Abstract
Aims
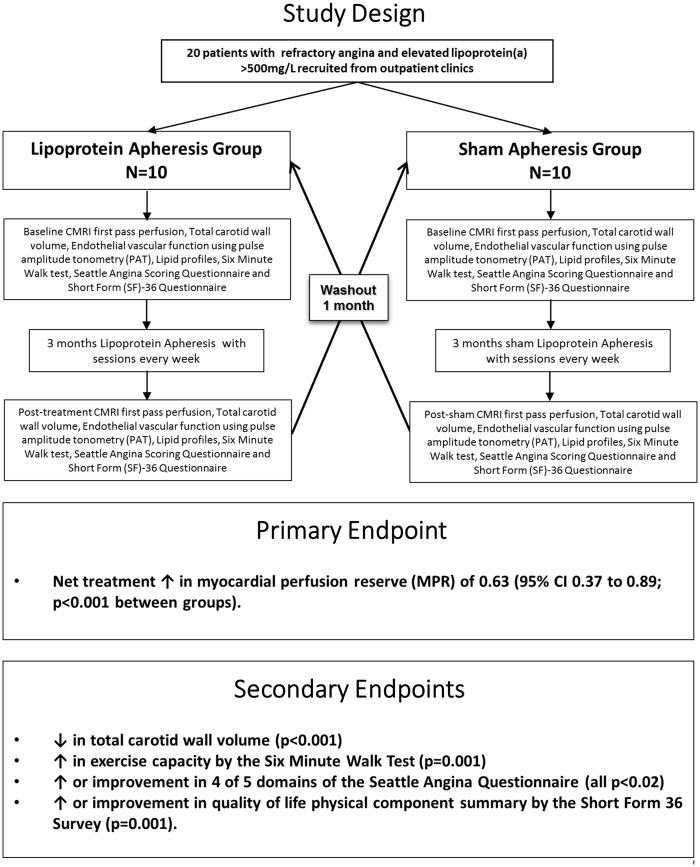
To determine the clinical impact of lipoprotein apheresis in patients with refractory angina and raised lipoprotein(a) > 500 mg/L on the primary end point of quantitative myocardial perfusion, as well as secondary end points including atheroma burden, exercise capacity, symptoms, and quality of life.
Methods
We conducted a single-blinded randomized controlled trial in 20 patients with refractory angina and raised lipoprotein(a) > 500 mg/L, with 3 months of blinded weekly lipoprotein apheresis or sham, followed by crossover. The primary endpoint was change in quantitative myocardial perfusion reserve (MPR) assessed by cardiovascular magnetic resonance. Secondary endpoints included measures of atheroma burden, exercise capacity, symptoms and quality of life.
Results
The primary endpoint, namely MPR, increased following apheresis (0.47; 95% CI 0.31–0.63) compared with sham (−0.16; 95% CI − 0.33–0.02) yielding a net treatment increase of 0.63 (95% CI 0.37–0.89; P < 0.001 between groups). Improvements with apheresis compared with sham also occurred in atherosclerotic burden as assessed by total carotid wall volume (P < 0.001), exercise capacity by the 6 min walk test (P = 0.001), 4 of 5 domains of the Seattle angina questionnaire (all P < 0.02) and quality of life physical component summary by the short form 36 survey (P = 0.001).
Conclusion
Lipoprotein apheresis may represent an effective novel treatment for patients with refractory angina and raised lipoprotein(a) improving myocardial perfusion, atheroma burden, exercise capacity and symptoms.
Keywords: Refractory angina, Lipoprotein(a), Apheresis, Cardiovascular magnetic resonance, Myocardial perfusion
Introduction
Refractory angina is a debilitating condition that is increasing in frequency as mortality from coronary artery disease (CAD) decreases. Although the exact incidence of refractory angina is unknown, according to a Swedish study based on a registry of patients referred for coronary angiography; the incidence is estimated to be 30 000–50 000 patients per year in Europe.1 These patients experience frequent angina despite optimized medical therapy, and treatment options are limited as the condition is not amenable to further surgical or percutaneous coronary revascularization.2 There is a pressing need for novel treatments for these patients. One possible contributing factor to the pathogenesis of refractory angina that has not been substantially investigated is lipoprotein(a), abbreviated as Lp(a).
Lp(a) is a genetically determined form of LDL-cholesterol consisting of a cholesterol rich LDL particle with apolipoprotein B (ApoB) and an additional protein apolipoprotein A (ApoA), attached via a disulphide bond.3 Lp(a) may enhance intimal lipoprotein deposition, and potentially affects myocardial perfusion, microvascular function, plasma viscosity, and endothelial function.4 Lp(a) may also promote thrombosis by inhibiting fibrinolysis and the tissue factor pathway inhibitor.4 Substantial evidence suggests that elevated Lp(a) is an independent cardiovascular risk factor.5,6 There is currently no satisfactory pharmacological treatment available which lowers Lp(a), but it can be effectively lowered with lipoprotein apheresis, a lipid-lowering extracorporeal treatment by which atherogenic ApoB containing lipoproteins, including Lp(a) and LDL, are removed from blood or plasma.7
Raised Lp(a) is common in refractory angina,8 and some data suggest a significant role in refractory angina. Patients who commenced lipoprotein apheresis because of elevated Lp(a) and progressive cardiovascular disease showed a reduction in major adverse coronary events from 0.41–0.09 per year.9 Similar results were seen in another retrospective observational study.10 There is a paucity of prospective randomized controlled trial data which aims to examine the impact of aggressively treating raised Lp(a) in the context of established coronary heart disease. In fact, to the best of our knowledge there are just two studies with a randomized controlled design, which attempt to explore this question. A cardiovascular magnetic resonance (CMR) study showed improved myocardial perfusion after a single apheresis session in patients with elevated Lp(a) and CAD.11 A quantitative angiographic study demonstrated some coronary atherosclerosis regression in stable CAD patients with high Lp(a) levels, after 18 months of weekly Lp(a) apheresis, compared with statin therapy alone.12 A case report suggested possible benefit in a patient with refractory angina.13 These preliminary data led us to conduct a randomized controlled trial of lipoprotein apheresis in patients with refractory angina and raised Lp(a).
Methods
Study design
We conducted a prospective randomized, sham controlled, single-blinded, cross-over study of 20 patients with refractory angina and elevated Lp(a) >500 mg/L (normal <300 mg/L). Eligible patients were identified from cardiology outpatient clinics and cardiac catheterization lists of the Royal Brompton and Harefield NHS Foundation Trust, a tertiary cardiac centre in London, UK; and were recruited between 1 March 2013 and 1 April 2015. All patients completed the trial protocol by 9 November 2015. The diagnosis of refractory angina was confirmed by at least one consultant cardiologist, ensuring that there was truly no opportunity for revascularization at the point of recruitment and that the complaints were genuinely felt to be of ischaemic origin, in most cases with some evidence of reversible ischaemia. Participants were randomized to an initial treatment arm (lipoprotein apheresis treatment sessions weekly for 3 months), or to an initial control group (sham apheresis sessions including needle insertion weekly for 3 months). After the first treatment period, there was a wash-out period of 1 month before cross-over to the alternative arm. Baseline and post-intervention investigations were repeated before and after each three-month treatment period. No investigations were conducted in the wash-out period between cross-over.
Main hypothesis
Lipoprotein apheresis improves quantitative myocardial perfusion as assessed by myocardial perfusion reserve (MPR) detected by stress/rest CMR, in patients with Refractory Angina and raised lipoprotein(a).
Study participants
The inclusion criteria were: refractory angina for >3 months; two or more episodes of angina per week; previous myocardial infarction, bypass surgery, percutaneous coronary angioplasty (or any combination of these three criteria); optimal medical therapy with at least two anti-anginal drugs; hypercholesterolaemia with an elevated Lp(a) >500 mg/L and an LDL-cholesterol less than 4.0 mmol/L, despite optimal lipid lowering drug therapy. The exclusion criteria were: poor calibre veins for cannulation; other chronic systemic illness such as liver or renal failure, neoplastic disease, overt heart failure, unstable CAD with significant and prolonged episodes of chest pain occurring at rest, coronary revascularization or a myocardial infarction within the previous 8 weeks; pregnancy, untreated diabetes mellitus, untreated arterial hypertension, and those with general contraindications to CMR or adenosine.
Outcome measures
The primary outcome measure was the change in the MPR from baseline to after 3 months of lipoprotein apheresis or sham. Myocardial perfusion reserve was calculated as the ratio of quantitative global average perfusion at stress to rest and was measured using CMR. The secondary outcome measures included changes in: carotid atheroma burden measured by CMR; endothelial vascular function; angina symptoms assessed with the Seattle angina questionnaire (SAQ); quality of life assessed by short form (SF)-36 Questionnaire; and exercise capacity assessed by the 6 min walk test (6MWT).The SAQ and SF-36 scores range from 0 to 100 with improvement signified by an increase in score for all measures.
Other investigations included fasting blood samples for lipid profiling consisting of total cholesterol, Lp(a), LDL cholesterol, HDL cholesterol, total cholesterol to HDL ratio, triglycerides, ApoA and ApoB. Cardiovascular magnetic resonance was used to assess left ventricular volumes and function, myocardial perfusion, myocardial late gadolinium enhancement (LGE), and carotid artery atherosclerosis burden. Peripheral arterial tonometry (PAT) was used to measure digital pulse amplitude to assess endothelial vascular function using the natural logarithm of reactive hyperaemia index (lnRHI) as the outcome measure.14
Lipoprotein apheresis
Lipoprotein apheresis was performed according to clinical guidelines for active treatment.15 The control group had sham apheresis sessions with needle insertion but the tubing was not connected to the machine. The apheresis machine was run to simulate active treatment and patients were blinded to treatment allocation with the use of screens and drapes. Treatments were performed in the Apheresis Unit in Harefield Hospital using the DX21 DHP (Direct Hemo Perfusion) Lipoprotein Apheresis machine with the Liposorber DL-75 column, which utilises dextran sulphate to covalently bind ApoB containing lipoproteins to remove them directly from whole blood.
Study oversight and ethics committee approval
Ethical approval was obtained from the National Research Ethics Committee, REC reference: 11/LO/1976. The study complies with the Declaration of Helsinki1964 and later revisions. The trial was registered with ClinicalTrials.gov, Identifier: NCT01796912. The full trial protocol can be obtained from the trial sponsor Imperial College London via the Joint Research Compliance Office, CRO reference 1880. All participants gave written informed consent.
Image acquisition
Cardiovascular magnetic resonance was performed at 3T, which has higher spatial resolution than 1.5T with greater signal to noise and superior results for perfusion quantification.16 For CMR assessment of quantitative perfusion, we used a prototype saturation-recovery prepared balanced steady-state free precession (bSSFP) sequence that includes a low-resolution gradient echo (GRE) acquisition for estimation of arterial-input-function, a technique known as ‘dual-sequence acquisition’.17 For stress, adenosine was infused at 140 ug/kg/min for 3 min via a left arm cannula. Gadolinium (Gadovist; Schering, Germany) contrast at a dose of 0.05 mmol/kg body weight was injected at 3.5 mL/s using a right arm cannula, followed by a 25 mL saline flush at 7 mL/s. Rest imaging was performed >20 min later using the same contrast injection methods. For CMR assessment of the carotid arteries, GRE bright blood localizers were acquired to locate the patient’s neck within the scanner. A stack of time-of flight (TOF) images was then acquired perpendicular to the long axis of the carotid arteries. Using these localizing images, high-resolution cross-sectional images were acquired at 2 mm intervals for 20 mm above, and 20 mm below the bifurcation of the common carotid artery on both sides using a locally established and validated protocol.18 All other CMR was performed using standardized protocols.19
Image analysis
Image analysis was performed blinded to treatment allocation on anonomized scans. All three short-axis slices of perfusion images covering the basal, mid and apical LV were analysed. Perfusion quantification was performed using previously validated software.20 Automated image processing steps included motion artifacts and signal intensity bias correction. Endocardial and epicardial borders of the left ventricular myocardium were manually traced on the perfusion image series to define myocardial regions of interest (ROI) for pixel-wise myocardial blood flow (MBF) estimation. An additional blood pool ROI was drawn on the low-resolution GRE image series to extract the arterial input function. Finally, pixel-wise myocardial time-signal intensity curves were extracted and quantified using a model-constrained deconvolution to estimate MBF. Carotid analysis was performed using Atheroma-Tools, a plug-in of CMRtools (Cardiovascular Imaging Solutions, London, UK) to derive carotid artery wall volume as a measure of atherosclerosis burden. For each cross-sectional image slice, the operator contoured the internal and external carotid arterial surfaces 20 mm above and below the bifurcation of the common carotid arteries on both sides. Total carotid wall volume was calculated for both sides.
Randomization and blinding
The randomization process was conducted by the trial statistician and was done with the computer software Stata using the command option ‘ralloc’. As the sample size was relatively small and the design was a crossover, blocking was used. All trial participants remained strictly blinded to treatment allocation throughout the entire protocol. All CMR image analysis was performed blinded to treatment allocation. Unblinding of the data and all statistical analysis was performed after the conclusion of the trial and after completing blinded analysis of the imaging end points.
Statistical analysis including power calculation
With the cross-over design, assuming the inter-study reproducibility for MPR to have a standard deviation (SD) of 0.15 with a postulated change in MPR of 0.2 between the groups, a sample size of 20 patients was required to achieve 99% power at a P-value of 0.05. Continuous data are presented as mean (SD) and (upper and lower 95% confidence interval [CI]) or median [interquartile range]. Comparisons between groups were performed using Student’s paired t-test for normally distributed data or the Wilcoxon matched-pairs signed-ranks test for other data. Linear mixed models were used to determine the treatment effect after adjustment for baseline MPR together with the sequence and period of treatment. From the paired t-tests treatment effect was estimated by the difference in means between the two treatment groups while for the non-parametric tests, the Hodges–Lehman estimator and its associated 95% CI was computed as the treatment effect. All of the analyses were done using the statistical software Stata 14.1 (Statacorp, Texas USA).
Role of the funding source
The study sponsors (National Institute for Health Research and Imperial College London) and the providers of the apheresis equipment (Kaneka Pharma Europe and LINC Medical), did not have any involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Results
Study population
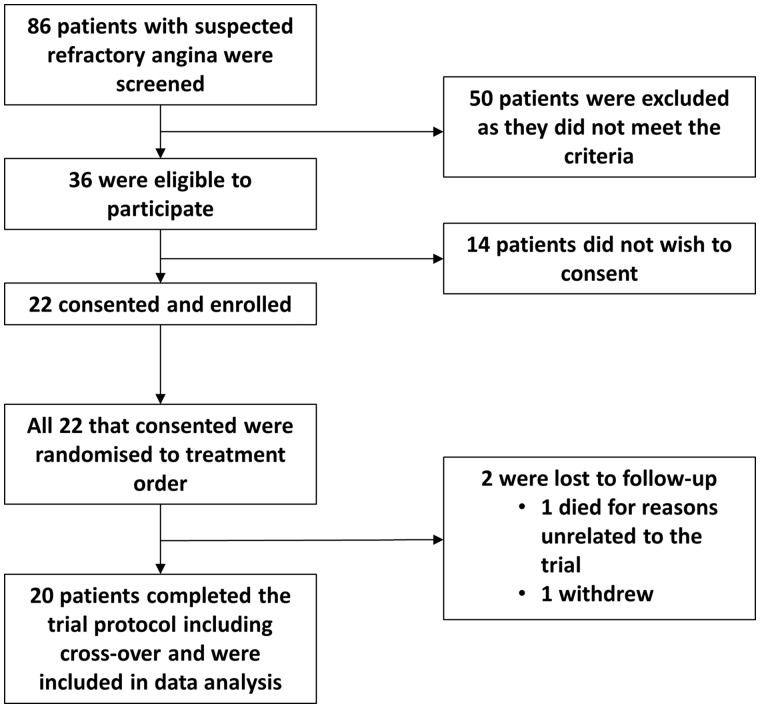
Of 86 patients with refractory angina formally screened for the trial, 36 met the eligibility criteria. Of these, 22 patients consented to participate. One patient withdrew during the study for personal reasons. One patient died during their cross-over wash-out period. Specifically, the patient was undergoing a nuclear stress perfusion scan unrelated to the trial and developed cardiogenic shock and acute renal failure and concurrently he also developed sepsis felt to be caused by a dental abscess. Therefore, 20 patients completed the trial protocol including cross-over (Figure 1). There was 98% attendance for the active sessions and 96% attendance for the sham sessions. One patient was unable to tolerate the DX 21 DHP system and was switched to a double filtration HF440 lipoprotein apheresis system. Baseline characteristics of all trial patients and the order in which treatment was randomized is described in Table 1. Twelve out of the 20 (60%) patients had prior bypass graft surgery and of those, 3 of 12 (25%) had undergone redo surgery. According to angiographic studies that had been performed for clinical purposes prior to recruitment, 9 of 12 (75%) with prior bypass surgery had at least 1 occluded graft, none had occluded all grafts i.e. all 12 had at least 1 patent graft remaining. Coronary stents had been inserted in 16 out of the total 20 (80%) patients. Amongst those with prior stents the average number of stents performed was 4. Of the 16 with prior stents, 11 (69%) had evidence of occlusion of at least one stent at the time of recruitment.
Figure 1.
Consort diagram.
Table 1.
Patient baseline characteristics
| Variable | Apheresis/Sham | Sham/Apheresis | All subjects |
|---|---|---|---|
| n | 9 | 11 | 20 |
| Age years | 59.1 (10.4) | 62.4 (9.0) | 60.9 (9.5) |
| Male | 9 (100) | 10 (91) | 19 (95) |
| Ethnicity: | |||
| White | 4 (44.4) | 3 (27.3) | 7 (35.0) |
| Asian | 5 (55.6) | 8 (72.7) | 13 (65.0) |
| Body-mass index (kg/m2) | 27.3 (1.9) | 27.5 (4.1) | 27.4 (3.2) |
| Systolic blood pressure (mmHg) | 125.6 (8.5) | 125.5 (9.1) | 125.5 (8.6) |
| Diastolic blood pressure (mmHg) | 72.2 (9.4) | 71.4 (2.3) | 71.8 (6.3) |
| Recruitment Lp(a) (mg/L) | 1120 (771, 1660) | 1080 (902, 1520) | 1100 (771, 1590) |
| Total cholesterol (mmol/L) | 3.46 (0.82) | 4.25 (0.74) | 3.90 (0.86) |
| LDL cholesterol (mmol/L) | 1.85 (0.74) | 2.41 (0.64) | 2.16 (0.73) |
| Diabetes | 2 (22.2) | 1 (9.1) | 3 (15.0) |
| Hypertension | 4 (44.4) | 8 (72.7) | 12 (60.0) |
| Smoker | |||
| No | 3 (37.3) | 7 (63.6) | 10 (50.0) |
| Ex | 4 (44.4) | 2 (18.2) | 6 (30.0) |
| Current | 2 (22.2) | 2 (18.2) | 4 (20.0) |
| Family history of CAD | 7 (77.8) | 9 (81.8) | 16 (80.0) |
| Anti-anginal drugs | |||
| Oral Nitrates | 7 (77.8) | 7 (63.6) | 14 (70.0) |
| Beta Blockers | 7 (77.8) | 11 (100) | 18 (90.0) |
| Ca channel blockers | 3 (33.3) | 5 (45.5) | 8 (40.0) |
| Ivabradine | 2 (22.2) | 2 (18.2) | 4 (20.0) |
| Ranolazine | 1 (11.1) | 0 | 1 (5.0) |
| Statin | 9 (100.0) | 11 (100.0) | 20 (100.0) |
| Prior coronary artery bypass graft surgery | 6 (66.7) | 6 (54.6) | 12 (60.0) |
| Prior percutaneous coronary intervention | 7 (77.8) | 9 (81.8) | 16 (80.0) |
| Prior myocardial infarction | 8 (88.9) | 9 (81.8) | 17 (85.0) |
Data are mean (SD), n (%), median (interquartile range).
LDL, low-density lipoprotein; CAD, coronary artery disease.
The response of lipids to apheresis is summarized in Table 2. There were significant reductions in the apheresis group in Lp(a), LDL, HDL, triglycerides, and ApoB.
Table 2.
Change in endpoints before and after apheresis and sham
| Variable | Apheresis | Sham | Treatment effect | P |
|---|---|---|---|---|
| Parametric Test: Mean (95% CI) | ||||
| Primary Outcome | ||||
| MPR | 0.47 (0.31, 0.63) | −0.16 (−0.33, 0.02) | 0.63 (0.37, 0.89) | 0.0001 |
| Secondary Outcomes | ||||
| Rest myocardial perfusion mL/min/g | 0.002 (−0.09, 0.10) | 0.06 (−0.05, 0.17) | −0.06 (−0.05, 0.18) | 0.42 |
| LVEF % | 1.50 (−0.78, 3.76) | 0.70 (−3.52, 3.92) | 0.80 (−2.96, 4.56) | 0.66 |
| Left carotid distensibility % | 4.90 (0.60, 9.20) | −0.80 (−4.40, 2.80) | 5.70 (0.44, 10.98) | 0.035 |
| Right carotid distensibility % | 7.10 (3.30, 10.90) | −0.80 (−3.80, 2.10) | 7.90 (2.48, 13.47) | 0.007 |
| EndoPat LnRHI | −0.05 (−0.08, 0.19) | −0.03 (−0.18, 0.11) | 0.08 (−0.03, 0.21) | 0.14 |
| SAQ—Angina stability | 17.50 (6.70, 28.30) | −1.75 (−17.10, 9.55) | 21.25 (4.58, 37.92) | 0.016 |
| SAQ—QoL | 25.80 (17.50, 34.10) | 4.60 (−6.10, 15.30) | 21.20 (7.08, 35.42) | 0.005 |
| SF-36—Mental component score (MCS) | 6.40 (2.50, 16.20) | 1.60 (−3.80, 7.00) | 4.80 (−2.63, 6.99) | 0.19 |
| HDL cholesterol mmol/L | −0.12 (−0.21, −0.04) | −0.002 (−0.08, 0.07) | −0.12 (−0.21, −0.04) | 0.006 |
| Triglycerides mmol/L | −0.28 (−0.49, −0.07) | 0.18 (−0.02, 0.37) | −0.46 (−0.77, −0.14) | 0.007 |
| Apolipoprotein A (g/L) | −0.09 (−0.17, −0.00) | −0.01 (−0.06, 0.04) | −0.08 (−0.16, −0.008) | 0.074 |
| Apolipoprotein B (g/L) | −0.41 (−0.47, −0.34) | −0.04 (−0.11, 0.03) | −0.37 (−0.45, −0.29) | <0.0001 |
| Non-parametric test: median [IQR] | ||||
| Stress myocardial perfusion mL/min/g | 0.44 [0.18, 0.67] | −0.07 [−0.14, 0.09] | 0.45 (0.28, 0.64) | 0.0004 |
| Total carotid wall volume (left & right) mm3 | −335 [−423, −247] | 127.35 [72.2, 183] | −564 (−729, −416) | <0.0001 |
| Total carotid wall volume (left) mm3 | −200 [−268, −133] | 93.40 [40.0, 147] | −337 (−487, −218) | <0.0001 |
| Total carotid wall volume (right) mm3 | −135 [−187, −81.8] | 34.0 [−19.3, 87.2] | −249 (−349, −141) | 0.0004 |
| 6MWT distance m | 70.5 [41.5, 105.5] | 3.5 [–15.1, 30.8] | 70.6 (39.0, 150) | 0.001 |
| SAQ—Physical limitation | 27.8 [16.7, 43.1] | −4.2 [−11.1, 6.9] | 28.5 (19.4, 40.3) | 0.003 |
| SAQ—Angina frequency | 35.0 [20.0, 50.0] | −5.0 [−20.0, 5.0] | 30.0 (15.0, 55.0) | 0.005 |
| SAQ—Treatment satisfaction | 6.25 [0.0, 18.75] | 0 [−3.125, 6.25] | 6.25 (0.0, 18.80) | 0.14 |
| SF-36—Physical component score (PCS) | 7.5 [5.0, 13.0] | −2.0 [−4.5, 1.0] | 11.0 (7.0, 14.0) | 0.001 |
| Lp(a) mg/L | −679.5 [−1102, −453] | −5.5 [−48.85, 51.5] | −912 (−1381, −597) | 0.0001 |
| LDL cholesterol mmol/L | −1.55 [−1.90, −1.17] | −0.03 [−0.04, 0.07] | −1.42 (−1.80, −1.15) | 0.0001 |
Data are mean (lower 95% CI, upper 95% CI) or median [lower quartile, upper quartile].
LVEF, left ventricular ejection fraction; 6MWT, 6-min walk test; LnRHI, natural logarithm of reactive hyperaemia index; SAQ, Seattle angina questionnaire; SF-36, short form-36 questionnaire; Lp(a), lipoprotein (a); LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Primary endpoint
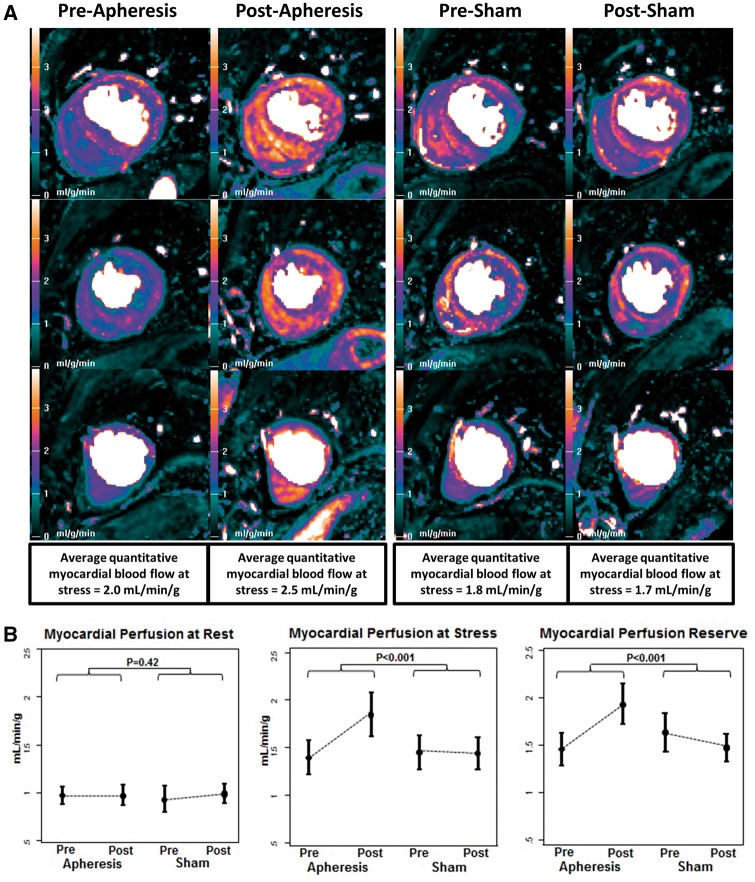
Myocardial perfusion reserve increased by 0.47 (0.31–0.63) from 1.45 (0.36) to 1.93 (0.45) with apheresis, but was unchanged during sham with a change of −0.16 (−0.33–0.02) from 1.63 (0.43) to 1.47 (0.30). The net treatment effect was an increase in MPR with apheresis by 0.63 (0.37–0.89) (P < 0.001 between groups). Rest myocardial flow (mL/min/g) did not change significantly during apheresis from 0.97 (0.18) to 0.97 (0.22), or during sham from 0.93 (0.29) to 0.99 (0.21) (P = 0.42 between groups). Stress myocardial flow (mL/min/g) increased by 0.44 [0.18–0.67] from 1.40 (0.39) prior to apheresis to 1.85 (0.50) following apheresis but did not change during sham from 1.45 (0.38) to 1.44 (0.36) (P < 0.001 between groups). Example images are illustrated in Figure 2A, with group data in Figure 2B and summary data in Table 2.
Figure 2.
Quantitative CMR perfusion pixel maps pre and post apheresis and pre and post sham (A) and group data from myocardial perfusion at rest (left), perfusion with stress (middle) and the myocardial perfusion reserve (right) (B). (A) Quantitative CMR perfusion pixel maps pre- and post-apheresis and pre- and post-sham. The colour scale shows perfusion from 0–4 mL/g/min as low (black-green), medium (mauve-pink) and high (orange-white), therefore brighter colours represent greater perfusion. In this single patient example, there is clear improvement in stress perfusion after apheresis compared with baseline, but no change is seen during sham treatment. (B) Group data are shown from myocardial perfusion at rest (left), perfusion with stress (middle) and the myocardial perfusion reserve (right). There are no changes in rest perfusion with apheresis or sham, but stress perfusion increases significantly with apheresis compared with sham. The myocardial perfusion reserve increases with apheresis because of the improved stress perfusion.
For the primary endpoint, a linear mixed effects model was used to assess the effect of the treatment with baseline MPR, sequence of treatment and period included in the model as fixed effects as well as testing for any treatment/period interaction. There was no effect of sequence −0.01 (−0.20, 0.22) P = 0.92 and more importantly there was no period/treatment interaction −0.02 (−0.43, 0.39) P = 0.92. The treatment effect from the model was 0.56 (0.29, 0.84) P < 0.0001.
Secondary endpoints
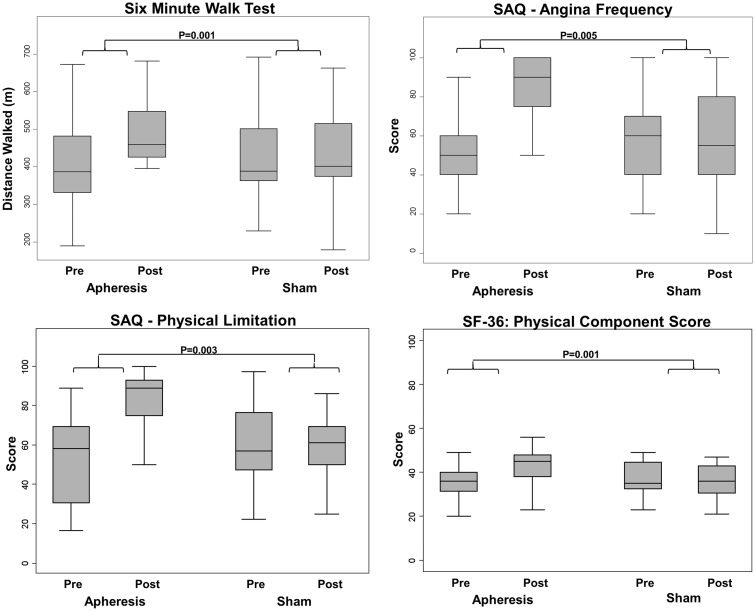
Median total carotid wall volume (mm3) reduced during apheresis by −335 [−423, −247] from 2482 [1910, 2836] before apheresis to 2251 [1719, 2437] after apheresis, but during sham total carotid wall volume increased from 2342 [1997, 2644] pre-sham to 2455 [2166, 2831] post-sham (P < 0.001 between groups, Figure 3). Mean left carotid distensibility (%) increased by 4.9 (0.6–9.2) during apheresis but did not change during sham −0.8 (−4.4–2.8) (P = 0.035 between groups). Similarly, mean right carotid distensibility (%) increased by 7.1 (3.3–10.9) during apheresis but did not change during sham −0.8 (−3.8–2.1) (P = 0.007 between groups).
Figure 3.
Improvements during apheresis compared with sham. Graphs showing improvements during apheresis compared with sham in: distance walked on 6 min walk test (top left); angina (top right); physical limitation (bottom left); overall physical wellbeing (bottom right).
Endothelial function, assessed peripherally via using the EndoPAT device did not change significantly during apheresis or sham: lnRHI changed during apheresis by 0.05 (−0.08, 0.19) and during sham by −0.03 (−0.18, 0.11) (P = 0.14 between groups). The 6MWT distance(m) improved during apheresis by 70.5 [41.5, 106] but did not change during sham: 3.5 [−15.1, 30.8] (P = 0.001 between groups, Figure 3).
Improvements occurred in 4 of 5 domains of the SAQ, indicating amelioration of angina symptoms during apheresis, which did not occur during sham (Figure 3). Seattle angina questionnaire—Physical limitation score improved by a median increase of 27.8 [16.7, 43.1] with a median change of −4.2 [−11.1, 6.9] during sham (P = 0.003 between groups). SAQ-Angina stability score improved by a mean increase of 17.5 (6.70, 28.3) with a mean change of −3.75 (−17.1, 9.55) during sham (P = 0.016 between groups). SAQ- Angina frequency score improved by 35.0 [20.0, 50.0] with a change during sham of −5.0 [−20.0, 5.0] (P = 0.005 between groups). SAQ- Treatment satisfaction score improved by a median increase of 6.25 [0.0, 18.75] with a median change during sham of 0.0 [−3.125, 6.25] (P = 0.14 between groups). SAQ- Quality of life score improved by a mean increase of 25.8 (17.5, 34.1) with a mean change during sham of 4.6 (−6.1, 15.3) (P = 0.005 between groups). Also, for quality of life measures, there was an improvement in the SF-36 physical component summary (PCS), with an increase in PCS score of 7.5 [5.0, 13.0] during apheresis and a median change of −2.0 [−4.5, 1.0] during sham (P = 0.001 between groups, Figure 3). SF-36 mental component summary (MCS) showed a mean change of 6.4 (2.5, 10.2) during apheresis and a change of 1.6 (−3.8, 7.0) during sham, but this did not reach statistical significance (P = 0.19 between groups).
Discussion
This is the first randomized controlled trial to examine the impact of lipoprotein apheresis in patients with refractory angina and raised Lp(a). There was clear improvement in the primary endpoint of MPR, as well as the secondary endpoints of exercise capacity, angina symptoms, quality of life and atheroma burden (Figure 4). This indicates that lipoprotein apheresis yields significant clinical improvement in this difficult to treat patient group, and is a welcome and much needed novel treatment option.
Figure 4.
Summary illustration of the trial design and the key findings.
Refractory angina is a growing problem worldwide due to improving survival rates owing to improved revascularization techniques, causing an expanding population of patients with treatment resistant angina.1,2 The healthcare burden of this condition is significant and the management of affected patients is challenging.1 According to the 2013 ESC guidelines on the management of stable CAD and specifically refractory angina;21 among non-pharmacological treatments, enhanced external counterpulsation therapy and neurostimulatory techniques have shown that they can ameliorate symptoms and improve quality of life, although robust evidence regarding reduction in both ischaemic burden and mortality is still lacking. Conversely, transmyocardial, or percutaneous myocardial revascularization have been abandoned because they are ineffective.21 The use of apheresis offers a new avenue to explore in refractory angina patients.
There are some limitations with regard to our results. First is the proportion of patients with refractory angina that may benefit. The prevalence of raised Lp(a) in refractory angina is reported in one study as 60%,8 suggesting a high proportion of patients with refractory angina could benefit from apheresis, but whether there is a lower threshold of Lp(a) than 500 mg/L which would yield clinical benefit is unknown. Second is the role that Lp(a) lowering plays in the treatment effect. Lipoprotein apheresis removes ApoB containing lipoproteins from whole blood which lowers Lp(a), but also lowers LDL. Whilst the treatment efficacy of apheresis remains clear, it leaves open to interpretation whether the effect is mediated by Lp(a) reduction, LDL reduction or both. Further trials to address this issue could use Lp(a) specific apheresis columns, or the alternative technology of oligonucleotide knock-down of Lp(a) mRNA.22 Third is the role of apheresis in removal from blood of factors other than lipoproteins, including fibrinogen, coagulation factors, thrombogenic factors, complement factors, inflammatory factors and adhesion molecules.23 This may mediate reduced coagulation and improvements in endothelial function or atherogenesis. Finally, the small sample size of this trial deserves mention, as although statistically significant findings were observed for the primary endpoint as well as the majority of secondary endpoints; to some extent, this may ultimately limit the generalizability of the results. Furthermore, ideally a larger study in patients with refractory angina and raised Lp(a) incorporating the impact of apheresis on major adverse cardiovascular event rates would help to validate the findings. In addition, the mechanisms of the numerous improvements in the observed endpoints of vessel wall, perfusion and symptoms need to be elucidated with further research.
A challenge for the provision of apheresis for patients with refractory angina is that apheresis availability is generally limited and costly.4 Expansion of these services to treat a significant cohort of patients with refractory and raised Lp(a) would require significant healthcare planning.
Conclusion
Lipoprotein apheresis may represent an effective novel treatment for refractory angina in the context of raised Lp(a) with improvements demonstrated in myocardial perfusion, exercise capacity, angina symptoms, quality of life and atheroma burden. This new treatment has potential to benefit the growing cohort of these patients worldwide and throw new light onto the pathogenic role of Lp(a) in atherosclerosis.
Funding
National Institute for Health Research Cardiovascular Biomedical Research Unit at Royal Brompton and Harefield Hospital NHS Foundation Trust; and Imperial College London. Apheresis equipment used in the trial was provided by Kaneka Pharma Europe and LINC Medical.
Conflict of interest: D.J.P. is a consultant to Bayer, a stockholder and director of Cardiovascular Imaging Solutions, and receives research support from Siemens. S.G. is a Siemens employee. P.C. is a consultant to Itamar Medical. The other authors have no conflicts to declare.
References
- 1. Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Lüscher T, Pasic M, Thelle D.. The problem of chronic refractory angina; report from the ESC joint study group on the treatment of refractory angina. Eur Heart J 2002;23:355–370. [DOI] [PubMed] [Google Scholar]
- 2. Henry TD, Satran D, Hodges JS, Johnson RK, Poulose AK, Campbell AR, Garberich RF, Bart BA, Olson RE, Boisjolie CR, Harvey KL, Arndt TL, Traverse JH.. Long-term survival in patients with refractory angina. Eur Heart J 2013;34:2683–2688. [DOI] [PubMed] [Google Scholar]
- 3. Berg K. A new serum type system in man: the Lp system. Acta Pathol Microbiol Scand 1963;59:369–382. [DOI] [PubMed] [Google Scholar]
- 4. Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgözoglu L, Tybjærg-Hansen A; European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 2010;31:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cremer P, Nagel D, Labrot B, Mann H, Muche R, Elster H, Seidel D.. Lipoprotein(a) as predictor of myocardial infarction in comparison to fibrinogen, LDL cholesterol and other risk factors: results from the prospective Gottingen Risk Incidence and Prevalence Study (GRIPS). Eur J Clin Invest 1994;24:444–453. [DOI] [PubMed] [Google Scholar]
- 6. Danesh J, Collins R, Peto R.. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 2000;102:1082–1085. [DOI] [PubMed] [Google Scholar]
- 7. Borberg H. Comparison of different Lp(a) elimination techniques: a retrospective evaluation. Trans Apheres Sci 2009;41:61–65. [DOI] [PubMed] [Google Scholar]
- 8. Khan TZ, Rhodes S, Pottle A, Banya W, Smith R, Kabir T, Ilsley C, Pennell DJ, Barbir M.. High prevalence of raised lipoprotein(a) in patients with refractory angina. Glob Cardiol Sci Pract 2015;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leebmann J, Roseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, Maerz W, Lehmacher W, Heibges A, Klingel R; for the Pro(a)LiFe Study Group. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease. Prospective observational multicenter study. Circulation 2013;128:2567–2576. [DOI] [PubMed] [Google Scholar]
- 10. Jaeger BR, Richter Y, Nagel D, Heigl F, Vogt A, Roeseler E, Parhofer K, Ramlow W, Koch M, Utermann G, Labarrere CA, Seidel D; Group of Clinical Investigators. Longitudinal cohort study on the effectiveness of apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Clin Pract Cardiovasc Med 2009;6:229–239. [DOI] [PubMed] [Google Scholar]
- 11. Bohl S, Kassner U, Eckardt R, Utz W, Mueller-Nordhorn J, Busjahn A, Thomas HP, Abdel-Aty H, Klingel R, Marcovina S, Dietz R, Steinhagen-Thiessen E, Schulz-Menger J, Vogt A.. Single lipoprotein apheresis session improves cardiac microvascular function in patients with elevated lipoprotein(a): detection by stress/rest perfusion magnetic resonance imaging. Ther Apher Dial 2009;13:129–137. [DOI] [PubMed] [Google Scholar]
- 12. Safarova MS, Ezhov MV, Afanasieva OI, Matchin YG, Atanesyan RV, Adamova IY, Utkina EA, Konovalov GA, Pokrovsky SN.. Effect of specific lipoprotein(a) apheresis on coronary atherosclerosis regression assessed by quantitative coronary angiography. Atheroscler Suppl 2013;14:93–99. [DOI] [PubMed] [Google Scholar]
- 13. Ibrahim M, Ussen B, Pottle A, Barbir M.. Low-density lipoprotein apheresis is effective in reducing lipoprotein(a) levels and in improving symptoms in a patient with refractory angina secondary to accelerated coronary artery disease. J Clin Lipidol 2012;6:192–194. [DOI] [PubMed] [Google Scholar]
- 14. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A.. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 15. Thompson GR; HEART-UK LDL Apheresis Working Group. Recommendations for the use of LDL apheresis. Atherosclerosis 2008;198:247–255. [DOI] [PubMed] [Google Scholar]
- 16. Cheng AS, Pegg TJ, Karamitsos TD, Searle N, Jerosch-Herold M, Choudhury RP, Banning AP, Neubauer S, Robson MD, Selvanayagam JB.. Cardiovascular magnetic resonance perfusion imaging at 3-Tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol 2007;49:2440–2449. [DOI] [PubMed] [Google Scholar]
- 17. Gatehouse PD, Elkington AG, Ablitt NA, Yang GZ, Pennell DJ, Firmin DN.. Accurate assessment of the arterial input function during high-dose myocardial perfusion cardiovascular magnetic resonance. J Magn Reson Imaging 2004;20:39–45. [DOI] [PubMed] [Google Scholar]
- 18. Varghese A, Crowe LA, Mohiaddin RH, Gatehouse PD, Yang GZ, Nott DM, McCall JM, Firmin DN, Pennell DJ.. Interstudy reproducibility of three-dimensional volume-selective fast spin echo magnetic resonance for quantifying carotid artery wall volume. J Cardiovasc Magn Reson 2005;21:187–191. [DOI] [PubMed] [Google Scholar]
- 19. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu LY, Groves DW, Aletras AH, Kellman P, Arai AE.. A quantitative pixel-wise measurement of myocardial blood flow by contrast-enhanced first-pass CMR perfusion imaging: microsphere validation in dogs and feasibility study in humans. JACC Cardiovasc Imaging 2012;5:154–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, DiMario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJM.. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 22. Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, Burkey JL, Yang Q, Marcovina SM, Geary RS, Crooke RM, Witztum JL.. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015;386:1472–1483. [DOI] [PubMed] [Google Scholar]
- 23. Yuasa Y, Osaki T, Makino H, Iwamoto N, Kishimoto I, Usami M, Minamino N, Harada-Shiba M.. Proteomic analysis of proteins eliminated by low-density lipoprotein apheresis. Ther Apher Dial 2014;18:93–102. [DOI] [PubMed] [Google Scholar]