Abstract
Objective
Despite the challenges of managing type 1 diabetes, many adolescents achieve optimal outcomes. A validated measure of diabetes-specific strengths is needed to measure adaptive behaviors and attitudes associated with overcoming challenges and achieving “resilient” outcomes
Methods
Baseline data from 260 adolescents (age 14–18 years, M = 15.7 ± 1.1, 60% female, 33% Non-Caucasian, M A1c = 9.1 ± 1.9%) and caregivers in a behavioral intervention trial were analyzed to evaluate psychometric properties of the 12-item self-report Diabetes Strengths and Resilience measure for adolescents (DSTAR-Teen). Reliability and validity were examined in relation to measures of related constructs, regimen adherence, and glycemic outcomes, and confirmatory factor analysis was conducted
Results
Reliability was good (internal consistency: α = .89; item-total correlations: r range = .55–.78). Significant correlations demonstrated construct and criterion validity. A two-factor structure reflecting intrapersonal and interpersonal processes fit the data better than a one-factor solution
Conclusions
The DSTAR-Teen has strong psychometric properties, captures adaptive aspects of adolescents’ diabetes management (i.e., “strengths”), and is related to clinical outcomes.
Keywords: adolescent, behavioral research, diabetes mellitus, type 1, outcome and process assessment (health care), resilience, psychological
Psychological, social, and physical development introduces numerous challenges to achieving optimal outcomes for adolescents with type 1 diabetes (T1D), making adherence and in-range glycemic outcomes a challenge (Hood etal., 2014; Miller etal., 2015; Rausch etal., 2012). Increasing insulin needs owing to puberty, a growing desire for autonomy, incomplete cognitive/executive functioning development, and spending more time away from home can disrupt diabetes management routines and contribute to suboptimal outcomes (Markowitz, Garvey, & Laffel, 2015; Wasserman, Hilliard, Schwartz, & Anderson, 2015). Additionally, changes in parent–adolescent relationships, including shifting diabetes management responsibility from parents to youth and arguments related to daily diabetes tasks, may challenge optimal parental involvement and adherence (Hilliard etal., 2013; Wiebe etal., 2014).
Despite the many challenges inherent to living with and managing diabetes in adolescence, many youth have good psychosocial and health outcomes, known as achieving “diabetes resilience” (Hilliard, Harris, & Weissberg-Benchell, 2012). There is a growing interest in evaluating not only the relative lack of problems but also the explicit strengths of youth who achieve positive outcomes and live well with demanding chronic conditions. Identification of the strengths and protective processes that youth and families use to overcome risks and achieve optimal outcomes is necessary for research to advance understanding and promotion of resilience in pediatric populations, including T1D (Hilliard, McQuaid, Nabors, & Hood, 2015). Strengths are defined as adaptive processes, behaviors, and attitudes that facilitate achievement of resilient outcomes when faced with disease-related challenges (Hilliard etal., 2012; Hilliard etal., 2015). For youth with T1D, these include supportive family communication, collaborative parent involvement, diabetes self-efficacy, and adaptive problem-solving skills (Wiebe etal., 2014; Wysocki etal., 2009; Jaser & White, 2010).
Despite recognizing the importance of supporting youth in overcoming challenges, assessment of condition-specific strengths is not routinely incorporated into clinical research or practice for youth with T1D or other conditions. This is largely owing to the inadequacy of existing measures. Currently, strengths-based research and resilience-promotion interventions tend to evaluate a single construct (e.g., optimism, self-efficacy) or use a battery of general measures of positive processes (Rosenberg etal., 2015; Jaser, Patel, Rothman, Choi, & Whittemore, 2014), which are important but may not capture the specific adaptive behaviors and attitudes unique to successfully managing a challenging chronic condition like diabetes. In practice, ambulatory diabetes care tends to focus on identifying and reducing barriers to improved outcomes rather than on measuring and enhancing facilitators or strengths (Kichler, Harris, & Weissberg-Benchell, 2015; Powell, Corathers, Raymond, & Streisand, 2015), perhaps owing to having no standardized tools to assess diabetes-related strengths (Hilliard, McQuaid, Nabors, & Hood, 2015).
There are few instruments that assess strengths rather than problems in diabetes or other populations. Limitations of those that touch on related constructs (e.g., self-efficacy, support from others) and are validated with adolescents with T1D (Iannotti etal., 2006; La Greca etal., 1995; Schilling etal., 2009) include being too long or complex for routine use in clinical research or care settings, measuring only a single construct, or focusing on specific technical tasks of diabetes management. These limitations make them inefficient or inappropriate for focused, theoretically grounded assessment of T1D-related strengths. Thus, for both research purposes and clinical applications, a brief, validated measure of diabetes-specific strengths is needed.
This study evaluated the psychometric properties of a newly developed measure of diabetes-specific strengths in adolescents with T1D, following guidelines for measure development and validation (Holmbeck & Devine, 2009). It was hypothesized that the measure would be psychometrically sound, with indications of adequate reliability and validity including internal consistency (α > .80), statistically significant (p< .05) item-total correlations, and significant (p< .05) bivariate correlations with measures of similar and dissimilar constructs (i.e., construct validity) and key clinical outcomes (i.e., criterion validity). A secondary aim was to evaluate the factor structure of the measure. Based on previous literature from pediatric and developmental psychology identifying multi-level risk and protective factors for youth with diabetes (Anderson, 2011; Bonanno & Mancini, 2008; Hilliard etal., 2012; Jaser etal., 2010; Mackey etal., 2011; Masten & Obradovic, 2006; Prince-Embury, 2007; Wiebe etal., 2014; Wysocki etal., 2009; Yi-Frazier etal., 2015), we expected measure items to load on two factors reflecting positive intrapersonal processes such as confidence and coping and positive interpersonal processes such as support from others and effective parental involvement.
Methods
Participants and Procedures
Participants were 260 adolescents with T1D (age range: 14–18 years) and one parent/caregiver, enrolled in a multisite behavioral clinical trial aimed at preventing the onset of depressive symptoms, described in detail elsewhere (Weissberg-Benchell, Rausch, Iturralde, Jedraszko, & Hood, 2016). At both sites, study information was sent to local diabetes practices and associations and high school nurses, who distributed the materials to families. To enroll a diverse and representative sample for this study, efforts were made to oversample participants from racial/ethnic minority backgrounds and from families with lower socioeconomic status by distributing study information to sites across two large metropolitan areas in California and Illinois. Interested families contacted the study team, and were screened for eligibility via brief telephone interviews. Study eligibility criteria included age 14–18 years, T1D diagnosis ≥12 months, insulin dosing ≥0.5 units/kg/day (to confirm clinical diagnosis per guidelines of the American Diabetes Association), and English fluency. Because the purpose of the intervention trial was to prevent deteriorations in mood among adolescents with T1D, exclusions included current diagnosis of Major Depressive Disorder and/or treatment with antidepressant medications. Additional exclusions included diagnosis of another major mental/developmental disorder that would impede one’s capacity to complete questionnaires or participate in study activities (e.g., thought disorder) or another chronic condition except celiac or thyroid disease and ward of the state. Across two sites, 665 families contacted the study teams: 264 provided informed assent/consent and enrolled, 169 were ineligible, 144 declined owing to time or distance, 79 were unreachable after initial call, and 9 called after recruitment closed. Cross-sectional baseline data from all participants with complete data (N = 260) were used in this report. Participant characteristics are summarized in Table I. Informed consent and assent were obtained, per each site’s institutional review board approved protocol.
Table I.
Participant Demographic and Clinical Characteristics
| Percent (n) | Mean (SD) | |
|---|---|---|
| Adolescent age, years | 15.7 (1.1) | |
| Adolescent gender, % female | 59 (158) | |
| Annual family income | ||
| <$50,000 | 16 (182) | |
| $51,000–100,000 | 33 (389) | |
| $101,000–150,000 | 23 (269) | |
| >$151,000 | 28 (322) | |
| Highest parental education | ||
| <High school diploma | 1 (18) | |
| High school diploma/GED | 27 (359) | |
| 2- or 4-year college degree | 38 (496) | |
| Advanced graduate or professional degree | 34 (451) | |
| Diabetes duration, years | 6.9 (4.0) | |
| Insulin administration, % insulin pump | 68 (177) | |
| Glycemic control, percent hemoglobin A1c | 9.1 (1.9) | |
| Mean daily BGMF, from meter | 3.7 (2.4) | |
| Adolescent race/ethnicity (parent-report) | ||
| White, non-Hispanic | 67 (171) | |
| Black, non-Hispanic | 13 (34) | |
| Hispanic | 11 (27) | |
| Asian or Pacific Islander | 2 (6) | |
| “Other” | 6 (14) |
Note: A1c = glycosylated hemoglobin A1c; BGMF = blood glucose monitoring frequency.
Measures
Adolescents and parents completed measures via HIPAA-compliant electronic data capture system accessible through the Internet at baseline before randomization or participating in any intervention sessions. The system did not permit respondents to skip items, resulting in no missing data for survey items.
Diabetes strengths were assessed using the Diabetes Strengths and Resilience measure for adolescents (DSTAR-Teen), created for this study. A review of developmental psychology (Bonanno & Mancini, 2008; Masten & Obradovic, 2006) and diabetes behavioral (Anderson, 2011; Mackey etal., 2011; Wysocki & Greco, 2006; Yi-Frazier etal., 2015) literature informed the content of items assessing adolescents’ perceptions of potential diabetes strengths in two major areas: (1) perceived capacity to manage the demands and adapt to the unpredictability of diabetes, and (2) perceived availability of and access to support from others, including family, friends, and health care providers. One diabetes psychologist generated items that reflected adaptive behaviors or attitudes related to positive diabetes outcomes based on observational and interventional behavioral diabetes literature, existing measures of related (i.e., general resilience, diabetes-specific risk factors) constructs, clinical experience, and resilience theory literature. Items were then reviewed and refined by two additional diabetes psychologists with expertise in measurement of diabetes-related risks and in clinical approaches to resilience promotion. Measure brevity was prioritized to develop an instrument that would be low-burden for use in clinical research and practice settings. To achieve this, the measure included a single item for each potential strength behavior or attitude, and efforts were made to minimize overlap among constructs. To fill the gap in diabetes-specific strengths-based measures, the items were all worded as positive statements; because numerous measures exist that measure diabetes-related risk factors, it was not felt that integrating items with negative wording was necessary. Potential items were reviewed by the investigators for relevance to the two domains of strengths (i.e., intrapersonal strengths and interpersonal strengths), to avoid redundancy among items, to ensure a variety of strengths were assessed based on theory and previous literature, and to maintain a brief length suitable for use in clinic or research. This resulted in a 12-item self-report questionnaire with items assessing youths’ perceptions of their own competence for managing the demanding diabetes management regimen and adapting to the unpredictability of diabetes, and for seeking help and support with diabetes challenges.
The format mirrored a validated measure of general resilience and strengths, the Resiliency Scales for Children and Adolescents (RSCA; Prince-Embury, 2007). DSTAR-Teen items were written in simple, readable language suitable for adolescents, confirmed by readability statistics (Flesch Reading Ease score = 76.7; Fleisch-Kincaid Grade Level = 5.9). Respondents were instructed to select “the answer that tells about you best” using a 5-point scale (1 = never to 5 = almost always). There was no specific recall period provided to capture youths’ perceptions of typical behaviors and attitudes about diabetes management. Responses on each item were summed to calculate a total score with a possible range of 12–60 because there were no missing data. For the total score, higher scores indicated youth perception of having greater T1D strengths. A parent proxy version was not developed because data suggest that parents are more accurate reporters of children’s observable behaviors and less accurate reporters of children’s internal states, such as emotions, beliefs, or perceptions (De Los Reyes & Kazdin, 2005; Eiser & Morse, 2001).
Construct validity was evaluated in relation to similar constructs (general resilience and adaptive coping) and dissimilar constructs (family conflict and diabetes burden). The RSCA (Prince-Embury, 2007) was used to measure general resilience-related attitudes and behaviors. This is a 44-item self-report measure of perceived mastery of everyday activities and social relatedness using a 5-point scale (never to almost always). Higher scores indicate youth perception of having more general strengths. The RSCA is a validated measure of general resilience and had excellent reliability in this sample (α = .97). The Coping Efficacy Questionnaire (CE; Sanders, Tein, Mehta, Wolchik, & Ayers, 2000) was used to assess coping strategies used previously and expectations for future use. Higher scores indicate youth perception of using more effective coping strategies. This eight-item measure has strong psychometric properties and had good reliability in this sample (α = .90).
Adolescents and parents each completed the Diabetes Family Conflict Scale-Revised (DFCS-R; Hood, Anderson, Butler, & Laffel, 2007), a 19-item self-report measure of the frequency of family conflict about diabetes over the past month. Higher scores indicate youth and parent perceptions of having more family conflict about aspects of T1D management. The Problem Areas in Diabetes–Teen (PAID-T) measure (Weissberg-Benchell & Antisdel-Lomaglio, 2011), a 26-item youth-report instrument measured diabetes-related distress or burden. Higher scores indicate youth perception of feeling more burdened related to T1D. Both are validated measures with strong psychometric properties (20–21), and excellent internal consistency in this sample (DFCS-R α = .91, PAID-T α = .95).
Criterion validity was evaluated in relation to key diabetes outcomes. Overall diabetes treatment adherence was assessed using the Self-Care Inventory-Revised (SCI-R; Weinger, Welch, Butler, & La Greca, 2005), a self-report instrument that measures the frequency with which respondents engaged in 15 diabetes management tasks over the previous 1–2 months. Higher scores indicate youth perception of engaging more frequently in T1D self-management behaviors. The SCI-R has been validated for use in a variety of populations with diabetes, and had adequate reliability in this sample, α = .78. Adherence was also assessed via mean blood glucose monitoring frequency (BGMF). Study staff downloaded electronic data including the date, time, and glucose value of all blood glucose monitoring events over the previous 14 days and calculated the mean daily BGMF. Higher values indicate completing more daily blood glucose checks, on average, over the previous 2 weeks. Glycemic control was measured via hemoglobin A1c collected at baseline using fingerstick capillary blood samples analyzed at Diabetes Diagnostic Laboratory at the University of Missouri (central laboratory). Higher values indicate higher average blood glucose over the past 2–3 months. This variable was treated both continuously and categorically according to the American Diabetes Association A1c target guidelines (<7.5% vs. ≥7.5%).
Demographic and clinical characteristics including adolescent age, gender, grade, race/ethnicity, family composition, diabetes duration, mode of insulin administration, and parent’s education and annual family income were provided by the participant’s primary caregiver.
Statistical Analyses
Descriptive, reliability, and validity analyses were conducted using SPSS software, version 24. Confirmatory factor analyses were performed in MPlus, version 7. Descriptive statistics were conducted to characterize the sample and the DSTAR-Teen summary data. There were no missing data, as the survey required all items to be answered. Reliability, validity, and factor analyses were conducted in accordance with the measure development and validation guidelines of Holmbeck and Devine (2009).
Characteristics of the DSTAR-Teen were evaluated with descriptive statistics of central tendency, and associations with key demographic and clinical variables were assessed with bivariate Pearson correlations (age, diabetes duration, A1c, BGMF), independent samples t tests (gender, insulin regimen, current use of continuous glucose monitor, A1c target categories), and ANOVAs (race/ethnicity, annual family income categories, highest parental education categories). Reliability of the DSTAR-Teen was assessed via item-total correlations and Cronbach’s alpha. Validity was assessed via bivariate Pearson correlations with each measure of similar and dissimilar constructs, and with key diabetes outcomes, to assess construct and criterion validity, respectively.
Confirmatory factor analysis (CFA), a theory-driven approach used to examine the latent structure underlying a group of items, was conducted. The hypothesis was that a two-factor structure would fit the data best and would reflect two distinct but related domains of positive intrapersonal processes and positive interpersonal processes reflecting diabetes strengths. A one-factor model was also evaluated as an alternative possible factor structure. The ordered nature and number of response options raised the possibility of an ordinal rather than continuous scale of measurement; inappropriate use of continuous maximum likelihood estimation can lead to biased estimates in CFA (Rhemtulla, Brosseau-Liard, & Savalei, 2012). To evaluate this, models that assumed continuous and ordinal categorical scales were compared. Estimation methods appropriate for skewed item distributions were used: maximum likelihood with robust standard errors (MLR) and weighted least squares with mean and variance-adjusted standard errors (WLSMV). Goodness of fit was evaluated according to recommended cutoffs for fit indices (Hu & Bentler, 1999; Yu, 2002): Bentler’s comparative fit index (CFI ≥ 0.95); the Tucker–Lewis Index (TLI ≥ 0.95); the root mean squared error of approximation (RMSEA ≤ 0.06); the standardized root mean squared residual (SRMR ≤ 0.08; for continuous scales); and the weighted root-mean-square residual (WRMR < 1.00; for noncontinuous scales). Given uncertainties about using strict cutoffs with small samples and noncontinuous data (Garrido, Abad, & Ponsoda, 2016), fit indices were interpreted cautiously along with the magnitude of factor loadings while balancing theoretical considerations. Following factor analyses, the psychometric properties of the resulting factors were evaluated in relation to the sample demographic and clinical characteristics, and in relation to the measures of construct and criterion validity.
Results
Descriptive Characteristics
Participant characteristics are summarized in Table I. The majority of adolescents were female (59%) and non-Hispanic White (67%), and family income was approximately evenly split (48% ≤$100,000/year). Clinically, approximately two-thirds (68%) received insulin through subcutaneous insulin infusion, the mean A1c was 9.1 ± 1.9% (17% under American Diabetes Association glycemic target of 7.5%), and mean daily BGMF was 3.7 ± 2.4 checks/day.
Of the possible score range on the DSTAR-Teen of 12–60, the mean score in this sample was 49.0 ± 7.9 (range = 12.0–60.0). On each of the 12 items, respondents endorsed the full range of responses, yet ratings were positively skewed with the majority of respondents endorsing “Often/4” or “Almost Always/5” on every item. Item skewness ranged from −1.21 to −0.38, and kurtosis ranged from −0.95 to 1.06; total score skewness = −0.63, kurtosis = 0.11. See Table II for the frequencies of responses to each item.
Table II.
Items and Response Options for the Diabetes Strengths and Resilience Measure for Adolescents Age 14–18 With Type 1 Diabetes (DSTAR-Teen). Frequencies of Endorsed Responses for Each Item are Indicated in Columns
| Instructions: Here is a list of things that people with diabetes sometimes think. Read each sentence carefully and circle the answer that tells about you best. There are no right or wrong answers. | ||||||
|---|---|---|---|---|---|---|
| 1 Never (%) | 2 Rarely (%) | 3 Sometimes (%) | 4 Often (%) | 5 Almost always (%) | Mean ± SD | |
| 1. I am able to take care of my diabetes pretty well. | 0.4 | 5.4 | 21.5 | 41.5 | 31.2 | 4.0 ± 0.9 |
| 2. I tell my friends about diabetes. | 2.7 | 8.5 | 21.2 | 24.2 | 43.5 | 4.0 ± 1.1 |
| 3. I am good at responding to high or low blood sugars. | 0.4 | 0.4 | 16.2 | 34.6 | 48.5 | 4.3 ± 0.8 |
| 4. I am able to ask my nurse or doctor questions about how to manage my diabetes. | 0.4 | 3.0 | 16.2 | 28.5 | 51.9 | 4.3 ± 0.9 |
| 5. I am good at figuring out what to do for my diabetes care when problems come up. | 0.4 | 2.3 | 15.0 | 35.0 | 47.3 | 4.3 ± 0.8 |
| 6. My parent(s) help me take care of my diabetes. | 1.2 | 5.4 | 17.3 | 24.2 | 51.9 | 4.2 ± 1.0 |
| 7. I can ask for help with my diabetes management when I need to. | 1.2 | 2.7 | 13.8 | 29.2 | 53.1 | 4.3 ± 0.9 |
| 8. If I try hard to do everything I need to do for my diabetes, it makes a difference. | 1.2 | 0.8 | 13.5 | 33.8 | 50.8 | 4.3 ± 0.8 |
| 9. I can count on my friends to help me take care of diabetes if I need help. | 8.8 | 13.5 | 24.6 | 25.4 | 27.7 | 3.5 ± 1.3 |
| 10. I can figure out ways to take care of my diabetes even when I am busy or other things make diabetes hard to manage. | 1.2 | 8.5 | 24.6 | 31.5 | 34.2 | 3.9 ± 1.0 |
| 11. There is someone I can always ask for help with my diabetes. | 1.5 | 5.4 | 17.3 | 25.8 | 50.0 | 4.2 ± 1.0 |
| 12. I talk to my parent(s) calmly about diabetes, like talking about my A1c or remembering to do blood sugar checks. | 3.5 | 11.9 | 25.4 | 23.1 | 36.2 | 4.0 ± 1.2 |
Note: Please contact the first author with requests to use this measure in any research or clinical setting.
The DSTAR-Teen scores were not significantly correlated with youth age or diabetes duration (Table III), and did not differ significantly across gender, insulin regimen, use of continuous glucose monitoring, child race/ethnicity, highest parental education, or family salary bracket.
Table III.
Correlation Matrix of Associations Among DSTAR-Teen Total Score, Demographic and Clinical Characteristics, and Measures of Construct and Criterion Validity
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. DSTAR-Teen | ||||||||||
| 2. Age | −.06 | |||||||||
| 3. Duration | .08 | .20** | ||||||||
| 4. A1c | −.35** | .06 | .00 | |||||||
| 5. BGMF | .22** | −.10 | .11 | −.36** | ||||||
| 6. RSCA | .73** | −.01 | .13* | −.28** | .21** | |||||
| 7. CE | .64** | .06 | .16* | −.28** | .16* | .67** | ||||
| 8. DFCS-R-T | −.29** | −.14* | −.04 | .31** | −.22** | −.27** | −.30** | |||
| 9. DFCS-R-P | −.17** | −.06 | .00 | .29** | −.24** | −.20** | −.21** | .34** | ||
| 10. PAID-T | −.57** | .03 | −.04 | .36** | −.15* | −.49** | −.61** | .35** | .26** | |
| 11. SCI-R | .57** | −.18** | −.04 | −.32** | .29** | .46** | .47** | −.19** | −.26** | −.46** |
Note: DSTAR-Teen = Diabetes Strengths and Resilience measure for adolescents; A1c = glycosylated hemoglobin A1c; BGMF = blood glucose monitoring frequency; RSCA = Resiliency Scales for Children and Adolescents; CE = Coping Efficacy Questionnaire; DFCS-R-T = Diabetes Family Conflict Scale-Revised-Teen; DFCS-R-P = Diabetes Family Conflict Scale-Revised-Parent; PAID-T = Problem Areas in Diabetes–Teen; SCI-R = Self-Care Inventory-Revised. **p < .01, *p<.05.
Reliability and Validity
Cronbach’s alpha indicated good internal consistency, α = .89. All corrected item-total correlations were significant, r range = .44−.73, p < .001, and the Cronbach’s alpha did not improve if any items were deleted. Inter-item correlations ranged from .20 to .69, and the average correlation between individual items and all other items was .42. Thus, all items were eligible to be retained in the final version of the DSTAR-Teen. Significant (p < .01) bivariate correlations (Table III) provided evidence of construct (RSCA: r = .73; CE: r = .64; DFCS-R-Teen: r=−.29; DFCS-R-Parent: r= .17; PAID-T: r=−.57) and criterion (SCI-R: r= .57; BGMF: r = .22; A1c: r = −.35) validity. Criterion validity was further supported because youth with A1c values within the target range <7.5% scored significantly higher on the DSTAR-Teen (M = 52.7 ± 6.2) compared with those with above target glycemic control (M = 48.2 ± 8.0), t(258) = 3.60, p < .001.
Factor Structure
A series of confirmatory factor analyses tested the hypothesized two-factor structure. Items reflecting positive intrapersonal processes were allowed to load on the first factor. These assessed respondents’ perceptions of overall diabetes management self-efficacy (Item 1), ability to successfully manage extreme blood glucose values (Item 3), self-efficacy for diabetes-related problem solving (Item 5), belief that trying hard makes a difference in diabetes management (Item 8), and ability to prioritize diabetes management (Item 10). This factor was labeled “Diabetes-related Confidence.” The second factor reflected positive interpersonal processes. Items loading on this factor assessed comfort disclosing diabetes to peers (Item 2), perceived availability and helpfulness of parental, peer, and other assistance with diabetes (Items 6, 9, and 11), and youths’ ability to calmly communicate with parents about diabetes (Item 12). This factor was labeled “Help with Diabetes Management.” Items 4 and 7, which reflected aspects of both confidence in one’s own abilities and availability of diabetes-related support, were explored on both factors with consideration of factor loadings and overall model fit.
Table IV presents fit statistics for one- and two-factor CFA models using both estimation methods (MLR and WLSMV). One-factor models fit poorly (CFIs and TLIs generally < 0.90; RMSEAs > 0.10; WRMR > 1.00). Two-factor models exhibited improved fit, with the WLSMV model showing variable fit across indices (CFI = 0.95; TLI = 0.93; RMSEA = 0.11; WRMR = 1.15). Item 4 (ability to ask medical providers questions) and Item 7 (ability to ask for help when needed) showed marginally stronger loadings on Factor 1 And Factor 2, respectively. Overall model fit worsened when these items were switched (e.g., for the WLSMV two-factor model: CFI = 0.92; TLI = 0.90; RMSEA = 0.14; WRMR = 1.42; not shown in Table IV).
Table IV.
Confirmatory Factor Analyses With Fit Statistics
| Fit indices | X2 (df) | CFI | TLI | RMSEA (90% CI) | SRMR | WRMR |
|---|---|---|---|---|---|---|
| One-factor models | ||||||
| 1.a. Continuous with MLR | 273.99 (54) | 0.81 | 0.77 | 0.13 (0.11, 0.14) | 0.07 | – |
| 1.b. Ordinal categorical with WLSMV | 354.68 (54) | 0.91 | 0.89 | 0.15 (0.13, 0.16) | – | 1.47 |
| Two-factor models | ||||||
| 2.a. Continuous with MLR | 181.98 (53) | 0.89 | 0.86 | 0.10 (0.08, 0.11) | 0.07 | – |
| 2.b. Ordinal categorical with WLSMV | 232.99 (53) | 0.95 | 0.93 | 0.11 (0.10, 0.13) | – | 1.15 |
| 2.a.i. MLR model with correlated residuals | 132.44 (51) | 0.93 | 0.91 | 0.08 (0.06, 0.10) | 0.06 | – |
| 2.b.i. WLSMV model with correlated residuals | 181.68 (51) | 0.96 | 0.95 | 0.10 (0.08, 0.12) | – | 0.99 |
Note. CFI = Bentler’s comparative fit index; TLI = Tucker–Lewis Index; RMSEA = root mean square error of approximation; CI = confidence interval; SRMR = standardized root mean squared residual; WRMR = weighted root-mean-square residual; MLR = maximum likelihood with robust standard errors; WLSMV = weighted least squares with mean and variance-adjusted standard errors.
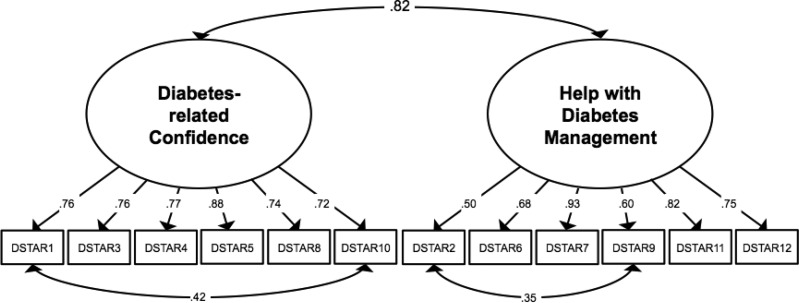
Inspection of the WLSMV model showed strong factor loadings (standardized estimates of 0.55–0.93). To further improve model fit, MPlus modification indices highlighted two pairs of items with significant shared error variance (Items 1 and 10, and Items 2 and 9), which indicated systematic similarities in how respondents answered these items that were not fully explained by the two underlying factors. Ignoring these similarities can lead to biased estimation of the latent factors and problems with model fit (Cole, Ciesla, & Steiger, 2007). The similar content in these pairs of items (ability to “take care” of diabetes for Items 1 and 10, friend relationships for Items 2 and 9) provided a rationale, based on possible shared measurement variance, to justify adding a correlation term for these pairs in subsequent models. The WLSMV structure with these correlated residuals (Table IV, Model 2.b.i.) was selected as the final model owing to strong factor loadings (standardized estimates of 0.50–0.93), acceptable fit indices (CFI = 0.96; TLI = 0.95; RMSEA = 0.10; WRMR = 0.99), and a significantly improved fit compared with the more restricted model that did not allow for correlated residuals (ΔX2(2) = 41.34, p < .001; based on robust chi-square difference testing as described by Asparouhov & Muthén, 2006). Factor loadings and correlated residuals for the final factor model are shown in Figure 1.
Figure 1.
Factor structure of the DSTAR-Teen.
Note. Standardized estimates. All ps < .001.
The two resulting factors were strongly correlated with one another, r = .82, p < .001, and each showed strong internal consistency, α = .86 and .80, respectively, demonstrating good reliability. Each factor demonstrated similar bivariate correlations with the measures of construct and criterion validity as the total score. Based on mean scores, Diabetes-related Confidence and Help with Diabetes Management were each significantly (p < .001) correlated with the measures of construct (RSCA: r = .65, .68; CE: r = .67, .52; DFCS-R-Teen: r = −.31, −.23; PAID-T: r = −.59, −.46) and criterion (SCI-R: r = .61, .44; A1c: r = −.44, −.21) validity. The correlations with BGMF were significant at the p < .001 level for the Diabetes-related Confidence factor (r= .26) and at the p < .05 level for the Help with Diabetes Management factor (r = .14). The correlations with parent-reported family conflict about diabetes (DFCS-R-Parent) were significant at the p < .01 level for Diabetes-related Confidence (r = −.21) and marginally related at p = .074 for Help with Diabetes Management (r = −.11). As with the total score, the two factor scores were each higher among youth with A1c values within the target range <7.5% compared with those with A1c values above target (Diabetes-related Confidence t = 4.17, p < .001; Help with Diabetes Management t = 2.72, p < .01). Thus, both the total score and each subscale demonstrate strong psychometric properties and would be appropriate for use.
Discussion
The psychometric properties of the DSTAR-Teen indicate that this newly developed self-report instrument reliably and validly measures adaptive aspects of adolescents’ diabetes management during a vulnerable developmental period. The strong association with general strengths and coping skills confirms that this measure captures similar protective processes. Negative correlations with measures of common diabetes-related challenges, as rated by both youth and parents, further support validity. DSTAR-Teen items were associated with key clinical outcomes, including a combination of objective and self-reported measures of higher regimen adherence, more frequent blood glucose monitoring, and lower A1c. These results demonstrate that the behaviors and attitudes assessed in this measure represent adaptive processes, or strengths, related to key components of living with and managing the demands of T1D.
The factor analysis results indicate that this measure assesses two related subsets of diabetes-related strengths, including confidence in one’s own abilities and access to help from close others. These factors support the hypothesized two-factor structure including intrapersonal and interpersonal strengths. Both the total score and each subscale score demonstrated good psychometric properties including reliability and multiple aspects of validity. Thus, the measure can be used either as a total score or as the two subscales for evaluating adolescents’ current sets of diabetes strengths in research and practice. Clinically, subscale scores may help identify areas of strength for patients and providers to build on in developing strategies to overcome challenges and promote resilient outcomes. For example, youth with high scores on Diabetes-related Confidence may be well-equipped to use cognitive strategies to address diabetes-related stressors, and those with high scores on Help with Diabetes Management may do well to engage family members in handling challenges that arise. High scores on specific items may also point to specific strengths to take advantage of, such as using peer support to address social stressors with diabetes or using one’s optimistic outlook to think about difficult diabetes situations.
On an item level, responses skewed high, with mean scores on each item around 4, equating to participants “often” endorsing the strengths. However, every item included responses ranging from “never” to “almost always” and the total scores spanned the whole possible range. Therefore, even though this sample comprised adolescents without Major Depressive Disorder (owing to the eligibility criteria of the trial from which these baseline data were drawn), participants represented a range of strengths, including some who endorsed few strengths and a larger proportion who endorsed many strengths. It is likely that a sample without this exclusion would result in a more evenly distributed frequency of responses, with less skew toward the top of the scale, and confirmatory psychometric analyses should be conducted in another such sample.
This study provided psychometric data on a measure of adaptive behaviors and attitudes, or strengths, but it did not evaluate whether these strengths are linked with achievement of resilient outcomes above and beyond the impact of risk factors. This measure will need to be evaluated in relation to individuals and families with a range of risk exposure, such as youth with extremely high A1c or greater psychosocial or socioeconomic adversity. Over 30% of participants were from a non-Caucasian racial/ethnic background and approximately one-half came from families with incomes <$100,000/year. This diversity in a large, multisite sample is a strength of this study and exceeds rates typically reported in behavioral diabetes research and national prevalence (Wysocki etal., 2009, Wiebe etal., 2014; Miller etal., 2015), which speaks to generalizability of findings. The lack of differences across demographic and diabetes-related clinical variables (e.g., use of diabetes management devices) supports this conclusion, and suggests that diabetes-related strengths may be relatively consistent across groups. Adaptations for different ages and parent-report are needed to evaluate diabetes-related strengths across the pediatric years.
Another consideration of this analysis is the cross-sectional nature of the baseline trial data. This precluded any conclusions that can be drawn about causal links between diabetes strengths and any of the other constructs or glycemic outcomes, as well as analysis of the DSTAR-Teen’s sensitivity to change or test–retest reliability. Ultimately, the intervention trial’s outcome data will be an ideal data set in which to evaluate whether the measure is sensitive to change related to an intensive, group-based resilience-promotion intervention taking place over nine biweekly sessions. A prospective, longitudinal sample with assessments closer in time will be needed to evaluate test–retest reliability without an intervening influence such as a behavioral trial.
As with all behavioral research relying on self-report measures, there is a potential that youth and parent answers on questionnaires may have been impacted by response bias. When possible, objective measures were used in conjunction with subjective measures to strengthen assessment of key constructs (e.g., blood glucose meter download and SCI-R scores both measured aspects of adherence). Future research would benefit from including additional sources of objective data (e.g., downloads from insulin pumps), responses from additional raters (e.g., health care providers), and measures of other related constructs (e.g., diabetes health-related quality of life) to reduce risk of bias and ensure comprehensive assessment of relevant constructs.
This measure was evaluated in accordance with guidelines (Holmbeck & Devine, 2009), which lends methodological strength to the measure. However, some guidelines were unable to be met. Construct and criterion validity were evaluated, but discriminant validity was unable to be assessed in relation to theoretically unrelated constructs, incremental validity was not compared with measures of other constructs for clinical judgement, and test–retest reliability and sensitivity to change were not able to be assessed with cross-sectional data. Additionally, the factor structure and psychometric properties were unable to be confirmed in a second sample. Replication of these analyses in another sample will enhance the robustness of the findings and inform conclusions about generalizability and utility of this measure.
Finally, the diabetes-specific content of the DSTAR-Teen was developed by three psychologists with a combined >40 years of personal and professional, clinical and research experience with T1D. As recommended by Holmbeck and Devine (2009), feedback from other health care providers and youth and families living with T1D may help to confirm or refine the appropriateness of the measure for future use.
The DSTAR-Teen may have applications for use in preventive and intervention trials to assess mechanisms of change, including not only decreases in maladaptive processes (e.g., nonadherence, diabetes burden) but also increases in protective processes. In practice, this brief, self-report measure may also have applicability for use in routine, clinic-based assessment of adaptive diabetes management behaviors. Professional guidelines call for providers to monitor and support youths’ well-being and behavioral diabetes management (American Diabetes Association, 2015; Delamater, de Wit, McDarby, Malik, & Acerini, 2014; Young-Hyman etal., 2016). Given the relevance of the DSTAR-Teen to these constructs and its explicit emphasis on diabetes-related strengths, this measure has potential to complement measures of depressive symptoms and quality of life for comprehensive clinical assessment. Applied research integrating the DSTAR-Teen into clinical practice and behavioral intervention protocols is necessary to evaluate the feasibility, acceptability, and impact of these clinical applications. Moreover, recent research demonstrates the value of monitoring aspects of behavioral functioning and well-being and discussing the findings during clinical care (De Wit etal., 2010; Herzer, Ramey, Rohan, & Cortina, 2012)—research is underway to evaluate personalized monitoring and feedback of diabetes-related strengths using the DSTAR-Teen as a part of routine diabetes care. While this measure was developed for and validated with youth with T1D, there is growing interest in strengths-based approaches to clinical research and practice across pediatric chronic conditions (Hilliard etal., 2015). Thus, similar measures of strengths will be needed that are specifically relevant for the adaptive behaviors and processes that youth with cancer, asthma, sickle cell disease, and other chronic conditions engage in to achieve optimal outcomes despite exposure to adversity and risk.
Funding
This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (R01 DK 901470). Dr Hilliard is also supported by K12 DK 097696 (PI: B Anderson).
Conflicts of interest: None declared.
References
- American Diabetes Association. (2015). Foundations of care: Education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes Care, 38(Suppl. 1), S20–S30. [DOI] [PubMed] [Google Scholar]
- Anderson B. J. (2011). Parenting styles and parenting practices in pediatric diabetes. Diabetes Care, 34, 1885–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T., Muthén В. (2006). Robust chi square difference testing with mean and variance adjusted test statistics. Mplus Web Notes: No. 10. Retrieved from http://www.statmodel.com/download/webnotes/webnotel0.pdf.
- Bonanno G. A., Mancini A. D. (2008). The human capacity to thrive in the face of potential trauma. Pediatrics, 121, 369–375. [DOI] [PubMed] [Google Scholar]
- Cole D. A., Ciesla J. A., Steiger J. H. (2007). The insidious effects of failing to include design-driven correlated residuals in latent-variable covariance structure analysis. Psychological Methods, 12, 381–398. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A., Kazdin A. E. (2005). Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131, 483–509. [DOI] [PubMed] [Google Scholar]
- De Wit M., Delemarre-Van de Waal H. A., Bokma J. A., Haasnoot K., Houdijk M. C., Gemke R. J., Snoek F. J. (2010). Follow-up results on monitoring and discussing health-related quality of life in adolescent diabetes care: Benefits do not sustain in routine practice. Pediatrics Diabetes, 11, 175–181. [DOI] [PubMed] [Google Scholar]
- Delamater A. M., de Wit M., McDarby V., Malik J., Acerini C. L. (2014). Psychological care of children and adolescents with type 1 diabetes. Pediatric Diabetes, 15 (Suppl. 20), 232–244. [DOI] [PubMed] [Google Scholar]
- Eiser C., Morse R. (2001). Can parents rate their child’s health-related quality of life? Results of a systematic review. Quality of Life Research, 10, 347–357. [DOI] [PubMed] [Google Scholar]
- Garrido L. E., Abad F. J., Ponsoda V. (2016). Are fit indices really fit to estimate the number of factors with categorical variables? Some cautionary findings via Monte Carlo simulation. Psychological Methods, 21, 93–111. [DOI] [PubMed] [Google Scholar]
- Herzer M., Ramey C., Rohan J., Cortina S. (2012). Incorporating electronic monitoring feedback into clinical care: A novel and promising adherence promotion approach. Clinical Child Psychology and Psychiatry, 17, 505–518. [DOI] [PubMed] [Google Scholar]
- Hilliard M. E., Harris M. A., Weissberg-Benchell J. (2012). Diabetes resilience: A model of risk and protection in type 1 diabetes. Current Diabetes Reports, 12, 739–748. [DOI] [PubMed] [Google Scholar]
- Hilliard M. E., Holmes C. S., Chen R., Maher K., Robinson E., Streisand R. (2013). Disentangling the roles of parental monitoring and family conflict in adolescents’ management of type 1 diabetes. Health Psychology, 32, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., McQuaid E. L., Nabors L., Hood K. K. (2015). Resilience in youth and families living with pediatric health and developmental conditions: Introduction to the special issue on resilience. Journal of Pediatric Psychology, 40, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G. N., Devine K. A. (2009). An author’s checklist for measure development and validation manuscripts. Journal of Pediatric Psychology, 34, 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood K. K., Anderson B. J., Butler D. A., Laffel L. M. B. (2007). Updated and revised Diabetes Family Conflict Scale. Diabetes Care, 30, 1764–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood K. K., Beavers D. P., Yi-Frazier J., Bell R., Dabelea D., Mckeown R. E., Lawrence J. M. (2014). Psychosocial burden and glycemic control during the first 6 years of diabetes: Results from the SEARCH for diabetes in youth study. Journal of Adolescent Health, 55, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Bentler P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. [Google Scholar]
- Iannotti R. J., Schneider S., Nansel T. R., Haynie D. L., Plotnick L. P., Clark L. M., Sobel D. O., Simons-Morton B. (2006). Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. Developmental and Behavioral Pediatrics, 27, 98–105. [DOI] [PubMed] [Google Scholar]
- Jaser S. S., White L. E. (2010). Coping and resilience in adolescents with type 1 diabetes. Child Care Health and Development, 37, 3355–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser S. S., Patel N., Rothman R. L., Choi L., Whittemore R. (2014). Check it! A randomized pilot of a positive psychology intervention to improve adherence in adolescents with type 1 diabetes. Diabetes Educator, 40, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichler J. C., Harris M. A., Weissberg-Benchell J. (2015). Contemporary roles of the pediatric psychologist in diabetes care. Current Diabetes Reviews, 11, 210–221. [DOI] [PubMed] [Google Scholar]
- La Greca A. M., Auslander W. F., Greco P., Spetter D., Fisher E. B., Santiago J. V. (1995). I get by with a little help from my family and friends: Adolescents’ support for diabetes care. Journal of Pediatric Psychology, 20, 449–476. [DOI] [PubMed] [Google Scholar]
- Mackey E. R., Hilliard M. E., Berger S. S., Streisand R., Chen R., Holmes C. (2011). Individual and family strengths: An examination of the relation to disease management and metabolic control in youth with type 1 diabetes. Families, Systems, and Health, 29, 314–326. [DOI] [PubMed] [Google Scholar]
- Markowitz J. T., Garvey K. C., Laffel L. M. B. (2015). Developmental changes in the roles of patients and families in type 1 diabetes management. Current Diabetes Reviews, 11, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten A. S., Obradovic J. (2006). Competence and resilience in development. Annals of the New York Academy of Sciences, 1094, 13–27. [DOI] [PubMed] [Google Scholar]
- Miller K. M., Foster N. C., Beck R. W., Bergenstal R. M., DuBose S. N., DiMeglio L. A., Maahs D. M., Tamborlane W. V.; T1D Exchange Clinic Network. (2015). Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D exchange clinic registry. Diabetes Care, 38, 971–978. [DOI] [PubMed] [Google Scholar]
- Powell P. W., Corathers S. D., Raymond J., Streisand R. (2015). New approaches to providing individualized diabetes care in the 21st century. Current Diabetes Reviews, 11, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince-Embury S. (2007). Resilience scales for children and adolescents, a profile of personal strengths: Manual. Pearson: Bloomington, MN. [Google Scholar]
- Rausch J., Hood K. K., Delamater A., Shroff Pendley J., Rohan J. M., Reeves G., Dolan L., Drotar D. (2012). Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care, 35, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhemtulla M., Brosseau-Liard P. E., Savalei V. (2012). When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychological Methods, 17, 354–373. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. R., Yi-Frazier J. P., Eaton L., Wharton C., Cochrane K., Pihoker C., Bakr K. S., McCauley E. (2015). Promoting resilience in stress management: A pilot study of a novel resilience-promoting intervention for adolescents and young adults with serious illness. Journal of Pediatric Psychology, 40, 992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders I. N., Tein J. Y., Mehta P., Wolchik S., Ayers T. (2000). Coping efficacy and psychological problems of children of divorce. Child Development, 71, 1099–1118. [DOI] [PubMed] [Google Scholar]
- Schilling L. S., Dixon J. K., Knafl K. A., Lynn M. R., Murphy K., Dumser S., Grey M. (2009). A new self-report measure of self-management of type 1 diabetes for adolescents. Nursing Research, 58, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman R. M., Hilliard M. E., Schwartz D. D., Anderson B. J. (2015). Practical strategies to enhance executive functioning and strengthen diabetes management across the lifespan. Current Diabetes Reports, 15, 622.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger K., Welch G. W., Butler H. A., La Greca A. M. (2005). Measuring diabetes self-care: A psychometric analysis of the Self-Care Inventory-revised with adults. Diabetes Care, 28, 1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg-Benchell J., Antisdel-Lomaglio J. (2011). Diabetes-specific emotional distress among adolescents: Feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatric Diabetes, 12, 341–344. [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J., Rausch J., Iturralde E., Jedrasko A., Hood K. (2016). A randomized clinical trial aimed at preventing poor psychosocial and glycemic outcomes in teens with type 1 diabetes (T1D). Contemporary Clinical Trials, 49, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe D. J., Chow C. M., Palmer D. L., Butner J., Butler J. M., Osborn P., Berg C. A. (2014). Developmental processes associated with longitudinal declines in parental responsibility and adherence to type 1 diabetes management across adolescence. Journal of Pediatric Psychology, 39, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T., Greco P. (2006). Social support and diabetes management in childhood and adolescence: Influence of parents and friends. Current Diabetes Reports, 6, 117–122. [DOI] [PubMed] [Google Scholar]
- Wysocki T., Nansel T. R., Holmbeck G. N., Chen R., Laffel L., Anderson B. J., Weissberg-Benchell J.. Steering Committee of the Family Management of Childhood Diabetes Study (2009). Collaborative involvement of primary and secondary caregivers: Associations with youths’ diabetes outcomes. Journal of Pediatric Psychology, 34, 869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi-Frazier J. P., Yaptangco M., Semana S., Buscaino E., Thompson V., Cochrane K., Tabile M., Alving E., Rosenberg A. R. (2015). The association of personal resilience with stress, coping, and diabetes outcomes in adolescents with type 1 diabetes: Variable- and person-focused approaches. Journal of Health Psychology, 20, 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Hyman D., de Groot M., Hill-Briggs F., Gonzalez J. S., Hood K., Peyrot M. (2016). Psychosocial care for people with diabetes: A position statement of the American Diabetes Association. Diabetes Care, 39: 2126, 40, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. -Y. (2002). Evaluating cutoff criteria of model fit indices for latent variable models with binary and continuous outcomes (Unpublished doctoral dissertation). University of California, Los Angeles.