Abstract
Phthalates are used in building materials, medical devices, and personal care products. Most studies on phthalates have focused on single phthalates, but it is important to study mixtures of phthalates because humans are exposed to such mixtures daily. We tested the hypothesis that phthalate mixture exposure decreases antral follicle growth, compromises steroidogenic capacity, and induces atresia. Antral follicles from adult CD-1 mice were cultured with vehicle control or phthalate mixture (1–500 µg/ml) for 96 h. The mixture was made of 35% diethyl phthalate, 21% di(2-ethylhexyl) phthalate, 15% dibutyl phthalate, 15% diisononyl phthalate, 8% diisobutyl phthalate, and 5% benzylbutyl phthalate. During culture, antral follicle diameters were measured every 24 h to monitor growth. After culture, media were subjected to measurements of sex steroid hormones and follicles were subjected to evaluation of gene expression and atresia. The phthalate mixture (100 and 500 µg/ml) decreased antral follicle growth starting at 24 h compared to controls. The mixture at 10, 100, and 500 µg/ml also decreased androstenedione, testosterone, estrone, and estradiol levels compared to control. The mixture (10, 100, and 500 µg/ml) reduced atresia rating, but it induced more oocyte fragmentation compared to control. The phthalate mixture at different doses adversely affected cell cycle regulators, antioxidant enzymes, apoptotic factors, steroidogenic enzymes, and receptors. Collectively, these data indicate that exposure to an environmentally relevant phthalate mixture reduces antral follicle growth, induces oocyte fragmentation, and decreases hormone production by adversely affecting the expression of cell cycle regulators, apoptotic factors, steroidogenic enzymes, and receptors.
Keywords: phthalates, mixture, antral follicles.
Phthalates are a family chemicals used as plasticizers and additives in common products, including plastics, personal care products (PCPs), polyvinyl chloride pipes, and vinyl flooring (Koniecki et al., 2011; Xu et al., 2010). As a result, humans and other animals are exposed to phthalates through ingestion, inhalation, and dermal contact. Metabolites of phthalates are commonly detected in human urine samples. They also can be detected in serum, milk, and saliva (Hines et al., 2009). Interestingly, phthalate metabolites have been detected in follicular fluid (Du et al., 2016; Krotz et al., 2012), indicating that oocytes are directly exposed to phthalates.
The most commonly used phthalates are diethyl phthalate (DEP), di-n-butyl phthalate (DBP), diisobutyl phthalate (DiBP), butyl benzyl phthalate (BBzP), di(2-ethylhexyl) phthalate (DEHP), diisononyl phthalate (DiNP), and diisodecyl phthalate (DiDP) (Johns et al., 2015). Food intake is considered to be a major route of phthalate exposure (Serrano et al., 2014). Phthalates are also commonly used in PCPs and urinary metabolite levels are correlated with the usage of PCPs (Bao et al., 2015; Braun et al., 2014; Cantonwine et al., 2014; Parlett et al., 2013; Romero-Franco et al., 2011). Phthalate exposure is of concern because studies have shown that it is associated with increased human health risks (Hauser and Calafat, 2005). Specifically, urinary DEHP metabolite (mono-ethylhexyl phthalate, MEHP) levels have been associated with pregnancy loss (Toft et al., 2012) and preterm birth (Ferguson et al., 2014a). Moreover, urinary levels of monobenzyl phthalate (MBzP), a metabolite of BBzP and mono-n-butyl phthalate (MBP), a metabolite of DBP, were associated with preterm birth (Meeker et al., 2009b). Childhood exposure to BBzP, DBP, DiBP, and DEHP has been associated with decreased testosterone and dehydroepiandrosterone (DHEA) levels in boys (Ferguson et al., 2014b). Urinary MEHP levels have also been inversely associated with testosterone and estradiol levels in adult male patients from an infertility clinic (Meeker et al., 2009a).
Numerous experimental studies have indicated that phthalates impair reproductive function, but such studies have mainly focused on male reproduction [reviewed in (Kay et al., 2014)]. Exposure to phthalates during gestation results in adverse effects on the male rat reproductive tract by suppressing the androgen pathway; thus, phthalates are known anti-androgenic chemicals (Gray et al., 2006). In male rats, prenatal exposure to a number of phthalates causes “phthalate syndrome”, which is characterized by malformations in the male reproductive organs, retention of nipples, and reduced anogenital distance (Foster, 2006). The effects of phthalates on female reproduction are less extensively studied [reviewed in Kay et al., 2013], but they indicate phthalates reduce the number of follicles, accelerate follicle recruitment, inhibit follicle growth, disrupt ovulation and corpus luteum formation, decrease steroidogenic enzyme expression, and alter sex steroid hormone production (Hannon and Flaws, 2015).
Most previous studies have only examined a single phthalate, and it is well known that humans and animals are exposed to mixtures of phthalates (Ferguson et al., 2014b; Meeker and Ferguson, 2014; Watkins et al., 2014). Available mixture studies have focused on male animals. Exposure to a mixture of 5 phthalates from gestational day (GD) 8–18 in rats reduced male fetal testosterone levels in a dose-additive manner (Howdeshell et al., 2008). Exposure to a mixture of anti-androgens including phthalates from GD 14–18 in rats disrupted male reproductive tract differentiation, induced hypospadias and epididymal agenesis, and caused undescended testes (Rider et al., 2008). Other studies on mixtures of different anti-androgens, including phthalates, also showed that mixture exposure reduced fetal testis testosterone production compared to control in rats (Hannas et al., 2011; Rider et al., 2009).
Information on the effects of a mixture of phthalates on female reproduction is very limited. Prenatal exposure (GD 8-19) to a phthalate mixture caused uterine and vaginal malformations in rats (Hannas et al., 2013). Gestational and lactational exposure to a mixture of endocrine disrupting chemicals (including phthalates) significantly reduced follicle numbers, disrupted estrous cycles, and caused adverse effects in reproductive organs (Johansson et al., 2016). To our knowledge, no studies have been conducted to examine the direct effects of a phthalate mixture on ovarian antral follicles. The antral follicle is the only follicle type that is capable of releasing an egg for fertilization and synthesizing sex steroid hormones such as estrogens (Hirshfield, 1991). Thus, in this study, we tested the hypothesis that phthalate mixture exposure decreases antral follicle growth, compromises steroidogenic capacity, and induces atresia.
METHODS
Chemicals
The mixture we used in our study contains 21% DEHP, 35% DEP, 15% DBP, 8% DiBP, 5% BBzP, and 15% DiNP. This mixture is based on estimates of phthalate exposure in women, which were derived from levels of phthalate metabolites measured in urine samples from pregnant women in Illinois (unpublished data from the iKids study). DEP, DEHP, DBP, DiBP, DiNP, BBzP (>98% purity) were purchased from Sigma-Aldrich (St. Louis, Missouri). To make 500 mg of pure phthalate mixture, weights of each phthalate were calculated according to the percentages. Then, the desired amount of each phthalate was carefully weighed and mixed thoroughly to make the mixture. The intended amount and actual amount of each of the phthalates are shown in Supplemental Material (Supplementary Data). The pure mixture solution was stored at –20 °C for up to 1 month before experimental use. A stock solution of pure mixture was dissolved and diluted in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) to achieve the selected phthalate mixture treatment concentrations (1.33, 13.3, 133, and 665 mg/ml) for final working concentrations of 1, 10, 100, and 500 µg of phthalate mixture per ml of culture medium. Using these treatment, concentrations allowed each working concentration to contain the same volume of chemical and vehicle solution (0.75 µl of phthalate mixture/DMSO solution per ml of culture media).
This is the first time this phthalate mixture has been tested. Thus, concentrations of phthalate mixture were chosen to cover a relatively wide range (1–500 µg/ml), to include a dose that mimics human exposure, and to include some of the doses of individual phthalates that have been shown to disrupt folliculogenesis and steroidogenesis (Craig et al., 2013; Hannon et al., 2015b). Currently, 2 studies measured phthalate concentrations in the follicular fluid (Du et al., 2016; Krotz et al., 2012), but only one of them included a large sample size (Du et al., 2016). In both studies, the median phthalate metabolite levels were below 10 ng/ml of follicular fluid, which is 100 times lower than our lowest dose (1 µg/ml) (Du et al., 2016; Krotz et al., 2012). However, in the study conducted by Du et al., the maximum detection of the single phthalates reached 415 ng/ml for MBP, a level higher than in our lowest dose (1 µg/ml of mixture contains 150 ng/ml of DBP, the parent compound for MBP) (Du et al., 2016). Our lowest doses is therefore within the high end of phthalate detection levels in humans. Previous studies have shown that in cultured antral follicles, DBP at 10 µg/ml disrupted the expression of cell cycle regulators Ccne1 and Cdkn1a (Craig et al., 2013), and DEHP at 10 µg/ml or higher disrupted expression of cell cycle regulators, apoptotic factors, and steroidogenic enzymes, and it induced atresia and decreased hormone production (Hannon et al., 2015b). Further, studies have shown that medical exposure to DEHP can result in relatively high phthalate concentrations in blood compared to concentrations before medical procedures (Kavlock et al., 2002). For example, DEHP concentrations in blood can be as high as 21.6 µg/ml after medical procedures in infants, and can reach 14 µg/ml in adults after medical procedures (Kavlock et al., 2002). In our study, the chosen mixture doses contain approximately 0.21, 2.1, 21, and 105 µg/ml of DEHP; thus our lower doses were within the range of medical exposure levels to phthalates.
Animals
Adult, cycling female CD-1 mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and allowed to acclimate to the facility for at least 2 days before use. The mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility. Food (Harlan Teklad 8604) and water were provided for ad libitum consumption. Temperature was maintained at 22 ± 1 °C, and animals were subjected to 12-h light-dark cycles. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
In vitro antral follicle culture
Adult cycling female CD-1 mice were euthanized and their ovaries were carefully removed to isolate antral follicles. Antral follicles were isolated mechanically from ovaries and interstitial tissue was removed using fine watchmaker forceps as described previously (Gupta et al., 2006; Miller et al., 2005). Antral follicles, diameters ranging from 250 to 400 µm, were selected to be placed individually in wells of 96-well culture plates. At least 2–3 mice were used for each separate culture to provide 60–90 follicles per culture. Individual follicles were randomly selected for each treatment group (n = 6 separate cultures, 6–12 follicles/treatment/culture).
Treatment groups for the antral follicle cultures included vehicle control (DMSO) and phthalate mixture (1, 10, 100, and 500 µg/ml) prepared in supplemented α-MEM. Supplemented α-MEM was prepared with: 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium, Sigma-Aldrich), 100 U/ml penicillin (Sigma-Aldrich), 100 mg/ml streptomycin (Sigma-Aldrich), 5 IU/ml recombinant follicle-stimulating hormone (FSH, Dr. A.F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, California), and 5% fetal bovine serum (Atlanta Biologicals, Lawrenceville, Georgia). The same volume of chemical (0.75 µg/ml of media) was added to each dose to control the amount of vehicle in each culture well to be 0.075%. Each follicle was cultured in 150 µl of medium for 96 h in an incubator at 37 °C with 5% CO2. At the end of the 96-h cultures, media were collected and stored at –80 °C. Some antral follicles were collected, snap frozen, and then stored at –80 °C for gene expression analyses, others were fixed in Dietrich’s solution for histological evaluation of atresia as described below.
Analysis of antral follicle growth
Follicles were cultured for 96 h and the growth was examined every 24 h by measuring follicle diameters. Follicle diameters were measured across perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. The sizes of follicles in each treatment group within each experiment were averaged first and then converted to percentage of the initial follicle size at the start of the culture. Then, percent data were averaged and analyzed across different experiments (n = 6 separate cultures).
Antral follicle culture hormone measurements
Culture media were collected at 96 h from 6 separate antral follicle cultures, and subjected to enzyme-linked immunosorbent assays (ELISAs, DRG International Inc., Springfield, New Jersey) for measurement of progesterone, androstenedione, testosterone, estrone, and estradiol. Assays were run according to the manufacturer’s instructions. Samples were diluted and run in duplicate for measurements of progesterone (1:5 dilution), androstenedione (1:5 dilution), testosterone (1:5 dilution), estrone (no dilution), and estradiol (1:10 dilution) to match the dynamic range of each ELISA kit.
Histological evaluation of antral follicles and atresia rating
After 96 h of culture, some antral follicles were collected for histological evaluation. Supplemented α-MEM was removed from each well and Dietrich’s solution (for 5100 ml final volume, 250 ml of 40% formaldehyde, 712.5 ml of 100% ethanol, 1537.5 mg water, and 50 ml glacial acetic acid) was added to the wells to fix the follicles. Then, the follicles were processed for histological analyses of atresia as described previously (Basavarajappa et al., 2012; Miller et al., 2005). Follicles were dehydrated and prepared for plastic embedding using the Technovit 7100 plastic embedding system (Electron Microscopy Science), and serial sectioned at 2 µm. Sections were mounted on glass slides and stained with Lee’s methylene blue-basic fuchsin. Sections with oocytes were randomly selected and scored for apoptotic bodies and oocyte fragmentation (5 sections per follicle, n = 12 follicles/treatment group, from 3 separate cultures). Based on the amount of apoptotic bodies present in the follicles, follicles were scored on a 1–4 scale as described previously (Basavarajappa et al., 2012; Miller et al., 2005). A score of 1 indicated the presence of apoptotic bodies encompassing 0–3% of the total area of follicle, a score of 2 indicated the presence of apoptotic bodies encompassing 4–10% of total area of follicle, a score of 3 indicated the presence of apoptotic bodies encompassing 11–30% of total area of follicle, and a score of 4 indicated >30% of apoptotic bodies of the total area of follicle. Atresia scores were reported based on the average of all 5 scores from the same follicle, and then analyzed across different treatment groups. Oocyte fragmentation was scored as “Yes” or “No” for each follicle. The percentage of follicles with fragmented oocytes compared to the total number of follicles was calculated. All scoring of atresia and oocyte fragmentation was performed without knowledge of treatment group.
Gene expression analysis
For quantitative polymerase chain reaction (qPCR) analyses, follicles were collected after culture, snap-frozen in liquid nitrogen, and then stored at −80 °C until RNA extraction. Total RNA was then isolated from follicles (n = 3, 9–12 follicles per pool for antral follicle cultures) using an RNeasy Micro kit (Qiagen, Inc., Valencia, California) following the manufacturer's instructions. RNA was eluted in 14 μl of RNase-free water and the concentration was determined using a NanoDrop (λ = 260/280 nm; ND 1000; Nanodrop Technologies Inc., Wilmington, Delaware). Total RNA (100 ng) was reverse transcribed to complementary DNA (cDNA) using the iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, California) according to the manufacturer’s protocol. Analysis of qPCR was conducted using the C and FX96 Real-Time PCR Detection System (Bio-Rad Laboratories) and accompanying CFX Manager Software according to the manufacturer’s protocol. The machine quantifies the amount of PCR product generated by measuring SsoFastEvaGreen dye (Bio-Rad Laboratories) that fluoresces when bound to double-stranded DNA. Specific qPCR primers (Integrated DNA Technologies, Coralville, Iowa) for all the genes of interest are listed in Supplemental Material (Supplementary Data). All qPCR reactions were conducted in triplicate using 1 μl cDNA, forward and reverse primers (5 pmol) for 18S ribosomal RNA (Rn 18S), superoxide dismutase 1 (Sod1), catalase (Cat), glutathione peroxidase (Gpx), glutathione reductase (Gsr), B cell leukemia/lymphoma 2 (Bcl2), Bcl2-associated X protein (Bax), caspase 3 (Casp3), caspase 8 (Casp8), steroidogenic acute regulatory protein (Star), cytochrome-P450 cholesterol side-chain cleavage (Cyp11a1), cytochrome P450 steroid 17-α-hydroxylase 1 (Cyp17a1), 3β-hydroxysteroid dehydrogenase (Hsd3b1), 17β-hydroxysteroid dehydrogenase 1 (Hsd17b1), cytochrome P450 aromatase (Cyp19a1), cyclin A2 (Ccna2), cyclin B1 (Ccnb1), cyclin D2 (Ccdn2), cyclin E1 (Ccne1), cyclin-dependent kinase 4 (Cdk4), cyclin-dependent kinase inhibitor 1a (Cdkn1a), cyclin-dependent kinase inhibitor 1c (Cdkn1c), cyclin-dependent kinase inhibitor 2a (Cdkn2a), estrogen receptor α (Esr1), estrogen receptor β (Esr2), follicle stimulating hormone receptor (Fshr), androgen receptor (Ar), peroxisome proliferator-activated receptor α (Pparα), and peroxisome proliferator-activated receptor γ (Pparγ) and SsoFastEvaGreen Supermix for a final reaction volume of 10 μl. The qPCR program consisted of an enzyme activation step (95 °C for 1 min), an amplification and quantification program (40 cycles of 95 °C for 10 s, 60 °C for 10 s, single fluorescence reading), a step of 72 °C for 5 min, a melt curve (65 °C–95 °C heating, 0.5 °C/sec with continuous fluorescence readings), and a final step at 72 °C for 5 min per the manufacturer's protocol. Rn 18S mRNA expression was used in qPCR analyses as a housekeeping gene. All gene expression data were normalized to the housekeeping gene. Relative fold changes were calculated as the ratio to DMSO group level and were analyzed using a mathematical model for relative quantification of real-time PCR data developed by Pfaffl (2001).
Statistical analysis
All data analyses were performed using SPSS statistical software (SPSS Inc., Chicago, Illinois). Data were expressed as means ± SEM from at least 3 separate experiments. Multiple comparisons between normally distributed experimental groups were made using 1-way analysis of variance (ANOVA) followed by Dunnett post hoc comparison, if equal variances were assumed, or Games–Howell post hoc comparisons if equal variances were not assumed. If data were not normally distributed, comparison between 2 groups was done using Kruskal–Wallis H test for several independent samples and Mann–Whitney U 2-independent sample tests. For oocyte fragmentation, we assigned each follicle a score of “0” or “1”. “0” indicates no oocyte fragmentation was observed in all sections examined and “1” indicates at least 1 section of the follicle had a fragmented oocyte. Then, we utilized Chi-square test to compared treatment groups to control. Statistical significance was assigned at P < .05.
RESULTS
Effects of the Mixture on Antral Follicle Growth
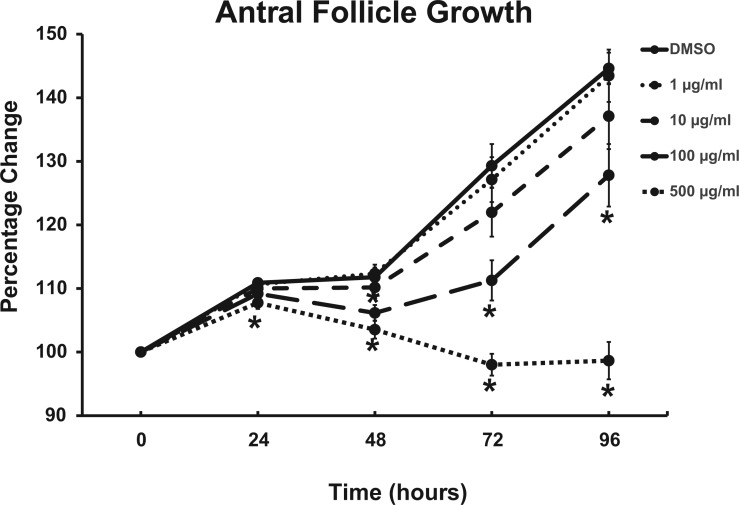
To determine whether phthalate mixture reduces antral follicle growth, individual antral follicles were cultured with either vehicle control (DMSO) or different doses of phthalate mixture (1–500 µg/ml). Follicles in control groups showed significant growth at 96 hours (Figure 1, n = 6 cultures, 6–12 antral follicles/treatment/culture). Phthalate mixture at 1 and 10 µg/ml did not significantly affect growth compared to control at any time point (Figure 1, n = 6 cultures, 6-12 antral follicles/treatment/culture). However, phthalate mixture at 100 µg/ml significantly inhibited growth starting at 48 hours and mixture at 500 µg/ml significantly inhibited antral follicle growth starting at 24 hours when compared to control (Figure 1, n = 6 cultures, 6-12 antral follicles/treatment/culture). The phthalate mixture-induced inhibition of follicle growth persisted throughout the 96-hour culture (Figure 1).
FIG. 1.
Effects of the phthalate mixture on antral follicle growth in culture. Antral follicles were isolated from mouse ovaries and treated with vehicle (DMSO) or phthalate mixture (1–500 µg/ml) for 96 h. Follicle growth was measured every 24 h. The graphs represent means ± SEM from 6 separate cultures (n = 6–12 follicles/treatment/culture). Asterisks (*) indicate significant differences from the control (P < .05).
Effects of the Mixture on Cell Cycle Regulators
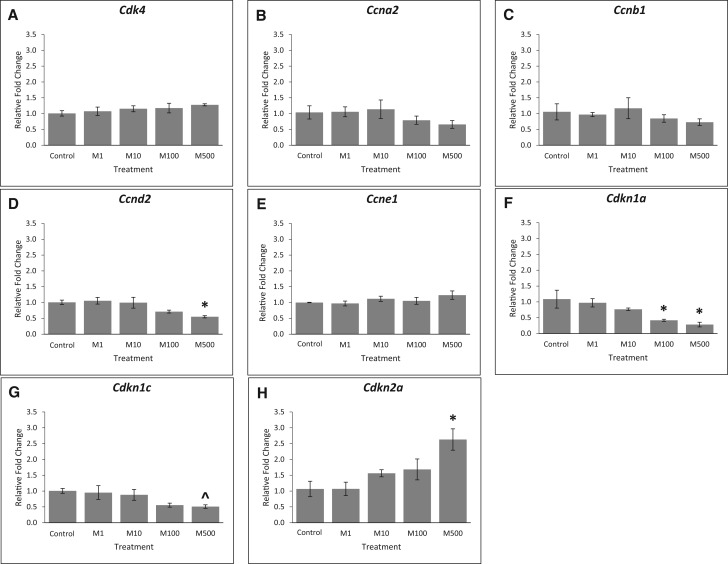
Cell cycle regulators play an important role in regulating follicle growth, and many cell cycle regulators are also the targets of phthalates [reviewed in Hannon and Flaws (2015)]. Thus, we compared expression of several cell cycle regulators in control and phthalate mixture-treated follicles. Our results show that phthalate mixture exposure did not affect the expression of Cdk4, Ccna2, Ccnb1, and Ccne1 (Figs. 2A, B, C, and E, n = 3, P > .05), but it (500 µg/ml) decreased the expression of Ccnd2 compared to control (Figure 2D, n = 3, P < .05). For selected cell cycle inhibitors, phthalate mixture at 100 and 500 µg/ml significantly decreased expression of Cdkn1a (Figure 2F, n = 3, P < .05), it (500 µg/ml) borderline decreased expression of Cdkn1c (Figure 2G, n = 3, P = .08), and it (500 µg/ml) significantly increased the expression of Cdkn2a compared to control (Figure 2H, n = 3, P < .05).
FIG. 2.
Effects of the phthalate mixture on the expression of cell cycle regulators. Antral follicles were isolated from mouse ovaries and treated with vehicle (DMSO) or phthalate mixture (1–500 µg/ml) for 96 h. Follicles were collected and subjected to qPCR analyses for the expression of Cdk4 (A), Ccna2 (B), Ccnb1 (C), Ccnd2 (D), Ccne1 (E), Cdkn1a (F), Cdkn1c (G), and Cdkn2a (H). Relative fold changes of each gene normalized to Rn18s are shown. Graphs represent means ± SEM from 3 separate experiments (n = 9–12 follicles/treatment/experiment). Asterisks (*) indicate significant differences from the control (P < .05). ^ indicates borderline significance compared to control, P = .08. M = mixture.
Effects of the Mixture on Antral Follicle Histology
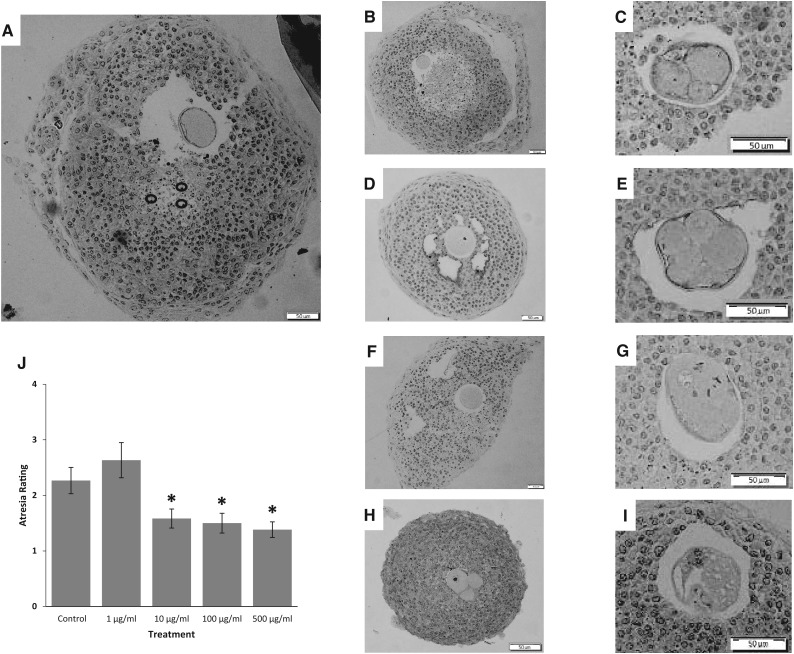
The phthalate mixture-induced reduction in follicle growth may also be the result of follicle atresia. Thus, we evaluated atresia in control and phthalate mixture treated follicles. In control follicles, some apoptotic bodies were normally present after 96 hours of culture, and the oocytes appeared to be intact (Figure 3A). In the phthalate mixture-treated follicles, the oocytes were frequently fragmented (Figure 3C, E, G, H, and I). When we compared the percentage of follicles with fragmented oocytes in control and phthalate mixture-treated follicles, our results indicated that 33.3% of the follicles in the control group, 16.7% of the follicles in the 1 µg/ml group, 33.3% follicles in the 10 µg/ml group, 58.3% in the 100 µg/ml group, and 75% in the 500 µg/ml group had fragmented oocytes (n = 12 different follicles/treatment group; P < .05 for control vs. 500 µg/ml). Interestingly, in phthalate mixture-treated follicles, the presence of apoptotic bodies decreased with the occurrence of oocyte fragmentation (Figure 3). Specifically, the presence of apoptotic bodies for phthalate mixture-treated follicles (10, 100, and 500 µg/ml) was lower than control follicles as indicated by the atresia ratings (Figure 3J, n = 12, P < .05).
FIG. 3.
Effect of the phthalate mixture on antral follicle atresia. Antral follicles were isolated from mouse ovaries and treated with vehicle (DMSO) or phthalate mixture (1–500 µg/ml) for 96 h. Following culture, antral follicles were processed for histological evaluation of atresia. A representative image of a DMSO treated follicle is found in panel A, representative apoptotic bodies are circled. Representative images of mixture at 1, 10, and 100 μg/ml with intact oocytes are shown in B, D, and F, respectively. Representative images of mixture at 1, 10, and 100 μg/ml with fragmented oocytes are shown in C, E, and G, respectively, Panel H and I are images of oocytes in the 500 μg/ml treatment group. All scale bars indicate 50 μm. Atresia scores are shown in panel J. A score of 1 indicates the presence of apoptotic bodies encompassing 0–3% of the total area of follicle, a score of 2 indicates the presence of apoptotic bodies encompassing 4–10% of total area of follicle, a score of 3 indicates the presence of apoptotic bodies encompassing 11–30% of total area of follicle, and a score of 4 indicates >30% of apoptotic bodies of the total area of follicle. Graph represents means ± SEM from 3 separate experiments, with 12 follicles/treatment group in each experiment. Asterisks (*) represent significant difference from the control (P < .05).
Effects of the Mixture on Apoptotic Factors
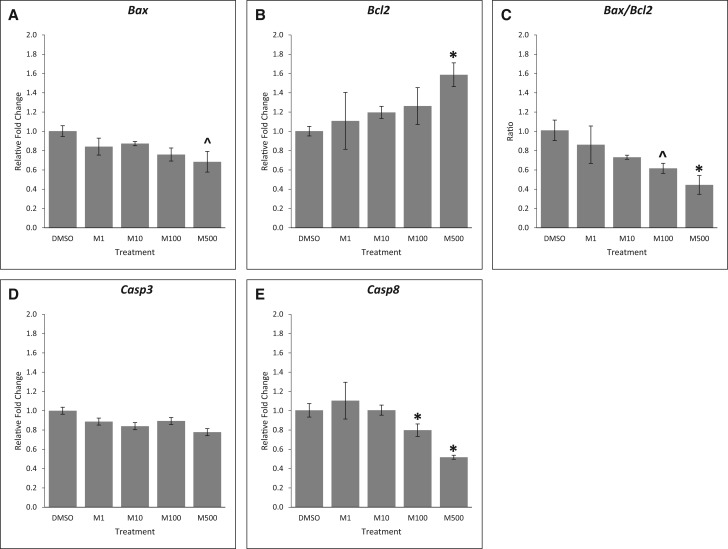
Next, we expanded our findings on the effects of the mixture on atresia by comparing the expression of key apoptotic factors in control and mixture-treated follicles. Our results show that phthalate mixture at the 3 lowest doses did not affect the expression of the pro-apoptotic factor Bax and the anti-apoptotic factor Bcl2 (Figure 4A and B, n = 3, P > .05), but phthalate mixture at 500 µg/ml decreased the expression of Bax (Figure 4A, n = 3, borderline significance, P = .058) and significantly increased the expression of Bcl2 compared to control (Figure 4B, n = 3, P < .05). The mixture at 500 µg/ml also decreased the ratio of Bax/Bcl2, indicating decreased apoptosis (Figure 4C, n = 3, *P < .05, ^P = .09). Further, although the phthalate mixture did not affect the expression of pro-apoptotic factor Casp3 (Figure 4D, n = 3, P > .05), it (100 and 500 µg/ml) significantly reduced the expression of pro-apoptotic factor Casp8 (Figure 4E, n = 3, P < .05), indicating reduced apoptosis in these follicles compared to control.
FIG. 4.
Effects of the phthalate mixture on the expression of apoptotic factors. Antral follicles were isolated from mouse ovaries and treated with vehicle (DMSO) or phthalate mixture (1–500 µg/ml) for 96 h. Follicles were collected and subjected to qPCR analyses for the expression of Bax (A), Bcl2 (B), Casp3 (D), and Casp8 (E). Relative fold changes of each gene normalized to Rn18s are shown. Ratio of the gene expression was calculated and is shown for Bax/Bcl2 (C). Graphs represent means ± SEM from 3 separate experiments (n = 9–12 follicles/treatment/experiment). Asterisks (*) indicate significant differences from the control (P < .05). ^ indicates borderline significance compared to control, P = .06 for panel A, P = .09 for (C). M = mixture.
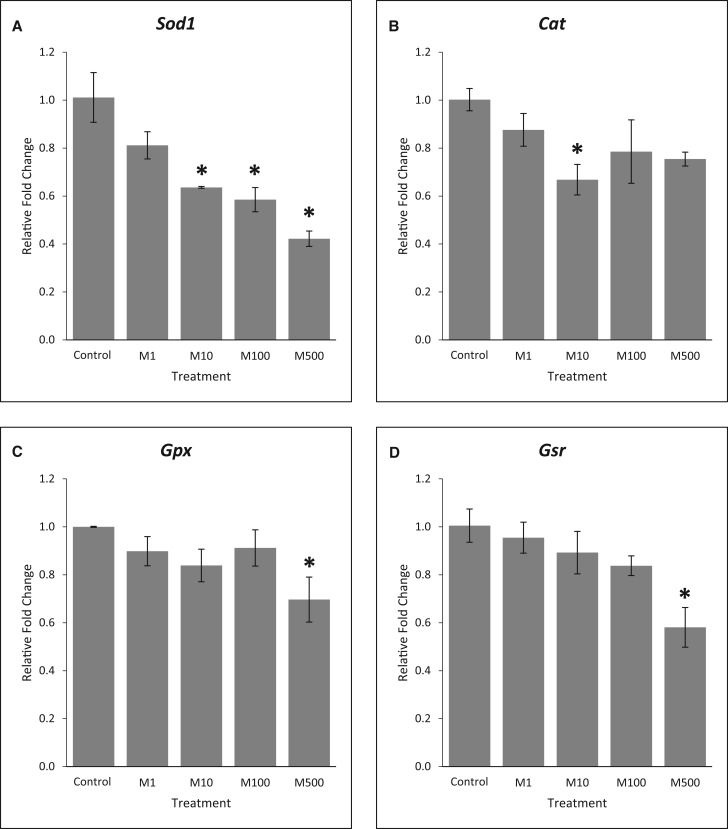
Effects of the Mixture on Antioxidant Enzymes
Phthalates have been shown to cause oxidative stress in the ovary (reviewed in Hannon and Flaws (2015)) and oxidative stress has been shown to regulate atresia (Behrman et al. 2001). To determine whether the phthalate mixture altered oxidative stress, the expression of 4 key antioxidant enzymes were compared between control and mixture-treated follicles. Our results show that phthalate mixture reduced expression of all the selected antioxidant enzymes (Figure 5, n = 3, P < .05). Specifically, at 96 h, phthalate mixture at 10, 100, and 500 µg/ml significantly reduced the expression of Sod1 (Figure 5A), phthalate mixture at 10 µg/ml reduced expression of Cat (Figure 5B), and phthalate mixture at 500 µg/ml significantly reduced the expression of Gpx (Figure 5C) and Gsr (Figure 5D) compared to control (n = 3, P < .05).
FIG. 5.
Effects of the phthalate mixture on the expression of antioxidant enzymes. Antral follicles were isolated from mouse ovaries and treated with vehicle (DMSO) or phthalate mixture (1–500 µg/ml) for 96 h. Follicles were collected and subjected to qPCR analyses for the expression of Sod1 (A), Cat (B), Gpx (C), and Gsr (D). Relative fold changes of each gene normalized to Rn18s are shown. Graphs represent means ± SEM from 3 separate experiments (n = 9–12 follicles/treatment/experiment). Asterisks (*) indicate significant differences from the control (P < .05). M = mixture.
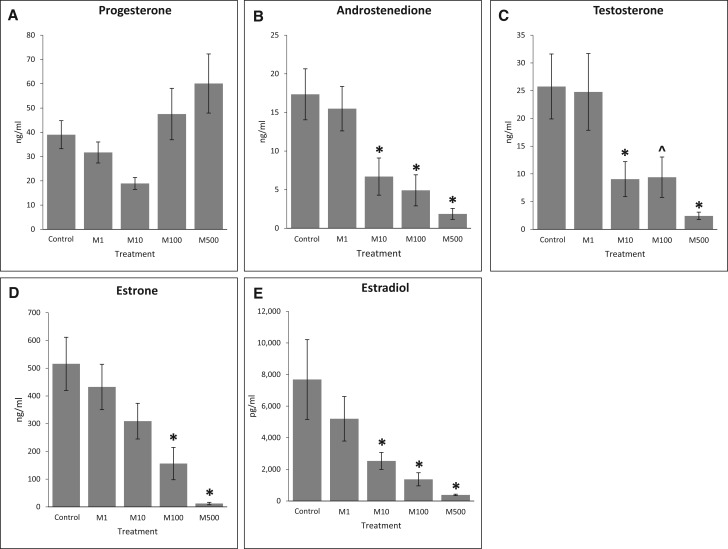
Effects of the Mixture on Hormone Production
The effects of phthalate mixture on steroidogenesis in antral follicles were tested by comparing the sex steroid hormone levels in culture media from control and mixture-treated follicles after 96 h of culture. The most upstream precursor sex steroid hormone that we were able to measure was progesterone. The phthalate mixture did not affect the level of progesterone compared to control (Figure 6A, n = 6). However, the phthalate mixture at 10, 100, and 500 µg/ml significantly reduced the levels of androstenedione compared to control (Figure 6B, n = 6, P < .05). Further, the phthalate mixture at 10 and 500 µg/ml significantly reduced testosterone levels and the mixture at 100 µg/ml showed a borderline significance of reducing testosterone levels compared to control (Figure 6C, n = 6, *P < .05, ^P = .06). Moreover, phthalate mixture at 100 and 500 µg/ml significantly reduced estrone production compared to control (Figure 6D, n = 6, P < .05). Finally, phthalate mixture at 10, 100, and 500 µg/ml significantly reduced estradiol levels compared to control (Figure 6E, n = 6, P < .05).
FIG. 6.
Effect of the phthalate mixture on antral follicle-produced sex steroid hormone levels. Antral follicles were isolated from mouse ovaries and treated with vehicle (DMSO) or phthalate mixture (1–500 µg/ml) for 96 h. Following culture, media were pooled per treatment group and were subjected to ELISAs for the measurements of progesterone (A), androstenedione (B), testosterone (C), estrone (D), and estradiol (E). Graph represents means ± SEM from 6 separate experiments, with medium from 6 to 12 wells/treatment group in each experiment. Asterisks (*) indicate significant differences from the control (P < .05). ^ indicates borderline significance compared to control, P = .06. M = mixture.
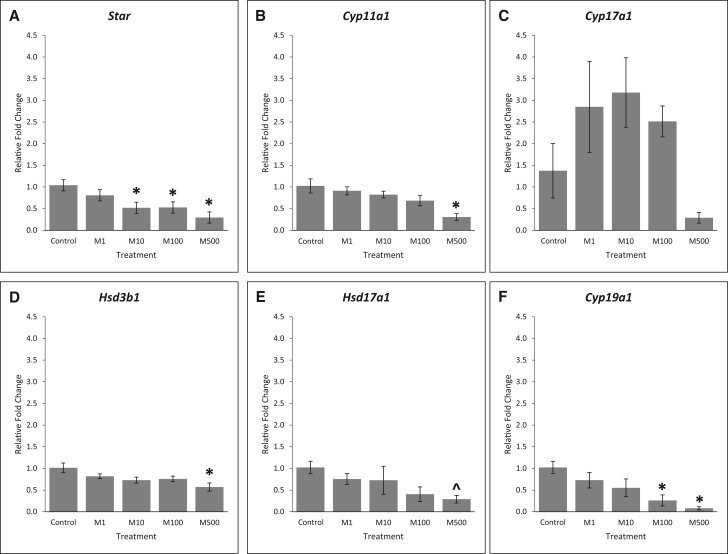
Effects of the Mixture on Steroidogenic Enzymes
Given that the phthalate mixture reduced several sex steroid hormones, we next compared the expression of steroidogenic enzymes in control and phthalate mixture-treated follicles. STAR is the enzyme that transports cholesterol into thecal and granulosa cells. Our results show that phthalate mixture at 10, 100, and 500 µg/ml significantly reduced the expression of Star compared to control (Figure 7A, n = 3, P < .05). CYP11A1 converts cholesterol into pregnenolone. The phthalate mixture at 500 µg/ml significantly decreased the expression of Cyp11a1 compared to control (Figure 7B, n = 3, P < .05). Pregnenolone is converted to DHEA by CYP17A1 in the granulosa cells. The phthalate mixture did not affect the expression of Cyp17a1 compared to control (Figure 7C, n = 3, P > .05). HSD3B1 converts pregnenolone to progesterone in both thecal and granulosa cells and it converts DHEA into androstenedione in the thecal cells. Our results showed that phthalate mixture at 500 µg/ml significantly reduced the expression of Hsd3b1 compared to control (Figure 7D, n = 3, P < .05). HSD17A1 is the enzyme that converts androstenedione to testosterone in the thecal cell and converts estrone into estradiol in the granulosa cells. The phthalate mixture did not significantly affect the expression of Hsd17a1 compared to control (Figure 7E, n = 3, P > .05, ^P = .075). In the granulosa cells, CYP19A1 is responsible for converting androstenedione into estrone and testosterone into estradiol. Phthalate mixture at 100 and 500 µg/ml significantly reduced the expression of Cyp19a1 compared to control (Figure 7F, n = 3, P < .05).
FIG. 7.
Effects of the phthalate mixture on the expression of steroidogenic enzymes. Antral follicles were isolated from mouse ovaries and treated with vehicle (DMSO) or phthalate mixture (1–500 µg/ml) for 96 h. Follicles were collected and subjected to qPCR analyses for the expression of Star (A), Cyp11a1 (B), Cyp17a1 (C), Hsd3b1 (D), Hsd17a1 (E), and Cyp19a1 (F). Relative fold changes of each gene normalized to Rn18s are shown. Graphs represent means ± SEM from 3 separate experiments (n = 9–12 follicles/treatment/experiment). Asterisks (*) indicate significant differences from the control (P < .05) ^ indicates borderline significance compared to control, P = .075. M = mixture.
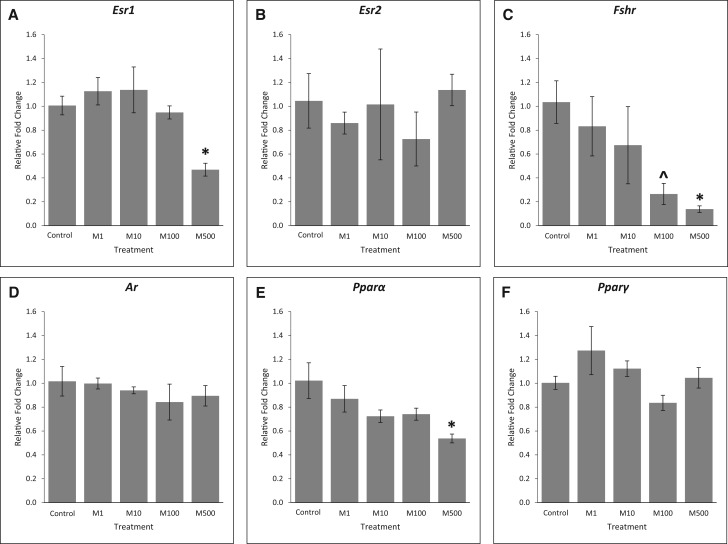
Effects of the Mixture on Receptors
Previous studies have shown that several hormone receptors and peroxisome proliferator-activated receptors (PPARs) are targets of some phthalates [reviewed in Hannon and Flaws (2015)]. Therefore, we compared the expression of several key receptors in control and phthalate mixture-treated follicles. Our results show that after 96 h of culture, phthalate mixture exposure at 500 µg/ml significantly reduced the expression of Esr1 (Figure 8A, n = 3, P < .05), but it did not affect the expression of Esr2 compared to control (Figure 8B, n = 3, P > .05). Moreover, phthalate mixture at 500 µg/ml significantly reduced the expression of Fshr and phthalate mixture at 100 µg/ml trended to reduce the expression compared to control (Figure 8C, n = 3, *P < .05, ^P = .07). The phthalate mixture did not significantly affect Ar expression compared to control (Figure 8D, n = 3, P > .05), but it (500 µg/ml) significantly reduced Pparα expression (Figure 8E, n = 3, P < .05). Finally, phthalate mixture did not affect the expression of Pparγ at any doses compared to control (Figure 8F, n = 3, P > .05).
FIG. 8.
Effects of the phthalate mixture on the expression of receptors. Antral follicles were isolated from mouse ovaries and treated with vehicle (DMSO) or phthalate mixture (1–500 µg/ml) for 96 h. Follicles were collected and subjected to qPCR analyses for the expression of Esr1 (A), Esr2 (B), Fshr (C), Ar (D), Pparα (E), and Pparγ (F). Relative fold changes of each gene normalized to Rn18s are shown. Graphs represent means ± SEM from 3 separate experiments (n = 9–12 follicles/treatment/experiment). Asterisks (*) indicate significant differences from the control (P < .05). ^ indicates borderline significance compared to control, P = .07. M = mixture.
DISCUSSION
In this study, we developed a phthalate mixture based on the phthalate levels detected in women and our results show that this mixture reduces follicle growth, steroidogenic capacity, and the health of antral follicles in mice. Previous studies indicate that exposure to single phthalates, like DEHP, inhibits antral follicle growth by 48 h (Gupta et al. 2010; Hannon and Flaws, 2015). Thus, we expected that the phthalate mixture would also reduce antral follicle growth in culture. Interestingly, our results showed that the phthalate mixture significantly decreased antral follicle growth as early as 24 h in culture. It is likely that we observed decreased follicle growth at an earlier time point than with single phthalates because our highest dose of the mixture contains a higher amount of DEHP than in the DEHP study mentioned above. In the 500 µg/ml mixture, the DEHP concentration is approximately 105 µg/ml. Thus, the concentration of DEHP is higher than that used in studies with DEHP alone (10 and 100 µg/ml) (Gupta et al., 2010).
Reduced follicle growth is likely due to disrupted cell cycle regulation. DBP and DEHP cause defects in the cell cycle by affecting the gene expression levels of cell cycle regulators (reviewed in Hannon and Flaws (2015)). Therefore, we tested the gene expression levels of the key cell cycle regulators. In antral follicles, the majority of the cells are granulosa cells and granulosa cells are actively proliferating in growing antral follicles. As a result, the expression of the selected genes reflects the expression in granulosa cells. Our results show that phthalate mixture exposure decreased Ccnd2 expression. Cyclin D2 is crucial for granulosa cell proliferation in response to FSH stimulation (Sicinski et al., 1996). In our experiments, we added equal amounts of FSH to each culture well. Thus, it is likely that the reduced expression of Ccnd2 decreases the response of granulosa cells to FSH, leading to inhibition of granulosa cell proliferation. Moreover, phthalate mixture exposure decreased the expression of Cdkn1a and increased the expression of Cdkn2a. CDKN1A (p21) inhibits the CDK1-cyclin A complex to establish the G1 phase (De Clercq and Inze, 2006). Our results showed that the mixture reduced Cdkn1a expression, indicating that the establishment of the G1 phase for follicular cells in these follicles was reduced compared to control. CDKN2A (p16) binds to CDK4 and CDK6 to prevent G1-S transition (De Clercq and Inze, 2006). Phthalate mixture significantly increased the expression of Cdkn2a compared to control, indicating that the cell cycle was arrested and follicular cells were not able to transit from G1 to S phase. This finding is similar to those of Craig et al., in which more DBP-treated follicular cells were arrested at G1 stage compared to controls (Craig et al., 2013).Taken together, these data suggest that because phthalate mixture dramatically increased the expression of Cdkn2a and decreased the expression of Ccnd2, follicular cells were likely arrested at G1 phase, the cell cycle could not progress, and the expression of Cdkn1a was decreased to try to possibly compensate for the effects of Ccnd2 and Cdkn2a. However, this compensatory effect was probably not enough to overcome the cell cycle arrest, resulting in reduced proliferation of follicular cells, which further led to reduced antral follicle growth.
Previous studies suggest that reduced antral follicle growth may also due to increased atresia (Craig et al., 2013; Hannon et al., 2015b). The reduction of antral follicle growth in the 500 µg/ml group was dramatic; thus, we expected that follicles in this treatment group were undergoing atresia. Surprisingly, we observed fewer apoptotic bodies in the 3 higher treatment groups (10, 100, and 500 µg/ml) compared to control. However, the presence of oocyte fragmentation in these groups was more frequent compared to control. Our gene expression results confirmed these histological findings. We observed an increased expression of anti-apoptotic factor Bcl2 and decreased ratio of Bax/Bcl2, indicating a dominant effect of anti-apoptotic factor Bcl2, which further protects the cell from apoptosis. Moreover, our results on the expression of Casp8 also support the histological findings, indicating reduced cell death. These findings differ from those reported in studies using single phthalates. DEHP (10 and 100 µg/ml) and DBP (100 µg/ml) increase antral follicle atresia in culture (Craig et al., 2013; Hannon et al., 2015b). Thus, it is likely that the phthalate mixture acts via different mechanisms to induce different effects than single phthalates. The reason that we observed decreased atresia in the mixture treated follicles may be because studies have shown that cells at G1/S transition are susceptible to undergoing apoptosis and cells at G1 stage are relatively resistant to apoptosis (Quirk et al., 2004). In our experiments, granulosa cells of antral follicles in the phthalate treatment groups were arrested at the G1 stage, whereas the granulosa cells of antral follicles in the control groups were not arrested in G1. As a result, control follicles increased in size and acquired apoptotic bodies due to normal granulosa cell proliferation and G1/S transition. The absence or reduced amount of atretic bodies in phthalate mixture treated groups is likely due to the resistance of apoptosis because of the cell cycle arrest.
In vitro antral follicle studies also showed that oxidative stress is likely a mechanism via which individual phthalates inhibit follicle growth [reviewed in Hannon and Flaws (2015)]. We measured expression of important antioxidant enzymes to determine whether phthalate mixture induces oxidative stress in our study. Our results show that phthalate mixture significantly reduced expression of antioxidant enzymes compared to control, indicating less availability of antioxidant enzymes in the exposed follicles, which could potentially increase oxidative stress in these follicles. Future examinations of reactive oxygen species levels are needed to further confirm that the phthalate mixture induces oxidative stress.
Antral follicles are the major producers of the sex steroid hormones required for female reproductive and non-reproductive health (Cauley, 2015; dos Santos et al., 2014; Toffoletto et al., 2014). Thus, we examined the effects of the mixture of steroidogenic capacity. The phthalate mixture reduced androstenedione, testosterone, estrone, and estradiol production compared to control. Moreover, the mixture disrupted the enzymes involved in steroidogenesis pathway. Collectively, these data indicate that the phthalate mixture disrupts the steroidogenesis pathway by affecting steroidogenic enzymes.
We also examined the effects of phthalate mixture on selected receptors. Estrogen receptors are important in folliculogenesis. Estrogens act through ESR1 to induce follicular cell proliferation and act via ESR2 to promote differentiation (Britt and Findlay, 2002). The phthalate mixture significantly reduced the expression of Esr1 compared to control, but it did not affect on the expression of Esr2. Reduced expression of Esr1 could lead to reduced follicular cell proliferation, a scenario observed in our study. In addition, phthalate mixture reduced the expression of Fshr compared to control, indicating that follicles in these groups had impaired FSH-stimulated downstream effects. These impaired FSH-stimulated downstream effects include reduced granulosa cell proliferation and sex steroid hormone production, which have also been observed in single phthalate treatment (Lovekamp-Swan and Davis, 2003; Wang et al., 2016). Androgen receptors also play an important role in folliculogenesis and steroidogenesis (reviewed in (Prizant et al., 2014)). Our results show that the phthalate mixture did not affect the expression of Ar. Thus, it is likely that phthalate mixture did not act through the androgen receptor to induce adverse effects. PPARs regulate transcription of several genes in the ovary and studies have shown that MEHP acts through PPARα and PPARγ to decrease the hormone production in rats (Lovekamp-Swan and Davis, 2003). The phthalate mixture decreased expression of Pparα, indicating that it is likely that some doses of the phthalate mixture act on the PPARs to reduce steroidogenesis in mouse antral follicles.
In summary, our study shows that direct exposure to a phthalate mixture adversely affects antral follicle health in vitro. Specifically, the phthalate mixture decreases antral follicle growth, induces oocyte fragmentation, and disrupts steroidogenesis by arresting cell cycle, inducing oxidative stress, reducing sex steroid hormone production and steroidogenic enzyme expression, and decreasing receptor expression. We hypothesize that phthalate mixture disrupts cell cycle and hormone receptor expression to reduce the antral follicle growth and induce oxidative stress, which further reduces steroidogenesis and induces oocyte fragmentation. However, further investigations are needed to test our hypothesis. Future studies should investigate the effects of this phthalate mixture in vivo because phthalates are quickly transformed into their metabolites after they enter the body (Frederiksen et al., 2007; Hauser and Calafat, 2005). Moreover, metabolites of some phthalates have been shown to act via different mechanisms than the parent compounds and to induce adverse effects at lower concentrations (Gupta et al., 2010; Hannon et al., 2015a; Wang et al., 2012). We also observed that the phthalate mixture causes oocyte fragmentation. Oocyte fragmentation has been reported to occur because of disruption in intracellular Ca2+ concentrations, the PI3K/Akt/mTOR pathway, and cytoplasmic polyadenylation element (Chen et al., 2016; Racki and Richter, 2006; Tiwari et al., 2015). Future studies should examine that if phthalate mixture interferes with these processes to cause oocyte fragmentation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all the members in Dr Flaws’ lab for technical assistance.
FUNDING
This work was supported by a National Institute Health grant [P01 ES022848 and R56 ES025147 to JAF]; an Environmental Protection Agency grant [RD-83459301 to JAF], and an Environmental Toxicology Fellowship to [CZ].
REFERENCES
- Bao J., Wang M., Ning X., Zhou Y., He Y., Yang J., et al. (2015). Phthalate concentrations in personal care products and the cumulative exposure to female adults and infants in shanghai. J. Toxicol. Environ. Health Part A 78, 325–341. [DOI] [PubMed] [Google Scholar]
- Basavarajappa M. S., Hernandez-Ochoa I., Wang W., Flaws J. A. (2012). Methoxychlor inhibits growth and induces atresia through the aryl hydrocarbon receptor pathway in mouse ovarian antral follicles. Reprod. Toxicol. 34, 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman H. R., Kodaman P. H., Preston S. L., Gao S. (2001). Oxidative stress and the ovary. J. Soc. Gynecol. Invest. 8, S40–S42. [DOI] [PubMed] [Google Scholar]
- Braun J. M., Just A. C., Williams P. L., Smith K. W., Calafat A. M., Hauser R. (2014). Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J. Exposure Sci. Environ. Epidemiol. 24, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt K. L., Findlay J. K. (2002). Estrogen actions in the ovary revisited. J. Endocrinol. 175, 269–276. [DOI] [PubMed] [Google Scholar]
- Cantonwine D. E., Cordero J. F., Rivera-Gonzalez L. O., Anzalota Del Toro L. V., Ferguson K. K., Mukherjee B., et al. (2014). Urinary phthalate metabolite concentrations among pregnant women in northern puerto rico: Distribution, temporal variability, and predictors. Environ. Int. 62, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley J. A. (2015). Estrogen and bone health in men and women. Steroids 99, 11–15. [DOI] [PubMed] [Google Scholar]
- Chen X. Y., Xia H. X., Guan H. Y., Li B., Zhang W. (2016). Follicle loss and apoptosis in cyclophosphamide-treated mice: What's the matter? Int. J. Mol. Sci. 17, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig Z. R., Hannon P. R., Wang W., Ziv-Gal A., Flaws J. A. (2013). Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol. Reprod. 88, 23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq A., Inze D. (2006). Cyclin-dependent kinase inhibitors in yeast, animals, and plants: A functional comparison. Critic. Rev. Biochem. Mol. Biol. 41, 293–313. [DOI] [PubMed] [Google Scholar]
- dos Santos R. L., da Silva F. B., Ribeiro R. F. Jr, Stefanon I. (2014). Sex hormones in the cardiovascular system. Hormone Mol. Biol. Clin. Invest. 18, 89–103. [DOI] [PubMed] [Google Scholar]
- Du Y. Y., Fang Y. L., Wang Y. X., Zeng Q., Guo N., Zhao H., et al. (2016). Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod. Toxicol. (Elmsford, NY) 61, 142–150. [DOI] [PubMed] [Google Scholar]
- Ferguson K. K., McElrath T. F., Ko Y. A., Mukherjee B., Meeker J. D. (2014a). Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int. 70, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K. K., Peterson K. E., Lee J. M., Mercado-Garcia A., Blank-Goldenberg C., Tellez-Rojo M. M., et al. (2014b). Prenatal and peripubertal phthalates and bisphenol a in relation to sex hormones and puberty in boys. Reprod. Toxicol. (Elmsford, NY) 47, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. M. (2006). Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 29, 140–147. discussion 181-145. [DOI] [PubMed] [Google Scholar]
- Frederiksen H., Skakkebaek N. E., Andersson A. M. (2007). Metabolism of phthalates in humans. Mol. Nutr. Food Res. 51, 899–911. [DOI] [PubMed] [Google Scholar]
- Gray L. E. Jr, Wilson V. S., Stoker T., Lambright C., Furr J., Noriega N., et al. (2006). Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int. J. Androl. 29, 96–104. discussion 105-108. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Miller K. P., Babus J. K., Flaws J. A. (2006). Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol. Sci. 93, 382–389. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Singh J. M., Leslie T. C., Meachum S., Flaws J. A., Yao H. H. (2010). Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol. Appl. Pharmacol. 242, 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R., Lambright C. S., Furr J., Howdeshell K. L., Wilson V. S., Gray L. E. Jr. (2011). Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol. Sci. 123, 206–216. [DOI] [PubMed] [Google Scholar]
- Hannas B. R., Howdeshell K. L., Furr J., Gray L. E. Jr. (2013). In utero phthalate effects in the female rat: a model for mrkh syndrome. Toxicol. Lett. 223, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Flaws J. A. (2015a). Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol. Reprod. 92, 120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Gupta R. K., Flaws J. A. (2015b). Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 284, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Flaws J. A. (2015). The effects of phthalates on the ovary. Front. Endocrinol. 6, 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R., Calafat A. M. (2005). Phthalates and human health. Occup. Environ. Med. 62, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines E. P., Calafat A. M., Silva M. J., Mendola P., Fenton S. E. (2009). Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating north carolina women. Environ. Health Perspect. 117, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield A. N. (1991). Development of follicles in the mammalian ovary. Int. Rev. Cytol. 124, 43–101. [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Wilson V. S., Furr J., Lambright C. R., Rider C. V., Blystone C. R., et al. (2008). A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol. Sci. 105, 153–165. [DOI] [PubMed] [Google Scholar]
- Johansson H. K., Jacobsen P. R., Hass U., Svingen T., Vinggaard A. M., Isling L. K., et al. (2016). Perinatal exposure to mixtures of endocrine disrupting chemicals reduces female rat follicle reserves and accelerates reproductive aging. Reprod. Toxicol. (Elmsford, NY) 61, 186–194. [DOI] [PubMed] [Google Scholar]
- Johns L. E., Cooper G. S., Galizia A., Meeker J. D. (2015). Exposure assessment issues in epidemiology studies of phthalates. Environ. Int. 85, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R., Boekelheide K., Chapin R., Cunningham M., Faustman E., Foster P., et al. (2002). Ntp center for the evaluation of risks to human reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. (Elmsford, NY) 16, 529–653. [DOI] [PubMed] [Google Scholar]
- Kay V. R., Chambers C., Foster W. G. (2013). Reproductive and developmental effects of phthalate diesters in females. Critic. Rev. Toxicol. 43, 200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay V. R., Bloom M. S., Foster W. G. (2014). Reproductive and developmental effects of phthalate diesters in males. Critic. Rev. Toxicol. 44, 467–498. [DOI] [PubMed] [Google Scholar]
- Koniecki D., Wang R., Moody R. P., Zhu J. (2011). Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 111, 329–336. [DOI] [PubMed] [Google Scholar]
- Krotz S. P., Carson S. A., Tomey C., Buster J. E. (2012). Phthalates and bisphenol do not accumulate in human follicular fluid. J. Assist. Reprod. Genet. 29, 773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp-Swan T., Davis B. J. (2003). Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 111, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Calafat A. M., Hauser R. (2009a). Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J. Androl. 30, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Hu H., Cantonwine D. E., Lamadrid-Figueroa H., Calafat A. M., Ettinger A. S., et al. (2009b). Urinary phthalate metabolites in relation to preterm birth in mexico city. Environ. Health Perspect. 117, 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Ferguson K. K. (2014). Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from nhanes 2011-2012. J.Clin. Endocrinol. Metabol. 99, 4346–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. P., Gupta R. K., Greenfeld C. R., Babus J. K., Flaws J. A. (2005). Methoxychlor directly affects ovarian antral follicle growth and atresia through bcl-2- and bax-mediated pathways. Toxicol. Sci. 88, 213–221. [DOI] [PubMed] [Google Scholar]
- Parlett L. E., Calafat A. M., Swan S. H. (2013). Women's exposure to phthalates in relation to use of personal care products. J. Exposure Sci. Environ. Epidemiol. 23, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time rt-pcr. Nucleic Acids Res. 29, e45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prizant H., Gleicher N., Sen A. (2014). Androgen actions in the ovary: Balance is key. J. Endocrinol. 222, R141–R151. [DOI] [PubMed] [Google Scholar]
- Quirk S. M., Cowan R. G., Harman R. M., Hu C. L., Porter D. A. (2004). Ovarian follicular growth and atresia: The relationship between cell proliferation and survival. J. Anim. Sci. 82 E-Suppl, E40–E52. [DOI] [PubMed] [Google Scholar]
- Racki W. J., Richter J. D. (2006). Cpeb controls oocyte growth and follicle development in the mouse. Dev. (Cambridge, England) 133, 4527–4537. [DOI] [PubMed] [Google Scholar]
- Rider C. V., Furr J., Wilson V. S., Gray L. E. Jr. (2008). A mixture of seven antiandrogens induces reproductive malformations in rats. Int. J. Androl. 31, 249–262. [DOI] [PubMed] [Google Scholar]
- Rider C. V., Wilson V. S., Howdeshell K. L., Hotchkiss A. K., Furr J. R., Lambright C. R., et al. (2009). Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicol. Pathol. 37, 100–113. [DOI] [PubMed] [Google Scholar]
- Romero-Franco M., Hernandez-Ramirez R. U., Calafat A. M., Cebrian M. E., Needham L. L., Teitelbaum S., et al. (2011). Personal care product use and urinary levels of phthalate metabolites in mexican women. Environ. Int. 37, 867–871. [DOI] [PubMed] [Google Scholar]
- Serrano S. E., Braun J., Trasande L., Dills R., Sathyanarayana S. (2014). Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 13, 43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P., Donaher J. L., Geng Y., Parker S. B., Gardner H., Park M. Y., et al. (1996). Cyclin d2 is an fsh-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384, 470–474. [DOI] [PubMed] [Google Scholar]
- Tiwari M., Prasad S., Tripathi A., Pandey A. N., Ali I., Singh A. K., et al. (2015). Apoptosis in mammalian oocytes: a review. Apoptosis. 20, 1019–1025. [DOI] [PubMed] [Google Scholar]
- Toffoletto S., Lanzenberger R., Gingnell M., Sundstrom-Poromaa I., Comasco E. (2014). Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology 50, 28–52. [DOI] [PubMed] [Google Scholar]
- Toft G., Jonsson B. A., Lindh C. H., Jensen T. K., Hjollund N. H., Vested A., et al. (2012). Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ. Health Perspect. 120, 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Craig Z. R., Basavarajappa M. S., Hafner K. S., Flaws J. A. (2012). Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol. Reprod. 87, 152.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. J., Xiong G. P., Luo X. M., Huang S. Z., Liu J., Huang X. L., et al. (2016). Dibutyl phthalate inhibits the effects of follicle-stimulating hormone on rat granulosa cells through down-regulation of follicle-stimulating hormone receptor. Biol. Reprod. 94, 144.. [DOI] [PubMed] [Google Scholar]
- Watkins D. J., Tellez-Rojo M. M., Ferguson K. K., Lee J. M., Solano-Gonzalez M., Blank-Goldenberg C., et al. (2014). In utero and peripubertal exposure to phthalates and bpa in relation to female sexual maturation. Environm. Res. 134, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Cohen Hubal E. A., Little J. C. (2010). Predicting residential exposure to phthalate plasticizer emitted from vinyl flooring: Sensitivity, uncertainty, and implications for biomonitoring. Environ. Health Perspect. 118, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.