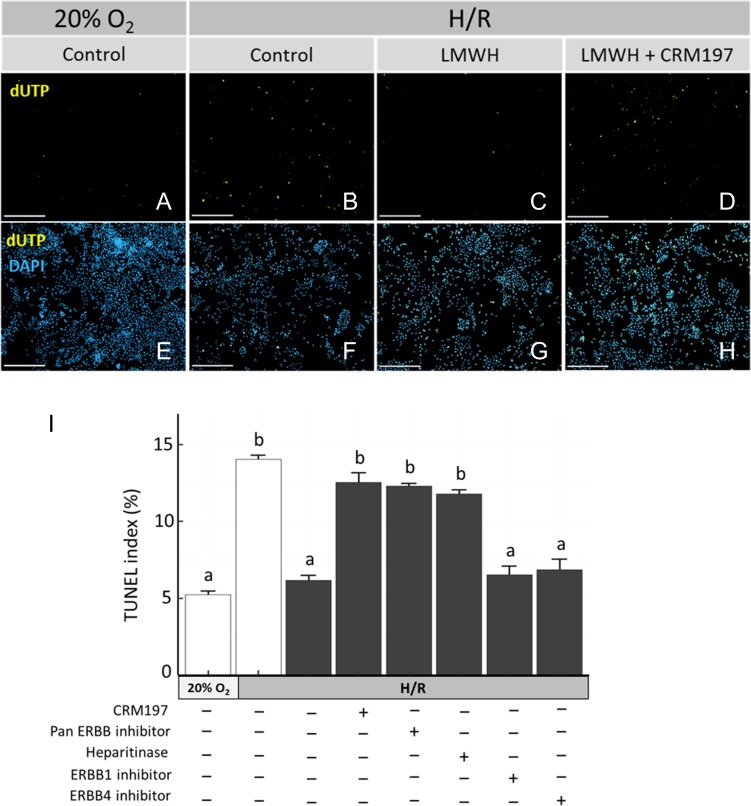
Finally, we determined whether HBEGF signaling is required to inhibit apoptosis. HTR-8/SVneo cells exposed to H/R exhibited elevated cell death, as detected by TUNEL, and compared to ambient (20% O2) culture (Fig. 5A, B). LMWH attenuated apoptosis during H/R to levels comparable to ambient controls (Fig. 5C), which was reversed in the presence of CRM197 (Fig. 5D). DAPI nuclear staining indicated equivalent amounts of cells present for each treatment (Fig. 5 E–H). Quantification of TUNEL (Fig. 5I) demonstrated that H/R increased (P < 0.05) the TUNEL index more than 2-fold over the control (5 ± 0.3–14 ± 0.2%). Supplementing the medium with 10 IU/ml of LMWH during H/R exposure reduced (P < 0.05) TUNEL to 6 ± 0.3%. Cell treatment with CRM197, pan-ERBB inhibitor and heparitinase each prevented the LMWH-induced rescue from cell death. Inhibition of either ERBB1 or ERBB4 alone had no effect on LMWH's ability to rescue from cell death (Fig. 5I).
Figure 5.
LMWH rescue from cell death requires HBEGF. HTR-8/SVneo cells were cultured without treatment at ambient (20%) O2, or exposed to H/R with no treatment (Control), supplementation with 10 IU/ml LMWH, or combinations of LMWH and either 10 μg/ml CRM197, pan-ERBB inhibitor, heparitinase I, ERBB1 blocking antibody or ERBB4 blocking antibody. HTR-8/SVneo cells were fluorescently double-labeled after treatment to visualize cell death by TUNEL (green fluorescence) and nuclei with DAPI (blue fluorescence). Representative fluorescent images are shown of TUNEL (A–D) or merged TUNEL/DAPI (E–H) in cells cultured at ambient O2 (A, E), with H/R (B, F), with H/R + LMWH (C, G) and with H/R + LMWH + CRM197 (D, H). Bars = 100 μM. TUNEL Index is shown graphically for the indicated culture conditions and treatments, which did (solid bars) or did not (open bars) include LMWH (I). Error bars denote SEM. Bars labeled with dissimilar letters indicate differences at P < 0.05.
Discussion
In this study, we provide for the first time, direct evidence for a mechanism by which LMWH exerts its anti-thrombin-independent effects on trophoblast survival and invasion through HBEGF signaling. In addition to anticoagulation properties, LMWH promotes villous cytotrophoblast proliferation and syncytiotrophoblast (STB) differentiation (Drewlo et al., 2011), increases extravillous trophoblast invasion (Di Simone et al., 2007) and exerts a cytoprotective effect (Chen et al., 2012). LMWH could also limit maternal disease through mechanisms independent of trophoblast function, as recently proposed in a study of its positive impact on endothelial function and angiogenesis (McLaughlin et al., 2016). HBEGF is important for embryonic implantation, uterine function (Jessmon et al., 2009) and trophoblast physiology (Hung and Burton, 2006; Leach et al., 2008; Jessmon et al., 2009; Chen et al., 2012). HBEGF signaling activates integrin switching to initiate trophoblast invasion, and exerts a cytoprotective effect during the first trimester (Leach et al., 2004; Armant et al., 2006; Wolff et al., 2007; Leach et al., 2008).
Our results illustrate that LMWH increases HBEGF protein expression and secretion, which is sustained through autocrine signaling in both a human first-trimester trophoblast cell line, HTR-8/SVneo, and first-trimester villous explants. We found that LMWH reduced apoptosis of villous explants and human trophoblast cells exposed to H/R through a mechanism dependent on HBEGF signaling. Additionally, LMWH enhanced extravillous trophoblast outgrowth in first-trimester villous explants, and directly induced trophoblast phenotypic differentiation at the molecular level by switching cells from α6β4 to α1β1 integrin protein expression. These changes increased cellular invasive activity in both villous explants and first-trimester cytotrophoblast cells. Inhibition of HBEGF during trophoblast culture using the antagonist CRM197, or specific inhibitors of its two receptors, ERBB1 and ERBB4, prevented the effects of LMWH, indicating that LMWH acts through and requires HBEGF signaling. Although each inhibitor of HBEGF signaling could have off-target effects, the very similar results found with each, supports our interpretation. Similar to HBEGF (Leach et al., 2004; Armant et al., 2006; Leach et al., 2008), the effects of LMWH on trophoblast survival and invasion were mediated by either one of the HBEGF-binding ERBB receptors, as inhibition of one receptor alone was not sufficient to prevent the effects of LMWH. Cell surface proteoglycans that contain heparin or heparan sulfate are required cofactors for HBEGF signaling (Nishi and Klagsbrun, 2004). However, addition of LMWH did not rescue from H/R after cell surface heparin was removed, suggesting LMWH and cell surface heparin work through different mechanisms that are each essential for reducing apoptosis. LMWH likely increases HBEGF accumulation, rather than contributing directly to HBEGF activation of ERBB receptors. HBEGF expression has been correlated with the beneficial effects of LMWH on trophoblast functions (Chen et al., 2012); however, our findings now provide a mechanism by which HBEGF contributes to the physiological effects of LMWH on trophoblasts.
Previous studies suggested that LMWHs might affect extravillous trophoblast invasion through increased matrix metalloproteinase-2 activity, and by increasing HBEGF and Cyr61 expression (Di Simone et al., 2007; Chen et al., 2012; D'Ippolito et al., 2012) based only on correlative data. We have extended this idea by demonstrating that inhibition of HBEGF signaling attenuates the effect of LMWH on extravillous trophoblast invasion, as well its cytoprotective activity. Although it is difficult to eliminate the potential role of proliferation in the higher numbers of HTR-8/SVneo cells found in the lower chamber after a 72-h invasion assay, LMWH treatment caused no increase in cell proliferation, as assessed by Ki-67 labeling. HBEGF transiently activates the mitogen-activated protein kinases (MAPKs) JNK, ERK and p38, as well as the PIK3/AKT pathway, downstream of its ERBB receptors in HTR-8/SVneo trophoblast cells (Jessmon et al., 2010). Of these kinases, p38 prevents apoptosis induced by oxygen fluctuations. We speculate that this signaling suppresses the cleavage of caspase 3 in trophoblast cells. Further, it appears to increase BCL-2α expression, but only under stress (H/R) conditions. The p38 pathway functions in combination with the ERK, JNK and PIK3 pathways to induce trophoblast extravillous differentiation (Jessmon et al., 2010). Additionally, it has been demonstrated that heparin, like EGF and HBEGF, elicits phosphorylation of the EGF receptor and activation of PI3K, ERK1/2 and JNK signal transduction pathways in primary villous trophoblast (Hills et al., 2006). Our findings strongly support a mechanism in which LMWH stimulation of HBEGF activity induces extravillous trophoblast differentiation and provides cytoprotection through ERBB1 and ERBB4, suggesting HBEGF is a necessary downstream target for LMWH.
The cellular mechanism that increases HBEGF in response to LMWH remains to be determined. We previously reported that HBEGF biosynthesis in HTR-8/SVneo cells is upregulated post-transcriptionally in response to low (2%) O2 levels (Armant et al., 2006). The accumulation of HBEGF under those conditions is dependent on HBEGF signaling through the ERBB and MAPK pathways (Armant et al., 2006; Jessmon et al., 2010). The similar dependence of LMWH-mediated accumulation of HBEGF on the ERBB pathway suggests a comparable mechanism. Experiments using the metalloproteinase inhibitor GM6001 suggest that HBEGF shedding might be essential for its upregulation in trophoblast cells cultured at low O2 (Armant et al., 2006). Produced initially as a transmembrane protein, HBEGF requires shedding of its ectodomain for paracrine and autocrine signaling, which can occur as cross-talk with other cellular signaling pathways that activate the appropriate sheddase (Umata et al., 2001). Therefore, it is plausible that LMWH exposure activates the HBEGF sheddase to initiate autocrine signaling and HBEGF biosynthesis.
Trophoblast survival and differentiation are compromised during placental dysfunction and have been hypothesized to contribute to preeclampsia and other adverse pregnancy outcomes (Hung et al., 2002; Hung and Burton, 2006). Placental-mediated disorders such as preeclampsia (Pennington et al., 2012), small for gestational age fetuses and APS, are all conditions associated with decreased trophoblast invasion (Khong et al., 1986) and increased levels of trophoblast apoptosis (DiFederico et al., 1999; Allaire et al., 2000). Studies indicate that LMWH can restore in vitro invasion and differentiation functions of trophoblast inhibited by anticardiolipan antibodies and has a direct influence on the trophoblast (Girardi et al., 2004). Antiphospholipid antibodies alter the invasiveness of trophoblast cells, causing defective placentation and thrombophilia associated with complications of pregnancy in patients with APS (Di Simone et al., 2000). HBEGF expression is also impaired in placental tissue from women with antiphospholipid antibody-mediated APS, and can reduce the effects of antiphospholipid antibodies on trophoblast cells (Di Simone et al., 2010).
Heparins are widely used in clinical practice to prevent deep vein thrombosis and reoccurring pregnancy loss, as well as improve blood flow to the implantation site (Simon and Laufer, 2012; Wang et al., 2016). In addition, heparins improve outcome in patients with repeated implantation failure during ART (Qublan et al., 2008; Akhtar et al., 2015). In a group of non-thrombotic patients, LMWH improved pregnancy outcomes (Rey et al., 2009). This work initiated many studies to understand the molecular anti-thrombin independent properties of heparins on placental function. The important interplay of HBEGF and LMWH provide new insights into the potential beneficial effects of LMWH in the treatment of placental-mediated disorders that may be useful in the management of at-risk patients, even in the absence of an identifiable thrombophilia or pro-thrombotic disorders.
Supplementary data
Supplementary data are available at Humrep Reproduction online.
Supplementary Material
Acknowledgments
The authors thank the Northland Family Planning Centers of Michigan for participating in this research study.
Authors’ roles
The study was designed, analyzed and written by A.D.B, J.M.B., B.A.K., H.-R.K.-G., J.D., M.P.D., D.R.A. and S.D., It was executed by A.D.B, J.M.B., B.A.K., O.J.P. and H,-R.K.-G. All authors read and approved the manuscript.
Funding
The National Institutes of Health (HD071408 and HL128628), the March of Dimes and the W. K. Kellogg Foundation.
Conflict of interest
There were no conflicts or competing interests.
References
- Adiguzel C, Jeske WP, Hoppensteadt D, Walenga JM, Bansal V, Fareed J. Structural and functional characterization of low-molecular-weight heparins: impact on the development of guidelines for generic products. Clin Appl Thromb Hemost 2009;15:137–144. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Shen S, Rojas AM, Fisher SJ, Irwin JC, Giudice LC. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod 2012;86:1–21. [DOI] [PubMed] [Google Scholar]
- Akhtar MA, Sur S, Raine-Fenning N, Jayaprakasan K, Thornton J, Quenby S, Marjoribanks J. Heparin for assisted reproduction: summary of a Cochrane review. Fertil Steril 2015;103:33–34. [DOI] [PubMed] [Google Scholar]
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol 2000;96:271–276. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kilburn BA, Petkova A, Edwin SS, Duniec-Dmuchowski ZM, Edwards HJ, Romero R, Leach RE. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development 2006;133:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczyk D, Dunk C, Huppertz B, Maxwell C, Reister F, Giannoulias D, Kingdom JC. Bi-potential behaviour of cytotrophoblasts in first trimester chorionic villi. Placenta 2006;27:367–374. [DOI] [PubMed] [Google Scholar]
- Bolnick JM, Kilburn BA, Bolnick AD, Diamond MP, Singh M, Hertz M, Dai J, Armant DR. Sildenafil stimulates human trophoblast invasion through nitric oxide and guanosine 3’,5’-cyclic monophosphate signaling. Fertil Steril 2015;103:1587–1595. e1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose P, Black S, Kadyrov M, Bartz C, Shlebak A, Regan L, Huppertz B. Adverse effects of lupus anticoagulant positive blood sera on placental viability can be prevented by heparin in vitro. Am J Obstet Gynecol 2004;191:2125–2131. [DOI] [PubMed] [Google Scholar]
- Bose P, Black S, Kadyrov M, Weissenborn U, Neulen J, Regan L, Huppertz B. Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am J Obstet Gynecol 2005;192:23–30. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 2004;11:342–352. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Lye SJ, Cross JC. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology 1997. a;138:3976–3986. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology 1997. b;138:4977–4988. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wu XX, Tan JP, Liu ML, Liu YL, Zhang JP. Effects of low molecular weight heparin and heparin-binding epidermal growth factor on human trophoblast in first trimester. Fertil Steril 2012;97:764–770. [DOI] [PubMed] [Google Scholar]
- Chobotova K, Karpovich N, Carver J, Manek S, Gullick WJ, Barlow DH, Mardon HJ. Heparin-binding epidermal growth factor and its receptors mediate decidualization and potentiate survival of human endometrial stromal cells. J Clin Endocrinol Metab 2005;90:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ippolito S, Di Nicuolo F, Marana R, Castellani R, Stinson J, Tersigni C, Scambia G, Di Simone N. Emerging nonanticoagulant role of low molecular weight heparins on extravillous trophoblast functions and on heparin binding-epidermal growth factor and cystein-rich angiogenic inducer 61 expression. Fertil Steril 2012;98:1028–1036. e1021–1022. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development 1994;120:3657–3666. [DOI] [PubMed] [Google Scholar]
- Di Prima FA, Valenti O, Hyseni E, Giorgio E, Faraci M, Renda E, De Domenico R, Monte S. Antiphospholipid Syndrome during pregnancy: the state of the art. J Prenat Med 2011;5:41–53. [PMC free article] [PubMed] [Google Scholar]
- Di Simone N, Di Nicuolo F, Sanguinetti M, Ferrazzani S, D'Alessio MC, Castellani R, Bompiani A, Caruso A. Low-molecular weight heparin induces in vitro trophoblast invasiveness: role of matrix metalloproteinases and tissue inhibitors. Placenta 2007;28:298–304. [DOI] [PubMed] [Google Scholar]
- Di Simone N, Marana R, Castellani R, Di Nicuolo F, D'Alessio MC, Raschi E, Borghi MO, Chen PP, Sanguinetti M, Caruso A et al. Decreased expression of heparin-binding epidermal growth factor-like growth factor as a newly identified pathogenic mechanism of antiphospholipid-mediated defective placentation. Arthritis Rheum 2010;62:1504–1512. [DOI] [PubMed] [Google Scholar]
- Di Simone N, Meroni PL, de Papa N, Raschi E, Caliandro D, De Carolis CS, Khamashta MA, Atsumi T, Hughes GR, Balestrieri G et al. Antiphospholipid antibodies affect trophoblast gonadotropin secretion and invasiveness by binding directly and through adhered beta2-glycoprotein I. Arthritis Rheum 2000;43:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol 1999;155:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JM, McLeod A, Windrim RC, Kingdom J. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database Syst Rev 2013;7:CD006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewlo S, Levytska K, Sobel M, Baczyk D, Lye SJ, Kingdom JC. Heparin promotes soluble VEGF receptor expression in human placental villi to impair endothelial VEGF signaling. J Thromb Haemost 2011;9:2486–2497. [DOI] [PubMed] [Google Scholar]
- Ganapathy R, Whitley GS, Cartwright JE, Dash PR, Thilaganathan B. Effect of heparin and fractionated heparin on trophoblast invasion. Hum Reprod 2007;22:2523–2527. [DOI] [PubMed] [Google Scholar]
- Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med 2004;10:1222–1226. [DOI] [PubMed] [Google Scholar]
- Hills FA, Abrahams VM, Gonzalez-Timon B, Francis J, Cloke B, Hinkson L, Rai R, Mor G, Regan L, Sullivan M et al. Heparin prevents programmed cell death in human trophoblast. Mol Hum Reprod 2006;12:237–243. [DOI] [PubMed] [Google Scholar]
- Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 2004;44:195–217. [DOI] [PubMed] [Google Scholar]
- Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol 2006;45:189–200. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res 2002;90:1274–1281. [DOI] [PubMed] [Google Scholar]
- Ignjatovic V, Summerhayes R, Gan A, Than J, Chan A, Cochrane A, Bennett M, Horton S, Shann F, Lane G et al. Monitoring unfractionated heparin (UFH) therapy: which Anti-Factor Xa assay is appropriate. Thromb Res 2007;120:347–351. [DOI] [PubMed] [Google Scholar]
- Jessmon P, Kilburn BA, Romero R, Leach RE, Armant DR. Function-specific intracellular signaling pathways downstream of heparin-binding EGF-like growth factor utilized by human trophoblasts. Biol Reprod 2010;82:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessmon P, Leach RE, Armant DR. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev 2009;76:1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986;93:1049–1059. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod 2000;62:739–747. [DOI] [PubMed] [Google Scholar]
- Kingdom JC, Drewlo S. Is heparin a placental anticoagulant in high-risk pregnancies. Blood 2011;118:4780–4788. [DOI] [PubMed] [Google Scholar]
- Leach RE, Jessmon P, Coutifaris C, Kruger M, Myers ER, Ali-Fehmi R, Carson SA, Legro RS, Schlaff WD, Carr BR et al. High throughput, cell type-specific analysis of key proteins in human endometrial biopsies of women from fertile and infertile couples. Hum Reprod 2012;27:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol 2004;266:223–237. [DOI] [PubMed] [Google Scholar]
- Leach RE, Kilburn BA, Petkova A, Romero R, Armant DR. Diminished survival of human cytotrophoblast cells exposed to hypoxia/reoxygenation injury and associated reduction of heparin-binding epidermal growth factor-like growth factor. Am J Obstet Gynecol 2008;198:471 e471–471 e477. discussion 471 e477–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach RE, Kilburn BA, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol 2004;266:223–237. [DOI] [PubMed] [Google Scholar]
- Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn B, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S et al. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet 2002;360:1215–1219. [DOI] [PubMed] [Google Scholar]
- McLaughlin K, Baczyk D, Potts A, Hladunewich M, Parker JD, Kingdom JC. Low molecular weight heparin improves endothelial function in pregnant women at high risk of preeclampsia. Hypertension 2016;69:180–188. [DOI] [PubMed] [Google Scholar]
- Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 1995;270:1015–1019. [DOI] [PubMed] [Google Scholar]
- Nishi E, Klagsbrun M. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a mediator of multiple physiological and pathological pathways. Growth Factors 2004;22:253–260. [DOI] [PubMed] [Google Scholar]
- Oberkersch R, Attorresi AI, Calabrese GC. Low-molecular-weight heparin inhibition in classical complement activation pathway during pregnancy. Thromb Res 2010;125:e240–e245. [DOI] [PubMed] [Google Scholar]
- Patel S, Kilburn B, Imudia A, Armant DR, Skafar DF. Estradiol elicits proapoptotic and antiproliferative effects in human trophoblast cells. Biol Reprod 2015;93:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech 2012;5:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qublan H, Amarin Z, Dabbas M, Farraj AE, Beni-Merei Z, Al-Akash H, Bdoor AN, Nawasreh M, Malkawi S, Diab F et al. Low-molecular-weight heparin in the treatment of recurrent IVF-ET failure and thrombophilia: a prospective randomized placebo-controlled trial. Hum Fertil (Camb) 2008;11:246–253. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta 2000;21:597–602. [DOI] [PubMed] [Google Scholar]
- Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, Morin F, Demers C, Kahn SR, Magee LA et al. Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. J Thromb Haemost 2009;7:58–64. [DOI] [PubMed] [Google Scholar]
- Riese DJ II, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays 1998;20:41–48. [DOI] [PubMed] [Google Scholar]
- Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 1998;16:5–15. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Pearson GD, Cutler JA, Lindheimer MD, National Heart L Blood I . Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertens Pregnancy 2003;22:109–127. [DOI] [PubMed] [Google Scholar]
- Rodger MA, Carrier M, Le Gal G, Martinelli I, Perna A, Rey E, de Vries JI, Gris JC. Low-Molecular-Weight Heparin for Placenta-Mediated Pregnancy Complications Study G . Meta-analysis of low-molecular-weight heparin to prevent recurrent placenta-mediated pregnancy complications. Blood 2014;123:822–828. [DOI] [PubMed] [Google Scholar]
- Schwenke M, Knofler M, Velicky P, Weimar CH, Kruse M, Samalecos A, Wolf A, Macklon NS, Bamberger AM, Gellersen B. Control of human endometrial stromal cell motility by PDGF-BB, HB-EGF and trophoblast-secreted factors. PLoS One 2013;8:e54336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox LE, Ormesher L, Tower C, Greer IA. Thrombophilia and pregnancy complications. Int J Mol Sci 2015;16:28418–28428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Laufer N. Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet 2012;29:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 2010;376:631–644. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science 1998;282:2072–2075. [DOI] [PubMed] [Google Scholar]
- Tong M, Viall CA, Chamley LW. Antiphospholipid antibodies and the placenta: a systematic review of their in vitro effects and modulation by treatment. Hum Reprod Update 2015;21:97–118. [DOI] [PubMed] [Google Scholar]
- Umata T, Hirata M, Takahashi T, Ryu F, Shida S, Takahashi Y, Tsuneoka M, Miura Y, Masuda M, Horiguchi Y et al. A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J Biol Chem 2001;276:30475–30482. [DOI] [PubMed] [Google Scholar]
- Wang KL, Chu PH, Lee CH, Pai PY, Lin PY, Shyu KG, Chang WT, Chiu KM, Huang CL, Lee CY et al. Management of venous thromboembolisms: part I. The consensus for deep vein thrombosis. Zhonghua Minguo Xin Zang Xue Hui Za Zhi 2016;32:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff GS, Chiang PJ, Smith SM, Romero R, Armant DR. Epidermal growth factor-like growth factors prevent apoptosis of alcohol-exposed human placental cytotrophoblast cells. Biol Reprod 2007;77:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinon Y, Ben Meir E, Margolis L, Lipitz S, Schiff E, Mazaki-Tovi S, Simchen MJ. Low molecular weight heparin therapy during pregnancy is associated with elevated circulatory levels of placental growth factor. Placenta 2015;36:121–124. [DOI] [PubMed] [Google Scholar]
- Yuksel H, Kayatas S, Boza AT, Api M, Ertekin AA, Cam C. Low molecular weight heparin use in unexplained recurrent miscarriage. Pak J Med Sci 2014;30:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.