Abstract
Cardiomyopathy caused by lamin A/C gene (LMNA) mutations (hereafter referred as LMNA cardiomyopathy) is characterized by cardiac conduction abnormalities and left ventricular systolic dysfunction predisposing to heart failure. Previous cardiac transcriptional profiling of LmnaH222P/H222P mouse, a small animal model of LMNA cardiomyopathy, suggested decreased WNT/β-catenin signalling. We confirmed decreased WNT/β-catenin signalling in the hearts of these mice by demonstrating decreased β-catenin and WNT proteins. This was correlated with increased expression of soluble Frizzled-related proteins that modulate the WNT/β-catenin signalling pathway. Hearts of LmnaH222P/H222P mice also demonstrated lowered expression of the gap junction connexin 43. Activation of WNT/β-catenin activity with 6-bromoindirubin-3’-oxime improved cardiac contractility and ameliorated intraventricular conduction defects in LmnaH222P/H222P mice, which was associated with increased expression of myocardial connexin 43. These results indicate that decreased WNT/β-catenin contributes to the pathophysiology of LMNA cardiomyopathy and that drugs activating β-catenin may be beneficial in affected individuals.
Introduction
Dominant mutations in the lamin A/C gene (LMNA), which encodes A-type nuclear lamins, cause dilated cardiomyopathy (herein referred to as LMNA cardiomyopathy), often associated with skeletal myopathy such as Emery-Dreifuss muscular dystrophy (1,2). LMNA cardiomyopathy is characterized by early conduction defects, impaired myocardial contractility and ventricular dilation, eventually causing heart failure (3–5). It has a more aggressive course than other inherited dilated cardiomyopathies due to the high incidence of heart block and ventricular arrhythmias (5). While sudden death from arrhythmias may be prevented by implantation of a pacemaker and/or defibrillator, the progressive heart failure eventually becomes resistant to treatment and heart transplantation is often the only therapeutic option (4).
To decipher mechanistic events underlying the pathogenesis of LMNA cardiomyopathy, we have studied LmnaH222P/H222P mice, which recapitulate the cardiac pathology that occurs in human subjects. These mice develop left ventricular dilatation and conduction defects in adulthood (6). Despite normal heart histology and the absence of left ventricular dilatation at early ages, cardiac transcriptional profiling has identified alterations in several cellular signalling pathways, including WNT/β-catenin (7,8).
WNT proteins are a family of secreted cysteine-rich glycoproteins implicated in a variety of cellular processes, including proliferation, differentiation, apoptosis, polarity, and senescence (9,10). There are 19 human WNT genes, which encode proteins that have been grouped into two classes: those in the canonical and non-canonical WNT pathways. In the canonical WNT signalling cascade, the expression level of β-catenin, the key effector functioning as a transcriptional co-activator, is critical for target gene expression. In the absence of WNT ligand, β-catenin is captured by the scaffold protein Axin, which facilitates its phosphorylation by glycogen synthase kinase 3-β (GSK3-β) in a destruction complex. E3-ubiquitin ligase β-TrCP then catalyzes the ubiquitination of phosphorylated β-catenin, which is subsequently rapidly degraded by the proteasome. Upon WNT ligand binding to the Frizzled and low-density lipoprotein receptor 5/6 complex, the β-catenin destruction complex becomes dysfunctional by a mechanism that is not fully understood. As a result, the newly synthesized β-catenin accumulates in the cytosol, translocates to the nucleus and forms a complex with transcription factor TCF/LEF, leading to activation of target genes.
Frizzled-related proteins and Dickkopfs are modulators of WNT/β-catenin signalling. These proteins have been shown to play a role in various cardiac pathophysiological processes (11–13). Given the alterations of WNT/β-catenin signalling in hearts of LmnaH222P/H222P mice suggested by transcriptional profiling (7,8), we explored the potential involvement of this pathway in the pathophysiology of LMNA cardiomyopathy.
Results
The canonical WNT/β-catenin signalling pathway is impaired in LMNA cardiomyopathy
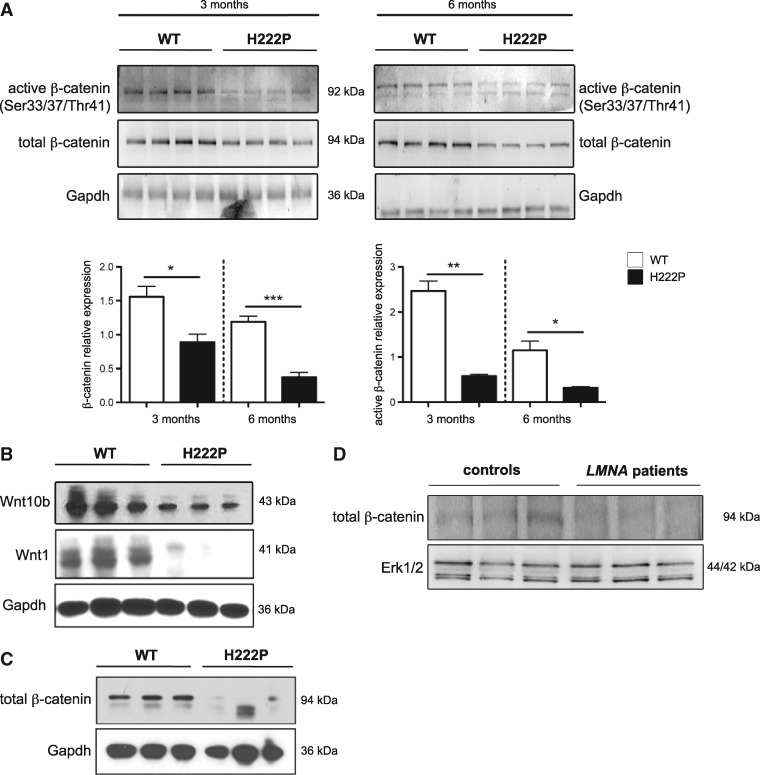
Lmna H222P/H222P mice develop cardiomyopathy that recapitulates human LMNA cardiomyopathy (6). For these experiments, we reconfirmed that male LmnaH222P/H222P mice develop decreased left ventricular fractional shortening (Supplementary Material Fig. S1A,) and first degree heart block (Supplementary Material, Fig. S1B) starting at approximately 3 months and progressing to approximately 6 months of age, which is their median survival. Female LmnaH222P/H222P mice develop signs and symptoms at significantly later ages (6). We therefore evaluated cardiac expression of total β-catenin and its active form (non-phosphorylated Ser33/37/Thr41) in male LmnaH222P/H222P mice. Immunoblotting of heart lysates from 3 month-old and 6 month-old LmnaH222P/H222P mice demonstrated a decrease in both total and active forms of β-catenin compared to wild type mice (Fig. 1A). Given that β-catenin expression was lowered in hearts from LmnaH222P/H222P mice, we next assessed the canonical WNT/β-catenin signalling activity. We observed significantly decreased expression of WNT1, and WNT10b in hearts from 6 month-old LmnaH222P/H222P mice compared to wild type mice (Fig. 1B). We next showed that β-catenin expression was significantly lowered in isolated cardiomyocytes from 6-month old LmnaH222P/H222P mice compared to wild type mice (Fig. 1C). We also analysed left ventricle tissue from three human subjects with LMNA cardiomyopathy obtained after cardiac transplantation. Immunoblotting using antibody against total β-catenin showed decreases in this protein in heart tissue of the patients with LMNA mutations compared with controls (Fig. 1D). These results demonstrated decreased WNT/β-catenin signalling in hearts of LmnaH222P/H222P mice and human subjects with LMNA cardiomyopathy.
Figure 1.
Altered WNT/β-catenin signalling in hearts of LmnaH222P/H222P mice and human subjects with LMNA cardiomyopathy. (A) Representative immunoblots showing active and total β-catenin expression in hearts from 3 month-old and 6 month-old male LmnaH222P/H222P (H222P) and wild type (WT) mice. Each lane contains protein extracts from a different mouse. Gapdh is the loading control. Error bars represent means ± standard errors of means (n = 4) for total β-catenin/gapdh and active β-catenin/total β-catenin relative expression. *P < 0.05, **P < 0.005, ***P < 0.0005. (B) Representative immunoblots showing WNT10b and WNT1, expression in hearts from 6 month-old male Lmna H222P mice compared to Lmna WT mice. Each lane contains protein extracts from a different mouse. Gapdh is the loading control. (C) Representative immunoblot showing total β-catenin expression in isolated cardiomyocytes from 6 month-old male Lmna H222P mice compared to Lmna WT mice. (D) Representative immunoblots showing total β-catenin expression in explanted hearts from control human subjects and human subjects with cardiomyopathy and LMNA point mutations (LMNA patients). Erk1/2 is the loading control (45). Migrations of molecular mass standards in kilodaltons (kDa) are indicated at the right of the blots.
Increased expression of soluble frizzled-related proteins modulate the WNT/β-catenin signalling pathway in LMNA cardiomyopathy
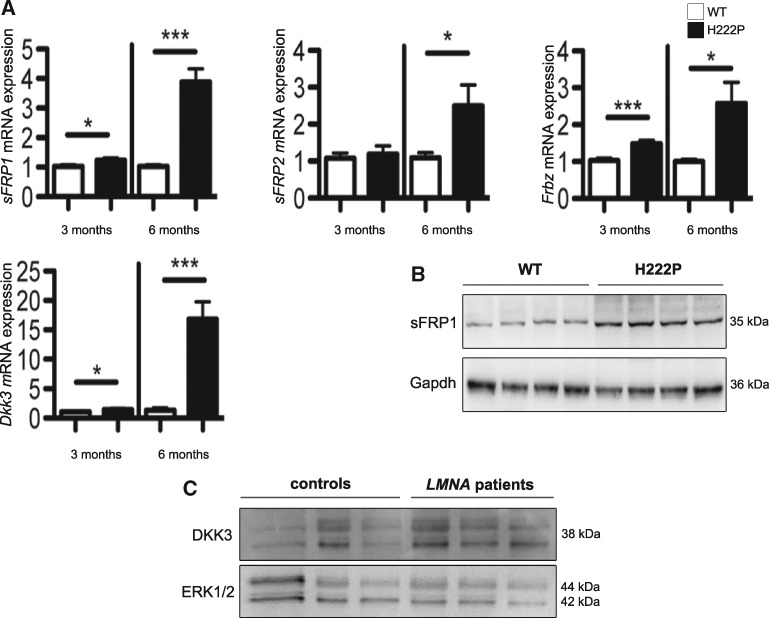
Soluble Frizzled-related proteins are inhibitors of WNT/β-catenin signalling and interact with WNT proteins. We measured the expression of genes encoding members of soluble Frizzled-related protein family (sFrp1, sFrp2, Frzb) and Dickkopf-related protein 3 (Dkk3). Compared to wild type mice, 3-month old LmnaH222P/H222P mice exhibited significantly increased cardiac sFrp1 and Frzb expression as well increased cardiac Dkk3 expression (Fig. 2A). At 6 months of age, LmnaH222P/H222P mice exhibited significantly increased cardiac sFrp1 (∼ 4 fold), sFrp2 (∼ 2.5 fold) and Frzb (∼ 3 fold) mRNA expression as well increased cardiac Dkk3 (∼ 17 fold) mRNA expression (Fig. 2A). We confirmed that the expression of sFRP1 protein was increased in hearts from 6-month old LmnaH222P/H222P mice compared to wild type mice (Fig. 2B). The expression of Dkk3 protein was similarly increased in heart tissues from subjects with LMNA cardiomyopathy (Fig. 2C). These data suggested that activation of extracellular inhibitors could trigger the inhibition of cardiac WNT/β-catenin signalling in LMNA cardiomyopathy.
Figure 2.
Increased expression of secreted antagonists of WNT/β-catenin signalling in hearts of LmnaH222P/H222P mice and human subjects with LMNA cardiomyopathy. (A) Expression of sFRP1, sFRP2, Frbz and Dkk3 mRNA in hearts from 3 month-old and 6 month-old male LmnaH222P/H222P (H222P) and wild type (WT) mice. Error bars represent means ± standard errors of means (n = 7). *P < 0.05, ***P < 0.0005. (B) Representative immunoblots showing sFRP1 expression in hearts from 6 month-old male H222P and WT mice. (C) Representative immunoblot showing Dkk3 expression in explanted hearts from control human subjects and human subjects with cardiomyopathy and LMNA point mutations (LMNA patients). Erk1/2 is the loading control (45). Migrations of molecular mass standards in kilodaltons (kDa) are indicated at the right of the blots in panels B and C.
Altered gap junction structure in LMNA cardiomyopathy
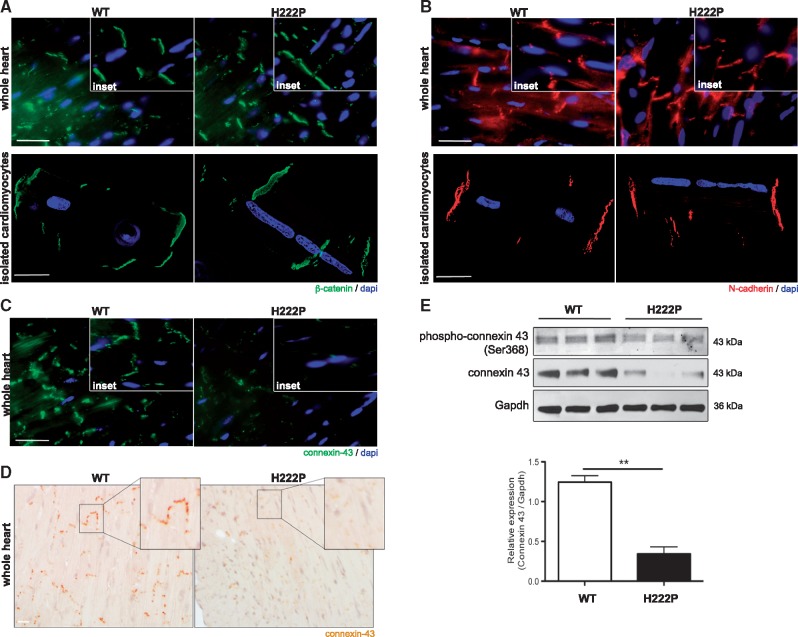
β-catenin is also located in intercalated discs (ICD). This cellular junction is a tightly regulated part of cardiomyocytes and composed of desmosomes, adherens junctions and gap junctions. Therefore, we assessed the architecture of ICDs in LmnaH222P/H222P mice. Immunofluorescence microscopic analyses of specific junctional components were performed on sections of heart tissue from 3 month-old mice as well as in isolated cardiomyocytes. The localization of β-catenin, a component of adherens junctions, was not altered in LmnaH222P/H222P mice compared to wild type mice (Fig. 3A). Similarly, the localization of N-cadherin, a component of both adherens junction and desmosomes, was not altered in LmnaH222P/H222P mice (Fig. 3B). Because of the cardiac conduction abnormalities that occur in LMNA cardiomyopathy, we examined the localization and expression of connexin 43, a central protein component of myocardial gap junctions. We observed decreased connexin 43 expression by immunofluorescence microscopy (Fig. 3C) and immunohistochemistry (Fig. 3D) in hearts from LmnaH222P/H222P mice compared to wild type mice. Given that the phosphorylation status of connexin 43 has been implicated in cardiovascular disease pathology (14), we next assessed the level of phosphorylated (Ser368) connexin 43 and confirmed the diminution of its expression in the hearts of 3 month-old LmnaH222P/H222P mice. Connexin 43 phosphorylation and total expression were both decreased in hearts from LmnaH222P/H222P mice compared to wild type mice (Fig. 3E). These data showed altered expression and phosphorylation of connexin 43 in LMNA cardiomyopathy.
Figure 3.
Decreased expression of connexin 43 in hearts of LmnaH222P/H222P mice. (A) Micrographs showing β-catenin labelling (green) in heart and isolated cardiomyocytes from male LmnaH222P/H222P (H222P) and wild type (WT) mice. Nuclei are counter-stained blue with 4′,6-diamidino-2-phenylindole (dapi). Scale bar, 25 μm. Insets are a representative area. (B) Micrographs showing N-cadherin labelling (red) in heart and isolated cardiomyocytes from H222P and WT mice. Nuclei are counter-stained with dapi. Scale bar, 25 μm. Insets are a representative area. (C) Micrographs showing connexin 43 labelling (green) in heart from male H222P mice and WT mice. Nuclei are counter-stained with dapi. Scale bar, 25 μm. Insets are a representative area. (D) Immunohistochemical labelling for connexin 43 in heart from H222P and WT mice. Scale bar, 25 μm. Insets are a representative area. (E) Representative immunoblot showing phospho-connexin 43 (ser368) and total connexin 43 expression in hearts from 6 month-old male H222P and WT mice. Migrations of molecular mass standards in kilodaltons (kDa) are indicated at the right of the blots. The bar graph shows connexin 43 relative expression compared to Gapdh (means ± standard errors of means) in hearts from WT (n = 3) and H222P (n = 3) mice. **P < 0.005.
Activating WNT/β-catenin signalling improves cardiac function in LMNAH222P/H222P mice
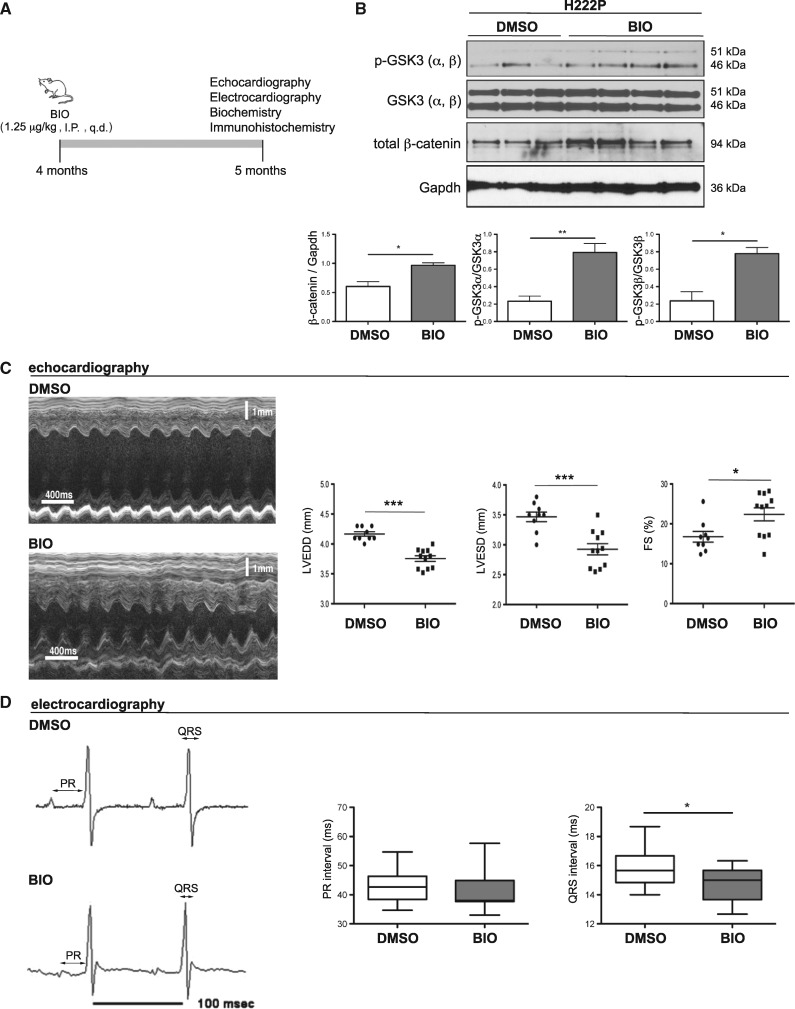
Given the altered WNT/β-catenin signalling in hearts of LmnaH222P/H222P mice, we hypothesized that increasing this pathway’s activity would prevent the progression of left ventricular dysfunction and conduction defects. We therefore treated male LmnaH222P/H222P mice with daily intra-peritoneal injections of 1.25 μg/kg/day of 6-bromoindirubin-3’-oxime (BIO) (Fig. 4A). BIO is a highly potent, selective ATP-competitive inhibitor of GSK3-β that activates the WNT/β-catenin signalling (15). After 1 month of treatment starting at 4 months of age, LmnaH222P/H222P mice were analysed using echocardiography and electrocardiography and then sacrificed for biochemical and histological analyses. BIO treatment increased GSK3-β phosphorylation (inactive form) and total β-catenin in hearts of LmnaH222P/H222P mice compared to dimethyl sulfoxide (DMSO) placebo treatment (Fig. 4B). This demonstrated that BIO positively regulated WNT/β-catenin signalling. M-mode echocardiography showed that left ventricular end-diastolic and end-systolic diameters in LmnaH222P/H222P mice treated with BIO were significantly smaller and fractional shortening was significantly increased compared to placebo-treated mice (Fig. 4C;Table 1). Treating LmnaH222P/H222P mice with BIO also lead to a significant reduction of mRNA levels of NppA and NppB, genes that encode natriuretic peptide precursors that are markers for heart failure, as well as the mRNA levels of Col1a1, encoding type I collagen of the extracellular matrix (Supplementary Material, Fig. S2). The prolonged QRS interval, but not PR interval, was also significantly decreased in BIO-treated LmnaH222P/H222P mice compared to DMSO-treated mice (Fig. 4D). Treating LmnaH222P/H222P mice with increased concentrations of BIO (2.5 or 5 μg/kg, daily) did not improve the left ventricular diameters and fractional shortening (Supplementary Material, Fig. S3), suggesting a therapeutic window.
Figure 4.
Activation of WNT/β-catenin signalling using BIO improves left ventricular function and intraventricular conduction in LmnaH222P/H222P mice. (A) Schematic representation of the treatment protocol of LmnaH222P/H222P (H222P) with BIO. I.P., intra-peritoneal; q.d., daily. (B) Representative immunoblots showing phospho-GSK3, total GSK3 and total β-catenin expression in hearts from 6 month-old male H222P mice treated with DMSO placebo or BIO (1.25 μg/kg/daily). Migrations of molecular mass standards in kilodaltons (kDa) are indicated at the right of the blots. Error bars represent means ± standard errors of means for total β-catenin/gapdh, p − GSK3/total GSK3 and p − GSK3/total GSK3 relative expression in 20-week-old male H222P mice treated with BIO (n = 4) or DMSO (n = 3). *P < 0.05, **P < 0.005. (C) Representative M-mode transthoracic echocardiographic tracings from 20-week-old male H222P mice treated with DMSO or BIO. Graphs show mean left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), and left ventricular fractional shortening (FS) in 20-week-old male H222P mice treated with BIO (n = 5) or DMSO (n = 8). Values for each individual mouse as well as means ± standard errors of means are shown. *P < 0.05, ***P < 0.0005. (D) Representative electrocardiogram tracings from 20-week-old H222P mice treated with DMSO or BIO. Graphs showing mean PR and QRS intervals in 20-week-old H222P mice treated with BIO (n = 5) or DMSO (n = 8). Data are represented as means ± standard errors of means. Values are shown as 25th to 75th percentiles of data values. The line in the middle is the median. Whiskers (Tukey method) extend down to the minimum value and up to the maximum value. *P < 0.05.
Table 1.
Echocardiographic parameters for male wild type (WT) and LmnaH222P/H222P (H222P) mice treated with either BIO or DMSO
| Genotype | Treatment | n | Heart rate (bpm) | LVEDD (mm) | LVESD (mm) | FS (%) |
|---|---|---|---|---|---|---|
| WT | none | 5 | 534.8 ± 31.8 | 3.2 ± 0.5 | 1.8 ± 0.3 | 45.7 ± 1.5 |
| H222P | DMSO | 8 | 501.7 ± 2.9 | 4.2 ± 0.1 | 3.5 ± 0.2 | 15.7 ± 2.4 |
| H222P | BIO | 5 | 496.3 ± 9.3 | 3.6 ± 0.2*** | 2.8 ± 0.3*** | 24.5 ± 5.2** |
LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; FS, fractional shortening. Values are means ± standard errors of means.
P < 0.005, ***P < 0.0005 between DMSO-treated and BIO-treated H222P mice.
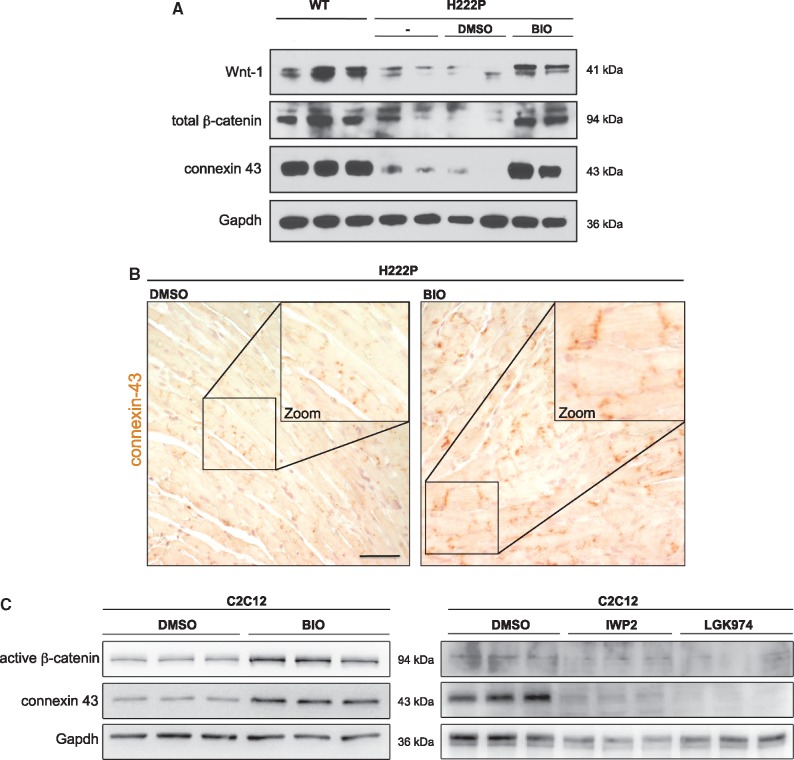
The QRS interval represents the time taken for the excitatory impulse to propagate throughout the ventricles, which is partly determined by the resistance of the intercellular connections between myocytes (16). Decreased expression of connexin 43 is associated with intraventricular conduction slowing and QRS prolongation (17–19). BIO treatment of LmnaH222P/H222P mice lead to increased expression of cardiac connexin 43, which occurs in parallel with re-expression β-catenin and WNT-1 (Fig. 5A). We confirmed this re-expression of connexin 43 by immunohistochemistry on heart from BIO-treated LmnaH222P/H222P mice compared to DMSO-treated mice (Fig. 5B). Given that correct localization of connexin 43 at gap junctions is important for myocyte-myocyte junctions and functions, we hypothesized that BIO treatment improves intraventricular conduction by normalizing expression of connexin 43 in hearts of LmnaH222P/H222P mice. To confirm that WNT/β-catenin signalling can modulate the expression of connexin 43, we treated C2C12 cells with BIO. Immunoblot analysis of cellular protein extracts showed that treatment with BIO compared to DMSO lead to an increase of connexin 43 expression concurrent with increased β-catenin signalling (Fig. 5C). Conversely, C2C12 cells treated with IWP2 or LGK974, which inhibit WNT/β-catenin signalling, showed decreased connexin 43 expression compared to DMSO-treated cells (Fig. 5D). These results demonstrated that WNT/β-catenin signalling triggers the expression of connexin 43, which is expressed at abnormally low levels in LMNA cardiomyopathy.
Figure 5.
Expression and localization of connexin 43 are under WNT/β-catenin signalling regulation in hearts from LmnaH222P/H222P mice and cultured C2C12 cells. (A) Representative immunoblots showing WNT-1, total β-catenin and connexin 43 expression in hearts from 20-week-old male wild type (WT) and LmnaH222P/H222P (H222P) mice untreated (-), treated with BIO or with DMSO. (B) Immunohistochemistry for connexin 43 labelling in hearts from H222P mice treated with BIO or with DMSO. Scale bar, 50 μm. (C) Representative immunoblot showing connexin 43 and active β-catenin expression in C2C12 cells treated with BIO to activate Wnt/β-catenin signalling, or IWP2 and LGK974 to inhibit Wnt/β-catenin signalling. Migrations of molecular mass standards in kilodaltons (kDa) are indicated between the blots.
Discussion
Our findings suggest a physiological role for A-type lamins as a modifier of WNT/β-catenin signalling in the heart. This is consistent with work from Hernandez and colleagues that showed deficient WNT/β-catenin signalling activity in LmnaL530P/530P mice (20), a small animal model for premature ageing with cardiac defects (21). Some evidence suggests that WNT/β-catenin signalling is regulated by emerin, another protein of the inner nuclear membrane, which interacts with A-type lamins (22–24). Mutations in EMD, the gene encoding emerin, also cause dilated cardiomyopathy (25). Interaction of β-catenin with emerin might inhibit TCF/LEF-dependent transcription by restriction access of β-catenin to the nucleus. A variant of nesprin 2, a protein of the Linker of Nucleoskeleton and Cytoskeleton (LINC) complex that physically couples the cytoplasm and the nucleoplasm, has also been shown to anchor β-catenin at cell-cell junctions and negatively regulate WNT/β-catenin signalling (26). Nesprins may also be involved in the pathogenesis of dilated cardiomyopathy (27–29). Hence, A-type lamins, emerin and nesprins may form a complex that modulates, at least to some extent, similar cellular signalling pathways that are involved in the pathogenesis of dilated cardiomyopathy.
Activation of WNT/β-catenin signalling has been reported to be involved in cardiac hypertrophy (30). There is increasing evidence that WNT/β-catenin signalling is also involved in cardiac remodelling and the progression to heart failure. We observed an up-regulation of soluble modulators of WNT/β-catenin signalling in hearts of LmnaH222P/H222P mice and human subjects with LMNA cardiomyopathy. Enhanced expression of Frizzled transcripts has been reported in several heart diseases and cardiomyopathies (30). Therefore, we can speculate that the up-regulation of soluble modulators of WNT/β-catenin signalling could be a consequence of molecular defects leading to cardiomyopathy and heart failure, with no specificity to LMNA cardiomyopathy. The mRNA levels of sFRP3 and sFRP4 are elevated in failing ventricles compared with control donor hearts (29). However, this finding contradicts other work showing that there is a decreased level of sFRPs in heart in an animal model of cardiac failure and in human cardiomyopathies (31). Accordingly, administration of sFRP2 improves cardiac function in a rat model of myocardial infarction (32). Similarly, overexpression of sFRP1 improves infarct healing and cardiac function in mice (11). This effect appears to occur by altering type I procollagen processing by inhibiting bone morphogenic protein 1 in primary cardiac fibroblasts and therefore reducing fibrosis. Our observation showing Dkk3 overexpression in hearts from LmnaH222P/H222P mice is in contrast to other published work showing that Dkk3 attenuated pressure overload-induced cardiac remodelling (12) and protected against cardiac dysfunction and ventricular remodelling following myocardial infarction (13). However, the pathogenic processes leading to cardiac dysfunction after infarction and in primary cardiomyopathies are likely to be very different.
Several converging lines of evidence have suggested that the effects of WNT/β-catenin signalling may result in part via modulation of gap junction channel activity. In response to WNT signalling, β-catenin interacts with the gene encoding connexin 43 to increase its transcription and also appears to interact with connexin 43, likely as part of a complex with the ICD (33). Whether this interaction is direct or requires other components of cell junctions remains to be determined. Several groups have documented downregulation or abnormal localization of connexins in experimental and human cardiomyopathies (34,35). This process, referred to as gap junction remodelling, is thought to predispose to cardiac conduction alterations. The robust response of connexin 43 expression to WNT/β-catenin signalling (33,36,37) suggests that alteration in WNT/β-catenin signalling might influence gap junction channel gene expression in the heart. This is supported by recent data showing that LRP6 deficiency disrupts gap junction formation and function (38). Our results showing a concordant decrease in β-catenin and connexin 43 levels in hearts from LmnaH222P/H222P mice are consistent with this hypothesis.
We have shown that the pharmacological activation of WNT/β-catenin signalling using a GSK3-β inhibitor induces connexin 43 expression and improves left ventricular dysfunction in LmnaH222P/H222P mice. Identified in the late 1970s as a protein kinase that inactivates glycogen synthase, GSK3 became a drug target when its role in insulin signal transduction and its potential importance for Alzheimer disease started to emerge (39). Several potent inhibitors of GSK3 have been identified with therapeutic potential (40). However, long-term use of GSK3 inhibitors may have potential problems. GSK3 inhibitors might be expected to mimic the WNT/β-catenin signalling and therefore could be potentially carcinogenic (41). Nonetheless, lithium, which is a potent GSK3 inhibitors, have been used to treat bipolar disorder, and their long-term use is not known to be associated with an increased risk of cancer (42). Careful adjustment of WNT/β-catenin signalling activity in LMNA cardiomyopathy using small molecule activators might therefore have beneficial effects in humans with LMNA cardiomyopathy. We have previously shown that pharmacological blockade of other signalling cascades - ERK1/2 (43–45), AKT/mTOR (46), TGF-β (47), CTGF (47), JNK (48), p38α (8) - also has beneficial effects on left ventricular function in LmnaH222P/H222P mice. Future studies could be designed to assess the therapeutic benefit of blocking combinations of these signalling pathways in LMNA cardiomyopathy.
Materials and Methods
Mice
Lmna H222P/H222P mice were bred and genotyped as previously described. Mice were fed chow and housed in a disease-free barrier facility with 12h/12h light/dark cycles. The Institutional Animal Care and Use Committee at Columbia University Medical Center approved the use of animals and the study protocols.
Isolation of mouse cardiomyocytes
Wild type and LmnaH222P/H222P mice (16 weeks of age) were anaesthetized with pentofurane. Ventricular cardiomyocytes were isolated as described in the Alliance for Cellular Signaling procedure protocol PP00000125 (http://www.signaling-gateway.org/data/ProtocolLinks.html). Briefly, hearts were removed and the aorta cannulated. After Ca2+-free buffer was perfused for two minutes, 0.25 mg/ml collagenase I/II (Roche) solution was perfused through the coronary arteries for 6 min with 12.5 μM Ca2+. Left ventricular tissue was teased apart and pipetted to release individual cells. After enzymatic dispersion, Ca2+ concentration in the buffer containing bovine serum albumin was elevated in three steps up to 500 μM.
Human tissue samples
Sections of explanted hearts from human subjects with LMNA mutations were obtained from Myobank-AFM of l'lnstitut de Myologie. The subjects were a 23 year-old man with cardiomyopathy associated with muscular dystrophy and LMNA delK61 mutation, a 47 year-old woman with cardiomyopathy and LMNA R60G mutation and from a 62 year-old woman with cardiomyopathy associated with muscular dystrophy and LMNA c.IVS9 + 1g sup a mutation. Control human heart samples were obtained from the National Disease Research Interchange from a 57 year-old man with an intracranial bleed and a 15 year-old woman who died of a drug overdose. All tissue samples were obtained with appropriate approvals and consent from l’lnstitut de Myologie and the National Disease Research Interchange and provided without patient identifiers.
Cell culture and reagents
C2C12 mouse myoblasts (ATCC) were maintained in DMEM supplemented with 10% Fetal Bovine Serum. Cells were incubated with BIO (10 μM) for 24 h, IWP2 (20 μM) for 24 h and LGK974 (8 μM) for 24 h.
Quantitative real-time RT-PCR analysis
Total RNA was extracted (Rneasy isolation kit, Qiagen) and cDNA was synthesized using Superscript first strand synthesis system according to the manufacturer’s instructions (Invitrogen). For each replicate in each experiment, RNA from tissue samples of different animals was used. Primers were designed corresponding to mouse RNA sequences using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Real-time quantitative RT-PCR reactions contained HotStart-IT SYBR green qPCR Master Mix (Affymetrix), 200 nM of each primer and 0.2 µl of template in a 25 µl reaction volume. Amplification was carried out using the ABI 7300 Real-Time PCR System (Applied Biosystems). Relative levels of mRNA expression were calculated using the ΔΔCT method. Individual expression values were normalized by comparison to Gapdh mRNA.
Protein extraction and immunoblotting
Human or mouse heart tissue was homogenized in sample extraction buffer (Cell Signalling) as previously described (8). Extracted proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and blotted with primary antibodies against β-catenin (No ab16051, Abcam), non-phosphorylated (active) β-catenin (No 8814, Cell Signalling), WNT1 (No sc-5630, Santa Cruz Biotechnologies), WNT10b (No sc-25524, Santa Cruz Biotechnologies), sFRP1 (No ab4193, Abcam), DKK3 (No sc-14956, Santa Cruz Biotechnologies), connexin 43 (No 3512, Cell Signalling), phosphorylated (ser368) connexin 43 (No 3511, Cell Signalling) and Gapdh (No sc-25778, Santa Cruz Biotechnologies). Secondary antibodies were horseradish peroxidate–conjugated (GE Healthcare). Recognized proteins were visualized by enhanced chemiluminescence (GE Healthcare).
Immunofluorescence microscopy
For immunofluorescence microscopy, frozen tissues were cut in 8 μm-thick sections. Cryosections were fixed (15 min, 4% paraformaldehyde in phosphate-buffered saline [PBS] at room temperature), permeabilized (10 min, 0.5% Triton X-100 in PBS) and blocked (1 h, PBS with 0.3% Triton X-100, 5% bovine serum albumin). Sections were incubated with primary antibodies against β-catenin (No ab16051, Abcam), connexin 43 (No ab11370, Abcam) and N-cadherin (No 33-3900, Invitrogen) (overnight, 4 °C, in PBS with 0.1% Triton X-100 and 1% bovine serum albumin) and washed in PBS. The sections were then incubated for 1 h with secondary antibodies. Sections were washed with PBS and slides were mounted in Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories).
Immunohistochemistry
Sections of heart tissue were fixed (24 h, 4% paraformaldehyde in PBS, pH 7.4 at 4 °C), dehydrated (EtOH 80%, 95%, and 100%, sequentially), embedded in paraffin and sectioned at 5 μm. Sections were incubated with primary antibodies against mouse anti-connexin 43 (No ab11370, Abcam).
Mouse treatment protocols
BIO was dissolved in DMSO and delivered at doses of 1.25, 2.5 or 5 μg/kg, daily. BIO and DMSO were administered by intra-peritoneal injections starting when the mice were 16 weeks of age and continued until 20 weeks of age.
Thansthoracic echocardiography
Mice were anaesthetized with 1.5% isoflurane in O2 and placed on a heating pad (37 °C). Echocardiography was performed using a Visualsonics Vevo 770 ultrasound with a 30 MHz transducer applied to the chest wall. Cardiac ventricular dimensions and fractional shortening were measured in 2D mode and M-mode 3 times for the number of animals indicated.
Electrocardiography
Electrocardiograms were recorded from mice sedated with low-dose inhaled isoflurane using the standard four limb leads and a B08 amplifier (Emka Technologies) with minimal filtering. Waveforms were recorded using Iox Software v1.8.9.18 and intervals were measured manually with ECG Auto v1.5.12.50, using the average of three representative consecutive beats. The electrocardiographer was blinded to mouse genotype.
Statistics
Values for real-time quantitative RT-PCR were compared using an unpaired Student t-test. Comparisons of echocardiographic parameters between BIO-treated and DMSO-treated LmnaH222P/H222P mice were performed using a Welch t-test; to validate these results, a non-parametric test (Mann-Whitney) was performed and concordance checked. Statistical analyses were performed using GraphPad Prism software.
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
Funding
This work was is supported by the National Institutes of Health [AR048997 to H.J.W.], a grant from the Association Française contre les Myopathies to C.L.D. and from the Institut National de la Sante et de la Recherche Médicale; the Université Pierre et Marie Curie-Paris 6, the Centre National de la Recherche Scientifique and the Association Française contre les Myopathies to A.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
References
- 1. Bonne G., Di Barletta M.R., Varnous S., Becane H.M., Hammouda E.H., Merlini L., Muntoni F., Greenberg C.R., Gary F., Urtizberea J.A., et al. (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet., 21, 285–288. [DOI] [PubMed] [Google Scholar]
- 2. Fatkin D., MacRae C., Sasaki T., Wolff M.R., Porcu M., Frenneaux M., Atherton J., Vidaillet H.J. Jr, Spudich S., et al. (1999) Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med., 341, 1715–1724. [DOI] [PubMed] [Google Scholar]
- 3. Meune C., Van Berlo J.H., Anselme F., Bonne P., Pinto Y.M., Duboc D. (2006) Primary prevention of sudden death in patients with lamin A/C gene mutations. N. Engl. J. Med., 354, 209–210. [DOI] [PubMed] [Google Scholar]
- 4. Ben Yaou R., Gueneau L., Demay L., Stora S., Chikhaoui K., Richard P., Bonne G. (2006) Heart involvement in lamin A/C related diseases. Arch. Mal. Coeur Vaiss., 99, 848–855. [PubMed] [Google Scholar]
- 5. Lu J.T., Muchir A., Nagy P.L., Worman H.J. (2011) LMNA cardiomyopathy: cell biology and genetics meet clinical medicine. Dis. Model. Mech., 4, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arimura T., Helbling-Leclerc A., Massart C., Varnous S., Niel F., Lacène E., Fromes Y., Toussaint M., Mura A.M., Keller D.I., et al. (2005) Mouse model carrying H222P-Lmna mutation develops muscular dystrophyand dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet., 14, 155–169. [DOI] [PubMed] [Google Scholar]
- 7. Muchir A., Pavlidis P., Decostre V., Herron A.J., Arimura T., Bonne G., Worman H.J. (2007) Activation of MAPK Pathway Links LMNA Mutations to Cardiomyopathy in Emery-Dreifuss Muscular Dystrophy. J. Clin. Invest., 117, 1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muchir A., Wu W., Choi J.C., Iwata S., Morrow J., Homma S., Worman H.J. (2012) Abnormal p38a mitogen-activated protein kinase signalling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet., 21, 4325–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moon R.T., Kohn A.D., De Ferrari G.V., Kaykas A. (2004) WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet., 5, 691–701. [DOI] [PubMed] [Google Scholar]
- 10. Reya T., Clevers H. (2005) Wnt signaling in stem cells and cancer. Nature, 6, 314–322. [DOI] [PubMed] [Google Scholar]
- 11. Barandon L., Couffinhal T., Ezan J., Dufourcq P., Costet P., Alzieu P., Leroux L., Moreau C., Dare D., Duplàa C. (2003) Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation, 108, 2282–2289. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y., Liu Y., Zhu X.H., Zhang X.D., Jiang D.S., Bian Z.Y., Zhang X.F., Chen K., Wei X., Gao L., et al. (2014) Dickkopf-3 attenuates pressure overload-induced cardiac remodeling. Cardiovasc. Res., 102, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bao M.W., Cai Z., Zhang X.J., Li L., Liu X., Wan N., Hu G., Wan F., Zhang R., Zhu X., et al. (2015) Dickkopf-3 protects against cardiac dysfunction and ventricular remodeling following myocardial infarction. Basic Res. Cardiol., 110, 25–42. [DOI] [PubMed] [Google Scholar]
- 14. Hatanaka K., Kawata H., Toyofuku T., Yoshida K. (2004) Down-regulation of connexin43 in early myocardial ischemia and protective effect by ischemic preconditioning in rat hearts in vivo. Jpn Heart J., 45, 1007–1019. [DOI] [PubMed] [Google Scholar]
- 15. Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A.H. (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med., 10, 55–63. [DOI] [PubMed] [Google Scholar]
- 16. Rohr S. (2004) Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc. Res., 62, 309–322. [DOI] [PubMed] [Google Scholar]
- 17. de Groot J.R., Veenstra T., Verkerk A.O., Wilders R., Smits J.P., Wilms-Schopman F.J., Wiegerinck R.F., Bourier J., Belterman C.N., Coronel R., et al. (2003) Conduction slowing by the gap junctional uncoupler carbenoxolone. Cardiovasc. Res., 60, 288–297. [DOI] [PubMed] [Google Scholar]
- 18. Thomas S.A., Schuessler R.B., Berul C.I., Beardslee M.A., Beyer E.C., Mendelsohn M.E., Saffitz J.E. (1998) Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction: evidence for chamber-specific molecular determinants of conduction. Circulation, 97, 686–691. [DOI] [PubMed] [Google Scholar]
- 19. Gutstein D.E., Morley G.E., Tamaddon H., Vaidya D., Schneider M.D., Chen J., Chien K.R., Stuhlmann H., Fishman G.I. (2001) Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ. Res., 88, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernandez L., Roux K.J., Wong E.S., Mounkes L.C., Mutalif R., Navasankari R., Rai B., Cool S., Jeong J.W., Wang H., et al. (2010) Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev, Cell, 19, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mounkes L.C., Kozlov S., Hermandez L., Sullivan T., Stewart C.L. (2003) A progeroid syndrome in mice is caused by defects in A-type lamins. Nature, 423, 298–301. [DOI] [PubMed] [Google Scholar]
- 22. Markiewicz E., Tilgner K., Barker N., van de Wetering M., Clevers H., Dorobek M., Hausmanova-Petrusewicz I., Ramaekers F.C., Broers J.L., Blankesteijn W.M., et al. (2006) The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. Embo J., 25, 3275–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wheeler M.A., Warley A., Roberts R.G., Ehler E., Ellis J.A. (2010) Identification of an emerin-beta-catenin complex in the heart important for intercalated disc architecture and beta-catenin localisation. Cell. Mol. Life Sci., 67, 781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stubenvoll A., Rice M., Wietelmann A., Wheeler M., Braun T. (2015) Attenuation of Wnt/β-catenin activity reverses enhanced generation of cardiomyocytes and cardiac defects caused by the loss of emerin. Hum. Mol. Genet., 24, 802–813. [DOI] [PubMed] [Google Scholar]
- 25. Bione S., Maestrini E., Rivella S., Mancini M., Regis S., Romeo G., Toniol0 D. (1994) Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet., 8, 323–327. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Q., Minaisah R.-M., Ferraro E., Li C., Porter L.J., Zhou C., Gao F., Zhang J., Rajgor D., Autore F., et al. (2016) N-terminal nesprin-2 variants regulate β-catenin signalling. Exp. Cell Res., 345, 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Puckelwartz M.J., Kessler E.J., Kim G., DeWitt M.M., Zhang Y., Earley J.U., Depreux F.F.S., Holaska J., Mewborn S.K., Pytel P., et al. (2010) Nesprin-1 mutations in human and murin cardiomyopathy. J. Mol. Cell. Cardiol., 48, 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banerjee I., Zhang J., Moore-Morris T., Pfeiffer E., Buchholz K.S., Liu A., Ouyang K., Stroud M.J., Gerace L., Evans S.M., et al. (2014) Targeted ablation of nesprin 1 and nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biochemical gene response. PLoS Genet., 10, e1004114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schumann H., Holtz J., Zerkowski H.R., Hatzfeld M. (2000) Expression of secreted frizzled related proteins 3 and 4 in human ventricular myocardium correlates with apoptosis related gene expression. Cardiovasc. Res., 45, 720–728. [DOI] [PubMed] [Google Scholar]
- 30. Hermans K.C.M., Blankesteijn W.M. (2015) Wnt signaling in cardiac disease. Compr. Physiol., 5, 1183–1209. [DOI] [PubMed] [Google Scholar]
- 31. Sklepkiewicz P., Shiomi T., Kaur R., Sun J., Kwon S., Mercer B., Bodine P., Schermuly R.T., George I., Schulze P.C., et al. (2015) Loss of secreted frizzled-related protein-1 leads to deterioration of cardiac function in mice and plays a role in human cardiomyopathy. Circ. Heart Fail., 8, 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He W., Zhang L., Ni A., Zhang Z., Mirotsou M., Mao L., Pratt R.E., Dzau V.J. (2010) Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc. Natl. Acad. Sci. USA, 107, 21110–21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van der Heyden M.A., Rook M.B., Hermans M.M., Rijksen G., Boonstra J., Defize L.H., Destrée O.H. (1998) Identification of connexin43 as a functional target fro Wnt signaling. J Cell. Sci., 111, 1741–1749. [DOI] [PubMed] [Google Scholar]
- 34. Severs N.J., Bruce A.F., Dupont E., Rothery S. (2008) Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc. Res., 80, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kostin S., Rieger M., Dammer S., Hein S., Richter M., Klövekorn W.P., Bauer E.P., Schaper J. (2003) Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol. Cell. Biochem., 242, 135–144. [PubMed] [Google Scholar]
- 36. Olson D.J., Christian J.L., Moon R.T. (1991) Effect of wnt-1 and related proteins on gap junctional communication in Xenopus embryos. Science, 252, 1173–1176. [DOI] [PubMed] [Google Scholar]
- 37. Ai Z., Fischer A., Spray D.C., Brown A.M., Fishman G.I. (2000) Wnt-a regulation of connexin 43 in cardiac myocytes. J. Clin. Invest., 105, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J., Li C., Liang D., Lv F., Yuan T., The E., Ma X., Wu Y., Zhen L., Xie D., et al. (2016) LRP6 acts as a scaffold protein in cardiac gap junction assembly. Nat. Comm., 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen P. (1979) The fifteenth Colworth Medal Lecture: The hormaonal control of glycogen metabolism in mammalian muscle by multivalent phosphorylation. Biochem. Soc. Trans., 7, 459–480. [DOI] [PubMed] [Google Scholar]
- 40. Cohen P., Goedert M. (2004) GSK3 inhibitors: development and therapeutic potential. Nat. Rev., 3, 479–487. [DOI] [PubMed] [Google Scholar]
- 41. Coghlan M.P., Culbert A.A., Cross D.A., Corcoran S.L., Yates J.W., Pearce N.J., Rausch O.L., Murphy G.J., Carter P.S., Roxbee Cox L., et al. (2000) Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol., 7, 793–803. [DOI] [PubMed] [Google Scholar]
- 42. Cohen Y., Chetrit A., Cohen Y., Sirota P., Modan B. (1998) Cancer morbidity in psychiatric patients: influence of lithium carbonate treatment. Med. Oncol., 15, 32–36. [DOI] [PubMed] [Google Scholar]
- 43. Muchir A., Shan J., Bonne G., Lehnart S.E., Worman H.J. (2009) Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum. Mol. Genet., 19, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu W., Muchir A., Shan J., Bonne G., Worman H.J. (2011) Mitogen-activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by mutation in lamin A/C gene. Circulation, 123, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muchir A., Reilly S.A., Wu W., Iwata S., Homma S., Bonne G., Worman H.J. (2012) Treatment with selumetinib preserves cardiac function and improves survival in cardiomyopathy caused by mutation in the lamin A/C gene. Cardiovasc. Res., 93, 311–319. [DOI] [PubMed] [Google Scholar]
- 46. Choi J.C., Muchir A., Wu W., Iwata S., Homma S., Morrow J.P., Worman H.J. (2012) Temsirolimus activates autophagy and ameliorates cardiomyopathy caused by lamin A/C gene mutation. Sc. Transl. Med., 4, 144ra102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chatzifrangkeskou M., Le Dour C., Wu W., Morrow J.P., Joseph L.C., Beuvin M., Sera F., Homma S., Vignier N., Mougenot N., et al. (2016) ERK1/2 directly acts on CTGF/CCN2 expression to mediate myocardial fibrosis in cardiomyopathy caused by mutations in the lamin A/C gene. Hum. Mol. Genet., pii, ddw090.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu W., Shan J., Bonne G., Worman H.J., Muchir A. (2016) Pharmacological inhibition of c-Jun N-terminal kinase signaling prevents cardiomyopathy caused by mutation in LMNA gene. Bioch. Biophys. Acta, 1802, 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.