Abstract
Background
Previously, we demonstrated similar human papillomavirus (HPV)16/18 vaccine efficacy estimates and stable HPV16/18 antibody levels four years postvaccination in a nonrandomized analysis of women who received a varying number of doses of the bivalent HPV16/18 vaccine. Here we extend data to seven years following initial vaccination.
Methods
We evaluated HPV16/18-vaccinated women who received one (n = 134), two (n0/1 = 193, n0/6 = 79), or three doses (n = 2043) to a median of 6.9 years postvaccination. Cervical HPV DNA was measured with the SPF10- DEIA-LiPA PCR system; HPV16/18-specific antibody levels were measured using enzyme-linked immunosorbent assays (n = 486). Infection and immunological measures were compared across vaccine dose groups. Prevalent HPV infection at year 7 was also compared with an unvaccinated control group (UCG). All statistical tests were two-sided.
Results
Among women in the three-dose, two-dose0/6, two-dose0/1, and one-dose groups, cumulative incident HPV16/18 infection rates (No. of events/No. of individuals) were 4.3% (88/2036, 95% confidence interval [CI] = 3.5% to 5.3%), 3.8% (3/78, 95% CI = 1.0% to 10.1%), 3.6% (7/192, 95% CI = 1.6% to 7.1%), and 1.5% (2/133, 95% CI = 0.3% to 4.9%; P = 1.00, .85, .17 comparing the two-dose0/6, two-dose0/1, and one-dose groups to the three-dose group, respectively). The prevalence of other carcinogenic and noncarcinogenic HPV types, excluding HPV16/18/31/33/45, were high and not statistically different among all dose groups, indicating that the low incidence of HPV16/18 in the one- and two-dose groups was not due to lack of exposure. At seven years, 100% of participants in all dose groups remained HPV16 and HPV18 seropositive. A non–statistically significant decrease in the geometric mean of the HPV16 antibody levels between years 4 and 7 was observed among women in the three-dose group: –10.8% (95% CI = –25.3% to 6.6%); two-dose (0/6 months) group: –17.3% (95% CI = –39.3% to 12.8%), two-dose (0/1 month) group: –6.9% (95% CI = –22.1% to 11.2%), and one-dose group: –5.5% (95% CI = –29.7% to 27.0%); results were similar for HPV18.
Conclusions
At an average of seven years of follow-up, we observed similar low rates of HPV16/18 infections and slight, if any, decreases in HPV16/18 antibody levels by dose group.
Cervical cancer affects more than half a million women annually, with 88% of mortality occurring in low-income nations (1). Human papillomavirus (HPV) vaccines were licensed and recommended a decade ago (2) in order to reduce individual- and population-level prevalence of HPV, a necessary cause of cervical carcinogenesis (3). These vaccines were initially tested and approved in three-dose regimens (2). Vaccine uptake has been poor in many world regions (4), likely the consequence of high costs and the intensive infrastructure required for administering three doses over a six-month period. In time, serological data provided consistent evidence that two doses administered among adolescents (age nine to 14 years) at least six months apart evoked immunological responses that were noninferior compared with three doses among the women age 16 to 26 years who experienced protection in the trials (5,6). Consequently, the European recommending bodies reduced the dosing recommendation for adolescents to two doses in 2014 (7); the United States made parallel recommendations in 2016 (8).
Despite this progress, global vaccination coverage is insufficient (4) and has increased only 1% since 2010 (9). The Costa Rica Vaccine Trial (CVT) (10) and PATRICIA Trial (11), both used the bivalent HPV vaccine, and in post hoc analyses showed similar vaccine efficacy over four years among women who received one, two, and three doses of the HPV16/18 vaccine, as well as stable antibody responses (12). Additionally, 36-month preliminary analysis of a postlicensure trial of the quadrivalent vaccine showed similar protection against HPV16/18 cervical infection regardless of the number of vaccine doses (13). At present, published data are available for one-dose efficacy for a maximum of four years (10,11). As durability of protection is an important determinant of the long-term impact of a vaccination program (14), the objective of this analysis was to extend our evaluation of reduced-dose HPV vaccine protection and immunogenicity out to seven years.
Methods
Study Population
Participants were from the publicly funded, four-year, community-based, randomized phase III CVT (registered with Clinicaltrials.gov NCT00128661) (15). Between 2004 and 2005, 7466 women were consented and randomly assigned to receive either the AS04-HPV-16/18 vaccine (Cervarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) or a control hepatitis A vaccine (GlaxoSmithKline Biologicals) in a 1:1 ratio at zero, one, and six months and were followed for four years. At enrollment and follow-up visits, participants provided a serum sample, and for sexually experienced women, a pelvic exam was performed, at which time cervical cells were collected for cytology and HPV DNA testing. At the end of the four-year trial, participants were offered the vaccine they had not received at enrollment (crossover vaccination) and were invited to stay in a long-term follow-up observational study (16). During this observational study, HPV-vaccinated participants were followed biannually, where each clinic visit consisted of a pelvic exam with collection of a cervical sample and a serum sample. To replace the original control group, this observational study recruited 2836 unvaccinated women, with similar characteristics to the trial participants, into an unvaccinated control group (UCG), who were also followed biannually. Protocols were approved by the Institutional Review Boards of the US National Cancer Institute (NCI) and the Costa Rican INCIENSA, and all participants signed informed consent.
Approximately 20% of women in the CVT received less than three doses of their assigned vaccine, even though all women were randomly assigned to receive three doses (10). Reasons for missing vaccine doses in both groups were independent of trial arm and largely involuntary (10), with major reasons being pregnancy and colposcopic referral.
For the present evaluation, all women contributed to analyses using virologic end points. We compared the following four groups: 1) women who received one HPV16/18 vaccine dose (n = 134); 2) women who received two HPV16/18 vaccine doses at enrollment and one month later (n = 193); 3) women who received two HPV16/18 vaccine doses at enrollment and six months later (n = 79); and 4) women who received all three HPV16/18 vaccine doses (n = 2043). Women from our unvaccinated control group were also included (n = 2382). For analyses using serologic end points, serum from a subset who received fewer than three doses (selected because they were tested in the previous round of testing [12] and had sufficient serum availability) and a random subset of women who received three doses was tested from years 4 and 7 (n = 104, 156, 61, and 165, respectively).
Laboratory Methods
HPV DNA detection and genotyping from cervical specimens were performed at DDL Diagnostic Laboratory (17–19). Extracted DNA was used for polymerase chain reaction amplification with the SPF10 primer sets. The same SPF10 amplimers were used on SPF10-DEIA-positive samples to identify HPV genotype by reverse hybridization on a line probe assay (LiPA; SPF10-DEIA/HPVLiPA25, version 1; Labo Bio-Medical Products, Rijswijk, the Netherlands), which detects 25 HPV genotypes.
HPV16 and HPV18 serum antibody levels were measured by virus-like particle (VLP) enzyme-linked immunosorbent assay at the NCI HPV Immunology Laboratory, as previously described (20). The laboratory-determined seropositivity cutoffs for HPV16 and HPV18 were 8 EU/mL and 7 EU/mL, respectively. Laboratory-blinded replicates (n = 116) were included as quality control samples, and the interplate coefficient of variation (CV) was observed to be 9.8% for HPV16 and 10.2% for HPV18. For a description of HPV16 avidity, which was also measured in serum (21), see the Supplementary Methods (available online).
Statistical Analysis
Demographic and clinical characteristics by dose groups were compared by standard contingency table methods. We report the percentage of women with an HPV infection in each of the four HPV vaccine groups (one dose; two doses0/1; two doses0/6; three doses) by three definitions of infection (end points): cumulative incident HPV infection, year 7 incident HPV infection, and year 7 prevalent HPV infection. Cumulative incident HPV infections were defined as a detectable infection at any visit up to and including year 7 among women who were (type-specific) negative at enrollment; case-counting began at the 12-month visit for all groups to include infections that occurred after the vaccination period; for each type individually, women were censored at first infection detection over follow-up. Year 7 incident infection was defined as an infection (type-specific) being present in year 7 that was not present in year 4 (in some cases, there may have been intervening visits; these were ignored). A year 7 prevalent infection was defined as an infection detected at the year 7 visit among all tested women. For this latter end point, we also report findings among women in our unvaccinated group as a comparison of circulating HPV infection.
For each of these end points, we report the number of women with the end point, the total number of eligible women, and corresponding percentage (%) by each of the four HPV vaccine groups. We also report the P values comparing rates in the two-dose (0/6 month), two-dose (0/1 months), and one-dose groups with the rate in the three-dose group using Fisher’s test. We report outcomes separately for each HPV type or group of HPV types.
We describe anti-HPV16 and anti-HPV18 antibody levels in each of the four HPV vaccine groups. Added to previously published results (12) describing antibody levels by number of doses received throughout the first four years of follow-up, we present new testing results describing antibody levels by number of doses received in years 4 and 7 of follow-up. We note that antibody levels were measured using two batches of VLP. The first batch (n = 391), performed for our initial paper (12), contained samples from baseline to year 4. The second batch (n = 486), performed for this paper, included samples from year 4 and year 7 among an overlapping (overlap n = 187), but not identical, set of participants. Because antigen characteristics vary by batch, direct comparisons can only be made within a batch. For antibody levels, we report geometric mean (EU/mL) of the serum antibody levels at the four- and seven-year visits and their ratio. We estimate the 95% confidence intervals (CIs) by modeling the log-level or the log-ratio by linear regression and exponentiating the 95% confidence interval for the intercept term. We further report the percentage of individuals who are seropositive at each visit. For HPV16 antibody avidity, we report the quartiles and the geometric mean of avidity levels at each visit. We compare antibody avidity levels between years 4 and 7.
All P values are two-sided, and a P value of less than .05 was considered statistically significant.
Results
Patient Characteristics
Differences were observed by dose group for most characteristics including enrollment variables of overall HPV infection, HPV16/18 seropositivity, number of sexual partners, smoking, and oral contraceptive use (Supplementary Table 1, available online). Median follow-up between enrollment and the year 7 visit was 6.9 years (IQR = 6.5–7.3 years) for trial participants.
Attack Rates
Cumulative incident HPV16 or HPV18 infections over the seven years were uniformly low and not different by number of doses, demonstrating sustained vaccine efficacy (HPV16: 3.2%, 95% CI = 2.5 to 4.0, among three-dose recipients; 1.7% among one-dose recipients, 95% CI = 0.3 to 5.4, P = .58; HPV18: 1.6%, 95% CI = 1.1 to 2.2, among three-dose recipients; 0% among one-dose recipients, 95% CI = 0.0 to 2.3, P = .26;(HPV16/18: 4.3%, 88/2036, 95% CI = 3.5% to 5.3%; 3.8%, 3/78, 95% CI = 1.0% to 10.1%; 3.6%, 7/192, 95% CI = 1.6% to 7.1%; and 1.5%, 2/133, 95% CI = 0.3% to 4.9%, P = 1.00, .85, .17 comparing the two-dose (0/6), two-dose (0/1), and one-dose groups to the three-dose group, respectively) (Table 1). The rates of high-risk HPV types not targeted by the vaccine but for which partial cross-protection has been reported (HPV31/33/45) were similar between three-dose, two-dose (0/6 months, P = .68), and one-dose groups (P = .87) and higher when comparing the two-dose group (0/1 months, P = .002) with the three-dose group (Table 1). The rates of other carcinogenic HPV types (excluding HPV16/18/31/33/45) and noncarcinogenic HPV types were high among all dose groups, indicating continued HPV exposure in all dose groups. Similar results were observed for rates of year 7 incident infections, except that the statistically significant increase in HPV31/33/45 infections in the two-dose group (0/1 month) compared with the three-dose group was no longer present (Table 2).
Table 1.
Cumulative incident HPV infections over 7 years*
| End point | 3 doses |
2 doses(0/6) |
2 doses(0/1) |
1 dose |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. with outcome/No. of women | % (95% CI) | No. with outcome/No. of women | % (95% CI) | P† | No. with outcome/No. of women | % (95% CI) | P† | No. with outcome/No. of women | % (95% CI) | P† | |
| HPV16 | 62/1943 | 3.2 (2.5 to 4.0) | 1/73 | 1.4 (0.1 to 6.6) | .73 | 5/180 | 2.8 (1.0 to 6.0) | 1.00 | 2/120 | 1.7 (0.3 to 5.4) | .58 |
| HPV18 | 31/1994 | 1.6 (1.1 to 2.2) | 2/78 | 2.6 (0.4 to 8.2) | .35 | 2/187 | 1.1 (0.2 to 3.5) | 1.00 | 0/127 | 0 (0.0 to 2.3) | .26 |
| HPV16/18 | 88/2036 | 4.3 (3.5 to 5.3) | 3/78 | 3.8 (1.0 to 10.1) | 1.00 | 7/192 | 3.6 (1.6 to 7.1) | .85 | 2/133 | 1.5 (0.3 to 4.9) | .17 |
| HPV31/33/45 | 164/2043 | 8.0 (6.9 to 9.3) | 7/79 | 8.9 (4.0 to 16.7) | .68 | 29/193 | 15.0 (10.5 to 20.6) | .002 | 11/134 | 8.2 (4.4 to 13.8) | .87 |
| Other carcinogenic‡ | 891/2043 | 43.6 (41.5 to 45.8) | 35/79 | 44.3 (33.7 to 55.4) | .91 | 89/193 | 46.1 (39.2 to 53.2) | .54 | 53/134 | 39.6 (31.5 to 48.0) | .37 |
| Noncarcinogenic | 943/2043 | 46.2 (44.0 to 48.3) | 42/79 | 53.2 (42.1 to 64.0) | .25 | 111/193 | 57.5 (50.5 to 64.4) | .003 | 59/134 | 44.0 (35.8 to 52.5) | .66 |
Seven-year cumulative incident infection: human papillomavirus (HPV)–negative (for the respective type) at enrollment, censored at first occurrence over follow-up (all data available from enrollment to seven years). Case counting began at the 12-month visit. CI = confidence interval; HPV = human papillomavirus.
P values are compared with the three-dose group by Fisher’s exact test and are two-sided.
Other carcinogenic: includes HPV types 35/39/51/52/56/58/59 and excludes HPV16/18/31/33/45.
Table 2.
Incident HPV infections detected at the 7-year follow-up visit*
| End point | 3 doses |
2 doses(0/6) |
2 doses(0/1) |
1 dose |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. with outcome/No. of women | % (95% CI) | No. with outcome/No. of women | % (95% CI) | P† | No. with outcome/No. of women | % (95% CI) | P† | No. with outcome/No. of women | % (95% CI) | P† | |
| HPV16 | 8/2012 | 0.4 (0.2 to 0.8) | 0/77 | 0.0 (0.0 to 3.8) | 1.00 | 1/191 | 0.5 (0.0 to 2.6) | .56 | 0/133 | 0.0 (0.0 to 2.2) | 1.00 |
| HPV18 | 1/2030 | 0.1 (0.0 to 0.2) | 0/78 | 0.0 (0.0 to 3.8) | 1.00 | 0/193 | 0.0 (0.0 to 1.5) | 1.00 | 0/134 | 0.0 (0.0 to 2.2) | 1.00 |
| HPV16/18 | 9/2042 | 0.4 (0.2 to 0.8) | 0/78 | 0.0 (0.0 to 3.8) | 1.00 | 1/193 | 0.5 (0.0 to 2.5) | .60 | 0/134 | 0.0 (0.0 to 2.2) | 1.00 |
| HPV31/33/45 | 31/2042 | 1.5 (1.1 to 2.1) | 0/78 | 0.0 (0.0 to 3.8) | .63 | 3/193 | 1.6 (0.4 to 4.2) | 1.00 | 2/134 | 1.5 (0.3 to 4.8) | 1.00 |
| Other carcinogenic‡ | 251/2042 | 12.3 (10.9 to 13.8) | 10/78 | 12.8 (6.7 to 21.7) | .86 | 26/193 | 13.5 (9.2 to 18.8) | .65 | 17/134 | 12.7 (7.8 to 19.2) | .89 |
| Noncarcinogenic | 255/2042 | 12.5 (11.1 to 14.0) | 8/78 | 10.3 (4.9 to 18.5) | .73 | 25/193 | 13.0 (8.8 to 18.3) | .82 | 12/134 | 9.0 (4.9 to 14.7) | .28 |
Infection incidentally detected at the seven-year follow-up visit but not at the four-year visit. CI = confidence interval; HPV = human papillomavirus.
P values are compared with the three-dose group by Fisher’s exact test and are two-sided.
Other carcinogenic: includes HPV types 35/39/51/52/56/58/59 and excludes HPV16/18/31/33/45.
We observed low prevalence of HPV16 and HPV18 at year 7 that was also not statistically significantly different by number of doses (Table 3). Only 1.0% (95% CI = 0.6% to 1.5%), 1.3% (95% CI = 0.1% to 6.1%), 1.0% (95% CI = 0.2% to 3.4%), and 0.0% (95% CI = 0.0% to 2.2%) of women in the three-dose, two-dose (0/6 month), two-dose (0/1 month), and one-dose groups had prevalent HPV16 or HPV18 at year 7. These percentages can be compared with the 6.6% (95% CI = 5.7% to 7.7%) of women in the UCG group who had an HPV16 or HPV18 infection at that time point. The prevalence of HPV31/33/45 was similarly low among vaccinated participants: 2.3% (95% CI = 1.8% to 3.1%), 0% (95% CI = 0.0% to 3.7%), 2.1% (95% CI = 0.7% to 4.9%), and 1.5% (95% CI = 0.3% to 4.8%) in three-dose, two-dose (0/6 month), two-dose (0/1 month), and one-dose groups, compared with 5.5% (95% CI = 4.7% to 6.5%) in the UCG group, suggesting some vaccine protection against phylogenetically related HPV types. For other carcinogenic and noncarcinogenic HPV types, the prevalent infection rates were similar in the vaccinated women and the UCG group, indicating comparable exposure with HPV in all groups evaluated.
Table 3.
One-time prevalent infection detected at the 7-year follow-up visit*
| End point | 3 doses |
2 doses(0/6) |
2 doses(0/1) |
1 dose |
UCG |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. with outcome/No. of women | % (95% CI) | No. with outcome/No. of women | % (95% CI) | P† | No. with outcome/No. of women | % (95% CI) | P† | No. with outcome/No. of women | % (95% CI) | P† | No. with outcome/No. of women | % (95% CI) | P† | |
| HPV16 | 16/2043 | 0.8 (0.5 to 1.2) | 1/79 | 1.3 (0.1 to 6.1) | 0.48 | 2/193 | 1.0 (0.2 to 3.4) | .66 | 0/134 | 0.0 (0.0 to 2.2) | .62 | 99/2382 | 4.2 (3.4 to 5.0) | <.001 |
| HPV18 | 4/2043 | 0.2 (0.1 to 0.5) | 0/79 | 0.0 (0.0 to 3.7) | 1.00 | 0/193 | 0.0 (0.0 to 1.5) | 1.00 | 0/134 | 0.0 (0.0 to 2.2) | 1.00 | 62/2382 | 2.6 (2.0 to 3.3) | <.001 |
| HPV16/18 | 20/2043 | 1.0 (0.6 to 1.5) | 1/79 | 1.3 (0.1 to 6.1) | .55 | 2/193 | 1.0 (0.2 to 3.4) | .71 | 0/134 | 0.0 (0.0 to 2.2) | .63 | 158/2382 | 6.6 (5.7 to 7.7) | <.001 |
| HPV31/33/45 | 48/2043 | 2.3 (1.8 to 3.1) | 0/79 | 0.0 (0.0 to 3.7) | .26 | 4/193 | 2.1 (0.7 to 4.9) | 1.00 | 2/134 | 1.5 (0.3 to 4.8) | .77 | 132/2382 | 5.5 (4.7 to 6.5) | <.001 |
| Other carcinogenic‡ | 310/2043 | 15.2 (13.7 to 16.8) | 11/79 | 13.9 (7.5 to 22.9) | .87 | 28/193 | 14.5 (10.1 to 20.0) | .92 | 18/134 | 13.4 (8.4 to 20.0) | .71 | 309/2382 | 13.0 (11.7 to 14.4) | .04 |
| Noncarcinogenic | 297/2043 | 14.5 (13.1 to 16.1) | 11/79 | 13.9 (7.5 to 22.9) | 1.00 | 30/193 | 15.5 (10.9 to 21.2) | .67 | 16/134 | 11.9 (7.2 to 18.3) | .45 | 320/2382 | 13.4 (12.1 to 14.9) | .30 |
Prevalent detection: one-time human papillomavirus (HPV)–DNA detection status at the seven-year follow-up visit. CI = confidence interval; UCG = unvaccinated control group.
P values are compared with the three-dose group by Fisher’s exact test and are two-sided.
Other carcinogenic: includes HPV types 35/39/51/52/56/58/59 and excludes HPV16/18/31/33/45.
Serum Antibody Patterns
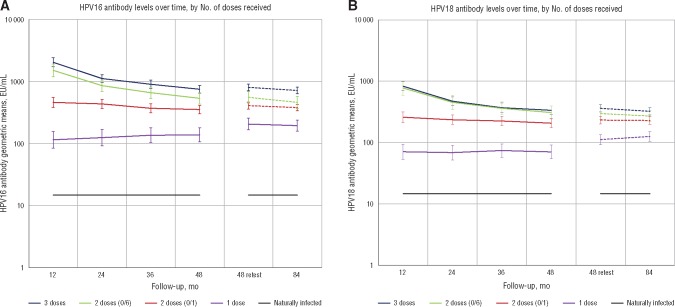
All women remained seropositive at year 7 regardless of number of doses received. Furthermore, for all dose groups, antibody levels for HPV16 and HPV18 remained relatively constant between years 4 and year 7, although geometric means did show a slight, but statistically nonsignificant, decrease during this time (Table 4; Figure 1). Among women in the three-dose, two-dose (0/6 month), two-dose (0/1 month), and one-dose groups, the decrease in the geometric means of the HPV16 antibody levels, respectively, were –10.8% (95% CI = –25.3% to 6.6%), –17.3% (95% CI = –39.3% to 12.8%), –6.9% (95% CI = –22.1% to 11.2%), and –5.5% (95% CI = –29.7% to 27%). In the same groups, the decrease in the geometric means of the HPV18 antibody levels, respectively, were –10.4% (95% CI = –26.2% to 8.7%), –8.9% (95% CI = –31.8% to 21.6%), –1.6% (95% CI = –19.9% to 20.9%), and, for the one–dose group, increased by 12.2% (95% CI = –13.3% to 45.3%). Additional details regarding the distribution of the change between years 4 and 7 are provided in Supplementary Figure 1, A and B (available online), for HPV16 and HPV18, respectively. Moreover, in addition to the geometric means, the distributions of antibody levels were similar at years 4 and 7 for all doses (Supplementary Figure 2, available online). Therefore, for any antibody threshold, the proportion of women below that threshold did not substantively change during this period. Antibody avidity increased with the number of HPV vaccine doses received, but within a dose level, avidity remained stable between years 4 and 7 (Table 4; Supplementary Figure 3, available online).
Table 4.
Distributions of serum antibody levels and serum avidity for HPV16 and HPV18 at years 4 and 7*
| Metric | 3 doses |
2 doses (0/6 mo) |
2 doses (0/1 mo) |
1 dose |
||||
|---|---|---|---|---|---|---|---|---|
| GM (95% CI) | IQR | GM (95% CI) | IQR | GM (95% CI) | IQR | GM (95% CI) | IQR | |
| HPV16 | ||||||||
| Antibody level, EU/mL | ||||||||
| Year 4 | 803 (708 to 909) | 440–1300 | 555 (447 to 690) | 329–954 | 407 (358 to 464) | 245–691 | 205 (165 to 255) | 98–367 |
| Year 7 | 716 (630 to 814) | 410–1158 | 460 (367 to 576) | 250–810 | 379 (335 to 429) | 256–386 | 194 (158 to 237) | 93–326 |
| % change (95% CI) | –10.8% (–25.3% to 6.6%) | –17.3% (–39.3% to 12.8%) | –6.9% (–22.1% to 11.2%) | –5.5% (–29.7% to 27.0%) | ||||
| Ratio of GM | 0.9 (0.7 to 1.1) | NA | 0.8 (0.6 to 1.1) | NA | 0.9 (0.8 to 1.1) | NA | 0.9 (0.7 to 1.3) | NA |
| Avidity level | ||||||||
| Year 4 | 2.5 (2.4 to 2.6) | 2.4–3.0 | 2.3 (2.1 to 2.6) | 2.2–2.9 | 2.3 (2.2 to 2.5) | 1.9–3.0 | 2.0 (1.8 to 2.2) | 1.7–2.7 |
| Year 7 | 2.5 (2.4 to 2.6) | 2.4–3.0 | 2.3 (2.1 to 2.6) | 2.1–2.9 | 2.3 (2.1 to 2.4) | 2.2–2.5 | 2.0 (1.8 to 2.2) | 1.9–2.7 |
| Ratio of GM | 1.0 (0.9 to 1.1) | NA | 1.0 (0.9 to 1.2) | NA | 1.0 (0.9 to 1.1) | NA | 1.0 (0.9 to 1.2) | NA |
| HPV18 | ||||||||
| Antibody level, EU/mL | ||||||||
| Year 4 | 360 (313 to 414) | 210–680 | 296 (240 to 366) | 154–560 | 232 (200 to 269) | 120–443 | 112 (93 to 134) | 56–210 |
| Year 7 | 322 (281 to 369) | 190–575 | 270 (221 to 330) | 138–522 | 228 (198 to 264) | 112–424 | 125 (105 to 150) | 66–235 |
| % change (95% CI) | –10.4% (–26.2% to 8.7%) | –8.9% (–31.8% to 21.6%) | –1.6% (–19.9% to 20.9%) | 12.2% (–13.3% to 45.3%) | ||||
| Ratio of GM | 0.9 (0.7 to 1.1) | NA | 0.9 (0.7 to 1.2) | NA | 1.0 (0.8 to 1.2) | NA | 1.1 (0.9 to 1.5) | NA |
CI = confidence interval; EU = enzyme-linked immunosorbent assay units; GM = geometric mean; IQR = interquartile range; NA = not applicable.
Figure 1.
Human papilloma virus (HPV) antibody levels over time by number of doses received. A) Anti-HPV16 and (B) anti-HPV18 antibody levels are presented in each of the four HPV vaccine groups. Added to previously published results (12) describing antibody levels by number of doses received throughout the first four years of follow-up (shown in solid lines), the new data describe antibody levels by number of doses received in years 4 and 7 of follow-up (shown in dashed lines). We note that antibody levels were measured using two batches of virus-like particles; because antigen characteristics vary by batch, direct comparisons can only be made within a batch. EU = enzyme-linked immunosorbent assay units; HPV = human papilloma virus.
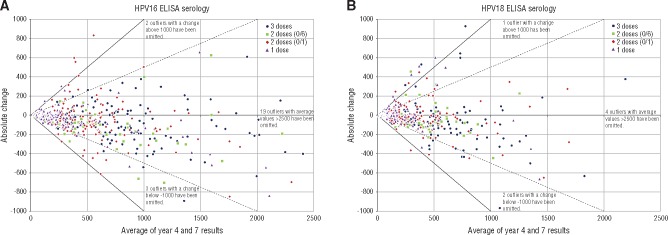
We compared the average antibody level against the absolute change from year 4 to 7 and benchmarked this at twofold and 1.5-fold change (Figure 2). These allow us to visualize the magnitude of the antibody relative to the individual level. As an example, for HPV16, in the one-dose group, zero (0%) had a twofold decline between years 4 and 7, and six (5.8%) experienced at least a 1.5-fold decline; in the two-dose (0/1 month) group, one (0.6%) had a twofold decline between years 4 and 7, and 11 (7.1%) experienced at least a 1.5-fold decline; in the two-dose group (0/6 month), zero (0%) had a twofold decline between years 4 and 7, and six (9.8%) experienced at least a 1.5-fold decline; and in the three-dose group, zero (0%) had a twofold decline between years 4 and 7, and eight (4.8%) experienced at least a 1.5-fold decline (Figure 2A). Similar results were observed for HPV18 (Figure 2B). Supplementary Figure 4 (available online) shows that subjects with decreasing antibody levels during this period tended to have levels that increased between years 3 and 4, suggesting that the observed decreases are more likely attributable to random variability around relatively constant levels than to a subset of individuals with continually decreasing levels.
Figure 2.
Absolute change in human papilloma virus (HPV) antibody levels by dose. Results for (A) HPV16 and (B) HPV18 are shown. The x-axis is the average level of serum antibody: (level at year 4 + level year 7)/2. The y-axis is the absolute change in antibody level between year 4 and year 7; each point represents an individual, and the color of that point indicates dose group. The solid line indicates a 2× change; the dashed line indicates a 1.5× change. ELISA = enzyme-linked immunosorbent assay.
Discussion
We extended our post hoc evaluation of HPV vaccine protection for women who received three, two, and one doses out to seven years following initial HPV vaccination. A low prevalence of HPV16/18 infections was observed for all dose groups, suggesting that the protection afforded by even a single dose may be long lived. While the HPV-vaccinated women had a deficit of HPV16/18 infections, they continued to test positive for noncarcinogenic HPV types, indicating continued HPV exposure. This contrasts with the unvaccinated women in our study, who had expected levels of HPV16/18 infection, observational evidence of continued circulation of HPV16/18 infections in the population.
Interestingly, in this evaluation at seven years, we observed some evidence for possible cross-protection against HPV31/33/45 in women receiving one or two doses of the vaccine, as evidenced by the lower rates of prevalent infection with these HPV types in our unvaccinated compared with vaccinated groups and largely comparable infection rates across dose groups among vaccinated women. This is noteworthy because we previously reported higher rates of cumulative HPV31/33/45 infections among two-dose women who received their vaccines one month apart, which we interpreted as a lack of evidence for protection against these HPV types (11); in this previous work, underlying group differences may have contributed to these observed differences. Thus, the question of whether reduced dosage regimens of the HPV16/18 vaccine retain partial cross-protection against HPV31/33/45 deserves further investigation. Regardless of what is ultimately determined regarding cross-protection, it is important to note that protection against the primary vaccine types (ie, HPV16 and HPV18) in reduced dosage schedules would provide a clear benefit, given that these two HPV types account for approximately 70% of all HPV-associated cancers worldwide.
The suggestion of sustained protection against HPV16/18 infection by a single dose of the HPV vaccine was supported by our immunological assessments. All of the HPV-vaccinated women, regardless of number of doses received, remained HPV16 and 18 seropositive seven years post–dose 1, and the average drop in antibody levels between years 4 and 7 was small and not statistically significant. Furthermore, within each dose, individuals with the lowest antibody levels did not experience a decline in antibody titers (on a relative scale) greater than those who received more doses. Mean antibody avidities among those receiving fewer than three doses were stable and within 75% to 90% of those in the three-dose group, suggesting considerable affinity maturation for all dose groups. It is interesting to note that avidities continued to increase between years 1 and 4 (21) but then stabilized between years 4 and 7 for all dose groups. These findings could reflect sustained germinal center reactions for four years after vaccination, but not longer. Alternatively, they could reflect preferential survival over the first four years of plasma cells that received stronger signaling through their B cell receptor upon initial engagement of the VLPs, followed by long-term persistence of the plasma cells that survive past that time point (22). Finally, they could also reflect anamnestic responses to continued natural exposure to HPV through sexual contact in the initial years, followed by reduced exposure as women age.
To our knowledge, there are no studies to date among individuals receiving a single dose of an HPV vaccine that have documented sustained protection beyond four years. Such data are needed and unlikely to come from phase IV surveillance studies given their inherent biases (23). Individuals who receive a single dose in population-vaccine programs tend to be older and initiate sexual activity at younger ages (inferred from age at first Pap test), which resulted in a higher number of prevalent HPV infections at vaccination, which reduced observed vaccine efficacy. To overcome these biases, analyses of efficacy should be restricted to the groups who are youngest at the time of vaccination, and events should be counted starting one or more years after vaccination. At present, these birth cohorts have not yet aged sufficiently to be at risk for HPV16/18-related diseases. Thus, current analyses should rely on nonrandomized data from efficacy trials to generate the most informative results. Post hoc analyses in efficacy trials suggest a potential longer-term efficacy of one dose of prophylactic HPV vaccines. Our data from CVT now extend those findings to seven years.
Our current data on seven-year protection afforded by one dose of the HPV vaccine challenge the prevailing dogma that protein-based subunit vaccines require a prime-boost regimen and instead suggest that a single dose may provide durable protection. Our leading hypothesis for why a single dose of the HPV vaccine is breaking this long-held principle is that the structure of the HPV VLPs, the key component of HPV prophylactic vaccines, present closely spaced, repetitive epitopes to the immune system that induce highly potent and durable protective antibody responses (24), which may reduce or eliminate the need for booster doses. Additionally, the immune-stimulatory effects of a toll-like receptor agonist adjuvant in the bivalent vaccine may also contribute to the magnitude and durability of the immune response to this vaccine. Work in this area is important for public health: if durable efficacy, in addition to durable antibody responses, could be unequivocally demonstrated after a single dose of an HPV VLP vaccine, it may encourage the development of one-dose vaccines targeting other pathogens, based on the principle of virus-like display of antigen.
Our study is not without limitations. First, women in this analysis were not randomly assigned to receive a single dose. While extensive work has been done to rule out bias, including the documentation of similar antibody responses by dose group one month after the initial dose, balance in the attack rate of non-16/18 HPV types by dose at the four-year randomized blinded study visit, and now similar rates of HPV incidence by dose group for nonvaccine HPV types out to seven years, the data do not afford the same protection against selection bias and level of evidence as a randomized trial comparing a single dose to more doses. Further, we are limited to a fixed number of women in the one-dose group and thus have limited power to detect small differences in HPV attack rates by dose. Further, as the HPV attack rate declines with age, our ability to document protection against virologic end points will decrease over time.
From the global perspective, women who are at the greatest lifetime risk of cervical cancer are simply not being vaccinated. Our data that a single dose of the HPV vaccine continued to protect against HPV infection, with documented stabilization of antibody and avidity up to seven years, adds to other data supporting the hypothesis that one dose may be sufficient. Continued demonstration of the protection afforded by one dose will be needed to document duration of protection. Yet, robust determination of the minimum number of doses needed in a formalized randomized controlled trial will likely be needed to provide the level of evidence that recommending bodies require to justify changes in current vaccine recommendations.
Funding
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women's Health. GlaxoSmithKline Biologicals (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomized blinded phase of our study.
Notes
The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. John T. Schiller and Douglas R. Lowy report that they are named inventors on US Government–owned human papillomavirus (HPV) vaccine patents that are licensed to GlaxoSmithKline and Merck and for which the National Cancer Institute receives licensing fees. They are entitled to limited royalties as specified by federal law. The other authors declare that they have no conflicts of interest.
Investigators in the Costa Rica HPV Vaccine Trial (CVT) Group: Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica—Bernal Cortés (specimen and repository manager), Paula González (LTFU: co-principal investigator), Rolando Herrero (CVT: co-principal investigator), Silvia E. Jiménez (trial coordinator), Carolina Porras (co-investigator), Ana Cecilia Rodríguez (co-investigator); United States National Cancer Institute, Bethesda, MD—Allan Hildesheim (co-principal investigator and NCI co-project officer), Aimée R. Kreimer (LTFU: co-principal investigator and NCI co-project officer), Douglas R. Lowy (HPV virologist), Mark Schiffman (CVT: medical monitor and NCI co-project officer), John T. Schiller (HPV virologist), Mark Sherman (CVT: QC pathologist), Sholom Wacholder (statistician); Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD (HPV Immunology Laboratory)—Ligia A. Pinto, Troy J. Kemp; Georgetown University, Washington, DC—Mary K. Sidawy (CVT: histopathologist); DDL Diagnostic Laboratory, Netherlands (HPV DNA Testing)—Wim Quint, Leen-Jan van Doorn, Linda Struijk; University of California, San Francisco, CA—Joel M. Palefsky (expert on anal HPV infection and disease diagnosis and management), Teresa M. Darragh (pathologist and clinical management); University of Virginia, Charlottesville, VA—Mark H. Stoler (QC pathologist).
We extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions by Loreto Carvajal, Rebeca Ocampo, Carlos Avila, Cristian Montero, Diego Guillen, Jorge Morales, and Mario Alfaro. In the United States, we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort, especially Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr. Diane Solomon (CVT: medical monitor and QC pathologist) for her invaluable contributions during the randomized blinded phase of the trial and the design of the LTFU and Nora Macklin (CVT) and Kate Torres (LTFU) for their expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants during the randomized, blinded phase of our study (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Anne Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Luis Rosero-Bixby, Kristen Suthers, Gypsyamber D’Souza, and Richard Roden).
Supplementary Material
Contributor Information
for the Costa Rica HPV Vaccine Trial (CVT) Group:
González Paula, Rolando Herrero, Silvia E. Jiménez, Carolina Porras, Ana Cecilia Rodríguez, Allan Hildesheim, Aimée R. Kreimer, Douglas R. Lowy, Mark Schiffman, John T. Schiller, Mark Sherman, Sholom Wacholder, Ligia A. Pinto, Troy J. Kemp, Mary K. Sidawy, Wim Quint, Leen-Jan van Doorn, Linda Struijk, Joel M. Palefsky, Teresa M. Darragh, and Mark H. Stoler
References
- 1. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. [DOI] [PubMed] [Google Scholar]
- 2. Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 3. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—part B: Biological agents. Lancet Oncol. 2009;10(4):321–322. [DOI] [PubMed] [Google Scholar]
- 4. Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob Health. 2016;4(7):e453–e463. [DOI] [PubMed] [Google Scholar]
- 5. Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: A randomized clinical trial. JAMA. 2013;309(17):1793–1802. [DOI] [PubMed] [Google Scholar]
- 6. Romanowski B, Schwarz TF, Ferguson LM, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: Results from a randomized study. Hum Vaccin. 2011;7(12):1374–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Weekly epidemiological record. Relevé épidémiologique hebdomadaire. In: Weekly Epidemiological Record, Geneva, Switzerland: World Health Organization; No. 43. 2014;465–492. [Google Scholar]
- 8. Centers for Disease Control and Prevention. CDC recommends only two HPV shots for younger adolescents In: Fewer Shots Offer More Incentive to Prevent HPV Cancers. US Department of Health and Human Services; Atlanta, GA: Center for Disease Control; 2016. [Google Scholar]
- 9. World Health Organization. Summary of the SAGE April 2016 meeting. In: The World Health Organization's (WHO) Strategic Advisory Group of Experts (SAGE). Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 10. Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: Combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015;16(7):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Safaeian M, Porras C, Pan Y, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila). 2013;6(11):1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sankaranarayanan R, Prabhu PR, Pawlita M, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre prospective cohort study. Lancet Oncol. 2016;17(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laprise JF, Markowitz LE, Chesson HW, et al. Comparison of 2-dose and 3-dose 9-valent human papillomavirus vaccine schedules in the United States: A cost-effectiveness analysis. J Infect Dis. 2016;214(5):685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez P, Hildesheim A, Herrero R, et al. Rationale and design of a long term follow-up study of women who did and did not receive HPV 16/18 vaccination in Guanacaste, Costa Rica. Vaccine. 2015;33(18):2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153(6):1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Doorn LJ, Molijn A, Kleter B, et al. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44(9):3292–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425–434. [DOI] [PubMed] [Google Scholar]
- 21. Safaeian M, Kemp TJ, Pan DY, et al. Cross-protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: Results from the Costa Rica Vaccine Trial. Hum Vaccin Immunother. 2013;9(7):1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amanna IJ, Slifka MK.. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreimer AR, Sherman ME, Sahasrabuddhe VV, et al. The case for conducting a randomized clinical trial to assess the efficacy of a single dose of prophylactic HPV vaccines among adolescents. J Natl Cancer Inst. 2015;107(3):dju436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schiller JT, Lowy DR.. Raising expectations for subunit vaccine. J Infect Dis. 2015;211(9):1373–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.