Abstract
Understanding how brain development normally proceeds is a premise of understanding neurodevelopmental disorders. This has sparked a wealth of magnetic resonance imaging (MRI) studies. Unfortunately, they are in marked disagreement on how the cerebral cortex matures. While cortical thickness increases for the first 8–9 years of life have repeatedly been reported, others find continuous cortical thinning from early childhood, at least from age 3 or 4 years. We review these inconsistencies, and discuss possible reasons, including the use of different scanners, recording parameters and analysis tools, and possible effects of variables such as head motion. When tested on the same subsample, 2 popular thickness estimation methods (CIVET and FreeSurfer) both yielded a continuous thickness decrease from 3 years. Importantly, MRI-derived measures of cortical development are merely our best current approximations, hence the term “apparent cortical thickness” may be preferable. We recommend strategies for reaching consensus in the field, including multimodal neuroimaging to measure phenomena using different techniques, for example, the use of T1 / T2 ratio, and data sharing to allow replication across analysis methods. As neurodevelopmental origins of early- and late-onset disease are increasingly recognized, resolving inconsistencies in brain maturation trajectories is important.
Keywords: childhood, cortical thickness, maturation, MRI, neurodevelopment
Introduction
In this feature article, we wish to address what we perceive as basic inconsistencies in current studies of human brain development. Through more than 2 decades, there has been a substantial increase in studies characterizing human neurodevelopmental trajectories, both in health and disease ( Giedd and Rapoport 2010 ; Raznahan, Lerch, et al. 2011 ). This most welcome ( Lee et al. 2014 ) research focus has been sparked by multiple factors. One is the advent and widespread use of magnetic resonance imaging (MRI), allowing noninvasive, safe characterization of brain macro- and microstructure and function in vivo. Another is the recognition that in order to understand deviant brain development, we must know how development normally proceeds, and so studies of large groups of healthy children and adolescents have been initiated alongside studies of neurodevelopmental disorders such as attention deficit hyperactivity disorder (ADHD) and schizophrenia ( Giedd and Rapoport 2010 ). The latter is clearly exemplified in the strategy of the Child Psychiatry Branch of the National Institute of Mental Health in the USA, where such studies have been ongoing since 1989 ( Giedd et al. 2015 ). By now, the neurodevelopmental origins of both early- and late-onset diseases are increasingly being recognized, and hence, resolving inconsistencies in reported brain maturation trajectories should be of utmost importance.
The human cerebral cortex is arguably the crown of development, phylogenetically and ontogenetically, and essential to higher-order cognitive function. Not surprisingly then, the quest to identify the fundaments of disordered and healthy human function in MRI studies has focused on cortical development. Human cortical development is protracted and heterogeneous, not only across regions, but also with respect to the characteristics that determine gray matter (GM) volume—namely cortical surface area and cortical thickness. These characteristics differ widely across development ( Amlien et al. 2014 ) and adult age ( Hogstrom et al. 2013 ), as they are shaped by independent genes ( Rakic 1988 ; Panizzon et al. 2009 ) and neurobiological events ( Rakic 2009 ). Although both surface area and thickness have been studied with increasing frequency, cortical thickness has thus far received the most attention in developmental studies, going back >10 years. However, and in our opinion largely unrecognized, current results regarding maturational trajectories of cortical thickness in particular are inconsistent. In the following, we review the discrepant views of human brain development that have emerged, focusing on the maturational trajectory of cortical thickness. We then proceed to discuss possible reasons for the divergent results across studies, and conclude with suggestions for how we might come to a consensus in the field about how cortical thickness and other related brain characteristics develop.
The Early-Emerging View of Cortical Thickness Development: Increases Peaking in Early School Age
Early human brain imaging studies reporting measures of cortical thickness appeared to generally agree in showing marked developmental “increases” in thickness within large areas through the preschool and early school-age years, with the age of peak thickness depending on the brain region ( Sowell et al. 2003 , 2004 ; Shaw, Greenstein, et al. 2006 , Shaw et al. 2007 , 2008 ; Raznahan, Shaw, et al. 2011 ). An overview of some of these studies is provided in Table 1 . Besides the rates of regionally varying thickness change, attention also has been paid, and significance attached to, the age at which cortical thickness “peaks.” Differences in developmental trajectories of cortical thickness have been mapped and associated with a wide variety of functional outcomes, spanning from general intellectual development ( Shaw, Greenstein, et al. 2006 ) and normal behavioral problems ( Shaw et al. 2011 ) to ADHD ( Shaw et al. 2007 ) and childhood schizophrenia ( Alexander-Bloch et al. 2014 ). For instance, the median age by which half of the cortical points attained peak thickness was observed to be 7.5 years for healthy children, while it appeared delayed by about 3 years for children with ADHD ( Shaw et al. 2007 ), and nonlinear alterations in cortical development have been found in children with schizophrenia but not normally developing children ( Alexander-Bloch et al. 2014 ).
Table 1.
Selection of studies showing increases and peaks in cortical thickness during school age
| N /scans | Age range (years) | Cross versus long | Processing | Measure unit | Central finding | Google acholar citations | |
|---|---|---|---|---|---|---|---|
| Shaw et al. (2008) , J Neurosci. | 375/764 | 3–33 | Long | CIVET | Vertex | Age at thickness peak is important; table of mean peak given for 56 ROIs | 658 |
| Raznahan, Lerch, et al. (2011 ), Neuron. | 108/376 | 9–22 | Long | CIVET | Vertex | Rates of cortical thickness change differ by region | 68 |
| Raznahan, Shaw, et al. (2011 ), J Neurosci. | 647/1274 | 3–30 | Long | CIVET | Vertex and total | Sex differences in cortical thickness | 128 |
| Shaw, Greenstein et al. (2006) , Nature. | 307/307 | 7–19 | Long | CIVET | Vertex | Increasing relation between thickness and IQ | 878 |
| Sowell et al. (2004) , J Neurosci. | 45/45 | 5–11 | Long | LONI | Vertex | Thickening in bilateral peri-Sylvian, related to vocabulary change | 833 |
| Sowell et al. (2003) a , Nat Neurosci. | 176/176 | 7–87 | Cross | LONI | Vertex | Posterior temporal cortex most protracted maturation | 1316 |
| Shaw et al. (2007) , Proc Natl Acad Sci USA | 223 ADHD, 223 CTRL/824 scans | 5–20+ | Both | CIVET | Vertex | Peak of cortical thickness differs by group | 716 |
| Sowell et al. (2007 ), Cereb Cortex | 176/176 | 7–87 | Cross | Thompson et al. (2004) , LONI | Vertex | Thickness differs by sex | 322 |
Note: Samples across publications are in some cases overlapping. Google scholar citations retrieved April 2015.
CIVET: http://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET . MacDonald et al. (2000 ) often used as a primary reference for thickness studies.
LONI, Laboratory for Neuroimaging, UCLA; ADHD, Attention Deficit Hyperactivity Disorder; ROI, regions of interest.
a GM density is measured, and said to “represent cortical thickness.”
From the results described above, it appeared that individual differences in cortical thickness maturation held promise as a potential biomarker for risk of severe neurodevelopmental disorders. It is problematic then that the general developmental trajectory—including the existence of any increase or peak in cortical thickness after infancy—is by now not agreed upon across studies. Numerous recent and large-scale studies have now failed to identify such peaks in any cortical region and rather have observed near-uniform monotonic thinning of the entire cortex extending from preschool through school age and adolescence into the young adult years.
The Later-Emerging View of Cortical Thickness Development: Monotonic Thinning From Early Childhood
Since about 2012, a growing set of studies have reported monotonic thinning of the cortex across an age range extending from the preschool years and into adolescence. In 2012, the first results from the large USA-based multicenter Pediatric Imaging, Neurocognition, and Genetics (PING) study ( pingstudy.ucsd.edu ), spanning 10 different sites across the country, surfaced showing continuously thinner cortex with higher age ( Brown and Jernigan 2012 ; Brown et al. 2012 ; Jernigan et al. 2015 ). This study scanned children from age 3 years, which would make it sensitive to the early increases previously observed around middle to late school age. Another cross-sectional single-site study came to the same conclusion based on scans from children 4 years and older ( Amlien et al. 2014 ). In these studies, both vertex-wise and region of interest (ROI) analyses were used without finding any evidence of initial thickness increases. Several longitudinal studies have now come to the same conclusion. Many of these were ROI-based, but often covering the entire cortex, making it unlikely they would have failed to detect regional increases in cortical thickness. These studies sampled children from as low as age 3 ( Zielinski et al. 2014 ) or 4 ( Nguyen et al. 2013 ) years and up to 6 ( Wendelken et al. 2011 ; Mutlu et al. 2013 ) or 7 years ( Mills et al. 2014 ; Wierenga et al. 2014 ), which should be sufficient to detect early peaks in cortical thickness. Sample sizes have been more than adequate, relying on from just above 200 scans ( Shaw, Lerch, et al. 2006 ; Gogtay et al. 2007 ; van Soelen et al. 2012 ; Mutlu et al. 2013 ; Wierenga et al. 2014 ) to beyond 850 ( Mills et al. 2014 ). An overview of select studies finding monotonic decreases in cortical thickness is provided in Table 2 .
Table 2.
Selection of recent studies showing monotonic reduction of cortical thickness
| N /scans | Age range (years) | Cross versus long | Processing | Measure unit | Central finding | Google scholar citations | |
|---|---|---|---|---|---|---|---|
| Amlien et al. (2014) , Cereb Cortex. | 331/331 | 4–30 | Cross | FS | Vertex, ROI | Continuous decrease entire cortex | 0 |
| Brown and Jernigan (2012) , Curr Biol. | 885/885 | 3–20 | Cross | FS | Vertex | Continuous decrease entire cortex | 32 |
| Brown et al. (2012) , Neuropsychol Rev. | 202/202 | 4–20 | Cross | FS | ROI | Continuous decrease total cortex | 63 |
| Mills et al. (2014) , Soc Cogn Affect Neurosci. | 288/857 | 7–30 | Long | FS | ROI | Linear decrease in 3 of 4 ROIs, increased thickness of the temporal pole until ≈20 years | 35 |
| Mutlu et al. (2013) , Neuroimage. | 137/209 | 6–30 | Long | FS | Vertex, ROI | Vertex-wise effects divided into linear, quadratic, cubic, and none show trajectories for 3 ROIs, all continuously decreasing | 18 |
| Zielinski et al. (2014) a , Brain. | 157/345 | 3–39 | Long | FS | ROI | Continuous decrease entire cortex | 16 |
| Nguyen et al. (2013) , Cereb Cortex. | 281/281 | 4–22 | Cross | CIVET | Vertex | Linear decrease entire cortex | 12 |
| Wierenga et al. (2014) , NeuroImage. | 135/201 | 7–23 | Long | FS | ROI | Linear decrease most ROIs, few quadratic, or cubic, mostly U-shaped | 16 |
| Wendelken et al. (2011) , J Neurosci. | 85/85 | 6–18 | Cross | FS | ROI | Linear decrease | 26 |
| van Soelen et al. (2012) , NeuroImage. | 190/315 | 9 and 12 | Long | CLASP | Vertex | No areas of thickening | 42 |
| Shaw, Lerch et al. (2006) , Arch Gen Psychiatr. | 163 ADHD, 166 CTRL | Mean 8.9 at entry | Long | CIVET | Vertex | Different (through all declining) slopes of thickness | 404 |
| Gogtay et al. (2007) , Arch Gen Psychiatr. | 52/113 | 8–28 | Long | CIVET | Vertex | “Cortical deficits” relate to GAS scores; siblings catch up at 20 years | 113 |
Note: Samples across publications are in some cases overlapping. Google scholar citations retrieved April 2015.
FS, FreeSurfer; ROI, regions of interest; GAS, Global Assessment Scale.
a Ninety-seven autistic and 60 normal males, both groups showed thinning.
In summary, these generally more recent reports are difficult to reconcile with the earlier, arguably more influential, view of cortical thickening and peaking during school age. But, the number and character of these more recent studies make them impossible to dismiss. They are from independent laboratories with independent samples, from different countries in Europe and the USA, some single-scanner and single-center studies, some multiscanner, multisite studies, using both cross-sectional and longitudinal designs, with samples spanning from 3 years of age to well into adulthood, using both ROI and anatomically unconstrained vertex-wise approaches. Complementary to these findings, recent infant studies have suggested that cortical development after the first 2 years of life is mainly caused by the expansion of surface area ( Li et al. 2013 ). By age 2, cortical thickness has been reported to be on average 97% of young adult values, compared with a total surface area of only 69% of expected young adult size ( Lyall et al. 2015 ).
We find it pivotal that the discrepancies in reported cortical thickness maturation are addressed and that steps are taken to reach consensus on general developmental trends, given the enormous scientific interest and the clinical and cognitive significance attached to cortical maturation.
Possible Reasons for Discrepant Findings Across Studies
There are multiple possible explanations for the inconsistent findings, including (1) sample heterogeneity and cohort effects, (2) curve fitting methods, and (3) methodological, conceptual, and terminological differences in measuring cortical thickness from MRI images. These studies do to a large extent sample similar and overlapping age ranges, but effects of other between-samples differences in demographic factors cannot with certainty be ruled out. The use of the popular higher-order polynomial fits has the potential for imposing peaks where there may be none ( Fjell et al. 2010 ). Also, since some studies do not show individual data points, it is difficult to get an impression of the appropriateness of the fits. Still, this is at least unlikely to be the sole reason for the discrepancies reported, since several studies finding both early increases and several studies reporting monotonic decreases have employed other fit models. However, the choice of fit model is worthy of consideration in future studies. Hence, we believe that methodological differences in measuring cortical thickness, which have themselves evolved over time, are a most likely and parsimonious explanation for the inconsistencies that have been observed. Over the last 3 decades, there have been vast differences in MRI methodology used in human developmental studies, pertaining to multiple levels, including both acquisition and analyses. For instance, it was recently found that motion has a negative effect on cortical thickness estimates even after removal of low-quality scans by regular manual inspection ( Reuter et al. 2015 ). Since movement is expected to correlate negatively with age during childhood development, this can potentially reduce the slope of the age trajectories of thickness. If movement due to unknown factors was higher in some parts of the age range than others, this could, in principle, also cause seemingly nonlinear developmental trajectories. Perhaps, even greater differences can be produced by the specific processing and analysis steps involved in the measurement of cortical thickness from MR images.
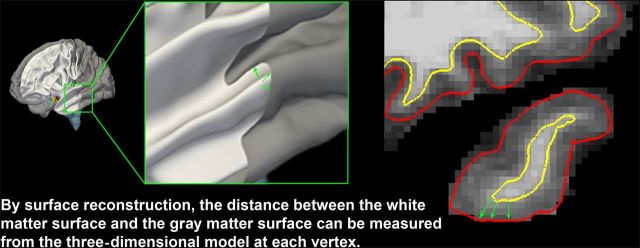
A decade ago and earlier, the term “cortical thickness” appeared relatively rarely in published MRI studies. There was a predominance of processing techniques measuring GM “density” or volume, including the cortex, which grew out of processing pipelines based on voxel-based morphometry (VBM) methods, such as within SPM ( Ashburner and Friston 2000 ) and Structural Image Evaluation, using Normalization, of Atrophy (SIENA) in FSL ( Smith et al. 2001 ). GM density refers to the probability that a given voxel consists of GM, and is often interpreted as GM volume within the VBM terminology. In contrast, processing streams using reconstructions of the inner and outer cortical surfaces recently have become more widely used. Examples of such analysis tools include Freesurfer ( Dale and Sereno 1993 ; Dale et al. 1999 ; Fischl and Dale 2000 ) and CIVET ( www.bic.mni.mcgill.ca/ServicesSoftware/CIVET ). Importantly, the measurement of cortical thickness requires that the analysis is performed at the nodes of a three-dimensional (3D) polygonal mesh rather than on a 3D voxel grid ( Lerch and Evans 2005 ), capturing the distance between the white matter (WM) surface and the intersection between the outer cortical boundary and the cerebrospinal fluid (CSF) (Fig. 1 ), according to a geometric definition which can vary across methods. It follows that the way cortical thickness is quantified is of importance.
Figure 1.
Estimating cortical thickness from MRI. Accurate measurement of cortical thickness requires three-dimensional reconstructions of the WM and the GM surfaces based on MR images.
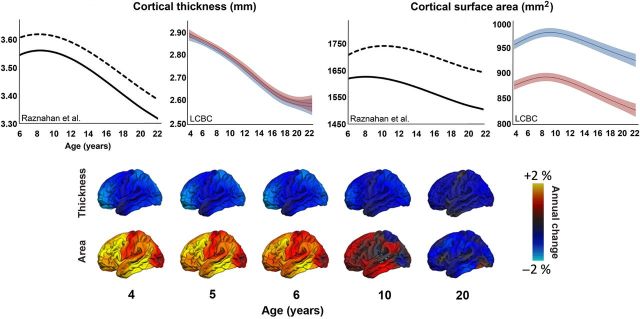
A major issue in this regard is the need to consistently distinguish among cortical thickness, cortical surface area, and cortical volume. As mentioned, the 2 former entities seem to show consistently different relationships to age ( Hogstrom et al. 2013 ) and are shaped by independent genes ( Rakic 1988 ; Panizzon et al. 2009 ) and neurobiological events ( Rakic 2009 ). Importantly, while studies disagree on the trajectories of cortical thickness, they seem to converge on developmental increases in cortical surface area. A striking, but nevertheless typical, example is illustrated in Figure 2 . Here, we see that in one large study of 647 healthy participants from 3 to 30 years, with >1250 longitudinally acquired brain scans, increases in cortical thickness are observed until about 8.5 years ( Raznahan, Shaw, et al. 2011 ). The trajectory for cortical thickness and surface area both followed the same basic cubic shape, although the peak for thickness was attained somewhat earlier than for area. In comparison, in a sample of a similar size ( n > 700, scans in total >1125, age range 4.1–30 years) from the Research group for Lifespan Changes in Brain and Cognition (LCBC), University of Oslo, only monotonic decreases in cortical thickness are evident. In contrast, the area trajectories resemble those seen in Raznahan, Shaw, et al. (2011 ), showing increases well into school age.
Figure 2.
Developmental trajectories of cortical thickness and area. Upper panel: Comparison of the developmental trajectories reported in Raznahan, Shaw, et al. (2011) to some of our own data (LCBC – Research group for Lifespan Changes in Brain and Cognition, University of Oslo) illustrates the discrepancies across studies. While surface area show similar inverse U-shaped trajectories, with larger absolute area for boys (dotted and blue lines) than for girls (solid and red lines), thickness results differ markedly between studies. In Raznahan, Shaw, et al. (2011 ), thickness increases until approximately 8.5 years, and boys have thicker cortex than girls throughout the age range. In the LCBC data, thickness shows a monotonic decrease from 4 years, with comparable absolute thickness estimates for boys and girls. Of note, thickness and area are more different in terms of trajectory and sex effects in the LCBC data than in Raznahan et al. Error bars for the LCBC curves represent 95% confidence interval. Lower panel: Vertex-wise annualized rates of change in cortical thickness and surface area computed in 778 subjects aged 3–20 years from the PING study. As can be seen, cortical thickness decreases monotonically within this age range, while area shows an inverted U-shaped pattern of increase followed by a decrease.
These discrepancies are puzzling. Both studies are done on 1.5-T MRI, and neither is performed at multiple sites using different scanner types. Although different scanners and T1 -weighted sequences were used, we believe that it is not likely that this caused the divergent results, as studies comparing results across scanners have not indicated biases of such a magnitude ( Fennema-Notestine et al. 2007 ; Dickerson et al. 2008 ), and current large-scale multicenter studies of both development (PING) and Alzheimer's disease (Alzheimer Disease Neuroimaging Initiative, ADNI) are based on different scanners and vendors. Different statistical models were used to fit the data, with Raznahan, Shaw, et al. (2011 ) using quadratic age terms to model the trajectories of thickness and area, while generalized additive mixed models with a nonparametric spline function were used to model the trajectories in the LCBC study. However, testing a quadratic model in the LCBC data did not reveal any evidence of thickening, so the different model fits cannot account for the differences seen. However, an indication of differences in how cortical thickness is measured comes from comparing the thickness and area trajectories, and the absolute differences between the females and males. In Raznahan et al., thickness and surface area trajectories take the same inverted U-shaped form, and males have both thicker cortex and larger surface area than females. Sex differences in thickness were found in an earlier study that also reported thickness increases, but larger for females ( Sowell et al. 2007 ). In the LCBC data, thickness and surface area follow very different trajectories, and absolute sex differences are found for area only. This is also seen in the large PING sample from the USA (Fig. 2 ), where the same approach to thickness estimation is used (FreeSurfer, FS). Thus, it seems reasonable to assume that a key to the differences observed in thickness trajectories between studies relates to how thickness and area are disentangled in the processing of MRI scans, with these 2 entities being much more different in some studies than in others. Inspection of Tables 1 and 2 shows that many, but not all, studies that found monotonic decreases in thickness have used FreeSurfer, and also that none of the studies that found increases in thickness used the FreeSurfer method.
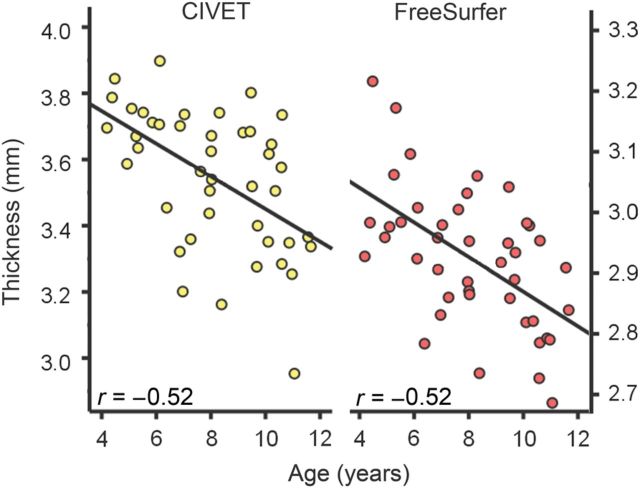
To investigate whether different software packages yielded different trajectories for cortical thickness with age, we analyzed MRI data from the same 43 children in the age range 3–11 years from the PING study with FreeSurfer and CIVET. The raw scans were run separately through FreeSurfer and CIVET, with no common preprocessing steps performed in advance. The results are shown in Figure 3 . For neither method did the trajectories estimated show any sign of cortical thickness increase with age. While there were differences in absolute cortical thickness estimates across these analysis methods, the correlations between age and cortical thickness were virtually identical (CIVET: r = −0.52 and FS: r = −0.56, both P < 0.0005). This suggests that at least with these recent versions of the software packages (FreeSurfer 4.5 and CIVET as of May 2012), the processing of MRI data per se would not be the causal factor causing discrepant age trajectories for cortical thickness development. It should be noted that these analyses are preliminary, and that a more thorough test would include more participants and comparisons between different versions of the same software.
Figure 3.
Cortical thickness averaged across hemispheres ( y -axis) as estimated by CIVET (left panel) and FreeSurfer (right panel) plotted against age ( x -axis) for 43 children (23 females) in the PING study. While absolute thickness estimates differed by about 0.6 mm, the shape and betas of the age functions were similar across image analysis methods (CIVET r = −0.52 and FreeSurfer = −0.56), both showing monotonically thinner cortex with age.
Possible Paths to a Consensus and a Cautionary Tale
With the different cortical thickness maturation trajectories reported, an obvious question may be “Which is the correct one?” However, we do not think that it is possible at the current time to give a definitive answer to this question. As with all in vivo imaging methods, our studies provide merely representations of the underlying neurobiology and inherently require some level of interpretation. It is important to be aware of the fact that the MRI-derived measures are merely our best current approximations, where reconstructions of the cortex are based on signal intensities and contrast properties that are prone to the influence of multiple factors. Hence, in principle, the term “apparent cortical thickness” should be used, much like one in diffusion-weighted imaging speaks of “apparent diffusion coefficient.” And with regard to cortical thickness, available histological data cannot with certainty inform us which MRI studies most accurately capture the true developmental trajectories. One postmortem study found that cortical thickness did not correlate with brain size ( Pakkenberg and Gundersen 1997 ), indicating that these metrics, in principle, could follow divergent developmental paths. However, accurately measuring cortical thickness in postmortem samples is very difficult without 3D reconstructions of the entire surfaces, as the measurements inherently depend on the orientation of the brain and the slicing direction. Thus, there is a need for further combined histological and MRI studies to better establish the relationships between available imaging-derived measures and the underlying neurobiology.
Histological studies, though scarce and not covering regional developmental differences in cortical thickness specifically, point to some general age differences in brain and cortical development ( Dekaban 1978 ; Huttenlocher 1979 , 1984 , 1990 ; Huttenlocher et al. 1982 ; Huttenlocher and de Courten 1987 ; Huttenlocher and Dabholkar 1997 ). The underlying mechanisms of cortical thickness differences are complex and believed to involve, for growth, proliferation of dendrites, dendritic spines, axonal sprouting, vascular elaboration, and for thinning, possibly synaptic pruning as well as intracortical myelination ( Huttenlocher 1979 ; Huttenlocher et al. 1982 ; Huttenlocher and Dabholkar 1997 ). Furthermore, the neocortex is essential to much of complex cognitive function, and many developmental milestones appear to be correspondingly ordered across individuals ( Sheldrick and Perrin 2013 ). On this background, the major differences that have been reported in the shape of brain developmental trajectories across healthy human children appear less likely.
There is evidence of a burst in synaptogenesis in the human visual cortex (area 17) around 4 months, with synaptic density reached at about 8 months and beginning to decline after age 1 year to an adult value of about 60% of maximum, which is reached by age 11 years ( Huttenlocher et al. 1982 ; Huttenlocher and de Courten 1987 ; Huttenlocher 1990 ). The available data indicate that there is a slower postnatal increase in synaptic density in the frontal cortex, where maximum density in layer III is reached at about 1 year, with subsequent declines, but slower than in the visual cortex, becoming evident around 7 years of age and reaching adult level by 16 years of age ( Huttenlocher 1979 ). While these data may not yield definitive evidence on differential regional timing of cortical maturation ( Goldman-Rakic 1987 , 1997 ), they are often cited to yield a cellular foundation for both the identified cortical thickness peaks in childhood and the continuous thinning in MRI studies. However, this link has never been established beyond a rather vague correlation; it does not appear that these synaptic density studies can be used to deem which is the more likely to reflect the true cortical thickness changes as observed longitudinally. What these synaptic density studies do support, to some extent, is a posterior-to-anterior and/or sensory-to-association gradient of maturation, but that is in fact a point on which imaging studies identifying peaks and continuous thinning largely agree.
Unfortunately, as pointed out by Huttenlocher (1990 ), the histological quantitative anatomy approach to cortical development has clear limitations too. One is the static nature of the measures, providing only a glimpse of one point in time for a few cases, which may even be affected by illness, injury, or postmortem changes. Development is dynamic, and growth and regressive changes may occur at the same time and balance estimated changes in histological studies ( Huttenlocher 1990 ), just like they may do in imaging studies. While neuronal count appears to change little in the cortex from end of gestation to late adult life, not enough is known about how estimates of synaptic density, dendritic length, and so forth are affected by, for example, cortical areal expansion in development in restricted histological samples. In the representations of the cortex created based on MR images, for example, intracortical myelination and regressive changes may affect the signal intensities, contrast, and quantitative measures derived in different ways. For these reasons, we do not believe that it is yet possible to say with confidence that either representation of the cortical thickness, from any study, is “the correct one.” Rather, we would suggest some steps to reach a greater consensus, especially along 2 lines.
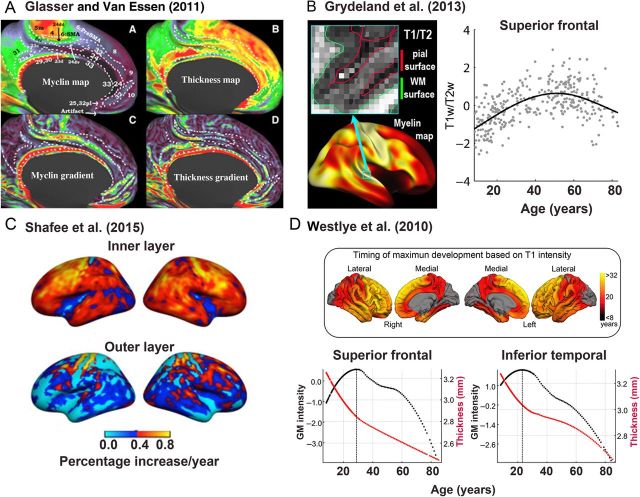
First, while morphometric measurements usually are derived from T1 -weighted images, a variety of different MRI modalities can be used to obtain relevant information about cortical maturation (Fig. 4 ). For instance, the definition of WM and GM boundaries can be improved by inclusion of T2 -weighted images, and the use of T1 / T2 ratio ( Glasser and Van Essen 2011 ; Fjell et al. 2013 ; Glasser et al. 2014 ; Grydeland et al. 2015 ; Shafee et al. 2015 ). The T1 / T2 ratio method has been shown to improve localization by increasing the contrast to noise ratio between heavily and lightly myelinated areas, and by canceling the MR-related intensity bias field ( Glasser and Van Essen 2011 ). This could potentially be used to improve the definition of the GM/WM boundary, which is critical for accurate estimation of cortical thickness, and also provide additional information about neurobiological events ongoing within the cerebral cortex and in adjacent WM during development. Examples of detailed apparent myelin and thickness maps obtained by using the T1 / T2 ratio are shown in Figure 4A . In Figure 4B , delineation of the GM/WM border from the superior temporal gyrus in a T1 / T2 contrast image is shown, along with the age trajectory obtained by sampling the ratio values from within the cortex, interpreted as reflecting intracortical myelin content. This inverted U-shape, with a peak in the late middle-age, differs from the typical declining thickness curve.
Figure 4.
Multimodal imaging as a path to increased knowledge about cortical development. We argue that utilization of additional imaging modalities, other than T1 -weighted scans, and additional imaging parameters, other than morphometric, will increase our understanding of cortical development and help to solve the deep discrepancies in the literature. ( A ) Glasser and Van Essen (2011 ) have convincingly shown that additional information can be obtained by taking advantage of both T1 -weighted and T2 -weighted MRI scans, likely related to myelin. This can be used to measure apparent intracortical myelin content, which can be studied in relationship to thickness changes in development. Clockwise from top left are maps of apparent cortical myelin content, thickness, thickness gradients, and myelin gradients. ( B ) Grydeland et al. ( Fjell et al. 2013 ) used the methods proposed by Glasser and Van Essen to track apparent myelin content from childhood and into old age, showing prolonged increases before age-related decline was seen in the last part of the lifespan. T1 / T2 contrast from a section of the superior temporal gyrus is shown, with the delineation of the GM/WM boundary (green line) and the GM/CSF boundary (red line) imposed on the image. The scatterplot shows the trajectory of the T1 / T2 ratio from the superior frontal cortex across 8 to 80+ years. ( C ) Shafee et al. (2015 ) found that estimated changes in myelin content, based on T1 / T2 ratio measures, from 18 to 35 years, were primarily driven by effects in the inner cortical layers, while almost no developmental effects were seen in the outer cortical layers. The upper brain surfaces are mostly red or yellow, indicating a 0.5% increase in apparent myelin content annually, whereas the changes in the outer layer appear mostly blue or cyan, indicating much lower estimated changes. These observations are relevant to the discussion of what drives the apparent cortical thinning in development seen in reconstructed MR images. ( D ) Taking advantage of the variation in signal intensities that can be found in T1 -weighted MR images, Westlye et al. (2010 ) found that while cortical thickness was negatively related to development, cortical intensity increased. The ages at peak intensity are color-coded and projected onto a brain surface in the upper part of ( D ). The lower part shows the age trajectories for T1 -signal intensity (black line) and thickness (red line) from 2 different cortical regions. In several regions were T1 -intensity increases observed until the age at which the apparent thickness reductions seem to proceed at a slower pace, again pointing to a potentially interesting interplay between cortical thinning and other neurobiological events that can be quantified in vivo by MRI.
Other signal intensity measures from the T1 -weighted images themselves can provide additional important information that is still not commonly used in developmental studies ( Salat et al. 2009 ; Westlye et al. 2009 , 2010 ; Fjell et al. 2013 ). For instance, Westlye et al. (2010 ) observed that even though thickness decreased monotonically from childhood, T1 signal intensity, normalized to ventricular intensity, both in GM and WM increased until almost 30 years, before age-related reductions were evident. This can be seen in Figure 4D . It was further suggested that the overlap in age between the peak of the T1 -signal intensity curve and the inflection point in the thickness curve could represent a “maturational milestone” in cortical development. In this way, combining thickness measures with the intensity measure yielded a more complete picture of cortical development both with regard to trajectories and with regard to dynamic regional variability. Shafee et al. (2015 ) used the T1 / T2 ratio to estimate intracortical myelin content, and found that this metric increased from 18 to 35 years, with mainly the inner layer of the cortex accounting for this effect. This is illustrated in Figure 4C , where the estimated annual increase exceeds 0.5% in most inner layer regions of the cortex, while changes in the outer layer are much lower. Both of these studies found a relationship between cortical thickness and the alternative measures, demonstrating the power of these multimodal approaches to enlighten us about the potential mechanisms for cortical thickness changes both in development and in adulthood. Also methods from other fields, such as the newly proposed method CLARITY ( Chung et al. 2013 ), may aid in providing a better understanding of the neurobiological underpinnings of MRI-derived measures.
Second, a greater degree of data sharing and development and shared use of publicly available databases would most certainly contribute massively to reconciling differences due to the effects of various processing and analysis methods. One very successful example of this has been the ADNI ( adni-info.org ), an open-access data repository with thousands of scans available for researchers in the area of aging and dementia. A similar initiative, but within the childhood age range, is the described PING study, which has produced a publicly available repository of imaging, cognitive behavioral, and genomic data, which includes raw and processed images ( Jernigan et al. 2016 ). When data and processing methods are made openly available to other laboratories for scrutiny and independent analysis, any inconsistencies that arise in the empirical observations or interpretations can be more accurately attributed to specific procedures or methods and can be more definitively reconciled.
Conclusion
The aim of this feature article was to address a critical inconsistency in current structural neuroimaging studies of human brain development—an early view that cortical thickness in some regions increases well into school age, and a more recent, different, view that monotonic thinning occurs across the cortex from an early age, at least from 3 or 4 years of age or earlier. The ongoing narrative about cortical thickness development represents a major sticking point for our understanding of both normal and aberrant cortical development. Although at this time there appears to be no final verdict about the likely causes of the discrepant findings, available information suggests that the differences may lie largely within the imaging methods that have previously been used for measuring cortical thickness from MR images. Going forward, we have suggested 2 strategies, among arguably many, that we believe can advance the limits of our knowledge on cortical development further. First, by integrating multimodal neuroimaging signals to focus on the same and related phenomenon from different angles, we can attain insights into cortical development, which hopefully will lead to reconciliation of the diverging views. Second, by promoting data sharing and encouraging independent groups to work on the same datasets, we believe consensus or at least a deeper understanding of the causes of the divergent results that will be strongly facilitated. Our hope in bringing attention to this discrepancy is to promote acknowledgment and discussion within the field, and hopefully to contribute to its resolution. In focusing on this particular issue within the study of human brain development, we are reminded of the inherent limitations of our brain imaging methods for getting at the underlying neurobiology and would do well to conduct more research that promotes a deeper understanding of their relationship.
Funding
This work was supported by the Department of Psychology, University of Oslo (to K.B.W. and A.M.F.), the Norwegian Research Council (to K.B.W. and A.M.F.), the European Research Council's Starting Grant scheme (ERC grant agreement 313440 to K.B.W. and 283634 to A.M.F.), and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to T.T.B.; R24 HD075489) and the National Institute on Drug Abuse (to T.T.B.; R01 DA038958).
Notes
Conflict of Interest : A.M.D. has ownership interest (including patents) in CorTech Laboratories, Inc.
References
- Alexander-Bloch AF , Reiss PT , Rapoport J , McAdams H , Giedd JN , Bullmore ET , Gogtay N . 2014. . Abnormal cortical growth in schizophrenia targets normative modules of synchronized development . Biol Psychiatry . 76 : 438 – 446 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlien IK , Fjell AM , Tamnes CK , Grydeland H , Krogsrud SK , Chaplin TA , Rosa MG , Walhovd KB . 2014. . Organizing principles of human cortical development-thickness and area from 4 to 30 years: insights from comparative primate neuroanatomy . Cereb Cortex . . [Epub ahead of print] 10.1093/cercor/bhu214 . [DOI] [PubMed] [Google Scholar]
- Ashburner J , Friston KJ . 2000. . Voxel-based morphometry—the methods . Neuroimage . 11 : 805 – 821 . [DOI] [PubMed] [Google Scholar]
- Brown TT , Jernigan TL . 2012. . Brain development during the preschool years . Neuropsychol Rev . 22 : 313 – 333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT , Kuperman JM , Chung Y , Erhart M , McCabe C , Hagler DJ Jr , Venkatraman VK , Akshoomoff N , Amaral DG , Bloss CS et al. . 2012. . Neuroanatomical assessment of biological maturity . Curr Biol . 22 : 1693 – 1698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K , Wallace J , Kim SY , Kalyanasundaram S , Andalman AS , Davidson TJ , Mirzabekov JJ , Zalocusky KA , Mattis J , Denisin AK et al. . 2013. . Structural and molecular interrogation of intact biological systems . Nature . 497 : 332 – 337 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM , Fischl B , Sereno MI . 1999. . Cortical surface-based analysis. I. Segmentation and surface reconstruction . Neuroimage . 9 : 179 – 194 . [DOI] [PubMed] [Google Scholar]
- Dale AM , Sereno MI . 1993. . Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach . J Cogn Neurosci . 5 : 162 – 176 . [DOI] [PubMed] [Google Scholar]
- Dekaban AS . 1978. . Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights . Ann Neurol . 4 : 345 – 356 . [DOI] [PubMed] [Google Scholar]
- Dickerson BC , Fenstermacher E , Salat DH , Wolk DA , Maguire RP , Desikan R , Pacheco J , Quinn BT , Van der Kouwe A , Greve DN et al. . 2008. . Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths . Neuroimage . 39 : 10 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C , Gamst AC , Quinn BT , Pacheco J , Jernigan TL , Thal L , Buckner R , Killiany R , Blacker D , Dale AM et al. . 2007. . Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data . Neuroinformatics . 5 : 235 – 245 . [DOI] [PubMed] [Google Scholar]
- Fischl B , Dale AM . 2000. . Measuring the thickness of the human cerebral cortex from magnetic resonance images . Proc Natl Acad Sci USA . 97 : 11050 – 11055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM , Walhovd KB , Westlye LT , Ostby Y , Tamnes CK , Jernigan TL , Gamst A , Dale AM . 2010. . When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies . Neuroimage . 50 : 1376 – 1383 . [DOI] [PubMed] [Google Scholar]
- Fjell AM , Westlye LT , Grydeland H , Amlien I , Espeseth T , Reinvang I , Raz N , Holland D , Dale AM , Walhovd KB , Alzheimer Disease Neuroimaging Initiative . 2013. . Critical ages in the life course of the adult brain: nonlinear subcortical aging . Neurobiol Aging . 34 : 2239 – 2247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN , Rapoport JL . 2010. . Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron . 67 : 728 – 734 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN , Raznahan A , Alexander-Bloch A , Schmitt E , Gogtay N , Rapoport JL . 2015. . Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development . Neuropsychopharmacology . 40 : 43 – 49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF , Goyal MS , Preuss TM , Raichle ME , Van Essen DC . 2014. . Trends and properties of human cerebral cortex: correlations with cortical myelin content . Neuroimage . 93(Pt 2) : 165 – 175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF , Van Essen DC . 2011. . Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI . J Neurosci . 31 : 11597 – 11616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N , Greenstein D , Lenane M , Clasen L , Sharp W , Gochman P , Butler P , Evans A , Rapoport J . 2007. . Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia . Arch Gen Psychiatry . 64 : 772 – 780 . [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS . 1987. . Development of cortical circuitry and cognitive function . Child Dev . 58 : 601 – 622 . [PubMed] [Google Scholar]
- Goldman-Rakic PS , Bourgeois J-P , Rakic P . 1997. . Synaptic substrate of cognitive development . In: Krasnegor NA , Lyon GR , Goldman-Rakic PS , editors. Development of the prefrontal cortex: evolution, neurobiology, and behavior . Baltimore: : Brookes Publishing Company; . [Google Scholar]
- Grydeland H , Westlye LT , Walhovd KB , Fjell AM . 2015. . Intracortical posterior cingulate myelin content relates to error processing: results from T1- and T2-weighted MRI myelin mapping and electrophysiology in healthy adults . Cereb Cortex . . [Epub ahead of print] 10.1093/cercor/bhv065 . [DOI] [PubMed] [Google Scholar]
- Hogstrom LJ , Westlye LT , Walhovd KB , Fjell AM . 2013. . The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification . Cereb Cortex . 23 : 2521 – 2530 . [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR . 1990. . Morphometric study of human cerebral cortex development . Neuropsychologia . 28 : 517 – 527 . [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR . 1979. . Synaptic density in human frontal cortex—developmental changes and effects of aging . Brain Res . 163 : 195 – 205 . [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR . 1984. . Synapse elimination and plasticity in developing human cerebral cortex . Am J Ment Def . 88 : 488 – 496 . [PubMed] [Google Scholar]
- Huttenlocher PR , Dabholkar AS . 1997. . Regional differences in synaptogenesis in human cerebral cortex . J Comp Neurol . 387 : 167 – 178 . [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR , de Courten C . 1987. . The development of synapses in striate cortex of man . Hum Neurobiol . 6 : 1 – 9 . [PubMed] [Google Scholar]
- Huttenlocher PR , De Courten C , Garey LJ , Van der Loos H . 1982. . Synaptic development in human cerebral cortex . Int J Neurol . 16–17 : 144 – 154 . [PubMed] [Google Scholar]
- Jernigan TL , Brown TT , Bartsch H , Dale AM . 2015. . Toward an integrative science of the developing human mind and brain: focus on the developing cortex . Dev Cogn Neurosci . pii: S1878-9293(15)30021-9. . [Epub ahead of print] 10.1016/j.dcn.2015.07.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL , Brown TT , Hagler DJ Jr , Akshoomoff N , Bartsch H , Newman E , Thompson WK , Bloss CS , Murray SS , Schork N et al. . 2016. . The Pediatric Imaging, Neurocognition, and Genetics (PING) data repository . Neuroimage . 124(Pt B) : 1149 – 1154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS , Heimer H , Giedd JN , Lein ES , Sestan N , Weinberger DR , Casey BJ . 2014. . Mental health. Adolescent mental health—opportunity and obligation . Science . 346 : 547 – 549 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP , Evans AC . 2005. . Cortical thickness analysis examined through power analysis and a population simulation . Neuroimage . 24 : 163 – 173 . [DOI] [PubMed] [Google Scholar]
- Li G , Nie J , Wang L , Shi F , Lin W , Gilmore JH , Shen D . 2013. . Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age . Cereb Cortex . 23 : 2724 – 2733 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall AE , Shi F , Geng X , Woolson S , Li G , Wang L , Hamer RM , Shen D , Gilmore JH . 2015. . Dynamic development of regional cortical thickness and surface area in early childhood . Cereb Cortex . 25 : 2204 – 2212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D , Kabani N , Avis D , Evans AC . 2000. . Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI . Neuroimage . 12 : 340 – 356 . [DOI] [PubMed] [Google Scholar]
- Mills KL , Lalonde F , Clasen LS , Giedd JN , Blakemore SJ . 2014. . Developmental changes in the structure of the social brain in late childhood and adolescence . Soc Cogn Affect Neurosci . 9 : 123 – 131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu AK , Schneider M , Debbane M , Badoud D , Eliez S , Schaer M . 2013. . Sex differences in thickness, and folding developments throughout the cortex . Neuroimage . 82 : 200 – 207 . [DOI] [PubMed] [Google Scholar]
- Nguyen TV , McCracken J , Ducharme S , Botteron KN , Mahabir M , Johnson W , Israel M , Evans AC , Karama S , Brain Development Cooperative Group . 2013. . Testosterone-related cortical maturation across childhood and adolescence . Cereb Cortex . 23 : 1424 – 1432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B , Gundersen HJ . 1997. . Neocortical neuron number in humans: effect of sex and age . J Comp Neurol . 384 : 312 – 320 . [PubMed] [Google Scholar]
- Panizzon MS , Fennema-Notestine C , Eyler LT , Jernigan TL , Prom-Wormley E , Neale M , Jacobson K , Lyons MJ , Grant MD , Franz CE et al. . 2009. . Distinct genetic influences on cortical surface area and cortical thickness . Cereb Cortex . 19 : 2728 – 2735 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P . 2009. . Evolution of the neocortex: a perspective from developmental biology . Nat Rev Neurosci . 10 : 724 – 735 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P . 1988. . Specification of cerebral cortical areas . Science . 241 : 170 – 176 . [DOI] [PubMed] [Google Scholar]
- Raznahan A , Lerch JP , Lee N , Greenstein D , Wallace GL , Stockman M , Clasen L , Shaw PW , Giedd JN . 2011. . Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling . Neuron . 72 : 873 – 884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A , Shaw P , Lalonde F , Stockman M , Wallace GL , Greenstein D , Clasen L , Gogtay N , Giedd JN . 2011. . How does your cortex grow? J Neurosci . 31 : 7174 – 7177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M , Tisdall MD , Qureshi A , Buckner RL , van der Kouwe AJ , Fischl B . 2015. . Head motion during MRI acquisition reduces gray matter volume and thickness estimates . Neuroimage . 107 : 107 – 115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH , Lee SY , van der Kouwe AJ , Greve DN , Fischl B , Rosas HD . 2009. . Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast . Neuroimage . 48 : 21 – 28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafee R , Buckner RL , Fischl B . 2015. . Gray matter myelination of 1555 human brains using partial volume corrected MRI images . Neuroimage . 105 : 473 – 485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P , Eckstrand K , Sharp W , Blumenthal J , Lerch JP , Greenstein D , Clasen L , Evans A , Giedd J , Rapoport JL . 2007. . Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation . Proc Natl Acad Sci USA . 104 : 19649 – 19654 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P , Gilliam M , Liverpool M , Weddle C , Malek M , Sharp W , Greenstein D , Evans A , Rapoport J , Giedd J . 2011. . Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder . Am J Psychiatry . 168 : 143 – 151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P , Greenstein D , Lerch J , Clasen L , Lenroot R , Gogtay N , Evans A , Rapoport J , Giedd J . 2006. . Intellectual ability and cortical development in children and adolescents . Nature . 440 : 676 – 679 . [DOI] [PubMed] [Google Scholar]
- Shaw P , Kabani NJ , Lerch JP , Eckstrand K , Lenroot R , Gogtay N , Greenstein D , Clasen L , Evans A , Rapoport JL et al. . 2008. . Neurodevelopmental trajectories of the human cerebral cortex . J Neurosci . 28 : 3586 – 3594 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P , Lerch J , Greenstein D , Sharp W , Clasen L , Evans A , Giedd J , Castellanos FX , Rapoport J . 2006. . Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder . Arch Gen Psychiatry . 63 : 540 – 549 . [DOI] [PubMed] [Google Scholar]
- Sheldrick RC , Perrin EC . 2013. . Evidence-based milestones for surveillance of cognitive, language, and motor development . Acad Pediatr . 13 : 577 – 586 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM , De Stefano N , Jenkinson M , Matthews PM . 2001. . Normalized accurate measurement of longitudinal brain change . J Comput Assist Tomogr . 25 : 466 – 475 . [DOI] [PubMed] [Google Scholar]
- Sowell ER , Peterson BS , Kan E , Woods RP , Yoshii J , Bansal R , Xu D , Zhu H , Thompson PM , Toga AW . 2007. . Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age . Cereb Cortex . 17 : 1550 – 1560 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER , Peterson BS , Thompson PM , Welcome SE , Henkenius AL , Toga AW . 2003. . Mapping cortical change across the human life span . Nat Neurosci . 6 : 309 – 315 . [DOI] [PubMed] [Google Scholar]
- Sowell ER , Thompson PM , Leonard CM , Welcome SE , Kan E , Toga AW . 2004. . Longitudinal mapping of cortical thickness and brain growth in normal children . J Neurosci . 24 : 8223 – 8231 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM , Hayashi KM , Sowell ER , Gogtay N , Giedd JN , Rapoport JL , de Zubicaray GI , Janke AL , Rose SE , Semple J et al. . 2004. . Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia . Neuroimage . 23(Suppl 1) : S2 – S18 . [DOI] [PubMed] [Google Scholar]
- van Soelen IL , Brouwer RM , van Baal GC , Schnack HG , Peper JS , Collins DL , Evans AC , Kahn RS , Boomsma DI , Hulshoff Pol HE . 2012. . Genetic influences on thinning of the cerebral cortex during development . Neuroimage . 59 : 3871 – 3880 . [DOI] [PubMed] [Google Scholar]
- Wendelken C , O'Hare ED , Whitaker KJ , Ferrer E , Bunge SA . 2011. . Increased functional selectivity over development in rostrolateral prefrontal cortex . J Neurosci . 31 : 17260 – 17268 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT , Walhovd KB , Dale AM , Bjornerud A , Due-Tonnessen P , Engvig A , Grydeland H , Tamnes CK , Ostby Y , Fjell AM . 2010. . Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity . Neuroimage . 52 : 172 – 185 . [DOI] [PubMed] [Google Scholar]
- Westlye LT , Walhovd KB , Dale AM , Espeseth T , Reinvang I , Raz N , Agartz I , Greve DN , Fischl B , Fjell AM . 2009. . Increased sensitivity to effects of normal aging and Alzheimer's disease on cortical thickness by adjustment for local variability in gray/white contrast: a multi-sample MRI study . Neuroimage . 47 : 1545 – 1557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM , Langen M , Oranje B , Durston S . 2014. . Unique developmental trajectories of cortical thickness and surface area . Neuroimage . 87 : 120 – 126 . [DOI] [PubMed] [Google Scholar]
- Zielinski BA , Prigge MB , Nielsen JA , Froehlich AL , Abildskov TJ , Anderson JS , Fletcher PT , Zygmunt KM , Travers BG , Lange N et al. . 2014. . Longitudinal changes in cortical thickness in autism and typical development . Brain . 137 : 1799 – 1812 . [DOI] [PMC free article] [PubMed] [Google Scholar]