Abstract
Di-(2-ethylhexyl) phthalate (DEHP) is the most commonly used phthalate, and it is an endocrine-disrupting chemical. This study tested a hypothesis that prenatal exposure to DEHP lays the foundation for premature gonadal dysfunction and subsequent reproductive senescence in male mice. Pregnant female CD-1 mice were orally dosed with vehicle control (tocopherol-stripped corn oil) or with 20 μg/kg/day, 200 μg/kg/day, 500 mg/kg/day, or 750 mg/kg/day of DEHP from gestational day 11 to birth. Overall, the prenatal DEHP exposure did not cause any overt physical health problems in male offspring, as no significant differences in their body nor gonadal weight were seen up to the age of 23 months. However, an age- and dose-dependent gonadal dysfunction was observed. As early as 7 months of age, the 750 mg/kg/day group of mice exhibited significantly reduced fertility. At 19 months of age, 86% of the 750 mg/kg/day mice became infertile, whereas only 25% of the control mice were infertile. At this age, all of the DEHP-exposed mice had lower serum testosterone levels, higher serum estradiol levels, and higher LH levels compared with control mice. Histological evaluations showed that mice prenatally exposed to DEHP displayed a wide array of gonadal and epididymal abnormalities such as increased germ cell apoptosis, degenerative seminiferous tubules, oligozoospermia, asthenozoospermia, and teratozoospermia in comparison to age-matching control mice. In summary, this study shows that prenatal exposure to DEHP induces premature reproductive senescence in male mice.
Keywords: DEHP, endocrine disruptor, fertility, testes, semen.
Phthalates are a family of synthetic chemicals that have been widely used in medical, automotive, and consumer product industries (Zota et al., 2014). Phthalate-free polyvinyl chloride (PVC) is rigid, but with the addition of phthalates it becomes soft and flexible so that it can be molded and used for a wide variety of products such as personal care products, baby toys, food wrappings, medical devices, intravenous injection and blood transfusion bags, lubricants, waxes, and insecticides (Schettler, 2006). Di-(2-ethylhexyl) phthalate (DEHP) is the most commonly used phthalate, with 3 million metric tons produced each year worldwide. DEHP is non-covalently bounded to the PVC polymer, and therefore, it easily leaches out into the environment and comes into contact with humans through dermal exposure, oral ingestion (Frederiksen et al., 2007), or inhalation (Huang et al., 2011).
DEHP is a high-molecular-weight compound that is hydrolyzed by intestinal lipases and liver esterases (Rusyn et al., 2006), forming mono (2-ethylhexyl) phthalate (MEHP), which is ten times more potent than DEHP and responsible for much of DEHP’s toxicity (Gupta et al., 2010). DEHP and its metabolites are rapidly removed from the circulation; the half-life is about 5–24 hours (Koch et al., 2005). In humans, MEHP is mostly conjugated with glucuronic acid and then excreted in urine (Koch et al., 2004), which is often used as a biomarker for estimating the level of DEHP exposure (Hauser, 2005).
Phthalates have been detected in amniotic fluid (Silva et al., 2004), umbilical cord blood (Latini et al., 2003), and other bodily fluids (Silva et al., 2005), indicating that humans are exposed to phthalates as early as the fetal developmental stage (Adibi et al., 2009). Babies may be continuously exposed to phthalates after birth through breast feeding (Main et al., 2006) and infant food sources (Zhu et al., 2006). DEHP exposure during the fetal period increases the chances of long-lasting endocrine disruption. Of particular concern, phthalates act as anti-androgens (Gray et al., 2001), and phthalate exposure has been implicated in decreased anogenital distance in humans (Swan et al., 2005), reduced testosterone levels (Pan et al., 2006), and poor semen quality (Pant et al., 2008). These earlier findings led us to hypothesize that fetal DEHP exposure induces premature reproductive senescence in males. In this study, we tested this hypothesis by assessing and following up the reproductive parameters of prenatally DEHP-exposed male mice.
MATERIALS AND METHODS
Chemicals
DEHP (99% purity) was purchased from Sigma-Aldrich (St. Louis, USA). Tocopherol-stripped corn oil was purchased from MP Bio Medicals (Solon, Ohio) and was used as a vehicle. Stock solutions of DEHP were prepared by diluting it in tocopherol-stripped corn oil to obtain the desired concentrations.
Animals and dosing regimen
CD-1 male and female mice were used in this study. Mice were housed at the University of Illinois at Urbana-Champaign (UIUC) animal care facility under 12-h light/dark cycles. The mice were provided with Teklad Rodent Diet 8604 (Harlan) and had free access to food and water. Animal handling and procedures were approved by the UIUC Institutional Animal Care and Use Committee (Protocol ID #: 14144). Pregnant female dams were prepared by mating 2-month-old females with proven breeder males. The pregnant female mice were orally dosed with vehicle control (tocopherol-stripped corn oil), or 20 μg/kg/day, 200 μg/kg/day, 500 mg/kg/day, or 750 mg/kg/day of DEHP from gestational day (GD) 11 to the day of birth by placing a pipette tip into the mouth as previously described (Niermann et al., 2015). This dosing protocol was used to mimic oral exposure in humans.
Previous studies have shown that DEHP appears to have a non-monotonic dose-response curve (Do et al., 2012). The lowest DEHP dose (20 µg/kg/day) was selected because it is the US Environmental Protection Agency (EPA) reference dose for human exposure (ATSDR, 2002) and this dose previously showing effects on female reproductive parameters (Niermann et al., 2015). The 200 and 750 mg/kg/day doses were selected because exposure to these levels during adulthood has been shown to affect reproduction in adult female mice (Hannon et al., 2014); whereas the 500 mg/kg/day dose was selected because it has been shown, with a dosing regimen from GD 7 to GD 14, to have adverse effects on male mouse reproduction (Doyle et al., 2013).
Weight and anogenital distance measurements
Body weight was measured during the entire experimental period from postnatal day (PND) eight until 22 months of age, and anogenital distance (AGD) was determined at PND 21 and 60 and at 16 months of age using a caliper to measure the distance from the urethral opening to the cranial opening of the anus. AGD was normalized to the body weight of each mouse and expressed as mm/100 g body weight.
Measurement of serum testosterone, estradiol, and LH concentration
Peripheral blood was collected at PND 21 and at 22 months of age by cardiac puncture, and blood was collected from the facial vein at 16 months of age. The blood was centrifuged at 2000 × g, and then serum was collected and preserved at −20°C until further analyses. The concentrations of circulating testosterone were measured at PND 21 and 16 months using ELISA kits (DRG Diagnostic) with a reportable range of 0.06–25 ng/ml. Serum estradiol concentrations were measured at 16 months using ELISA kits (DRG Diagnostic) with a reportable range of 0–200 pg/ml. Serum luteinizing hormone (LH) concentrations were measured at 22 months of age, by a sandwich immunoassay using monoclonal antibodies against both the bovine LH (no. 581B7) and the human LH-beta subunit (no. 5303: Medix Biochemica, Kauniainen, Finland), at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, as previously described (Haavisto et al., 1993) with a reportable range of 0.04–37.4 ng/mL.
Fertility test (mating study)
To assess fertility, 3-month-old breeder proven female CD-1 mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and given a week-long acclimation period. Each male mouse at the ages of 4, 5, 7, and 19 months, respectively, was housed with a breeder female for 2 weeks or until a vaginal sperm plug was observed. The fertility % (number of male that produce litter/total number of males × 100), litter size (number of pups per litter), and sex ratio (numbers of female/numbers of male pups) were recorded.
Semen analysis
For semen analysis, the cauda of the left epididymis was excised and minced with fine scissors in a warm (37 °C) phosphate buffered saline. The sperm suspension was incubated at 37 °C for 10 min to allow spermatozoa to swim out of the minced epididymis. Sperm motility was then analyzed by a computer-assisted sperm analyzer (CASA; Sperm Vision II, Minitube of America, Vernon, Wisconsin, USA). At least ten microscopic fields, covering the entire viewable area of the semen analysis chamber without overlapping successive fields, were examined.
Sperm motility was measured by the percentage of motile sperm, percent of progressive motile sperm, and percentage of immotile sperm. Also the curvilinear velocity (VCL; μm/sec), average path velocity (VAP; lm/s), straight line velocity (VSL; lm/s), beat cross frequency (BCF; Hz), amplitude of lateral head displacement (ALH; lm), linearity (LIN; VSL/VCL × 100), and straightness (STR; VSL/VAP × 100) were measured.
For total sperm counts, 2 aliquots of semen samples were collected from each mouse and diluted in 1:200 of formalin for immobilization. Sperm numbers were counted using a hemocytometer, and the average number of sperm concentration per milliliter was calculated and reported as million sperm/mL. To determine the degree of morphological abnormalities, wet mount sperm slides were prepared on clean, grease-free slides containing buffered formalin with eosin nigrosine stain. Then examined 100 sperm per sample under oil immersion lens using a light microscope (Otubanjo and Mosuro, 2001).
Tissue collection and testicular histopathology
Mice were euthanized by CO2 asphyxiation followed by cervical dislocation. The testis and epididymis were collected at postnatal days (PND) 21, 60, and at 22 months of age, then fixed in Bouins solution (Ricca chemical Co.) for 24 h. Then, transferred to 70% ethyl alcohol until tissue processing. The tissues were embedded in paraffin, sectioned at 7 µm thickness, stained with hematoxylin and eosin, and examined using light microscopy (Olympus BX 51).
TUNEL assay
Mice were euthanized at 22 months of age and testes was collected and examined to determine the degree of cell apoptosis. Apoptotic assay was performed using an in situ apoptosis detection kit S7100 (Millipore) stained with DAB chromogen (diaminobenzidine) and counterstained with hematoxylin according to the manufacturer’s instructions. The frequency of apoptotic cells within seminiferous tubules was expressed as the average number of apoptotic cells (brown nucleus) within 20 seminiferous tubules (Russell et al., 1990).
Statistical analysis
The data were analyzed using the statistical software package SPSS. The comparison was between control and treated groups and the same age point and the statistical sampling unit was litter. Multiple comparisons between normally distributed continuous experimental groups were analyzed by the one-way analysis of variance (ANOVA) as a parametric test followed by the Dunnett (2-sided) post hoc test. Multiple comparisons between non-normally distributed experimental groups were analyzed by Kruskal–Wallis as a non-parametric test. Fertility data was compared using Fisher’s exact test for each treatment group against control group. The number of animals used for statistical analyses ranged between 4 to 7 mice during the whole experimental period, except for data analyzed after 16 months of age when there were only 2 mice in the 20 μg/kg/day DEHP-treated group. Therefore, that group was excluded from the statistical analyses. The data are expressed as mean ± SEM. Statistical significance was assigned as P ≤ .05 and marked as (*), whereas statistical tendency was set as P ≤ .09 and marked as (#).
RESULTS
Body Weight Growth and Gonadal Weights
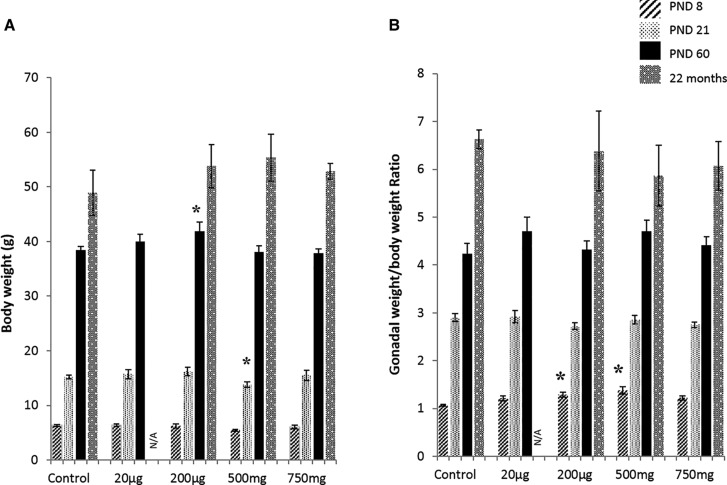
Pregnant females were orally dosed with vehicle control or 20 μg/kg/day, 200 μg/kg/day, 500 mg/kg/day, or 750 mg/kg/day of DEHP daily from gestational day 11 to the day of giving birth. The dosing period is the temporal window of gonadal development (Hirshfield, 1991). When the dams gave birth to pups, body weight, gonadal weight, and gonadal weight to body weight ratio of F1 male mice were measured at PND 8, 21, 60 (Figure 1A) and at postnatal months 4, 11, and 16, 22 (Supplementary Table 1). Body weight of the F1 male mice exposed to 200 µg/kg/day DEHP was significantly higher at PND 60 (P = .05) compared with control mice, but the difference was not seen later. Mice exposed to 500 mg/kg/day DEHP had a body weight that was transiently lower at PND 21 (P = .03) compared with the control group (Figure 1A). This newly born male mouse did not show any phenotypic malformation in the genital tract.
FIG. 1.
Effects of prenatal DEHP exposure on the body and gonadal weights. Body weight growth (A), and gonadal (testis and epididymis) weights to body weight ratio (B) were measured at the ages of postnatal day (PND) 8, 21, 60, and 22 months of age. Graphs show mean ± SEM. Asterisks indicate P ≤ .05, (n = 4–7 per treatment group). N/A: all of mice of 20 µg/kg/day group dead at age of 22 months.
The testis and epididymis were collected and weighed together at PND 8, 21, 60, and 22 months (Supplementary Table 1), and the gonadal weight to body weight ratio was determined (Figure 1B). The 200 µg/kg/day DEHP and 500 mg/kg/day DEHP mice showed a significantly higher gonadal weight to body weight ratio compared with the control group at PND 8 (P = .05 and P = .01, respectively), but at later ages, the difference was no longer significant. The testis weight was also measured separately at 22 months, there were no significant changes between control and DEHP treated groups (Supplementary Table 2).
Mortality incidence in all groups was followed to 22 months of age (Supplementary Figure 1). Interestingly, those 20 µg/kg/day DEHP mice showed a marked decrease in the survival rate over time. All of the mice in this group died by age 20 months, whereas the control mice had a survival rate above 50%.
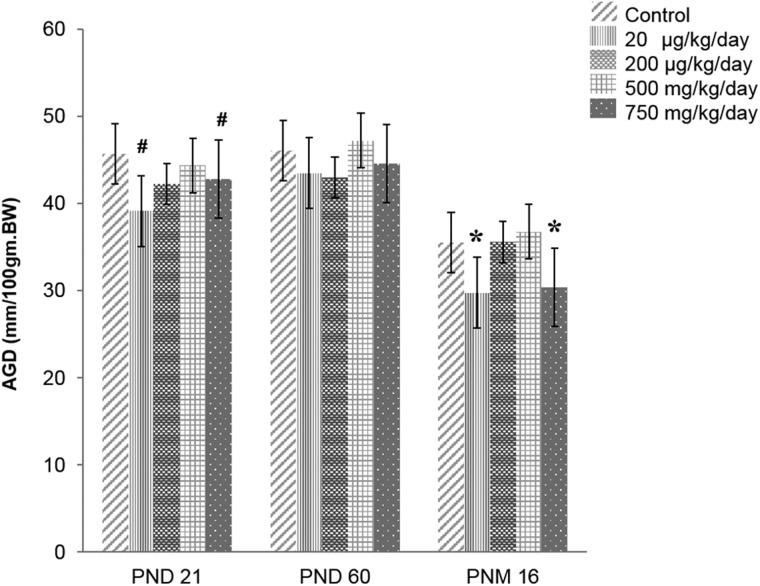
Anogenital Distance
Anogenital distance (AGD) is often used as an indicator of fetal and postnatal testosterone environment. The AGD was measured and normalized to body weight (mm/100 mg) at PND 21, PND 60, and 16 months of age (Figure 2). The 20 μg/kg/day and 750 mg/kg/day DEHP mice exhibited shorter AGD compared with the control group (P = .07 and P = .09) at PND21 and at 16 months of age (P = .03 and P = .04). AGD was also measured at 22 months, and it was the same as AGD at 16 months.
FIG. 2.
Effect of prenatal DEHP exposure on anogenital distance (AGD) at PND 21, PND 60 and 16 months of age. The AGD was normalized to body weight as mm/100gm BW (n = 4–7 per treatment group). Asterisks indicates P ≤ .05 when compared with control group, # indicate P ≤ .09. Note that AGD was also measured at 22 months, it was the same as AGD at 16 months of old.
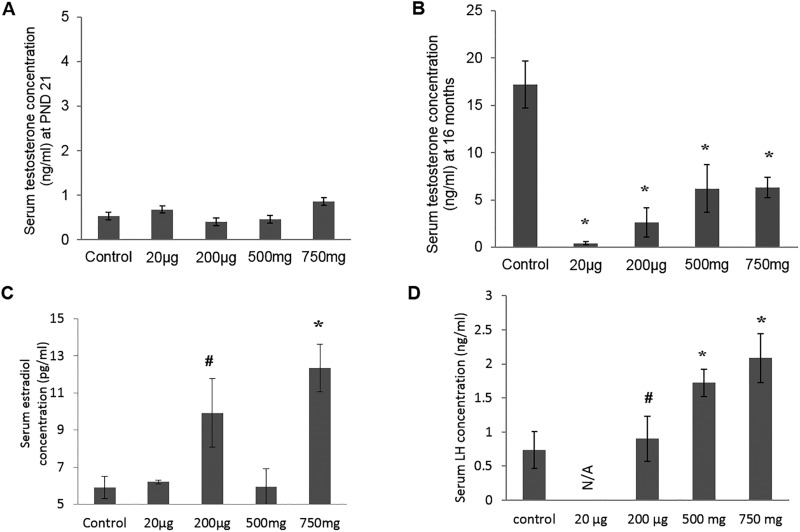
Serum Testosterone, Estradiol and LH Levels
At PND 21, serum testosterone levels of DEHP-treated mice were not statistically different from those of the control group (Figure 3A). However, when examined at age 16 months, DEHP-treated mice had significantly lower serum testosterone levels (P < .05) (Figure 3B) and higher serum estradiol level in the 200 μg/kg/day and 750 mg/kg/day groups when compared with control mice (P < .02) (Figure 3C). LH concentrations were measured at 22 months (Figure 3D); a significantly higher LH level was observed in the 500 mg and 750 mg/kg/day DEHP mice compared with the controls (P = .03 and .04, respectively).
FIG. 3.
Effect of prenatal DEHP exposure on hormonal level. Serum testosterone concentrations was measured at the ages of PND 21 (A) and at 16 months of age (B), serum estradiol concentrations was measured at 16 months of age (C), serum LH concentrations was measured at 22 months of age (D). Graphs show mean ± SEM, asterisks indicates P ≤ .05 when compared with control group, # indicate P ≤ .09. (n = 4–7 per treatment group), N/A: all of mice of 20 µg/kg/day group dead at 22 months of age.
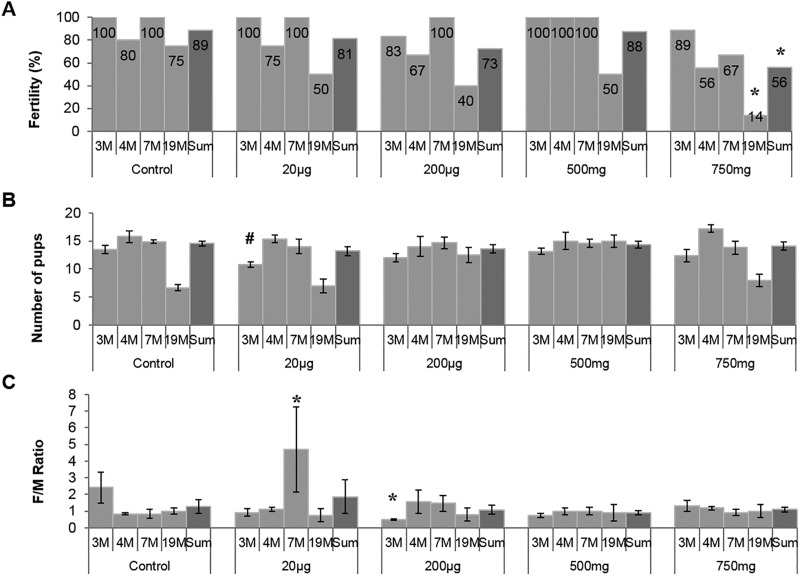
Fertility
To determine the effects of prenatal DEHP exposure on overall gonadal function, fertility, litter size, and sex ratio of offspring were assessed. These parameters were measured at 4 different ages (3, 4, 7, and 19 months) to see if DEHP exposure led to an age-dependent gonadal dysfunction (Figure 4A). No significant differences in fertility were seen among the treatment groups at the ages of 3 and 4 months. At 7 months, the highest DEHP-dose group (750 mg/kg/day) showed a decrease in fertility compared with the control group. At the last fertility test performed at 19 months of age, this highest dosage group showed a significant drop in fertility (14%), whereas the fertility in the control group was 75% (Figure 4A). When the fertility outcomes of the 4 trials were combined, the mice exposed to 750 mg/kg/day DEHP dose showed a significantly lower fertility compared with the control group (P = .028).
FIG. 4.
Effect of prenatal DEHP exposure on fertility outcomes. Four fertility tests were done at 3, 4, 7, and 19 months of age, this graph shows the fertility % (percent of males that produced a litter at each trial, A), litter size (numbers of pups per litter, B), and sex ratio (number of female to male pups produced in each litter, C). The numbers of mice used for this experiment (n = 4–7) and the ages of mice that were used at each fertility trial are indicated in months (M). Graphs show mean ± SEM, asterisks indicate P ≤ .05 when compared with control group, # indicate P ≤ .09. Fertility data was compared using Fisher’s exact test for each treatment group against control group.
Litter sizes were measured to determine if fecundity was affected by DEHP exposure (Figure 4B). Interestingly, only the 200 µg/kg/day DEHP-treated group produced significantly smaller litter numbers at 3 months of age compared with the control group (P = .08), but there was no significant difference later. No other DEHP group exhibited differences in litter size at any fertility trial.
To see if prenatal DEHP exposure impacted the sex ratios of the next generation, the female-to-male ratio was determined (Figure 4C). The 200 μg/kg/day DEHP mice had a significantly lower female-to-male ratio compared with the control group (P = .007) during the first round of fertility tests (3 months). In contrast, the 20 µg/kg/day DEHP-treated group had higher female-to-male ratio than the control group (P = .01) at age 7 months. Other than that, no other significant difference in sex ratio was observed between the control group and any treatment group.
Semen Quality
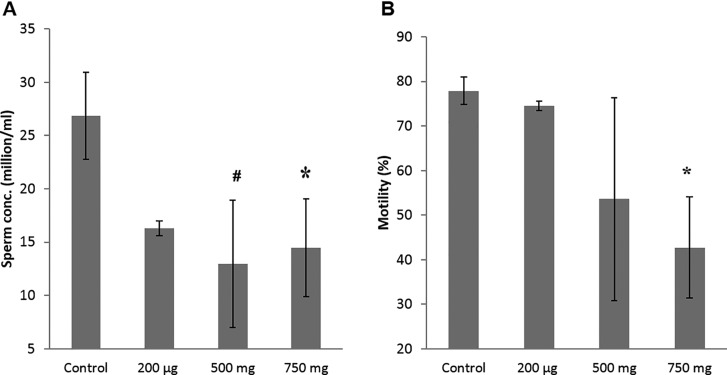
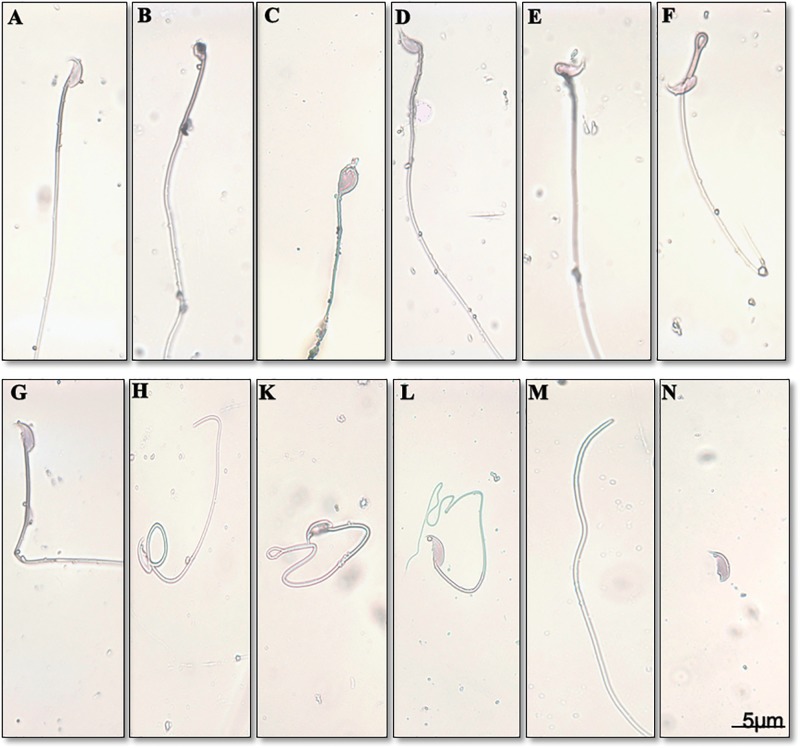
To determine whether sperm count and motility were affected by prenatal DEHP exposure, sperm analysis was used to assess sperm quality at age 22 months. Sperm concentration (millions/mL) was significantly lower in the 750 mg/kg/day DEHP-treated group compared with the control (Figure 5A). Also, the 500 mg/kg/day DEHP-treated group had a moderately lower sperm concentration compared with the control (P = .08). Interestingly, a dose-dependent reduction in the percentage of motile sperm was seen in the DEHP mice (Figure 5B), with the lowest motility observed in the highest dosage group. The percentage of progressive motile sperm was significantly decreased in the 750 mg/kg/day DEHP mice (P = .02) (Table 1). These 750 mg/kg/day DEHP mice also had a higher percentage of locally motile and immotile sperm compared with the control group, although these differences did not reach statistical significance (P = .21, P = .32, respectively). However, no differences in velocity parameters such as curvilinear velocity (VCL), average path velocity (VAP), and straight line velocity (VSL) were seen in mobile sperm (Table 1). Also, no differences were observed in the swimming pattern and head movement such as beat cross frequency (BCF), amplitude of lateral head displacement (ALH), linearity (LIN), and straightness (STR) of sperm between the control and any of the DEHP mice. Interestingly, the percentage of morphologically abnormal sperm was increased in the DEHP mice (Figure 6 and Table 2), e.g., sperm with abnormal heads, abnormal hooked-shape heads, abnormal mid-pieces or tails, or headless sperm.
FIG. 5.
Effect of prenatal DEHP exposure on sperm quality at 22 months of age. Sperm concentrations (millions/mL, A), sperm motility % (percent of motile sperm, B) are shown. Graphs show mean ± SEM, asterisks indicates P ≤ .05 when compared with control group, # indicate P ≤ .09 (n = 4–7 per treatment group). All of mice of the 20 µg/kg/day group were dead at this time point.
TABLE 1.
Effect of Prenatal DEHP Exposure on Different Sperm Parameters at 22 Months of Age
| Parameters | Control | 200 µg/kg/day | 500 mg/kg/day | 750 mg/kg/day |
|---|---|---|---|---|
| Progressive motility % | 47.81 ± 9.63 | 24.23 ± 1.84 | 32.13 ± 14.32 | 17.78 ± 4.61* |
| Local motility % | 28.76 ± 8.92 | 41.63 ± 10.27 | 29.76 ± 5.03 | 41.22 ± 4.69 |
| Immotile % | 23.43 ± 0.69 | 34.13 ± 9.46 | 38.06 ± 19.38 | 36.57 ± 3.84 |
| Track curvilinear velocity (VCL) | 118.68 ± 25.43 | 119.93 ± 14.23 | 107.76 ± 7.65 | 106.77 ± 23.64 |
| Average path velocity (VAP) | 68.27 ±15.76 | 69.57 ± 9.06 | 59.44 ± 3.51 | 60.60 ± 13.71 |
| Straight line velocity (VSL) | 49.45 ± 12.11 | 51.46 ± 7.10 | 44.11 ± 1.50 | 41.84 ± 8.84 |
| Linearity (LIN) | 0.40 ± 0.23 | 0.43 ± 0.35 | 0.41 ± 0.16 | 0.40 ± 0.27 |
| Straightness (STR) | 0.72 ± 0.02 | 0.74 ± 0.03 | 0.74 ± 0.01 | 0.70 ± 0.05 |
| Beat cross frequency (BCF) | 20.68 ± 2.47 | 19.23 ± 1.49 | 20.18 ± 0.23 | 18.42 ± 1.62 |
| Lateral head displacement (ALH) | 5.31 ± 0.57 | 5.86 ± 0.27 | 5.90 ± 0.44 | 4.59 ± 0.78 |
Sperm concentration (million/ml), Motility % (percent of motile sperm), Different pattern of motility % (Progressive motility, local motility and immotile %), different sperm velocity parameters. Table show mean ± SEM. asterisks indicates P ≤ .05 when compared with control group (n = 4–7 per treatment group). Table show mean ± SEM (n = 4–7 per treatment group). All of mice of the 20 µg/kg/day group were dead at this time point.
FIG. 6.
Effect of prenatal DEHP exposure on sperm morphology at 22 months of age. Normal sperm (A), knobbed hook sperm (B), sperm with amorphous head (C), bent head sperm (D), sperm with incorrect head neck connection (E), folded midpiece (F), bent tail (G), looping midpiece (H), folded tail (K), coiled tail (L), headless sperm (M), detached head (N).
TABLE 2.
Effect of Prenatal DEHP Exposure on Sperm Morphological Abnormalities
| Parameters | Control | 200 µg/kg/day | 500 mg/kg/day | 750 mg/kg/day |
|---|---|---|---|---|
| Normal sperm (%) | 76.43 ± 1.69 | 59.13 ± 2.32 * | 45.85 ± 2.01* | 19.52 ± 0.26 * |
| Total sperm abnormalities (%) | 23.54 ± 0.66 | 41.09 ± 3.92 * | 54.27 ± 0.99* | 80.55 ± 0.11* |
| Head abnormality (%) | 9.13 ± 1.89 | 18.56 ± 1.55* | 25.13 ± 2.11* | 31.90 ±1.70* |
| Neck and Midpiece abnormality (%) | 7.33 ± 0.54 | 11.18 ± 2.42 | 15.21 ± 0.89* | 23.51 ± 2.01* |
| Tail abnormality (%) | 6.01 ± 0.81 | 9.31 ± 1.71 | 11.34 ± 0.67 | 21.88 ± 0.44* |
| Double tail and head (%) | 1.62 ± 1.10 | 2.01 ± 1.12 | 2.11 ± 1.45 | 3.30 ± 1.99* |
Count 100 sperm from each group. Showed percent of normal sperm, percent of total sperm abnormalities, percent of sperm with head abnormalities, percent of sperm with neck and midpiece abnormalities, percent of sperm with tail abnormalities and percent of double tail and head sperm. Asterisks indicate P ≤ .05 compared with control group, data are mean ± SEM (n = 4–7 per treatment group) except 20µg/kg/day group all of mice was dead at 22 months of age.
Testis and Epididymis
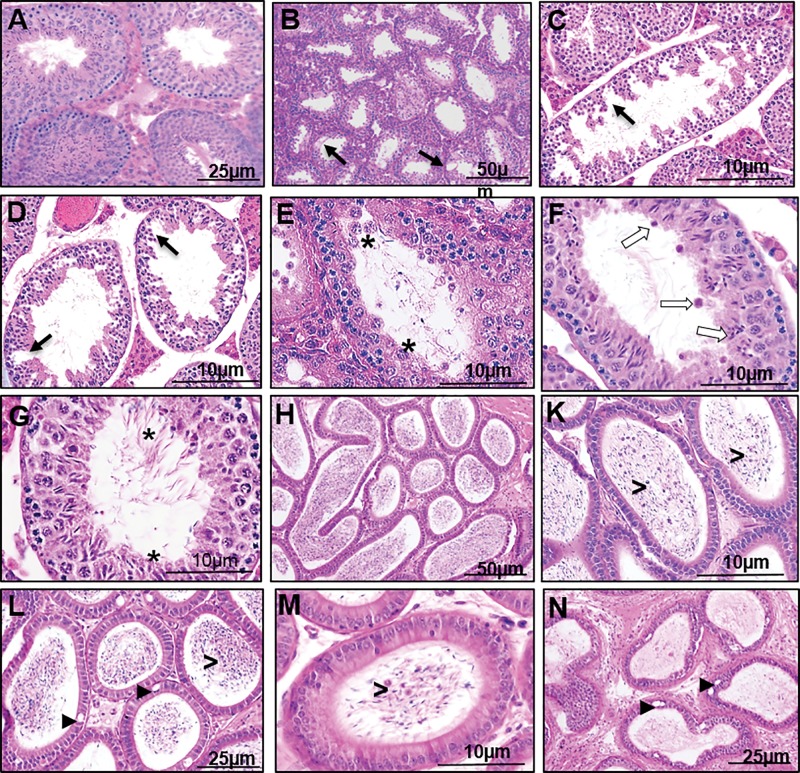
To determine if prenatal DEHP exposure impacted gonadal and epididymal development or function, testes and epididymis were collected at PND 21, 60, and at 22 months of age, and the seminiferous tubule, rete testis, testicular interstitium, efferent ductule, and epididymis were microscopically examined. No histopathological changes were detected between control and DEHP mice at PND 21 and 60 (Supplementary Figure 2). At 22 months of age, severe pathological abnormalities were found in the DEHP-treated mice (Figure 7). The control mice had normal seminiferous tubules and Leydig cell organization (Figure 7A) and densely populated sperm in the epididymis (Figure 7H). However, the testes of DEHP-treated mice had hypospermatogenesis with degenerative changes in the seminiferous tubules showing germ cell degeneration and fewer developing spermatids (Figure 7B–D). Desquamation of large multinucleated cells into the lumen was also observed in the DEHP testes (Figure 7E) as well as failure of spermiation and formation of abnormal residual bodies in the lumen (Figure 7F), which lead to the appearance of abnormal mixing of spermatogenic stages (Figure 7G). Of note, one mouse from the 750 mg/kg/day DEHP group had testicular cancer in the seminiferous tubules (germ cell tumor) with complete absence of any sperm production in the epididymis (Supplementary Figure 3A and B).
FIG. 7.
Effect of DEHP exposure on the histology of the testes and epididymis at 22 months of age. Testis of a control group (A). Testes of DEHP treated groups (B–G). Epididymis of a control group (H). Epididymis of DEHP treated groups (K–N). Note hypospermatogenesis with degenerative changes in the seminiferous tubules and germ cell degeneration (black arrows), desquamation of large multinucleated cell into the lumen (asterisks), failure of spermiation with abnormal residual bodies found in the lumen (white arrows), abnormal stages of spermatogenesis (stars), vacuoles in the epididymis epithelium (arrow heads), desquamated germ cell the in lumen of epididymis (>), ductal atrophy of the epididymis (N).
The epididymis of the DEHP-treated mice had a cribriform appearance of vacuoles in the epithelial lining (Figure 7L and N), desquamated germ cells the in the lumen (Figure 7K and M), and ductal atrophy with decreased sperm volume (Figure 7N). The epididymis of a mouse from the 500 mg/kg/day DEHP group had an occlusion of the efferent ductule that contained highly congested sperm in the lumen (Supplementary Figure 3C and D), and the initial segment contained residual bodies of spermatid cytoplasm and inflammatory cells without any sperm presence in the entire epididymal duct (Supplementary Figure 3E and F). The highest dosed group had a higher percentage of abnormalities in all categories examined (Table 3).
TABLE 3.
Quantitative Analysis of Histopathological Abnormalities of DEHP Treated Groups
| Histopathological abnormalities | Control | 200µg/kg/day | 500 mg/kg/day | 750 mg/kg/day |
|---|---|---|---|---|
| Testis | ||||
| −Hypospermatogenesis | 0% (0/4) | 50% (2/4) | 75% (3/4) | 80% (4/5) |
| −Germ cell degeneration | 25% (1/4) | 50% (2/4) | 75% (3/4) | 80% (4/5) |
| −Failure of spermiation. | 0% (0/4) | 25% (1/4) | 50% (2/4) | 40% (2/5) |
| −Abnormal residual bodies | 0% (0/4) | 25% (1/4) | 50% (2/4) | 40% (2/5) |
| −Spermatocele | 0% (0/4) | 0% (0/4) | 25% (1/4) | 0% (0/5) |
| −Testicular cancer. | 0% (0/4) | 0% (0/4) | 0% (0/4) | 20% (1/5) |
| Epididymis | ||||
| −Epididymal vacuoles. | 25% (1/4) | 25% (1/4) | 75% (3/4) | 100% (5/5) |
| −Germ cell in lumen of epididymis | 25% (1/4) | 50% (2/4) | 75% (3/4) | 80% (4/5) |
Count each histological abnormality in testis and epididymis from each mouse in all groups, and calculated the percent of affected mice for each abnormality (affected litters/total number of litters). Showed percent of each abnormality, percent of mice testis suffering from hypospermatogenesis, germ cell degeneration, failure of spermiation, abnormal residual bodies, spermatocele and testicular cancer. Also calculated the percentage of mice showing vacuoles in epithelium lining of epididymis and germ cell found in lumen of epididymis. (n = 4–5 per treatment group). All of mice of 20 µg/kg/day group were dead at this time point.
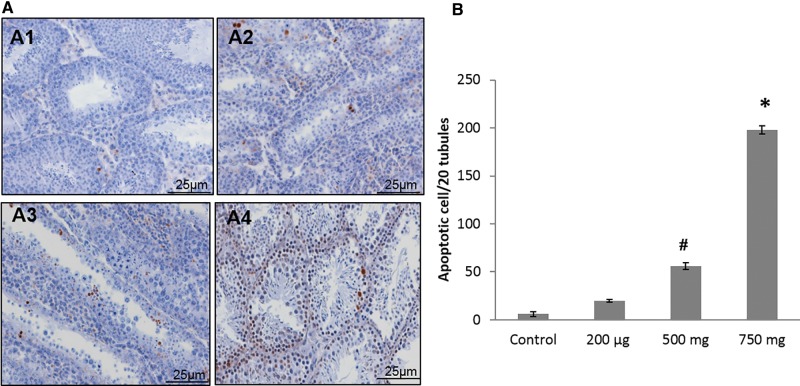
Testicular Germ Cell Apoptosis
To determine whether prenatal DEHP exposure increased testicular germ cell apoptosis, TUNEL staining was performed at 22 months of age. In the testis of DEHP-treated mice, a significantly higher numbers of apoptotic germ cells were present when compared with control mice (Figure 8A). In particular, the 500 and 750 mg/kg/day DEHP mice had significantly more apoptotic germ cells than the control mice (Figure 8B).
FIG. 8.
Effect of DEHP exposure on cell death at 22 months of age. Shown are representative images of each treatment group (A) examined by high power (20×), testis of control group (A1), testis of 200 µg/kg/day DEHP treated group (A2), testis of 500mg/kg/day DEHP treated group (A3), testis of 750mg/kg/day DEHP treated group (A4). Number of apoptotic cell/20 seminiferous tubules for all treated groups (B). Graphs show mean ± SEM, asterisks indicates P ≤ .05 when compared with control group, # indicate P ≤ .09. (n = 4–7 per treatment group). All of mice of 20 µg/kg/day group were dead at this time point.
DISCUSSION
A number of studies have assessed the impact of prenatal DEHP exposure on the male reproductive function and reported that DEHP induces reproductive dysfunction during adolescence or young adulthood (Doyle et al., 2013; Fisher, 2004; Pant et al., 2008; Swan et al., 2005). However, the long-term deleterious effects of the prenatal DEHP exposure on the male reproduction have not been explored. In this study, the impact of prenatal exposure of DEHP was assessed by a 2-year-long follow-up study focusing on possible effects on gonadal function in mature mice.
Previous studies have reported dissimilar impacts of DEHP exposure on body weight and therefore, effects on general health. A factor that is likely to contribute to the observed differences in those studies is the difference in the temporal window of DEHP administration. When the DEHP dosing period was limited to the gestational period, DEHP exposure did not affect body weight (Jarfelt et al., 2005; Kavlock et al., 2006), whereas if the dosing period was extended to the postnatal period and therefore, the animals were orally exposed to DEHP, body weight was affected (Tanaka, 2002; Hao et al. 2013). Consistent with these previous studies, the DEHP-treated mice did not show any significant difference in their body weights at any measurement time, indicating that the DEHP doses used in this study were not overly toxic to these mice. In addition, potential impacts on gonadal development would not be a major contributing factor to the reproductive pathologies observed in this study because mice from all of the DEHP dosing group exhibited a normal range of fertility up to the ages of 7 months (Figure 4), indicating normally functioning gonads by this age. However, it is noteworthy that the 200 μg/kg/day and 500 mg/kg/day DEHP mice had higher gonadal weight to body weight ratios at PND 8 compared with the control mice, even though the statistical significance disappeared later, indicating that the impact was either minor or transient, consistent with previous reports that DEHP increased testis weight (5–135 mg/Kg/day DEHP) in weaning rats and had no effect on adult testis (Andrade et al., 2006).
Interestingly, the 20 µg/kg/day DEHP mice had markedly lower survival rates over time, and at age 20 months, all mice in this group died, whereas the control mice had a survival rate above 50%, indicating a long-term impact of DEHP on health. In this study, no overt cause of the deaths of the 20 µg/kg/day DEHP mice was identified. A previous study reported that chronic exposure to DEHP in rats decreased their survival rate (David et al., 2000) and identified mononuclear cell leukemia as the most frequent cause of deaths.
The prenatal exposure to DEHP of 20 μg/kg/day and 750 mg/kg/day significantly decreased anogenital distance compared with the control group. This finding is not surprising because DEHP is suspected to be an anti-androgen (Jarfelt et al., 2005) and reportedly shortens the anogenital distance in humans (Swan et al., 2005) and animals (Andrade et al. 2006). Many anti-androgenic effects of DEHP are attributed to decreased perinatal androgen synthesis or systemic impairment in responsiveness to androgens after birth (Do et al., 2012). Overall, the consistent impact of DEHP on anogenital distance seen in our study and others demonstrates that the dosing paradigm used in this study was effective and comparable to the previous studies.
DEHP is thought to decrease fetal testosterone synthesis by interfering with the expression of steroidogenic enzymes involved in androgen biosynthesis (Martinez-Arguelles et al., 2009; Sekaran and Jagadeesan, 2015). Interestingly, testosterone concentrations of DEHP mice were not different from those of control mice when examined at PND 21 (Figure 3A). However, when testosterone levels were examined at 16 months of age, all of the DEHP mice had substantially lower serum testosterone levels (Figure 3B) than the control group, showing a long-term effect of DEHP exposure on testosterone levels. Fetal and adult-type Leydig cells have different lineages (Martinez-Arguelles and Papadopoulos, 2015), so our findings indicate that DEHP may alter steroidogenic capacity or the development of adult-type Leydig cell progenitors. Also, the decreased testosterone in the adult DEHP mice likely contributed to the abnormal reproductive phenotypes and shorter anogenital distance seen in the adult DEHP mice.
Testosterone is converted to estradiol by Cyp19A1 (aromatase), so decreased testosterone may lead to a reduced estradiol synthesis. However, serum estradiol levels were elevated in DEHP mice compared with the control group (Figure 3C), which may be attributed to abnormal hepatic estrogenic metabolism. Testosterone is critical for maintaining rapid estrogen metabolism and excretion in the liver (Eagon et al., 1994); decreased testosterone levels lead to lower estrogen metabolism and thus, increased serum estradiol levels. Also, DEHP has been shown to induce liver hyperplasia and disrupt estrogen metabolism (Eagon et al., 1994). Serum LH levels were elevated in DEHP mice compared with the control mice. This may be due to a direct impact on the LH secretion from the pituitary or an indirect consequence of decreased testosterone levels that normally suppresses LH secretion from the pituitary.
The male reproductive performance during the experiment was assessed by 4 fertility tests. No statistically significant differences in fertility were seen in any of the DEHP mice at the ages of 3 and 4 months; however, fertility of the 750 mg/kg/day DEHP mice decreased at 7 months of age and continued to drop to 14% at 19 months compared with 75% fertility in control mice, demonstrating that DEHP decreased fertility prematurely. It is plausible that decreased testosterone (Figure 3B) may have caused a premature reproductive senescence, as a proper level of testosterone would be required to maintain fertility. It will be interesting to determine whether testosterone therapy would restore the fertility in DEHP-treated mice. Interestingly, the percentage of female pups/litter was significantly lower in the 200 μg/kg/day group compared with controls (P = .007) when measured at 3 months of age. However, no significant difference in the sex ratio of pups was seen in the mice when examined at older ages. Furthermore, no significant differences in sex ratios were seen in other groups of mice at this age and fertility tests performed at older ages. Results from this study are consistent with the report by Tanaka, who showed that DEHP induced no adverse effects in the litter size, weight, or sex ratio (Tanaka, 2002).
The epididymal sperm parameters and the histopathological changes seen in seminiferous tubules of the mature DEHP mice may explain decreased fertility and premature reproductive senescence. As at early ages (PND 21 and 60), the testes and epididymis of DEHP mice did not show any histopathological aberrations compared with control mice, but at 22 months, the testes of DEHP mice displayed multiple abnormal testicular morphologies, including hypospermatogenesis with degenerative changes in the seminiferous tubules and germ cell degeneration with fewer developing sperm and the presence of sloughed large multinucleated cells in the lumen. Because adequate testosterone levels are required for germ cell attachment in seminiferous tubules (Blanco-Rodríguez and Martínez-García, 1997), the decreased testosterone levels might contribute to the germ cell detachment in the DEHP-treated mice and subsequent germ cell apoptosis seen in previous studies (Barlow and Foster, 2003; Doyle et al., 2013; Shirota et al., 2005). Although a low background level of germ cell apoptosis occurs normally in the testis and is essential for maintaining spermatogenesis (Tripathi et al., 2009), an increase in germ cell apoptosis is often observed in the experimental animals exposed to an array of testicular toxicants (Kasahara et al., 2002; Li et al., 2009; Ryu et al., 2007; Tinwell et al., 2007). Consistent with the reduced testosterone level and pathological abnormalities detected in testis, a TUNEL assay showed elevated numbers of apoptotic testicular germ cells, especially in the 750 mg/kg/day DEHP group. Sloughing of immature germ cells, which were found in the epididymal lumen, has been associated with increased germ cell apoptosis.
In addition, abnormal residual bodies found in the lumen of seminiferous tubules of the DEHP mice testes, which are considered sensitive indicators of testosterone depletion (Saito et al., 2000), indicated failure of spermiation. The epididymis of DEHP-treated groups showed intraepithelial cysts or cribriform changes (La Perle et al., 2002) similar to the effects of diethylstilbestrol (DES) exposure (Atanassova et al., 2005).
Desquamated germ cells were also found in the epididymal lumen, and ductal atrophy with decreased sperm volume was a sporadic observation at 22 months compared with control mice (Figure 7). This indicates that testicular abnormalities were found to be progressively severe as the mice aged. Furthermore, a mouse from 750 mg/kg/day DEHP group had a testicular cancer in the seminiferous tubules with complete absence of any sperm production in the epididymis. It will be interesting to see whether the incidence of testicular cancer increases in older DEHP mice.
The changes observed in sperm quality were consistent with the higher incidence of pathological abnormalities observed in the testes of DEHP-treated groups compared with the controls. DEHP males showed decreases in sperm concentration (oligozoospermia) and a dose-dependent reduction of motile sperm (asthenozoospermia). These findings are consistent with previous reports of decreases in epididymal sperm concentration following DEHP exposures (Gray et al., 2000; Moore et al., 2001). The percentage of progressive motile sperm was also lower in the DEHP mice, with a higher percentage of locally motile and immotile sperm compared with the control group. In the DEHP-treated mice, morphologically abnormal sperm (teratozoospermia) were common: abnormal heads, mid-pieces, tails; bent necks; headless sperm. These conditions may either be a result of DEHP effects on Sertoli cells, which help to regulate the production of spermatozoa, or on the epididymis, which is responsible for the storage and maturation of sperm.
In conclusion, this study discovered that prenatal exposure to DEHP induces premature reproductive senescence that is consistent with an impairment of testosterone production and decline in sperm quality. Further studies are needed to identify the mechanisms responsible for these DEHP effects on male reproduction. One possibility is a prenatal DEHP-induced epigenetic modification in the adult Leydig cell precursors during pregnancy, which could result in the premature decline in general reproductive health later in life. Therefore, we plan to test the epigenetic alterations in testis resulting from prenatal exposure to DEHP.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for measuring serum hormone levels and Susan Flanegin for her kind help in revising manuscript. The authors declare no conflict of interest here.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institute of Environmental Health Sciences grant (P01-ES022848 to C.K and J.A.F); Environmental Protection Agency grant (RD-83459301 to C.K and J.A.F.), Tox training grant (T32 ES007326 to S.R.) and Egyptian Mission Sector (JS-3041); Higher Ministry of Education to R.B..
REFERENCES
- Adibi J. J., Hauser R., Williams P. L., Whyatt R. M., Calafat A. M., Nelson H., Robert H., Swan S. H. (2009). Maternal urinary metabolites of Di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am. J. Epidemiol. 169, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry ( 2002). Toxicological profile: di(2-ethylhexyl)phthalate (DEHP). ATSDR, Atlanta, GA. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp9.html. [PubMed]
- Andrade A. J., Grande S. W., Talsness C. E., Grote K., Golombiewski A., Sterner-Kock A., Chahoud I. (2006). A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Effects on androgenic status, developmental landmarks and testicular histology in male offspring rats. Toxicology 225, 64–74. [DOI] [PubMed] [Google Scholar]
- Atanassova N., McKinnell C., Fisher J., Sharpe R. M. (2005). Neonatal treatment of rats with diethylstilboestrol (DES) induces stromal-epithelial abnormalities of the vas deferens and cauda epididymis in adulthood following delayed basal cell development. Reproduction 129, 589–601. [DOI] [PubMed] [Google Scholar]
- Barlow N. J., Foster P. M. D. (2003). Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol. Pathol. 31, 397–410. [DOI] [PubMed] [Google Scholar]
- Blanco-Rodríguez J., Martínez-García C. (1997). Apoptosis pattern elicited by oestradiol treatment of the seminiferous epithelium of the adult rat. J. Reprod. Fertil. 110, 61–70. [DOI] [PubMed] [Google Scholar]
- David R. M., Moore M. R., Finney D. C., Guest D. (2000). Chronic toxicity of Di(2-ethylhexyl)phthalate in mice. Toxicol. Sci. 58, 377–385. [DOI] [PubMed] [Google Scholar]
- Doyle T. J., Bowman J. L., Windell V. L., McLean D. J., Kim K. H. (2013). Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol. Reprod. 88, 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagon P. K., Chandar N., Epley M. J., Elm M. S., Brady E. P., Rao K. N. (1994). Di(2-ethylhexyl)phthalate-induced changes in liver estrogen metabolism and hyperplasia. Int. J. Cancer 58, 736–743. [DOI] [PubMed] [Google Scholar]
- Do R. P., Stahlhut R. W., Ponzi D., Saal F. S., Taylor J. A. (2012). Non-monotonic dose effects of in utero exposure to di(2- ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod. Toxicol. 34, 614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. S. (2004). Environmental anti-androgens and male reproductive health: Focus on phthalates and testicular dysgenesis syndrome. Reproduction 127, 305–315. [DOI] [PubMed] [Google Scholar]
- Frederiksen H., Skakkebaek N. E., Andersson A. M. (2007). Metabolism of phthalates in humans. Mol. Nutr. Food Res. 51, 899–911. [DOI] [PubMed] [Google Scholar]
- Gray L. E., et al. (2001). Effects of environmental antiandrogens on reproductive development in experimental animals. Hum. Reprod. Update 7, 248–264. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Ostby J., Furr J., Price M., Veeramachaneni D. N. R., Parks L. (2000). Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 58, 350–365. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Singh J. M., Leslie T. C., Meachum S., Flaws J. A., Yao H. H. C. (2010). Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol. Appl. Pharmacol. 242, 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavisto A. M., Pettersson K., Bergendahl M., Perheentupa A., Roser J. F., Huhtaniemi I. (1993). A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology 132, 1687–1691. [DOI] [PubMed] [Google Scholar]
- Hannon P. R., Peretz J., Flaws J. A. (2014). Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-Kinase signaling pathway in adult mice. Biol. Reprod. 90, 136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C., Cheng X., Guo J., Xia H., Ma X. (2013). Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Front. Biosci. 5, 725–733. [DOI] [PubMed] [Google Scholar]
- Hauser R. (2005). Phthalates and human health. Occup. Environ. Med. 62, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield A. N. (1991). Development of follicles in the mammalian ovary. Int. Rev. Cytol. 124, 43–101. [DOI] [PubMed] [Google Scholar]
- Huang L. P., Lee C. C., Hsu P. C., Shih T. S. (2011). The association between semen quality in workers and the concentration of di(2-ethylhexyl) phthalate in polyvinyl chloride pellet plant air. Fertil. Steril. 96, 90–94. [DOI] [PubMed] [Google Scholar]
- Jarfelt K., Dalgaard M., Hass U., Borch J., Jacobsen H., Ladefoged O. (2005). Antiandrogenic effects in male rats perinatally exposed to a mixture of di(2-ethylhexyl) phthalate and di(2-ethylhexyl) adipate. Reprod. Toxicol. 19, 505–515. [DOI] [PubMed] [Google Scholar]
- Kasahara E., Sato E. F., Miyoshi M., Konaka R., Hiramoto K., Sasaki J., Tokuda M., Nakano Y., Inoue M. (2002). Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem. J. 365, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R., et al. (2006). NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. 22, 291–399. [DOI] [PubMed] [Google Scholar]
- Koch H. M., Bolt H. M., Angerer J. (2004). Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch. Toxicol. 78, 123–130. [DOI] [PubMed] [Google Scholar]
- Koch H. M., Bolt H. M., Preuss R., Angerer J. (2005). New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol. 79, 367–376. [DOI] [PubMed] [Google Scholar]
- Latini G., De Felice C., Presta G., Del Vecchio A., Paris I., Ruggieri F., Mazzeo P. (2003). In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 111, 1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. J., Song T. B., Cai Y. Y., Zhou J. S., Song X., Zhao X., Wu Z. L. (2009). Bisphenol A exposure induces apoptosis and upregulation of fas/fasl and caspase-3 expression in the testes of mice. Toxicol. Sci. 108, 427–436. [DOI] [PubMed] [Google Scholar]
- Main K. M., et al. (2006). Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 114, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles D. B., Culty M., Zirkin B. R., Papadopoulos V. (2009). In utero exposure to di-(2-ethylhexyl) phthalate decreases mineralocorticoid receptor expression in the adult testis. Endocrinology 150, 5575–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles D. B., Papadopoulos V. (2015). Mechanisms mediating environmental chemical-induced endocrine disruption in the adrenal gland. Front. Endocrinol. 6, 29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. W., Rudy T. A., Lin T. M., Ko K., Peterson R. E. (2001). Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer di(2-ethylhexyl) phthalate. Environ. Health Perspect. 109, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermann S., Rattan S., Brehm E., Flaws J. A. (2015). Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod. Toxicol. 53, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otubanjo O. A., Mosuro A. A. (2001). An in vivo evaluation of induction of abnormal sperm morphology by some anthelmintic drugs in mice. Mutat. Res. 497, 131–138. [DOI] [PubMed] [Google Scholar]
- Pan G., Hanaoka T., Yoshimura M., Zhang S., Wang P., Tsukino H., Inoue K., Nakazawa H., Tsugane S., Takahashi K. (2006). Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ. Health Perspect. 114, 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant N., Shukla M., Patel D. K., Shukla Y., Mathur N., Gupta Y. k., Saxena D. K. (2008). Correlation of phthalate exposures with semen quality. Toxicol. Appl. Pharmacol. 231, 112–116. [DOI] [PubMed] [Google Scholar]
- La Perle K. M., Blomme E. A., Sagartz J. E., Capen C. C. (2002). Epididymal cribriform hyperplasia with nuclear atypia in p53 homozygous knockout mice on a mixed 129/Sv-FVB/N background. Comp. Med. 52, 568–571. [PubMed] [Google Scholar]
- Russell L. D., Ettlin R. A., Sinha A. P., Clegg E. D. (1990). Histological and histopathological evaluation of the testis. Int. J. Androl. 16, 83–83. [Google Scholar]
- Rusyn I., Peters J. M., Cunningham M. L. (2006). Effects of DEHP in the liver: modes of action and species-specific differences. Crit. Rev. Toxicol. 36, 459–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J. Y., Whang J., Park H., Im J. Y., Kim J., Ahn M. Y., Lee J., Kim H. S., Lee B. M., Yoo S. D., et al. (2007). Di(2-ethylhexyl) phthalate induces apoptosis through peroxisome proliferators-activated receptor-gamma and ERK 1/2 activation in testis of Sprague-Dawley rats. J. Toxicol. Environ. Health. A 70, 1296–1303. [DOI] [PubMed] [Google Scholar]
- Saito K., O'Donnell L., McLachlan R. I., Robertson D. M. (2000). Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology 141, 2779–2785. [DOI] [PubMed] [Google Scholar]
- Schettler T. (2006). Human exposure to phthalates via consumer products. Int. J. Androl. 29, 134–139. [DOI] [PubMed] [Google Scholar]
- Sekaran S., Jagadeesan A. (2015). In utero exposure to phthalate downregulates critical genes in Leydig cells of F1 male progeny. J. Cell. Biochem. 116, 1466–1477. [DOI] [PubMed] [Google Scholar]
- Shirota M., Saito Y., Imai K., Horiuchi S., Yoshimura S., Sato M., Nagao T., Ono H., Katoh M. (2005). Influence of di-(2-ethylhexyl)phthalate on fetal testicular development by oral administration to pregnant rats. J. Toxicol. Sci. 30, 175–194. [DOI] [PubMed] [Google Scholar]
- Silva M. J., Reidy J. A., Herbert A. R., Preau J. R., Needham L. L., Calafat A. M. (2004). Detection of phthalate metabolites in human amniotic fluid. Bull. Environ. Contam. Toxicol. 72, 1226–1231. [DOI] [PubMed] [Google Scholar]
- Silva M. J., Reidy J. A., Samandar E., Herbert A. R., Needham L. L., Calafat A. M. (2005). Detection of phthalate metabolites in human saliva. Arch. Toxicol. 79, 647–652. [DOI] [PubMed] [Google Scholar]
- Swan S. H., et al. (2005). Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 113, 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. (2002). Reproductive and neurobehavioural effects of lac dye administered in the diet to mice. Food Addit. Contam. 14, 373–380. [DOI] [PubMed] [Google Scholar]
- Tinwell H., Friry-Santini C., Rouquie D., Belluco S., Elies L., Pallen C., Bars R. (2007). Evaluation of the antiandrogenic effects of flutamide, DDE, and linuron in the weanling rat assay using organ weight, histopathological, and proteomic approaches. Toxicol. Sci. 100, 54–65. [DOI] [PubMed] [Google Scholar]
- Tripathi R., Mishra D. P., Shaha C. (2009). Male germ cell development: turning on the apoptotic pathways. J. Reprod. Immunol. 83, 31–35. [DOI] [PubMed] [Google Scholar]
- Zhu J., Phillips S. P., Feng Y. L., Yang X. (2006). Phthalate esters in human milk: Concentration variations over a 6-month postpartum time. Environ. Sci. Technol. 40, 5276–5281. [DOI] [PubMed] [Google Scholar]
- Zota A. R., Calafat A. M., Woodruff T. J. (2014). Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001-2010. Environ. Health Perspect. 122, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.