Abstract
In this work, we report synthesis and characterization of DenTimol, a dendrimer-based polymeric timolol analog as glaucoma medication. A timolol precursor (S)-4-[4-(oxiranylmethoxy)-1,2,5-thiadiazol-3-yl]morpholine (OTM) was reacted with the heterobifunctional amine polyethylene glycol acetic acid (Amine-PEG-Acetic Acid, Mn=2000 g/mol) via the ring opening reaction of epoxide by an amine to form OTM-PEG conjugate. OTM-PEG was then coupled to ethylenediamine (EDA) core polyamidoamine (PAMAM) dendrimer G3 to generate DenTimol using the N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS) coupling reaction. MALDI mass spectrometry, 1H NMR spectroscopy, and HPLC were applied to characterize the intermediate and final products. Ex vivo corneal permeation of DenTimol was assessed using the Franz diffusion cell system mounted with freshly extracted rabbit cornea. Cytotoxicity of DenTimol was assessed using WST-1 assay. Our results show that DenTimol is nontoxic up to an OTM equivalent concentration of 100 μM. DenTimol is efficient crossing the cornea. About 8% of the dendrimeric drug permeated through the cornea in 4 h. Its IOP-lowering effect was observed in normotensive adult Brown Norway male rats. Compared to the undosed eye, an IOP reduction by an average of 7.3 mmHg (~30% reduction from baseline) was observed in the eye topically treated with DenTimol in less than 30 min. Daily dosing of DenTimol for a week did not cause any irritation or toxicity as confirmed by the histological examination of ocular tissues including the cornea, ciliary body, and retina.
Keywords: β-blocker, 3-amino-1, 2-propanediol, nanomedicine, glaucoma, polymeric drug
TOC image
INTRODUCTION
Ocular hypertension, i.e., elevated intraocular pressure (IOP), is the characteristics of glaucoma, a chronic ocular disease that can lead to degenerative and irreparable vision loss. Although the pathophysiology leading to glaucoma is multifactorial, the immediate aim of treatment is to lower IOP back to normal levels and prevent disease progression. Pharmacologic therapies are usually the first treatment option because they are less costly and less invasive than surgical interventions. Beta-adrenergic antagonists, commonly known as β-blockers, reduce IOP by slowing the production of aqueous humor. Timolol maleate is a β-blocker developed in the 1970s.1 It selectively binds to adrenergic receptors in the ciliary body, making it a more potent IOP lowering drug than other β-blockers.2 Despite the existence of these potent IOP lowering drugs, glaucoma remains the second leading cause of blindness worldwide.3 This is because the impact of current treatments is severely limited by inefficient drug delivery formulations. For example, when timolol is delivered as a topical solution, only about 5% of the total drug at best makes it to the target organ with each dose.4 Because of this, the period after a dose where the drug is within its therapeutic concentration window in the anterior chamber is narrow, typically 4-5 hours.5, 6 The remaining volume is flushed into systemic circulation where it can cause cardiovascular side effects of varying severity.7–9
New antiglaucoma drugs are being actively pursued by targeting different therapeutic targets to acquire IOP reduction and neuroprotective benefits, for example via increasing optic nerve head blood flow or trabecular meshwork outflow.10 Like current antiglaucoma drugs or other small molecule drugs, new drugs may eventually need an enabling delivery system to achieve sustained and effective delivery and improved bioavailability.11 In our opinion, a compelling proactive approach for antiglaucoma drug development is to build molecules with a known therapeutic effect or established therapeutic potential into a polymeric backbone to form pharmacologically active polymeric drugs. While much advances have been made in the development of polymeric drugs for some diseases,12–16 it is rarely used in the rational design of antiglaucoma drugs. This approach potentially accelerates new antiglaucoma therapy development by capitalizing on benefits of polymeric drugs such as macromolecular multivalency and flexible structures allowing for accommodation of additional moieties to have desirable physicochemical properties for delivery.
Polymeric vehicles that can improve the delivery efficiency of this drug could reduce either the necessary concentration or dosing regimen, representing a significant improvement in the clinical management of glaucoma. The study of nanoparticle vehicles to improve the bioavailability of topical ocular drugs has received considerable attention in the past decade.17–19 Most drug loss from eye drops occurs due to pre-corneal mechanisms.4 Generally, most drugs penetrate the corneal epithelium slowly relative to the rate of tear drainage and turnover.20 Polyamidoamine (PAMAM) dendrimers have the potential to overcome these factors because they have appropriate size and mucoadhesiveness to both slow washout and increase cornea permeability.21, 22 PAMAM dendrimers have been complexed with other ocular therapeutics, resulting in improved drug delivery efficiency, but these methods rely on the formation of metastable dendrimer-drug complexes.23–27 In the past, however, no success has been reported in developing polymeric drugs for glaucoma.25, 28 In this work, we synthesized and characterized a dendrimer-based polymeric timolol analog (DenTimol) as a glaucoma medication. Its efficacy and safety were assessed in normotensive adult Brown Norway male rats. Developing IOP-lowering polymeric drugs is complementary to traditional small molecule antiglaucoma drug development and offers unique features by simultaneously tackling pharmacological potency and physiological barriers to delivery. It is novel to develop DenTimol as a glaucoma medication.
EXPERIMENTAL SECTION
Synthesis
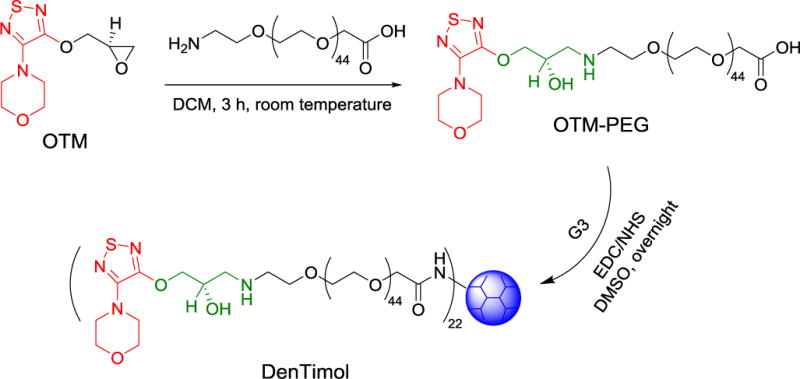
(S)-4-[4-(Oxiranylmethoxy)-1,2,5-thiadiazol-3-yl]morpholine (OTM) (Toronto Research Chemicals, Toronto, Canada, cat#O847080) was dissolved in dichloromethane (DCM) and reacted with an equimolar amount of amine polyethylene glycol acetic acid (Amine-PEG-Acetic Acid, Mn=2000 g/mol) (JenKem, Plano, TX) for 3 h at room temperature. The resulting OTM-PEG was recovered by rotary evaporation and purified by dialysis in water with a 3.5 kDa dialysis membrane and lyophilized. OTM-PEG (32 equiv.) was then reacted with EDA core polyamidoamine dendrimer generation 3.0 (G3.0) (1 equiv.) (Dendritech, Midland, MI) in DMSO overnight in the presence of a large molar excess of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS). The product DenTimol was purified by dialysis with a 7.5 kDa dialysis membrane for 48 h and lyophilized.
HPLC analysis
The reactants and products were analyzed with a Waters reverse phase HPLC system equipped with a Waters 2487 dual absorbance detector and an XTerra C18 column (4.6 mm×150 mm, particle size 5 μm). The mobile phase constituted water/acetonitrile (50:50, v/v). UV absorbance was monitored at 220 and 300 nm.
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS)
MALDI MS analysis was performed on an Applied Biosystems Voyager matrix-assisted laser desorption/ionization time-of-flight mass spectrometer. Spectra were calibrated internally for the AARS screening assay to 756.2352 (the mass of the 4-formylphenoxypropyl triphenylphosphonium AMP derivative). The reactant Amine-PEG-Acetic Acid and the intermediate OTM-PEG were dissolved in deionized water and spotted on a MALDI plate before analysis.
1H NMR spectroscopy
1H NMR spectrum of DenTimol was collected on a Bruker 600 MHz NMR spectrometer. 1H NMR (600 MHz, CD3OD): δ (ppm) 3.89 (s, 2H), 3.80–3.73 (m, 4H), 3.73–3.59 (m, 223H), 3.52 (d, J = 4.6 Hz, 2H), 3.47–3.35 (m, 5H), 3.27 (s, 3H), 3.18 (dd, J = 10.0, 4.7 Hz, 2H), 2.85 (d, J = 57.9 Hz, 9H), 2.60 (s, 3H), 2.38 (s, 7H).
Ex vivo corneal permeation study
Corneal permeability was determined using Franz diffusion cells (PermeGear, Hellertown, PA).29 Corneas were extracted from fresh rabbit eyes and placed immediately into diffusion cells with the endothelial surface facing the acceptor chamber and the epithelial surface facing the donor chamber. The acceptor chamber was filled with 5 mL of glutathione buffered Ringer’s solution, and the donor chamber with 100 μL of a solution containing 1 mg of DenTimol (3.7 mM OTM equivalent). For comparison, permeation of OTM and OTM-PEG across the cornea were also tested at the same OTM equivalent concentration. The diffusion cells were placed in a water bath at 37 °C. At various time points, 250 μL samples were withdrawn from the acceptor chamber, and drug concentrations were measured using the reverse phase HPLC system.
Cytotoxicity study
NIH 3T3 fibroblasts were seeded in a 96-well plate at 5000 cells per well and allowed 24 h to attach. The cells were maintained in DMEM medium supplemented with 10% serum, streptomycin (100 U/mL) and penicillin (100 U/mL) at 37 °C in 95% air/5% CO2. Upon the removal of the medium, the cells were incubated with fresh DMEM medium (control) or fresh medium containing various amounts of OTM, OTM-PEG, or DenTimol (n=8) for 24 h. Cell viability relative to untreated cells was determined using the WST-1 cell proliferation assay.
In vivo efficacy assessment
Normotensive adult brown Norway rats (Charles River Labs, Wilmington MA) were used for all animal experiments in this study. They were housed under proper conditions at Virginia Commonwealth University (VCU). The rats were kept under a cycle of 12-h light and 12-h dark. The animal procedures were approved by the VCU IACUC. Drug efficacy was measured in vivo by dosing brown Norway rats (n=4) in the right eye with timolol solution (2×5 μL, 0.5% w/v) or an equivalent DenTimol solution. IOP was measured using a TonoLab rebound tonometer (Icare, Finland) at various time points in both eyes. Change in IOP was referenced to the contralateral (left) eye. No anesthetics or chemical restraints were employed during measurements. All measurements were taken by the same operator at the same location using the hand corresponding to that eye. Time 0 for all experiments (and baseline IOP readings) occurred at approximately 10 a.m. The reported IOP values are the average of 18-30 individual instrument readings of each eye.
In vivo safety assessment
To assess in vivo ocular tolerance, rats received OTM, timolol, or DenTimol (2×5 μL, 0.5% w/v OTM equivalent) in the right eye once daily for seven days. At the conclusion of the experiment, the rats were euthanized. The eyes were enucleated and immediately fixed in Davidson’s solution, and 5 μm sections prepared for histological evaluation.
Statistical analysis
All the data are expressed as means ± standard deviation. Student’s t-test was performed for comparison. A value of p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Synthesis and characterization
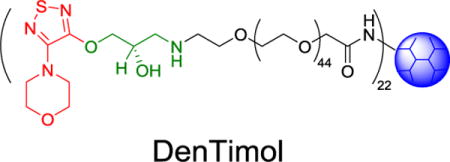
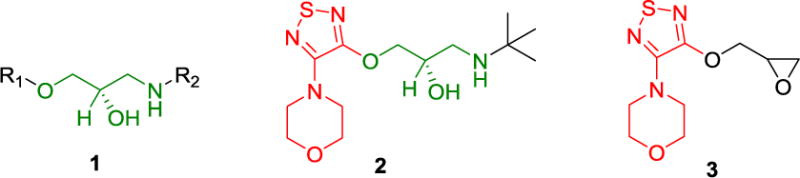
Most β-blockers contain 3-amino-1,2-propanediol (APD) (1 in Scheme 1), a key structural element responsible for antiglaucoma effects. Timolol (2) is a commonly prescribed β-blocker as a glaucoma medication. However, it lacks reactive groups that can directly conjugate with a polymer to form a polymeric antiglaucoma drug. To make a polymeric (dendrimer-based) timolol drug, we used a timolol precursor (S)-4-[4-(oxiranylmethoxy)-1,2,5-thiadiazol-3-yl]morpholine (OTM, 3),30 which has not only the same key chemical structure as timolol (highlighted in red) but also a reactive epoxy end-group for functionalization. As shown in Scheme 2, the terminal amine group of the heterobifunctional PEG spacer Amine-PEG-Acetic Acid was reacted with the epoxy of OTM to generate OTM-PEG conjugate containing an APD moiety. MALDI mass spectrometry analysis shows that m/z value is 2057.7 (Figure 1A), suggesting a successful one-to-one coupling of OTM to PEG (PEG m/z=1880.0, Figure S1). OTM-PEG was then conjugated to PAMAM dendrimer G3 via the NHS/EDC coupling reaction. HPLC analysis (Figure S2) confirmed that DenTimol was purified successfully and there was no unreacted OTM in the final conjugate. 1H NMR spectrum (Figure 1B) shows that DenTimol has both PEG and OTM on the surface, and an average of 22 OTM-PEG were coupled to dendrimer according to integrals.
Scheme 1.
Chemical structures of common β-blockers (1), timolol (2), and OTM (3). R1 and R2 in 1 stand for different groups.
Scheme 2.
Synthesis of DenTimol.
Figure 1.
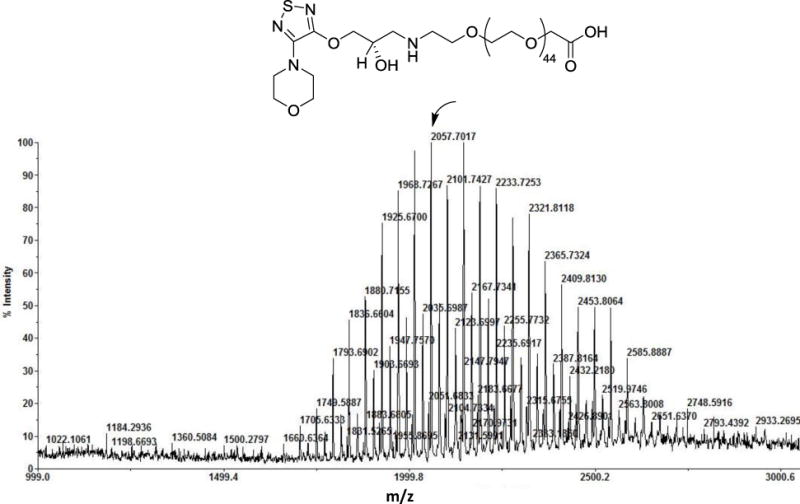
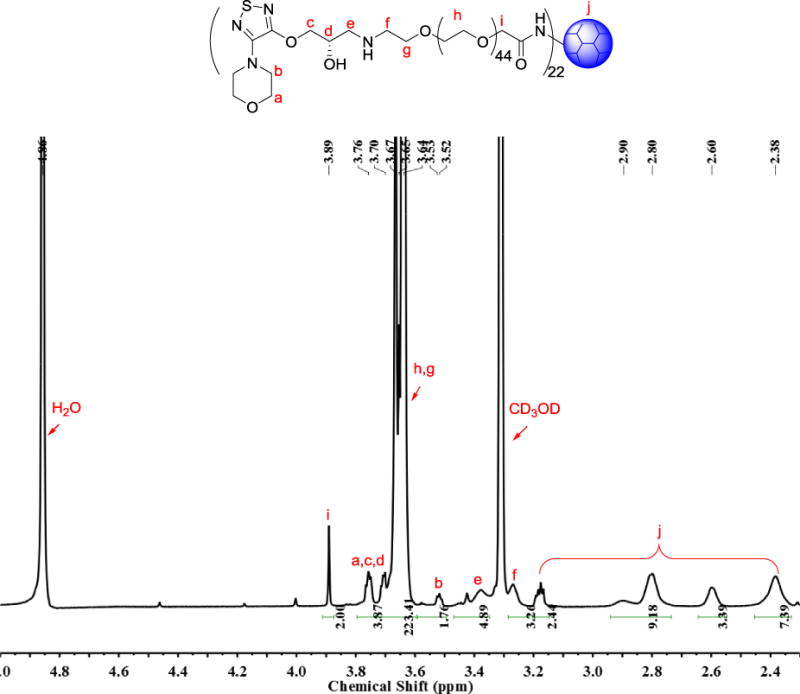
Structural characterization of the intermediate product OTM-PEG and final product DenTimol. (A) MALDI mass spectrum of OTM-PEG; (B) 1H NMR spectrum of DenTimol.
In vitro assessment
Covering a large portion of the dendrimer surface with PEG chains significantly improves dendrimer cytocompatibility.31 Indeed, our cytotoxicity results indicate that DenTimol shows no signs of cytotoxicity at the OTM equivalent concentration of 100 μM (Figure 2A). Because of the high drug payload per dendrimer, this suggests DenTimol can be more effective in maintaining therapeutic concentrations for timolol.
Figure 2.
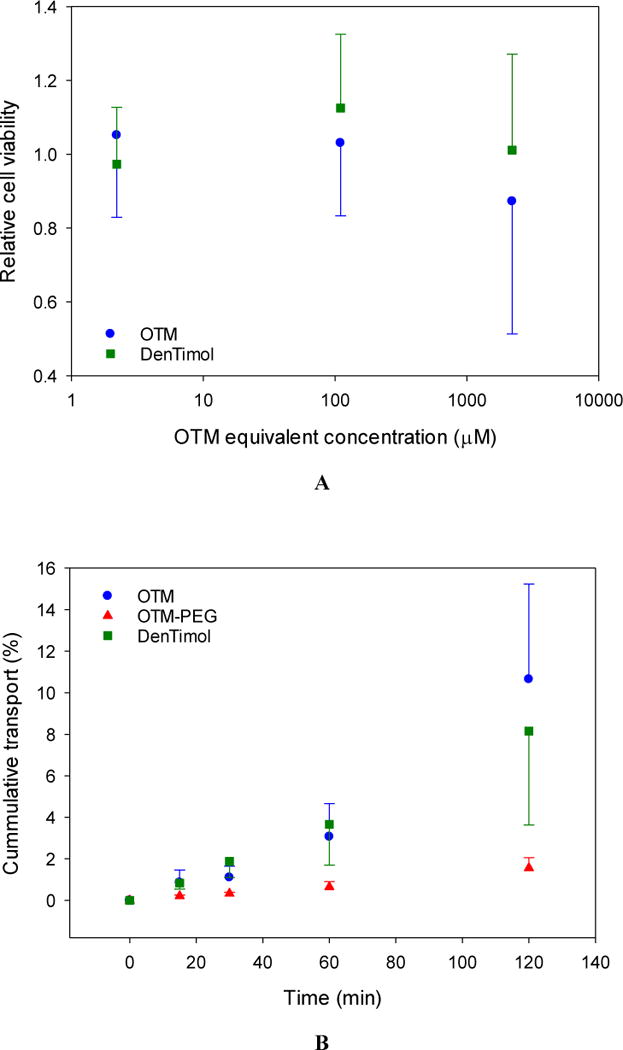
In vitro assessment of DenTimol. (A) Dose-dependent cytotoxicity of OTM and DenTimol. (B) Ex vivo permeation of OTM, OTM-PEG and DenTimol across rabbit cornea.
OTM has similar bioactivity to timolol, but it is hydrophobic and has even lower ocular bioavailability than timolol maleate.32 Coupling to a linear hydrophilic polymer such as PEG can solubilize the compound, but as our corneal permeability results show this structure does not readily penetrate the cornea (<2% in 2 h) likely due to its high hydrophilicity (Figure 2B). We found that DenTimol is efficient crossing the cornea: 8% of the drug permeated through the cornea in 4 h, which was 2.3-fold and 4-fold higher than timolol (3.5% in 4 h33) and OTM-PEG (<2% in 2 h) (Figure 2B), respectively. DenTimol’s high corneal permeation is attributed to its hydrophilic-lipophilic balance with timolol being the outermost layer. PAMAM dendrimers are also highly hydrophilic, but they are also compact and strongly cationic. This unique architecture allows them to penetrate the cornea relatively easily even when covered with drug moieties. Direct coupling of OTM to dendrimer without a spacer is theoretically possible, and would likely result in an even higher level of bioavailability.34 However, without the flexibility provided by the polymeric linker it is likely bioactivity of each prodrug moiety would be reduced, either due to steric hindrance from the dendrimer, or folding in of the dendrimer end groups towards the core of the molecule.35
In vivo efficacy and safety assessment
One-time topical application of DenTimol (10 μl of 0.5% w/v timolol) in normotensive adult Brown Norway male rats resulted in an IOP reduction by an average of 7.3 mmHg (~30%) in less than 30 min, which was significantly stronger than timolol PBS eye drops (Figure 3) (both showed a peak effect in less than 30 min). The repeated application of DenTimol did not show any signs of toxicity or ocular irritation in vivo. H&E stained sections of ocular tissues confirmed that repeated dosing of DenTimol, OTM, or OTM-PEG did not alter the structures of the cornea, ciliary body, and retina (Figure 4).
Figure 3.
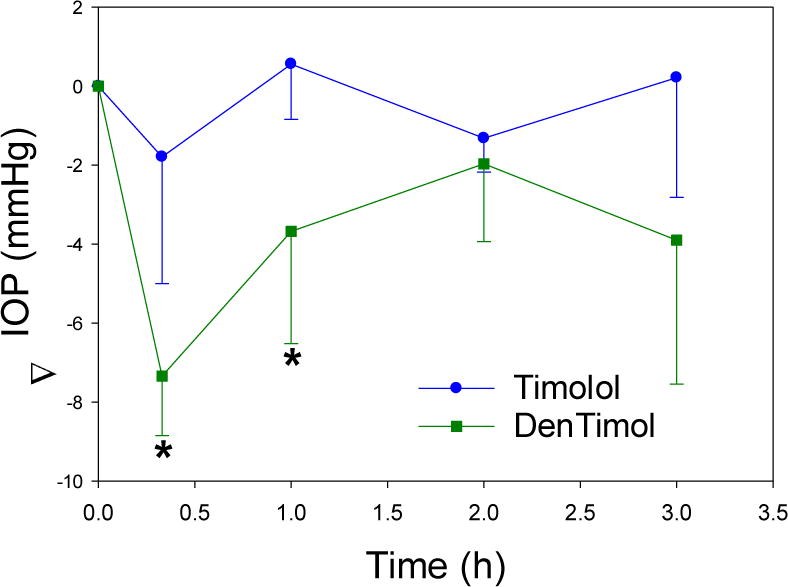
In vivo IOP-lowering assessment of DenTimol in normotensive rats after one-time topical administration.* indicates p<0.05 vs. timolol PBS eye drops.
Figure 4.
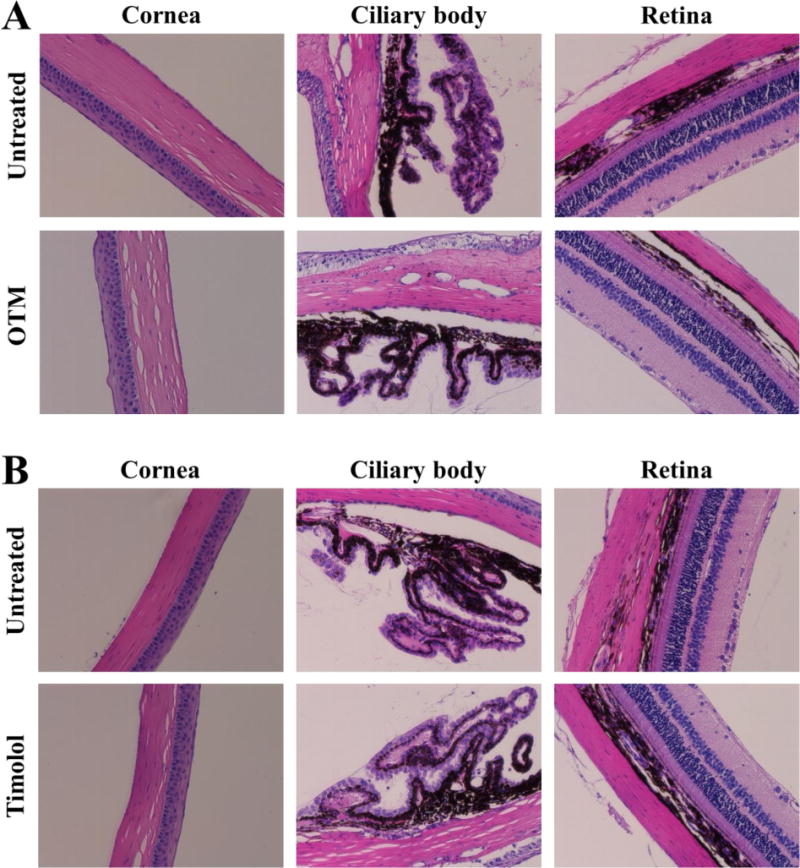
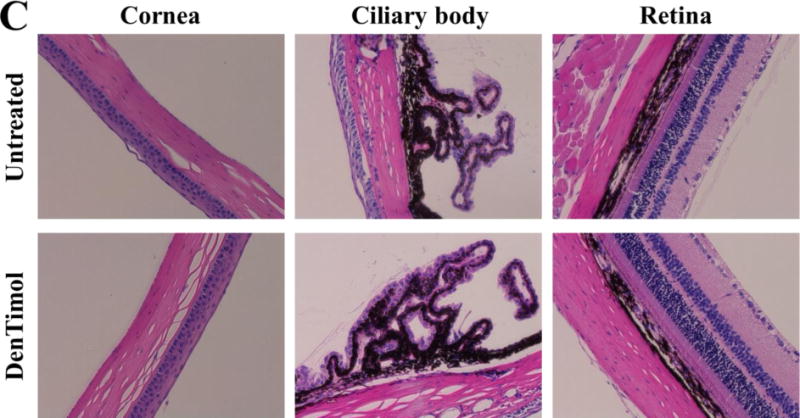
Ocular tissues stained with H&E following chronic application of OTM (A), timolol (B), or DenTimol (C) (magnification 200×).
Although the use of fully awake animals provides a more philologically relevant model, it produces more noise in the measurement of pressures by rebound tonometry. This noise makes it unclear at this point if potency is increased over timolol. The IOP response profile observed for DenTimol is roughly equivalent to literature reported values for timolol in rodents.36 The effect of the chronic application has also not yet been studied. Clinical IOP lowering treatments rely on an additive effect of repeated drug dosing. We expect, at minimum, for this phenomenon to be present with DenTimol treatment if not greater due to the mucoadhesive quality of the dendrimeric drug. Future work will be focused on building a complete pharmacokinetic profile for DenTimol. The efficacy and safety of DenTimol will be validated in animal models of glaucoma. We believe DenTimol can extend the therapeutic window for timolol sufficiently to reduce dosing frequency to once a day or less. DenTimol has three distinct structural features: 1) it retains the effective structure of timolol, thus gaining the potency as an antiglaucoma drug; 2) the incorporation of PEG spacer can enhance the biocompatibility and water solubility of the new polymeric drug; 3) DenTimol is a novel self-transporting “smart” drug as PAMAM dendrimers can serve as a powerful ocular drug delivery vehicle with adaptable features and cornea transport into in the anterior chamber once applied to the eye.
CONCLUSIONS
In this work, we developed DenTimol, a dendrimer-based polymeric timolol analog as glaucoma medication. DenTimol is highly water soluble and shows no signs of toxicity or ocular irritation in vitro or in vivo. We observed its IOP-lowering effect, supporting us to fully examine its PK/PD and study how to use the multivalency of dendrimers to improve its potency and safety. Optimization of drug loading and spacer length may further improve its IOP-lowering effect. Such studies will be conducted in future work.
Supplementary Material
Acknowledgments
This work was supported, in part, by the National Institutes of Health (R01EY024072). The facility is supported, in part, by funding from the NIH-NCI Cancer Center Support Grant P30CA016059.
Footnotes
Supporting Information
ORCID
Hu Yang: 0000-0003-3030-004X
Notes
The authors declare no competing interest.
REFERENCES CITED
- 1.Franciosa JA, Freis ED, Conway J. Antihypertensive and hemodynamic properties of the new beta adrenergic blocking agent timolol. Circulation. 1973;48(1):118–24. doi: 10.1161/01.cir.48.1.118. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman TJ, Kaufman HE. Timolol. A beta-adrenergic blocking agent for the treatment of glaucoma. Arch Ophthalmol. 1977;95(4):601–4. doi: 10.1001/archopht.1977.04450040067008. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British journal of ophthalmology. 2006;90(3):262. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies NM. Biopharmaceutical considerations in topical ocular drug delivery. Clinical and experimental pharmacology and physiology. 2000;27(7):558. doi: 10.1046/j.1440-1681.2000.03288.x. [DOI] [PubMed] [Google Scholar]
- 5.Coakes RL, Brubaker RF. The mechanism of timolol in lowering intraocular pressure. In the normal eye. Arch Ophthalmol. 1978;96(11):2045–8. doi: 10.1001/archopht.1978.03910060433007. [DOI] [PubMed] [Google Scholar]
- 6.Büker E, Dinç E. A New UPLC Method with Chemometric Design-Optimization Approach for the Simultaneous Quantitation of Brimonidine Tartrate and Timolol Maleate in an Eye Drop Preparation. J Chromatogr Sci. 2017;55(2):154–161. doi: 10.1093/chromsci/bmw160. [DOI] [PubMed] [Google Scholar]
- 7.Nieminen T, Lehtimäki T, Mäenpää J, Ropo A, Uusitalo H, Kähönen M. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand J Clin Lab Invest. 2007;67(2):237–45. doi: 10.1080/00365510601034736. [DOI] [PubMed] [Google Scholar]
- 8.Rana MA, Mady AF, Rehman BA, Alharthy A, Huwait B, Riaz A, Aletreby WT. From Eye Drops to ICU, a Case Report of Three Side Effects of Ophthalmic Timolol Maleate in the Same Patient. Case Rep Crit Care. 2015;2015:714919. doi: 10.1155/2015/714919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korte JM, Kaila T, Saari MK. Systemic bioavailability and cardiopulmonary effects of 0.5% timolol eyedrops. Graefe’s archive for clinical and experimental ophthalmology. 2002;240(6):430. doi: 10.1007/s00417-002-0462-2. [DOI] [PubMed] [Google Scholar]
- 10.Donegan RK, Lieberman RL. Discovery of Molecular Therapeutics for Glaucoma: Challenges, Successes, and Promising Directions. J Med Chem. 2016;59(3):788–809. doi: 10.1021/acs.jmedchem.5b00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheybani ND, Yang H. Pediatric ocular nanomedicines: Challenges and opportunities. Chin Chem Lett. 2017;28(9):1817–1821. doi: 10.1016/j.cclet.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K, Yang PP, Zhang JP, Hao LW. Recent advances of transformable nanoparticles for theranostics. Chinese Chemical Letters. 2017;28(9):1808–1816. [Google Scholar]
- 13.Qi X, Qin J, Fan Y, Qin X, Jiang Y, Wu Z. Carboxymethyl Chitosan-Modified Polyamidoamine Dendrimer Enables Progressive Drug Targeting of Tumors via pH-Sensitive Charge Inversion. J Biomed Nanotechnol. 2016;12(4):667–78. doi: 10.1166/jbn.2016.2206. [DOI] [PubMed] [Google Scholar]
- 14.Qu Y, Chu B, Shi K, Peng J, Qian Z. Recent Progress in Functional Micellar Carriers with Intrinsic Therapeutic Activities for Anticancer Drug DeliveryRecent Progress. J Biomed Nanotechnol. 2017;13(12):1598–1618. doi: 10.1166/jbn.2017.2475. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Zhu J, Chu B, Chen L, Shi K, Zhang L, Chen X, Qian Z. Preparation, Characterization and In Vivo Antitumor Evaluation of a Micellar Formulation of Camptothecin Prodrug. Nanoscience and Nanotechnology Letters. 2017;9(11):1755–1766. [Google Scholar]
- 16.Li J, Yu F, Chen Y, Oupický D. Polymeric drugs: Advances in the development of pharmacologically active polymers. J Control Release. 2015;219:369–382. doi: 10.1016/j.jconrel.2015.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Advanced Drug Delivery Reviews. 1995;16(1):61. [Google Scholar]
- 18.Diebold Y, Calonge M. Applications of nanoparticles in ophthalmology. Progress in retinal and eye research. 2010;29(6):596. doi: 10.1016/j.preteyeres.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Almeida H, Amaral MH, Lobao P, Frigerio C, Sousa Lobo JM. Nanoparticles in Ocular Drug Delivery Systems for Topical Administration: Promises and Challenges. Curr Pharm Des. 2015;21(36):5212–24. doi: 10.2174/1381612821666150923095155. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim MM, Abd-Elgawad AH, Soliman OA, Jablonski MM. Natural Bioadhesive Biodegradable Nanoparticle-Based Topical Ophthalmic Formulations for Management of Glaucoma. Transl Vis Sci Technol. 2015;4(3):12. doi: 10.1167/tvst.4.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravo-Osuna I, Woodward A, Argueso P, Martínez ITM, Gómez R, de la Mata FJ, Navarro MM, Noiray M, Ponchel G, Herrero-Vanrell R. Linear Polymers Versus PAMAM Dendrimers In The Interaction With Transmembrane Ocular Mucins: Analysis By Biosensor Technology. Investigative ophthalmology & visual science. 2012;53(14):1845. [Google Scholar]
- 22.Kannan R, Nance E, Kannan S, Tomalia D. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. Journal of Internal Medicine. 2014;276(6):579–617. doi: 10.1111/joim.12280. [DOI] [PubMed] [Google Scholar]
- 23.Yao W, Sun K, Mu H, Liang N, Liu Y, Yao C, Liang R, Wang A. Preparation and characterization of puerarin–dendrimer complexes as an ocular drug delivery system. Drug development and industrial pharmacy. 2010;36(9):1027. doi: 10.3109/03639041003610799. [DOI] [PubMed] [Google Scholar]
- 24.Yavuz B, Pehlivan SB, Vural I, Unlu N. In Vitro/In Vivo Evaluation of Dexamethasone–PAMAM Dendrimer Complexes for Retinal Drug Delivery. J Pharm Sci. 2015;104(11):3814–23. doi: 10.1002/jps.24588. [DOI] [PubMed] [Google Scholar]
- 25.Kambhampati SP, Mishra MK, Mastorakos P, Oh Y, Lutty GA, Kannan RM. Intracellular delivery of dendrimer triamcinolone acetonide conjugates into microglial and human retinal pigment epithelial cells. European Journal of Pharmaceutics and Biopharmaceutics. 2015;95:239–249. doi: 10.1016/j.ejpb.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra V, Jain NK. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int J Pharm. 2014;461(1–2):380–90. doi: 10.1016/j.ijpharm.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 27.Lancina MG, Singh S, Kompella UB, Husain S, Yang H. Fast Dissolving Dendrimer Nanofiber Mats as Alternative to Eye Drops for More Efficient Antiglaucoma Drug Delivery. ACS Biomater Sci Eng. 2017;3(8):1861–1868. doi: 10.1021/acsbiomaterials.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lancina M, Yang H. Dendrimers for ocular drug delivery. Canadian Journal of Chemistry. 2017;95(9):897–902. doi: 10.1139/cjc-2017-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Williamson G, Lancina M, Yang H. Mildly Cross-Linked Dendrimer Hydrogel Prepared via Aza-Michael Addition Reaction for Topical Brimonidine Delivery. Journal of Biomedical Nanotechnology. 2017;13(9):1089–1096. doi: 10.1166/jbn.2017.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bredikhin AA, Zakharychev DV, Gubaidullin AT, Fayzullin RR, Pashagin AV, Bredikhina ZA. Crystallization Features of the Chiral Drug Timolol Precursor: The Rare Case of Conglomerate with Partial Solid Solutions. Crystal Growth & Design. 2014;14(4):1676–1683. [Google Scholar]
- 31.Wang W, Xiong W, Wan J, Sun X, Xu H, Yang X. The decrease of PAMAM dendrimer-induced cytotoxicity by PEGylation via attenuation of oxidative stress. Nanotechnology. 2009;20(10):105103. doi: 10.1088/0957-4484/20/10/105103. [DOI] [PubMed] [Google Scholar]
- 32.Pech B, Duval O, Richomme P, Benoit J. A timolol prodrug for improved ocular delivery: Synthesis, conformational study and hydrolysis of palmitoyl timolol malonate. International Journal of Pharmaceutics. 1996;128(1–2):179–188. [Google Scholar]
- 33.Holden CA, Tyagi P, Thakur A, Kadam R, Jadhav G, Kompella UB, Yang H. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine. 2012;8(5):776–83. doi: 10.1016/j.nano.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Sk UH, Kambhampati SP, Mishra MK, Lesniak WG, Zhang F, Kannan RM. Enhancing the efficacy of Ara-C through conjugation with PAMAM dendrimer and linear PEG: a comparative study. Biomacromolecules. 2013;14(3):801–10. doi: 10.1021/bm3018615. [DOI] [PubMed] [Google Scholar]
- 35.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug discovery today. 2010;15(5):171. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Akaishi T, Odani-Kawabata N, Ishida N, Nakamura M. Ocular hypotensive effects of anti-glaucoma agents in mice. J Ocul Pharmacol Ther. 2009;25(5):401–8. doi: 10.1089/jop.2009.0006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


