Abstract
Head movements are typically viewed as a nuisance to functional magnetic resonance imaging (fMRI) analysis, and are particularly problematic for resting state fMRI. However, there is growing evidence that head motion is a behavioral trait with neural and genetic underpinnings. Using data from a large randomly ascertained extended pedigree sample of Mexican Americans (n = 689), we modeled the genetic structure of head motion during resting state fMRI and its relation to 48 other demographic and behavioral phenotypes. A replication analysis was performed using data from the Human Connectome Project, which uses an extended twin design (n = 864). In both samples, head motion was significantly heritable (h2 = 0.313 and 0.427, respectively), and phenotypically correlated with numerous traits. The most strongly replicated relationship was between head motion and body mass index, which showed evidence of shared genetic influences in both data sets. These results highlight the need to view head motion in fMRI as a complex neurobehavioral trait correlated with a number of other demographic and behavioral phenotypes. Given this, when examining individual differences in functional connectivity, the confounding of head motion with other traits of interest needs to be taken into consideration alongside the critical important of addressing head motion artifacts.
Keywords: BMI, head motion, MRI
Introduction
Head movements during functional magnetic resonance imaging (fMRI) can be detected in all subjects, even with head restraint. These movements shift the position of the brain in space, disrupting the spatial and temporal precision of blood oxygen level dependent signal acquisition (Jiang et al. 1995; Friston et al. 1996; Thacker et al. 1999; Oakes et al. 2005). While data from subjects who display large head motion are often removed from analyses, even small movements within the scanner can be problematic, particularly for functional connectivity studies (Power et al. 2012; Satterthwaite et al. 2012; Van Dijk et al. 2012). Measure of functional connectivity are often based on the covariance of signals between voxels over time; head motion can confound between voxels covariance patterns, depending on the type of motion and the position of each voxel. Power and colleagues demonstrated that head motion typically leads to increases in covariance when voxels are proximal to one another, but often reduces covariance for more distant voxels, creating spurious spatially structured patterns of artifactual signal in functional connectivity data (Power et al. 2012, 2014). Importantly, even after standard motion correction methods, these artifacts can be pervasive (Satterthwaite et al. 2013), biasing functional connectivity analyses (Power et al. 2012; Satterthwaite et al. 2012; Van Dijk et al. 2012).
Whereas motion during scanning is customarily conceived as a source of artifact, it can also be conceptualized as a fine-grained behavioral measurement, given that many sources of gross head movement reflect actions that are under voluntary motor control (such as swallowing or fidgeting). Indeed, there is increasing evidence that the degree of motion during MRI scanning is trait-like. Among healthy young adults, scanner movement is moderately stable over time, with intra-individual correlations of r = 0.53–0.66 among subjects who completed at least 2 scanning sessions (Van Dijk et al. 2012; Couvy-Duchesne et al. 2014; Zeng et al. 2014). In samples of children, head motion tends to decrease with age (Satterthwaite et al. 2012), potentially reflecting the development of cognitive control processes, as head motion has been shown to correlate with impulsivity measures in both child and adult samples (Kong et al. 2014). Zeng and colleagues (2014) found that individuals with reduced long-range connectivity observed during high motion scans also had lower than expected connectivity during low motion scans, suggesting that the level of observed functional connectivity was not entirely due to motion artifacts and may reflect biological differences between individuals (Zeng et al. 2014). Furthermore, individual differences in head motion appear to be genetically mediated. Using a young adult twin sample (n = 462), motion was reported to be between 37% and 51% heritable, depending on the specific head motion measurement (Couvy-Duchesne et al. 2014). This suggests that at least a portion of the complex behavioral, motoric, or neurobiological factors related to remaining still during MRI scanning are due to genetic variation.
Given that head motion-related artifacts are ubiquitous in brain imaging studies and the initial evidence that one's ability to stay still for prolonged periods of time reflects true biologically based individual differences, we set out to further document the genetic basis of individual differences in head motion and determine if common environmental or genetic factors influence head motion and other demographic and behavioral traits. Using 689 individuals from randomly selected extended pedigrees who participated in the “Genetics of Brain Structure and Function” study (GOBS), and 864 individuals in an extended twin design from the “Human Connectome Project” (HCP), we confirm that head motion is heritable and show significant phenotypic correlations between head motion with body mass index (BMI), waist circumference, diabetes, hypertension, history of smoking, and performance on delay discounting tasks. The largest of these associations is with BMI, where there is strong evidence in both samples of pleiotropy between head motion and BMI.
Materials and Methods
The Genetics of Brain Structure and Function Study
Sample Details
Subjects participated in the GOBS, an extension of the San Antonio Family study (Puppala et al. 2006). This cohort recruited Mexican Americans who were part of a large family from the San Antonio region (Texas, USA) (for full recruitment details see Olvera et al. 2011; McKay et al. 2014). Exclusion criteria included MRI contraindications, documented history of neurological illness, or any major neurological event. All participants provided written informed consent approved by the institutional review boards at the University of Texas Health Science Center San Antonio and Yale University. This analysis includes 689 individuals with head motion data available from a 7.5 min resting state fMRI scan (see Supplementary Materials for imaging protocols). These individuals come from 69 pedigrees, with between 2 and 79 in each pedigree (mean pedigree size = 9.42), plus an additional 39 individuals who were genetically unrelated (see Supplementary Materials for details of familial relationships). The sample is 60.5% female, with an age range of 18–85 years (mean = 43.36).
Head Motion Measures in GOBS
The primary head motion measure was mean frame-wise displacement, calculated using the method described by Power et al. (2012), referred to as FD(Power) throughout the manuscript. To avoid gross head movements, we excluded all individuals where FD(Power) > 3 standard deviations from the mean (n = 5). Inclusion of these individuals does not change the pattern of results. To assess the robustness of our findings, we confirmed all significant findings using 3 additional measures of head motion: root mean square, maximum rotation, and maximum translation. We note that all measure of head motion show highly significant correlation with one another (r = 0.55–0.85, in all cases, P < 1 × 10–15), see Supplementary Materials.
Traits Available in the GOBS Sample
A total of 55 available traits were considered in GOBS, covering demographic details, medical and psychiatric history, neurocognitive traits, and blood-based biomarkers. ‘‘Body size measures’’—weight and height were used to calculate BMI, a measure of obesity based upon weight scaled to height. Waist circumference was measured as an index of central adiposity. ‘‘Blood-based biomarkers’’—14 blood-based biomarkers were assayed. Further details can be found elsewhere (Mitchell et al. 1996; Arar et al. 2008). Briefly, blood samples where collected following a 12 h fast. These samples were used to measure fasting plasma glucose, using an Abbott V/P Analyzer. A standardized 75 g oral glucose load (Orangedex or Koladex, Custom Laboratories, Baltimore, MD) was then given and plasma glucose levels were measured 2 h after administration. Total plasma cholesterol and triglycerides were assayed enzymatically (using commercial regents from Boehringer-Mannheim Diagnostics and Stanbio, respectively). High density lipoprotein (HDL) cholesterol measured after precipitation of apoB-containing particles with dextran sulfate-Mg2+ (Warnick et al. 1982). Very low density lipoprotein (VLDL) cholesterol was estimated as one fifth of total triglyceride levels (Friedewald et al. 1972) and low density lipoprotein (LDL) cholesterol derived by subtracting HDL cholesterol and VLDL cholesterol from total cholesterol. LDL cholesterol concentrations were computed only for those individuals with triglyceride levels of <400 mg/dL. Serum levels of creatinine, blood urea nitrogen, potassium, calcium, and sodium levels were estimated using the Beckman Synchron LX System. ‘‘Medical and psychiatric history’’—all subjects received face-to-face medical and psychiatric interviews in the subject's language of choice (English or Spanish). The psychiatric interview was conducted using the semi-structured Mini International Psychiatric Interview Plus (MINI-Plus; Sheehan et al. 1998), with additional questions to establish lifetime history of psychiatric disorders. History of smoking was defined as ever having smoked cigarettes, cigars or a pipe every day for a month or more. Psychiatric trait measures were also collected including the Beck Depression Inventory (Beck et al. 1961), State-Trait Anxiety Inventory (STAI; Spielberger 2010), and a global assessment of functioning score (according to DSM-IV criteria). ‘‘Neurocognitive traits’’—The South Texas Assessment of Neurocognition, a 90 min battery comprising of standardized and computerized measures was administered, which includes measures of attention/concentration, executive processing, working memory, declarative memory, language processing, intelligence, and emotional processing (Glahn et al. 2007, 2010).
The Human Connectome Project
Sample Details
For replication of significant findings, we used the WU-Minn consortium HCP S900 sample (humanconnectome.org), released in December 2015. This sample includes 900 healthy individuals from families with twin and non-twin siblings recruited through the Missouri Family and Twin Registry. All subjects provided written informed consent and the study was approved by the Institutional Review Board of Washington University in St Louis. Further details of recruitment protocols are given elsewhere (Van Essen et al. 2013). This analysis included 864 individuals with head motion measures from resting state fMRI scans (details and references for imagining protocols in the Supplementary Materials), from 380 pedigrees with 91 monozygotic and 89 dizygotic twin pairs (mean pedigree size = 2.33, maximum pedigree size = 6). The sample is 56.1% female, aged between 22 and 37 years (mean = 28.8 years) and racially and ethnically mixed (see Supplementary Materials for further details).
Head Motion Measures in HCP
In the HCP sample, we index head motion using frame-wise displacement as calculated in FSL McFlirt (Jenkinson et al. 2002) (FD(FSL)). Mean FD(FSL) was calculated across the 4 runs (2 sessions) resting state acquisition. The 2 sessions of data acquisition were typically conducted on Day 1 and Day 2 of the 2-day data collection protocol.
Replication Traits in the HCP
The HCP includes data from a broad range of behavioral and demographic measures for replication of the significant findings we observe in GOBS. As such, we extracted information on BMI, hypertension and history of smoking for individuals with head motion measures. History of smoking was defined as those who have at one point in their lives been regular smokers (compared with those who had never smoked or experimented less that 100 times). There were insufficient numbers of individuals with diabetes in the HCP to conduct an analysis; instead we used the available HbA1C (glycated hemoglobin) measures to index the 3-month average plasma glucose concentration. Waist circumference was not measured in the HCP.
We also sought the replicate the previously published finding that links head motion to impulsivity (specifically self-control) (Kong et al. 2014), using the behavioral measures of self-regulation that are included in the HCP data set; delay discounting using a low ($200) and a high ($40 000) monetary amount were assessed. Lower monetary amounts are generally discounted more steeply than larger amounts. AUC values were calculated (Myerson et al. 2001), whereby smaller AUCs indicate greater discounting by delay, or reduced self-control. No equivalent measure of self-control is available in the GOBS data set.
Analysis
All analyses were conducted within SOLAR (Almasy and Blangero 1998), which uses maximum likelihood variance component methods to partition trait covariance between family members into genetic and environmental components, as a function of genetic proximity. First, we calculated the heritability of each trait in GOBS, which represents the proportion of phenotypic variance accounted for by additive genetic variance. Then, bivariate analyses were conducted for all traits with significant heritability estimates (n = 48 in GOBS), calculating the phenotypic correlation with head motion (FDPower) for each trait. These phenotypic correlations were decomposed into their genetic and environmental components utilizing all available pedigree information; genetic correlations indicate shared genetic influences (pleiotropy). A multivariate normal threshold model was used for analysis of combined dichotomous and continuous traits (Williams et al. 1999). As 48 traits were considered in a pairwise fashion with respect to head motion in GOBS, a false discovery rate (Benjamini and Hochberg 1995) of FDR < 0.05 was used to determine significant correlations. Next, we applied this analysis framework in the HCP sample, using FD(FSL) to index head motion and using an FDR < 0.05 correction across the 6 heritable replication traits in the HCP.
Prior to analysis, all continuous traits were transformed using an inverse normal transformation. The covariates of age, age2, sex, and the interactions between these were included in all analyses (with race and ethnicity also included for analyses in the HCP sample).
Results
Head Motion is a Reliable and Heritable Trait
Descriptive statistics and heritability estimates for head motion in the GOBS and HCP samples are described in Table 1. Across the 2 scanning sessions in the HCP, there is a high test-retest reliability of head motion (FD(FSL)) estimates (ICC agreement = 0.814, 95% CI = 0.790–0.836, F(838,839) = 9.76 , P = 1.67–200), indicating trait stability across days. Heritability estimates were significant in both samples, and of a similar magnitude. Sex was a significant covariate in GOBS (P = 0.012, males show greater head motion than females), while age was a significant covariate in the HCP (P = 9.73 × 10–3), with older subjects moving more (the age range for the HCP is 22–37 years). All other covariates included in the heritability models were not significant.
Table 1.
Head motion measures in GOBS and HCP data sets
| GOBS | HCP | |||
|---|---|---|---|---|
| N | 684 | 864 | ||
| Age mean | 43.46 years | 28.8 years | ||
| Age range | 18–85 years | 22–37 years | ||
| % Female | 60.5% | 56.1% | ||
| Head motion measure | FD(Power) | FD(FSL) | ||
| Head motion mean | 0.125 | 0.162 | ||
| Head motion SD | 0.049 | 0.050 | ||
| Head motion range | 0.052–0.381 | 0.073–0.339 | ||
| Heritability of head motion | ||||
| H2 | 0.313 | 0.427 | ||
| P value | 5.94 × 10–4 | 2.42 × 10–9 | ||
| SE | 0.106 | 0.071 | ||
| Heritability covariates | Beta | P value | Beta | P value |
| Age | 0.001 | 0.809 | 0.040 | 0.010 |
| Sex | −0.258 | 0.012 | −0.060 | 0.544 |
| Age × sex | −2.16 × 10–5 | 0.997 | −0.007 | 0.723 |
| Age2 | −2.16 × 10–5 | 0.533 | −0.004 | 0.329 |
| Age2 × sex | −1.32 × 10–4 | 0.673 | 0.007 | 0.153 |
Note that given differences in image acquisition and motion calculation protocols between samples, the values cannot be compared directly.
Phenotypic and Genetic Correlations of Traits with Head Motion
Correlations within GOBS
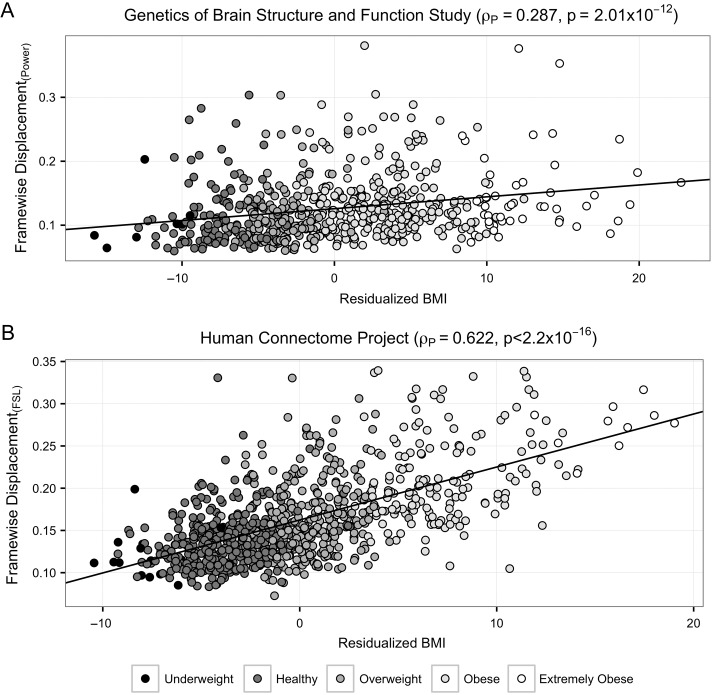
Among the 48 demographic and behavioral traits with significant heritability estimates, 5 showed significant phenotypic correlation with head motion after controlling for multiple comparisons (Table 2; see Supplementary Results for full description). Increased levels of head motion was linked to greater BMI, waist circumference, and the presence of hypertension, diabetes or smoking history. The relationship between head motion and BMI for this sample (the strongest phenotypic correlation observed) is depicted in Figure 1A. Decomposing these phenotypic relationships using a bivariate genetic model, substantial genetic correlations with head motion were seen for BMI and waist circumference. No environmental correlations were statistically significant. Confirming the robustness of these results within GOBS, we observed a similar pattern of results for these 5 traits across 3 alternative head motion measures in the sample (see Supplementary Materials). We note that BMI is significantly phenotypically correlated with waist circumference, diabetes, and hypertension, but not smoking (see Supplementary Materials). Intracranial volume was unrelated to either head motion (ρp = 0.045, P = 0.262) or BMI (ρp = −0.030, P = 0.484) and inclusion of intracranial volume as a covariate in our analyses did not change the pattern of results shown.
Table 2.
Traits showing significant correlation with head motion. Significant correlations (FDR < 0.05) are bolded
| Trait | N | Mean (SD)/cases | Heritability (P values) | Phenotypic correlation with FD(Power) | Genetic correlation with FD(Power) | ||||
|---|---|---|---|---|---|---|---|---|---|
| ρp | P valueP | FDRP | ρG | P valueG | FDRG | ||||
| GOBS sample | |||||||||
| BMI | 606 | 30.93 (6.56) | 0.445 (5.31E-5) | 0.287 | 2.01E-12 | 9.65E-11 | 0.843 | 6.34E-05 | 3.04E-03 |
| Waist circum. (cm) | 586 | 101.40 (14.701) | 0.430 (3.34E-5) | 0.279 | 8.69E-12 | 2.09E-10 | 0.687 | 3.53E-04 | 8.47E-03 |
| Diabetes | 688 | 108 cases | 0.781 (1.86E-5) | 0.189 | 2.71E-03 | 0.026 | −0.026 | 0.914 | 0.953 |
| Hypertension | 690 | 201 cases | 0.393 (7.96E-3) | 0.169 | 1.81E-03 | 0.023 | 0.296 | 0.329 | 0.953 |
| Smoking | 689 | 233 cases | 0.719 (2.90E-6) | 0.162 | 1.93E-03 | 0.023 | 0.108 | 0.613 | 0.953 |
| HCP sample | |||||||||
| BMI | 863 | 26.37 (5.03) | 0.683 (9.88E-20) | 0.622 | <2.2E-16 | 1.32E-15 | 0.797 | 1.11E-13 | 6.66E-13 |
| HbA1C | 583 | 5.25 (0.35) | 0.729 (6.97E-16) | 0.167 | 2.32E-04 | 4.64E-04 | 0.164 | 0.138 | 0.166 |
| Hypertension | 856 | 109 cases | 0.622 (5.33E-05) | 0.264 | 1.73E-5 | 5.19E-05 | 0.717 | 2.61E-03 | 5.22E-03 |
| Smoking | 858 | 216 cases | 0.753 (1.11E-12) | 0.130 | 8.35E-3 | 0.010 | 0.164 | 0.183 | 0.183 |
| Delay discounting (AUC $200) | 858 | 0.253 (0.201) | 0.361 (1.00E-7) | −0.089 | 0.013 | 0.013 | −0.481 | 6.89E-04 | 2.07E-03 |
| Delay discounting (AUC $40 000) | 858 | 0.499 (0.287) | 0.496 (1.54E-13) | −0.120 | 8.72E-4 | 1.31E-03 | −0.297 | 0.011 | 0.017 |
Figure 1.
Phenotypic relationship between head motion and BMI (adjusted for covariates) in (A) the GOBS (n = 606) and (B) the HCP (n = 863). BMI category indicated by shading.
Replicating Correlations within HCP
All of the 6 traits analyzed in the HCP (BMI, HbA1C, showing glycated hemoglobin related to the average plasma glucose concentration over 3 months, hypertension, history of smoking, and 2 measures of delayed discounting) were heritable and showed significant phenotypic correlations with head motion (Table 2). The strongest phenotypic relationship is observed between head motion and BMI, as depicted in Figure 1B. The negative correlations between the delay discounting measures and head motion indicate that increased head motion is correlated with greater discounting of delayed monetary rewards (an index reflecting greater impulsivity). All traits except for history of smoking show significant genetic correlations with head motion, but only BMI shows a significant environmental correlation (ρE = 0.430, P = 4.29 × 10–7, FDR = 2.57 × 10–6).
Further analysis within the HCP data (shown in Supplementary Materials) demonstrates that BMI is correlated with both hypertension and HbA1c. For delay discounting with higher amounts ($40 000) there is a small phenotypic association with BMI, although this does not pass the FDR threshold for significance (ρP = −0.078, PP = 0.038, FDRP = 0.063). BMI is not correlated with delay discounting with lower monetary values ($200), or with history of smoking.
We used a gene by sex interaction model to test for sex differences in the heritability of head motion in each data set. These methods are described elsewhere in detail (Almasy et al. 2001; Glahn et al. 2013). Briefly we test sex as an environmental variable within a gene by environment model and assess whether there are heritability differences in head motion between the sexes (either in terms of the magnitude of the genetic influence or in the specific set of genes involved). We found no evidence of gene by sex interactions in either GOBS or the HCP, indicating that while there may be phenotypic differences between the sexes in terms of head motion, in these samples there is no evidence that the genetic influences on head motion vary between males and females.
Neither GOBS nor the HCP included data on eye tracking or wakefulness during the resting state scanning protocol, so it is not possible to directly examine the effect of wakefulness on head motion. However, in the HCP is there a general measure of sleep quality in the form of the Pittsburgh Sleep Questionnaire Index, which could be considered a proxy of general alertness. Including this trait as a covariate did not alter the pattern of our results (see Supplementary Materials). We also considered the effect of respiration, using the data available in the HCP collected using a respiration belt (no such data was collected in the GOBS sample). Again, including this trait as a covariate did not alter the pattern of results (as shown in the Supplementary Materials).
Discussion
We demonstrate that head motion during an MRI scan is heritable and correlated with an individual's BMI, waist circumference, hypertension, diabetes (or plasma glucose concentration), history of smoking, and impulsivity. These findings are in line with a growing body of literature indicating head motion is a stable and consistent phenotype, with a genetic basis and biological correlates (Couvy-Duchesne et al. 2014; Kong et al. 2014; Zeng et al. 2014). The strongest association is between head motion and BMI; this is observed in 2 independent samples and using bivariate genetic analyses we show that this phenotypic relationship is underpinned by shared genetic factors influencing both head motion and BMI.
Given the relationship between BMI with waist circumference, diabetes/plasma glucose concentration and hypertension, it seems likely that BMI drives the observed associations between these traits with head motion. However, history of smoking is not correlated with BMI in either samples, which suggests the phenotypic relationship between head motion and smoking is independent of any BMI-linked effect.
In the HCP, the association we find between head motion and behavioral impulsivity measures (as indexed by delay discounting tasks) replicate previous findings using self-report measures of impulsivity (specifically self-control; Kong et al. 2014), and extend this work to show genetic correlations between the traits. However, the correlations between head motion and delay discounting is much smaller in magnitude that that between head motion and BMI. Further, the relationship between BMI and delay discounting is unclear; BMI is not related to delay discounting with a low monetary amount, but for high monetary amounts, the results were not clear-cut. As previous work has shown robust relationships between BMI and delay discounting tasks (e.g., see Jarmolowicz et al. 2014), we suggest that further work is needed (potentially using a range of measures of self-control) to investigate the interrelationships between BMI, impulsivity, and head motion.
Head motion is an important confound in the analysis of resting state fMRI connectivity data (Power et al. 2012; Satterthwaite et al. 2012; Van Dijk et al. 2012). However, our findings demonstrate that head motion is not only a nuisance variable that produces technical artifact in an imaging data set but also a stable trait with a genetic basis and strong interrelationships, both phenotypic and genetic, to other measures of interest. If head motion is not only an incidental source of noise in the data, but varies between subjects in a way that is interconnected to their genetics and other traits, the way in which motion should be modeled within a data set should be carefully considered. Apparent motion-related artifact could in fact be a result of motion-correlated variables such as BMI, history of smoking, or impulsivity. In other words differences in connectivity between high and low motion groups might be a result of the technical effects of head motion on the imaging data collection (e.g., motion-induced bias). However, it is also possible that some independent factor is associated with both reduced connectivity and an increased likelihood for motion (e.g., including genetic differences and/or differences in BMI). This possibility is further supported by a previous report showing neurobiological correlates of head motion (Zeng et al. 2014). Indeed the study design used in that report, comparing high and low motion scans (or scanning periods) from the same individuals, may be fruitful for attempting to disentangle motion-induced artifact from connectivity differences resulting from motion-correlated traits, though this may also reflect habituation to the MRI scanning environment over time.
If the nature and relationship between motion-induced artifact and connectivity differences resulting from motion-correlated traits can be better characterized using such within-subject designs, then researchers can begin developing appropriate modeling methods to handle any potential confounding effects, aiming to remove the artifactual effects of motion while accounting for connectivity differences driven by motion-correlated variables.
In terms of the observed correlations observed between traits, there are a number of possible models that could explain these patterns. For example the genetic correlations between head motion and BMI could result from an overlapping set of genes exerting independent effects on each trait, or genetic influences acting via BMI to increase head motion (for instance, higher BMI might increase discomfort in the scanner and so increase movement). Alternatively, genes might act via a third heritable trait that influences the likelihood of both increased head motion and BMI. In the HCP data set, we also report significant environmental correlations between head motion and BMI, suggesting that non-genetic factors also contribute to the patterns of covariance between head motion and BMI. Unfortunately, few environmental measures were included in the HCP data set, making it difficult to determine the extent to which any single factor influences the environmental correlation. To disentangle the mechanisms underlying the observed genetic and environmental correlations, it may be useful to consider measurements of potential intermediary traits of interest (such as discomfort within the scanner or respiration effects) in subsequent studies
Taken together, our results imply that the investigation of functional connectivity in samples where BMI, waist circumference, diabetes, hypertension, history of smoking, and/or impulsivity/cognitive control potentially differ between individuals or groups must carefully consider the most appropriate method of correcting for motion artifacts. Correction for true motion artifact remains critical but if apparently motion-related signals are in fact due to the effects of motion-correlated traits, inappropriate corrections could bias results in unknown ways.
Despite the similarities between findings in the GOBS and HCP cohorts, the samples do differ. Due to variation in both calculation methods and MRI data collection protocols, we do not directly compare values for head motion variables in GOBS versus the HCP (Power et al. 2014). However, we do observe differences in the relationship between head motion and covariates; head motion is significantly associated with sex but not age in GOBS, while in the HCP it is significantly associated with age but not sex. Additionally in both GOBS and HCP, there is a highly significant phenotypic relationship between head motion and BMI, but the magnitude of this correlation differs between the 2 samples (GOBS ρp = 0.287, HCP ρp = 0.622). Despite these differences in phenotypic correlations, the genetic correlation between head motion and BMI is both substantial and significant in both data sets (GOBS ρG = 0.843, HCP ρG = 0.797).
Differences such as these may be explained in part by differences between the 2 samples. First, while GOBS focuses solely on Mexican Americans from the San Antonio region (USA), the HCP protocol did not restrict recruitment by race or ethnicity in its recruitment from Missouri (USA). Second, both data sets are adult samples but the HCP data set includes only a restricted age range (22–37 years). Third, there are minor differences in the way in which frame-wise displacement is calculated in each sample, although Yan et al. (2013) show these values are highly correlated. Fourth, the GOBS data show significantly higher levels of obesity, hypertension, and smoking than HCP (see Supplementary Materials for statistics). Finally, GOBS recruitment was focused on extended pedigrees, while HCP recruited much smaller sibling/twin pedigrees. This difference in pedigree structure may affect genetic estimations, though both samples were analyzed with the same statistical framework. Direct estimation of common environmental influences are less informative in large extended pedigrees, as members of the same pedigree typically live in different households. As previous analyses within a classical twin sample ruled out shared environmental influences on head motion measures (Couvy-Duchesne et al. 2014), this information may not be fundamental for understanding genetic influence upon head motion. Finally, the inclusion of multigenerational families in GOBS mean that the confounding of shared environmental effects with genetic effects are less likely in this sample (Gur et al. 2007).
Head motion is an important source of bias in fMRI analysis, causing artifactual patterns of signal in resting state connectivity analyses. However, there is growing evidence that movement in the scanner is not a randomly occurring nuisance variable; it has a biological and genetic basis and here we present the first evidence that this genetic basis is pleiotropic with BMI. While we did not identify the biological pathways linking head motion and other phenotypes, the current report should help researchers formulate the best way to consider and account for the biasing effects of head motion within resting state connectivity data, given its correlation with other traits. Our findings specifically highlight the need to consider BMI in the analyses of connectivity and motion differences and more generally the complexities of dealing with head motion effects on connectivity analyses, particularly when genetic approaches are used.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Supplementary Material
Notes
We are grateful to all of the participants involved in the Genetics of Brain Structure and Function Study and the Human Connectome Project. Conflict of Interest: None declared.
Funding
Financial support for the Genetics of Brain Structure and Function Study was provided by the National Institute of Mental Health grants MH078143 (PI: DC Glahn), MH078111 (PI: J Blangero), and MH083824 (PI: DC Glahn). SOLAR is supported by NIMH grant MH059490 (J Blangero). Data provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) was financially supported by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research and by the McDonnell Center for Systems Neuroscience at Washington University.
References
- Almasy L, Blangero J. 1998. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 62:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Towne B, Peterson C, Blangero J. 2001. Detecting genotype x age interaction. Genet Epidemiol. 21 (Suppl 1):S819–S824. [DOI] [PubMed] [Google Scholar]
- Arar NH, Voruganti VS, Nath SD, Thameem F, Bauer R, Cole SA, Blangero J, MacCluer JW, Comuzzie AG, Abboud HE. 2008. A genome-wide search for linkage to chronic kidney disease in a community-based sample: the SAFHS. Nephrol Dial Transplant. 23:3184–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. 1961. An inventory for measuring depression. Arch Gen Psychiatry. 4:561–571. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple. Testing. J R Stat Soc Ser B. 57:289–300. [Google Scholar]
- Couvy-Duchesne B, Blokland GAM, Hickie IB, Thompson PM, Martin NG, de Zubicaray GI, McMahon KL, Wright MJ. 2014. Heritability of head motion during resting state functional MRI in 462 healthy twins. Neuroimage. 102 (Pt 2):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 18:499–502. [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. 1996. Movement-related effects in fMRI time-series. Magn Reson Med. 35:346–355. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Almasy L, Barguil M, Hare E, Peralta JM, Kent JW, Dassori A, Contreras J, Pacheco A, Lanzagorta N, et al. 2010. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry. 67:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, Meyenberg N, Castro MP, Barrett J, Nicolini H, et al. 2007. Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 144B:242–249. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Kent JW, Sprooten E, Diego VP, Winkler AM, Curran JE, McKay DR, Knowles EE, Carless MA, Göring HHH, et al. 2013. Genetic basis of neurocognitive decline and reduced white-matter integrity in normal human brain aging. Proc Natl Acad Sci USA. 110:19006–19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur R. 2007. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 164:813–819. [DOI] [PubMed] [Google Scholar]
- Jarmolowicz DP, Cherry JBC, Reed DD, Bruce JM, Crespi JM, Lusk JL, Bruce AS. 2014. Robust relation between temporal discounting rates and body mass. Appetite. 78:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jiang A, Kennedy DN, Baker JR, Weisskoff RM, Tootell RBH, Woods RP, Benson RR, Kwong KK, Brady TJ, Rosen BR, et al. 1995. Motion detection and correction in functional MR imaging. Hum Brain Mapp. 3:224–235. [Google Scholar]
- Kong X-Z, Zhen Z, Li X, Lu H-H, Wang R, Liu L, He Y, Zang Y, Liu J. 2014. Individual differences in impulsivity predict head motion during magnetic resonance imaging. PLoS One. 9:e104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay DR, Knowles EEM, Winkler AAM, Sprooten E, Kochunov P, Olvera RL, Curran JE, Kent JW, Carless MA, Göring HHH, et al. 2014. Influence of age, sex and genetic factors on the human brain. Brain Imaging Behav. 8:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BD, Kammerer CM, Blangero J, Mahaney MC, Rainwater DL, Dyke B, Hixson JE, Henkel RD, Sharp RM, Comuzzie AG, et al. 1996. Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans. The San Antonio Family Heart Study. Circulation. 94:2159–2170. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. 2001. Area under the curve as a measure of discounting. J Exp Anal Behav. 76:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes TR, Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ. 2005. Comparison of fMRI motion correction software tools. Neuroimage. 28:529–543. [DOI] [PubMed] [Google Scholar]
- Olvera RL, Bearden CE, Velligan DI, Almasy L, Carless MA, Curran JE, Williamson DE, Duggirala R, Blangero J, Glahn DC. 2011. Common genetic influences on depression, alcohol, and substance use disorders in Mexican-American families. Am J Med Genet B Neuropsychiatr Genet. 156B:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. 2014. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 105:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppala S, Dodd GD, Fowler S, Arya R, Schneider J, Farook VS, Granato R, Dyer TD, Almasy L, Jenkinson CP, et al. 2006. A genomewide search finds major susceptibility loci for gallbladder disease on chromosome 1 in Mexican Americans. Am J Hum Genet. 78:377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, et al. 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. 2012. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59 (Suppl 2):22–33;quiz 34–57. [PubMed] [Google Scholar]
- Spielberger CD. 2010State-trait anxiety inventory. In The Corsini Encyclopedia of Psychology. Hoboken, NJ, USA: John Wiley & Sons, Inc. [Google Scholar]
- Thacker NA, Burton E, Lacey AJ, Jackson A. 1999. The effects of motion on parametric fMRI analysis techniques. Physiol Meas. 20:251–263. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. 2012. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. 2013. The WU-minn human connectome project: an overview. Neuroimage. 80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnick GR, Benderson J, Albers JJ. 1982. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 28:1379–1388. [PubMed] [Google Scholar]
- Williams JT, Begleiter H, Porjesz B, Edenberg HJ, Foroud T, Reich T, Goate A, Van Eerdewegh P, Almasy L, Blangero J. 1999. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. II. Alcoholism and event-related potentials. Am J Hum Genet. 65:1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X-N, Castellanos FX, Milham MP. 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L-L, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, Buckner RL, Liu H. 2014. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci USA. 111:6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.